Abstract
The human T-cell leukemia virus type 1 (HTLV-1) is a complex deltaretrovirus linked to adult T-cell leukemia/lymphoma (ATLL), a fatal CD4+ malignancy in 3–5% of infected individuals. The HTLV-1 Tax regulatory protein plays indispensable roles in regulating viral gene expression and activating cellular signaling pathways that drive the proliferation and clonal expansion of T cells bearing HTLV-1 proviral integrations. Tax is a potent activator of NF-κB, a key signaling pathway that is essential for the survival and proliferation of HTLV-1 infected T cells. However, constitutive NF-κB activation by Tax also triggers a senescence response, suggesting the possibility that only T cells capable of overcoming NF-κB-induced senescence can selectively undergo clonal expansion after HTLV-1 infection. Tax expression is often silenced in the majority of ATLL due to genetic alterations in the tax gene or DNA hypermethylation of the 5′-LTR. Despite the loss of Tax, NF-κB activation remains persistently activated in ATLL due to somatic mutations in genes in the T/B-cell receptor (T/BCR) and NF-κB signaling pathways. In this review, we focus on the key events driving Tax-dependent and independent mechanisms of NF-κB activation during the multi-step process leading to ATLL.
Keywords: HTLV-1, NF-κB, Tax, senescence, leukemia
Graphical abstract
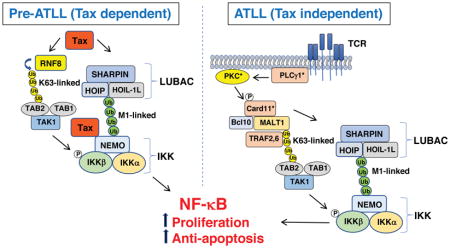
Chronic activation of the NF-κB pathway is a key event in HTLV-1-induced leukemia. HTLV-1 Tax directs RNF8 and LUBAC to assemble hybrid polyubiquitin chains to promote TAK1, IKK and NF-κB activation, and clonal expansion. Tax expression frequently becomes silenced by genetic/epigenetic mechanisms. Somatic mutations in B/T cell receptor signaling pathways then develop to drive Tax-independent NF-κB activation in ATLL cells.
Introduction
The human T-cell leukemia virus type 1 (HTLV-1) is a complex deltaretrovirus that infects between 10–20 million people worldwide [1]. The vast majority of HTLV-1 infected individuals remain asymptomatic; however, 3–5% develop adult T-cell leukemia/lymphoma (ATLL) after a long latent period spanning 4–6 decades after the initial infection [2]. In addition, ~3% of infected individuals develop one of a spectrum of inflammatory diseases, such as the neuroinflammatory disease HAM/TSP (HTLV-1 associated myelopathy/tropical spastic paraparesis) [3]. There are well established viral and host factors that determine host susceptibility to ATLL or HAM/TSP including the route of infection (i.e. peripheral blood or mucosal), HLA (human leukocyte antigen) alleles and the magnitude of the antiviral immune response [4, 5]. A high proviral load represents a significant risk factor for progression to ATLL or HAM/TSP [6, 7].
Although HTLV-1 exhibits a broad tropism in vitro, the majority of the cells harboring HTLV-1 provirus in vivo are CD4+ T cells (~90%) with the remainder derived from CD8+ T cells and monocytes [8, 9]. HTLV-1 can infect hematopoietic stem cells (HSCs) in the bone marrow, which can then differentiate into diverse immune cell lineages [10]. Neuropilin 1 (NRP1), glucose transporter 1 (GLUT-1) and heparin sulfate proteoglycans (HSPGs) all serve as HTLV-1 receptors [11–13]. In contrast to the human immunodeficiency virus 1 (HIV-1), HTLV-1 viral particles are poorly infectious and the proviral load in HTLV-1 infected individuals is maintained by both the division of infected cells (i.e. mitotic spread) and periodic viral reactivation and de novo infection [14]. Therefore, drugs that target retroviral replication, such as reverse transcriptase inhibitors, exert little, if any, effect on the HTLV-1 proviral load. HTLV-1 infection requires cell-to-cell contact and is mediated by a virological synapse supported by interactions between the transmembrane protein ICAM-1 and the integrin LFA-1 [15]. The virological synapse is initiated by the infected cell and mediates the transfer of core proteins and the HTLV-1 genome to an uninfected cell [15].
The HTLV-1 genome is ~10 kilobases in length and consists of Gag, Pol and Env genes encoding essential retroviral structural and enzymatic proteins flanked on both ends by long terminal repeats (LTRs). The 5′-LTR contains cis sequences for binding by both viral and cellular proteins that regulate the expression of Gag, Pol, Env and regulatory/accessory genes in the pX region (Tax, Rex, p12, p13 and p30) [16]. The 3′-LTR regulates expression of the hbz gene in the antisense direction. Both Tax and HBZ play key roles in the pathogenesis of HTLV-1 associated diseases [17]. Recent studies have quantified Tax and HBZ expression in single cell clones revealing Tax and HBZ expression occurring dynamically in transcriptional bursts [18]. Tax expression can be rapidly induced due to cytotoxic stress or hypoxia to promote cell survival and/or viral reactivation [19, 20].
Recent studies have described a new mechanism by which HTLV-1 insertion may influence host gene expression. The HTLV-1 provirus interacts with CCCTC-binding factor (CTCF), which can dimerize and form chromatin loops with distant CTCF sites in the genome [21]. The strong HTLV-1 promoter/enhancer may therefore exert effects on host gene expression at distant sites from the integrated provirus [22].
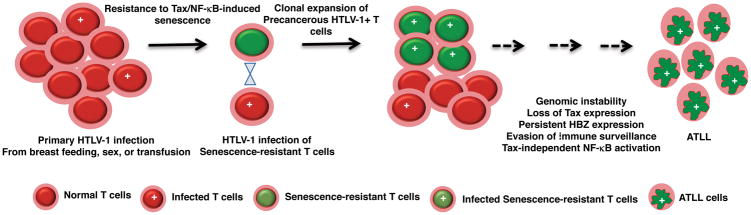
Tax exerts pleiotropic functions in the HTLV-1 life cycle by regulating viral gene expression and promoting the survival and clonal expansion of infected cells. Tax interacts with CREB and CREB binding protein (CBP) at the 5′-LTR to drive viral gene expression [23, 24]. Tax dysregulates cell cycle checkpoints by complex mechanisms with premature activation of the anaphase promoting complex (APCcdc20) and delayed mitosis [25, 26]. Tax also targets and inactivates key tumor suppressors p53 and Rb to promote cell cycle progression and the survival of HTLV-1 infected cells [27, 28]. Furthermore, Tax promotes genomic instability and aneuploidy by inducing reactive oxygen species (ROS) and inhibiting repair of DNA double-strand breaks through homologous recombination [29–31]. These collective activities endow Tax with oncogenic functions, and as such Tax can immortalize primary T cells, albeit with low efficiency, suggesting that Tax requires other viral proteins or specific cellular alterations for efficient transformation [32]. Tax transgenic mice can also develop specific tumors depending on the promoter used to drive Tax expression [33]. Tax promotes the chronic activation of the canonical and noncanonical NF-κB pathways (described below) to upregulate the expression of anti-apoptotic, proliferation and cell cycle progression genes [34]. Counterintuitively, chronic activation of NF-κB by Tax also induces a p53-independent senescence response mediated by cyclin-dependent kinase inhibitors, p21CIP1/WAF1 (p21) and p27KIP1 (p27), suggesting that the senescence response triggered by NF-κB would have to be overcome in order for persistently active NF-κB to serve as a cancer driver (Fig. 1). The NF-κB pathway is critical in both early (Tax-dependent) and later (Tax independent) steps in the multi-step progression to ATLL (Fig. 1). In this review, we will focus on the key molecular events leading to Tax-dependent and independent NF-κB activation in ATLL.
Figure 1. A model for how HTLV-1 infection progresses to ATLL.
Key events that drive ATLL development include acquisition of senescence-blocking genetic and/or epigenetic alterations by naïve T cells prior to viral infection, clonal expansion of HTLV-1-infected senescence-resistant precancerous T cells, genomic instability, loss of Tax expression, evasion of immune surveillance, persistent HBZ expression, and development of Tax-independent NF-κB activation.
Tax-dependent NF-κB activation
The canonical NF-κB pathway is activated by a wide range of stimuli including proinflammatory cytokines, antigens and stress responses. NF-κB dimers such as RelA/p50 are sequestered in the cytoplasm as inactive complexes by the IκB inhibitory proteins. The IKK (IκB kinase) complex, consisting of the catalytic subunits IKKα and IKKβ and the regulatory subunit NEMO (also known as IKKγ), phosphorylates IκB proteins in a stimulus-dependent manner to trigger their proteasomal degradation and allowing the nuclear translocation of NF-κB to regulate gene expression [35]. Early studies revealed that NF-κB was persistently activated and localized in the nucleus in HTLV-1 transformed cell lines [36]. Consistent with these observations, IKK was also chronically activated in HTLV-1 transformed cell lines and primary ATLL cells [36, 37]. Inhibition of IKK/NF-κB with small molecule inhibitors triggers the apoptosis of HTLV-1-transformed cell lines and ATLL cells, thus demonstrating the requirement for NF-κB in ATLL cell survival and the maintenance of the transformed phenotype [38, 39]. Targeted inhibition of IKK in HTLV-1 transformed cell lines and ATLL cells results in downregulated expression of genes involved in anti-apoptosis and cell cycle progression [40–42].
Tax directly interacts with NEMO to persistently activate IKK kinase activity [43–45]. Tax requires NEMO to activate NF-κB since Tax activation of NF-κB is impaired in a NEMO-deficient Jurkat T cell line [46]. Furthermore, the Tax point mutant M22 (T130A and L131S) is defective in NEMO binding and NF-κB activation whereas M47 (L319R and L320S) is defective in CREB activation [43]. Tax also activates IKK upstream kinases such as TAK1 and promotes the interaction of TAK1 with IKK [47]. Tax interaction with NEMO is essential to promote the chronic phosphorylation and oligomerization of IKK.
