Summary
Each year approximately one million people living with HIV (PLWH) globally develop tuberculosis. While the drug regimens used to treat tuberculosis in PLWH are the same as those used in HIV-negative patients, there are a number of challenges with co-treatment of antituberculosis and antiretroviral therapy: the optimal timing of antiretroviral initiation, drug-drug interactions, drug tolerability, and the prevention and treatment of the tuberculosis-associated immune reconstitution syndrome. Furthermore, mortality is high in PLWH diagnosed with tuberculosis during a hospital admission and in those with tuberculous meningitis. Recent studies in this field have better characterized these challenges and informed optimal management and guideline revisions.
Introduction
The World Health Organization (WHO) estimates that 920,000 people living with HIV (PLWH) developed tuberculosis disease in 2017, representing 9% of the 10 million incident cases of tuberculosis globally.1 Africa is severely affected by the convergence of these two epidemics - over 75% of the global burden of HIV-associated tuberculosis occurs in the WHO Africa region, and in southern Africa over 50% of patients with tuberculosis disease are HIV-positive.1
Tuberculosis is the leading cause of death (40%), hospitalization (18%) and in-hospital death (25%) in PLWH.2,3 HIV-associated tuberculosis is associated with substantially higher mortality than tuberculosis in HIV-negative people; HIV-associated tuberculosis accounted for 300,000 of the total 1.6 million tuberculosis deaths in 2016 (19%).1 Mortality among patients sick enough to require hospital admission at the time of diagnosis of HIV-associated tuberculosis in Africa is 11%−32%.4–7 Many patients die before diagnosis or early during tuberculosis treatment. There are two key reasons for the higher mortality in PLWH with tuberculosis. First, tuberculosis progresses more rapidly as HIV-related immunosuppression worsens and severe disease, notably disseminated tuberculosis, becomes common. Disseminated tuberculosis was found in 88% of autopsies of HIV-positive adults dying of tuberculosis in resource-limited settings.8 Second, diagnosis is more difficult due to lower bacillary load in sputum, because lung cavities are less common, and there is a high frequency of extra-pulmonary disease. Advances in diagnostic yield have resulted from introduction of the Xpert MTB/RIF, Xpert MTB/RIF Ultra, and the urine lipo-arabinomannan (LAM) assay. In clinical trials, urine LAM testing has been shown to reduce mortality in inpatients with advanced HIV.9,10
In a Ugandan study almost 25% of adult patients with HIV infection hospitalized with severe sepsis had Mycobacterium tuberculosis grown on blood culture. Sputum-based tests for tuberculosis performed poorly in these patients.11 The poor performance of sputum diagnostics results in delayed diagnosis of tuberculosis and initiation of treatment, which contributes to the high mortality observed in these severely ill patients. The use of clinical prediction scores and urine-based diagnostics to facilitate more rapid treatment initiation in these patients may improve outcomes.10,11 In addition, novel treatment strategies need to be evaluated in severely ill hospitalised patients diagnosed with HIV-associated tuberculosis.
All PLWH are now eligible for ART regardless of CD4 count - in 2017 WHO estimated that 84% of notified tuberculosis patients known to be HIV-positive were on or started antiretroviral therapy (ART).1 The co-treatment of tuberculosis and HIV presents substantial challenges including drug-drug interactions, immune reconstitution inflammatory syndrome (TB-IRIS) and shared side effects of medication. Good evidence has been generated to address these challenges and optimise management of patients diagnosed with HIV-associated tuberculosis, which is the focus of our review. The focus is management of adults; an overview of paediatric management issues is presented in the Supplementary Panel (see Appendix).
Drugs and duration of treatment
Drug-susceptible tuberculosis
Treatment of tuberculosis in PLWH is largely the same as in HIV-negative patients but does require several additional considerations. The recommended regimen for drug-susceptible disease is a combination of isoniazid, rifampicin, ethambutol, and pyrazinamide for 2 months, followed by 4 additional months of isoniazid and rifampicin.12 The clinical trials supporting this regimen were performed decades ago in HIV-negative patients,13 but many studies have demonstrated its effectiveness in PLWH. Pregnant women are treated with standard first line treatment for tuberculosis.
In PLWH not on ART, studies demonstrated a lower risk of relapse if antituberculosis treatment was extended to 9–12 months, but there was no survival advantage.14,15 In the ART era, there is debate as to whether PLWH are more prone to relapse; while some authors have advocated for a longer duration of treatment, international guidelines have not adopted this recommendation. Tuberculosis in PLWH should be treated with daily rather than intermittent dosing regimens. Intermittent dosing was associated with an increased risk of treatment failure, relapse, and the development of rifamycin resistance.16–18
Higher doses of rifampicin (up to 50mg/kg/day) are currently being evaluated in HIV-negative tuberculosis patients to assess whether this would allow treatment shortening.19,20 If found to be effective and safe in these patients, these higher doses would need to be assessed in PLWH as there may be unique safety and drug-drug interaction considerations in the patients.
Rifampicin-resistant tuberculosis
As with drug-susceptible tuberculosis, treatment of rifampicin-resistant disease is the same irrespective of HIV status. WHO has issued guidelines for the management of multidrug drug-resistant tuberculosis (MDR-TB, defined as resistance to at least isoniazid and rifampicin). Historically, this involved a 20–24 month regimen consisting of at least 5 drugs with known or presumed activity,21 but in 2016, WHO revised these guidelines to recommend a 9–12 month short-course clofazimine-based regimen for selected patients, including PLWH.22 The effectiveness of this short-course regimen was first demonstrated in several observational studies in south Asia and central/west Africa.23 The STREAM I randomised controlled trial reported a higher mortality rate with the short-course regimen compared with the control 20–24 month regimen in PLWH, but this did not reach statistical significance.24
In studies conducted before the widespread availability of ART, treatment outcomes for patients with MDR tuberculosis or extensively drug-resistant tuberculosis (XDR-TB i.e., MDR-TB with additional resistance to a fluoroquinolone and a second-line injectable agent) were much worse in PLWH, with 5-year survival rates of 10–20%.25,26 A recent large meta-analysis of MDR-TB treatment studies published since 2009 found that pooled treatment success was considerably higher among PLWH who were receiving ART compared with those who were not on ART (55% vs. 34%), but treatment success was higher in HIV-negative patients (68%) - death during treatment was 26% versus 29% versus 9%, respectively.27
After a 40-year drought, several new and repurposed medications are now available to treat drug-resistant disease, including bedaquiline, delamanid, linezolid, and clofazimine. Because of cost and registration issues these drugs have limited availability in many high DR-TB burden settings. Outcomes of patients treated with bedaquiline and linezolid containing regimens appear to be superior to older regimens.28–31 However, in a phase 3 trial time to sputum culture conversion was not more rapid when delamanid was added to an MDR-TB regimen.32 In the NiX-TB single-arm trial, patients (the majority of whom had XDR-TB) were treated with a 6-month regimen containing pretomanid, bedaquiline and linezolid (n=75, 51% HIV infected). At follow-up 6 months after completion of treatment, 66 participants (89%) were classified as having a favourable outcome.28
A number of ongoing and planned clinical trials are evaluating the use of these and other investigational medications in different combinations and patient populations, including PLWH. In August 2018, WHO issued a new recommendation that the 20–24 month long MDR-TB regimen be shortened to 18 months with an all-oral regimen of bedaquiline, linezolid, moxifloxacin/levofloxacin with cycloserine/terizidone and/or clofazimine.33 This regimen pertains to patients in whom fluoroquinolone resistance has been excluded. The recommendation for this new “long course” regimen was based on an individual patient data meta-analysis of mainly observational data that evaluated the outcomes associated with the individual drugs in various regimens, rather than a randomised controlled trial evaluating this specific regimen.27 There are controversial aspects to this recommendation, including the exclusion of high-dose isoniazid despite a clinical trial demonstrating its efficacy when included in an MDR-TB regimen.34
It is our view that going forward in all clinical trials of new drugs and regimens in drug-resistant tuberculosis, PLWH should be included in sufficient numbers to adequately assess efficacy, safety and potential drug-drug interactions in this subgroup.
Drug absorption and exposure
Among patients treated with first-line antituberculosis therapy, there is considerable variability in drug exposure and lower drug exposure has been shown to correlate with poor treatment outcomes.35,36 While genetics are known to explain some of this variability (e.g., slow vs. fast acetylators of isoniazid), it has been hypothesized that PLWH may have lower drug exposure. Data from studies attempting to examine this effect, however, have had conflicting results.37–41 In a recent meta-analysis, there was no significant difference in rifampicin area under the curve comparing PLWH and HIV-negative individuals when limited to measurements taken at steady state.42 A recent trial (the RAFA trial) comparing a higher dose of rifampicin (15 mg/kg) to the standard dose (10 mg/kg) in the treatment of HIV-associated tuberculosis found a mortality benefit in the subgroup of participants with a CD4 count <100 cells/µL.43 To date, guidelines have not recommended any changes in dose of antituberculosis medications in patients co-infected with HIV. Further studies to evaluate the safety and efficacy of high dose rifampicin in PLWH are needed. While the RAFA trial suggested that higher doses of rifampicin may reduce mortality in a subgroup analysis, this needs to be further evaluated in a sufficiently powered trial to derive definitive conclusions and more safety data
Extrapulmonary TB
PLWH—particularly those with advanced immunosuppression—are more likely to have extrapulmonary and disseminated tuberculosis. Management of extrapulmonary tuberculosis, including tuberculous meningitis, is identical to that in HIV-negative patients. There is increasing evidence that standard oral doses of rifampicin (10 mg/kg/day) do not reach therapeutic concentrations in CSF. 44,45 In PLWH and TB meningitis, mortality on conventional treatment is extremely high. The safety of rifampicin doses up to 35 mg/kg/day have been demonstrated in a phase 2 study19 and several studies are testing whether such high doses with or without higher doses of isoniazid, the addition of linezolid (a drug with good central nervous system penetration) and/or adjuvant aspirin improve survival in tuberculous meningitis.
