Abstract
Background:
Growing evidence showed that high pretreatment plasma fibrinogen could be used as a potential prognostic marker in colorectal cancer (CRC). However, the conclusions were controversial. Therefore, this meta-analysis was conducted to evaluate the prognostic value of pretreatment plasma fibrinogen in patients with CRC.
Methods:
Relevant studies were searched in the databases including PubMed, EMBASE, Web of Science, Cochrane library, and China National Knowledge Infrastructure up until December 10th, 2018. Pooled hazard ratios (HRs) with their 95% confidence intervals (CIs) were used to estimate the effects.
Results:
A total of 17 articles with 6863 patients were included in this meta-analysis. The results revealed that elevated pretreatment plasma fibrinogen was significantly associated with both poor overall survival (univariate analysis: HR = 1.69, 95% CI 1.47–1.95, P = .000; multivariate analysis: HR = 1.50, 95% CI 1.28–1.77, P = .000) and poor disease-free survival (univariate analysis: HR = 1.90, 95% CI 1.49–2.41, P = .000; multivariate analysis: HR = 2.08, 95% CI 1.52–2.86, P = .000) in patients with CRC.
Conclusions:
High pretreatment plasma fibrinogen level is significantly associated with worse survival outcomes in CRC patients. Plasma fibrinogen may be used as an effective prognostic marker and potential therapeutic target. Further studies are required to support these results.
Keywords: colorectal cancer, fibrinogen, meta-analysis, prognosis
1. Introduction
Colorectal cancer (CRC) is a common digestive system cancer and it is the third most common malignant tumor among all cancer types following breast/prostate, lung, and bronchus cancer all over the world.[1,2] About 1.2 million new cases and 600,000 deaths occur each year.[3] A number of studies have shown that early screening and treatment can reduce patients’ mortality, so it is urgent to find more relevant prognostic factors and apply them to clinical treatment to improve the prognosis of patients.
Chronic inflammation is relevant to the estimated 15% of the cancer-related death,[4] and a large amount of evidence showed that chronic inflammation was one of the most important causes of the metastasis of CRC.[5] What's more, a large number of factors of the hemostatic system have been linked to the progression of CRC, especially metastasis.[6] Fibrinolytic mechanisms are important for tumor growth and proliferation, and they are involved in important steps of cancer progression such as tumor cell invasion, fibrin network remodeling, and capillary hyperplasia.[7] On the contrary, the presence of tumor may affect the coagulation and hemostasis systems of the host by altering the molecular environment and promoting the growth, progression, and metastasis of tumor cells.[8] Fibrinogen is a dimer with 340 kDa consisting of 3 pairs of nonidentical polypeptide chains, termed α-, β-, and γ-chains.[9] It consists of 2964 amino acids, which are connected by disulfide bonds and synthesized by the liver, but also exists in megakaryocytes.[10] Fibrinogen is an important factor of both chronic inflammation and hemostatic system.[11] Angiogenesis is considered to be important for the growth and metastasis of tumors and fibrinogen is also involved in the regulation of angiogenic mechanisms.[12] In addition, fibrinogen can also protect tumor cells from the host's antitumor cell immune surveillance mechanism.[13]
So far, many studies have shown that plasma fibrinogen can be used as an inflammatory marker and also as a prognostic marker of CRC, but controversy persists. Therefore, this meta-analysis was performed to investigate comprehensively prognostic value of pretreatment plasma fibrinogen in CRC patients.
2. Materials and methods
2.1. Study selection
Two authors (ML and YW) conducted independently a comprehensive literature retrieval using the PubMed, EMBASE, Web of Science, Cochrane library, and China National Knowledge Infrastructure (CNKI). The search time was up to December 10th, 2018. The following keywords and their combinations were used in searching: “colorectal” or “rectal” or “colon” or “bowel,” and “cancer” or “carcinoma” or “adenocarcinoma” or “neoplasms,” and “fibrinogen.” In addition, we also retrieved the references of the relevant articles for data extraction and analysis.
2.2. Inclusion and exclusion
Literature inclusion criteria included the following: all primary CRCs were all histopathologically diagnosed; assessment of the prognostic effect of pretreatment plasma fibrinogen on overall survival (OS), disease-free survival (DFS), recurrence-free survival (RFS), or progression-free survival (PFS); the hazard ratio (HR) with its 95% confidence interval (CI) for survival outcomes were provided directly, or these values could be extracted by reading Kaplan-Meier (KM) curve in original articles; the HR and its 95% CI extracted from the adjusted KM curve were regarded as the results of multivariate analysis; otherwise, they were regarded as the results of univariate analysis; reports were published in English or Chinese. Studies that did not meet the inclusion criteria and reported in case reports, reviews, conference abstracts, letters, or systematic reviews were excluded. The report published earlier was included when duplicate publications came from the same cohort study. Discrepancies were resolved by discussion with the third appraiser.
2.3. Data collection and quality assessment
Two authors (ML and YW) extracted independently the following information from each included study: the first author, publication year, country, number of patients, age, cancer stage, metastasis, cutoff value (per 100 U of concentration) of plasma fibrinogen, survival outcome, the HR with its 95% CI, HR source, follow-up time, and quality assessment score. The outcome measures were OS and/or DFS. In case HRs and their 95% CIs for survival outcomes were not provided directly, the method introduced by Tierney et al[14] was used to extract HRs by reading KM curve.
A quality assessment of the included studies was assessed by using the Newcastle–Ottawa Quality Assessment Scale (NOS). The stars obtained from the NOS, which consist of selection, comparability, and outcome, varied from 0 to 9. A study that scored ≥6 stars was defined as high-quality.
2.4. Statistical analysis
Statistical analyses in this meta-analysis were performed using STATA 12.0. Pooled HRs with CIs were used to evaluate the prognostic role of pretreatment plasma fibrinogen on OS/DFS in colocrectal cancer patients. Statistical heterogeneity among studies was tested using χ2 test and I2 statistics. A fixed-effect model was implemented if there was no obvious heterogeneity (I2 < 50% and P > .1); otherwise, a random-effect model was implemented. Sensitivity analysis was carried out to evaluate the influence of single study on pooled results and to find the causes of heterogeneity. Both the Begg funnel plot and Egger linear regression test were used to check the potential publication bias. P < .05 was considered as statistically significant.
2.5. Ethics approval
Because no patient participates in this meta-analysis directly, ethical approval was not necessary.