The pathogenicity of Tax mutants defective for NF-κB activation have been examined both in vitro and in vivo, clearly revealing roles for NF-κB in tumorigenesis and/or inflammation. In the context of a proviral clone, the Tax M47 mutant retains the ability for the immortalization of primary human T cells; however, Tax M22 is impaired in T-cell immortalization [48]. Transgenic mice conditionally expressing wild-type Tax or Tax M47, but not Tax M22, develop a lethal, inflammatory CD4+ T cell-mediated skin disease [49].
Noncanonical NF-κB signaling regulates the inducible processing of NF-κB2/p100 to p52, which can heterodimerize with RelB to regulate a distinct group of genes controlled by canonical NF-κB. Noncanonical NF-κB signaling regulates B lymphocyte survival, lymphoid organogenesis, and the development of specific immune cell subsets [50]. The processing of p100 to p52 is tightly regulated by extracellular signals mediated by tumor necrosis factor receptor (TNFR) superfamily members BAFF, lymphotoxin-β, CD40 and others [50]. Signaling through these receptors inactivate an E3 ligase complex consisting of TRAF2, TRAF3, cIAP1 and cIAP2 which together promote the ubiquitination and proteasomal degradation of the NF-κB inducing kinase (NIK) [51]. NIK phosphorylates IKKα, which in turn phosphorylates p100 to trigger its processing to p52 [52, 53]. Somatic inactivating mutations in TRAF2, TRAF3, cIAP1 and cIAP2 have been linked to NIK stabilization and constitutive noncanonical NF-κB signaling in multiple myeloma [54, 55].
Tax also functions as a potent activator of the noncanonical NF-κB pathway. Early studies suggested that Tax directly interacts with p100 [56]. Interestingly, Tax activation of noncanonical NF-κB occurs independently of TNF receptors, and requires IKKα, and possibly NIK [57, 58]. Tax-mediated transformation has been shown to depend on p100 and noncanonical NF-κB signaling [59]. Amino acids 225–232 in Tax were shown to be important for the processing of p100 and cell transformation [60]. Furthermore, genetic loss of p100/p52 delayed tumorigenesis in Tax transgenic mice [61]. Together, it appears that the noncanonical NF-κB pathway plays a critical role in the oncogenic properties of Tax.
Tax is modified by various post-translational modifications (PTMs) that not only regulate Tax stability and cellular localization, but also NF-κB activation. In this regard, ubiquitination has been shown to play a prominent role in the functions of Tax [62, 63]. Tax can undergo both degradative lysine 48 (K48)-linked polyubiquitination and nondegradative K63-linked polyubiquitination [64]. Mutagenesis and mapping studies have revealed that lysine residues K263, K280 and K284 play key roles as acceptor sites for Tax ubiquitination [65, 66]. Monoubiquitination of Tax occurs in response to genotoxic stress and DNA damage and mediates the nuclear export of Tax [67]. However, K63-linked polyubiquitination of Tax is thought to lead to NF-κB activation. Indeed, the E2 ubiquitin conjugating enzyme Ubc13 was shown to be required for Tax to activate IKK and NF-κB [64]. Knockdown of Ubc13 with siRNA or genetic deletion of Ubc13 impaired the ability of Tax to undergo K63-linked polyubiquitination and activate NF-κB [64]. The mechanistic roles of K63-linked polyubiquitination of Tax have yet to be fully understood, but may function as a scaffold for recruitment of proteins with K63-Ub binding modules, possibly TAB2/3. While overexpression of TRAF2, 5 and 6 augment Tax polyubiquitination [68], there is no compelling genetic evidence that these E3 ligases are actually involved in Tax K63-linked polyubiquitination. Indeed, the identity of the E3 ligase(s) that directly conjugates Tax with K63-linked polyubiquitin chains remains unknown. Tax polyubiquitination, as well as TRAF6 polyubiquitination, can be countered by the USP20 deubiquitinase [69]. Tax-mediated NF-κB activation can also be indirectly regulated by the STAMBPL1 deubiquitinase, which promotes DNA damage-induced Tax nuclear export [70]. Tax has also been shown to be modified by SUMOylation [66]. Although one study reported a key role for Tax SUMOylation in NF-κB activation [71], a conflicting study found that a non-SUMOylated Tax protein retains the capacity for NF-κB activation [72]. More work is clearly needed in this area to understand if Tax SUMOylation affects NF-κB signaling.
Tax can promote the relocalization of NEMO and the IKK complex to Golgi-associated structures in T lymphocytes in a ubiquitin-dependent manner [73], where interactions occur with the selective autophagy receptors TAX1BP1 and Optineurin [74]. Given that Tax activates IKK in lipid rafts [75], Tax may initially assemble an IKK signaling complex in lipid rafts in the Golgi. It is unclear if the Tax-IKK-TAX1BP1-Optineurin complex in the Golgi is linked to autophagy/autophagosomes involved in NF-κB activation (see below).
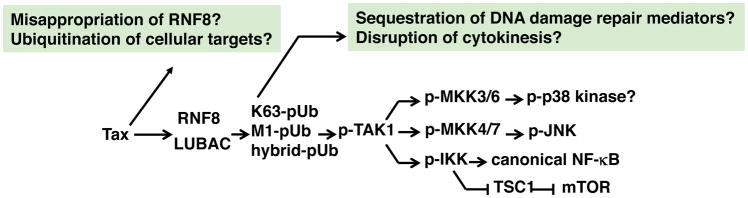
Although an E3 ligase directly ubiquitinating Tax with K63-linked poly-Ub chains has yet to be identified, Ho et al. have recently used in vitro reconstitution to demonstrate that Tax activates the E3 ligase, ring finger protein 8 (RNF8), for canonical IKK and NF-κB activation [76]. In the presence of Tax, RNF8 and Ubc13/Uev1a/Uev2 become greatly stimulated both in vitro and in vivo and assemble long K63-linked polyubiquitin chains, which activate TAK1 and IKK, and downstream kinases including JNK and mTOR [76, 77]. Interestingly, Tax has been previously demonstrated to activate mTOR to transform mouse T cells [78]. These results support the model that Tax hijacks RNF8, a cellular E3 ligase involved in the early signaling events of the DNA double-stranded break repair pathway, for not only canonical NF-κB activation, but also activation of a large ensemble of kinases downstream of TAK1 to facilitate viral replication (Fig. 2). The pleiotropic effects of Tax on cell signaling can thus be explained nicely by this model. The K63-linked polyubiquitin chains assembled by Tax and RNF8 in vitro are unanchored [76]. Whether substrate-anchored polyubiquitin chains are formed by the Tax-RNF8 complex remains to be determined [79]. RNF8 is localized primarily in the nucleus and becomes released into the cytosol during mitosis where it regulates cytokinesis. In the presence of Tax a significant fraction of RNF8 becomes localized to the cytoplasm. As Tax is expected to stimulate both nuclear and cytosolic RNF8 for K63-linked polyubiquitin chain assembly, whether the interaction between Tax and RNF8, the aberrant cytosolic localization of RNF8, or the dysregulated assembly of mislocalized polyubiquitin chains contributes to the cytopathic effects of Tax remains to be elucidated. In addition to RNF8, Tax can also activate the E3 ligase TRAF6, and Tax can directly interact with TRAF6 through a TRAF6 binding motif in its C-terminus [80]. A ubiquitin proteomics approach to survey for host proteins ubiquitinated upon Tax expression in Jurkat T cells identified the anti-apoptotic BCL-2 family member Mcl-1 which can be subject to K63-linked polyubiquitination by TRAF6 as a mechanism to enhance Mcl-1 stability and prevent apoptosis [80].
Figure 2. Tax hijacks RNF8 and LUBAC to activate TAK1 and IKK, and multiple downstream signaling pathways.
Tax interacts with and stimulates RNF8 and LUBAC to assemble hybrid K63-linked and M1-linked (linear) polyubiquitin chains as signaling scaffolds for TAK1 and IKK recruitment and activation. TAK1, in turn, activates mitogen-activated protein kinase kinases (MKKs) and IKK, and downstream p38 kinase, c-Jun kinase (JNK), canonical NF-κB. and mammalian target of rapamycin (mTOR) pathways. Whether the mislocalization and aberrant activation of RNF8 and LUBAC lead to covalent modifications of cellular proteins by K63-linked and M1-linked (linear) polyubiquitin chains remains unclear. Whether the over-abundance of K63-linked, M1-linked, and hybrid polyubiquitin chains cause sequestration/disruption of critical cellular processes such as DNA damage repair and cytokinesis also remain to be determined.
A recent study has claimed that Tax itself may function as an E3 ligase, which can synthesize free mixed-linkage polyubiquitin chains that bind to NEMO and activate IKK [81]. However, a recent study was unable to replicate these results by in vitro ubiquitination assays with recombinant Tax together with either UbcH5c, UbcH7 or Ubc13/Uev1A [79]. Wang et al. [81] also reported that K63-linked polyubiquitin chains do not play a role in IKK activation by Tax, in direct contrast to multiple published reports on the importance of K63-linked polyubiquitination in Tax-NF-κB signaling [64, 76, 79]. It is highly improbable that Tax actually functions as an E3 ligase; however, additional studies are required to further examine this notion. Tax was previously shown to bind zinc via a putative zinc finger domain [82]; however, this particular domain is not typical of a RING-type zinc finger domain found in a class of E3 ligases. Amino acid substitutions in the cysteine and histidine residues (C29, C36, C49, H52) comprising the putative zinc finger domain severely impaired NF-κB and LTR activation [79, 82]. It is important to note that the zinc finger domain also plays an important role in Tax dimerization [83], therefore mutation or deletion of the zinc finger may indirectly inhibit Tax function.
