Abstract
Rationale:
The prevalence of obesity has increased over the past few years, becoming a public health problem. Generally, the primary therapeutic remedies are diet, physical exercise, medication, and bariatric surgery. However, an increased number of obese and overweight people are using complementary and herbal slimming supplements.
Patient concerns:
A 70-years-old Caucasian woman presented to the outpatient clinic with tachycardia (>100 bpm), insomnia, anxiety, and recent weight loss (6 kilos in 3 months). She had no previous thyroid disease, but she presented transient hyperthyroidism at 3 months after ingestion of tablets containing kelp seaweeds.
Diagnoses:
Hypertensive and obese patient, without previous thyroid disease, presented with transient hyperthyroidism at 3 months following ingestion of tablets containing kelp seaweed.
Interventions:
The kelp-containing tablets were discontinued, and antithyroid therapy with Methimazole was initiated as follows: Methimazole at 15 mg/day for 1 month, at 10 mg/day in the second month, and 5 mg/day for the third month.
Outcomes:
After 3 months of antithyroid therapy and without the consumption of kelp - containing tablets, normal thyroid function was regained. Further analysis revealed normal thyroid function, so the hyperthyroidism reversed completely.
Lessons:
Adults who consume complementary medication based on kelp seaweed should be informed of the risk of developing thyroid dysfunction also in the absence of any pre-existing thyroid disease. Due to the high iodine content, supplements containing kelp should be taken with the supervision of a physician and with monitoring of thyroid function.
Keywords: complementary medication, iodine, kelp seaweed, obesity, thyroid disease
1. Introduction
The prevalence of obesity has continuously increased over the last few years becoming a public health problem. Generally, the primary therapeutic remedies are diet, physical exercise, medication, and bariatric surgery. However, a greater number of obese and overweight people are utilizing complementary and herbal slimming medicines.
Herbal medications consumed for weight loss are available in pharmacies, herbal stores and on the Internet. Most of these products used for weight loss contain kelp seaweed. Kelp has many therapeutic and nutritional benefits: it is a rich in iodine, minerals, antioxidants, fiber, proteins, and complex carbohydrates. Because kelp seaweed is rich in iodine, depending on its quantity, ingestion may lead to thyroid dysfunction.
We report a case of thyroid dysfunction due to the consumption of kelp containing tablets. Informed written consent was obtained from the patients for publication of this case report and accompanying images. Approval from the ethics committee of the University Emergency Clinical Hospital “Pius Brinzeu” Timisoara was obtained.
2. Case presentation
2.1. Patient information
A 70-years-old Caucasian woman presented to the outpatient clinic with tachycardia (>100 bpm), insomnia, anxiety, and recent weight loss (6 kilos in three months). Her social history was negative for alcohol and smoking. Her medical history and familial history were significant for hypertension and cardiac disorders (both parents). There was no history of thyroid diseases. She took antihypertensive medications (converse enzyme inhibitors and beta blockers). Due to her hypertension, she had replaced salt with iodized salt in her diet.
Previous endocrine tests revealed normal thyroid hormones and thyroid-stimulating hormone (TSH) plasma levels and absence of thyroid antibodies (AB). A detailed medical history revealed that for the past 3 months, she had self-administrated 2 tablets of a dietary supplement containing kelp for weight loss daily.
The patient has provided informed consent for publication of this case.
2.2. Investigations
At our initial exam, the patient weighed 84 kg, and was 157 cm tall, with a body mass index (BMI) of 34.14 (obesity grade I). She displayed clinical signs of hyperthyroidism: a regular pulse of 100 bpm, warm moist skin, trembling hands, anxiety, and insomnia. Initially, she was obese grade II (90 kg, BMI = 36.58). Physical examination revealed a healthy thyroid gland. Thyroid ultrasound: thyroid aspect – homogenous, hypoechogenic, volume 3.7 ml for right lobe and 2.7 ml for left lobe, thyroid isthmus = 4.3 mm, but with small nodular formations in both lobes.