3. Results
3.1. Summary of enrolled studies
At first, a total of 773 studies were available from above databases. After 327 duplicated studies were excluded, 446 studies remained. A total of 425 studies were excluded as case reports, reviews, irrelevant topics, animal studies, and not English or Chinese language studies by screening the titles and abstracts, and 21 articles were left for full-text review. In the end, 17 articles with 6863 patients were included in this quantitative synthesis. Details of identifying studies were described in Figure 1.
Figure 1.
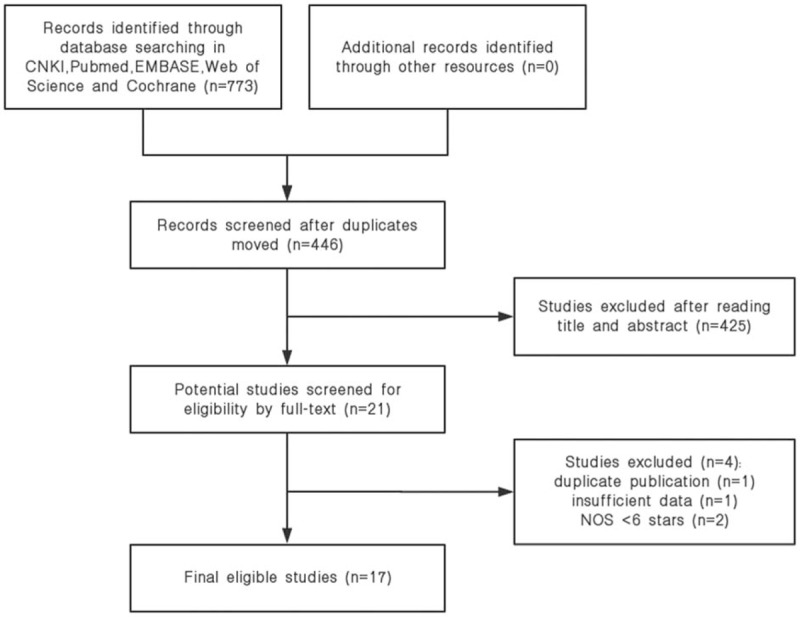
Flow diagram of this study selection process.
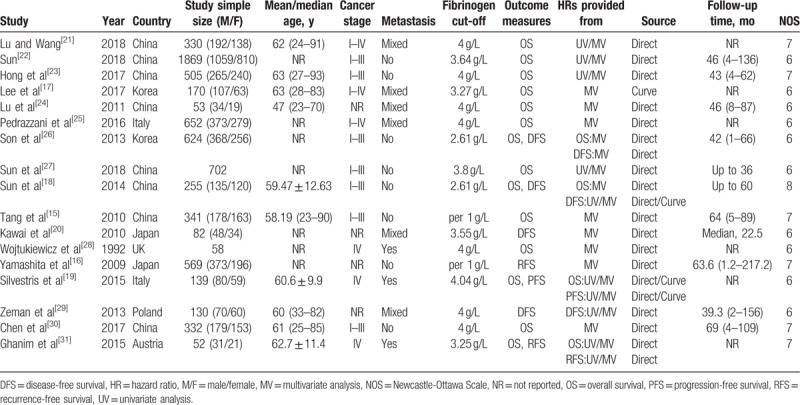
A total of 8 studies were from China, 2 studies were performed in Korea, 2 studies were carried out in Italy, 2 studies were actualized in Japan, the remaining 3 studies were performed in UK, Poland, and Austria, respectively. Seventeen studies with the numbers of participants ranging from 52 to 1869 were published between 1992 and 2018. Except for 2 studies,[15,16] the others studies reported the cutoff values of pretreatment plasma fibrinogen range from 2.61 to 4.04 g/L. As to survival outcomes, OS results were reported in 14 studies, DFS results were reported in 4 studies, RFS results were reported in 2 studies, and PFS results were reported in 1 study. All HRs with their 95% CIs were reported directly in original studies except 3 studies,[17–19] in which HRs with their 95% CIs were calculated through KM curve in the method introduced by Tierney et al indirectly. The studies[15,18,20] calculated relative risks (RRs) with their 95% CIs by using the Cox proportional hazards model, which were pooled with HRs together in this meta-analysis. The characteristics of these studies were shown in Table 1.[15–31]
Table 1.
Main characteristics of 18 studies included in the meta-analysis.
3.2. Prognostic value of pretreatment plasma fibrinogen on OS in CRC
According to univariate analysis results, there were 6 studies including 3597 patients investigating the relationship between pretreatment plasma fibrinogen and OS in CRC. Among those studies, towing to no obvious heterogeneity (I2 = 0.0%, P = .501), fixed-effect model was used to calculate the pooled HR and its 95% CI (HR = 1.69, 95% CI 1.47–1.95, P = .000) (Fig. 2A). In addition, 14 studies including 6082 patients investigated the relationship between pretreatment plasma fibrinogen and OS in CRC according to multivariate analysis results. Owing to the severe heterogeneity (I2 = 45.8%, P = .031), random-effect model was used to calculate the pooled HR and its 95% CI (HR = 1.50, 95% CI 1.28–1.77, P = .000) (Fig. 2B). The pooled analysis showed that high pretreatment plasma fibrinogen was significantly associated with worse OS in CRC patients.
Figure 2.
