Abstract
Host-related immunodeficiency is known to play a role in the development of multiple myeloma (MM) from its precursor conditions (monoclonal gammopathy of undetermined significance, MGUS, smoldering multiple myeloma, SMM). In order to understand the underlying immune changes in this process, we characterized immune patterns from MGUS to SMM to MM. We further sought to identify potential novel immune biomarkers that may predict progression of SMM to MM. We characterized patterns of circulating lymphocytes in 181 patients using multiparametric flow cytometry. We found decreased B- (p = .0003), increased T- (p = .037) and unaltered NK cell proportions from MGUS to SMM to MM. To gain insights into functional variability, we further characterized immunophenotypic lymphocyte subsets, which uncovered differences in CD57 subsets. Specifically, we found that SMM patients who eventually progressed to MM showed decreased proportions of CD57-CD56 + (p = .0061) and CD57-CD16 + (p = .035) lymphocyte subsets. We thus report novel data characterizing the nature of host-related immunodeficiency in the development of MM. We show sequential changes in lymphocyte subsets from MGUS to SMM to MM. We further suggest that CD57 subsets may serve as potential markers of progression from SMM to MM. Our findings support the study of lymphocyte subsets in the search for immune biomarkers. Such markers could provide clinical guidance in managing myeloma precursor disease.
Keywords: Immunology, neoplasia myeloma and other plasma cell dyscrasias, cancer biology, tumor markers
Introduction
Multiple myeloma (MM) is consistently preceded by a precursor state, monoclonal gammopathy of undetermined significance (MGUS) or smoldering multiple myeloma (SMM) [1]. Although the average annual risk of MM transformation from MGUS and SMM is about 0.5–1% and 10%, respectively, the risk of transformation for individual patients varies greatly [2]. There thus continues to be a need for a better understanding of the pathogenesis of the development of MM from these precursor conditions, the study of which would also assist in the search for markers to predict this transformation [3].
It is currently understood that host-related immunodeficiency plays a role in the development of MM. For example, the suppression of uninvolved immunoglobulins is one of the known adverse markers of transformation from MGUS/SMM to MM and is termed as immunoparesis [4]. Moreover, MM patients have been found to have a depletion in circulating B cells and this has been linked to the immunodeficiency associated with the disease [5].
In order to expand on these findings and better understand the nature of the immune system in MM and its precursor states, and with the goal of identifying novel biomarkers for MM transformation; we designed a study to characterize patterns of immune cells in MGUS, SMM and MM patients. We assessed peripheral blood (PB) lymphocyte profiles, including B-, T- and NK-cell subsets, in a large prospective cohort that included 181 patients. We hypothesized that immune changes over the course of myelomagenesis would be reflected in changes in lymphocyte subsets.
Methods
Patients
Baseline PB samples of MGUS (N = 85) and SMM (N = 85) and MM (N = 11) patients were collected at enrollment in the NCI Natural History Study of MGUS and SMM prospective clinical trial (). All patients received regular 3–6 month clinical follow-ups for up to 5 years on study. Among SMM patients with consistent follow-up, progression analysis using baseline samples was performed between those who subsequently progressed to MM, that is, ‘SMM progressors’ (N = 8) versus those who did not progress to MM, that is, ‘SMM non-progressors’ (N = 71).
Flow cytometry
Multiparametric flow cytometric analysis of lymphocyte subsets was performed using the following phenotypic markers: CD3, CD4, CD8, CD16, CD56, CD57, CD19, TCRαβ and TCRγδ. B cells, T cells, NK cells, NKT cells and γδT cells were defined as CD19+, CD3+, CD3-CD16+ CD56+, CD3+ CD16+ CD56+and CD3+TCRγδ+ lymphocytes, respectively. Multicolor acquisition and analysis was performed using BD FACS CANTO and DIVA software (Becton Dickinson, San Jose, CA).
Statistical methods
Jonckheere–Terpstra trend test was used for ordered trend analyses. Wilcoxon rank-sum test was used for two group comparisons. Fisher’s exact test was used for the analysis of 2 × 2 contingency tables. All p values are two-tailed and unadjusted for multiple comparisons.