Tolerability of drugs used in co-treatment of HIV and tuberculosis
Adverse events from antituberculosis therapy occur more commonly in PLWH, notably drug-induced liver injury and cutaneous adverse drug reactions, both of which can be life-threatening. HIV infection increases the risk of cutaneous adverse drug reactions to many drugs, including antituberculosis drugs; rifampicin followed by isoniazid are the most common offending drugs.46 It is prudent to add pyridoxine to all PLWH on isoniazid as they have a high prevalence of peripheral neuropathy. PLWH are at increased risk of ototoxicity from long term aminoglycoside use for drug-resistant tuberculosis.47
Concerns about the tolerability of co-treatment with ART and antituberculosis therapy were dispelled by randomised controlled trials examining timing of ART initiation during antituberculosis therapy. Two of these trials, SAPIT and TB-HAART, evaluated starting ART during or after antituberculosis therapy - both trials showed no difference in treatment emergent grade 3 or 4 adverse events by arm (Table 1).48,49 Two randomised controlled trials evaluated the role of empiric antituberculosis therapy irrespective of symptoms in patients starting ART with severe immune suppression: REMEMBER found no differences in treatment-emergent grade 3 or 4 adverse events, but STATIS reported that the incidence of grade 3 or 4 drug-related toxicity was higher in participants on co-treatment (Table 1).50,51 The proportions with grade 3 or 4 liver function test abnormalities were similar in TB-HAART and REMEMBER, but SAPIT reported more grade 3 or 4 unspecified “liver abnormalities” in participants on ART with antituberculosis therapy (36/429 versus 8/213). Taken together, the randomised controlled trials show that co-treatment with ART and antituberculosis therapy is generally well tolerated.
Table 1.
Tolerability of combining antiretroviral and antituberculosis therapy in randomized controlled trials that had comparator arms without combined therapy in patients with tuberculosis (SAPIT and TB-HAART) or trials of empiric antituberculosis therapy in patients starting ART with severe immune suppression (REMEMBER and STATIS).
| Study | N | Toxicity measure | Combined TB therapy and ART | Comparator arm | Difference (95% confidence intervals) |
|---|---|---|---|---|---|
| Timing of ART initiation in participants with TB | |||||
| ART started during TB therapy | ART not started during TB therapy | ||||
| SAPIT48 | 642 | Grade 3–4 events (excluding IRIS) |
30/100 py | 32/100 py | P = 0.69 |
| TB-HAART49 | 1675 | Grade 3–4 events | 149/834 (18%) | 174/841 (21%) | IRR 0.905 (0.72, 1.13) clinical AEs; IRR 1.026 (0.90, 1.17) laboratory AEs |
| Empiric TB therapy in participants with severe immune suppression | |||||
| Empiric TB therapy with ART | No empiric TB therapy with ART | ||||
| REMEMBER51$ | 851 | Grade 3–4 laboratory events | 26/424 (6%) | 29/427 (7%)# | P = 0.696 |
| STATIS50 | 1047 | Grade 3–4 drug-related toxicity | 16.3% by week 24 | 6.5%* by week 24 | HR 2.70 (1.80, 4.04) |
All participants in this arm received isoniazid preventive therapy
16.4% started started antituberculosis therapy within 24 weeks
In the REMEMBER trial, participants were randomised to empirical four drug antituberculosis treatment or isoniazid preventive therapy. Full antituberculosis treatment did not reduce mortality, but surprisingly, was associated with an increased risk of tuberculosis.
Abbreviations: TB = tuberculosis; py = person years of observation; IRIS = immune reconstitution inflammatory syndrome; IRR = incidence rate ratio; AEs = adverse events; HR = hazard ratio
Managing suspected adverse drug reactions in PLWH on co-treatment for HIV- associated tuberculosis is difficult due to co-morbidities and use of multiple drugs with overlapping toxicities. For example, patients developing symptomatic hepatitis could have TB-IRIS, exacerbation of chronic hepatitis B or C, sepsis, or drug-induced liver injury from ART (efavirenz, protease inhibitors, or integrase inhibitors), antituberculosis therapy (rifampicin, isoniazid, or pyrazinamide), or prophylactic therapy (fluconazole or co-trimoxazole). If drug-induced liver injury is thought to be the cause then all potentially hepatotoxic drugs should be stopped and at least three antituberculosis drugs (e.g. ethambutol, moxifloxacin or levofloxacin, and amikacin) given while waiting for improvement in the liver function tests; once these have improved (bilirubin to less than twice upper limit of normal and alanine aminotransferase to less than 2.5 times upper limit of normal) rechallenge with rifampicin followed by isoniazid three to seven days later should be done with close monitoring of liver function tests. Patients who developed acute liver failure due to antituberculosis drug-induced hepatitis should not be rechallenged with rifampicin, isoniazid or pyrazinamide; they should be treated with second-line drugs. Modification in composition and duration of the antituberculosis drug regimen should be discussed with an expert. ART should only be rechallenged once the antituberculosis drug regimen has been decided. Future research in this field should aim to define the optimal drug combination and rechallenge strategy of antituberculosis drugs after drug-induced liver injury or skin reactions that minimizes the duration of treatment interruption. There may be a role for the new antituberculosis drugs in rechallenge regimens, but this can only be addressed through prospective research.
Several drugs now used in the treatment of DR-TB have the potential for prolongation of the QT interval, including bedaquiline, delamanid, clofazimine and fluoroquinolones. Electrocardiogram monitoring should be performed in patients on combinations of these drugs.
Antiretroviral-antituberculosis drug-drug interactions
Additive toxicity, which is a pharmacodynamic drug-drug interaction, is seldom seen with antituberculosis therapy and currently used ART, which is well tolerated. However, there are many important pharmacokinetic drug-drug interactions between ART and antituberculosis therapy, which can be bidirectional.
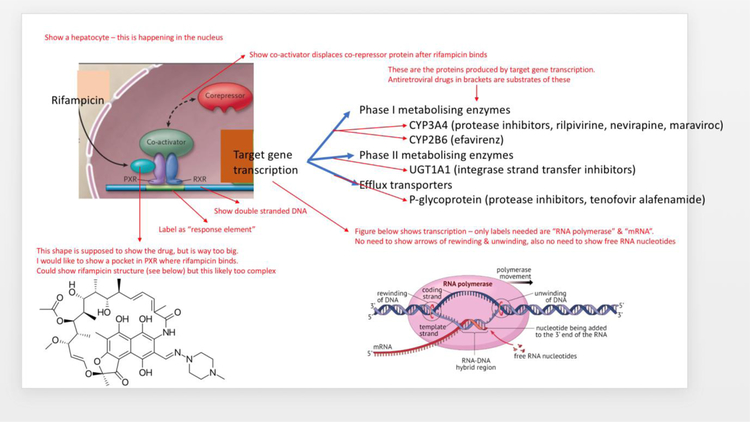
Rifampicin is one of the most potent activators of the nuclear pregnane X receptor, which increases the transcriptional activation of many genes involved in the metabolism and efflux of antiretroviral drugs (Figure 1).52 The induction of metabolizing enzymes and efflux transporters is maximal about two weeks after starting rifampicin and persists for up to four weeks after stopping. The magnitude of drug-drug interactions between rifampicin and substrates of the induced efflux transporters and metabolizing enzymes (Table 2a) depends in part on the extent of the induction, which is greatest for the cytochrome P450 (CYP) enzyme 3A4. Antiretroviral drugs that are substrates of both CYP3A4 and P-glycoprotein, like the protease inhibitors, are most affected by the interaction with rifampicin.
Figure 1:
Rifampicin is a potent agonist of the pregnane X receptor (PXR), which forms a heterodimer with retinoid X receptor (RXR) to form a transcriptional activation factor for many target genes involved in the metabolism and efflux of drugs and xenobiotics. The activated genes that reduce exposure to antiretroviral drugs are shown. PXR is primarily expressed in the liver, intestine, and kidney.
CYP = cytochrome P450; UGT = UDP-glucuronosyltransferase
Table 2a.
