Abstract
A thyroid cancer ultrasonography screening for all residents 18 years old or younger living in the Fukushima prefecture started in October 2011 to investigate the possible effect of the radiological contamination after the Fukushima Daiichi Nuclear Power Plant accidents as of March 12 to 15, 2011. Thyroid cancer in 184 cases was reported by February 2017. The question arises to which extent those cancer cases are a biological consequence of the radiation exposure or an artefactual result of the intense screening of a large population.
Experiences with the Chernobyl accident suggest that the external dose may be considered a valid surrogate for the internal dose of the thyroid gland. We, therefore, calculated the average external effective dose-rate (μSv/h) for the 59 municipalities of the Fukushima prefecture based on published data of air and soil radiation. We further determined the municipality-specific absolute numbers of thyroid cancers found by each of the two screening rounds in the corresponding municipality-specific exposed person-time observed. A possible association between the radiation exposure and the thyroid cancer detection rate was analyzed with Poisson regression assuming Poisson distributed thyroid cancer cases in the exposed person-time observed per municipality.
The target populations consisted of 367,674 and 381,286 children and adolescents for the 1st and the 2nd screening rounds, respectively. In the 1st screening, 300,476 persons participated and 270,489 in the 2nd round. From October 2011 to March 2016, a total of 184 cancer cases were found in 1,079,786 person-years counted from the onset of the exposure to the corresponding examination periods in the municipalities. A significant association between the external effective dose-rate and the thyroid cancer detection rate exists: detection rate ratio (DRR) per μSv/h 1.065 (1.013, 1.119). Restricting the analysis to the 53 municipalities that received less than 2 μSv/h, and which represent 176 of the total 184 cancer cases, the association appears to be considerably stronger: DRR per μSv/h 1.555 (1.096, 2.206).
The average radiation dose-rates in the 59 municipalities of the Fukushima prefecture in June 2011 and the corresponding thyroid cancer detection rates in the period October 2011 to March 2016 show statistically significant relationships.
Keywords: children and adolescents, ecological study, exposed person-time, ionizing radiation, radiation induced health effects
1. Introduction
A large amount of radioactive material was released after the Fukushima Daiichi Nuclear Power Plant accidents. The activity emitted is estimated to be 900 pBq (131-I: 500 pBq, 137-Cs: 10 pBq). Compared to the Chernobyl accident, the discharged quantities of 131-I and 137-Cs are 10% and 30%, respectively.[1]
The sensitivity of the thyroid to radiation was already known since the atmospheric nuclear weapons tests, for example by studies in the Marshall islanders affected by the fallout from the atomic bombing of the Bikini Atoll,[2] or after low-dose X-ray exposure in children treated for tinea capitis with about 90mSv thyroid dose.[3–6] The association between the radiation exposure after the Chernobyl nuclear power plant accident and the prevalence or incidence of thyroid cancer has been investigated by many ecological, cohort, and case control studies. Numerous investigations show the existence of distinct positive dose-response relationships between the radiation exposure and the presence or the occurrence of thyroid cancer.[7–24] Nagataki and Yamashita describe the historical evolution of the thinking about thyroid cancer and the causal role of radiation, from the initial conviction that there can be no (statistically detectable) radiation-induced Chernobyl health effects, to the currently recognized causal link between Chernobyl fallout and thyroid cancer.[25] Consequently, thyroid cancer was expected to increase after the nuclear power plant accidents in Fukushima.
1.1. The Fukushima Health Management Survey (FHMS)
A thyroid sonography screening for children and adolescents living in Fukushima Prefecture at the time of the accident began in October 2011. For a detailed description of the FHMS see Akiba.[26] The FHMS is organized in 4 rounds. The data of the 1st and the 2nd round will be scrutinized and analyzed in the present investigation. The third and the fourth rounds of examinations are in progress, but because these are not yet finished, and since less spatially differentiated protocols compared to the previous screenings are being employed, they will be excluded from our investigation.[27,28]
1.2. Epidemiological assessments of the FHMS
In the interim summary of the Prefectural People's Health Council in March 2016, the observed thyroid cancer prevalence in the Fukushima prefecture was roughly quantified as to be “an order several tens of times as high as the prevalence estimated from thyroid cancer prevalence statistics”.[29] However, in the same summary, regarding the relationship between radiation exposure and thyroid cancer, it is claimed that “because the radiation dose is considered to be less than that of Chernobyl at that time, it cannot be made a decision on the relationship between radiation exposure and thyroid cancer. And there is the opinion that the excess of thyroid cancer would be due to over-diagnosis”.[29] We emphasize that the presumption of possible ‘over-diagnosis’ in this summary report has not been substantiated with data or references.
Several studies evaluated the thyroid cancer data of the FHMS. Tsuda et al analyzed the 1st and the 2nd round by internal and external comparisons;[30] they observed a 50-fold increase of the thyroid cancer incidence in several contaminated areas compared to the national annual incidence and a difference in the prevalence by area in the Fukushima prefecture. Therefore, Tsuda et al provided evidence for a causal relation between increased thyroid cancer incidence and the nuclear catastrophes. For the debate following the publication by Tsuda et al see[31–40] as well as the rejoinder by Tsuda et al.[41]
Suzuki et al[42,43] questioned the association between the increased incidence of thyroid cancer and the nuclear accidents on the basis of a presumed ‘screening effect’. Ahn et al provide an interesting example of a strong screening effect.[44] Katanoda et al[45] questioned the correlation between cancer and radioactivity on the account of ‘over-diagnosis’. Suzuki, Katanoda, and colleagues claim no relation between the prevalence of thyroid cancer and the external exposure assessed by a self-reported questionnaire in the FHMS. However, these reports do not exhaust the available information sufficiently. In particular, the observed spatially and temporally structured person-time (from the onset of the exposure to the municipality-specific examination periods) as an appropriate reference parameter for the observed cancer cases in the municipalities was not determined and accounted for.
1.3. The objective of the present investigation
As a refinement of previously published analyses, the aim of our study is to estimate the detection rate of thyroid cancer in the screenings after the Fukushima nuclear power plant accidents for each municipality using the screened cancer cases in the municipalities as Poisson distributed events and the corresponding observed approximate municipality-specific exposed person-time as the reference quantity to examine a possible association between the thyroid cancer detection rate and the effective dose-rate. The null hypothesis is ‘no association between the external effective dose-rate and the thyroid cancer detection rate’. If the null hypothesis can be rejected, this can be interpreted as evidence of a causal effect of radiation exposure.
2. Methods
2.1. Study population and medical examination
The health effect information used in the present investigation is exclusively confined to data published by the Fukushima Health Management Survey.[28,46,47,48] The first round of the thyroid cancer screening was titled ‘Preliminary Baseline Survey’ and was conducted from October 2011 to March 2014 targeting at all 367,674 Fukushima residents born from April 2, 1992 to April 1, 2011. There were 300,476 (81.7%) participants in the first round. The first round began in the most contaminated area closest to the nuclear power plant at October 9, 2011 in the FY 2011, see dark area in Figure 1. In the FY 2012, the screening was performed mainly in the central Fukushima prefecture, see medium gray level area in Figure 1; and in the FY 2013, the inland municipalities relatively far from the nuclear power plant have been covered, see light area in Figure 1.
Figure 1.
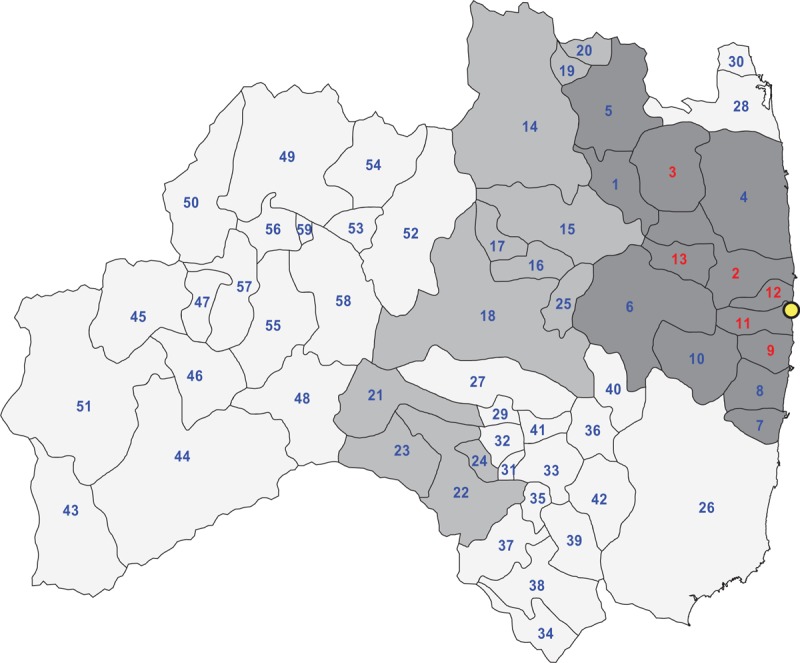
Target municipalities (n = 59) of the 1st round of the Fukushima Health Management Survey partitioned into 3 organizational strata; dark: FY 2011 area, No. 1–13; medium: FY 2012 area, No. 14–25; light: FY 2013 area, No. 26–59; the red indexed municipalities are subject to an average dose-rate greater than 2.0 μSv/h; see Table 1 for a list of the municipalities by consecutive number; http://fmu-global.jp/?wpdmdl=1563.
The second round, which was reported by Fukushima Prefecture as a ‘First Full-Scale Screening Program’, began in April 2014. This round of examinations comprised in principle all residents/participants from the first round and added children born April 2, 2011 to April 1, 2012. It was labeled ‘Full-Scale Survey’. The second round was aimed at 381,286 residents and was carried out from May 2014 to March 2016. There were 270,489 (70.9%) participants in the second round.[49,50] The first screening of the second round was conducted in the two areas of the first round ‘FY 2011 area’ and ‘FY 2012 area’ combined and carried out in the FY 2014. Similarly, the first round ‘FY 2013 area’ was covered as the ‘FY 2015 area’ in the second round. The first screening of this area was scheduled from May 2015 to June 2016.[47]
The diagnostic procedure was performed in 2 steps: a ‘Primary Examination’ was followed by a ‘Confirmatory Examination’. Participants with abnormalities such as a nodule with a diameter of 5.1 mm or more and cysts with a diameter of 20.1 mm or more at the ‘Primary Examination’ were sent to the ‘Confirmatory Examination’ where they were subjected to a precise echography, and if judged necessary, to a fine needle aspiration cytology (FNAC), see Figure 2.[28] The most suspicious or malignant cases as a result of the FNAC underwent surgery. Of those who underwent surgery, 1 person was excluded from the analysis since her tumor was benign, although the initial needle biopsy was positive. Nevertheless, all patients with suspicious or malignant status who did not undergo surgery were counted as cancer. Eventually, as reported at the 26th survey meeting on February 20, 2017, 184 cancer cases were detected, 115 in the 1st round and 69 in the 2nd round. Ethics approval and consent to participate are not required, since only publicly available data and previously published information is being used.
Figure 2.
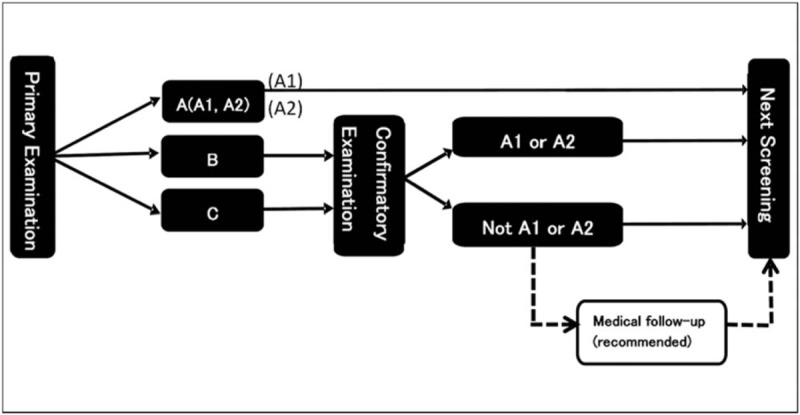
Flow chart of the thyroid cancer examination in the FHMS.[28] Decision criteria: A1: no nodules or cysts; A2: nodules ≤5.0 mm or cysts ≤20.0 mm; B: nodules ≥ 5.1 mm or cysts ≥ 20.1 mm, some A2 test results may be re-classified as B results when clinically indicated; C: need for immediate confirmed examination.