Results
Distribution of B-, T- and NK-cell lymphocyte subsets in SMM progressors versus SMM non-progressors
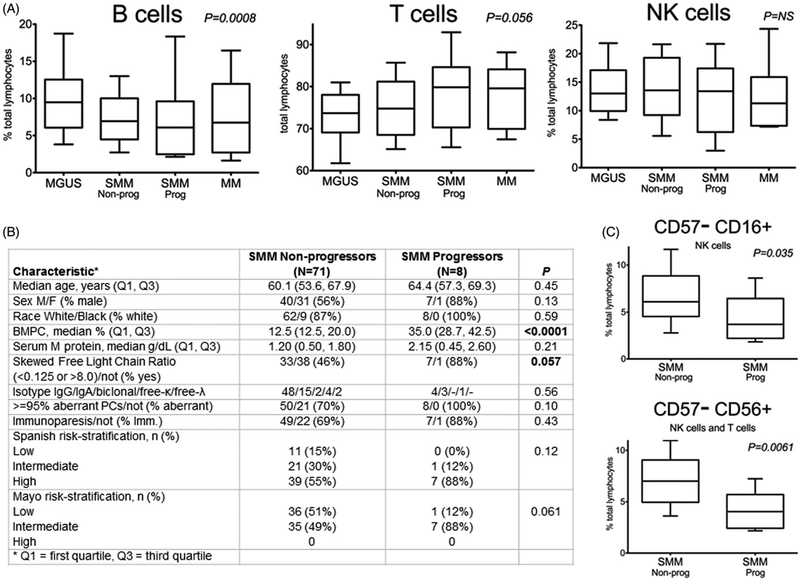
We stratified SMM patients based on their progression status as described in the ‘Patients‘ section above. As expected, [2] SMM progressors (versus nonprogressors) demonstrated distinct baseline clinical characteristics (Figure 1(B)). In particular, they demonstrated higher proportions of bone marrow plasma cells (%BMPC) (p < .0001) and were more likely to have a skewed free light chain (FLC) ratio (p = .057) at baseline. Using flow cytometric analysis of lymphocyte subsets, we initially compared samples from MGUS, SMM and MM patients. We found depletion (i.e. a lower proportion in relation to all lymphocyte subsets) in PB B cells (p = .0003; Figure 2(A)) and increases in T cells (p = .037;Figure 2(B)) but saw no trends in NK cells (data not shown) from MGUS to SMM to MM. We observed the same trends in B- (p = .0008), T- (p = .056) and NK cells upon stratifying SMM patients based on progression status (Figure 1(A)).
Figure 1.
Clinical characteristics and peripheral blood lymphocyte profiles of SMM nonprogressors versus SMM progressors (A) B and T cells, but not NK cells, demonstrate changes over the course of myelomagenesis. (B) Clinical characteristics between SMM nonprogressors versus SMM progressors reveal differences in %BMPC and skewed FLC ratios at baseline. For SMM progressors, the median time to progression was 9.4 months (min: 5.2 months, max: 16.6 months). The median follow-up for SMM nonprogressors was 31.4 months. SMM risk-stratified via Mayo Clinic criteria as low, intermediate or high risk based on the presence of one, two or three of the following, respectively: BMPC ≥10%, serum M-protein ≥3 g/dL and serum Ig FLC ratio <0.125 or >8; Similarly, Spanish PETHEMA risk stratification as low, intermediate, or high risk was based on the presence of zero, one or two of the following, respectively: ≥95% abnormal PCs (defined by multiparametric flow cytometry) and immunoparesis. (C) SMM progressors possessed distinct baseline lymphocyte profiles characterized by a decrease in CD57–CD56+ and CD57–CD16+ subsets. All box-and-whisker plots represent relative numbers (proportion of total PB lymphocytes), with whiskers extending to the 10th and 90th percentiles (values beyond whiskers not shown). Sample sizes and statistical analyses as described in the text.