Drug-drug interactions between rifampicin and antiretroviral drugs (source: https://www.hiv-druginteractions.org).
| Antiretroviral drug | AUC change | Management of interaction |
|---|---|---|
| CCR5 inhibitor | ||
| Maraviroc | ↓63% | Increase maraviroc dose to 600 mg 12 hourly |
| Integrase inhibitors | ||
| Bictegravir | ↓75% | Not recommended with either rifampicin or rifabutin |
| Dolutegravir | ↓54% | Increase dolutegravir dose to 50 mg 12 hourly |
| Elvitegravir/cobicistat | Not studied | Not recommended with either rifampicin or rifabutin |
| Raltegravir | ↓40% | Standard or double dose had similar efficacy in phase 2 study.97 Standard dosing is being further evaluated in a current phase 3 study. |
| Non-nucleoside RTI | ||
| Efavirenz | No significant change | No dose adjustment |
| Etravirine | Not studied | Not recommended with either rifampicin or rifabutin |
| Nevirapine | ↓58% | Switch to rifabutin 300 mg daily |
| Rilpivirine | ↓80% | Switch to rifabutin 300 mg daily and double rilpivirine dose |
| Nucleotide RTI | ||
| Tenofovir alafenamide | ↓54% | Standard dose (still has higher intracellular active drug than TDF, suggesting that dose adjustment is unnecessary)98 |
| Protease inhibitors | ||
| Atazanavir/r | ↓72% | Switch to rifabutin 150 mg daily |
| Darunavir/r | ↓57% | Switch to rifabutin 150 mg daily |
| Lopinavir/r | ↓75% | Double dose (in young children on lopinavir/r oral solution, add ritonavir to ratio of 1:1) OR switch to rifabutin 150 mg daily |
Abbreviations: AUC = area under the concentration curve; CCR5 = chemokine co-receptor 5; RTI = reverse transcriptase inhibitor; TDF = tenofovir disoproxil fumarate; r = ritonavir
Efavirenz induces its own metabolism by CYP2B6 and rifampicin co-administration does not cause significant reductions in efavirenz exposure once steady state of efavirenz auto-induction has been reached. Efavirenz is predominantly metabolized by CYP2A6 in people with slow metaboliser CYP2B6 genotypes, which has a prevalence of about 20% in sub-Saharan Africa, India, and Thailand. Isoniazid inhibits CYP2A6, resulting in a 50% increase in efavirenz concentrations in people with slow metaboliser CYP2B6 genotypes, who already have high efavirenz concentrations – studies are needed to assess the clinical significance of this interaction.53 While efavirenz at a dose of 600mg daily can be co-administered with rifampicin, there are limited data on co-administration with efavirenz 400mg daily.
The integrase strand transfer inhibitor, dolutegravir, is increasingly being used in ART regimens in tuberculosis endemic settings. Rifampicin induction reduces dolutegravir exposure by 54%, which can be overcome by increasing the dolutegravir dose from 50mg daily to 50mg twice daily. In the INSPIRING trial in patients with HIV-associated TB treated with this dose, a similar proportion achieved viral suppression (75%) to those treated with an efavirenz regimen (82%) although the trial was not powered for formal statistical comparison.54
There are important drug-drug interactions between other rifamycins and antiretrovirals. Rifapentine is a potent inducer, similar to rifampicin in magnitude. Rifabutin, which is a weak inducer, can replace rifampicin in first-line antituberculosis therapy if there is a significant drug-drug interaction between rifampicin and an antiretroviral drug that cannot be overcome by dose adjustment, or if dose adjustment is not tolerated. Rifabutin is a substrate of CYP3A4; therefore, co-administration with ritonavir or cobicistat, which are strong inhibitors of CYP3A4, necessitates halving the standard dose of rifabutin to 150mg daily.55 However, the concentrations of the 25-O-desacetyl metabolite of rifabutin, which is both active and toxic, are higher with halved rifabutin dosing plus ritonavir or cobicistat compared with standard dosing without the inhibitors; therefore, it is important to closely monitor for toxicity (especially uveitis and neutropenia) if rifabutin is used with ritonavir or cobicistat. Further clinical trials of dose-adjusted rifabutin with boosted protease inhibitors assessing pharmacokinetics, safety and efficacy in terms of both HIV and tuberculosis outcomes are warranted. Rifabutin has limited availability in low-middle income countries, which severely limits options for co-treatment of tuberculosis and HIV in PLWH on second-line ART.
There are limited data on drug-drug interactions between antiretrovirals and drugs currently recommended for rifampicin-resistant tuberculosis – recommendations for co-treatment of the main currently recommended drugs with ART are given in Table 2b. The new antimycobacterial drug bedaquiline is a substrate of CYP3A4 and can be the victim of drug-drug interactions when co-administered with antiretroviral drugs that induce or inhibit CYP3A4. Lopinavir-ritonavir is a potent CYP3A4 inhibitor and substantially increases bedaquiline exposure, but reduces the concentrations of the M2 metabolite, which is responsible for the QT prolongation observed during bedaquiline therapy - therefore, this interaction may not cause increased toxicity, but this needs confirmation in a clinical study.56 No significant pharmacokinetic drug-drug interactions are expected between antiretroviral drugs and levofloxacin, linezolid, or delamanid.
Table 2b.
Drug-drug interactions between WHO group A and B drugs for rifampicin-resistant tuberculosis and antiretroviral drugs.
| Anti-TB drug | Interacting antiretroviral drug |
|---|---|
| Bedaquiline | Efavirenz approximately halves exposure - avoid co-administration99 Ritonavir or cobicistat markedly increase exposure – monitor ECG |
| Clofazimine | Potential additive QT effect with efavirenz – monitor ECG |
| Levofloxacin | No interactions |
| Linezolid | Avoid zidovudine (shared bone marrow toxicity) |
| Moxifloxacin | Efavirenz reduces AUC by 30% - the clinical significance of this interaction needs further study; consider using levofloxacin100 |
Abbreviations: AUC = Area under the curve; ECG = electrocardiogram
Drug-drug interaction studies are typically done in healthy volunteers, but this practice has resulted in several misleading findings. Concentrations of rifabutin are similar in healthy volunteers and PLWH, but are higher in healthy volunteers than PLWH when co-administered with protease inhibitors.55 Increased doses of protease inhibitors with rifampicin co-administration resulted in very high rates of symptomatic hepatitis in healthy volunteers, but double dose lopinavir/ritonavir with rifampicin was relatively well tolerated in PLWH.57 However, a study of adjusted doses of darunavir/ritonavir with rifampicin in PLWH was stopped early due to a high proportion of participants developing hepatoxicity.58 Whether atazanavir-ritonavir can be safely used in combination with rifampicin at higher doses to overcome the rifampicin induction, and what doses are optimal, are questions for future research. A study of the interaction between dolutegravir and weekly rifapentine plus isoniazid in healthy volunteers was stopped early due to high rates of systemic hypersensitivity.59 In a study of PLWH (n=60) dolutegravir with weekly rifapentine plus isoniazid was well tolerated.60
Tuberculosis-associated immune reconstitution inflammatory syndrome (TB-IRIS)
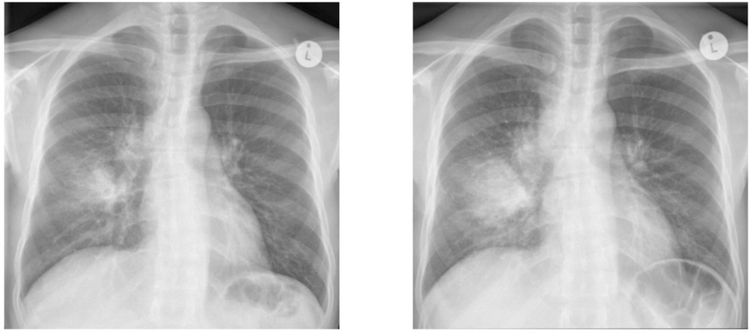
Patients with advanced HIV being treated for tuberculosis are at substantial risk for an immune-mediated deterioration in their clinical condition during the first weeks of initiating ART, re-initiating ART or switching from an ineffective to an effective ART regimen. This condition is referred to as paradoxical tuberculosis-associated immune reconstitution inflammatory syndrome (TB-IRIS) and manifests with inflammation at the sites of tuberculosis disease and features of systemic inflammation. The median time of onset of TB-IRIS symptoms is 14 days after starting or switching ART, but onset may be delayed up to 3 months.61 TB-IRIS is attributed to enhanced immune responses to Mycobacterium tuberculosis in the context of a rapid fall in HIV viral load and early immune recovery on ART. Common clinical features are recurrence of TB symptoms, enlargement and suppuration of lymph nodes, abscess formation, worsening of radiographic pulmonary infiltrates (example in Figure 2), new or enlarging effusions and granulomatous hepatitis. Features of systemic inflammation include fever, tachycardia and weight loss.62
Figure 2: Illustrative case of paradoxical TB-IRIS:
This 34 year-old man with a CD4 count of 23 cells/µL was diagnosed with tuberculosis (Xpert MTB/RIF showed Mycobacterium tuberculosis; rifampicin susceptible). His tuberculosis symptoms had largely resolved by the time he started ART, 12 days after starting antituberculosis treatment. Three days after starting ART, he developed anorexia, vomiting, nocturnal fevers and worsening cough. When assessed four days later he was febrile (38.6 degrees Celsius) and tachycardic (heart rate 129/minute) and had lost 2 kilograms in weight. His repeat chest radiograph (b) showed expansion of the right middle lobe consolidation with enlarging lymph nodes at the right tracheobronchial angle and a new infiltrate in the left lower lobe when compared with the radiograph performed prior to starting antiretrovirals (a). He was started on prednisone 80mg/day to treat TB-IRIS. His fever and symptoms resolved over the next 2–3 weeks and his prednisone was weaned and stopped.