2.2. Induction and latent period, point prevalence, incidence proportion and incidence rate, and detection rate
Induction period means the time from causal action to disease occurrence and latent period is the time from disease occurrence to detection.[51] In children and especially in newborns, minimum induction and latent periods for cancer can be rather short or may approach even zero. Certain types of cancers (lymphoproliferative and hematopoietic) can be present at birth or may occur shortly after birth.[52] Beach and Dolphin[53,54] and Schmitz-Feuerhake et al[55] provide data and evidence that the combined minimum induction and latent periods for thyroid cancer in children are in the order of magnitude of 1 year. In principle, the development of uncontrolled cell proliferation may start immediately after mutagenic or carcinogenic exposure (causal action). De Groot et al state “The data suggest that the abnormality induced by radiation occurs at or close to the time of original irradiation, and that carcinoma is detected earlier than benign lesions”.[56] More recently, John Howard compiled findings on ‘Minimum Latency & Types or Categories of Cancer’.[57] For thyroid cancers in children and adults he reports (average) minimum latency periods of 1.0 and 2.5 years, respectively.
Point prevalence is the proportion of a population with a disease at a specified time and incidence proportion is defined as the proportion of a closed population at risk that becomes diseased within a specified period of time.[51] Proper estimation of point prevalence means exclusion of newly diseased (i.e., incident) cases during the study period and accurate estimation of incidence proportion means exclusion of the cases already diseased (i.e. prevalent cases) at the onset of the study. In a strict sense, therefore, the FHMS is not suited for estimating the point prevalence or the incidence proportion[58] since there is no means of separating prevalent and incident cases based on one single medical examination per participant and screening round.
The problem of not being able to accurately distinguish between prevalent and incident cancer cases is frequently encountered in cancer screenings. In the literature on repeated screenings often ‘prevalence’ is used for the first screening round and ‘incidence’ is employed to the subsequent screenings.[59–61] As an alternative, and as prevalence and incidence cases cannot be separated, several authors consistently use the term ‘detection rate’ for both the first and subsequent screenings.[62–65] For the FHMS, it suggests itself to consider the detection rate of thyroid cancer using the screened cancer cases in each municipality as Poisson distributed events. The natural logarithm of the municipality-specific average person-time since the exposure (March 11, 2011) to the date of the medical examination defines the reference parameter (offset). Therefore, the detection rate (DR) quantifies the thyroid cancers detected in the corresponding exposed person-time observed. A possible relationship between the rate of detection of thyroid cancer and the effective dose rate can then be analyzed using Poisson regression and this relationship is expressed and estimated by the corresponding detection rate ratio (DRR) per μSv/h.
Evidence for the assumption of very rare thyroid cancers in un-exposed children is provided by several authors.[20,30,66,67] If it is true that only few thyroid cancer cases would have occurred in Fukushima without the nuclear accidents, then our detection rate would be conceptually identical to the incidence rate. We, however, prefer the term ‘detection rate’ over ‘incidence rate’ to indicate that the accurate point prevalence of thyroid cancer in Fukushima prior to the nuclear accidents is not known.
2.3. Person-time observed
The base population (among those aged 0–18 years) for the determination of the thyroid cancer detection rate is the cumulative group of the individual subjects by municipality who had undergone thyroid ultrasonography reported at the 26th survey meeting on February 20, 2017. For each of the 59 municipalities in the Fukushima prefecture, the person-time observed (PTO) was calculated as follows: We set the basic initial observation time from March 11, 2011 (T0), when the nuclear accidents were triggered, until the begin of the period when the thyroid ultrasonography in each municipality and screening round (r) was started (Si,r i = 1 to 59, r = 1 or r = 2, see Fig. 3). This basic period is extended by one quarter of the length of the interval between the start date Si,r and the first round-specific summary date t1,r in round r = 1 and in round r = 2. Similarly, the collective average observation time for any municipality (mi, i = 1 to 59) is incremented step by step by one quarter of the respective length of the consecutive summary date intervals of the FHMS. These incremental time periods are multiplied by the corresponding incremental number of cumulative participants reported in the respective FHMS meetings. Finally, these quantities are totaled to obtain the observed approximate municipality-specific and round-specific exposed person-time in years (PTOi,r). The detection rate is computed by dividing the screened or observed thyroid cancer cases by the person-years for all municipalities and for the 2 rounds. The combined person-years of the both rounds together result from incrementing the person-years in round 2 by the (small) fraction of the person-years in round 1 corresponding to participants in round 1, who did not participate in round 2. The combined person-years total is 1.045 times the person-years total in round 2. See Tables 1 and 2 for a list of the resulting cumulative participants, the exposed person-years observed, and the detection rates per 100,000 for the 2 screening rounds and the screening rounds combined. Note that the number of the incremental cumulative participants is zero while the summary date is not beyond the starting date in any municipality. The rationale behind this approach is the following: whereas we do not know the precise examination date of the individual participants in the FHMS, we, nevertheless, know this date up to an accuracy of approximately 90 days, since the medium interval length between the reported summary dates in round 1 and round 2 combined is precisely 91 days. Assuming an approximate natural exponential probability density of the participants’ time-to-examination within 2 successive summary dates, which density approaches zero at the end of the observed period of ca. 90 days, implies that the mean of this incremental observation time is assumed at 90/4 = 22.5 days, which is one quarter of the observed period of 90 days. In short, the average incremental person time in the period between two reporting dates (in any municipality) is approximately 1 quarter of that period-length. Note that this approach is rather robust, in as much as realistic alternative assumptions about the location of the mean examination date between two consecutive summary dates do not change our findings in principal. Altogether, our municipality-specific and round-specific framework and the formula for the determination of the exposed person-time observed (PTOi,r) are summarized in the following box:
T0 March 11, 2011, when the nuclear accidents were triggered
Si,r start of the FHMS in municipality mi i = 1 to 59 of round r = 1 or r = 2, see Figure 3
tj,r j = 1 to kr dates of the FHMS report summaries r = 1 or 2 and k1 = 16 and k2 = 11
ni,j,r cumulative number of participants for mi, at summary date tj,r j = 1 to kr of round r = 1 or r = 2
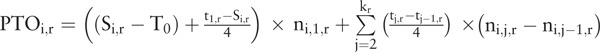
Figure 3.
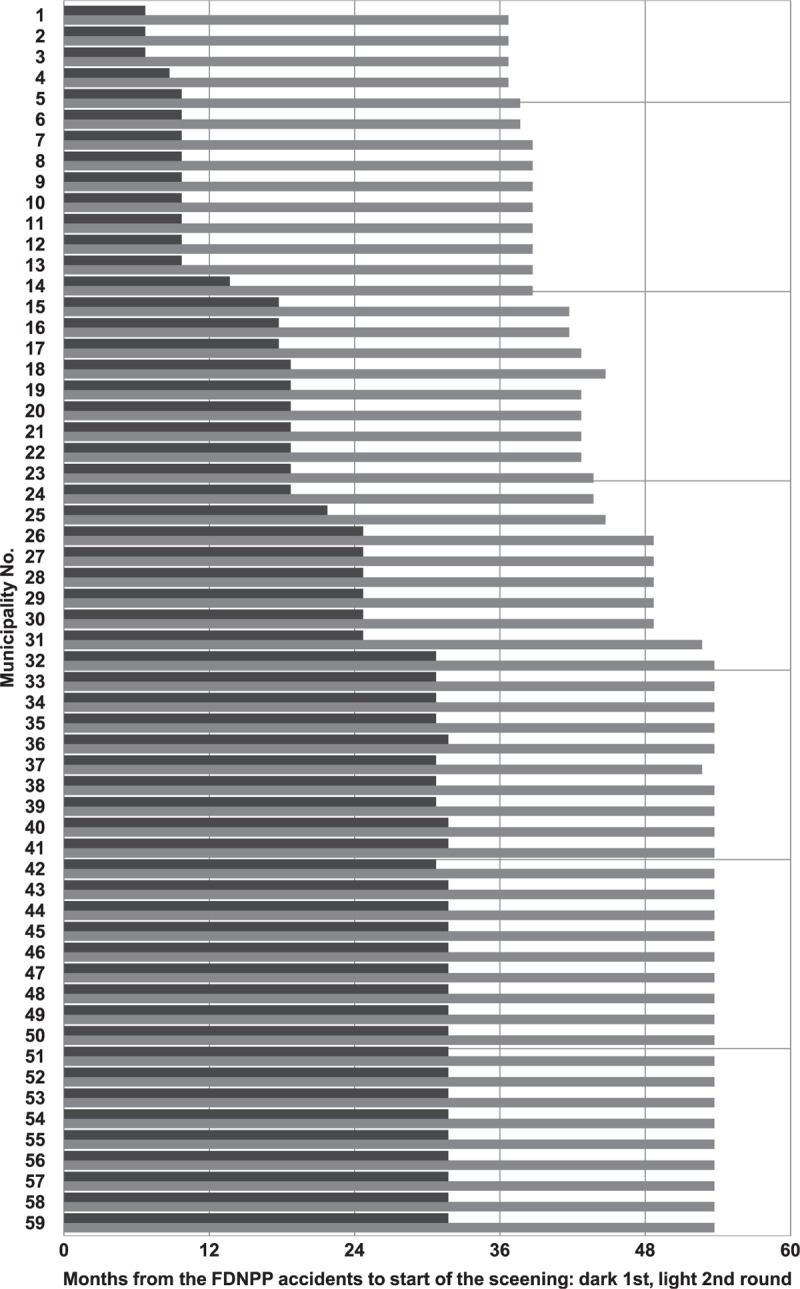
Time schedule of the 1st and the 2nd thyroid cancer screenings in units of 1 month relative to the date of the beginning of the nuclear accidents in the FDNPP in March 11, 2011; dark: 1st screening round, light: 2nd screening round; see Table 1 for the municipalities by index number.
Table 1.
Target population, participants, and the numbers of thyroid cancer cases for the 1st and the 2nd rounds of the TC screening in the 59 municipalities of the Fukushima prefecture, October 2011 to December 2016.