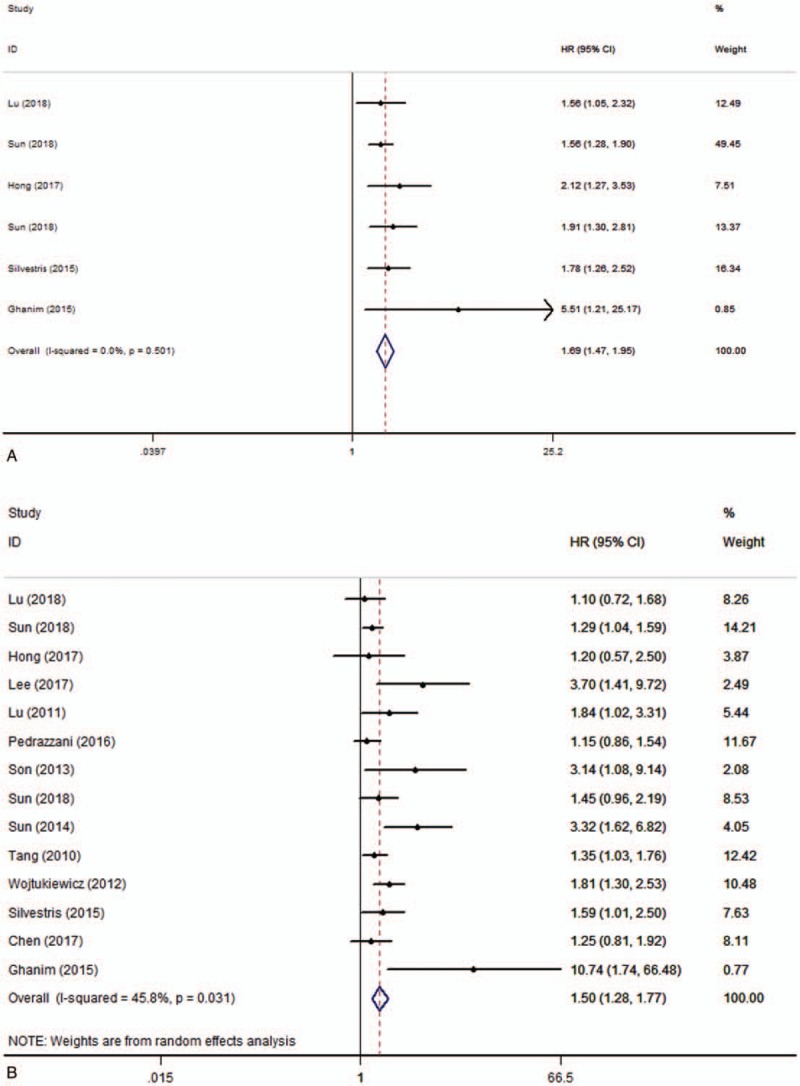
Forest plots of pooled hazard ratio (HR) for the association of pretreatment plasma fibrinogen with overall survival. (A) Univariate analysis, (B) multivariate analysis.
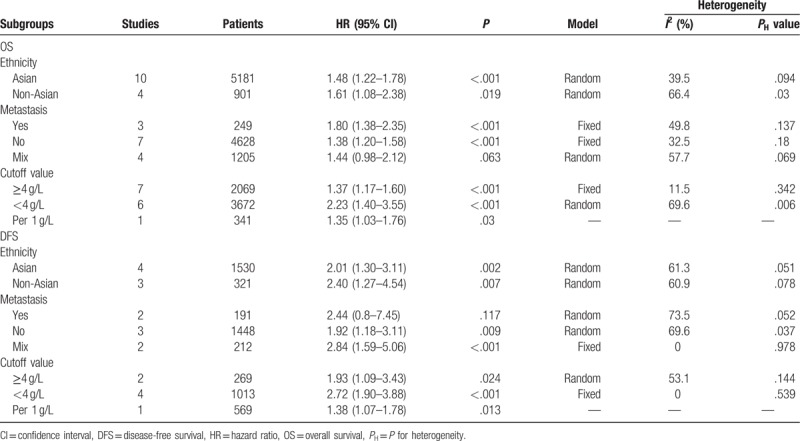
Owing to the obvious heterogeneity in multivariate analysis results, we performed subgroup analyses by ethnicity, metastasis, and cutoff value to explore the potential sources of heterogeneity. The results of subgroup analysis showed that a significant association between high pretreatment plasma fibrinogen level and shorter OS in Asian (HR = 1.48, 95% CI 1.22–1.78, P = 0.000) and non-Asian patients (HR = 1.61, 95% CI 1.08–2.38, P = .019). As for clinical setting of disease, the results showed that high pretreatment plasma fibrinogen was significantly associated with worse OS in patients with metastatic disease (HR = 1.80, 95% CI 1.38–2.35, P = .000) and nonmetastatic disease (HR = 1.38, 95% CI 1.20–1.58, P = .000). However, there was no significant association between high pretreatment plasma fibrinogen and OS of CRC patients in mixed subgroup (patients with both non-metastatic and metastatic disease). Subgroup analysis based on cut-off value revealed high pretreatment plasma fibrinogen was associated with poor OS in the ≥4 g/L (HR = 1.37,95%CI 1.17–1.60,P = 0.000) and <4 g/L groups (HR = 2.23,95%CI 1.40–3.55,P = 0.001). The results of subgroup analyses about OS were shown in Table 2.
Table 2.
Pooled hazard ratios for OS and DFS according to subgroup analyses in multivariate analysis.
3.3. Prognostic value of pretreatment plasma fibrinogen on DFS in CRC
There were 4 studies with 576 patients investigating the relationship between pretreatment plasma fibrinogen and DFS in CRC according to univariate analysis results. Because there was no obvious heterogeneity (I2 = 12.3%, P = .331), fixed-effect model was implemented to calculate the pooled HR and its 95% CI (HR = 1.90, 95% CI 1.49–2.41, P = .000) (Fig. 3A). Meanwhile, a total of 7 studies with 1851 patients investigated the relationship between pretreatment plasma fibrinogen and DFS in CRC according to multivariate analysis results. Owing to the obvious heterogeneity (I2 = 55.4%, P = .036), random-effect model was implemented to calculate the pooled HR and its 95% CI (HR = 2.08, 95% CI 1.52–2.86, P = .000) (Fig. 3B). These results showed that high pretreatment plasma fibrinogen was significantly related to a worse DFS in CRC patients.
Figure 3.
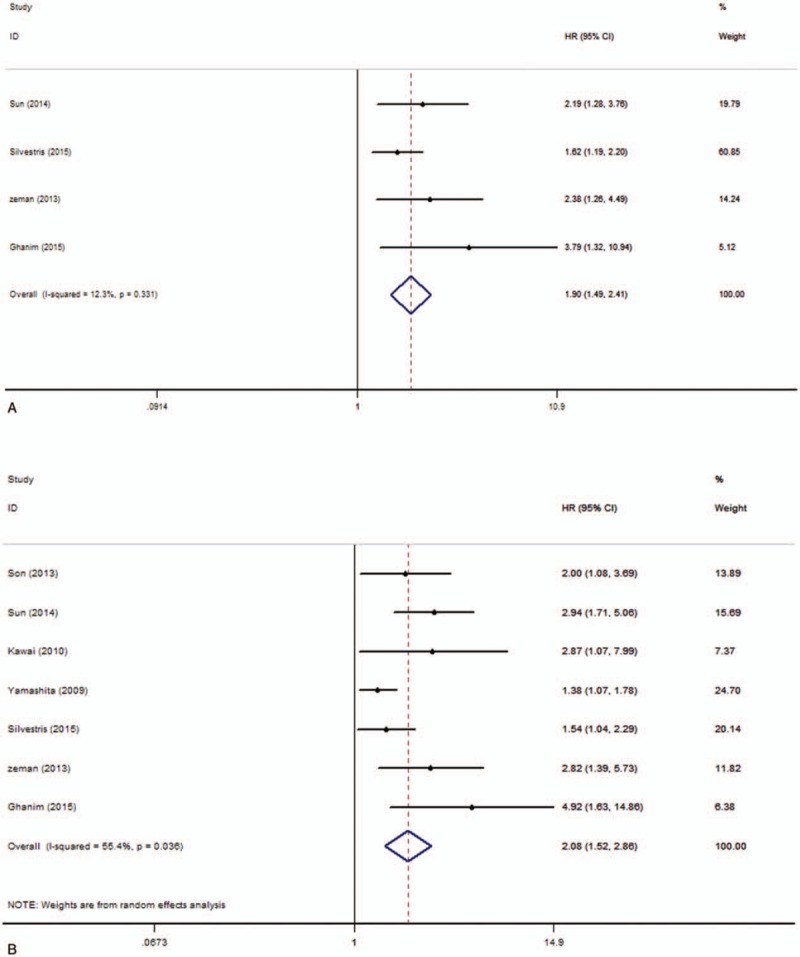
Forest plots of pooled hazard ratio (HR) for the association of pretreatment plasma fibrinogen with disease-free survival. (A) Univariate analysis, (B) multivariate analysis.