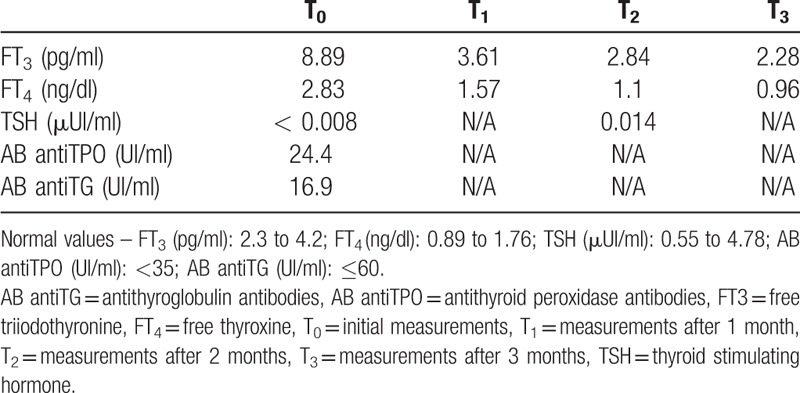
The hormonal profile was of overt hyperthyroidism, she was negative antithyroid-stimulating hormone receptor AB and also for antithyroid peroxidase (antiTPO) and antithyroglobulin (anti-TG) AB (Table 1).
Table 1.
The hormonal and antibodies profile.
2.3. Treatment
The kelp-containing tablets were discontinued, and we were initiated antithyroid therapy with Methimazole 15 mg/day for 1 month. After 1 month of treatment, the thyroid hormone levels returned to normal, and the Methimazole dose was reduced to 10 mg/day. After another month of drug therapy, the Methimazole dose was reduced to 5 mg/day. Following 3 months of drug therapy, the treatment was discontinued, and the patient maintained thyroid hormones within normal range.
2.4. Outcome and follow-up
Endocrine tests revealed normal range of thyroid hormones and thyroid stimulating hormone (TSH) at 1, 2, 3, 6, and 12 months following completion of Methimazole treatment (Table 2). In addition, the thyroid was further monitored, and the endocrine tests showed normal thyroid function, so the hyperthyroidism reversed completely.
Table 2.
The hormonal profile at follow-up.

3. Discussion
We have described a case of transient hyperthyroidism following the ingestion of kelp-containing tablets.
Hyperthyroidism is a condition that occurs due to excessive production of thyroid hormones by the thyroid gland. Typically blood tests show low TSH and elevated levels of the thyroid hormones free triiodothyronine (FT3) and free thyroxine (FT4).[1]
The principal causes of hyperthyroidism are Graves’ disease, toxic thyroid adenoma and toxic multinodular goiter. Other causes include Hashimoto's thyroiditis, subacute thyroiditis, oral consumption of excess thyroid hormone tablets, postpartum thyroiditis, and excess iodine consumption from algae, such as kelp, antiarrhythmic drugs, such as amiodarone, or radioactive substances containing iodine.[2] In our case, thyroid dysfunction developed following excess iodine consumption from kelp-algae.
The thyroid gland requires iodine to produce thyroid hormones. Generally, the amount of iodine needed is obtained from the diet (iodized salt, dairy products, bread, and seafood).[3] The quantity of iodine required for normal thyroid function is 150 mcg in adults who are not pregnant or lactating. Insufficient or excess iodine intake may cause hypothyroidism or hyperthyroidism with or without goiter and autoimmunity.[3]
Most individuals with normal thyroid function can tolerate excess iodine intake and maintain thyroid hormone levels within a normal range. Although serum FT4 and FT3 can usually be moderately reduced, TSH increase may cause a small goiter to develop.[3]
The thyroid gland has intrinsic regulatory mechanisms that maintain normal thyroid function when iodine intake is excessive. However, subjects with normal thyroid function who receive excess amounts of iodine, have been observed to have a transient decrease in thyroid hormones synthesis upwards of 48 hours. This reaction is called the acute Wolff-Chaikoff effect and is due to increased intra-thyroid iodine concentrations.[4]
The adaptation to or escape from the acute Wolff-Chaikoff effect is caused by a decrease in the expression of the sodium-iodide symporter. This reduces the active transport of iodide into the thyroid gland, thus restoring normal thyroid function.[5] Subjects remain euthyroid even in the case of excess of iodine administration (up to 500 mcg/day).[6]
Most individuals can tolerate chronic excess iodine without clinical symptoms, the normal thyroid gland can adapt to excess iodine by the mechanisms described above. Sometimes these mechanisms fail, and excess iodine leads to overt clinical hyperthyroidism or hypothyroidism. In some instances, such as in a multinodular goiter, the excess iodine can trigger hyperproduction of thyroid hormones leading to clinical signs of hyperthyroidism. Our patient presented small nodular formations in both lobes of her thyroid.