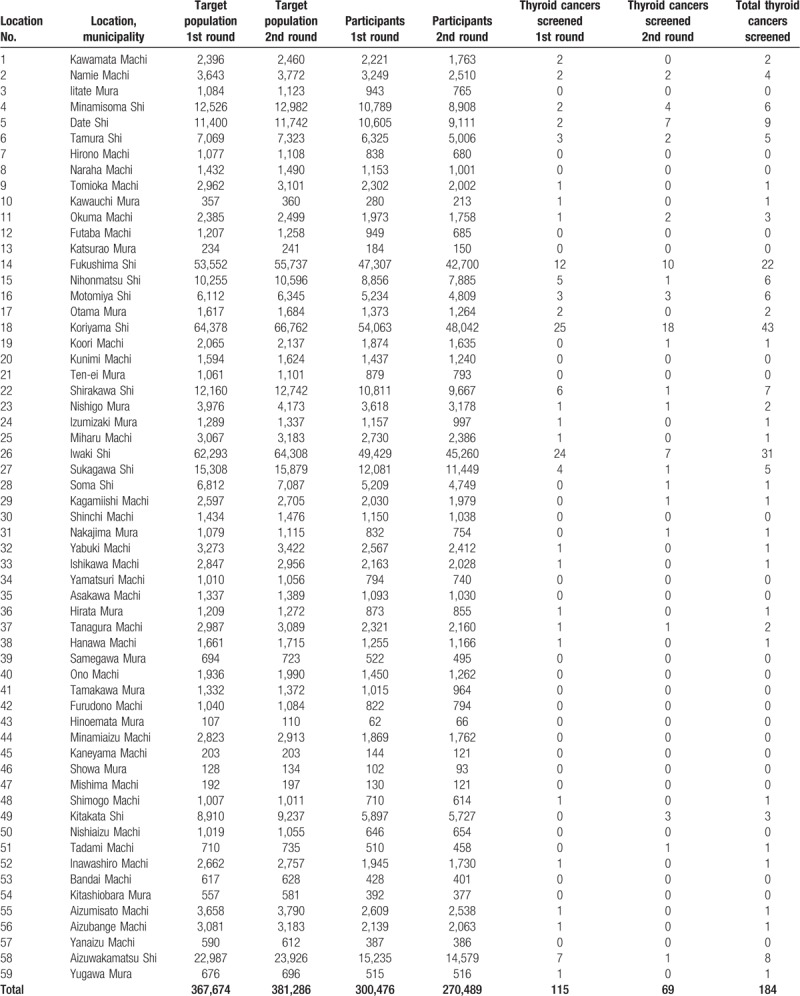
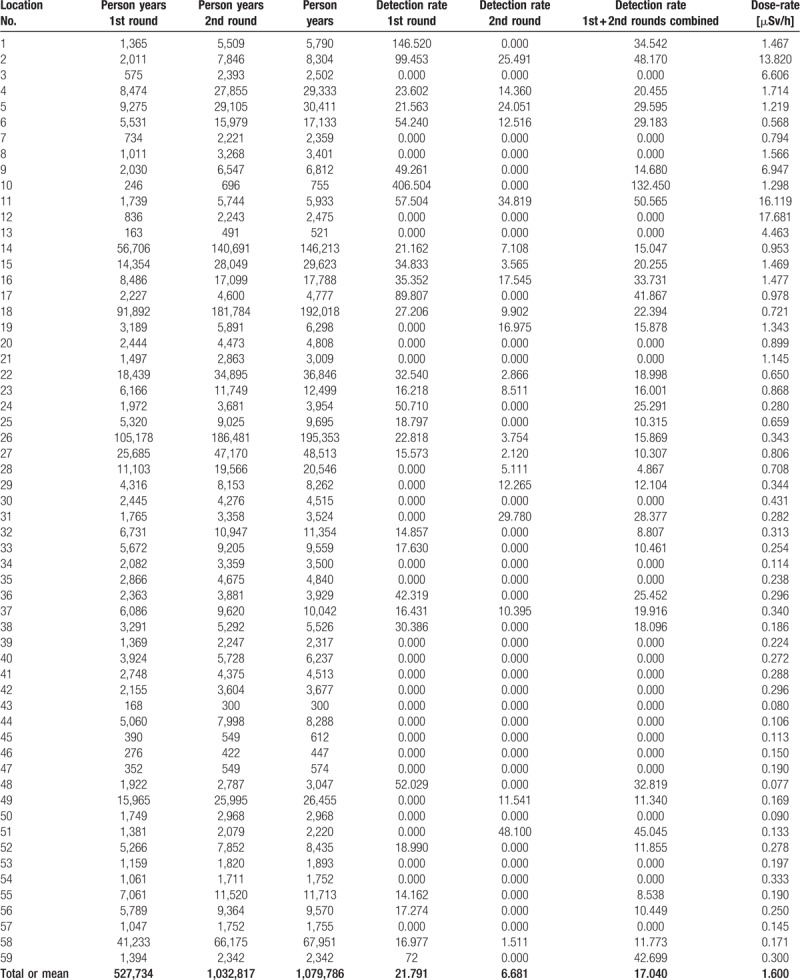
Table 2.
Person-time observed and thyroid cancer detection rate (100,000) for the 1st and the 2nd rounds of the TC screening and for both rounds combined in the 59 municipalities of the Fukushima prefecture, October 2011 to December 2016; average dose-rates [μSv/h] in the municipalities based on overall 1710 positive measurements in the Fukushima prefecture in June 2011[68].
2.4. Radiation dose assessment
Figure 4 shows the locations and a contour plot of 1710 positive dose-rate measurements published by the Ministry of Education, Culture, Sports, Science, and Technology in Japan (MEXT) in the United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR) 2013 report.[68] These data were provided by the Government of Japan as described in the report titled ‘Summarized version of the results of the research on distribution of radioactive substances discharged by the accident at TEPCO's Fukushima Daiichi NPP’. The Japan Atomic Energy Authority (JAEA) conducted the survey with cooperation of universities and research institutes. MEXT was responsible for the measurements and their validity. UNSCEAR reviewed, verified, and published the dataset submitted: www.unscear.org/docs/publications/2013/UNSCEAR_2013_Annex-A_Attach_C-7.xls. The single dose measurements range from 0.05 μSv/h to 50.00 μSv/h, with mean 1.47 μSv/h and median 0.51 μSv/h. The municipality-specific average dose-rates range from the practically normal minimum level of 0.08 μSv/h to a more than 200 fold elevated maximum level of 17.68 μSv/h, with the overall mean 1.60 μSv/h and the median 0.34 μSv/h. Gavrilin et al reconstructed the thyroid dose for the inhabitants of the Republic of Belarus after the Chernobyl accident based on the available area-wide fallout measurements.[69] With their equation (8), they provide a monotone relationship for estimating the thyroid dose based on ground deposition densities of iodine (I-131) and cesium (Cs-137). As the Cs-137 deposition as well as the I-131 deposition in Fukushima are linearly related to the external effective dose-rate, see Figure 5 A and B, we consider the average external effective dose-rate (μSv/h) per municipality of the Fukushima prefecture as an appropriate (proportional) surrogate exposure measure for the internal dose of the thyroid gland. In the UNSCEAR 2013 report in attachment C-9,[68] the ‘calculated Thyroid equivalent dose (mSv)’ is linearly related to the cesium fallout.
Figure 4.
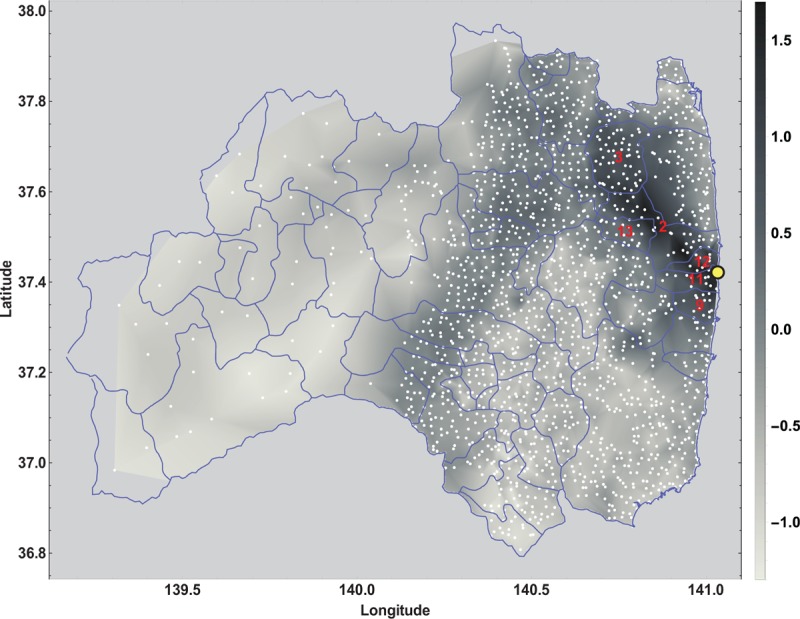
Contour plot of the dose-rate [μSv/h] on a log10 gray level scale for 1710 dose-rate measurements taken at locations (white dots) in the Fukushima prefecture in June 2011; the red indexed municipalities are subject to an average dose-rate of greater than 2.0 μSv/h, see the UNSCEAR 2013 report.[68]
Figure 5.
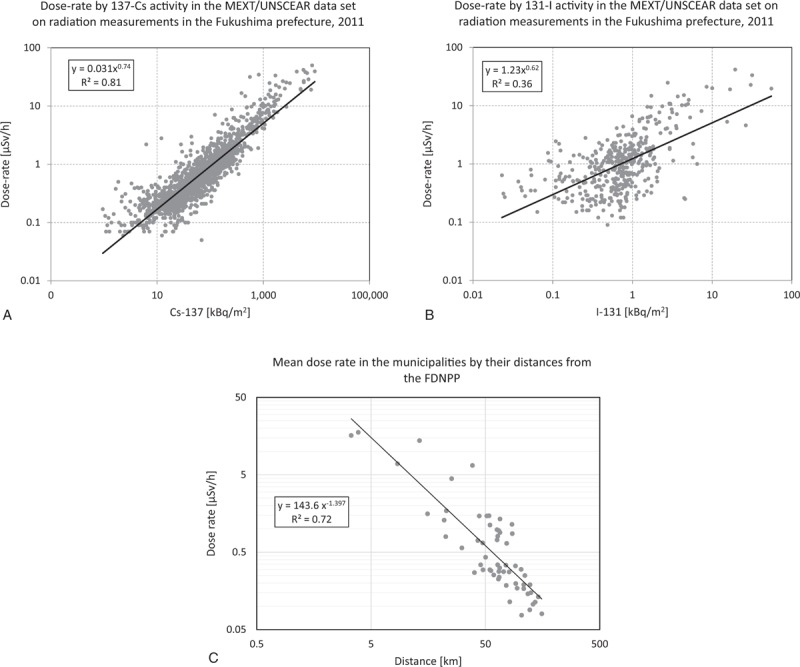
A. Association of the dose-rate [μSv/h] with the Cs-137 activity [kBq/m2] for 1710 positive radiation readings; B. Association of the dose-rate [μSv/h] with the I-131 activity [kBq/m2] for 418 positive radiation readings; C. Mean dose rate in the municipalities by their distances from the FDNPP; see the UNSCEAR 2013 report.[68]
2.5. Municipalities’ distances from the FDNPP and the dose rate
The distances of the municipalities from the power plant range from 3.4 km to 155.7 km with median and mean of 65.4 km and 69.0 km, respectively. As a proxy for the geographical location of a municipality we used the center of its area-polygon, see Figure 1. The 3 most contaminated prefectures (> 10 μSv/h) are within 15 km from the FDNPP. The 48 municipalities with distances over 40 km have mean dose rates below 1.5 μSv/h. There is a distinct negative association of the mean dose rate with distance (x), which can be described with a power function: dose(x) = 143.6 ∗ x−1.397; R2 = 0.72, see Figure 5C. Note that this is an interesting empirical confirmation of the theoretical distance law for the activity concentration Ca(x) ∼ x−1.42 proposed by UNSCEAR in 2017.[70,71]
2.6. Statistical analysis
The detected cancer cases in the 2 screening rounds separated and combined are assumed to be Poisson distributed in the municipalities within the respective exposed person-time observed. The detection rate (DR) is defined as the ratio of the cancer cases divided by the corresponding person-time. Therefore, a natural and straightforward approach is Poisson regression with the number of the observed cancer cases as the dependent variable and the average dose-rate of the municipality as the independent variable.[51] The natural logarithm of the person-years per municipality serves as the corresponding auxiliary offset variable in the Poisson regression (see the SAS 9.4 documentation of procedure GENMOD).
Note that the assumption of a Poisson distribution implies that the variances of the random deviations are determined by the Poisson parameter. In practice, the empirical variances are always smaller or larger than theory predicts, for mere randomness or for diverse unknown reasons, for example, unspecified or unknown co-variables. Therefore, the models can be generalized by introducing a heterogeneity parameter, and, to be conservative, we allow for this extension in case of overdispersion (extra-Poisson variation[51]) but not in case of underdispersion. In all Poisson regression models presented in this paper there was always minor underdispersion with the deviance/df in the range of 0.7 to 1.0. Consequently, there was no need for any heterogeneity correction. In other words, the analyzed Fukushima thyroid cancer data comply very well with the Poisson assumption. The parameter of main interest in the present context is the detection rate ratio (DRR) per unit dose-rate (1 μSv/h). For data processing, statistical analyses, and results display, we used Microsoft Excel 2016 (Office 365), R 3.5.1 (Version 2017-10-04), Wolfram MATHEMATICA 11.3, and mostly SAS/STAT software 9.4 (SAS Institute Inc: SAS/STAT User's Guide, Version 9.4, Cary NC: SAS Institute Inc, © 2002–2012).
3. Results
Table 1 shows the target population, the participants, and the detected thyroid cancers by municipality and examination round (PBLSP and FFSSP). Likewise, Table 2 lists the cumulative person-years from exposure to medical examination, the detection rate per 100,000 person-years and the dose-rate in μSv/h. As the exposure quantification and the exposure distribution may influence epidemiological findings,[51] we took a closer look at the distribution of the dose-rates in Table 2, with emphasis on possible outliers: the mean of the doses below 2 μSv/h is 0.54 (0.41, 0.67) whereas the remaining 6 doses above 2 μSv/h have a mean of 10.94 (5.05, 16.82), which is a highly significant contrast of more than one order of magnitude between those mean values. The doses in the 6 most extreme municipalities are single as well as collective outliers in the dose distribution over the 59 municipalities. Therefore, we considered it instructive to exclude those outliers by way of trial from the analyses. This led to the discovery that the dose specific effects (DRR per μSv/h) are consistently pronounced below 2 μSv/h compared to the overall analyses. Somewhat different population-protective mechanisms such as the evacuations and/or different biological or carcinogenic processes may be relevant in the dose ranges below and above 2 μSv/h in Fukushima. The following paragraphs detail the determination of the DRR per μSv/h using Poisson regression for the 1st, the 2nd, and the combined examination rounds. All analyses are characterized by the criterion ‘all data vs below 2 μSv/h’.