In a meta-analysis of 40 cohort studies, the pooled incidence of TB-IRIS among patients with tuberculosis initiating ART was 18% (95%CI=16–21% ).63 The most consistently identified risk factors are low CD4 count at ART initiation (especially < 50 cells/µL), extrapulmonary or disseminated TB, and a short interval between starting antituberculosis treatment and ART. In studies enrolling patients with these risk factors, the incidence of TB-IRIS reported has been over 50%.64,65
Mortality attributable to TB-IRIS is infrequent; death is attributable to TB-IRIS in 2%.63 Most cases where TB-IRIS is the cause of death have neurologic involvement.63 TB-IRIS may cause enlargement of tuberculomas and new or recurrent meningitis which can be complicated by strokes, cerebral oedema, and hydrocephalus.66
Several components of the immune system contribute to the inflammatory response and tissue pathology in TB-IRIS.67,68 Higher mycobacterial-specific T-lymphocyte effector responses have been reported in TB-IRIS patients as well as higher concentrations of serum cytokines involved in both the adaptive and innate immune response (especially TNF, IL-6 and IFN-gamma).69–71 Gene expression studies have described enhanced innate immune signaling (enriched for genes involved in pattern recognition receptor pathways, inflammasomes and the complement cascade) during early ART.72,73 In TB-IRIS complicating tuberculous meningitis, higher cerebrospinal fluid neutrophil count and levels of neutrophil-associated soluble inflammatory mediators were present both at TBM diagnosis and at the time of TB-IRIS.74 Together these data suggest a central role for innate immune cells in TB-IRIS pathogenesis.
The diagnosis of paradoxical TB-IRIS relies on typical clinical features and exclusion of alternative diagnoses that may mimic the presentation; there is no confirmatory test. Consensus case definitions for TB-IRIS have been published, with their main objective being standardization across research studies.62 Key components of the paradoxical TB-IRIS case definition include a reliable diagnosis of TB; initial response to TB treatment; deterioration with compatible symptoms, signs and/or radiographic features within 3 months of ART initiation, re-initiation or regimen change because of treatment failure; and exclusion of relevant alternative explanations such as antituberculosis drug resistance.62
One clinical trial has evaluated treatment of TB-IRIS in patients without immediately life-threatening manifestations. Participants were randomized to prednisone (1.5 mg/kg/day for 2 weeks followed by 0.75 mg/kg/day for 2 weeks) or placebo. Prednisone reduced cumulative days hospitalized and outpatient therapeutic procedures (the composite primary endpoint) and also resulted in more rapid resolution of symptoms, chest radiology score and C-reactive protein elevation.75 We suggest that patients with a clinical diagnosis of paradoxical TB-IRIS and without contra-indications to corticosteroids should be treated with a course of prednisone starting at 1.5mg/kg/day and weaning over 4 weeks. Some patients require longer courses of prednisone because their symptoms recur on weaning or stopping prednisone.
Corticosteroids also prevented TB-IRIS in a randomised placebo-controlled trial in patients with HIV-associated TB with a CD4 count ≤ 100 cells/µL and starting ART within 30 days of starting antituberculosis treatment.76 Prednisone (40mg/day for 2 weeks followed by 20mg/day for 2 weeks) reduced the incidence of TB-IRIS from 47% in the placebo arm to 33% in the prednisone arm (RR=0.70, 95%CI=0.51–0.96). In both trials, prednisone was well tolerated with no excess risk of severe infections or HIV-related malignancies. While delaying ART initiation to 8 weeks on TB treatment will reduce the risk of TB-IRIS, this is not advised in patients with very low CD4 counts because of the increased risk of mortality associated with such a delay (discussed below).
The diagnosis, common features, treatment and prevention of paradoxical TB-IRIS are summarized in the Panel. Research questions in this field include whether higher doses of prednisone could be more effective in preventing paradoxical TB-IRIS and whether such a strategy is safe; and whether there is a role for more directed immunomodulation for the prevention and treatment of TB-IRIS (eg. TNF or interleukin-6 blockade) particularly in the treatment of life-threatening neurologic TB-IRIS.
Another form of TB-IRIS (unmasking TB-IRIS) is recognized. This occurs in patients with undiagnosed active TB at the time of initiating ART, who then manifest exaggerated inflammatory presentations of TB after starting ART.62 Unmasking TB-IRIS is less well characterised than paradoxical TB-IRIS and there are no controlled studies of management strategies.
Timing of ART initiation in patients with tuberculosis
Given the challenges associated with concurrent antituberculosis treatment and ART, many clinicians were previously of the opinion that ART should be delayed in patients being treated for tuberculosis. A number of randomised controlled trials published over the last decade have clarified the optimal timing of ART in ART-naïve patients with HIV-associated TB.77
A meta-analysis of 8 clinical trials, cumulatively enrolling 4568 patients, showed that early ART initiation (1 to 4 weeks after starting antituberculosis treatment) reduced mortality by 19% (relative risk (RR) = 0.81, 95%CI= 0.66–0.99) when compared with delayed ART (8 to 12 weeks after starting antituberculosis treatment).77 In the subgroup of patients with CD4 count < 50 cells/µL, the reduction in mortality was of greater magnitude (RR=0.71, 95%CI=0.54–0.93). In those patients with a CD4 count > 50 cells/µL, no mortality benefit from earlier ART was evident. There was no difference in HIV viral suppression, TB cure or adverse events comparing early and delayed ART. Loss to follow-up, however, was 60% higher with early compared to delayed ART. Early ART was also associated with a two-fold higher incidence of TB-IRIS (RR=2.31, 95%CI=1.81–2.86), which was present in patients with CD4 count < 50 cells/µL and those with CD4 count > 50 cells/µL. Thus, while patients with low CD4 counts cannot afford to defer ART beyond 2 weeks because of increased mortality risk this comes at the cost of an increased risk of TB-IRIS. A question that should be explored in future research is whether immediate initiation of ART at the same time as antituberculosis treatment in severely ill patients with CD4 counts < 50 cells/μL, could improve outcomes.
A randomized placebo-controlled multi-country trial (TB-HAART) evaluated whether ART could be safely deferred until after completion of antituberculosis treatment in patients with a CD4 count > 220 cells/µL. The composite primary endpoint included tuberculosis treatment failure, tuberculosis recurrence and death within 12 months. Comparing patients who started at 2 weeks versus after 6 months of antituberculosis treatment, there was no significant difference in the primary endpoint, mortality, adverse events or TB-IRIS.49 While TB-HAART suggests that it is safe to defer ART until the end of antituberculosis treatment in PLWH who are not severely immune suppressed, in programmatic settings this misses the opportunity to initiate ART during antituberculosis treatment. In a large observational study in Cape Town, 22% of patients with HIV-associated tuberculosis not on ART at diagnosis did not start ART during antituberculosis treatment despite guidelines that all were eligible; failure to start ART was more common in patients with CD4 count > 500 cells/µL.78
WHO guidelines recommend that antituberculosis treatment should be initiated first, followed by ART within the first 8 weeks of treatment. Patients with CD4 count < 50 cells/µL should receive ART within 2 weeks of initiating antituberculosis treatment.79
No trials of ART timing have enrolled sufficient numbers of patients with drug-resistant tuberculosis to address when ART should be initiated. For patients with drug-resistant TB, we suggest the same guidelines for starting ART be followed. In tuberculous meningitis it is generally advised that ART initiation should be deferred a few weeks, the concern being the risk of neurologic TB-IRIS which can be fatal.79,80 In a clinical trial conducted in Vietnam, there was no significant difference in 9-month mortality or the time to new AIDS events or death when comparing immediate ART or deferral for 2 months. However, grade 4 adverse events were significantly more common in the immediate ART arm.81
Adjunctive corticosteroids
In a Cochrane meta-analysis, corticosteroids reduced deaths from tuberculous pericarditis among HIV-negative patients (RR 0.39, 95% CI = 0.19–0.80). In HIV-negative patients there was also a trend toward reduced all-cause death and need for repeat pericardiocentesis. In PLWH with tuberculous pericarditis, there was a trend towards reduced pericardial constriction when treated with adjunctive corticosteroids (risk ratio = 0.55, 95% CI = 0.26–1.16),82 but there was no discernable impact on death or need for repeat pericardiocentesis. However, in these trials only 20% of PLWH were on ART. In the Investigation of the Management of Pericarditis (IMPI) trial,83 which included 939 HIV-positive patients, prednisolone was associated with an increased risk of HIV-associated cancers (1.8% vs. 0.6%; hazard ratio = 3.27; 95% CI = 1.07–10.0), predominantly Kaposi sarcoma in participants not yet on ART (personal communication, Mpiko Ntsekhe). The risk of Kaposi sarcoma is likely to be lower in patients on ART. In HIV-positive patients not on ART, harms of high-dose corticosteroids appear to outweigh potential benefit. In those on ART, it has been suggested that management should be similar to HIV-negative patients, but there is insufficient clinical trial data to support this.82
In another Cochrane meta-analysis of patients with tuberculous meningitis, adjunctive corticosteroids were associated with reduced death in all patients (risk ratio = 0.75, 95%CI=0.65–0.87).84 There was little or no effect on disabling neurological deficit as a long-term complication. Only one trial included patients with HIV infection, but there were few PLWH enrolled (n=98).85 The results did not show heterogeneity with respect to HIV-status in this trial, but the point estimate for death (risk ratio = 0.90, 95%CI = 0.67–1.20) did not allow for a definitive conclusion that corticosteroids have survival benefit in PLWH with tuberculous meningitis. This question is being assessed in an ongoing trial in Vietnam and Indonesia in which PLWH with tuberculous meningitis are being randomised to 6–8 weeks of adjunctive dexamethasone or identical placebo (https://clinicaltrials.gov/ct2/show/NCT03092817). In the interim, and based on current evidence, many clinicians do use adjunctive corticosteroids in PLWH with tuberculous meningitis.