Over the past years, many countries (including Romania) have introduced iodized salt into foods and nutrition to prevent hypothyroidism and cardiovascular diseases. Research on the health benefits of iodized salt in food and diet are unclear. Several studies of populations in Denmark,[7] New Zealand,[8] Spain,[9] and Zimbabwe[10] have shown an increased prevalence of transient hyperthyroidism and thyrotoxicosis; however, a Bangladesh study did not note adverse effects on the thyroid gland.[11]
Kelp seaweed may contain high quantity of iodine depending on the harvest location and preparation. Iodine levels can range between 200 and 1500 mcg/g in a dried preparation depending on the kelp type.[12] Kelp seaweed was originally consumed in the diet of some Asian and European countries.[13]
The medical literature has documented many instances of iodine-induced hyperthyroidism following ingestion of kelp seaweed, including kelp-containing teas,[14] kelp containing marketed diets,[15] and kelp-containing dietary supplements.[16] In other studies, chronic kelp seaweed ingestion only caused a slight increase of TSH levels without overt thyroid dysfunction.[17,18]
In our case, the subject did not have pre-existing or underlying thyroid disease. For 3 months, she ingested 336 mcg iodine daily from complementary medication (Table 3). This is double the amount of iodine necessary for proper thyroid function. The total amount of iodine she consumed from her diet (milk, yogurt, bread, fruits, and vegetables), and supplements was greater than 500 mcg/day (Table 4). She developed hyperthyroidism after 3 months of taking kelp supplement. She recovered normal thyroid function after discontinuing the kelp seaweed supplement and starting antithyroid medication.
Table 3.
Components of tablets∗.
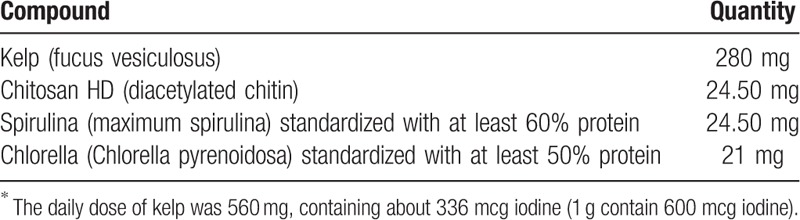
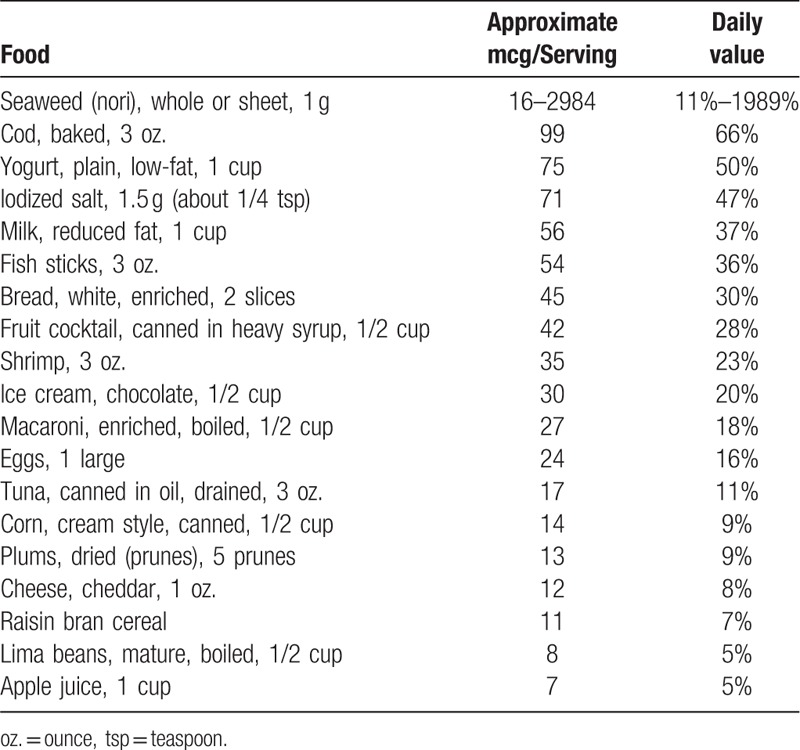
Table 4.