Figure 6 shows the thyroid cancer detection rate per 100,000 exposed person-years by dose-rate in the Preliminary Baseline Screening Program as well as the results of the Poisson regression analysis. Figure 6A displays the data and the regression line for all 59 municipalities with 115 cancer cases and 527,734 person-years. The detection rate ratio per μSv/h is 1.086 (1.012, 1.165). In Figure 6B, the analysis is restricted to the 53 municipalities with dose-rate < 2 μSv/h, see Figure 1. For the reduced data set, the detection rate ratio per μSv/h is 1.672 (1.062, 2.631) based on 111 cancer cases in 520,383 person-years.
Figure 6.
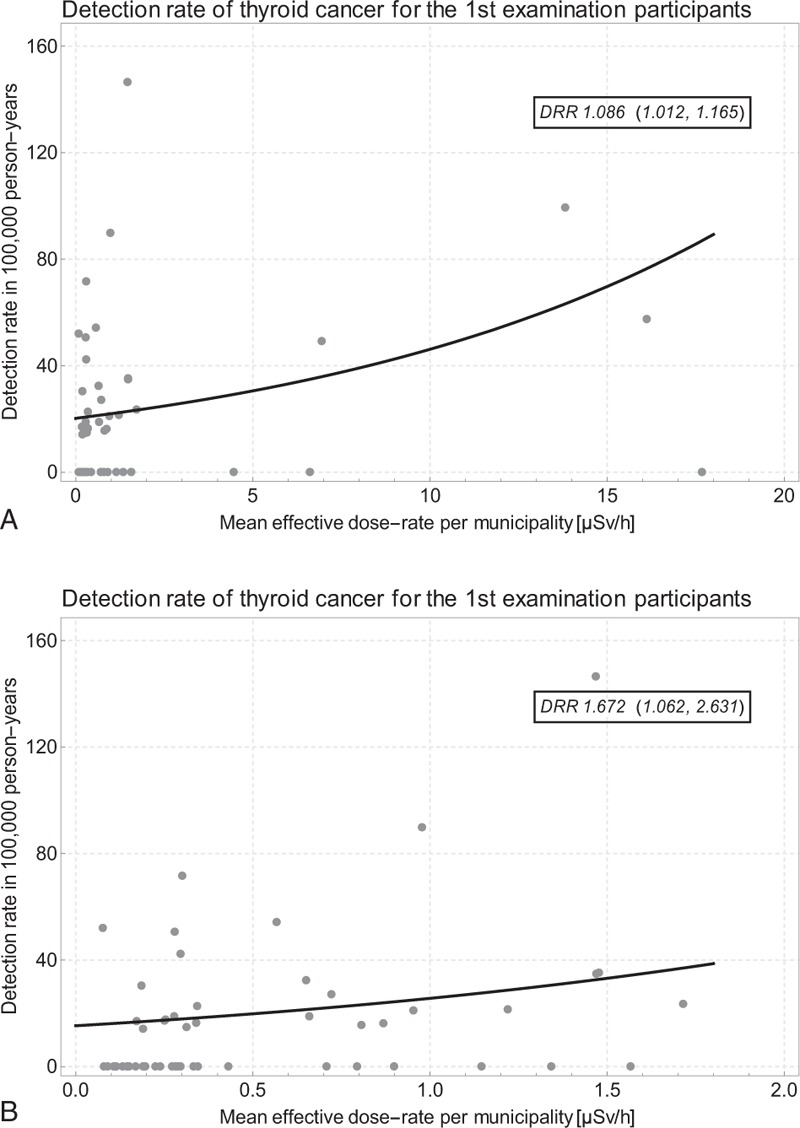
A. Thyroid cancer detection rate by dose-rate and Poisson regression line in the Preliminary Baseline Screening Program in all 59 municipalities; B. data restricted to 53 municipalities with dose-rate < 2.0 μSv/h; one outlying data point not shown for prefecture No. 10 Kawauchi Mura with 1 TC in 280 1st round participants.
Figure 7 displays the thyroid cancer detection rate per 100,000 exposed person-years by dose-rate in the First Full-Scale Screening Program, as well as the Poisson regression results. Figure 7A shows the data and the regression line for all 59 municipalities with 69 cancer cases and 1,032,817 person-years. The detection rate ratio per μSv/h is 1.101 (1.031, 1.176). In Figure 7B, the analysis is again restricted to the 53 municipalities with dose-rate < 2 μSv/h. For the reduced data set representing 65 cancer cases and 1,007,554 person-years, the detection rate ratio per μSv/h is 2.498 (1.446, 4.315).
Figure 7.
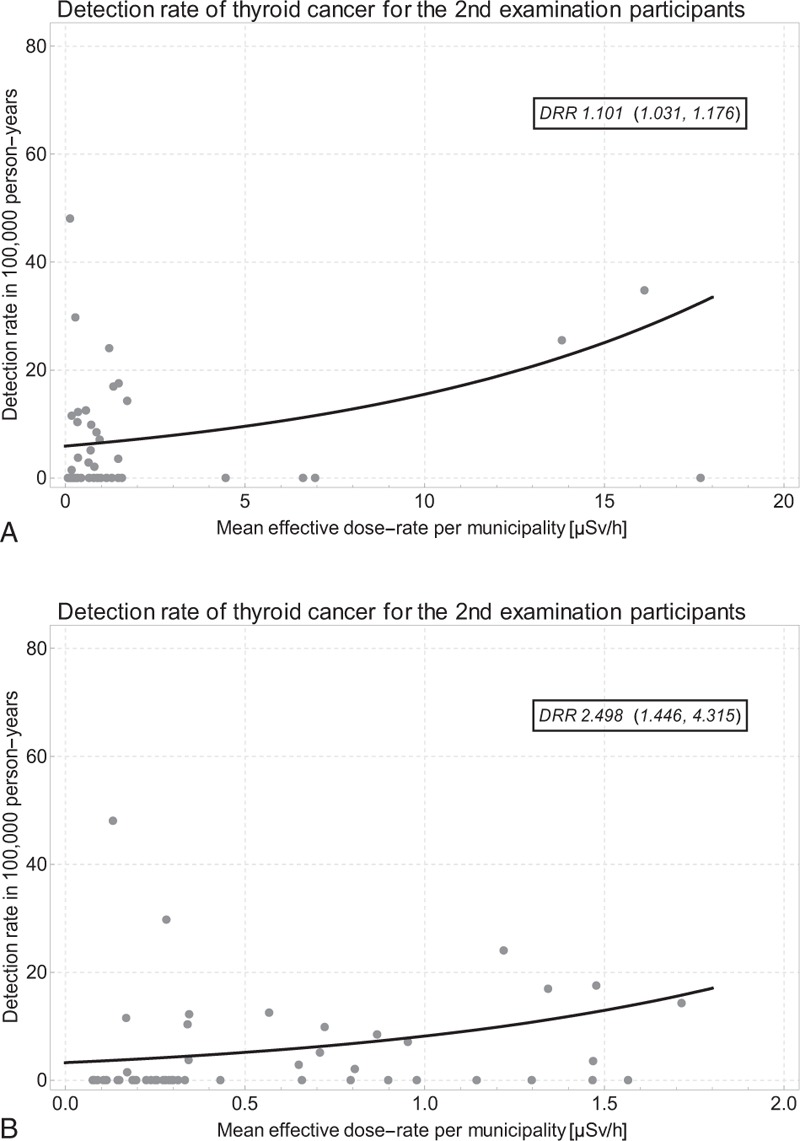
A. Thyroid cancer detection rate by dose-rate and Poisson regression line in the First Full-Scale Screening Program in all 59 municipalities; B. data restricted to 53 municipalities with dose-rate < 2.0 μSv/h.
Tables 1 and 2 contain the thyroid cancer data and the exposed person-years for the combined 1st and 2nd screening rounds. Figure 8 shows the thyroid cancer detection rate per 100,000 person-years by dose-rate in the combined data set as well as the parameters of the Poisson regression analyses. Figure 8A displays the data and the regression line for all 59 municipalities with 184 cancer cases and 1,079,786 person-years. The detection rate ratio per μSv/h is 1.065 (1.013, 1.119). In Figure 8B, the analysis is once more restricted to the 53 municipalities with dose-rate < 2 μSv/h. With the reduced data set, the detection rate ratio per μSv/h is 1.555 (1.096, 2.206) based on 176 cancer cases in 1,053,236 person-years.
Figure 8.
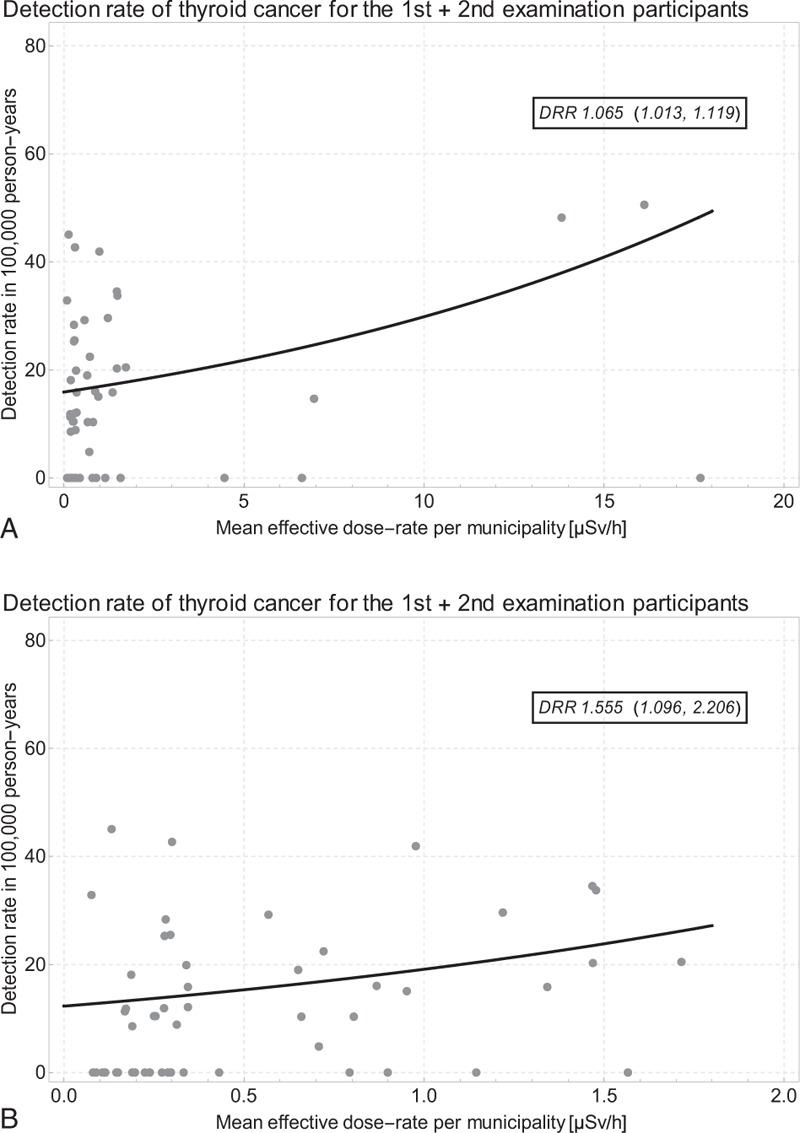
A. Thyroid cancer detection rate by dose-rate and Poisson regression line (Preliminary Baseline Screening and First Full-Scale Screening Programs combined) in all 59 municipalities; B. data restricted to 53 municipalities with dose-rate < 2.0 μSv/h; one outlying data point not shown for prefecture No. 10 Kawauchi Mura with 1 TC in 280 1st round participants and no TC in 213 2nd round participants.