As discussed above, prednisone has been shown to be of benefit in the treatment and prevention of paradoxical TB-IRIS without risk of excess severe infections or malignancy; all patients in those studies were on ART.75,76 Trials of prednisolone in patients with pulmonary and pleural TB in the pre-ART era in Africa showed a significant risk of adverse events, both metabolic and Kaposi sarcoma, and there is insufficient evidence of clinical benefit to justify their use for these indications.86–88
Secondary prophylaxis
HIV-infected patients with drug-susceptible tuberculosis generally respond well to treatment, but following therapy, remain at risk of recurrence—typically due to re-infection in high endemic settings. Two randomised trials and one observational cohort in high burden settings have examined the effectiveness of secondary prophylaxis with isoniazid following successful treatment with combination first-line antituberculosis therapy;89–91 PLWH who received secondary prophylaxis with isoniazid had a lower rate of recurrence than those who did not. Reductions in tuberculosis TB incidence ranged from 55% to 82%, but these results should be interpreted with caution. First, two of the studies used only clinical symptoms to diagnose TB. Second, and more importantly, all three studies were performed before the availability of ART. Given the known protective effect of ART and CD4 cell recovery in preventing TB disease, it is not clear that secondary prophylaxis with isoniazid would provide any additional benefit.92 Now that ART is widely available and recommended for all patients with HIV-associated tuberculosis, secondary isoniazid prophylaxis has not been common practice. Secondary prophylaxis is not currently recommended by the WHO.
Co-trimoxazole prophylaxis and ancillary management
Two randomised placebo-controlled trials in patients with HIV-associated tuberculosis from the pre-ART era showed that co-trimoxazole prophylaxis significantly reduced the incidence of mortality and hospitalisation.93,94 There were no CD4 inclusion or exclusion criteria in either study, but one study93 reported no benefit of co-trimoxazole with CD4 counts above 350 cells/µL. WHO recommends initiation of co-trimoxazole prophylaxis in patients with clinical stage 3 or 4 disease, which includes all patients with tuberculosis, irrespective of CD4 count.79 Co-trimoxazole can be discontinued when the CD4 count exceeds 350 cells/µL on ART, except in regions where severe bacterial infections and/or malaria are highly prevalent, where co-trimoxazole should be continued lifelong.
In patients with advanced HIV, it is important to consider other aspects of HIV management such as cryptococcal antigen screening in those with low CD4 counts (below 200 or 100 cells/µL dependent on national guidelines)95 and clinical screening for other opportunistic infections such as pneumocystis pneumonia. Superimposed bacterial infections may complicate HIV-associated tuberculosis, requiring close clinical monitoring. In hospitalised patients HIV-associated tuberculosis is associated with substantial risk for deep vein thrombosis and heparin prophylaxis is advised.96
Conclusions
Substantial progress has been made in characterising the challenges faced during co-treatment of tuberculosis and HIV, and in development of strategies to manage them. All patients with HIV-associated tuberculosis should start ART within 2 months of antituberculosis treatment and, in those with CD4 < 50 cells/µL, this should be within 2 weeks. Starting ART within 2 weeks of antituberculosis treatment has a survival benefit, limited to PLWH with CD4 < 50 cells/µL, but increases the risk of paradoxical TB-IRIS. Prophylactic prednisone has recently been shown to reduce the risk of TB-IRIS by 30%. Major drug-drug interactions exist between several antituberculosis and antiretroviral drugs, but strategies have been developed to appropriately manage these. Mortality remains high among PLWH diagnosed with tuberculosis during a hospital admission (many of whom have disseminated tuberculosis and mycobacteraemia) and those with tuberculous meningitis. Research to improve outcomes using more effective antituberculosis therapy and/or host directed therapies in these patients is a priority.
Supplementary Material
Search strategy and selection criteria.
We searched Pubmed for original research and reviews describing management and complications of managing HIV-associated tuberculosis. We used the following search terms in select combinations: “HIV”, “antiretroviral therapy” “tuberculosis”, “drug-resistant tuberculosis”, “drug interactions”, “drug reactions”, “drug toxicity”, “immune reconstitution inflammatory syndrome”, “drug resistant”. A systematic review of all publications was not conducted. Rather studies were selected for inclusion that were most pertinent to informing management recommendations, with an emphasis on randomised controlled trials and pharmacokinetic studies. We prioritised more recent original research (published in last 2 years) for review and included in our review the most recent relevant systematic reviews and meta-analyses published in this field. We restricted our search to English language publications. We also reviewed abstracts from the International AIDS Conference, the Conference on Retroviruses and Opportunistic Infections, the IAS Conference on HIV Science and the Union Conference on Lung Health in the last 2 years. We reviewed relevant sections of World Health Organization treatment guidelines on HIV, tuberculosis and drug-resistant tuberculosis.
Panel: Paradoxical tuberculosis-associated immune reconstitution inflammatory syndrome (TB-IRIS).
Diagnosis
Recurrent, new or worsening TB symptoms, signs and/or radiologic features after starting ART in a patient on treatment for TB
Onset of symptoms within 3 months of starting ART (in most patients within 1–2 weeks)
Exclusion of other diagnoses that could explain clinical deterioration (eg. drug resistant TB, another opportunistic infection)
There is no diagnostic test that confirms the diagnosis
Common features
Recurrent symptoms (e,g. cough, fever, night sweats, weight loss)
Enlargement of lymph nodes with or without suppuration
Worsening of chest radiograph infiltrates
New, recurrent or enlarging serous effusions (pleural, pericardial or ascites)
Abscess formation
Painful liver enlargement
New or recurrent meningitis
Enlarging cerebral tuberculomas
Prevention
Delaying ART to 8 weeks after starting antituberculosis treatment (but this cannot be recommended in patients with CD4 count ≤ 50 cells/µl due to higher mortality if ART is delayed)
Prednisone for 4 weeks (40mg daily for 2 weeks followed by 20mg daily for 2 weeks) in patients with TB and CD4 count ≤ 100 cells/µl starting ART reduced incidence of TB-IRIS by 30% in a clinical trial 76
Treatment
Prednisone 1.5mg/kg/day for 2 weeks followed by 0.75mg/kg/day for 2 weeks resulted in more rapid resolution of symptoms and reduced duration of hospitalization and therapeutic procedures in a clinical trial 75
Some patients require longer courses of prednisone because their symptoms recur on weaning or stopping prednisone.
Non-steroidal anti-inflammatories drugs have been used for mild manifestations
In refractory cases, thalidomide, TNF-blockers and interleukin-6 blockers have been used
Aspiration procedures (eg. lymph node aspirate, pericardiocentesis) may be required to relieve symptoms or mitigate complications
Interruption of ART is not advised
In the majority of patients with TB-IRIS, antituberculosis treatment should not be prolonged beyond 6 months
In patients with abscesses or tuberculomas that are present > 6 months after starting antituberculosis treatment most clinicians prolong the treatment
Acknowledgements
Graeme Meintjes was supported by the Wellcome Trust (098316 and 203135/Z/16/Z), the South African Research Chairs Initiative of the Department of Science and Technology and National Research Foundation (NRF) of South Africa (Grant No 64787), NRF incentive funding (UID: 85858) and the South African Medical Research Council through its TB and HIV Collaborating Centres Programme with funds received from the National Department of Health (RFA# SAMRC-RFA-CC: TB/HIV/AIDS-01–2014). James Brust was supported by the U.S. National Institutes of Health (R01AI114304 and P30AI124414). Gary Maartens was supported by the Wellcome Trust (203135/Z/16/Z) and NRF incentive funding (UID: 85810).
The funders had no role in the writing of this Review. The opinions, findings and conclusions expressed in this manuscript reflect those of the authors alone.
Footnotes
Declaration of interests
GMa served on an advisory board for ViiV. All other authors declare no competing interests.
Contributor Information
Graeme Meintjes, Wellcome Centre for Infectious Diseases Research in Africa, Institute of Infectious Disease and Molecular Medicine, University of Cape Town, South Africa, Division of Infectious Diseases and HIV Medicine, Department of Medicine, University of Cape Town, South Africa.
James C.M. Brust, Divisions of General Internal Medicine and Infectious Diseases, Albert Einstein College of Medicine, New York, United States.
James Nuttall, Department of Paediatrics and Child Health, University of Cape Town, South Africa.
