Abstract
Exposure to intense sound or noise can result in purely temporary threshold shift (TTS), or leave a residual permanent threshold shift (PTS) along with alterations in growth functions of auditory nerve output. Recent research has revealed a number of mechanisms that contribute to noise-induced hearing loss (NIHL). The principle cause of NIHL is damage to cochlear hair cells and associated synaptopathy. Contributions to TTS include reversible damage to hair cell (HC) stereocilia or synapses, while moderate TTS reflects protective purinergic hearing adaptation. PTS represents permanent damage to or loss of HCs and synapses. While the substrates of HC damage are complex, they include the accumulation of reactive oxygen species and the active stimulation of intracellular stress pathways, leading to programmed and/or necrotic cell death. Permanent damage to cochlear neurons can also contribute to the effects of NIHL, in addition to HC damage. These mechanisms have translational potential for pharmacological intervention and provide multiple opportunities to prevent HC damage or to rescue HCs and spiral ganglion neurons that have suffered injury. This paper reviews advances in our understanding of cellular mechanisms that contribute to NIHL and their potential for therapeutic manipulation.
Keywords: Noise-induced hearing loss, Hair cell, Damage signaling, Survival signaling, Apoptosis, Pharmacotherapy
1. Introduction
Hearing loss is a significant handicap, affecting communication and impacting quality of life. There are many causes of hearing loss. These include exposure to ototoxic compounds including drugs (Roland and Rutka, 2004), mutations in deafness genes (Vona and Haaf, 2016), infections such as labyrinthitis or prenatal cytomegalovirus (Furutate et al., 2011), and aging (Zhang et al., 2013). Exposure to excessive levels of sound, even for short time periods, can also produce loss of hearing sensitivity and auditory acuity. Noise can lead to temporary threshold shift (TTS) that fully recovers to normal. However, it can also produce losses that fail to return to pre-exposure levels. Such permanent threshold shift (PTS) can have a significant effect on communication and quality of life (World Health Organization, 2015; accessed 26/06/2016).
Intense sound is a significant cause of hearing loss in the general population, due to occupational and recreational acoustic overstimulation. In fact, noise is one of the most common occupational hazards in the United States. Noise-induced hearing loss (NIHL) significantly affects the military and veterans. Service in the armed forces often involves exposure to noise, and blast exposure has been an increasingly common hazard of military deployment (Bramble, 2009). Blast exposure substantially raises the risk for hearing loss (Muhr and Rosenhall, 2011; Wells et al., 2015; Yong and Wang, 2015). Veterans who served in the military during the period from 2001 to 2010 are four times more likely than age- and occupation-matched non-veterans to suffer severe hearing loss (Centers for Disease Control, 2011), and high numbers of active duty military and veterans suffer hearing loss due to service in Afghanistan and Iraq (Theodoroff et al., 2015). More than 775,000 veterans had significant hearing loss prior to 2009 (Fausti et al., 2009), and this number has certainly only increased. The impacts of hearing loss on quality of life, psychological status and employability discussed above are of profound importance to these veterans, impeding their return to civilian life (Theodoroff et al., 2015). NIHL also results in substantial disability and rehabilitation expenses.
Due to the impacts on quality of life, extensive attention has rightly been focused on devices that protect the ear from acoustic overstimulation. However, despite decades of efforts, the problem of NIHL continues to grow (e.g. Johansson and Arlinger, 2004), especially among those associated with the military (Bramble, 2009; Pearson, 2009; Wells et al., 2015; Yong and Wang, 2015). In part, this problem reflects the resistance of many individuals to wearing noise suppressors such as earplugs with noise-intensive recreation, as well as workplace and military operational constraints which may limit practical sound barrier use, such as combat conditions (Bramble, 2009).
It is therefore important to develop alternative means of NIHL prevention. NIHL primarily reflects damage to the sensorineural structures of the cochlea, especially the sensory hair cells (HCs), but also primary auditory neurons (Webster and Webster, 1981; Kujawa and Liberman, 2009).
There have been many significant advances in our understanding of the cellular processes that mediate the death and survival of HCs. These processes represent potential check-points in cochlear damage mechanisms, at which intervention should be protective. Pharmacological intervention to protect the cochlea therefore has considerable future potential for the protection of hearing from noise.