Figure 2.
B and T cells in myelomagenesis and association with immunoparesis (A) B cells over the course of myelomagenesis with SMM cohort delineated based on Spanish PETHEMA risk stratification. Immunoparesis is associated with decreased B cells in SMM patients. (B) T cells over the course of myelomagenesis, with SMM risk stratified as above. Similar trends for B and T cells were observed when SMM cohort was risk stratified using Mayo Clinic model or using immunoparesis (data not shown). Box plots in this figure use N = 6 instead of N = 11 for MM patients. Risk stratification of SMM patients and box plots as described in Figure 1 legend. Sample sizes and statistical analyses as described in the text.
Upon immunophenotypic analysis of these lymphocyte subsets, the increase in T cells was found to be due to an increase in both CD4+and CD8+ subsets, although these were not individually different; similarly, there were only small differences in NKT cells and γδT cells between all groups (data not shown). However, we did uncover differences in specific CD57 lymphocyte subsets upon progression of disease, which was most notable in our analysis of our stratified SMM patients (Figure 1(C)). Specifically, SMM progressors (versus nonprogressors) had decreased proportions of CD57-lymphocyte subsets, which included the CD57-CD16 + (p = .035) and CD57-CD56 + (p = .0061) subsets. Importantly, the former subset comprised only NK cells, while latter subset included both NK and T cells.
Distribution of B-, T-, and NK-cell lymphocyte subsets in risk-stratified SMM patients versus MGUS and MM patients
In order to expand our findings beyond SMM progressors versus SMM non-progressors, we also assessed patterns by currently available clinical risk models. Specifically, we delineated the SMM cohort into low-, intermediate- or high risk of progression per the Spanish PETHEMA [4] and Mayo Clinic [2,6] risk models. Consistent with the findings above, we found a prominent decrease in PB B cells (p < .0001) and an increase in T cells (p = .0056) by risk group, which we graphically demonstrate using the Spanish PETHEMA criteria (Figure 2). Moreover, the low-risk SMM cohort tended to correspond to MGUS while high-risk SMM corresponded to MM.
Effect of host immunoparesis on the distribution of B, T and NK cells in MGUS, SMM and MM patients
Previous findings have shown that immunoparesis is more prevalent in SMM than in MGUS [4]. With this knowledge, and after stratifying our SMM cohort based on the presence of immunoparesis, we studied lymphocyte subsets from MGUS to SMM to MM in order of increasing immunoparesis. We found that an increase in host immunoparesis also corresponds with a decrease in B cells (p = .0004) and an increase in T cells (p = .018) (data not shown). Moreover, the presence of immunoparesis particularly mirrored the depletion of B cells in our cohort of SMM patients (p = .027) (Figure 2(A)).
Discussion
We studied host-related immunodeficiency in the development of MM by describing differences in B-, T- and NK-cell lymphocyte subsets of MGUS, SMM and MM patients. The depletion in circulating PB B cells has been previously described in MM patients and is considered to play a role in the immunodeficiency associated with the disease [5]. In fact, a decrease in circulating B cells was found to be an independent prognostic factor in MM [5]. Importantly, our study is the first that expands these findings to myelomagenesis by demonstrating strong trends toward a depletion of B cells from MGUS to SMM to MM. A potential explanation for our findings could be that an increasing tumor burden over myelomagenesis leads to a depletion of both B-cell precursors and normal plasma cells in the bone marrow [7,8]. Depletion of the latter could manifest in immunoparesis, [8] which mirrored the depletion of B cells in our cohort of SMM patients. An alternative explanation of our findings would be that both immunoparesis and decreased B cells are a reflection of an increasingly dysfunctional immune system over myelomagenesis. Indeed, our findings of a concurrent increase in T cells show that immune changes are not limited to the humoral compartment.