Gary Maartens, Wellcome Centre for Infectious Diseases Research in Africa, Institute of Infectious Disease and Molecular Medicine, University of Cape Town, South Africa, Division of Clinical Pharmacology, Department of Medicine, University of Cape Town, South Africa.
References
- 1.Global Tuberculosis Report, 2018. World Health Organization; https://www.who.int/tb/publications/global_report/en/. [Google Scholar]
- 2.Ford N, Matteelli A, Shubber Z, et al. TB as a cause of hospitalization and in-hospital mortality among people living with HIV worldwide: a systematic review and meta-analysis. J Int AIDS Soc 2016; 19(1): 20714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ford N, Shubber Z, Meintjes G, et al. Causes of hospital admission among people living with HIV worldwide: a systematic review and meta-analysis. Lancet HIV 2015; 2(10): e438–44. [DOI] [PubMed] [Google Scholar]
- 4.Bigna JJ, Noubiap JJ, Agbor AA, et al. Early Mortality during Initial Treatment of Tuberculosis in Patients Co-Infected with HIV at the Yaounde Central Hospital, Cameroon: An 8-Year Retrospective Cohort Study (2006–2013). PLoS One 2015; 10(7): e0132394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kyeyune R, den Boon S, Cattamanchi A, et al. Causes of early mortality in HIV-infected TB suspects in an East African referral hospital. J Acquir Immune Defic Syndr 2010; 55(4): 446–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meintjes G, Kerkhoff AD, Burton R, et al. HIV-Related Medical Admissions to a South African District Hospital Remain Frequent Despite Effective Antiretroviral Therapy Scale-Up. Medicine (Baltimore) 2015; 94(50): e2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Subbarao S, Wilkinson KA, van Halsema CL, et al. Raised Venous Lactate and Markers of Intestinal Translocation Are Associated With Mortality Among In-Patients With HIV-Associated TB in Rural South Africa. J Acquir Immune Defic Syndr 2015; 70(4): 406–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gupta RK, Lucas SB, Fielding KL, Lawn SD. Prevalence of tuberculosis in post-mortem studies of HIV-infected adults and children in resource-limited settings: a systematic review and meta-analysis. AIDS 2015; 29(15): 1987–2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gupta-Wright A, Corbett EL, van Oosterhout JJ, et al. Rapid urine-based screening for tuberculosis in HIV-positive patients admitted to hospital in Africa (STAMP): a pragmatic, multicentre, parallel-group, double-blind, randomised controlled trial. Lancet 2018; 392(10144): 292–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peter JG, Zijenah LS, Chanda D, et al. Effect on mortality of point-of-care, urine-based lipoarabinomannan testing to guide tuberculosis treatment initiation in HIV-positive hospital inpatients: a pragmatic, parallel-group, multicountry, open-label, randomised controlled trial. Lancet 2016; 387(10024): 1187–97. [DOI] [PubMed] [Google Scholar]
- 11.Jacob ST, Pavlinac PB, Nakiyingi L, et al. Mycobacterium tuberculosis bacteremia in a cohort of hiv-infected patients hospitalized with severe sepsis in uganda-high frequency, low clinical suspicion [corrected] and derivation of a clinical prediction score. PLoS One 2013; 8(8): e70305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.World Health Organization, Guidelines for treatment of drug-susceptible tuberculosis and patient care 2017. UPDATE. WHO/HTM/TB/2017.05.
- 13.Fox W, Ellard GA, Mitchison DA. Studies on the treatment of tuberculosis undertaken by the British Medical Research Council tuberculosis units, 1946–1986, with relevant subsequent publications. Int J Tuberc Lung Dis 1999; 3(10 Suppl 2): S231–79. [PubMed] [Google Scholar]
- 14.Perriens JH, St Louis ME, Mukadi YB, et al. Pulmonary tuberculosis in HIV-infected patients in Zaire. A controlled trial of treatment for either 6 or 12 months. N Engl J Med 1995; 332(12): 779–84. [DOI] [PubMed] [Google Scholar]
- 15.Swaminathan S, Narendran G, Venkatesan P, et al. Efficacy of a 6-month versus 9-month intermittent treatment regimen in HIV-infected patients with tuberculosis: a randomized clinical trial. Am J Respir Crit Care Med 2010; 181(7): 743–51. [DOI] [PubMed] [Google Scholar]
- 16.Ahmad Khan F, Minion J, Al-Motairi A, Benedetti A, Harries AD, Menzies D. An updated systematic review and meta-analysis on the treatment of active tuberculosis in patients with HIV infection. Clin Infect Dis 2012; 55(8): 1154–63. [DOI] [PubMed] [Google Scholar]
- 17.Centers for Disease C, Prevention. Acquired rifamycin resistance in persons with advanced HIV disease being treated for active tuberculosis with intermittent rifamycin-based regimens. MMWR Morb Mortal Wkly Rep 2002; 51(10): 214–5. [PubMed] [Google Scholar]
- 18.Gopalan N, Santhanakrishnan RK, Palaniappan AN, et al. Daily vs Intermittent Antituberculosis Therapy for Pulmonary Tuberculosis in Patients With HIV: A Randomized Clinical Trial. JAMA Intern Med 2018; 178(4): 485–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boeree MJ, Heinrich N, Aarnoutse R, et al. High-dose rifampicin, moxifloxacin, and SQ109 for treating tuberculosis: a multi-arm, multi-stage randomised controlled trial. Lancet Infect Dis 2017; 17(1): 39–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tiberi S, du Plessis N, Walzl G, et al. Tuberculosis: progress and advances in development of new drugs, treatment regimens, and host-directed therapies. Lancet Infect Dis 2018; 18(7): e183–e98. [DOI] [PubMed] [Google Scholar]
- 21.Falzon D, Jaramillo E, Schunemann HJ, et al. WHO guidelines for the programmatic management of drug-resistant tuberculosis: 2011 update. Eur Respir J 2011; 38(3): 516–28. [DOI] [PubMed] [Google Scholar]
- 22.Falzon D, Schunemann HJ, Harausz E, et al. World Health Organization treatment guidelines for drug-resistant tuberculosis, 2016 update. Eur Respir J 2017; 49(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuaban C, Noeske J, Rieder HL, Ait-Khaled N, Abena Foe JL, Trebucq A. High effectiveness of a 12-month regimen for MDR-TB patients in Cameroon. Int J Tuberc Lung Dis 2015; 19(5): 517–24. [DOI] [PubMed] [Google Scholar]
- 24.Nunn AJ, Phillips PPJ, Meredith SK, et al. A Trial of a Shorter Regimen for Rifampin-Resistant Tuberculosis. N Engl J Med 2019. [DOI] [PubMed]
- 25.Gandhi NR, Shah NS, Andrews JR, et al. HIV coinfection in multidrug- and extensively drug-resistant tuberculosis results in high early mortality. Am J Respir Crit Care Med 2010; 181(1): 80–6. [DOI] [PubMed] [Google Scholar]
- 26.Pietersen E, Ignatius E, Streicher EM, et al. Long-term outcomes of patients with extensively drug-resistant tuberculosis in South Africa: a cohort study. Lancet 2014; 383(9924): 1230–9. [DOI] [PubMed] [Google Scholar]
- 27.Collaborative Group for the Meta-Analysis of Individual Patient Data in MDRTBt, Ahmad N, Ahuja SD, et al. Treatment correlates of successful outcomes in pulmonary multidrug-resistant tuberculosis: an individual patient data meta-analysis. Lancet 2018; 392(10150): 821–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Conradie F, Diacon A, Howell P, Everitt D, Crook A, Mendel C, Egizi E, Moreira J, Timm J, McHugh T, Wills G, Van Niekerk C, Li M, Olugbosi M, Spigelman M. Sustained high rate of successful treatment outcomes: Interim results of 75 patients in the Nix-TB clinical study of pretomanid, bedaquiline and linezolid. 49th Union World Conference on Lung Health. The Hague, Netherlands 24–27 October 2018 Abstract OA03–213-25. [Google Scholar]
- 29.Geiter LJ, Delamanid global clinical database and Phase 3 trial results. Satellite Session at the 48th Union World Conference on Lung Health; 11–14 October 2017; Guadalajara, Mexico. [Google Scholar]
- 30.Lee M, Lee J, Carroll MW, et al. Linezolid for treatment of chronic extensively drug-resistant tuberculosis. N Engl J Med 2012; 367(16): 1508–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schnippel K, Ndjeka N, Maartens G, et al. Effect of bedaquiline on mortality in South African patients with drug-resistant tuberculosis: a retrospective cohort study. Lancet Respir Med 2018. [DOI] [PubMed]
- 32.von Groote-Bidlingmaier F, Patientia R, Sanchez E, et al. Efficacy and safety of delamanid in combination with an optimised background regimen for treatment of multidrug-resistant tuberculosis: a multicentre, randomised, double-blind, placebo-controlled, parallel group phase 3 trial. Lancet Respir Med 2019; 7(3): 249–59. [DOI] [PubMed] [Google Scholar]
- 33.World Health Organization 2018, Rapid communication: key changes to treatment of multidrug- and rifampicin-resistant tuberculosis (MDR/RR-TB) WHO/CDS/TB/201818; Geneva: 2018. [Google Scholar]