4. Discussion
In brief, the detection rate of thyroid cancer in the FHMS was determined using the screened cancer cases and the approximate person-years between exposure onset and the medical examination periods in the municipalities. From the MEXT dataset,[68] we calculated the average radiation dose-rates for all 59 municipalities of the Fukushima prefecture after the nuclear accidents. In both the first and second rounds of investigation and in both cycles combined, we found clear positive and significant dose-response relationships between the thyroid cancer detection rate and the external effective dose-rate.
4.1. Necessity of the person-year method
The thyroid ultrasonography screening in the first round of examinations in Fukushima started 7 months after the accidents in some areas, but in other areas it started more than two years later, see Figure 3. Therefore, the period lengths between the accident and the timing of the screenings vary 3 to 4-fold depending on the municipality. Moreover, in areas with a higher contamination level the screening began earlier and the time between the accident and the screening was shorter, see Figure 1, Figs. 3 and 4. In such cases, since a person once screened is not monitored any longer in the FHMS, it is appropriate to use the effective exposed person-time observed to determine the municipality-specific detection rate. For the first time, we investigated the relationship between the detection rate of thyroid cancer and the average radiation dose-rate at the municipality level in the Fukushima prefecture.
4.2. In context with Chernobyl research
In the aftermath of Chernobyl, ecological spatiotemporal correlation studies,[7,11,12,23] case control studies,[13–15] and cohort studies[16–18] were carried out. These studies have demonstrated that radiation exposure of the thyroid gland and the thyroid cancer incidence rate are associated significantly. In the UNSCEAR 2008 report accounting for more than 1800 children with thyroid cancer, the statistical relationship between radiation and thyroid cancer after Chernobyl was confirmed. However, the causal association of ionizing radiation with the induction of thyroid cancers was still considered uncertain, and the increased thyroid cancer rates were attributed to the mass screening: “The estimates of radiation risk from these studies remain somewhat uncertain, however, and may have been influenced by variations in the use of ultrasonography and mass screening after the accident”.[72] In principle, it is possible that screening may detect thyroid cancers which progress slowly, and which would never have become clinically manifest.
Zablotska and colleagues repeated the thyroid screening examinations in Belarus with ultrasonography on a cohort whose exposure dose was known. The initial screening was called pre-screening, and it was distinguished from the regular second-time screening. As a result of both the pre-screening and the combined screenings, a dose-response relationship between thyroid cancer and radiation dose was established.[19] This is consistent with our result, indicating that the influence of radiation is stronger than a possible ‘screening effect’.
In several investigations after Chernobyl, the thyroid dose was estimated by personal interviews, radio-ecological models assessing the spatiotemporal variation of 131-I, as well as direct thyroid dose measurements. For Fukushima, there is the possibility that the true exposure was larger than the exposure estimated using the MEXT dataset.[73] Looking at the degree of soil pollution by 137-Cs in the Fukushima prefecture, 137,084 people lived in an area with a contamination level above 185kBq/m2. On the other hand, in the oblast Gomel in Belarus 218,303 people lived in an area with over 185kBq/m2 137-Cs, and 118,795 people lived in an area with over 185kBq/m2 137-Cs in the remainder of Belarus.[74] Regions where 137-Cs concentration exceeded 185kBq/m2 were designated as the ‘Zone of obligatory re-settlement’, in Belarus after Chernobyl.[75] Therefore, as concerns the 137-Cs soil contamination, highly populated areas in Fukushima are comparable with large areas in Belarus.
4.3. In context with Fukushima research
From the data provided by the ‘System for Prediction of Environmental Emergency Dose Information (SPEEDI)’, the accumulated thyroid equivalent dose in the evacuation area of Fukushima from March 12 to April 24 has exceeded approximately 100mSv: “Children in Iitate-mura and Iwaki Prefecture have been hypothesized to have thyroid radiation exposure possibly reaching 100mSv by SPEEDI, although they were residing outside the 20 km range”.[76] The UNSCEAR 2013 report estimates that the absorbed thyroid dose was 47–83mGy, and might have been as high as 36 to 795mGy in case inhabitants would not be or have not been evacuated.[68] In 2012, the WHO reports an estimate of 100 to 200mSv.[77] Therefore, the thyroid equivalent dose in Fukushima was considerably increased.
Most of the papers cited above dealing with thyroid cancers in Fukushima claim that the height of the radiation dose to the residents was extremely low, thus additional thyroid cancers could not have been induced. However, since the detection rate of thyroid cancers is abnormally high and cannot be ignored or discarded, there are the following two arguments to explain that nevertheless the (true) incidence rate of thyroid cancer has not changed. A typical claim is the ‘screening effect theory’ according to which thyroid cancers are found before any symptoms emerge simply since they are sought by applying an ultrasonic examination screening to a large population.[34,43] Since this point cannot explain the fact that many persons have already undergone surgery on semi-international standards, there are also publications maintaining that many of the cancers detected and operated were due to ‘over-diagnosis’.[45] Both lines of reasoning are extensively employed in the scientific literature to deny any possible causal relationship between the observed and widely accepted excesses of thyroid cancers after nuclear accidents and radiation exposure.[58] Moreover, before Fukushima, thyroid cancers have not been found in excess by ultrasonic screening in generations that were not exposed to radioactive iodine, or were exposed to only low doses after Chernobyl.[20] If there were really no biological effects from the elevated ionizing radiation, only few, if any, thyroid cancers should have been detected after the nuclear accidents in the Fukushima prefecture. Further evidence of possible radiation induced detrimental health effects arises from exposure-dependent increases in perinatal mortality and increased congenital malformations in Japan from 2012 onward.[78–81]
Hayashida and colleagues conducted thyroid ultrasonic screening on 4365 children and adolescents 3 to 18 years old at three university hospitals in Aomori, Yamanashi, and Nagasaki, and reported that one thyroid cancer was found.[82] This paper is often quoted as providing evidence that this discovery rate (1/4365) indicates about the same prevalence elsewhere and in Fukushima. However, although the thyroid cancer incidence in children is rare, that is, most recently estimated as 0.54/100,000,[83,84] it entails a prevalence of 10 in 100,000 children aged 1 to 18. Assuming a prevalence of 10 in 100,000 yields a probability as large as 0.354 of observing 1 or more diseased in 4365 children, as was the case in the Hayashida et al study.[82] Therefore, this study does not provide evidence against a background incidence rate in the order of magnitude of 1/200,000. Nevertheless, many people deny the excess thyroid cancers in Fukushima based on just this one single case obviously found by chance in the Hayashida et al study. For comparison, the actual period prevalence[85] in Fukushima is in the order of magnitude of 184/300,000∗100,000≈60, which is a 6-fold increase compared to normal levels. Consider also that for a stable long-term prevalence of 10 in 100,000 to increase 6-fold in 5 years, the change in the incidence rate must be relatively strong. Shibuya et al[86] and Katanoda et al[45] consider potential ‘over-diagnosis’ as a cause for the increased thyroid cancer prevalence in Fukushima since they assume rather unrealistic thyroid doses below 3mSv. However, they concede that it is difficult to support ‘over-diagnosis’ without appropriate control populations for comparison. Moreover, the claim of ‘over-diagnosis’ would call into question the quality and the reliability of the thyroid cancer diagnostics as well as the validity of surgery.
Tokonami and colleagues measured the equivalent dose to the thyroid by 131-I in 42 people who have been evacuated in April 2011, and they reported an average thyroid equivalent dose of 3.5mSv for 6 children aged 0 to 19 years.[87] In fact, the thyroid equivalent dose of two of the children was 21 and 23mSv. Because the number of subjects was small, Tokonami et al considered the possibility that the thyroid equivalent dose could have been up to 83mSv. In addition, the inhalation exposure by the plume was estimated during 4 hours on March 15, and the thyroid equivalent dose for that 4-hour period was evaluated.
Furuta et al reported the monitoring results of dose-rates, radioactivity concentrations (in air and fallout), and meteorological observations before June 1, 2011 at the Tokai Research and Development Center located about 130 km south of Fukushima. In addition, they estimated the child's thyroid equivalent dose during this period in the range of 20mSv.[88] If the same calculation is applied to the UNSCEAR Report 2013 data set in attachment C-9,[68] not only in the evacuation area, but also in Iwaki, the biggest city in the Fukushima prefecture, the thyroid equivalent doses exceeded 100mSv.
Kim and colleagues published examination results determining the radiation dose of 1080 children aged 0 to 15 in Iwaki City, Kawamata Town, and Iidate Village from March 24 to March 30, 2011 by contacting the thyroid gland with a dosimeter.[73] It was an examination with 0.2 μSv/h as a lower limit at places where the ambient doses were 0.07 to 0.17 μSv/h. It was assumed that 0.2 μSv/h were equivalent to a thyroid dose of 100mSv for a 1-year old child. In later work, Kim et al estimated the rounded 90th percentile values for a 1-year old child to be 30mSv in Futaba town, Itate village, and Iwaki city, which means that theoretically 10% of the children of that age and in these locations may have been exposed to thyroid doses above 30mSv.
Kamada and colleagues estimated external and internal radiation doses for 15 residents who lived approximately 37 km northwest of the FDNPP. Residents were interviewed on their behavior and their diet after the incident. Effective doses up to 54 days after the deposition were calculated. The average cumulative effective dose was 8.4 mSv for adults and 5.1 mSv for children. 131-I was measured in urinary samples of five residents. The equivalent doses for the thyroid gland were 27 to 66 mSv.[89]
Tsuda et al[30] analyzed the prefecture results from the first and the second round up to December 31, 2014 in comparison with the Japanese annual incidence and in comparison with the incidence in a reference area in the Fukushima prefecture. The highest incidence rate ratio, assuming a mean latency period of 4 years, was observed in the central middle district of the prefecture compared with the Japanese annual incidence: incidence rate ratio 50 (25, 90). In the second screening round, even under the assumption that the rest of the examinees were disease free, Tsuda et al found an incidence rate ratio of 12 (5.1, 23.0). They also concluded that the increase was unlikely to be explained by a screening surge. Tsuda et al emphasize that the length of the time-interval between the accident and the screenings should be considered. This is what we have done for the first time and even at the municipality level in the Fukushima prefecture in the present investigation.
4.4. Dose-response analyses concerning Fukushima
On the grounds that thyroid cancer patients had external exposure estimates of below 2.2mSv during the first 4 months, and that there was no significant difference in the prevalence between the evacuated and the non-evacuated areas, Suzuki et al explain the thyroid cancer increases in Fukushima by a screening effect.[42,43] Ohira and colleagues classified the Fukushima prefecture into groups based on the same dose data employed by Suzuki et al: highest, middle, and lowest dose areas. As a result of comparing the prevalence ORs between the groups, they also concluded that there was no dose-response relationship between thyroid cancer and radiation dose.[90] In a more recent letter, Ohira et al classified the FHMS participants into quintiles according to individual radiation doses. They employed an indirect exposure criterion, namely the proportions of persons in the municipalities with external doses above 1.0mSv. The odds ratios (ORs) and 95%-confidence intervals of thyroid cancer were calculated using logistic regression models adjusted for age, sex, and the duration from the accidents to the examinations. Ohira et al concluded “regional and individual differences in external radiation dose were not associated with thyroid cancer prevalence among children in the 4 years after the nuclear power plant accident”.[91] In their response, Tsuda et al criticize, among others, that Ohira et al considered only 1st screening round data.[92] What is common to those papers and letters is the utilization of a questionnaire-based individual radiation dose ascertainment (with 27% response rate) for the assessment of possible thyroid cancer prevalence differences between 3 to 5 groups of the 59 municipalities. Therefore, all these approaches do not exhaust the available quantitative spatiotemporal exposure-effect information.