Moreover, NK- and T-cell subsets are functionally variable [9] and may thus behave in differing ways during tumor evolution. For this reason, we analyzed lymphocyte subsets with a wide range of immunophenotypic markers. Similar to previous studies of immunophenotypic changes in lymphocytes in MM, [10,11] we focused on the relative numbers of such lymphocytes in the circulation. In our SMM cohort, we found a decrease in certain CD57-lymphocyte subsets in patients who were more likely to progress to MM (SMM progressors), suggesting that CD57 expression may be associated with increasing disease stage.
These findings are limited by the modest number of ‘SMM progressors,’ but warrant investigation of these subsets in larger cohorts. Of note, the findings were not different in SMM patients risk-stratified based on currently used models, which employ humoral immune markers. In fact, CD57 is expressed on mature T and NK lymphocytes, which are involved in cell-mediated immunity and CD57 + lymphocytes are often generated in response to consistent antigen exposure [12]. Several studies have demonstrated increased circulating CD57 + lymphocytes in both solid and hematological malignancies, likely due to persistent tumor-associated antigen stimulation of the subset in the absence of effective tumor clearance [12–15]. In MM, the majority of circulating T-cell clones are of the CD8 + CD57 + phenotype, which may be immunosuppressive and have been linked to the immunodeficiency observed in the condition [10,11,14]. The generation of these clones was independent of other causes of CD57 expansion such as hepatitis or human cytomegalovirus infection [11]. Interestingly, findings in patients with chronic HIV infection have also shown the loss of CD57-lymphocyte subsets, possibly due to a decline in less-differentiated NK cells [16]. While our study did not include functional assays, these studies have previously suggested the link between CD57 subsets and immunodeficiency. Although the exact nature of interactions between CD57 lymphocyte subsets and myeloma cells remains to be established, based on these previous findings, we hypothesize that a loss in CD57-subsets may be a reflection of increasing immunodeficiency over the course of myelomagenesis.
Our study provides novel data showing patterns of altered immunophenotypic characteristics from precursor disease to symptomatic myeloma. We expand on the knowledge of humoral immunodeficiency in the development of the condition by showing the sequential depletion of B lymphocytes from MGUS to SMM to MM and its relation to the host immune status. Prior studies have suggested changes in NK cells and T cells in MM [17,18] – using multiparametric flow cytometry of a wide range of immunophenotypes, we show that while there are only minor changes in total NK cells, CD4 + T cells, CD8 + T cells, γδT cells, or NKT cells from precursor disease to MM, there appears to be an altered expression of certain CD57 subsets. We thus suggest that CD57, which is expressed on mature T cells and NK cells in response to antigen exposure, may have a role as an immune biomarker, especially with regards to SMM progressors (versus nonprogressors) having decreased proportions of CD57-CD56+ and CD57-CD16+ lymphocytes. Our findings are novel and support the development of longitudinal studies with sequential collection of samples to define the dynamics of functional subsets of immune cells in individual patients over time.
Funding
This research was made possible through the National Institutes of Health (NIH) Medical Research Scholars Program, a public-private partnership supported jointly by the NIH and generous contributions to the Foundation for the NIH from Pfizer Inc, The Doris Duke Charitable Foundation, The Alexandria Real Estate Equities, Inc. and Mr. and Mrs. Joel S. Marcus, and the Howard Hughes Medical Institute, as well as other private donors. For a complete list, please visit the Foundation website at: http://fnih.org/work/education-training-0/medical-research-scholars-program. This work was also supported by Memorial Sloan Kettering Core Grant P30 CA008748 from the National Institutes of Health, National Cancer Institute.
Footnotes
Potential conflict of interest: Disclosure forms provided by the authors are available with the full text of this article online at http://10.1080/10428194.2017.1361026.