Because of the severe heterogeneity in multivariate analysis results, we carried out subgroup analyses by ethnicity, metastasis and cutoff value to explore the potential sources of heterogeneity. In subgroup analysis stratified by ethnicity, the results showed that a significant association between high pretreatment plasma fibrinogen and worse DFS in Asian (HR = 2.01, 95% CI 1.30–3.11, P = .002) and non-Asian patients (HR = 2.40, 95% CI 1.27–4.54, P = .007). Subgroup analysis performed by clinical setting of disease revealed high pretreatment plasma fibrinogen was significantly associated with poor DFS in patients with nonmetastatic disease (HR = 1.92, 95% CI 1.18–3.11, P = .009) and mixed subgroup (HR = 2.84, 95% CI 1.59–5.06, P = .000). However, there was no significant association between high fibrinogen level and DFS of CRC patients in metastatic disease. Subgroup analysis based on cutoff value demonstrated that a high fibrinogen level was associated with poor DFS in the ≥4 g/L (HR = 1.93, 95% CI 1.09–3.43, P = .024) and <4 g/L groups (HR = 2.72, 95% CI 1.90–3.88, P = .001). The results of subgroup analyses about DFS were shown in Table 2.
3.4. Publication bias
Begg funnel plots and Egger regression test were used to assess the potential publication bias in the meta-analysis. Publication bias was not obvious with both OS (P = .06, univariate analysis results) and DFS (P = .133, multivariate analysis results) by Begg test. However, statistically significant publication bias was found in OS according to multivariate analysis results (P = .016) by Begg test (Fig. 4). What's more, publication bias was identified with OS (P = .013, univariate analysis results; P = .004, multivariate analysis results) and DFS (P = .005, multivariate analysis results) by Egger test. Because the number of studies discussing the relationship between pretreatment plasma fibrinogen and DFS according to univariate analysis results was small and the heterogeneity was not obvious, publication bias was not assessed.
Figure 4.
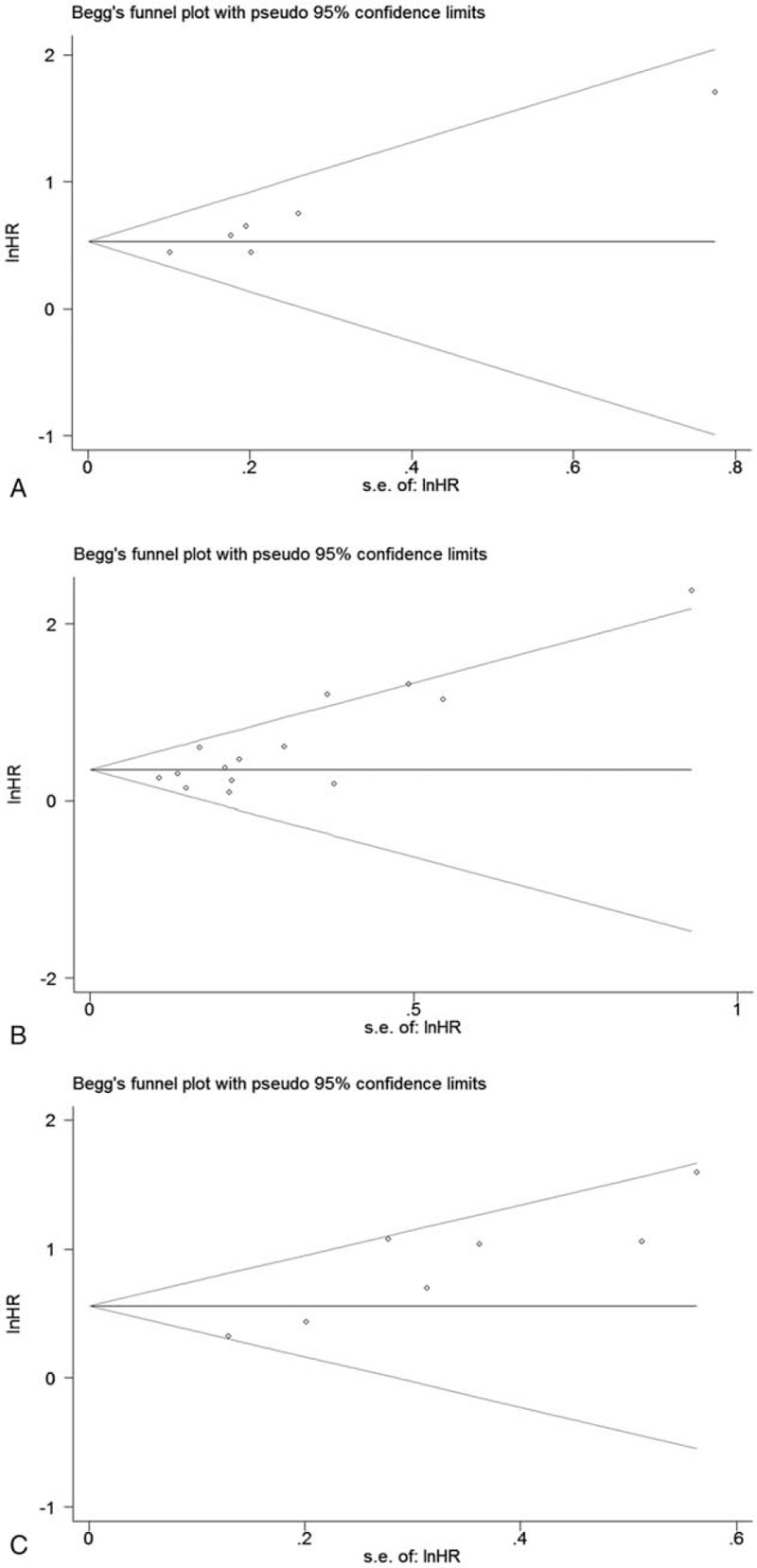
Begg funnel plots assessing the publication bias for the results. (A) Overall survival in univariate analysis, (B) overall survival in multivariate analysis, (C) disease-free survival in multivariate analysis.