4.5. Questionnaire-based assessment of the individual external radiation dose
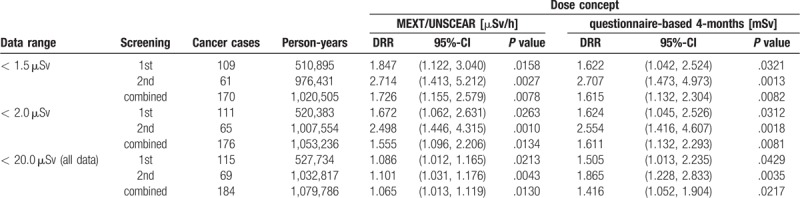
Employing a questionnaire-based method, the Fukushima Health Management Survey estimated the distribution of the external individual radiation doses in each municipality (see http://www.pref.fukushima.lg.jp/uploaded/attachment/201726.pdf). Although the response rate was as low as ca. 27%, this dose distribution takes into account that no or only few residents remained in the evacuation areas.[93] The eight municipalities with average dose-rates above 1.5 μSv/h Naraha Machi(1.6), Minamisoma Shi(1.7), Katsurao Mura(4.5), Iitate Mura(6.6), Tomioka Machi(6.9), Namie Machi(13.8), Okuma Machi(16.1), and Futaba Machi(17.7) comprise areas that have been evacuated (partially or completely) within a few days to a few weeks after the nuclear power plant accidents. It is, therefore, of interest to set the average municipality-specific MEXT/ UNSCEAR dose-rates [μSv/h] employed in our study in perspective to the average FHMS questionnaire-based cumulative doses [mSv] in the municipalities during the 4 months period from March 11 to July 11, 2011. Figure 9 reflects a plausible and relatively strong association between the two dose concepts in the 51 essentially non-evacuated municipalities. However, a restricted resolution of the FMHS dose-system below 0.4 μSv/h becomes apparent, which is due to the (reported) coarse 1.0mSv-steps of the dose distributions in the municipalities and the low participation rate. This low resolution can be considered an additional disadvantage of the questionnaire-based approach. Table 3 summarizes and compares the thyroid cancer detection rate ratios (DRR) for the single and the combined screenings stratified by the two different dose concepts and by the data ranges: < 1.5, < 2.0, and < 20.0 μSv/h, respectively. It becomes obvious that the two dose-concepts yield consistent and significant results especially in most of the data, that is, excluding the partly or completely evacuated areas. Therefore, the two dose concepts mutually corroborate each other and support each a distinct significant association between radiation-exposure and subsequent thyroid cancer detection.
Figure 9.

Comparison of the MEXT/UNSCEAR dose-rate and the questionnaire-based FHMS dose in 51 Fukushima municipalities with dose-rate < 1.5 μSv/h,[68,93] see also Table 3 and http://www.pref.fukushima.lg.jp/uploaded/attachment/201726.pdf.
Table 3.
Detection rate ratios (DRR), 95%-confidence intervals, and p-values for the Poisson regression of the thyroid cancer detection rate on dose in the FHMS by dose concept, data range, and screening rounds; see Figure 9.
4.6. Non-linearity of the dose-response association
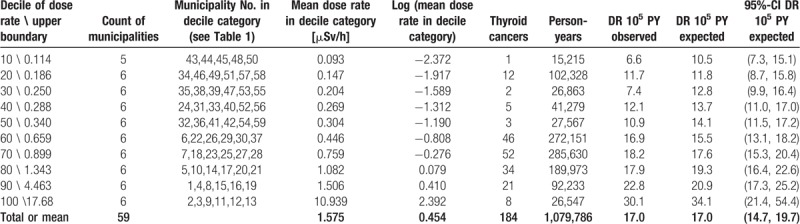
In the 3 analysis variants (first, second, and both rounds combined), the DRRs based on doses below 2 μSv/h are considerably larger than the DRRs based on the full dose range below 20 μSv/h. This is consistent by trend with an observation by Zablotska et al “The excess odds ratio per gray (EOR/Gy) was modelled using linear and linear-exponential functions. For thyroid doses <5Gy, the dose-response was linear (n = 87 EOR/Gy = 2.15, 95% confidence interval: 0.81–5.47), but at higher doses the excess risk fell”.[19] One may speculate that this is an artifact of better protection (such as evacuation), some other biases in the highly exposed sub-populations, or an intrinsic biological nonlinearity. A possible explanation for this nonlinear behavior might be that high doses or dose-rates of 131-I destroy thyroid tissue and thus reduce the risk of developing cancer.[94–96] To cope with this disproportionate behavior of the association between the widely scattered dose-rate and the detection rate, it might be interesting to consider the log transformed dose-rate. Figure 10 shows the Poisson regression of the thyroid cancer detection rate on the natural log of the mean dose-rates in the decile categories. A less scattered (compared to Figs. 6–8) and monotone increasing association between the log-transformed dose-rate and the thyroid cancer detection rate becomes evident. The association is described by a DRR per log (μSv/h) of 1.281 (1.088, 1.508), P value .0030. See Table 4 for the corresponding decile-based data and the according point estimates and interval estimates.
Figure 10.
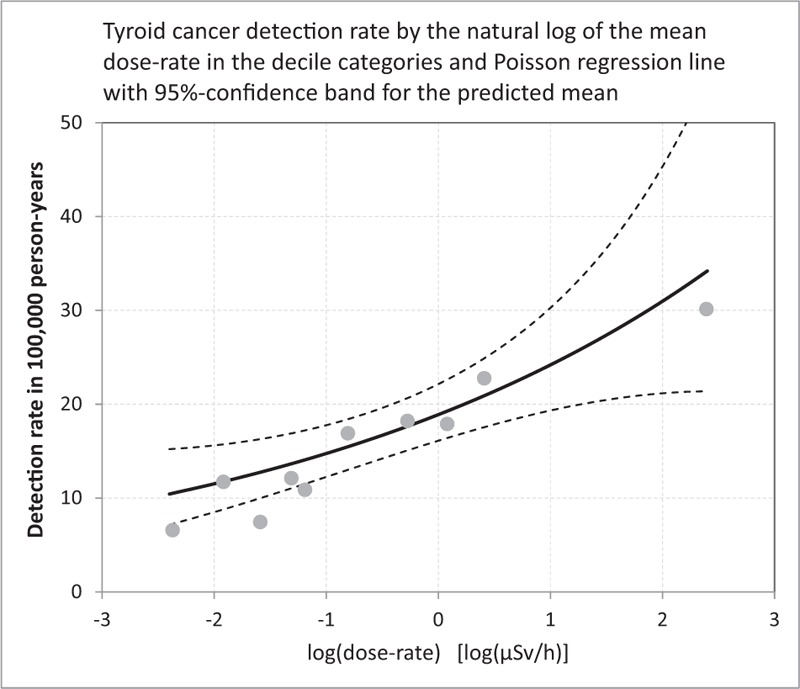
Thyroid cancer detection rate in 100,000 person-years (1st and 2nd screening rounds combined) by the natural log of the mean dose-rate in the 10 decile categories and Poisson trend with 95%-prediction bands for the mean; see Table 4.
Table 4.
Data and results of the decile-based Poisson regression analysis for the 1st and the 2nd rounds combined; data is obtained by calculating the dose-rate deciles and by totaling the thyroid cancers and the person-year counts in Tables 1 and 2 for the 10 strata (categories) defined by those deciles; see Figure 10.
4.7. Dependence of the detection rate on the participation rate
The participation rate per municipality (participants / target population, see Table 1) increases from 58% to 93% in the first screening round, and from 54% to 78% in the second screening round. The highest participation rate (93%) is found in Date Shi in the first screening and the lowest rate is observed in the second round in Futaba Machi (54%), which is the highest contaminated and evacuated municipality (17.7 μSv/h). It is true that the higher the participation rate the more cancer cases can be detected, which increases the numerator, but at the same time more participants increase the person-years in the denominator. Thus, theoretically, there is no (intrinsic) association between the participation rate and the detection rate. Hence, there is no need to adjust for the participation rate, which adjustment could lead to over-fitting and variance inflation decreasing the precision of the Poisson regression models.
4.8. Limitations
The precise (anonymized) individual data on where the participants lived, when the screenings were carried out, and when the diagnoses were established, have not yet been released. We requested this information from one of the directors of the Fukushima Medical University to carry out independent investigations and to publish epidemiological studies. Unfortunately, this data was not provided or made public. Note, the concealment of these data is partly due to restrictions outlined in the informed consent by the FHMS participants.[97] Therefore, we estimated the corresponding proxy information from the published screening schedule tables, the number of the municipality-specific cumulative examination participants, and the thyroid cancer cases in each municipality. This data was announced approximately once every three months, which is why we assumed that the date when an individual had been examined can be estimated up to a precision of 90 days. For more accurate analyses, it is required to provide open access to the (anonymized) information as to where any participating person lived and when he or she was examined and diagnosed. With this more precise data, our analyses can easily be replicated. Since our approach inevitably entails spatial-temporal non-differential misclassification, it is likely that ‘true’ effects based on individual location and individual exposed person-time observed will turn out to be stronger and more precise compared to the results reported in the present paper. From this point of view, our quantitative estimates may be considered conservative.
There are few data, if any, which allow estimating validly the internal thyroid exposure after the FDNPP accidents for larger numbers of residents. It is a disadvantage of our study that information on the external dose resulting from the initial plume, the inhalation, and the diet contamination could not be assessed directly. However, such information cannot be obtained by simple physical measurement; exposure measures are rather dependent on various conversion coefficients and many other factors that introduce considerable uncertainty.
It was not possible to analyze the data by comparing the radiation dose-rate between the individuals that developed thyroid cancer and the individuals that remained healthy. In other words, we were not able to carry out a case-control study, which is a powerful epidemiological instrument. The reason is that the geographical locations where the thyroid cancer cases and the healthy participants lived as well as the date when the participants were examined or diagnosed have not yet been made available. The confounding factors age and sex at the municipality level have not been disclosed by the Fukushima Health Management Survey as yet. So, it is not possible to adjust our analyses for age and sex. We are also not aware of representative BMI data and iodine intake statistics at the municipality level that could be used for our study. Nevertheless, we think that our approach and our analyses are valid and important and that our work may motivate more refined and better adjusted analyses in the future.
5. Conclusions
We suggest an innovative statistical technique to determine the municipality-specific average exposed person-time of the participants in the ’Fukushima Health Management Survey’. The knowledge of the exposed person-time enables the assessment of the association between the radiation dose rate and the thyroid cancer detection rate more precisely than in previous studies. The thyroid cancer detection rate and the radiation dose-rate in the 59 municipalities in the Fukushima prefecture show statistically significant dose-response relationships. The detection rate ratio per μSv/h was 1.065 (1.013, 1.119) based on all data in both examination rounds combined. In the 53 municipalities subjected to less than 2 μSv/h, the detection rate ratio was considerably higher: 1.555 (1.096, 2.206). Therefore, it became evident that the radiation contamination due to the Fukushima nuclear power plant accidents is positively associated with the thyroid cancer detection rate in children and adolescents. This corroborates previous studies providing evidence for a causal relation between nuclear accidents and the subsequent occurrence of thyroid cancer.
Acknowledgments
We are most grateful to five reviewers for many constructive comments on earlier drafts and for references to lesser known, however important literature.
Author contributions
Conceptualization: Hidehiko Yamamoto, Keiji Hayashi.
Data curation: Hidehiko Yamamoto, Keiji Hayashi.
Formal analysis: Hagen Scherb.
Investigation: Hidehiko Yamamoto, Keiji Hayashi, Hagen Scherb.
Methodology: Hidehiko Yamamoto, Keiji Hayashi, Hagen Scherb.
Software: Hagen Scherb.
Supervision: Hidehiko Yamamoto, Keiji Hayashi.
Validation: Hidehiko Yamamoto, Keiji Hayashi, Hagen Scherb.
Visualization: Keiji Hayashi, Hagen Scherb.
Writing – original draft: Hidehiko Yamamoto, Keiji Hayashi, Hagen Scherb.
Writing – review & editing: Hidehiko Yamamoto, Keiji Hayashi, Hagen Scherb.
Hagen Scherb orcid: 0000-0002-2730-5619.
Footnotes
Abbreviations: (.,.) = 95%-confidence interval (95%-CI), μSv/h = Micro-Sieverts per hour, 131-I = Iodine-131, 134-Cs = Cesium-134, 137-Cs = Cesium-137, BMI = body mass index, Bq = Becquerel, df = degrees of freedom, DR = detection rate, cases/person-time or cases per 100,000 person-years, DRR = detection rate ratio, FDNPP = Fukushima Daiichi Nuclear Power Plant, FFSSP = first full-scale screening program, FHMS = Fukushima Health Management Survey, FNAC = fine needle aspiration cytology, FY = Fiscal year in Japan: 1 April to 31 March, kBq/m2 = Kilo-Becquerel per square meter, MEXT = Ministry of Education, Culture, Sports, Science, and Technology in Japan, mGy = Milli-Gray, mSv/a = Milli-Sieverts/annum or milli-Sieverts/year, OR = odds ratio, PBLSP = preliminary baseline screening program, pBq = Peta-Becquerel, 1015 Bq, PTO = person-time observed, PY = person-years, RR = rate ratio, SAS = statistical analysis system, software produced by SAS Institute Inc., TC = thyroid cancer (ICD-10: C73-C75), TEPCO = Tokyo Electric Power Company, TUE = Thyroid Ultrasound Examination in the Fukushima prefecture, UNSCAER = United Nations Scientific Committee on the Effects of Atomic Radiation, WHO = World Health Organization.