It is our purpose, in this paper, to review current knowledge relevant to the biology of HC damage and its prevention. To accomplish this, we first review cellular mechanisms that have been found to contribute to NIHL. We then describe protective pathways that act in opposition to these damage pathways. Finally, we review the potential of damage and survival mechanisms as targets for pharmacological intervention to prevent or ameliorate NIHL.
The various molecules discussed in the paper are presented in Table 1.
Table 1.
Selected molecules relevant NIHL.
| Damage mediators |
| Free radicals |
| Reactive oxygen species (ROS) |
| Reactive nitrogen species (RNS) |
| Intracellular free Ca2+ |
| Nicotoinamide adenine dinucleotide phosphate oxidase (NADPH oxidase) |
| Pro-inflammatory cytokines |
| Interleukin 1 beta (IL-1β) |
| Interleukin 6 (IL-6) |
| Tumor necrosis factor alpha (TNFα) |
| Damage signaling molecules |
| Nuclear Factor kappa B (NF-κB) |
| Focal adhesion kinase (FAK) |
| Src |
| Kirsten rat sarcoma viral oncogene homolog (kRas) |
| Ras-related C3 botulinum toxin substrate (Rac) |
| Cell division control protein 42 (Cdc42) |
| Mixed lineage kinases (MLKs) |
| Jun amino-terminal kinase (JNK) |
| Jun |
| Activator protein 1 (AP-1) |
| Apoptosis pathway molecules |
| Bcl2 Associated X (Bax) |
| Bcl2 Associated death promoter (Bad) |
| B cell lymphoma 2 (Bcl2) |
| Bcl2 related gene (Bclx) |
| Cytochrome C |
| Apoptotic protease activating factor 1 (APAF) |
| Caspase 1 |
| Caspases 3,6,7 |
| Protective molecules |
| Antioxidants |
| Growth factors (GFs) |
| Harvey rat sarcoma oncogene (hRas) |
| Phosphinositol 3 kinase (PI3K) |
| Protein kinase B (PKB or AKT) |
| Extracellularly regulated kinase (ERK) |
| Pharmacological protectants |
| N-acetyl cysteine (NAC) (antioxidant) |
| FTI-277 (inhibitor of KRas at 10 μM; hRas at 1 μM) |
| Adenosine A1 receptor agonist adenosine amine congener (ADAC) |
| D-JNKI-1 (peptide JNK inhibitor) |
| Etanercept (TNFα inhibitor) |
| Anti-IL-6-receptor antibody |
| Dexamethasone (steroid) |
2. Mechanisms of sensorineural damage in the cochlea
2.1. Mechanisms of TTS
Temporary loss of hearing sensitivity is often viewed as a less severe form of the same changes that lead to permanent cochlear damage. However, recent evidence suggests that TTS may be mediated by distinct mechanisms. Housley et al. (2013) found that low-level TTS is mediated by ion channels that are activated by extracellular ATP, since mice deficient in a specific channel (P2RX2) do not experience TTS after noise exposure that normally causes about 15 dB of temporary sensitivity loss. This ATP receptor is a nonselective cation channel, expressed in cochlear HCs and epithelial cells lining the scala media. Noise is known to stimulate local ATP release in the cochlea (Telang et al., 2010). This ATP opens the P2RX2 channels, which then shunt endocochlear current away from the HC transduction channel (Thorne et al., 2004; Morton-Jones et al., 2015) and also activates longer-lasting sensitivity reduction via a yet uncharacterized mechanism. Both mice and humans (Housley et al., 2013; Yan et al., 2013) lacking the P2rx2 gene that encodes this receptor exhibit increased sensitivity to PTS when exposed to higher levels of noise or long periods of moderate level noise exposure. These findings suggest that low-level TTS, largely arising from P2RX2 receptor activation, may reflect hearing adaptation that extends the intensity range of hearing, and protects the cochlea from damage.
However more extensive, TTS (up to 50 dB) can also recover to normal threshold levels over time (Ryan and Bone, 1978), if not to normal levels of synaptic contact between HCs and spiral ganglion neurons (Kujawa and Liberman, 2009). These higher levels of TTS are thus due to additional mechanisms. Nordmann et al. (2000) noted that uncoupling of the outer HC stereocilia from the tectorial membrane was the primary morphological feature associated with 43 dB of TTS in animals. Other investigators have noted swelling of the afferent endings underneath the inner HCs after noise exposure, suggestive of excitotoxicity due to the release of excessive glutamate from overstimulated HCs (Puel et al., 1998). Supporting this mechanism, Puel et al. (1998) found that pre-treatment with the glutamate antagonist kynurenate not only prevented this swelling, but also reduced the amount of TTS. This finding suggests that reversible excitotoxicity to cochlear afferent neurons can also contribute to TTS.