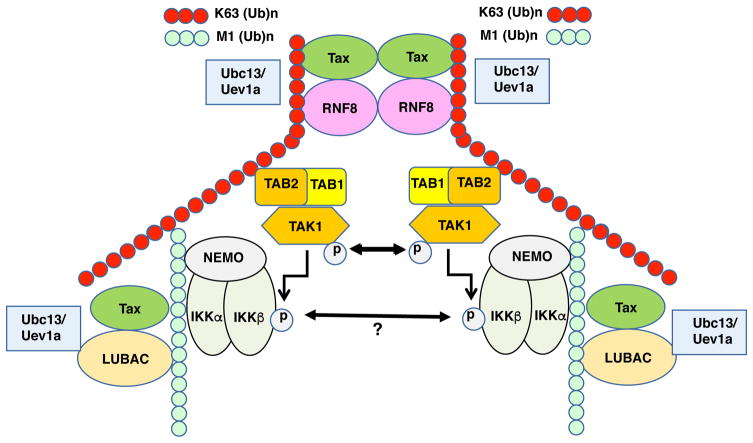
K63/M1-linked hybrid polyubiquitin chains are generated during IL-1 signaling and other innate immune signaling pathways as a mechanism to colocalize TAK1 and NEMO-containing IKK complexes [84, 85]. Upon recruitment of TAK1 and IKK to the hybrid polyubiquitin chains, TAK1 becomes activated by autophosphorylation, and then phosphorylates and activates IKK. The assembly of K63-linked polyubiqutin chains is a prerequisite for the recruitment of the linear ubiquitin assembly complex (LUBAC) E3 ligase, and the LUBAC subunit, heme-oxidized IRP2 ubiquitin ligase 1 interacting protein (HOIP), specifically interacts with K63-linked polyubiquitin chains [84, 86]. Given the importance of M1-linear polyubiquitination in various NF-κB signaling pathways, it is not surprising that the LUBAC complex, consisting of HOIP, HOIL-1L and Sharpin, has been implicated in Tax-induced NF-κB activation. Shibata et al. [79] have demonstrated that Tax interacts with and recruits LUBAC to NEMO and the IKK complex in HTLV-1 transformed T cell lines, and together with a K63-specific E3 ligase, generate K63/M1-linked hybrid polyubiquitin chains. The hybrid polyubiquitin chains bind to NEMO via the UBAN and NZF domains, which are thought to then promote the oligomerization and trans-autophosphorylation-mediated activation of IKK complexes [79]. Whether IKK complexes undergo auto-activation or are activated by TAK1 in the presence of Tax has not been fully resolved, although prevailing evidence supports the latter mechanism. Similar to NEMO, the selective autophagy receptor Optineurin has a UBAN domain that can bind to linear polyubiquitin chains [87], but it is unclear if Optineurin sensing of M1-linear polyubiquitin chains plays a role in Tax-induced IKK activation. These recent results are schematically summarized in Fig. 3.
Figure 3. Tax hijacks RNF8 and LUBAC to assemble hybrid K63- and M1-linked polyubiquitin chains for TAK1 and IKK activation.
Tax stimulates ubiquitin E3 ligases RNF8 and LUBAC, which together with E2 conjugating enzymes, Ubc13/Uev1a/Uev2 assemble hybrid K63-linked and M1-linked polyubiqutin chains to recruit TAK1 and IKK. The TAB2 subunit of TAK1 and the NEMO subunit of IKK preferentially interact with K63- and M1-polyubiquitin chains respectively. In this signaling scaffold, TAK1 undergoes auto-phosphorylation and auto-activation. Activated TAK1, in turn, phosphorylates and activates IKK. Whether the IKK assimilated to the signaling scaffold undergoes auto-activation is a matter of debate.
To maintain persistent NF-κB activation, Tax utilizes numerous mechanisms to counteract inhibitory checkpoints and negative regulators of IKK/NF-κB. The phosphatase PP2A negatively regulates IKK, however Tax forms a ternary complex with PP2A and IKK to impair PP2A regulation of IKK [88]. Tax also interacts with the NF-κB negative regulator TAX1BP1 and blocks its phosphorylation to suppress A20-mediated inhibition of IKK/NF-κB [89]. A20 can disrupt interactions between E2 and E3 ubiquitin ligase components to inhibit NF-κB signaling; however, Tax can block A20-Ubc13 binding to sustain Ubc13-dependent K63-linked polyubiquitination [90]. Tax also interacts with CADM1 (Cell Adhesion Molecule 1) in lipid rafts in HTLV-1 transformed cell lines as part of its mechanism to inhibit A20 [91]. The deubiquitinase and NF-κB inhibitor CYLD can interact with and inhibit Tax-mediated IKK activation; however, CYLD is constitutively phosphorylated and inactivated in HTLV-1 transformed cells [92]. Together, it appears that Tax not only interacts with and activates IKK but also effectively neutralizes IKK negative regulatory mechanisms for persistent NF-κB signaling.
The role of autophagy in Tax-mediated NF-κB activation
Autophagy, a homeostatic process for degradation of proteins or organelles, plays complex roles in regulating virus replication. Tax has been shown to promote autophagosome accumulation, resulting in increased HTLV-1 replication [93]. Tax increases the number of autophagosomes in cells by blocking the fusion of autophagosomes with lysosomes by an unknown mechanism. Tax itself appears to be a substrate for autophagic degradation since treatment with the autophagosome-lysosome fusion inhibitor bafilomycin A increased Tax protein stability [93]. Tax deregulation of autophagy appears to be dependent on IKK activation and relocalization of autophagy regulators Beclin and Bif-1 to lipid rafts [94]. Downregulation of Beclin and PI3 kinase class III impaired the growth of HTLV-1-transformed cell lines. Tax-induced autophagy activation exerts oncogenic functions by activating both IKK and STAT3 [95]. The colocalization of Tax, IKK and core autophagy proteins implies a role for autophagy and autophagosomes in IKK activation by Tax. Interestingly, the Tax-binding protein TAX1BP1 contains LC3 interaction motifs and has been implicated as a selective autophagy receptor [96, 97]. It is unknown if TAX1BP1 regulates Tax stability through autophagy or possibly Tax-induced IKK activation. In this regard, the autophagy receptors Optineurin and TAX1BP1 were both demonstrated to regulate Tax ubiquitination and NF-κB activation [74, 89]. Exactly how Tax utilizes autophagy/autophagosomes for IKK activation requires further study.
Tax activation of NF-κB leads to senescence
While Tax potently activates IKK/NF-κB, the principal driver of ATLL, Tax expression counter-intuitively induces a rapid cellular senescence response mediated by tumor suppressors p21 and p27 [98]. Tax-induced senescence occurs readily in cells that lack functional p53 and pRb [98], and is driven by chronically and persistently activated (hyperactivated) NF-κB as NF-κB inhibition by ΔN-IκBα, a degradation resistant mutant of IκBα, prevents senescence induction by Tax [99]. Curiously, Tax-induced senescence requires the transcriptional activity of NF-κB, and is prevented by shRNA-mediated silencing of p65/RelA, the trans-activator subunit of NF-κB; and silencing of IKKα, which phosphorylates/activates the transcriptional activity of p65/RelA [100]. How the transcriptional activity of NF-κB promotes senescence induction remains unclear. Importantly, HTLV-1 infection in cell culture predominantly triggers a senescence response in both lymphoid and non-lymphoid cells. Only a small fraction of infected cells that express very low levels of Tax and Rex can continue to grow and divide [101]. In these cells, HBZ represses Tax and Rex activities [101], down-regulates NF-κB activation by Tax and dampens senescence induction [99]. If NF-κB hyperactivation, be it Tax-dependent or mutation-driven, triggers a senescence response, then ATLL cells that are addicted to chronic NF-κB activation must have acquired genetic and/or epigenetic alterations that can prevent or attenuate Tax/NF-κB-induced senescence. Some of these changes may be identified among the recurrent gain-of-function and loss-of-function mutations found in ATLL genomes by whole-genome sequence analysis [102]. Finally, the senescence-mitigating genetic or epigenetic alterations likely occur early, and collaborate with Tax-mediated NF-κB activation to promote clonal expansion of HTLV-1-infected T cells. They subsequently predispose the development of Tax-independent NF-κB activation after viral gene expression becomes extinguished by cytotoxic lymphocytes. In this vein, the Kaposi’s sarcoma herpesvirus (KSHV) vCyclin has been shown to block NF-κB-associated senescence induced by Tax and KSHV vFLIP through the formation of a vCyclin/CDK6 complex that resists p21 and p27 inhibition, and at the same time targets p27 for degradation [103]. Functionally similar alterations are likely present in ATLL cells.