How to cite this article: Yamamoto H, Hayashi K, Scherb H. Association between the detection rate of thyroid cancer and the external radiation dose-rate after the nuclear power plant accidents in Fukushima, Japan. Medicine. 2019;98:37(e17165).
The authors have no funding and conflicts of interest to disclose.
The employed data has exclusively been published previously and/or it is contained in the Tables and in the Figures included in this paper.
References
- [1].2012. [Accessed August 30, 2019]. TEPCO. Estimation of Radioactive Material Released to the Atmosphere during the Fukushima Daiichi NPS Accident; http://www.tepco.co.jp/en/press/corp-com/release/betu12_e/images/120524e0205.pdf. [Google Scholar]
- [2].Hamilton TE, van Belle G, LoGerfo JP. Thyroid neoplasia in Marshall Islanders exposed to nuclear fallout. JAMA 1987;258:629–35. [PubMed] [Google Scholar]
- [3].Ron E, Modan B. Benign and malignant thyroid neoplasms after childhood irradiation for tinea capitis. J Natl Cancer Inst 1980;65:7–11. [PubMed] [Google Scholar]
- [4].Modan B, Ron E, Werner A. Thyroid cancer following scalp irradiation. Radiology 1977;123:741–4. [DOI] [PubMed] [Google Scholar]
- [5].Shore RE, Albert RE, Pasternack BS. Follow-up study of patients treated by X-ray epilation for Tinea capitis; resurvey of post-treatment illness and mortality experience. Arch Environ Health 1976;31:21–8. [DOI] [PubMed] [Google Scholar]
- [6].International Agency for Research on Cancer, International Agency for Research on Cancer Working Group on the Evaluation of the Carcinogenic Risks to Humans. Ionizing radiation, part 2: some internally deposited radionuclides. Lyon Geneva: IARC distrib. by the World Health Organization Distribution and Sales etc.; 2001. [Google Scholar]
- [7].Likhtarov I, Kovgan L, Vavilov S, et al. Post-Chernobyl thyroid cancers in Ukraine. Report 2: risk analysis. Radiat Res 2006;166:375–86. [DOI] [PubMed] [Google Scholar]
- [8].Radespiel-Troger M, Batzler WU, Holleczek B, et al. Rising incidence of papillary thyroid carcinoma in Germany. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz 2014;57:84–92. [DOI] [PubMed] [Google Scholar]
- [9].Mahoney MC, Lawvere S, Falkner KL, et al. Thyroid cancer incidence trends in Belarus: examining the impact of Chernobyl. Int J Epidemiol 2004;33:1025–33. [DOI] [PubMed] [Google Scholar]
- [10].Muerbeth S, Rousarova M, Scherb H, et al. Thyroid cancer has increased in the adult populations of countries moderately affected by Chernobyl fallout. Med Sci Monit 2004;10:CR300–6. [PubMed] [Google Scholar]
- [11].Jacob P, Kenigsberg Y, Zvonova I, et al. Childhood exposure due to the Chernobyl accident and thyroid cancer risk in contaminated areas of Belarus and Russia. Br J Cancer 1999;80:1461–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Heidenreich WF, Bogdanova TI, Jacob P, et al. Age and time patterns in thyroid cancer after the Chernobyl accidents in the Ukraine. Radiat Res 2000;154:731–2. discussion 734-735. [PubMed] [Google Scholar]
- [13].Astakhova LN, Anspaugh LR, Beebe GW, et al. Chernobyl-related thyroid cancer in children of Belarus: a case-control study. Radiat Res 1998;150:349–56. [PubMed] [Google Scholar]
- [14].Davis S, Stepanenko V, Rivkind N, et al. Risk of thyroid cancer in the Bryansk Oblast of the Russian Federation after the Chernobyl Power Station accident. Radiat Res 2004;162:241–8. [DOI] [PubMed] [Google Scholar]
- [15].Cardis E, Kesminiene A, Ivanov V, et al. Risk of thyroid cancer after exposure to 131I in childhood. J Natl Cancer Inst 2005;97:724–32. [DOI] [PubMed] [Google Scholar]
- [16].Stezhko VA, Buglova EE, Danilova LI, et al. A cohort study of thyroid cancer and other thyroid diseases after the Chornobyl accident: objectives, design and methods. Radiat Res 2004;161:481–92. [DOI] [PubMed] [Google Scholar]
- [17].Tronko MD, Howe GR, Bogdanova TI, et al. A cohort study of thyroid cancer and other thyroid diseases after the chornobyl accident: thyroid cancer in Ukraine detected during first screening. J Natl Cancer Inst 2006;98:897–903. [DOI] [PubMed] [Google Scholar]
- [18].Zablotska LB, Bogdanova TI, Ron E, et al. A cohort study of thyroid cancer and other thyroid diseases after the Chornobyl accident: dose-response analysis of thyroid follicular adenomas detected during first screening in Ukraine (1998–2000). Am J Epidemiol 2008;167:305–12. [DOI] [PubMed] [Google Scholar]
- [19].Zablotska LB, Ron E, Rozhko AV, et al. Thyroid cancer risk in Belarus among children and adolescents exposed to radioiodine after the Chornobyl accident. Br J Cancer 2011;104:181–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Demidchik YE, Saenko VA, Yamashita S. Childhood thyroid cancer in Belarus, Russia, and Ukraine after Chernobyl and at present. Arq Bras Endocrinol Metabol 2007;51:748–62. [DOI] [PubMed] [Google Scholar]
- [21].Preston DL, Shimizu Y, Pierce DA, et al. Studies of mortality of atomic bomb survivors. Report 13: solid cancer and noncancer disease mortality: 1950–1997. 2003. Radiat Res 2012;178:AV146–172. [DOI] [PubMed] [Google Scholar]
- [22].Ivanov VK, Kashcheev VV, Chekin SY, et al. Thyroid cancer: lessons of Chernobyl and projections for Fukushima. Radiat Risk 2016;25:15. [Google Scholar]
- [23].Buglova EE, Kenigsberg JE, Sergeeva NV. Cancer risk estimation in Belarussian children due to thyroid irradiation as a consequence of the Chernobyl nuclear accident. Health Phys 1996;71:45–9. [DOI] [PubMed] [Google Scholar]
- [24].Cardis E, Hatch M. The Chernobyl accident--an epidemiological perspective. Clin Oncol (R Coll Radiol) 2011;23:251–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Academic Press, Nagataki S, Yamashita S. Yamashita S, Thomas G. Chapter 2 - Thirty Years After the Chernobyl Nuclear Power Plant Accident: Contribution From Japan—“Confirming the Increase of Childhood Thyroid Cancer”. Thyroid Cancer and Nuclear Accidents: Long-Term Aftereffects of Chernobyl and Fukushima: 2017. 11–20. [Google Scholar]
- [26].Akiba S. Epidemiological studies of Fukushima residents exposed to ionising radiation from the Fukushima Daiichi Nuclear Power Plant prefecture--a preliminary review of current plans. J Radiol Prot 2012;32:1–0. [DOI] [PubMed] [Google Scholar]
- [27].FMU. Fukushima Medical University (FMU), Report of Second-round Thyroid Ultrasound Examinations, http://fmu-global.jp/download/thyroid-ultrasound-examination-first-full-scale-thyroid-screening-program-4/?wpdmdl=2199 2017. Accessed August 30, 2019. [Google Scholar]
- [28].FMU. Fukushima Medical University (FMU), Report of Third-Round Thyroid Ultrasound Examinations; http://fmu-global.jp/download/thyroid-ultrasound-examination-second-full-scale-thyroid-screening-program-7/?wpdmdl=2693 2017. Accessed August 30, 2019. [Google Scholar]
- [29].PPHS. The 19th Prefectural People's Health Survey (PPHS), Review Committee Interim summary of the Prefectural People's Health Council in March 2016 (Japanese) http://www.pref.fukushima.lg.jp/uploaded/attachment/174220.pdf 2016. 2017. Accessed August 30, 2019. [Google Scholar]
- [30].Tsuda T, Tokinobu A, Yamamoto E, et al. Thyroid Cancer Detection by Ultrasound Among Residents Ages 18 Years and Younger in Fukushima, Japan: 2011 to 2014. Epidemiology 2016;27:316–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Takahashi H, Ohira T, Ohtsuru A, et al. The authors respond. Epidemiology 2017;28:e5–6. [DOI] [PubMed] [Google Scholar]
- [32].Hamaoka Y. Re: Thyroid cancer among young people in Fukushima. Epidemiology 2017;28:e4–5. [DOI] [PubMed] [Google Scholar]
- [33].Takahashi H, Ohira T, Yasumura S, et al. Re: Thyroid cancer among young people in Fukushima. Epidemiology 2016;27:e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Suzuki S. Re: Thyroid cancer among young people in Fukushima. Epidemiology 2016;27:e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Takamura N. Re: Thyroid cancer among young people in Fukushima. Epidemiology 2016;27:e18. [DOI] [PubMed] [Google Scholar]
- [36].Shibata Y. Re: Thyroid cancer among young people in Fukushima. Epidemiology 2016;27:e19–20. [DOI] [PubMed] [Google Scholar]
- [37].Davis S. Commentary: screening for thyroid cancer after the Fukushima disaster: what do we learn from such an effort? Epidemiology 2016;27:323–5. [DOI] [PubMed] [Google Scholar]
- [38].Wakeford R, Auvinen A, Gent RN, et al. Re: Thyroid cancer among young people in Fukushima. Epidemiology 2016;27:e20–1. [DOI] [PubMed] [Google Scholar]
- [39].Jorgensen TJ. Re: Thyroid cancer among young people in Fukushima. Epidemiology 2016;27:e17. [DOI] [PubMed] [Google Scholar]
- [40].Korblein A. Re: Thyroid cancer among young people in Fukushima. Epidemiology 2016;27:e18–9. [DOI] [PubMed] [Google Scholar]
- [41].Tsuda T, Tokinobu A, Yamamoto E, et al. The authors respond. Epidemiology 2016;27:e21–3. [DOI] [PubMed] [Google Scholar]
- [42].Suzuki S, Suzuki S, Fukushima T, et al. Comprehensive survey results of childhood thyroid ultrasound examinations in fukushima in the first four years after the fukushima daiichi nuclear power plant accident. Thyroid 2016;26:843–51. [DOI] [PubMed] [Google Scholar]
- [43].Suzuki S. Childhood and adolescent thyroid cancer in fukushima after the fukushima daiichi nuclear power plant accident: 5 Years On. Clin Oncol (R Coll Radiol) 2016;28:263–71. [DOI] [PubMed] [Google Scholar]
- [44].Ahn HS, Kim HJ, Welch HG. Korea's thyroid-cancer “epidemic”--screening and overdiagnosis. N Engl J Med 2014;371:1765–7. [DOI] [PubMed] [Google Scholar]
- [45].Katanoda K, Kamo K, Tsugane S. Quantification of the increase in thyroid cancer prevalence in Fukushima after the nuclear disaster in 2011--a potential overdiagnosis? Jpn J Clin Oncol 2016;46:284–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].FMU. Fukushima Medical University (FMU), Thyroid Ultrasound Examination (Preliminary Baseline Screening), Supplemental Report of the FY2016 Survey; http://fmu-global.jp/fukushima-health-management-survey/ 2016. Accessed August 30, 2019. [Google Scholar]
- [47].FMU. Fukushima Medical University (FMU), Thyroid Ultrasound Examination (Second Full-Scale Thyroid Screening Program); http://fmu-global.jp/survey/proceedings-of-the-27th-prefectural-oversight-committee-meeting-for-fukushima-health-management-survey/ 2017. Accessed August 30, 2019. [Google Scholar]
- [48].FMU. Fukushima Medical University (FMU), Thyroid Ultrasound Examination (First Full-Scale Thyroid Screening Program); http://fmu-global.jp/survey/proceedings-of-the-27th-prefectural-oversight-committee-meeting-for-fukushima-health-management-survey/ 2017. Accessed August 30, 2019. [Google Scholar]
- [49].MPOC. The 23th Meeting of the Prefectural Oversight Committee (MPOC) for Fukushima Health Management Survey, http://fmu-global.jp/survey/the-23rd-prefectural-oversight-committee-meeting-for-fukushima-health-management-survey/ 2016. Accessed August 30, 2019. [Google Scholar]