Selected sources of dietary iodine20.
A UK-based study evaluated 224 seaweed-containing product labels for iodine content. Only 10% of the products evaluated (22) contained information regarding iodine content, and another 18% (40) only provided information sufficient to estimate the iodine content. Of these, 26 products may potentially lead to iodine intake in excess of accepted European levels.[19]
The quantity of iodine is not included on a product label unless the food has been fortified with it. Foods containing ≥20% of the necessary iodine quantity are considered a high source of the nutrient (Table 4).[20]
Treatment of hyperthyroidism requires ending the excess iodine exposure and use of an antithyroid medication. Generally, after a few months of treatment, the normal thyroid function is recovered. In our case, the patient discontinued taking the kelp containing tablets and was treated with Methimazole for 3 months. She regained normal thyroid function and maintained it for 1 year following treatment, thus completely reversing the hyperthyroidism.
The case we presented demonstrates that transient hyperthyroidism can be induced by excess iodine intake in persons with normal thyroid function. Healthy individuals with normal thyroid function should be cautioned to avoid kelp or other iodine-rich containing herbal medicines or diets for weight loss.
4. Conclusion
People who take complementary medication containing kelp seaweed should be informed of the risk of developing a thyroid dysfunction even in the absence of pre-existing thyroid disease. Due to the elevated iodine content, complementary medication containing kelp should only be taken under the supervision of a physician with monitoring of thyroid function.
Acknowledgments
We thank the patient for participating in this study.
Author contributions
Conceptualization: Adriana Gherbon.
Investigation: Adriana Gherbon.
Methodology: Mirela Frandes, Diana Lungeanu.
Resources: Marioara Nicula.
Supervision: Adriana Gherbon, Romulus Timar.
Validation: Adriana Gherbon, Mirela Frandes.
Writing – original draft: Adriana Gherbon, Mirela Frandes, Diana Lungeanu, Marioara Nicula, Romulus Timar.
Writing – review & editing: Adriana Gherbon, Mirela Frandes, Diana Lungeanu, Marioara Nicula, Romulus Timar.
Footnotes
How to cite this article: Gherbon A, Frandes M, Lungeanu D, Nicula M, Timar R. Transient Hyperthyroidism following the ingestion of complementary medications containing kelp seaweed. Medicine. 2019;98:37(e17058).
Abbreviations: AB = antibodies, antiTG = antithyroglobulin, antiTPO = antithyroid Peroxidase, BMI = body mass index, bpm = beats per minute, FT3 = free triiodothyronine, FT4 = free thyroxine, mcg = microgram, T0 = initial measurements, T1 = measurements after 1 month, T2 = measurements after 2 months, T3 = measurements after 3 months, T5 = measurements after 1 year, TSH = thyroid stimulating hormone, UK = United Kingdom.
Informed written consent was obtained from the patient for publication of this case report and the accompanying images.
The authors have no conflicts of interests to disclose.