Tax-independent NF-κB activation in ATLL
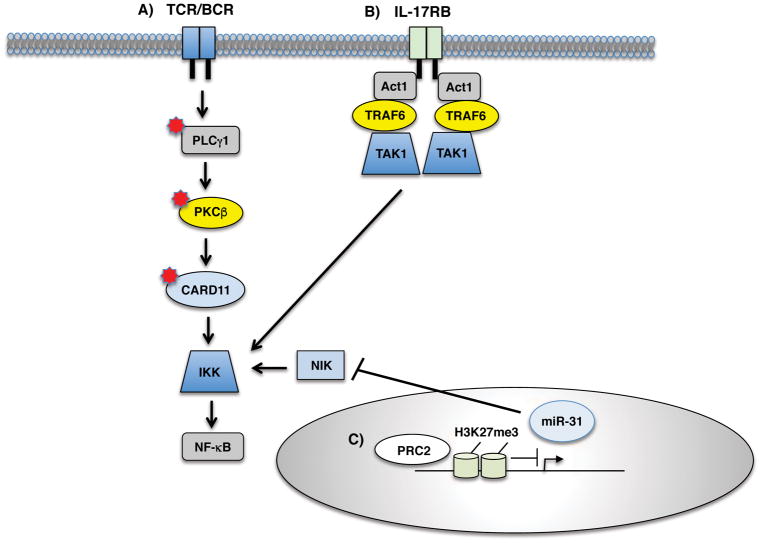
It has long been a mystery why ATLL cells not expressing Tax still exhibit constitutive IKK and NF-κB activity [37, 104]. Whole-genome, exome and transcriptome sequencing of ATLL patients have revealed frequent somatic mutations in genes involved in T/B cell receptor (T/BCR)-NF-κB signaling, many of which overlap with the Tax interactome [102] (Fig. 4). Activating mutations were found most frequently (36% of ATLL) in phospholipase Cγ1 (PLCγ1), a key component of proximal TCR signaling [102]. Mutations were also commonly found in PRKCB (PKCβ) and CARD11, which are likely to activate NF-κB persistently in ATLL cells. PKCβ is a kinase functioning downstream of PLCγ and critical for BCR signaling, therefore mutations in PKCβ in ATLL were somewhat surprising. CARD11 is a scaffold protein upstream of IKK in the TCR and BCR signaling pathways. Gain-of-function mutations in CARD11 are often associated with lymphoproliferative disorders [105]. Some of these mutations may occur together in ATLL and synergistically augment NF-κB activation. Finally, frequent mutations were also found in negative regulators of NF-κB such as TNFAIP3, TRAF3 and NFKB1A [102].
Figure 4. Tax-independent mechanisms of NF-κB activation in ATLL.
A) Somatic mutations in TCR/BCR signaling components including PLCγ1, PKCβ and CARD11 may promote persistent IKK/NF-κB activity in ATLL. B) Overexpression of IL-17RB can lead to IKK/NF-κB activation in a subset of ATLL cell lines. C) Polycomb repressive complex 2 (PRC2)-mediated epigenetic silencing of miR-31 can lead to the overexpression of NIK and noncanonical NF-κB signaling in the absence of Tax expression.
In addition to somatic mutations, other genetic and/or epigenetic changes can plausibly lead to persistent NF-κB signaling in ATLL. Epigenetic downregulation of the microRNA miR-31 results in NIK overexpression and noncanonical NF-κB activation [106] (Fig. 4). Overexpression of receptors (or potentially downstream signaling proteins) activating NF-κB may contribute to NF-κB signaling in Tax-negative ATLL cell lines. IL-17RB, the receptor for IL-25, is upregulated in an NF-κB-dependent feed-forward mechanism by Tax in T cells [107] (Fig. 4). IL-17RB can also mediate NF-κB signaling in a subset of Tax-negative ATLL cell lines including TL-OM1 and ATL43T. It remains to be determined if somatic mutations or potentially other overexpressed receptors and/or signaling mediators activate NF-κB in other ATLL cell lines. Notably, the IL-17RB locus is encoded on chromosome 3p21.1, one of the most frequently amplified chromosomal regions in acute ATLL [108]. Interestingly, aberrant IL-17RB expression and signaling has been linked to tumorigenicity and metastasis in breast and pancreatic cancers [109, 110].
Pending questions and concluding remarks
NF-κB is critical not only in the early stages of ATLL consisting of the proliferation and clonal expansion of HTLV-1-infected T cells, but is also important for the survival and proliferation of malignant ATLL clones. Tax-dependent NF-κB signaling drives the initial polyclonal expansion after HTLV-1 infection. However, constitutive activation of NF-κB triggers a cellular senescence response, and a majority of cells infected by HTLV-1 in culture become senescent. Thus, the present model for how HTLV-1 infection and NF-κB activation promote ATLL development requires significant revision. To reconcile these seemingly conflicting outcomes of persistent NF-κB activation, current evidence suggests that infected cells that express Tax at low or undetectable levels and where HBZ can effectively inhibit Tax-dependent NF-κB activation are able to survive and persist [101]. Furthermore, it would appear that only T cells with genetic and/or epigenetic alterations that can prevent or attenuate Tax/NF-κB-induced senescence will be able to proliferate and clonally expand after HTLV-1 infection. These cellular changes are expected to play a crucial role in facilitating the development of Tax-independent NF-κB activation seen in ATLL. This area is worthy of attention because the said cellular alterations may be therapeutically targeted to cause ATLL cells to arrest in senescence. As constitutive NF-κB activation is the hallmark of many hematological malignancies, what is learned from Tax and ATLL is likely to be broadly applicable. To date, many key questions remain to be addressed. How does NF-κB hyperactivation by Tax drive senescence induction? What might be the cellular alterations in ATLL that can blunt/prevent cellular senescence associated with NF-κB hyperactivation? Finally, what is the connection among NF-κB hyperactivation, senescence induction, and Tax-induced genomic instability and aneuploidy, which drive chromosomal imbalances and somatic mutations?
Tax expression is frequently lost in ATLL due to DNA hypermethylation of the 5′-LTR and mutations in tax. The activation of both canonical and noncanonical NF-κB by Tax requires Tax binding to NEMO and the IKK complex. Tax hijacks components of the ubiquitin-proteasome pathway including E3 ligases RNF8 and LUBAC to promote the ubiquitin-dependent oligomerization and auto- and trans-phosphorylation of TAK1 and IKK. Emerging studies indicate the involvement of lipid rafts and the core autophagy pathway in Tax-mediated IKK activation. However, the links between Tax-IKK, autophagy and ubiquitination remain poorly understood. How does Tax utilize autophagy components and potentially autophagosomes to activate IKK? How does Tax prevent autophagic degradation of Tax itself, IKK and other regulatory proteins involved in IKK activation? What is the identity of the E3 ligase that conjugates Tax with K63-linked polyubiquitin chains? Does Tax actually possess E3 ligase activity?
Upon Tax downregulation in ATLL, driver mutations in T/BCR signaling components may “compensate” for Tax and promote persistent NF-κB activation. The emergence of these mutations is likely aided by the senescence-blunting cellular alterations. However, more mechanistic studies are needed to determine how the recurrent mutations identified in ATLL yield dysregulated proteins that constitutively activate NF-κB and/or prevent the Tax/NF-κB-associated senescence response. This knowledge may provide a rationale for targeted therapies for ATLL patients. In addition to somatic mutations of TCR signaling proteins, there are also other potential mechanisms that can lead to NF-κB activation in the absence of Tax. Overexpression of NIK, IL-17RB and perhaps other signaling mediators may promote Tax-independent canonical and noncanonical NF-κB signaling. Unbiased genome-wide approaches to identify NF-κB regulators in ATLL may lead to the discovery of new mechanisms of Tax-independent NF-κB activation in ATLL.
Acknowledgments
The laboratory of EWH is supported by NIH grant R01CA135362. The laboratory of CZG is supported by NIH grant R21CA216660.
Abbreviations
- APC cdc20
anaphase promoting complex
- ATLL
adult T-cell leukemia/lymphoma
- BAFF
B-cell activating factor
- BCR
B cell receptor
- CADM1
cell adhesion molecule 1
- CARD11
caspase recruitment domain family member 11
- CBP
CREB binding protein
- CDK6
cyclin-dependent kinase 6
- cIAP1/2
cellular inhibitor of apoptosis 1/2
- CREB
cyclic AMP-responsive element-binding protein
- CTCF
CCCTC-binding factor
- CYLD
cylindromatosis lysine K63 deubiquitinase
- Env
envelope protein
- Gag
group antigens
- GLUT-1
glucose transporter 1
- HAM/TSP
HTLV-1 associated myelopathy/tropical spastic paraparesis
- HBZ
HTLV-1 bZIP factor
- HIV-1
human immunodeficiency virus 1
- HLA
human leukocyte antigen
- HOIL-1L
heme-oxidized iron-regulatory protein 2 ubiquitin ligase 1
- HOIP
heme-oxidized iron-regulatory protein 2 ubiquitin ligase 1 interacting protein
- HSC
hematopoietic stem cell
- HSPG
heparin sulfate proteoglycan
- HTLV-1
human T-cell leukemia virus type 1
- ICAM-1
intercellular adhesion molecule 1
- IκB
inhibitor of nuclear factor-kappa B
- IKK
inhibitor of nuclear factor-kappa B kinase
- IL-17RB
interleukin-17 receptor B
- JNK
c-Jun N-terminal kinase
- K48
lysine 48
- K63
lysine 63
- KSHV
Kaposi’s sarcoma herpesvirus
- LC3
light chain 3
- LFA-1
lymphocyte function-associated antigen 1
- LTR
long terminal repeat
- LUBAC
linear ubiquitin chain assembly complex
- M1
amino-terminal methionione
- Mcl-1
myeloid cell leukemia 1
- MKK
mitogen-activated protein kinase
- mTOR
mechanistic target of rapamycin kinase
- NEMO
NF-κB essential modulator
- NF-κB
nuclear factor-kappa B
- NIK
NF-κB inducing kinase
- NRP1
neuropilin 1
- NZF
Npl4 zinc finger
- p21
p21CIP1/WAF1
- p27
p27KIP1
- PI3 kinase
phosphoinositide 3-kinase
- PKCβ
protein kinase C beta
- PLCγ1
phospholipase Cγ1
- Pol
DNA polymerase
- PP2A
protein phosphatase 2A
- pRB
Retinoblastoma protein
- PRC2
polycomb repressive complex 2
- PTM
post-translational modification
- RING
really interesting new gene
- RNF8
RING finger protein 8
- ROS
reactive oxygen species
- SHARPIN
SHANK-associated RH domain-interacting protein
- STAMBPL1
STAM binding protein like 1
- TAB2/3
TGF-beta activated kinase 1 binding protein 2/3
- TAK1
transforming growth factor beta-activated kinase 1
- TAX1BP1
Tax1-binding protein 1
- TCR
T cell receptor
- TNF
tumor necrosis factor
- TNFAIP3
TNF alpha induced protein 3
- TNFR
tumor necrosis factor receptor
- TRAF2/3/5/6
TNF receptor-associated factor 2, 3, 5, 6
- UBAN
ubiquitin binding in ABIN and NEMO domain
- USP20
ubiquitin specific peptidase 20
Footnotes
Competing Financial interests
EWH and CZG do not have any competing financial interests.