References
- [1].Landgren O, Gridley G, Turesson I, et al. Monoclonal gammopathy of undetermined significance (MGUS) consistently precedes multiple myeloma: a prospective study. Blood. 2009;113:5412–5417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Kyle RA, Ellen D, Remstein MD, et al. Clinical course and prognosis of smoldering (asymptomatic) multiple myeloma. N Engl J Med. 2007;356:2582–2590. [DOI] [PubMed] [Google Scholar]
- [3].Ghobrial IM, Landgren O. How I treat smoldering multiple myeloma. Blood. 2014;124:3380–3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Perez-Persona E, Vidriales MB, Mateo G, et al. New criteria to identify risk of progression in monoclonal gammopathy of uncertain significance and smoldering multiple myeloma based on multiparameter flow cytometry analysis of bone marrow plasma cells. Blood. 2007;110:2586–2592. [DOI] [PubMed] [Google Scholar]
- [5].Kay NE, Leong T, Kyle RA, et al. Circulating blood B cells in multiple myeloma: analysis and relationship to circulating clonal cells and clinical parameters in a cohort of patients entered on the Eastern Cooperative Oncology Group phase III E9486 clinical trial. Blood. 1997;90:340–345. [PubMed] [Google Scholar]
- [6].Dispenzieri A, Kyle RA, Katzmann JA, et al. Immunoglobulin free light chain ratio is an independent risk factor for progression of smoldering (asymptomatic) multiple myeloma. Blood. 2008;111:785–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Rawstron AC, Davies FE, Owen RG, et al. B-lymphocyte suppression in multiple myeloma is a reversible phenomenon specific to normal B-cell progenitors and plasma cell precursors. Br J Haematol. 1998;100: 176–183. [DOI] [PubMed] [Google Scholar]
- [8].Paiva B, Pérez-Andrés M, Vídriales MB, et al. Competition between clonal plasma cells and normal cells for potentially overlapping bone marrow niches is associated with a progressively altered cellular distribution in MGUS vs myeloma. Leukemia. 2011;25: 697–706. [DOI] [PubMed] [Google Scholar]
- [9].Lopez-Verges S, Milush JM, Pandey S, et al. CD57 defines a functionally distinct population of mature NK cells in the human CD56dimCD16+ NK-cell subset. Blood. 2010;116:3865–3874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Frassanito MA, Silvestris F Cafforio P, et al. CD8+/CD57 cells and apoptosis suppress T-cell functions in multiple myeloma. Br J Haematol. 1998;100:469–477. [DOI] [PubMed] [Google Scholar]
- [11].Sze DM, Giesajtis G, Brown RD, et al. Clonal cytotoxic T cells are expanded in myeloma and reside in the CD8(+)CD57(+)CD28(−) compartment. Blood. 2001;98: 2817–2827. [DOI] [PubMed] [Google Scholar]
- [12].Nielsen CM, White MJ, Goodier MR, et al. Functional significance of CD57 expression on human NK cells and relevance to disease. Front Immunol. 2013;4:422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Van den Hove LE, Symons F, Vandenberghe P, et al. Peripheral blood lymphocyte subset shifts in patients with untreated hematological tumors: evidence for systemic activation of the T cell compartment. Leuk Res. 1998;22:175–184. [DOI] [PubMed] [Google Scholar]
- [14].Garcia-Sanz R, Gonz alez M, Orfão A, et al. Analysis of natural killer-associated antigens in peripheral blood and bone marrow of multiple myeloma patients and prognostic implications. Br J Haematol. 1996;93:81–88. [DOI] [PubMed] [Google Scholar]
- [15].Focosi D, Bestagno M, Burrone O, Petrini M. CD57+ T lymphocytes and functional immune deficiency. J Leukoc Biol. 2010;87:107–116. [DOI] [PubMed] [Google Scholar]
- [16].Hong HS, Eberhard JM, Keudel P, et al. HIV infection is associated with a preferential decline in less-differentiated CD56dim CD16+ NK cells. J Virol. 2010;84: 1183–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Dosani T, Carlsten M, Maric I, Landgren O. The cellular immune system in myelomagenesis: NK cells and T cells in the development of myeloma [corrected] and their uses in immunotherapies. Blood Cancer J. 2015;5:e306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Pratt G, Goodyear O, Moss P. Immunodeficiency and immunotherapy in multiple myeloma. Br J Haematol. 2007;138:563–579. [DOI] [PubMed] [Google Scholar]