References
- [1].De Leo S, Lee SY, Braverman LE. Hyperthyroidism. Lancet 2016;388:906–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Ross DS, Burch HB, Cooper DS, et al. 2016 American Thyroid Association Guidelines for Diagnosis and Management of Hyperthyroidism and Other Causes of Thyrotoxicosis. Thyroid 2016;26:1343–421. doi: 10.1089/thy.2016.0229 [DOI] [PubMed] [Google Scholar]
- [3].Wolmarans De W. Maintaining euthyroidism: fundamentals of thyroid hormone physiology, iodine metabolism and hypothyroidism. South Afr Fam Pract 2017;59:11–21. [Google Scholar]
- [4].Wolff J, Chaikoff IL, Goldberg Meier JR. The temporary nature of the inhibitory action of excess iodide on organic iodine synthesis in the normal thyroid. Endocrinology 1949;45:504–13. [DOI] [PubMed] [Google Scholar]
- [5].Eng PHK, Cardona GR, Fang SL, et al. Escape from the acute Wolff-Chaikoff effect is associated with a decrease in thyroid sodium/iodide symporter messenger ribonucleicacid and protein. Endocrinology 1999;140:3404–10. [DOI] [PubMed] [Google Scholar]
- [6].Paul T, Meyer B, Witorsch RJ, et al. The effect of small increases in dietary iodine on thyroid function in euthyroid subjects. Metabolism 1988;37:121–4. [DOI] [PubMed] [Google Scholar]
- [7].Laurberg P, et al. The Danish investigation on iodine intake and thyroid disease, Dan Thyr: status and perspectives. Eur J Endocrinol 2006;155:219–28. [DOI] [PubMed] [Google Scholar]
- [8].Thomson CD, Campbell JM, Miller J, et al. Minimal impact of excess iodate intake on thyroid hormones and selenium status in older New Zealanders. Eur J Endocrinol 2011;165:745–52. [DOI] [PubMed] [Google Scholar]
- [9].Galofre JC, Fernandez-Calvet L, Rios M, et al. Increased incidence of thyrotoxicosis after iodine supplementation in an iodine sufficient area. J Endocrinol Invest 1994;17:23–7. [DOI] [PubMed] [Google Scholar]
- [10].Todd CH, et al. Increase in thyrotoxicosis associated with iodine supplements in Zimbabwe. Lancet 1995;346:1563–4. [DOI] [PubMed] [Google Scholar]
- [11].Parveen S, Latif SA, Kamal MM, et al. Effects of long-term iodized salt consumption on serum T3, T4 and TSH in an iodine deficient area of Bangladesh. Mymensingh Med J 2007;16:57–60. [DOI] [PubMed] [Google Scholar]
- [12].Teas J, Pino S, Critchley A, et al. Variability of iodine content in common commercially available edible seaweeds thyroid 2004;14:836–41. [DOI] [PubMed] [Google Scholar]
- [13].Zava TT, Zava DT. Assessment of Japanese iodine intake based on seaweed consumption in Japan: a literature-based analysis. Thyroid Res 2011;4:14–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Mussig K, et al. Iodine-induced thyrotoxicosis after ingestion of kelp-containing tea. J Gen InternMed 2006;21:C11–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Di Matola T, Zeppa P, Gasperi M, et al. Thyroid dysfunction following a kelp-containing marketed diet. BMJ Case Rep 2014;ii: bcr2014206330. doi: 10.1136/bcr-2014-206330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Eliason BC. Transient hyperthyroidism in a patient taking dietary supplements containing kelp. JAm Board Fam Pract 1998;11:478–80. [DOI] [PubMed] [Google Scholar]
- [17].Teas J, et al. Seaweed and soy: companion foods in Asian cuisine and their effects on thyroid function in American women. J Med Food 2007;10:90–100. [DOI] [PubMed] [Google Scholar]
- [18].Miyai K, Tokushige T, Kondo M. Iodine Research Group. Suppression of thyroid function during ingestion of seaweed “Kombu” (Laminaria japonoca) in normal Japanese adults. Endocr J 2008;55:1103–8. [DOI] [PubMed] [Google Scholar]
- [19].Bouga M, Combet E. Emergence of seaweed and seaweed-containing foods in the UK: focus on labeling, iodine content, toxicity and nutrition. Foods 2015;4:240–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Wright KC. Update on Iodine. Today's Dietitian 2018;20:24. [Google Scholar]