Other evidence suggests that metabolic overstimulation may also contribute to temporary changes in threshold after noise. Cheng et al. (2008) found that treatment with the antioxidant D-methionine protected animals from TTS, implicating the generation of reactive oxygen species (ROS) by mitochondria, perhaps in response to metabolic overload. They also found that activity of the ion transporters Na,K-ATPase and Ca-ATPase was decreased, while free radicals were increased, in the cochlear lateral wall after TTS-inducing noise. Given the role of these transporters in generating the endocochlear potential (Mori et al., 2009), the decreased activity suggests that reversible reductions in the endocochlear potential may partially mediate TTS.
2.2. Mechanisms of PTS
Given sufficient noise exposure, the ability of the cochlea to recover is overwhelmed, and hearing loss becomes irreversible. Such permanent changes in auditory thresholds have primarily been linked to cochlear HC damage and loss, although damage to neurons and the lateral wall can also mediate long-term loss of hearing (Schuknecht, 1993). Sufficiently intense overstimulation of the cochlea, as can occur with blast exposure, will produce mechanical damage to the cochlea. This damage includes direct mechanical disruption of HC stereociliary arrays (Liberman and Beil, 1979; Slepecky, 1986; Patuzzi et al., 1989), which can reduce or even eliminate function. The most intense stimulation can even compromise the integrity of the sensory epithelium, disrupting HCs and supporting cells. Moreover, such breaching of the barrier between endolymph and perilymph can expose the basal poles of remaining intact HCs to high levels of potassium, leading to HC death. However, damaging levels of noise begin well below the threshold of such frank mechanical damage. The majority of NIHL reflects HC damage mediated by biochemical processes that occur within the cells themselves.
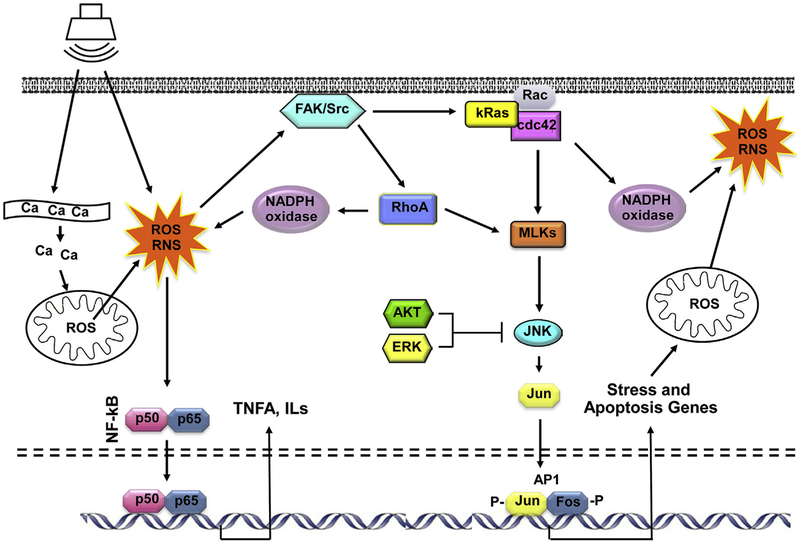
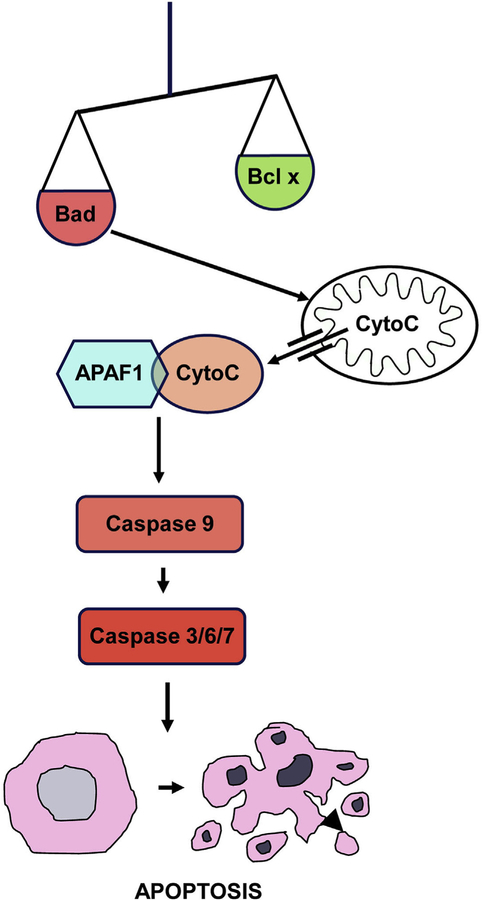
Until approximately 20 years ago, there was relatively little information on the cellular processes that mediate damage to HCs. However, intensive research on the mechanisms of cell death and survival was ongoing in other disciplines, especially cancer where the regulation of cell death is central to the search for cures. The knowledge gained provided the tools with which to begin understanding the cellular molecular pathways involved in HC damage and loss. More importantly, many of these processes are amenable to pharmacological intervention. Moreover, the cell biological processes that have been implicated in NIHL also appeared to be involved in other forms of HC damage, including ototoxicity (Schacht, 1986) and age-related hearing loss (Kujawa and Liberman, 2006; Wong and Ryan, 2015). Thus, any interventions developed for NIHL could also be potentially effective against other forms of sensorineural hearing loss (SNHL), as well. A diagrammatic representation of some of the documented processes that occur within HCs during damage is presented in Figs. 1 and 2.