Author contributions
EWH and CZG wrote the review and contributed the figures.
References
- 1.Gessain A, Cassar O. Epidemiological Aspects and World Distribution of HTLV-1 Infection. Frontiers in microbiology. 2012;3:388. doi: 10.3389/fmicb.2012.00388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Iwanaga M, Watanabe T, Yamaguchi K. Adult T-cell leukemia: a review of epidemiological evidence. Frontiers in microbiology. 2012;3:322. doi: 10.3389/fmicb.2012.00322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Enose-Akahata Y, Vellucci A, Jacobson S. Role of HTLV-1 Tax and HBZ in the Pathogenesis of HAM/TSP. Frontiers in microbiology. 2017;8:2563. doi: 10.3389/fmicb.2017.02563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fujino T, Nagata Y. HTLV-I transmission from mother to child. J Reprod Immunol. 2000;47:197–206. doi: 10.1016/s0165-0378(00)00054-1. [DOI] [PubMed] [Google Scholar]
- 5.Jeffery KJ, Usuku K, Hall SE, Matsumoto W, Taylor GP, Procter J, Bunce M, Ogg GS, Welsh KI, Weber JN, Lloyd AL, Nowak MA, Nagai M, Kodama D, Izumo S, Osame M, Bangham CR. HLA alleles determine human T-lymphotropic virus-I (HTLV-I) proviral load and the risk of HTLV-I-associated myelopathy. Proc Natl Acad Sci U S A. 1999;96:3848–53. doi: 10.1073/pnas.96.7.3848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iwanaga M, Watanabe T, Utsunomiya A, Okayama A, Uchimaru K, Koh KR, Ogata M, Kikuchi H, Sagara Y, Uozumi K, Mochizuki M, Tsukasaki K, Saburi Y, Yamamura M, Tanaka J, Moriuchi Y, Hino S, Kamihira S, Yamaguchi K. Human T-cell leukemia virus type I (HTLV-1) proviral load and disease progression in asymptomatic HTLV-1 carriers: a nationwide prospective study in Japan. Blood. 2010;116:1211–9. doi: 10.1182/blood-2009-12-257410. [DOI] [PubMed] [Google Scholar]
- 7.Nagai M, Usuku K, Matsumoto W, Kodama D, Takenouchi N, Moritoyo T, Hashiguchi S, Ichinose M, Bangham CR, Izumo S, Osame M. Analysis of HTLV-I proviral load in 202 HAM/TSP patients and 243 asymptomatic HTLV-I carriers: high proviral load strongly predisposes to HAM/TSP. J Neurovirol. 1998;4:586–93. doi: 10.3109/13550289809114225. [DOI] [PubMed] [Google Scholar]
- 8.Richardson JH, Edwards AJ, Cruickshank JK, Rudge P, Dalgleish AG. In vivo cellular tropism of human T-cell leukemia virus type 1. J Virol. 1990;64:5682–7. doi: 10.1128/jvi.64.11.5682-5687.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Castro-Amarante MF, Pise-Masison CA, McKinnon K, Washington Parks R, Galli V, Omsland M, Andresen V, Massoud R, Brunetto G, Caruso B, Venzon D, Jacobson S, Franchini G. Human T Cell Leukemia Virus Type 1 Infection of the Three Monocyte Subsets Contributes to Viral Burden in Humans. J Virol. 2015;90:2195–207. doi: 10.1128/JVI.02735-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Furuta R, Yasunaga JI, Miura M, Sugata K, Saito A, Akari H, Ueno T, Takenouchi N, Fujisawa JI, Koh KR, Higuchi Y, Mahgoub M, Shimizu M, Matsuda F, Melamed A, Bangham CR, Matsuoka M. Human T-cell leukemia virus type 1 infects multiple lineage hematopoietic cells in vivo. PLoS Pathog. 2017;13:e1006722. doi: 10.1371/journal.ppat.1006722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ghez D, Lepelletier Y, Lambert S, Fourneau JM, Blot V, Janvier S, Arnulf B, van Endert PM, Heveker N, Pique C, Hermine O. Neuropilin-1 is involved in human T-cell lymphotropic virus type 1 entry. J Virol. 2006;80:6844–54. doi: 10.1128/JVI.02719-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Manel N, Kim FJ, Kinet S, Taylor N, Sitbon M, Battini JL. The ubiquitous glucose transporter GLUT-1 is a receptor for HTLV. Cell. 2003;115:449–59. doi: 10.1016/s0092-8674(03)00881-x. [DOI] [PubMed] [Google Scholar]
- 13.Jones KS, Petrow-Sadowski C, Bertolette DC, Huang Y, Ruscetti FW. Heparan sulfate proteoglycans mediate attachment and entry of human T-cell leukemia virus type 1 virions into CD4+ T cells. J Virol. 2005;79:12692–702. doi: 10.1128/JVI.79.20.12692-12702.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bangham CR, Cook LB, Melamed A. HTLV-1 clonality in adult T-cell leukaemia and non-malignant HTLV-1 infection. Semin Cancer Biol. 2014;26:89–98. doi: 10.1016/j.semcancer.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Igakura T, Stinchcombe JC, Goon PK, Taylor GP, Weber JN, Griffiths GM, Tanaka Y, Osame M, Bangham CR. Spread of HTLV-I between lymphocytes by virus-induced polarization of the cytoskeleton. Science. 2003;299:1713–6. doi: 10.1126/science.1080115. [DOI] [PubMed] [Google Scholar]
- 16.Kannian P, Green PL. Human T Lymphotropic Virus Type 1 (HTLV-1): Molecular Biology and Oncogenesis. Viruses. 2010;2:2037–77. doi: 10.3390/v2092037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Giam CZ, Semmes OJ. HTLV-1 Infection and Adult T-Cell Leukemia/Lymphoma-A Tale of Two Proteins: Tax and HBZ. Viruses. 2016:8. doi: 10.3390/v8060161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Billman MR, Rueda D, Bangham CRM. Single-cell heterogeneity and cell-cycle-related viral gene bursts in the human leukaemia virus HTLV-1. Wellcome Open Res. 2017;2:87. doi: 10.12688/wellcomeopenres.12469.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kulkarni A, Mateus M, Thinnes CC, McCullagh JS, Schofield CJ, Taylor GP, Bangham CRM. Glucose Metabolism and Oxygen Availability Govern Reactivation of the Latent Human Retrovirus HTLV-1. Cell Chem Biol. 2017;24:1377–1387 e3. doi: 10.1016/j.chembiol.2017.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mahgoub M, Yasunaga JI, Iwami S, Nakaoka S, Koizumi Y, Shimura K, Matsuoka M. Sporadic on/off switching of HTLV-1 Tax expression is crucial to maintain the whole population of virus-induced leukemic cells. Proc Natl Acad Sci U S A. 2018 doi: 10.1073/pnas.1715724115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Satou Y, Miyazato P, Ishihara K, Yaguchi H, Melamed A, Miura M, Fukuda A, Nosaka K, Watanabe T, Rowan AG, Nakao M, Bangham CR. The retrovirus HTLV-1 inserts an ectopic CTCF-binding site into the human genome. Proc Natl Acad Sci U S A. 2016;113:3054–9. doi: 10.1073/pnas.1423199113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cook L, Melamed A, Yaguchi H, Bangham CR. The impact of HTLV-1 on the cellular genome. Curr Opin Virol. 2017;26:125–131. doi: 10.1016/j.coviro.2017.07.013. [DOI] [PubMed] [Google Scholar]
- 23.Zhao LJ, Giam CZ. Human T-cell lymphotropic virus type I (HTLV-I) transcriptional activator, Tax, enhances CREB binding to HTLV-I 21-base-pair repeats by protein-protein interaction. Proc Natl Acad Sci U S A. 1992;89:7070–4. doi: 10.1073/pnas.89.15.7070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kashanchi F, Duvall JF, Kwok RP, Lundblad JR, Goodman RH, Brady JN. The coactivator CBP stimulates human T-cell lymphotrophic virus type I Tax transactivation in vitro. J Biol Chem. 1998;273:34646–52. doi: 10.1074/jbc.273.51.34646. [DOI] [PubMed] [Google Scholar]
- 25.Liu B, Hong S, Tang Z, Yu H, Giam CZ. HTLV-I Tax directly binds the Cdc20-associated anaphase-promoting complex and activates it ahead of schedule. Proc Natl Acad Sci U S A. 2005;102:63–8. doi: 10.1073/pnas.0406424101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liang MH, Geisbert T, Yao Y, Hinrichs SH, Giam CZ. Human T-lymphotropic virus type 1 oncoprotein tax promotes S-phase entry but blocks mitosis. J Virol. 2002;76:4022–33. doi: 10.1128/JVI.76.8.4022-4033.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pise-Masison CA, Mahieux R, Jiang H, Ashcroft M, Radonovich M, Duvall J, Guillerm C, Brady JN. Inactivation of p53 by human T-cell lymphotropic virus type 1 Tax requires activation of the NF-kappaB pathway and is dependent on p53 phosphorylation. Mol Cell Biol. 2000;20:3377–86. doi: 10.1128/mcb.20.10.3377-3386.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kehn K, de Fuente CL, Strouss K, Berro R, Jiang H, Brady J, Mahieux R, Pumfery A, Bottazzi ME, Kashanchi F. The HTLV-I Tax oncoprotein targets the retinoblastoma protein for proteasomal degradation. Oncogene. 2005;24:525–40. doi: 10.1038/sj.onc.1208105. [DOI] [PubMed] [Google Scholar]