A trim-and-fill analysis was performed to explore whether the publication bias influenced the stability of the results in this meta-analysis. The updated results not changed significantly (OS: univariate analysis: HR = 1.609, 95% CI 1.387–1.866, P = .000; multivariate analysis: HR = 1.368, 95% CI 1.128–1.659, P = .001; DFS: multivariate analysis: HR = 1.693, 95% CI 1.239–2.312, P = .001) and the shapes of adjusted funnel plots for publication bias were not obvious asymmetrical. Therefore, the results in our meta-analysis were robust.
3.5. Sensitivity analysis
To further confirm the stability of the pooled results, sensitivity analyses were performed to assess the influence of each study on pooled HRs. The results showed that any individual study was close to the central line, so the heterogeneity was not caused by any single study result (Fig. 5). Because the number of studies discussing the relationship between pretreatment plasma fibrinogen and DFS according to univariate analysis results was limited and the heterogeneity was not obvious, sensitivity analysis was not performed.
Figure 5.
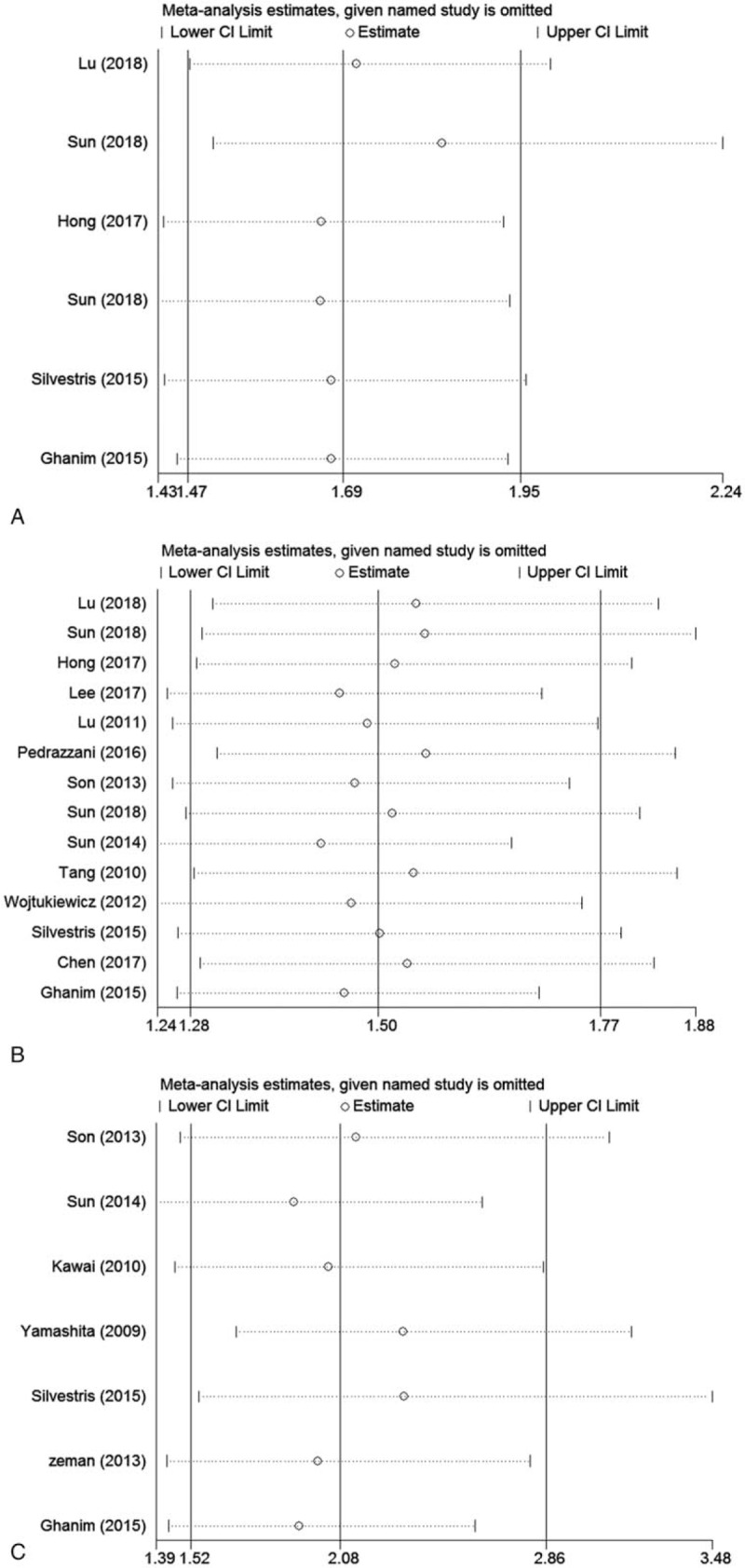
Sensitivity analysis for included studies in the meta-analysis with prognosis. (A) Overall survival in univariate analysis, (B) overall survival in multivariate analysis, (C) disease-free survival in multivariate analysis.
4. Discussion
It has been reported that malignant tumors might be associated with abnormal blood coagulation as early as >150 years ago,[32] which has been confirmed by following studies. In 1992, Wojtukiewicz et al[28] reported the prognostic value of blood coagulation tests in carcinoma of the lung and colon. The result of the study including 58 colon cancer patients showed that elevated pretreatment plasma fibrinogen was significantly associated with worse outcomes. In the past 20 years, the prognostic role of fibrinogen in CRC has been investigated in many studies, but the results remain controversial. There have been some meta-analyses investigating the prognostic value of pretreatment plasma fibrinogen in patients with hepatocellular carcinoma, esophageal carcinoma, urological cancers, digestive system tumors, and solid tumors,[33–38] but no meta-analysis has been performed specifically to study the prognostic role of pretreatment plasma fibrinogen in CRC. What's more, in some studies,[21,23,27] the prognostic role of high pretreatment plasma fibrinogen in CRC patients was significant according to univariate analysis results, but no significant according to multivariate analysis results, whereas in others,[19,22,31] both were significant. Therefore, on the basis of several newly published studies, we conducted this meta-analysis to confirm the prognostic role of pretreatment plasma fibrinogen in CRC.