Fig. 1. Diagram illustrating damage processes and pathways thought to contribute to HC loss due to acoustic overexposure.
Noise initiates the production of ROS via release of Ca2+ from the endoplasmic reticulum and/or entry from extracellular fluid, which induces release of ROS from mitochondria, and by activation of NADPH oxidases. ROS can activate NF-κB, leading to the production of pro-inflammatory cytokines, and also κRas/cdc42/JNK pathway leading to the expression of stress and apoptosis genes. Pro-apoptotic factors further increase mitochondrial membrane permeability, leading to the release of additional ROS. The JNK pathway can be inhibited by the ERK MAPK or AKT, signaling molecules that can be activated by growth factors.
Fig. 2. Diagram of the intrinsic pathway of apoptosis.
Apoptosis can be initiated when pro-apoptotic proteins such as BAX or BAD which overwhelm anti-apoptotic proteins of the BCL family. This destabilizes mitochondrial membranes, releasing Cytochrome c into the cytoplasm. Interaction of Cytochrome c with APAF forms an apoptosome, which enzymatically cleaves pro-caspase 1 into its active form. This initiator caspase in turn cleaves executor caspases (3, 6 and 7), which mediate programmed death and orderly fragmentation of the cell.
2.3. Reactive oxygen species (ROS) and reactive nitrogen species (RNS)
A widely accepted mediator of HC damage are ROS (see Fig. 2). These free radicals cause damage by chemically reacting with numerous constituents within cells, including DNA, proteins, cytosolic molecules, cell surface receptors, and membrane lipids, thereby affecting multiple intracellular processes (Dröge, 2002). Generation of free radical species has been observed in the cochlea after exposure to damaging levels of noise (Shi and Nuttall, 2003; Henderson et al., 2006; Hu et al., 2006; Vlajkovic et al., 2010), as well as following HC exposure to ototoxic drugs (Hirose et al., 1997; Kim et al., 2010). ROS have been detected in cochlear tissue immediately after noise exposure (Yamane et al., 1995), indicating that free radical accumulation is an early event in the HC damage process. Free radicals are observed within HCs well before any morphological signs of damage are obvious (Choung et al., 2009), further supporting a role in damage initiation. However, ROS can also persist in the cochlea for 7–10 days after noise exposure, spreading from the basal to the apex (Yamane et al., 1995). Such prolonged oxidative stress can be presumed to induce progressive cochlear injury. Further evidence of a role for ROS in NIHL was provided by Liu et al. (2010), who observed an association between sensitivity to occupational noise damage and polymorphisms of the gene encoding the endogenous antioxidant enzyme superoxide dismutase 1 (sod1) in Chinese workers. With respect to RNS, nitrotyrosine immunoreactivity is a marker of RNS production. It is found to be elevated in rat cochleae six days after PTS-level noise exposure (Vlajkovic et al., 2010).