- 29.Kinjo T, Ham-Terhune J, Peloponese JM, Jr, Jeang KT. Induction of reactive oxygen species by human T-cell leukemia virus type 1 tax correlates with DNA damage and expression of cellular senescence marker. J Virol. 2010;84:5431–7. doi: 10.1128/JVI.02460-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baydoun HH, Bai XT, Shelton S, Nicot C. HTLV-I tax increases genetic instability by inducing DNA double strand breaks during DNA replication and switching repair to NHEJ. PLoS One. 2012;7:e42226. doi: 10.1371/journal.pone.0042226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marriott SJ, Semmes OJ. Impact of HTLV-I Tax on cell cycle progression and the cellular DNA damage repair response. Oncogene. 2005;24:5986–95. doi: 10.1038/sj.onc.1208976. [DOI] [PubMed] [Google Scholar]
- 32.Bellon M, Baydoun HH, Yao Y, Nicot C. HTLV-I Tax-dependent and -independent events associated with immortalization of human primary T lymphocytes. Blood. 2010;115:2441–8. doi: 10.1182/blood-2009-08-241117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Niewiesk S. Animals Models of Human T Cell Leukemia Virus Type I Leukemogenesis. ILAR J. 2016;57:3–11. doi: 10.1093/ilar/ilv045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fochi S, Mutascio S, Bertazzoni U, Zipeto D, Romanelli MG. HTLV Deregulation of the NF-kappaB Pathway: An Update on Tax and Antisense Proteins Role. Frontiers in microbiology. 2018;9:285. doi: 10.3389/fmicb.2018.00285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hayden MS, Ghosh S. Shared principles in NF-kappaB signaling. Cell. 2008;132:344–62. doi: 10.1016/j.cell.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 36.Sun SC, Elwood J, Beraud C, Greene WC. Human T-cell leukemia virus type I Tax activation of NF-kappa B/Rel involves phosphorylation and degradation of I kappa B alpha and RelA (p65)-mediated induction of the c-rel gene. Mol Cell Biol. 1994;14:7377–84. doi: 10.1128/mcb.14.11.7377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mori N, Fujii M, Ikeda S, Yamada Y, Tomonaga M, Ballard DW, Yamamoto N. Constitutive activation of NF-kappaB in primary adult T-cell leukemia cells. Blood. 1999;93:2360–8. [PubMed] [Google Scholar]
- 38.Mori N, Yamada Y, Ikeda S, Yamasaki Y, Tsukasaki K, Tanaka Y, Tomonaga M, Yamamoto N, Fujii M. Bay 11-7082 inhibits transcription factor NF-kappaB and induces apoptosis of HTLV-I-infected T-cell lines and primary adult T-cell leukemia cells. Blood. 2002;100:1828–34. doi: 10.1182/blood-2002-01-0151. [DOI] [PubMed] [Google Scholar]
- 39.Watanabe M, Ohsugi T, Shoda M, Ishida T, Aizawa S, Maruyama-Nagai M, Utsunomiya A, Koga S, Yamada Y, Kamihira S, Okayama A, Kikuchi H, Uozumi K, Yamaguchi K, Higashihara M, Umezawa K, Watanabe T, Horie R. Dual targeting of transformed and untransformed HTLV-1-infected T cells by DHMEQ, a potent and selective inhibitor of NF-kappaB, as a strategy for chemoprevention and therapy of adult T-cell leukemia. Blood. 2005;106:2462–71. doi: 10.1182/blood-2004-09-3646. [DOI] [PubMed] [Google Scholar]
- 40.Macaire H, Riquet A, Moncollin V, Biemont-Trescol MC, Duc Dodon M, Hermine O, Debaud AL, Mahieux R, Mesnard JM, Pierre M, Gazzolo L, Bonnefoy N, Valentin H. Tax protein-induced expression of antiapoptotic Bfl-1 protein contributes to survival of human T-cell leukemia virus type 1 (HTLV-1)-infected T-cells. J Biol Chem. 2012;287:21357–70. doi: 10.1074/jbc.M112.340992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Waldele K, Silbermann K, Schneider G, Ruckes T, Cullen BR, Grassmann R. Requirement of the human T-cell leukemia virus (HTLV-1) tax-stimulated HIAP-1 gene for the survival of transformed lymphocytes. Blood. 2006;107:4491–9. doi: 10.1182/blood-2005-08-3138. [DOI] [PubMed] [Google Scholar]
- 42.Iwanaga R, Ohtani K, Hayashi T, Nakamura M. Molecular mechanism of cell cycle progression induced by the oncogene product Tax of human T-cell leukemia virus type I. Oncogene. 2001;20:2055–67. doi: 10.1038/sj.onc.1204304. [DOI] [PubMed] [Google Scholar]
- 43.Harhaj EW, Sun SC. IKKgamma serves as a docking subunit of the IkappaB kinase (IKK) and mediates interaction of IKK with the human T-cell leukemia virus Tax protein. J Biol Chem. 1999;274:22911–4. doi: 10.1074/jbc.274.33.22911. [DOI] [PubMed] [Google Scholar]
- 44.Chu ZL, Shin YA, Yang JM, DiDonato JA, Ballard DW. IKKgamma mediates the interaction of cellular IkappaB kinases with the tax transforming protein of human T cell leukemia virus type 1. J Biol Chem. 1999;274:15297–300. doi: 10.1074/jbc.274.22.15297. [DOI] [PubMed] [Google Scholar]
- 45.Jin DY, Giordano V, Kibler KV, Nakano H, Jeang KT. Role of adapter function in oncoprotein-mediated activation of NF-kappaB. Human T-cell leukemia virus type I Tax interacts directly with IkappaB kinase gamma. J Biol Chem. 1999;274:17402–5. doi: 10.1074/jbc.274.25.17402. [DOI] [PubMed] [Google Scholar]
- 46.Harhaj EW, Good L, Xiao G, Uhlik M, Cvijic ME, Rivera-Walsh I, Sun SC. Somatic mutagenesis studies of NF-kappa B signaling in human T cells: evidence for an essential role of IKK gamma in NF-kappa B activation by T-cell costimulatory signals and HTLV-I Tax protein. Oncogene. 2000;19:1448–56. doi: 10.1038/sj.onc.1203445. [DOI] [PubMed] [Google Scholar]
- 47.Wu X, Sun SC. Retroviral oncoprotein Tax deregulates NF-kappaB by activating Tak1 and mediating the physical association of Tak1-IKK. EMBO Rep. 2007;8:510–5. doi: 10.1038/sj.embor.7400931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Robek MD, Ratner L. Immortalization of CD4(+) and CD8(+) T lymphocytes by human T-cell leukemia virus type 1 Tax mutants expressed in a functional molecular clone. J Virol. 1999;73:4856–65. doi: 10.1128/jvi.73.6.4856-4865.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kwon H, Ogle L, Benitez B, Bohuslav J, Montano M, Felsher DW, Greene WC. Lethal cutaneous disease in transgenic mice conditionally expressing type I human T cell leukemia virus Tax. J Biol Chem. 2005;280:35713–22. doi: 10.1074/jbc.M504848200. [DOI] [PubMed] [Google Scholar]
- 50.Sun SC. The noncanonical NF-kappaB pathway. Immunol Rev. 2012;246:125–40. doi: 10.1111/j.1600-065X.2011.01088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zarnegar BJ, Wang Y, Mahoney DJ, Dempsey PW, Cheung HH, He J, Shiba T, Yang X, Yeh WC, Mak TW, Korneluk RG, Cheng G. Noncanonical NF-kappaB activation requires coordinated assembly of a regulatory complex of the adaptors cIAP1, cIAP2, TRAF2 and TRAF3 and the kinase NIK. Nat Immunol. 2008;9:1371–8. doi: 10.1038/ni.1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xiao G, Harhaj EW, Sun SC. NF-kappaB-inducing kinase regulates the processing of NF-kappaB2 p100. Mol Cell. 2001;7:401–9. doi: 10.1016/s1097-2765(01)00187-3. [DOI] [PubMed] [Google Scholar]
- 53.Senftleben U, Cao Y, Xiao G, Greten FR, Krahn G, Bonizzi G, Chen Y, Hu Y, Fong A, Sun SC, Karin M. Activation by IKKalpha of a second, evolutionary conserved, NF-kappa B signaling pathway. Science. 2001;293:1495–9. doi: 10.1126/science.1062677. [DOI] [PubMed] [Google Scholar]
- 54.Annunziata CM, Davis RE, Demchenko Y, Bellamy W, Gabrea A, Zhan F, Lenz G, Hanamura I, Wright G, Xiao W, Dave S, Hurt EM, Tan B, Zhao H, Stephens O, Santra M, Williams DR, Dang L, Barlogie B, Shaughnessy JD, Jr, Kuehl WM, Staudt LM. Frequent engagement of the classical and alternative NF-kappaB pathways by diverse genetic abnormalities in multiple myeloma. Cancer Cell. 2007;12:115–30. doi: 10.1016/j.ccr.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Keats JJ, Fonseca R, Chesi M, Schop R, Baker A, Chng WJ, Van Wier S, Tiedemann R, Shi CX, Sebag M, Braggio E, Henry T, Zhu YX, Fogle H, Price-Troska T, Ahmann G, Mancini C, Brents LA, Kumar S, Greipp P, Dispenzieri A, Bryant B, Mulligan G, Bruhn L, Barrett M, Valdez R, Trent J, Stewart AK, Carpten J, Bergsagel PL. Promiscuous mutations activate the noncanonical NF-kappaB pathway in multiple myeloma. Cancer Cell. 2007;12:131–44. doi: 10.1016/j.ccr.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Beraud C, Sun SC, Ganchi P, Ballard DW, Greene WC. Human T-cell leukemia virus type I Tax associates with and is negatively regulated by the NF-kappa B2 p100 gene product: implications for viral latency. Mol Cell Biol. 1994;14:1374–82. doi: 10.1128/mcb.14.2.1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Uhlik M, Good L, Xiao G, Harhaj EW, Zandi E, Karin M, Sun SC. NF-kappaB-inducing kinase and IkappaB kinase participate in human T-cell leukemia virus I Tax-mediated NF-kappaB activation. J Biol Chem. 1998;273:21132–6. doi: 10.1074/jbc.273.33.21132. [DOI] [PubMed] [Google Scholar]