A total of 17 studies with 6863 patients were included in this quantitative synthesis. In this meta-analysis, the results showed that high pretreatment plasma fibrinogen was associated with worse OS and DFS in CRC patients on the basis of both univariate and multivariate analysis results. In other words, we confirmed that the patients with high pretreatment plasma fibrinogen maybe had worse outcomes. Subgroup analyses were performed in multivariate analysis results of OS and DFS, respectively. The results provided evidence that elevated pretreatment plasma fibrinogen was significantly associated with worse OS and DFS in Asian and non-Asian patients. The results of subgroup analysis by clinical setting of disease showed a significant association between high fibrinogen level and shorter OS in patients with metastatic disease and nonmetastatic disease, but worse DFS in patients with nonmetastatic disease and mixed subgroup. When subgroup analysis based on cut-off value was carried out, we found that high pretreatment plasma fibrinogen was related to poor OS and DFS both in the ≥4 g/L and <4 g/L groups. Although the heterogeneity was not eliminated completely in subgroup analyses, most of the results showed that elevated pretreatment plasma fibrinogen was significantly linked to worse outcomes in patients with CRC. However, the number of studies included in subgroup analyses was limited, so more large-scale studies were needed to confirm the results. Because publication bias was found in some of the results, we also conducted a trim-and-fill analysis and sensitivity analysis in the results of OS and DFS to verify the stability of the results of this meta-analysis. All in all, the results of this meta-analysis were robust to a certain extent.
The proliferation and metastasis of cancer cells are related to 2 aspects, one is the genetic mutations and changes in gene expression of cancer cells, and the other is the change of tumor microenvironment.[39] CRC has similar characteristics, such as chromosomal instability, microsatellite instability, the GpG island methylator phenotype, and other mechanisms including microRNA and inflammation.[40] Among them, the mutation of mismatch repair and APC genes are more likely to cause hypercoagulation than other carcinogenic mutations. So far, a lot of studies have confirmed that abnormal coagulation and hemostasis system are linked to behaviors of malignant tumors. Fibrin is derived from fibrinogen and its deposition in the extracellular matrix provides a good angiogenic bed for tumor cells.[41] High fibrinogen level may stimulate primary macrophages and activated/differentiated monocytic cell lines to upregulate the expression of macrophage inflammatory protein-1α, macrophage inflammatory protein-1β, macrophage inflammatory protein-2, and monocyte chemoattractant protein-1.[42] Moreover, the expression of nuclear factor-κB and interleukin-1β also enhanced when exposed to high fibrinogen level.[43,44] So, changes of microenvironment may accelerate gene instability or carcinogenic mutations in cancer cells. Microthrombi formed by fibrin, tumor cells, and platelets adheres to the outside of tumor cells in the high coagulation state and wraps them up for protection, thus avoiding mechanical damage and fighting against host's immune monitoring system. In addition, fibrinogen, as a bridge between cancer cells, platelets, and vascular endothelial cells, increases the capabilities of adhesion and binding to each other, and plays an important role in the metastasis of tumor cells.[45] Two transplantable murine tumor cell lines, Lewis lung carcinoma and B16-BL6 melanoma, and fibrinogen-deficient transgenic mice were used to prove that fibrinogen can enhance the metastasis ability of circulating tumor cells, but in further studies, the conclusion that the presence of fibrinogen promoted tumor growth had not been confirmed.[46] These studies showed that fibrinogen played an important role in CRC patients.
The systematic evidence provided by this meta-analysis has profound and lasting clinical implications. At first, blood sample is obtained by peripheral venous puncture that patients tolerate well, and it can be repeated sampling for long-term monitoring. Plasma fibrinogen level is detected as a part of the clinical routine, so it is an inexpensive and widely available molecular marker. Second, the results of this meta-analysis suggested that relevant treatments to reduce plasma fibrinogen level were expected to prolong survival time in patients with CRC. It has been reported that changes in lifestyle such as smoking cessation, weight or stress reduction, and an increase in regular physical activity may assist in reducing plasma fibrinogen level and a regular moderate alcohol consumption may reduce fibrinogen level slightly.[47,48] In addition, many oral drugs such as antihypertensive drugs incluing β-adrenergic-receptor blockers and vasodilators, hypolipidemic drugs incluing fibrates and statins, niacin, curcuma longa, and nattokinase, may decrease plasma fibrinogen level too.[47–51] However, lifestyle modifications are regarded as a first-line strategy to reduce fibrinogen.[47] In the light of the role of fibrinogen in formation and metastasis of tumors, it can be used for initial screening or in combination with other biomarkers for the diagnosis, prevention, or treatment decision-making of CRC. Moreover, plasma fibrinogen can be used as a stratified variable in clinical trials of CRC.[38]
Although a rigorous and systematic retrieval was performed before pooling results of studies, several limitations should be considered. First, in Ghanim et al's study,[31] because all patients with pulmonary metastasis for CRC undergone pulmonary metastasectomy, pulmonary lesions might affect plasma fibrinogen level, and the study was limited by the small number of patients, so the study might be one of the sources of heterogeneity. In addition, clauss clotting method was selected to detect plasma fibrinogen in most studies, but the detection methods of plasma fibrinogen have not been clarified in some studies, so it might lead heterogeneity too. What's more, the cutoff values of pretreatment plasma fibrinogen and the treatment methods for CRC were different from each others, which also probably contributed to the heterogeneity. Meanwhile, the number of studies pooled in some results was too small, which was probably another factor contributing to heterogeneity. Second, several HRs and 95% CIs were not reported in original articles,so the HR and its 95% extracted from survival curve might not be accurate. Third, this meta-analysis only included studies published in English and Chinese, so the language bias might be caused. Fourth, a large portion of the included studies were from Asia, which might reduce the generalizability of the results. In addition, plasma fibrinogen values were inevitably influenced by non–tumor-related factors, such as infection, liver disease, and coagulation disorders. Therefore, more large-scale studies are required to confirm the prognostic value of high plasma fibrinogen in CRC patients.
5. Conclusions
In conclusion, this meta-analysis provides robust evidence that elevated pretreatment plasma fibrinogen level is significantly associated with worse survival outcomes in CRC patients, suggesting that it could be used as an effective prognostic marker and potential therapeutic target.