- 34.Katiyar SK, Bihari S, Prakash S, Mamtani M, Kulkarni H. A randomised controlled trial of high-dose isoniazid adjuvant therapy for multidrug-resistant tuberculosis. Int J Tuberc Lung Dis 2008; 12(2): 139–45. [PubMed] [Google Scholar]
- 35.Chigutsa E, Pasipanodya JG, Visser ME, et al. Impact of nonlinear interactions of pharmacokinetics and MICs on sputum bacillary kill rates as a marker of sterilizing effect in tuberculosis. Antimicrob Agents Chemother 2015; 59(1): 38–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pasipanodya JG, McIlleron H, Burger A, Wash PA, Smith P, Gumbo T. Serum drug concentrations predictive of pulmonary tuberculosis outcomes. J Infect Dis 2013; 208(9): 1464–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peloquin CA, Nitta AT, Burman WJ, et al. Low antituberculosis drug concentrations in patients with AIDS. Ann Pharmacother 1996; 30(9): 919–25. [DOI] [PubMed] [Google Scholar]
- 38.Gurumurthy P, Ramachandran G, Hemanth Kumar AK, et al. Decreased bioavailability of rifampin and other antituberculosis drugs in patients with advanced human immunodeficiency virus disease. Antimicrob Agents Chemother 2004; 48(11): 4473–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tappero JW, Bradford WZ, Agerton TB, et al. Serum concentrations of antimycobacterial drugs in patients with pulmonary tuberculosis in Botswana. Clin Infect Dis 2005; 41(4): 461–9. [DOI] [PubMed] [Google Scholar]
- 40.van Oosterhout JJ, Dzinjalamala FK, Dimba A, et al. Pharmacokinetics of Antituberculosis Drugs in HIV-Positive and HIV-Negative Adults in Malawi. Antimicrob Agents Chemother 2015; 59(10): 6175–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McIlleron H, Rustomjee R, Vahedi M, et al. Reduced antituberculosis drug concentrations in HIV-infected patients who are men or have low weight: implications for international dosing guidelines. Antimicrob Agents Chemother 2012; 56(6): 3232–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stott KE, Pertinez H, Sturkenboom MGG, et al. Pharmacokinetics of rifampicin in adult TB patients and healthy volunteers: a systematic review and meta-analysis. J Antimicrob Chemother 2018; 73(9): 2305–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Merle CS, Floyd S, Ndiaye A, Galperine T, Furco A, De Jong BC, McIlleron H, Glynn J, Sarr M, Bah-Sow O, Affolabi D, on behalf of the RAFA Team. High-dose rifampicin tuberculosis treatment regimen to reduce 12-month mortality of TB/HIV co-infected patients: the RAFA trial results. 21st International AIDS Conference, Durban, 2016. Abstract WEAB0205LB. [Google Scholar]
- 44.Te Brake L, Dian S, Ganiem AR, et al. Pharmacokinetic/pharmacodynamic analysis of an intensified regimen containing rifampicin and moxifloxacin for tuberculous meningitis. Int J Antimicrob Agents 2015; 45(5): 496–503. [DOI] [PubMed] [Google Scholar]
- 45.Yunivita V, Dian S, Ganiem AR, et al. Pharmacokinetics and safety/tolerability of higher oral and intravenous doses of rifampicin in adult tuberculous meningitis patients. Int J Antimicrob Agents 2016; 48(4): 415–21. [DOI] [PubMed] [Google Scholar]
- 46.Lehloenya RJ, Todd G, Badri M, Dheda K. Outcomes of reintroducing anti-tuberculosis drugs following cutaneous adverse drug reactions. Int J Tuberc Lung Dis 2011; 15(12): 1649–57. [DOI] [PubMed] [Google Scholar]
- 47.Hong H, Budhathoki C, Farley JE. Increased risk of aminoglycoside-induced hearing loss in MDR-TB patients with HIV coinfection. Int J Tuberc Lung Dis 2018; 22(6): 667–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Abdool Karim SS, Naidoo K, Grobler A, et al. Timing of initiation of antiretroviral drugs during tuberculosis therapy. N Engl J Med 2010; 362(8): 697–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mfinanga SG, Kirenga BJ, Chanda DM, et al. Early versus delayed initiation of highly active antiretroviral therapy for HIV-positive adults with newly diagnosed pulmonary tuberculosis (TB-HAART): a prospective, international, randomised, placebo-controlled trial. Lancet Infect Dis 2014; 14(7): 563–71. [DOI] [PubMed] [Google Scholar]
- 50.Blanc F, Badje A, Bonnet M, Gabillard D, Messou E, Muzoora C, Samreth S, Bang Nguyen D, Borand L, Domergue A, Ntukunda N, Eholi S, Domoua S, Anglaret X, Laureillard D. Systematic vs Test-guided tuberculosis treatment: data of the STATIS randomized trial. Conference on Retroviruses and Opportunistic Infections. Boston, US March 4–7 2018 Abstract 29LB. [Google Scholar]
- 51.Hosseinipour MC, Bisson GP, Miyahara S, et al. Empirical tuberculosis therapy versus isoniazid in adult outpatients with advanced HIV initiating antiretroviral therapy (REMEMBER): a multicountry open-label randomised controlled trial. Lancet 2016; 387(10024): 1198–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen J, Raymond K. Roles of rifampicin in drug-drug interactions: underlying molecular mechanisms involving the nuclear pregnane X receptor. Ann Clin Microbiol Antimicrob 2006; 5: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McIlleron HM, Schomaker M, Ren Y, et al. Effects of rifampin-based antituberculosis therapy on plasma efavirenz concentrations in children vary by CYP2B6 genotype. AIDS 2013; 27(12): 1933–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dooley K, Kaplan R, Mwelase T, Grinsztejn B, Ticona E, Lacerda M, Sued O, Belonosova E, Ait-Khaled M, Angelis K, Brown D, Singh R, Talarico C, Tenorio A, Keegan M, Aboud M. Safety and efficacy of dolutegravir-based ART in TB/HIV co-infected adults at week 48. AIDS 2018. Amsterdam 23–27 July 2018 Oral abstract TUAB0206. [Google Scholar]
- 55.Hennig S, Svensson EM, Niebecker R, et al. Population pharmacokinetic drug-drug interaction pooled analysis of existing data for rifabutin and HIV PIs. J Antimicrob Chemother 2016; 71(5): 1330–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brill MJ, Svensson EM, Pandie M, Maartens G, Karlsson MO. Confirming model-predicted pharmacokinetic interactions between bedaquiline and lopinavir/ritonavir or nevirapine in patients with HIV and drug-resistant tuberculosis. Int J Antimicrob Agents 2017; 49(2): 212–7. [DOI] [PubMed] [Google Scholar]
- 57.Decloedt EH, McIlleron H, Smith P, Merry C, Orrell C, Maartens G. Pharmacokinetics of lopinavir in HIV-infected adults receiving rifampin with adjusted doses of lopinavir-ritonavir tablets. Antimicrob Agents Chemother 2011; 55(7): 3195–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ebrahim I, Maartens G, Smythe W, Orrell C, Wiesner L, Mcilleron H. Pharmacokinetics and safety of adjusted darunavir/ritonavir with rifampin in PLWH. Conference on Retroviruses and Opportunistic Infections. March 4–7, 2019 Seattle, Washington, US Abstract 81LB. [Google Scholar]
- 59.Brooks KM, George JM, Pau AK, et al. Cytokine-Mediated Systemic Adverse Drug Reactions in a Drug-Drug Interaction Study of Dolutegravir With Once-Weekly Isoniazid and Rifapentine. Clin Infect Dis 2018; 67(2): 193–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dooley KE, Churchyard G, Savic RM, Gupte A, Marzinke MA, Zhang N, Edward V, Wolf L, Sebe M, Likoti M, Fyvie M, Shibambo I, Beattie T , Chaisson RE for the DOLPHIN Study Team. Safety and PK of weekly rifapentine/isoniazid (3HP) in adults with HIV on dolutegravir. Conference on Retroviruses and Opportunistic Infections. March 4–7, 2019 Seattle, Washington, US Abstract 80LB. [Google Scholar]
- 61.Bana TM, Lesosky M, Pepper DJ, et al. Prolonged tuberculosis-associated immune reconstitution inflammatory syndrome: characteristics and risk factors. BMC Infect Dis 2016; 16(1): 518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Meintjes G, Lawn SD, Scano F, et al. Tuberculosis-associated immune reconstitution inflammatory syndrome: case definitions for use in resource-limited settings. Lancet Infect Dis 2008; 8(8): 516–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Namale PE, Abdullahi LH, Fine S, Kamkuemah M, Wilkinson RJ, Meintjes G. Paradoxical TB-IRIS in HIV-infected adults: a systematic review and meta-analysis. Future Microbiol 2015; 10(6): 1077–99. [DOI] [PubMed] [Google Scholar]