- 58.Xiao G, Cvijic ME, Fong A, Harhaj EW, Uhlik MT, Waterfield M, Sun SC. Retroviral oncoprotein Tax induces processing of NF-kappaB2/p100 in T cells: evidence for the involvement of IKKalpha. EMBO J. 2001;20:6805–15. doi: 10.1093/emboj/20.23.6805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Higuchi M, Tsubata C, Kondo R, Yoshida S, Takahashi M, Oie M, Tanaka Y, Mahieux R, Matsuoka M, Fujii M. Cooperation of NF-kappaB2/p100 activation and the PDZ domain binding motif signal in human T-cell leukemia virus type 1 (HTLV-1) Tax1 but not HTLV-2 Tax2 is crucial for interleukin-2-independent growth transformation of a T-cell line. J Virol. 2007;81:11900–7. doi: 10.1128/JVI.00532-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shoji T, Higuchi M, Kondo R, Takahashi M, Oie M, Tanaka Y, Aoyagi Y, Fujii M. Identification of a novel motif responsible for the distinctive transforming activity of human T-cell leukemia virus (HTLV) type 1 Tax1 protein from HTLV-2 Tax2. Retrovirology. 2009;6:83. doi: 10.1186/1742-4690-6-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fu J, Qu Z, Yan P, Ishikawa C, Aqeilan RI, Rabson AB, Xiao G. The tumor suppressor gene WWOX links the canonical and noncanonical NF-kappaB pathways in HTLV-I Tax-mediated tumorigenesis. Blood. 2011;117:1652–61. doi: 10.1182/blood-2010-08-303073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Peloponese JM, Jr, Iha H, Yedavalli VR, Miyazato A, Li Y, Haller K, Benkirane M, Jeang KT. Ubiquitination of human T-cell leukemia virus type 1 tax modulates its activity. J Virol. 2004;78:11686–95. doi: 10.1128/JVI.78.21.11686-11695.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lavorgna A, Harhaj EW. Regulation of HTLV-1 tax stability, cellular trafficking and NF-kappaB activation by the ubiquitin-proteasome pathway. Viruses. 2014;6:3925–43. doi: 10.3390/v6103925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shembade N, Harhaj NS, Yamamoto M, Akira S, Harhaj EW. The human T-cell leukemia virus type 1 Tax oncoprotein requires the ubiquitin-conjugating enzyme Ubc13 for NF-kappaB activation. J Virol. 2007;81:13735–42. doi: 10.1128/JVI.01790-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chiari E, Lamsoul I, Lodewick J, Chopin C, Bex F, Pique C. Stable ubiquitination of human T-cell leukemia virus type 1 tax is required for proteasome binding. J Virol. 2004;78:11823–32. doi: 10.1128/JVI.78.21.11823-11832.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lamsoul I, Lodewick J, Lebrun S, Brasseur R, Burny A, Gaynor RB, Bex F. Exclusive ubiquitination and sumoylation on overlapping lysine residues mediate NF-kappaB activation by the human T-cell leukemia virus tax oncoprotein. Mol Cell Biol. 2005;25:10391–406. doi: 10.1128/MCB.25.23.10391-10406.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gatza ML, Dayaram T, Marriott SJ. Ubiquitination of HTLV-I Tax in response to DNA damage regulates nuclear complex formation and nuclear export. Retrovirology. 2007;4:95. doi: 10.1186/1742-4690-4-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yu Q, Minoda Y, Yoshida R, Yoshida H, Iha H, Kobayashi T, Yoshimura A, Takaesu G. HTLV-1 Tax-mediated TAK1 activation involves TAB2 adapter protein. Biochem Biophys Res Commun. 2008;365:189–94. doi: 10.1016/j.bbrc.2007.10.172. [DOI] [PubMed] [Google Scholar]
- 69.Yasunaga J, Lin FC, Lu X, Jeang KT. Ubiquitin-specific peptidase 20 targets TRAF6 and human T cell leukemia virus type 1 tax to negatively regulate NF-kappaB signaling. J Virol. 2011;85:6212–9. doi: 10.1128/JVI.00079-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lavorgna A, Harhaj EW. An RNA interference screen identifies the Deubiquitinase STAMBPL1 as a critical regulator of human T-cell leukemia virus type 1 tax nuclear export and NF-kappaB activation. J Virol. 2012;86:3357–69. doi: 10.1128/JVI.06456-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Turci M, Lodewick J, Di Gennaro G, Rinaldi AS, Marin O, Diani E, Sampaio C, Bex F, Bertazzoni U, Romanelli MG. Ubiquitination and sumoylation of the HTLV-2 Tax-2B protein regulate its NF-kappaB activity: a comparative study with the HTLV-1 Tax-1 protein. Retrovirology. 2012;9:102. doi: 10.1186/1742-4690-9-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pene S, Waast L, Bonnet A, Benit L, Pique C. A Non-SUMOylated Tax Protein Is Still Functional for NF-kappaB Pathway Activation. J Virol. 2014;88:10655–61. doi: 10.1128/JVI.01827-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Harhaj NS, Sun SC, Harhaj EW. Activation of NF-kappaB by the human T cell leukemia virus type I Tax oncoprotein is associated with ubiquitin-dependent relocalization of IkappaB kinase. J Biol Chem. 2007;282:4185–92. doi: 10.1074/jbc.M611031200. [DOI] [PubMed] [Google Scholar]
- 74.Journo C, Filipe J, About F, Chevalier SA, Afonso PV, Brady JN, Flynn D, Tangy F, Israel A, Vidalain PO, Mahieux R, Weil R. NRP/Optineurin Cooperates with TAX1BP1 to potentiate the activation of NF-kappaB by human T-lymphotropic virus type 1 tax protein. PLoS Pathog. 2009;5:e1000521. doi: 10.1371/journal.ppat.1000521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Huang J, Ren T, Guan H, Jiang Y, Cheng H. HTLV-1 Tax is a critical lipid raft modulator that hijacks IkappaB kinases to the microdomains for persistent activation of NF-kappaB. J Biol Chem. 2009;284:6208–17. doi: 10.1074/jbc.M806390200. [DOI] [PubMed] [Google Scholar]
- 76.Ho YK, Zhi H, Bowlin T, Dorjbal B, Philip S, Zahoor MA, Shih HM, Semmes OJ, Schaefer B, Glover JN, Giam CZ. HTLV-1 Tax Stimulates Ubiquitin E3 Ligase, Ring Finger Protein 8, to Assemble Lysine 63-Linked Polyubiquitin Chains for TAK1 and IKK Activation. PLoS Pathog. 2015;11:e1005102. doi: 10.1371/journal.ppat.1005102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lee DF, Kuo HP, Chen CT, Hsu JM, Chou CK, Wei Y, Sun HL, Li LY, Ping B, Huang WC, He X, Hung JY, Lai CC, Ding Q, Su JL, Yang JY, Sahin AA, Hortobagyi GN, Tsai FJ, Tsai CH, Hung MC. IKK beta suppression of TSC1 links inflammation and tumor angiogenesis via the mTOR pathway. Cell. 2007;130:440–55. doi: 10.1016/j.cell.2007.05.058. [DOI] [PubMed] [Google Scholar]
- 78.Yoshita M, Higuchi M, Takahashi M, Oie M, Tanaka Y, Fujii M. Activation of mTOR by human T-cell leukemia virus type 1 Tax is important for the transformation of mouse T cells to interleukin-2-independent growth. Cancer Sci. 2012;103:369–74. doi: 10.1111/j.1349-7006.2011.02123.x. [DOI] [PubMed] [Google Scholar]
- 79.Shibata Y, Tokunaga F, Goto E, Komatsu G, Gohda J, Saeki Y, Tanaka K, Takahashi H, Sawasaki T, Inoue S, Oshiumi H, Seya T, Nakano H, Tanaka Y, Iwai K, Inoue JI. HTLV-1 Tax Induces Formation of the Active Macromolecular IKK Complex by Generating Lys63- and Met1-Linked Hybrid Polyubiquitin Chains. PLoS Pathog. 2017;13:e1006162. doi: 10.1371/journal.ppat.1006162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Choi YB, Harhaj EW. HTLV-1 tax stabilizes MCL-1 via TRAF6-dependent K63-linked polyubiquitination to promote cell survival and transformation. PLoS Pathog. 2014;10:e1004458. doi: 10.1371/journal.ppat.1004458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wang C, Long W, Peng C, Hu L, Zhang Q, Wu A, Zhang X, Duan X, Wong CC, Tanaka Y, Xia Z. HTLV-1 Tax Functions as a Ubiquitin E3 Ligase for Direct IKK Activation via Synthesis of Mixed-Linkage Polyubiquitin Chains. PLoS Pathog. 2016;12:e1005584. doi: 10.1371/journal.ppat.1005584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Semmes OJ, Jeang KT. HTLV-I Tax is a zinc-binding protein: role of zinc in Tax structure and function. Virology. 1992;188:754–64. doi: 10.1016/0042-6822(92)90530-3. [DOI] [PubMed] [Google Scholar]