Author contributions
Conceptualization: Menglei Li, Yongqiang Yuan.
Data curation: Menglei Li, Yang Wu, Jiwang Zhang, Lijun Huang, Xianlan Wu.
Formal analysis: Menglei Li, Yang Wu, Jiwang Zhang, Lijun Huang, Xianlan Wu.
Funding acquisition: Menglei Li, Yang Wu, Jiwang Zhang, Lijun Huang, Xianlan Wu.
Investigation: Menglei Li, Lijun Huang.
Methodology: Menglei Li.
Project administration: Menglei Li, Yang Wu.
Resources: Menglei Li.
Software: Menglei Li.
Supervision: Menglei Li.
Validation: Menglei Li.
Visualization: Menglei Li.
Writing – original draft: Menglei Li.
Writing – review & editing: Menglei Li.
Footnotes
Abbreviations: 95% CI = 95% confidence interval, CRC = colorectal cancer, DFS = disease-free survival, HR = hazard ratio, NOS = Newcastle–Ottawa Quality Assessment Scale, OS = overall survival, PFS = progression-free survival, RFS = recurrence-free survival.
How to cite this article: Li M, Wu Y, Zhang J, Huang L, Wu X, Yuan Y. Prognostic value of pretreatment plasma fibrinogen in patients with colorectal cancer. Medicine. 2019;98:37(e16974).
The authors report no conflicts of interest.
References
- [1].Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. Cancer J Clin 2018;68:7–30. [DOI] [PubMed] [Google Scholar]
- [2].Smith RA, Manassaram-Baptiste D, Brooks D, et al. Cancer screening in the United States, 2015: a review of current American Cancer Society guidelines and current issues in cancer screening. CA Cancer J Clin 2015;65:30–54. [DOI] [PubMed] [Google Scholar]
- [3].Jemal A, Center MM, DeSantis C, et al. Global patterns of caner incidence and mortalily rates and trends. Cancer Epidemiol Biomarkers Prey 2010;19:1893–907. [DOI] [PubMed] [Google Scholar]
- [4].Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet 2001;357:539–45. [DOI] [PubMed] [Google Scholar]
- [5].Mantovani A, Allavena P, Sica A, et al. Cancer-related inflammation. Nature 2008;454:436–44. [DOI] [PubMed] [Google Scholar]
- [6].Palumbo JS. Mechanisms linking tumor cell-associated procoagulant function to tumor dissemination. Semin Thromb Hemost 2008;34:154–60. [DOI] [PubMed] [Google Scholar]
- [7].Südhoff T, Schneider W. Fibrinolytic mechanisms in tumor growth and spreading. Clin Investig 1992;70:631–6. [DOI] [PubMed] [Google Scholar]
- [8].Repetto O, De Re V. Coagulation and fibrinolysis in gastric cancer. Ann N Y Acad Sci 2017;1404:27–48. [DOI] [PubMed] [Google Scholar]
- [9].Blombäck B, Blombäck M. The molecular structure of fibrinogen. Ann NY Acad Sci 1972;8:77–97. [DOI] [PubMed] [Google Scholar]
- [10].Pulanić D, Rudan I. The past decade: fibrinogen. Coll Antropol 2005;29:341–9. [PubMed] [Google Scholar]
- [11].Davalos D, Akassoglou K. Fibrinogen as a key regulator of inflammation in disease. Semin Immunopathol 2012;34:43–62. [DOI] [PubMed] [Google Scholar]
- [12].Staton CA, Brown NJ, Lewis CE. The role of fibrinogen and related fragments in tumour angiogenesis and metastasis. Expert Opin Biol Ther 2003;3:1105–20. [DOI] [PubMed] [Google Scholar]
- [13].Yapijakis C, Bramos A, Nixon AM. The interplay between hemostasis and malignancy the oral cancer paradigm. Anticancer Res 2012;32:1791–800. [PubMed] [Google Scholar]
- [14].Tierney JF, Stewart LA, Ghersi D, et al. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007;8:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Tang L, Liu K, Wang J, et al. High preoperative plasma fibrinogen levels are associated with distant metastases and impaired prognosis after curative resection in patients with colorectal cancer. J Surg Oncol 2010;102:428–32. [DOI] [PubMed] [Google Scholar]
- [16].Yamashita H, Kitayama J, Taguri M, et al. Effect of preoperative hyperfibrinogenemia on recurrence of colorectal cancer without a systemic inflammatory response. World J Surg 2009;33:1298–305. [DOI] [PubMed] [Google Scholar]
- [17].Lee S, Huh SJ, Oh SY, et al. Clinical significance of coagulation factors in operable colorectal cancer. Oncol Lett 2017;13:4669–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Sun ZQ, Han XN, Wang HJ, et al. Prognostic significance of preoperative fibrinogen in patients with colon cancer. World J Gastroenterol 2014;20:8583–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Silvestris N, Scartozzi M, Graziano G, et al. Basal and bevacizumab-based therapy-induced changes of lactate dehydrogenases and fibrinogen levels and clinical outcome of previously untreated metastatic colorectal cancer patients a multicentric retrospective analysis. Expert Opin Biol Ther 2015;15:155–62. [DOI] [PubMed] [Google Scholar]
- [20].Kawai K, Kitayama J, Tsuno NH, et al. Hyperfibrinogenemia after preoperative chemoradiotherapy predicts poor response and poor prognosis in rectal cancer. Int J Colorectal Dis 2011;26:45–51. [DOI] [PubMed] [Google Scholar]
- [21].Lu TT, Wang ZB. Prognostic value of preoperative fibrinogen and D-dimer levels in patients with colorectal cancer. Journal of China Medical University 2018;47:513–8. (Article in Chinese). [Google Scholar]
- [22].Sun Y. Prognostic value of preoperative fibrinogen for predicting clinical outcome in patients with colorectal cancer. China Medical University 2018;(Article in Chinese). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Hong T, Shen D, Chen X, et al. Preoperative plasma fibrinogen, but not D-dimer might represent a prognostic factor in non-metastatic colorectal cancer: a prospective cohort study. Cancer Biomark 2017;19:103–11. [DOI] [PubMed] [Google Scholar]
- [24].Lu K, Zhu Y, Sheng L, et al. Serum fibrinogen level predicts the therapeutic response and prognosis in patients with locally advanced rectal cancer. Hepatogastroenterology 2011;58:1507–10. [DOI] [PubMed] [Google Scholar]