- [50].MPOC. The 26th Meeting of the Prefectural Oversight Committee (MPOC) for Fukushima Health Management Survey, http://fmu-global.jp/survey/the-26th-prefectural-oversight-committee-meeting-for-fukushima-health-management-survey-2/ 2017. Accessed August 30, 2019. [Google Scholar]
- [51].Rothman KJ, Greenland S, Lash TL. Modern Epidemiology, Third Edition. 3rd ed.Philadelphia, Baltimore, New York, London, Buenos Aires, Hongkong, Sydney, Tokyo: Lippincott Williams & Wilkins; 2008. [Google Scholar]
- [52].Kaatsch P, Spix C, Jung I, et al. Childhood leukemia in the vicinity of nuclear power plants in Germany. Dtsch Arztebl Int 2008;105:725–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Beach SA, Dolphin GW. A study of the relationship between X-ray dose delivered to the thyroids of children and the subsequent development of malignant tumours. Phys Med Biol 1962;6:16. [DOI] [PubMed] [Google Scholar]
- [54].Dolphin GW, Beach SA. The relationship between radiation dose delivered to the thyroids of children and the subsequent development of malignant tumours. Health Phys 1963;9:1385–90. [DOI] [PubMed] [Google Scholar]
- [55].Schmitz-Feuerhake I, Muschol E, Bätjer K, et al. Risk estimation of radiation-induced thyroid cancer in adults. VIENNE: IAEA - International Atomic Energy Agency; 1978. [Google Scholar]
- [56].DeGroot LJ, Reilly M, Pinnameneni K, et al. Retrospective and prospective study of radiation-induced thyroid disease. Am J Med 1983;74:852–62. [DOI] [PubMed] [Google Scholar]
- [57].Howard J. Minimum Latency & Types or Categories of Cancer. 9.11 Monitoring and Treatment, https://www.cdc.gov/wtc/pdfs/policies/wtchpminlatcancer2014-11-07-508.pdf; 2014. [Google Scholar]
- [58].Saenko VA, Thomas GA, Yamashita S. Meeting report: the 5th International expert symposIum in Fukushima on radiation and health. Environ Health 2017;16:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Duffy SW, Gabe R. What should the detection rates of cancers be in breast screening programmes? Br J Cancer 2005;92:597–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Duffy SW, Agbaje O, Tabar L, et al. Overdiagnosis and overtreatment of breast cancer: estimates of overdiagnosis from two trials of mammographic screening for breast cancer. Breast Cancer Res 2005;7:258–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Steele RJ, Kostourou I, McClements P, et al. Effect of repeated invitations on uptake of colorectal cancer screening using faecal occult blood testing: analysis of prevalence and incidence screening. BMJ 2010;341:c5531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Anttila A, Koskela J, Hakama M. Programme sensitivity and effectiveness of mammography service screening in Helsinki, Finland. J Med Screen 2002;9:153–8. [DOI] [PubMed] [Google Scholar]
- [63].Blanks RG, Moss SM, Patnick J. Results from the UK NHS breast screening programme 1994–1999. J Med Screen 2000;7:195–8. [DOI] [PubMed] [Google Scholar]
- [64].Otten JD, van Dijck JA, Peer PG, et al. Long term breast cancer screening in Nijmegen, The Netherlands: the nine rounds from 1975–92. J Epidemiol Community Health 1996;50:353–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Steele RJ, McClements PL, Libby G, et al. Results from the first three rounds of the Scottish demonstration pilot of FOBT screening for colorectal cancer. Gut 2009;58:530–5. [DOI] [PubMed] [Google Scholar]
- [66].Shibata Y, Yamashita S, Masyakin VB, et al. 15 years after Chernobyl: new evidence of thyroid cancer. Lancet 2001;358:1965–6. [DOI] [PubMed] [Google Scholar]
- [67].Ito M, Yamashita S, Ashizawa K, et al. Childhood thyroid diseases around Chernobyl evaluated by ultrasound examination and fine needle aspiration cytology. Thyroid 1995;5:365–8. [DOI] [PubMed] [Google Scholar]
- [68].UNSCEAR. UNSCEAR 2013 Report, Volume I, United Nations Scientific Committee on the Effects of Atomic Radiation, report to the general assembly, scientific annex A: Levels and effects of radiation exposure due to the nuclear accident after the 2011 great east-Japan earthquake and tsunami, http://www.unscear.org/docs/reports/2013/13-85418_Report_2013_Annex_A.pdf 2013. Accessed August 30, 2019. [Google Scholar]
- [69].Gavrilin YI, Khrouch VT, Shinkarev SM, et al. Chernobyl accident: reconstruction of thyroid dose for inhabitants of the Republic of Belarus. Health Phys 1999;76:105–19. [DOI] [PubMed] [Google Scholar]
- [70].United Nations. Scientific Committee on the Effects of Atomic Radiation. Sources, effects and risks of ionizing radiation: United Nations Scientific Committee on the Effects of Atomic Radiation UNSCEAR 2016 Report to the General Assembly, with scientific annexes: Annex A - Methodology for estimating public exposures due to radioactive discharges. New York: United Nations; 2017. [Google Scholar]
- [71].Scherb H, Kusmierz R, Voigt K. Secondary sex ratio and trends in the associated gender-specific births near nuclear facilities in France and Germany: Update of birth counts. Reprod Toxicol 2019;89:159–67. [DOI] [PubMed] [Google Scholar]
- [72].UNSCEAR. UNSCEAR 2008 Report, United Nations Scientific Committee on the Effects of Atomic Radiation, Annex D, Health effects due to radiation from the Chernobyl accident, http://www.unscear.org/docs/publications/2008/UNSCEAR_2008_Annex-D-CORR.pdf 2008. Accessed August 30, 2019. [Google Scholar]
- [73].Kim E, Kurihara O, Kunishima N, et al. Internal thyroid doses to Fukushima residents-estimation and issues remaining. J Radiat Res 2016;57Suppl 1:i118–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].UNSCEAR. UNSCEAR 2000 Report, United Nations Scientific Committee on the Effects of Atomic Radiation, Annex J, Exposure and effects of the Chernobyl accident, http://www.unscear.org/docs/publications/2000/UNSCEAR_2000_Annex-J.pdf 2000. Accessed August 30, 2019. [Google Scholar]
- [75].Yablokov AV, Nesterenko VB, Nesterenko AV. Chernobyl: Consequences of the Catastrophe for People and the Environment. Berlin Heidelberg New York Tokyo: John Wiley and Sons; 2010. [Google Scholar]
- [76].Yamashita S, Suzuki S. Risk of thyroid cancer after the Fukushima nuclear power plant accident. Respir Investig 2013;51:128–33. [DOI] [PubMed] [Google Scholar]
- [77].WHO. World Health Organisation (WHO), Preliminary dose estimation from the nuclear accident after the 2011 Great East Japan Earthquake and Tsunami, http://www.who.int/ionizing_radiation/pub_meet/fukushima_dose_assessment/en/ 2012. Accessed August 30, 2019. [Google Scholar]
- [78].Scherb H, Mori K, Hayashi K. Comment on ’Perinatal mortality after the Fukushima accident’. J Radiol Prot 2019;39:647–9. [DOI] [PubMed] [Google Scholar]
- [79].Murase K, Murase J, Mishima A. Nationwide increase in complex congenital heart diseases after the fukushima nuclear accident. J Am Heart Assoc 2019;8:e009486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Murase K, Murase J, Machidori K, et al. Nationwide increase in cryptorchidism after the fukushima nuclear accident. Urology 2018;118:65–70. [DOI] [PubMed] [Google Scholar]
- [81].Scherb H, Mori K, Hayashi K. Increases in perinatal mortality in prefectures contaminated by the Fukushima nuclear power plant accident in Japan: A spatially stratified longitudinal study. Medicine (Baltimore) 2016;95:e4958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Hayashida N, Imaizumi M, Shimura H, et al. Thyroid ultrasound findings in a follow-up survey of children from three Japanese prefectures: Aomori, Yamanashi, and Nagasaki. Sci Rep 2015;5:9046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Stephen LaFranchi M. UpToDate, Thyroid nodules and cancer in children, http://www.uptodate.com/contents/thyroid-nodules-and-cancer-in-children 2017. Accessed August 30, 2019. [Google Scholar]
- [84].Hogan AR, Zhuge Y, Perez EA, et al. Pediatric thyroid carcinoma: incidence and outcomes in 1753 patients. J Surg Res 2009;156:167–72. [DOI] [PubMed] [Google Scholar]
- [85].MacMahon B, Pugh TF. Epidemiolgy, Principles and Methods. 1st ed.Boston: Little, Brown and Company; 1970. [Google Scholar]
- [86].Shibuya K, Gilmour S, Oshima A. Time to reconsider thyroid cancer screening in Fukushima. Lancet 2014;383:1883–4. [DOI] [PubMed] [Google Scholar]
- [87].Tokonami S, Hosoda M, Akiba S, et al. Thyroid doses for evacuees from the Fukushima nuclear accident. Sci Rep 2012;2:507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Furuta S, Sumiya S, Watanabe H, et al. Results of the environmental radiation monitoring following the accident at the Fukushima Daiichi Nuclear Power Plant. JAEA-Review 2011-035 (2011). (in Japanese with English abstract). 2017 https://inis.iaea.org/search/search.aspx?orig_q=RN:43088311 Accessed July 23, 2018. [Google Scholar]
- [89].Kamada N, Saito O, Endo S, et al. Radiation doses among residents living 37 km northwest of the Fukushima Dai-ichi Nuclear Power Plant. J Environ Radioact 2012;110:84–9. [DOI] [PubMed] [Google Scholar]
- [90].Ohira T, Takahashi H, Yasumura S, et al. Comparison of childhood thyroid cancer prevalence among 3 areas based on external radiation dose after the Fukushima Daiichi nuclear power plant accident: The Fukushima health management survey. Medicine (Baltimore) 2016;95:e4472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Ohira T, Takahashi H, Yasumura S, et al. Associations between childhood thyroid cancer and external radiation dose after the fukushima daiichi nuclear power plant accident. Epidemiology 2018;29:e32–4. [DOI] [PubMed] [Google Scholar]
- [92].Tsuda T, Tokinobu A, Yamamoto E, et al. Re: Associations between childhood thyroid cancer and external radiation dose after the fukushima daiichi nuclear power plant accident. Epidemiology 2018;29:e56–7. [DOI] [PubMed] [Google Scholar]
- [93].Ishikawa T, Takahashi H, Yasumura S, et al. Representativeness of individual external doses estimated for one quarter of residents in the Fukushima Prefecture after the nuclear disaster: the Fukushima Health Management Survey. J Radiol Prot 2017;37:584–605. [DOI] [PubMed] [Google Scholar]
- [94].Schmitz-Feuerhake I, Batjer K, Muschol E. Evaluation of the somatic radiation risks and the recommendations in the IRCP publication no. 26 (1977) (author's transl). Rofo 1979;131:84–9. [DOI] [PubMed] [Google Scholar]
- [95].Doi SA, Woodhouse NJ, Thalib L, et al. Ablation of the thyroid remnant and I-131 dose in differentiated thyroid cancer: a meta-analysis revisited. Clin Med Res 2007;5:87–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Schmitz-Feuerhake I, Batjer K, Prevot H. Risk estimation for thyroid cancer induced by diagnostic radiation doses (authors transl). Rofo 1978;128:622–7. [DOI] [PubMed] [Google Scholar]
- [97].Shimura H, Sobue T, Takahashi H, et al. Findings of thyroid ultrasound examination within 3 years after the Fukushima nuclear power plant accident: The Fukushima health management survey. J Clin Endocrinol Metab 2018;103:861–9. [DOI] [PubMed] [Google Scholar]