- 83.Jin DY, Jeang KT. HTLV-I Tax self-association in optimal trans-activation function. Nucleic Acids Res. 1997;25:379–87. doi: 10.1093/nar/25.2.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Emmerich CH, Ordureau A, Strickson S, Arthur JS, Pedrioli PG, Komander D, Cohen P. Activation of the canonical IKK complex by K63/M1-linked hybrid ubiquitin chains. Proc Natl Acad Sci U S A. 2013;110:15247–52. doi: 10.1073/pnas.1314715110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Emmerich CH, Bakshi S, Kelsall IR, Ortiz-Guerrero J, Shpiro N, Cohen P. Lys63/Met1-hybrid ubiquitin chains are commonly formed during the activation of innate immune signalling. Biochem Biophys Res Commun. 2016;474:452–461. doi: 10.1016/j.bbrc.2016.04.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Haas TL, Emmerich CH, Gerlach B, Schmukle AC, Cordier SM, Rieser E, Feltham R, Vince J, Warnken U, Wenger T, Koschny R, Komander D, Silke J, Walczak H. Recruitment of the linear ubiquitin chain assembly complex stabilizes the TNF-R1 signaling complex and is required for TNF-mediated gene induction. Mol Cell. 2009;36:831–44. doi: 10.1016/j.molcel.2009.10.013. [DOI] [PubMed] [Google Scholar]
- 87.Nakazawa S, Oikawa D, Ishii R, Ayaki T, Takahashi H, Takeda H, Ishitani R, Kamei K, Takeyoshi I, Kawakami H, Iwai K, Hatada I, Sawasaki T, Ito H, Nureki O, Tokunaga F. Linear ubiquitination is involved in the pathogenesis of optineurin-associated amyotrophic lateral sclerosis. Nature communications. 2016;7:12547. doi: 10.1038/ncomms12547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fu DX, Kuo YL, Liu BY, Jeang KT, Giam CZ. Human T-lymphotropic virus type I tax activates I-kappa B kinase by inhibiting I-kappa B kinase-associated serine/threonine protein phosphatase 2A. J Biol Chem. 2003;278:1487–93. doi: 10.1074/jbc.M210631200. [DOI] [PubMed] [Google Scholar]
- 89.Shembade N, Pujari R, Harhaj NS, Abbott DW, Harhaj EW. The kinase IKKalpha inhibits activation of the transcription factor NF-kappaB by phosphorylating the regulatory molecule TAX1BP1. Nat Immunol. 2011;12:834–43. doi: 10.1038/ni.2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shembade N, Ma A, Harhaj EW. Inhibition of NF-kappaB signaling by A20 through disruption of ubiquitin enzyme complexes. Science. 2010;327:1135–9. doi: 10.1126/science.1182364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pujari R, Hunte R, Thomas R, van der Weyden L, Rauch D, Ratner L, Nyborg JK, Ramos JC, Takai Y, Shembade N. Human T-cell leukemia virus type 1 (HTLV-1) tax requires CADM1/TSLC1 for inactivation of the NF-kappaB inhibitor A20 and constitutive NF-kappaB signaling. PLoS Pathog. 2015;11:e1004721. doi: 10.1371/journal.ppat.1004721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wu X, Zhang M, Sun SC. Mutual regulation between deubiquitinase CYLD and retroviral oncoprotein Tax. Cell Biosci. 2011;1:27. doi: 10.1186/2045-3701-1-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tang SW, Chen CY, Klase Z, Zane L, Jeang KT. The cellular autophagy pathway modulates human T-cell leukemia virus type 1 replication. J Virol. 2013;87:1699–707. doi: 10.1128/JVI.02147-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ren T, Takahashi Y, Liu X, Loughran TP, Sun SC, Wang HG, Cheng H. HTLV-1 Tax deregulates autophagy by recruiting autophagic molecules into lipid raft microdomains. Oncogene. 2015;34:334–45. doi: 10.1038/onc.2013.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chen L, Liu D, Zhang Y, Zhang H, Cheng H. The autophagy molecule Beclin 1 maintains persistent activity of NF-kappaB and Stat3 in HTLV-1-transformed T lymphocytes. Biochem Biophys Res Commun. 2015;465:739–45. doi: 10.1016/j.bbrc.2015.08.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Newman AC, Scholefield CL, Kemp AJ, Newman M, McIver EG, Kamal A, Wilkinson S. TBK1 kinase addiction in lung cancer cells is mediated via autophagy of Tax1bp1/Ndp52 and non-canonical NF-kappaB signalling. PLoS ONE. 2012;7:e50672. doi: 10.1371/journal.pone.0050672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tumbarello DA, Manna PT, Allen M, Bycroft M, Arden SD, Kendrick-Jones J, Buss F. The Autophagy Receptor TAX1BP1 and the Molecular Motor Myosin VI Are Required for Clearance of Salmonella Typhimurium by Autophagy. PLoS Pathog. 2015;11:e1005174. doi: 10.1371/journal.ppat.1005174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kuo YL, Giam CZ. Activation of the anaphase promoting complex by HTLV-1 tax leads to senescence. EMBO J. 2006;25:1741–52. doi: 10.1038/sj.emboj.7601054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhi H, Yang L, Kuo YL, Ho YK, Shih HM, Giam CZ. NF-kappaB hyper-activation by HTLV-1 tax induces cellular senescence, but can be alleviated by the viral anti-sense protein HBZ. PLoS Pathog. 2011;7:e1002025. doi: 10.1371/journal.ppat.1002025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ho YK, Zhi H, DeBiaso D, Philip S, Shih HM, Giam CZ. HTLV-1 tax-induced rapid senescence is driven by the transcriptional activity of NF-kappaB and depends on chronically activated IKKalpha and p65/RelA. J Virol. 2012;86:9474–83. doi: 10.1128/JVI.00158-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Philip S, Zahoor MA, Zhi H, Ho YK, Giam CZ. Regulation of human T-lymphotropic virus type I latency and reactivation by HBZ and Rex. PLoS Pathog. 2014;10:e1004040. doi: 10.1371/journal.ppat.1004040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kataoka K, Nagata Y, Kitanaka A, Shiraishi Y, Shimamura T, Yasunaga J, Totoki Y, Chiba K, Sato-Otsubo A, Nagae G, Ishii R, Muto S, Kotani S, Watatani Y, Takeda J, Sanada M, Tanaka H, Suzuki H, Sato Y, Shiozawa Y, Yoshizato T, Yoshida K, Makishima H, Iwanaga M, Ma G, Nosaka K, Hishizawa M, Itonaga H, Imaizumi Y, Munakata W, Ogasawara H, Sato T, Sasai K, Muramoto K, Penova M, Kawaguchi T, Nakamura H, Hama N, Shide K, Kubuki Y, Hidaka T, Kameda T, Nakamaki T, Ishiyama K, Miyawaki S, Yoon SS, Tobinai K, Miyazaki Y, Takaori-Kondo A, Matsuda F, Takeuchi K, Nureki O, Aburatani H, Watanabe T, Shibata T, Matsuoka M, Miyano S, Shimoda K, Ogawa S. Integrated molecular analysis of adult T cell leukemia/lymphoma. Nat Genet. 2015;47:1304–15. doi: 10.1038/ng.3415. [DOI] [PubMed] [Google Scholar]
- 103.Zhi H, Zahoor MA, Shudofsky AM, Giam CZ. KSHV vCyclin counters the senescence/G1 arrest response triggered by NF-kappaB hyperactivation. Oncogene. 2015;34:496–505. doi: 10.1038/onc.2013.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hironaka N, Mochida K, Mori N, Maeda M, Yamamoto N, Yamaoka S. Tax-independent constitutive IkappaB kinase activation in adult T-cell leukemia cells. Neoplasia. 2004;6:266–78. doi: 10.1593/neo.3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Snow AL, Xiao W, Stinson JR, Lu W, Chaigne-Delalande B, Zheng L, Pittaluga S, Matthews HF, Schmitz R, Jhavar S, Kuchen S, Kardava L, Wang W, Lamborn IT, Jing H, Raffeld M, Moir S, Fleisher TA, Staudt LM, Su HC, Lenardo MJ. Congenital B cell lymphocytosis explained by novel germline CARD11 mutations. J Exp Med. 2012;209:2247–61. doi: 10.1084/jem.20120831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yamagishi M, Nakano K, Miyake A, Yamochi T, Kagami Y, Tsutsumi A, Matsuda Y, Sato-Otsubo A, Muto S, Utsunomiya A, Yamaguchi K, Uchimaru K, Ogawa S, Watanabe T. Polycomb-mediated loss of miR-31 activates NIK-dependent NF-kappaB pathway in adult T cell leukemia and other cancers. Cancer Cell. 2012;21:121–35. doi: 10.1016/j.ccr.2011.12.015. [DOI] [PubMed] [Google Scholar]
- 107.Lavorgna A, Matsuoka M, Harhaj EW. A critical role for IL-17RB signaling in HTLV-1 tax-induced NF-kappaB activation and T-cell transformation. PLoS Pathog. 2014;10:e1004418. doi: 10.1371/journal.ppat.1004418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Tsukasaki K, Krebs J, Nagai K, Tomonaga M, Koeffler HP, Bartram CR, Jauch A. Comparative genomic hybridization analysis in adult T-cell leukemia/lymphoma: correlation with clinical course. Blood. 2001;97:3875–81. doi: 10.1182/blood.v97.12.3875. [DOI] [PubMed] [Google Scholar]
- 109.Wu HH, Hwang-Verslues WW, Lee WH, Huang CK, Wei PC, Chen CL, Shew JY, Lee EY, Jeng YM, Tien YW, Ma C, Lee WH. Targeting IL-17B-IL-17RB signaling with an anti-IL-17RB antibody blocks pancreatic cancer metastasis by silencing multiple chemokines. J Exp Med. 2015;212:333–49. doi: 10.1084/jem.20141702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Huang CK, Yang CY, Jeng YM, Chen CL, Wu HH, Chang YC, Ma C, Kuo WH, Chang KJ, Shew JY, Lee WH. Autocrine/paracrine mechanism of interleukin-17B receptor promotes breast tumorigenesis through NF-kappaB-mediated antiapoptotic pathway. Oncogene. 2014;33:2968–77. doi: 10.1038/onc.2013.268. [DOI] [PubMed] [Google Scholar]