- 64.Walker NF, Wilkinson KA, Meintjes G, et al. Matrix Degradation in Human Immunodeficiency Virus Type 1-Associated Tuberculosis and Tuberculosis Immune Reconstitution Inflammatory Syndrome: A Prospective Observational Study. Clin Infect Dis 2017; 65(1): 121–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Narendran G, Andrade BB, Porter BO, et al. Paradoxical tuberculosis immune reconstitution inflammatory syndrome (TB-IRIS) in HIV patients with culture confirmed pulmonary tuberculosis in India and the potential role of IL-6 in prediction. PLoS One 2013; 8(5): e63541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pepper DJ, Marais S, Maartens G, et al. Neurologic manifestations of paradoxical tuberculosis-associated immune reconstitution inflammatory syndrome: a case series. Clin Infect Dis 2009; 48(11): e96–107. [DOI] [PubMed] [Google Scholar]
- 67.Walker NF, Stek C, Wasserman S, Wilkinson RJ, Meintjes G. The tuberculosis-associated immune reconstitution inflammatory syndrome: recent advances in clinical and pathogenesis research. Curr Opin HIV AIDS 2018. [DOI] [PMC free article] [PubMed]
- 68.Lai RP, Meintjes G, Wilkinson RJ. HIV-1 tuberculosis-associated immune reconstitution inflammatory syndrome. Semin Immunopathol 2016; 38(2): 185–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Meintjes G, Wilkinson KA, Rangaka MX, et al. Type 1 helper T cells and FoxP3-positive T cells in HIV-tuberculosis-associated immune reconstitution inflammatory syndrome. Am J Respir Crit Care Med 2008; 178(10): 1083–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bourgarit A, Carcelain G, Martinez V, et al. Explosion of tuberculin-specific Th1-responses induces immune restoration syndrome in tuberculosis and HIV co-infected patients. AIDS 2006; 20(2): F1–7. [DOI] [PubMed] [Google Scholar]
- 71.Tadokera R, Meintjes G, Skolimowska KH, et al. Hypercytokinaemia accompanies HIV-tuberculosis immune reconstitution inflammatory syndrome. Eur Respir J 2011; 37(5): 1248–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lai RP, Meintjes G, Wilkinson KA, et al. HIV-tuberculosis-associated immune reconstitution inflammatory syndrome is characterized by Toll-like receptor and inflammasome signalling. Nat Commun 2015; 6: 8451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tran HT, Van den Bergh R, Loembe MM, et al. Modulation of the complement system in monocytes contributes to tuberculosis-associated immune reconstitution inflammatory syndrome. AIDS 2013; 27(11): 1725–34. [DOI] [PubMed] [Google Scholar]
- 74.Marais S, Wilkinson KA, Lesosky M, et al. Neutrophil-associated central nervous system inflammation in tuberculous meningitis immune reconstitution inflammatory syndrome. Clin Infect Dis 2014; 59(11): 1638–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Meintjes G, Wilkinson RJ, Morroni C, et al. Randomized placebo-controlled trial of prednisone for paradoxical tuberculosis-associated immune reconstitution inflammatory syndrome. AIDS 2010; 24(15): 2381–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Meintjes G, Stek C, Blumenthal L, et al. Prednisone for the Prevention of Paradoxical Tuberculosis-Associated IRIS. N Engl J Med 2018; 379(20): 1915–25. [DOI] [PubMed] [Google Scholar]
- 77.Uthman OA, Okwundu C, Gbenga K, et al. Optimal Timing of Antiretroviral Therapy Initiation for HIV-Infected Adults With Newly Diagnosed Pulmonary Tuberculosis: A Systematic Review and Meta-analysis. Ann Intern Med 2015; 163(1): 32–9. [DOI] [PubMed] [Google Scholar]
- 78.Kaplan R, Hermans S, Caldwell J, Jennings K, Bekker LG, Wood R. HIV and TB co-infection in the ART era: CD4 count distributions and TB case fatality in Cape Town. BMC Infect Dis 2018; 18(1): 356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.World Helth Orgnisation, . Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. Recommndations for public health approach Second edition. 2016. [PubMed]
- 80.Marais S, Meintjes G, Pepper DJ, et al. Frequency, severity, and prediction of tuberculous meningitis immune reconstitution inflammatory syndrome. Clin Infect Dis 2013; 56(3): 450–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Torok ME, Yen NT, Chau TT, et al. Timing of initiation of antiretroviral therapy in human immunodeficiency virus (HIV)--associated tuberculous meningitis. Clin Infect Dis 2011; 52(11): 1374–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wiysonge CS, Ntsekhe M, Thabane L, et al. Interventions for treating tuberculous pericarditis. Cochrane Database Syst Rev 2017; 9: CD000526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mayosi BM, Ntsekhe M, Bosch J, et al. Prednisolone and Mycobacterium indicus pranii in tuberculous pericarditis. N Engl J Med 2014; 371(12): 1121–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Prasad K, Singh MB, Ryan H. Corticosteroids for managing tuberculous meningitis. Cochrane Database Syst Rev 2016; 4: CD002244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Thwaites GE, Nguyen DB, Nguyen HD, et al. Dexamethasone for the treatment of tuberculous meningitis in adolescents and adults. N Engl J Med 2004; 351(17): 1741–51. [DOI] [PubMed] [Google Scholar]
- 86.Elliott AM, Luzze H, Quigley MA, et al. A randomized, double-blind, placebo-controlled trial of the use of prednisolone as an adjunct to treatment in HIV-1-associated pleural tuberculosis. J Infect Dis 2004; 190(5): 869–78. [DOI] [PubMed] [Google Scholar]
- 87.Mayanja-Kizza H, Jones-Lopez E, Okwera A, et al. Immunoadjuvant prednisolone therapy for HIV-associated tuberculosis: a phase 2 clinical trial in Uganda. J Infect Dis 2005; 191(6): 856–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Elliott AM, Halwiindi B, Bagshawe A, et al. Use of prednisolone in the treatment of HIV-positive tuberculosis patients. Q J Med 1992; 85(307–308): 855–60. [PubMed] [Google Scholar]
- 89.Churchyard GJ, Fielding K, Charalambous S, et al. Efficacy of secondary isoniazid preventive therapy among HIV-infected Southern Africans: time to change policy? AIDS 2003; 17(14): 2063–70. [DOI] [PubMed] [Google Scholar]
- 90.Fitzgerald DW, Desvarieux M, Severe P, Joseph P, Johnson WD Jr., Pape JW. Effect of post-treatment isoniazid on prevention of recurrent tuberculosis in HIV-1-infected individuals: a randomised trial. Lancet 2000; 356(9240): 1470–4. [DOI] [PubMed] [Google Scholar]
- 91.Haller L, Sossouhounto R, Coulibaly IM, et al. Isoniazid plus sulphadoxine-pyrimethamine can reduce morbidity of HIV-positive patients treated for tuberculosis in Africa: a controlled clinical trial. Chemotherapy 1999; 45(6): 452–65. [DOI] [PubMed] [Google Scholar]
- 92.Suthar AB, Lawn SD, del Amo J, et al. Antiretroviral therapy for prevention of tuberculosis in adults with HIV: a systematic review and meta-analysis. PLoS Med 2012; 9(7): e1001270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wiktor SZ, Sassan-Morokro M, Grant AD, et al. Efficacy of trimethoprim-sulphamethoxazole prophylaxis to decrease morbidity and mortality in HIV-1-infected patients with tuberculosis in Abidjan, Cote d’Ivoire: a randomised controlled trial. Lancet 1999; 353(9163): 1469–75. [DOI] [PubMed] [Google Scholar]
- 94.Nunn AJ, Mwaba P, Chintu C, et al. Role of co-trimoxazole prophylaxis in reducing mortality in HIV infected adults being treated for tuberculosis: randomised clinical trial. BMJ 2008; 337: a257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ford N, Shubber Z, Jarvis JN, et al. CD4 Cell Count Threshold for Cryptococcal Antigen Screening of HIV-Infected Individuals: A Systematic Review and Meta-analysis. Clin Infect Dis 2018; 66(suppl_2): S152–S9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Awolesi D, Naidoo M, Cassimjee MH. The profile and frequency of known risk factors or comorbidities for deep vein thrombosis in an urban district hospital in KwaZulu-Natal. South Afr J HIV Med 2016; 17(1): 425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Grinsztejn B, De Castro N, Arnold V, et al. Raltegravir for the treatment of patients co-infected with HIV and tuberculosis (ANRS 12 180 Reflate TB): a multicentre, phase 2, non-comparative, open-label, randomised trial. Lancet Infect Dis 2014; 14(6): 459–67. [DOI] [PubMed] [Google Scholar]
- 98.Cerrone M, Alfarisi O, Neary M, et al. Rifampicin effect on intracellular and plasma pharmacokinetics of tenofovir alafenamide. J Antimicrob Chemother 2019. [DOI] [PMC free article] [PubMed]
- 99.Svensson EM, Aweeka F, Park JG, Marzan F, Dooley KE, Karlsson MO. Model-based estimates of the effects of efavirenz on bedaquiline pharmacokinetics and suggested dose adjustments for patients coinfected with HIV and tuberculosis. Antimicrob Agents Chemother 2013; 57(6): 2780–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Naidoo A, Chirehwa M, McIlleron H, et al. Effect of rifampicin and efavirenz on moxifloxacin concentrations when co-administered in patients with drug-susceptible TB. J Antimicrob Chemother 2017; 72(5): 1441–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.