Beyond direct biochemical damage, ROS can have indirect effects. Yamashita et al. (2004) found that ROS induced lipid peroxidation in the cochlea, including the production of highly toxic products. While lipid peroxidation products themselves can lead to apoptosis, vasoactive lipid peroxidation products such as isoprostanes can potentially lead to the reduced cochlear blood flow that can be associated with excessive noise (Thorne et al., 1987; Seidman et al., 1999; Ohinata et al., 2000; Jaumann et al., 2012). Noise-induced ischemia and subsequent re-perfusion might further potentiate the generation of ROS in a positive feedback loop. The reactive nitrogen product peroxynitrite (ONOO–), a particular dangerous free radical which is produced by the combination of nitric oxide (NO) with superoxide, has been observed in the cochlea after noise exposure (Yamashita et al., 2004). ROS can also lead to inflammation, including the production of pro-inflammatory cytokines such as interleukin-6 (IL-6; Wakabayashi et al., 2010) and tumor necrosis factor á (TNFá; Keithley et al., 2008), both of which have been observed after ROS generation in the cochlea. These pro-inflammatory mediators can themselves produce cochlear damage (Tan et al., 2016).
2.4. Calcium homeostasis and the generation of ROS
The above data strongly implicate ROS in HC damage, but they do not address the source of free radicals. A primary generator of ROS in cells is the mitochondrion, which generates reactive species as a byproduct of metabolism. Mitochondrial ROS are normally controlled by potent antioxidant enzymes within the mitochondrion, which rely on NADPH as a source of reducing equivalents. Egress of ROS into the cytoplasm is limited by the mitochondrial membrane, including its normal potential. Loss of mitochondrial membrane integrity and/or potential leads to the release of ROS into the cytoplasm, and can also lead to increased free radical production (Batandier et al., 2004). Calcium homeostasis can play a significant role in regulating this process.
Esterberg et al. (2013, 2014) found that aminoglycoside antibiotics can induce cytoplasmic ROS in HCs by disrupting calcium homeostasis between the endoplasmic reticulum (ER) and mitochondria. They found that aminoglycosides can enhance the flow of Ca2+ from endoplasmic reticulum into mitochondria. The Ca2+ release leads to loss of mitochondrial membrane potential and increased membrane permeability.
Free Ca2+ has also been found to increase in cochlear HCs immediately after exposure to damaging noise (Fridberger et al., 1998). This increase appears to have several causes, including entry from the extracellular compartment via ion channels such as L-type Ca2+ and P2X2 ATP-gated channels. Extracellular Ca2+ entry in turn can enhance the release of Ca2+ from intracellular stores (Orrenius et al., 2003), further elevating free Ca2+. Elevated calcium may not only induce cytoplasmic ROS accumulation, but may also trigger apoptotic and necrotic cell death pathways independent of ROS (Orrenius et al., 2003). In addition, free Ca2+ can modulate the activity of mitogen-activated protein kinase (MAPK) and other intracellular signaling cascades that mediate cell stress (e.g., Agell et al., 2002; Harr and Distelhorst, 2010). In fact, there is extensive evidence that MAPK cascades play a significant role in damage to HCs (Maeda et al., 2013).
Another source of ROS in noise are NADPH oxidases. These enzymes generate the ROS superoxide under conditions of cell stress. Bielefeld (2013) found that intracochlear treatment of animals with an NADPH oxidase inhibitor reduced noise-induced PTS.
2.5. Cell signaling networks as mediators of HC damage
Cell signaling pathways connect the processes of cells and ultimately link them to the cell nucleus, activating gene expression programs that can be powerful determinants of cell fate. Amongst the various cell signaling pathways, the MAPKs are important mediators of damage and survival signaling. They act downstream from plasma membrane receptors, intracellular receptors, and ROS (Wortzel and Seger, 2011; Torres, 2003), linked sequentially via intermediate signaling proteins, including members of the Src, Ras, Rac/cdc42, and mixed lineage kinase protein families, to the activation of gene expression. When phosphorylated, MAPKs in turn phosphorylate elements of the AP-1 transcriptional complex, leading to the transcription of diverse genes. They also interact with other target proteins that can directly regulate intracellular processes. MAPK activation can influence cell proliferation, differentiation, motility, cell death and cell survival. The MAPKs include the extracellular signal-regulated kinases 1, 2 and 5 (ERK1, 2 and 5), most often induced by growth factors and mediates tissue growth and survival. Stress-activated MAPKs include c-Jun-N-terminal kinase (JNK) isoforms 1–3, and p38 MAPK (isoforms á, â, ã and ä). These stress-activated MAPKs act in key pathways mediating cellular stress and inflammation responses evoked by a variety of physical, chemical and biological stress stimuli. Strong and sustained activation of the stress MAPKs can lead to apoptotic or necrotic cell death.