- [25].Pedrazzani C, Mantovani G, Salvagno GL, et al. Elevated fibrinogen plasma level is not an independent predictor of poor prognosis in a large cohort of Western patients undergoing surgery for colorectal cancer. World J Gastroenterol 2016;22:9994–10001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Son HJ, Park JW, Chang HJ, et al. Preoperative plasma hyperfibrinogenemia is predictive of poor prognosis in patients with nonmetastatic colon cancer. Ann Surg Oncol 2013;20:2908–13. [DOI] [PubMed] [Google Scholar]
- [27].Sun F, Peng HX, Gao QF, et al. Preoperative circulating FPR and CCF score are promising biomarkers for predicting clinical outcome of stage II-III colorectal cancer patients. Cancer Manag Res 2018;10:2151–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Wojtukiewicz MZ, Zacharski LR, Moritz TE, et al. Prognostic significance of blood coagulation tests in carcinoma of the lung and colon. Blood Coagul Fibrinolysis 1992;3:429–37. [PubMed] [Google Scholar]
- [29].Zeman M, Maciejewski A, Półtorak S, et al. Evaluation of outcomes and treatment safety of patients with metastatic colorectal cancer to the liver with estimation of prognostic factors. Pol Przegl Chir 2013;85:333–9. [DOI] [PubMed] [Google Scholar]
- [30].Chen WJ, Wu SS, Luo HQ, et al. Prognostic factors of patients with stagesI - III left-sided versus rightsided colon cancer receiving radical surgery. Tumor 2017;37:981–8. [Google Scholar]
- [31].Ghanim B, Schweiger T, Jedamzik J, et al. Elevated inflammatory parameters and inflammation scores are associated with poor prognosis in patients undergoing pulmonary metastasectomy for colorectal cancer. Interact Cardiovasc Thorac Surg 2015;21:616–23. [DOI] [PubMed] [Google Scholar]
- [32].Trousseau A. Phlegmansia alba dolens. Clin Med Hotel-dieu Paris 1865;3:654–712. [Google Scholar]
- [33].Huang G, jiang H, Lin Y, et al. Prognostic value of plasma fibrinogen in hepatocellular carcinoma: a meta-analysis. Cancer Manag Res 2018;10:5027–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Lv GY, Yu Y, An L, et al. Preoperative plasma fibrinogen is associated with poor prognosis in esophageal carcinoma: a meta-analysis. Clin Transl Oncol 2018;20:853–61. [DOI] [PubMed] [Google Scholar]
- [35].Song H, Kuang G, Zhang Z, et al. The prognostic value of pretreatment plasma fibrinogen in urological cancers: a systematic review and meta-analysis. J Cancer 2019;10:479–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Ji R, Ren Q, Bai S, et al. Prognostic significance of pretreatment plasma fibrinogen level in patients with digestive system tumors: a meta-analysis. Int J Biol Markers 2018;33:254–65. [DOI] [PubMed] [Google Scholar]
- [37].Lin Y, Liu Z, Qiu Y, et al. Clinical significance of plasma D-dimer and fibrinogen in digestive cancer: a systematic review and meta-analysis. Eur J Surg Oncol 2018;44:1494–503. [DOI] [PubMed] [Google Scholar]
- [38].Perisanidis C, Psyrri A, Cohen EE, et al. Prognostic role of pretreatment plasma fibrinogen in patients with solid tumors: a systematic review and meta-analysis. Cancer Treat Rev 2015;41:960–70. [DOI] [PubMed] [Google Scholar]
- [39].Sahai E. Mechanisms of cancer cell invasion. Curr Opin Genet Dev 2005;15:87–96. [DOI] [PubMed] [Google Scholar]
- [40].Hamzehzadeh L, Yousefi M, Ghaffari SH. Colorectal cancer screening: a comprehensive review to recent non-invasive methods. Int J Hematol Oncol Stem Cell Res 2017;11:250–61. [PMC free article] [PubMed] [Google Scholar]
- [41].Cronin-Fenton DP, Søndergaard F, Pedersen LA, et al. Hospitalisation for venous thromboembolism in cancer patients and the general population:a population-based cohort study in Denmark. Br J Cancer 2010;103:947–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Smiley ST, King JA, Hancock WW. Fibrinogen stimulates macrophage chemokine secretion through toll-like receptor 4. J Immunol 2001;167:2887–94. [DOI] [PubMed] [Google Scholar]
- [43].Sitrin R, Pan PM, Srikanth S, et al. Fibrinogen activates NF-κB transcription factors in mononuclear phagocytes. J Immunol 1998;161:1462–70. [PubMed] [Google Scholar]
- [44].Perez RL, Roman J. Fibrin enhances the expression of IL-1β by human peripheral blood mononuclear cells: implications in pulmonary inflammation. J Immunol 1995;154:1879–87. [PubMed] [Google Scholar]
- [45].Simanek R, Vormittag R, Ay C, et al. High platelet count associated with venous thromboembolism in cancer patients:results from the Vienna Cancer and Thrombosis Study (CATS). J Thromb Haemost 2010;8:114–20. [DOI] [PubMed] [Google Scholar]
- [46].Palumbo JS, Kombrinck KW, Drew AF, et al. Fibrinogen is an important determinant of the metastatic potential of circulating tumor cells. Blood 2000;96:3302–9. [PubMed] [Google Scholar]
- [47].Ernst E, Resch KL. Therapeutic interventions to lower plasma fibrinogen concentration. Eur Heart J 1995;16suppl A:47–52. discussion 52-53. [DOI] [PubMed] [Google Scholar]
- [48].Kamath S, Lip GY. Fibrinogen: biochemistry, epidemiology and determinants. QJM 2003;96:711–29. [DOI] [PubMed] [Google Scholar]
- [49].Philipp CS, Cisar LA, Saidi P, et al. Effect of niacin supplementation on fibrinogen levels in patients with peripheral vascular disease. Am J Cardiol 1998;82:697–9. A9. [DOI] [PubMed] [Google Scholar]
- [50].Ramirez Boscá A, Soler A, Carrión-Gutiérrez MA, et al. An hydroalcoholic extract of Curcuma longa lowers the abnormally high values of human-plasma fibrinoge. Mech Ageing Dev 2000;114:207–10. [DOI] [PubMed] [Google Scholar]
- [51].Hsia CH, Shen MC, Lin JS, et al. Nattokinase decreases plasma levels of fibrinogen, factor VII, and factor VIII in human subjects. Nutr Res 2009;29:190–6. [DOI] [PubMed] [Google Scholar]