Intense noise has been found to alter cochlear MAPK phosphorylation that is linked to HC death. Murai et al. (2008) observed JNK activation in the organ of Corti after impulse noise exposure, followed by positive TUNEL labeling indicative of apoptosis. Maeda et al. (2013) also noted increased organ of Corti JNK phosphorylation within hours of intense noise stimulation, leading to increased c-Jun phosphorylation. Increased HC c-Jun phosphorylation was also noted after noise exposure by Anttonen et al. (2016). Inhibitors of JNK have demonstrated protection against both noise-induced and aminoglycoside-induced HC loss (Pirvola et al., 2000; Wang et al., 2003, 2007). In addition, mice in which c-Jun phosphorylation sites were mutated showed partial protection against noise trauma (Anttonen et al., 2016). Inhibition of upstream activators of the JNK pathway, including KRas, Rac/cdc42 and mixed lineage kinases also provides HC protection (Bodmer et al., 2002a, b; Battaglia et al., 2003), delineating the pathway leading to JNK activation.
2.6. Programmed cell death
Following intense noise exposure activation of ROS, RNS and MAPK stress pathways, cochlear HCs can undergo apoptosis and/or necroptosis (Hu et al., 2000, 2002a, b; Nicotera et al., 2004; Wang et al., 2003; Yang et al., 2004). Apoptosis occurs through the sequential actions of caspases, initiated by their associated extrinsic and intrinsic pathways (Yakovlev and Faden, 2001). The extrinsic pathway is activated by extracellular stimuli such as TNFα through transmembrane death receptors, which cleave caspase-8 and activate downstream execution mediated by caspases 3 and 7. The intrinsic pathway (Fig. 2) is initiated by a change in mitochondrial membrane permeability. Increased permeability releases not only ROS, as discussed above, but also cytochrome C. Cytochrome C binds with Apaf-1 to form an apoptosome, which activates caspase-9 and the downstream apoptotic execution pathway. There are also caspase-independent processes that lead to apoptosis, mediated by other factors including receptor-interacting serine/threonine-protein kinase 1 (RIP-1) or AIF (Tait and Green, 2008). The Bcl-2 proteins play an important role in regulating apoptosis. Pro-apoptotic family members such as Bax and Bak, promote apoptosis, while anti-apoptotic members such as Bcl-2 and Bcl-xL inhibit apoptosis. RIP-1 can also initiate the process of necroptosis, which differs from apoptosis both in initiation and effect. Apoptosis results in the orderly disassembly of cells into membrane-packaged fragments that can be disposed of by phagocytes in a non-inflammatory process. Necroptosis permeabilizes intra- and extracellular membranes, releasing cellular and organelle contents into the extracellular medium, where they induce inflammation.
The caspase-mediated cell death pathway has been widely implicated in programmed death of HCs (Nicotera et al., 2004; Yang et al., 2004; Bohne et al., 2007; Tadros et al., 2008), with the preponderance of evidence implicating the intrinsic pathway (e.g., Tabuchi et al., 2007; Esterberg et al., 2013, 2014), but there is also evidence for extrinsic pathway involvement (Bodmer et al., 2002b), as well as the participation of necroptosis (Zheng et al., 2014). Yamashita et al. (2008) found that while TTS-inducing levels of noise up-regulated anti-apoptotic Bcl-xl in HCs, PTS-inducing levels of noise up-regulated pro-apoptotic Bak.
2.7. Inflammatory mediators
Stress signaling also regulates the expression of inflammatory mediators. Both apoptosis and ROS generation trigger inflammation. Noise exposure has been shown to up-regulate cochlear production of cytokines such as IL-6 (Fujioka et al., 2006; Wakabayashi et al., 2010) and chemotactic chemokines that attract inflammatory cells to the cochlea (Tornabene et al., 2006). Generation of these mediators can occur via activation of the nuclear factor kappa B (NF-κB) signaling cascade, leading to cytokine production (Yamamoto et al., 2009). Alternatively, noise induced HC ischemia can trigger the stabilization of cochlear HIF-1α (hypoxia inducible factor). This transcription factor in turn triggers the increased expression of pro-inflammatory TNFα and suppresses the protective factor IGF1 (Riva et al., 2007). The ability of cytokines such as TNFα to produce HC loss is well documented (e.g. Infante et al., 2012).
Another potential source of inflammatory mediators is the release of intracellular components into the extracellular environment. Such release is particularly the case with necrosis or necroptosis, both of which have been implicated in cochlear noise damage (Zheng et al., 2014). Some intracellular components comprise damage-associated molecular patterns (DAMPs), which can initiate inflammation through interaction with specific receptors. These include the Toll-like receptors (TLRs), which also mediate innate immunity to pathogens, and receptor for advanced glycation endproduct (RAGE). Binding of a variety of DAMPs to TLRs or RAGE leads to the activation and nuclear translocation of NF-κB, a transcription factor that produces the transcription of a variety of inflammatory cytokine genes. Masuda et al. (2006) found that NF-κB was activated 2–6 h after PTS-inducing noise, consistent with damage signaling. DAMP signaling can also activate the stress MAPKs JNK and p38. Vethanayagam et al. (2016) found that mice deficient in TLR4 exhibited reduced NIHL and HC damage after noise exposure. The levels of IL-6 production were also reduced within the organ of Corti, but not in the lateral wall. Since noise exposure presumably does not induce cochlear infection, this study strongly implicates inflammation induced by DAMP signaling as a contributor to NIHL. Interestingly, Duan et al. (2000) noted that preventing damage to cochlear synapses by treatment with an NMDA antagonist and a neurotrophin reduced noise-induced HC loss. At the time, the link between neural damage and HC survival was unclear (Ryan, 2000). However, it seems likely that DAMP signaling from injured cochlear synapses may contribute to HC damage.
Finally, the production of pro-apoptotic proteins can serve to amplify cellular damage, by de-stabilizing the mitochondrial membrane and increasing the release of ROS (Siddiqui et al., 2015), creating a positive feedback loop that enhances the transition of cells into programmed cell death.
3. Survival signaling and the negative regulation of HC damage
As one would expect, the cellular processes that lead to damage and death are tightly regulated. For virtually all of the processes that contribute to cell damage, there are opposing rescue processes that occur concurrently, aiming to restore physiological balance. Only when these survival-promoting mechanisms are overwhelmed that cellular damage leads to cell death.
One such balancing act is cochlear signaling via the P2X2 ATP receptor, as described above. It reduces the effects of noise just above the potentially damaging level, resulting in TTS and extending the range at which the cochlea can encode intensity without damage. This response also serves to protect the cochlea from higher levels of noise, resulting in reduced PTS (Housley et al., 2013). For example, Chinese families with a P2rx2 mutation that silences the P2X2 receptor channel experience accelerated hearing loss when exposed to elevated environmental noise levels (Yan et al., 2013).
The accumulation of ROS in cells is opposed by the action of native antioxidant enzyme systems, and HCs are no exception to this process. Glutathione, superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase, glutathione reductase and coenzyme Q10 have all been detected in cochlear tissues (Jacono et al., 1998; McFadden et al., 1999; Fetoni et al., 2013). These natural defenses must be overcome by ROS before damage is initiated. Interestingly, Sha et al. (2001) found that the antioxidant glutathione in the organ of Corti was distributed in a high-to-low gradient from apex to base. This distribution may help to explain the familiar pattern of HC damage due to several causes, with initial appearance in the base and progressive extension toward the apex. Fetoni et al. (2013) found cochlear coenzyme Q10 to be reduced for 10 days by exposure to 60 min of 100 dB SPL noise. They also noted that pre-administration of exogenous CoQ10 improved post-exposure hearing thresholds, and also reduced HC bundle disorientation and SGN loss.
Similarly, calcium homeostasis in HCs is maintained by intra- and extracellular active transport systems (Furuta et al., 1998; Kennedy, 2002) and Ca2+ buffering proteins such as oncomodulin, calbindin, calretinin, and parvalbumins, with which the HC is well supplied (Hackney et al., 2005). These resist the buildup of cytoplasmic calcium unless overwhelmed by release from mitochondria and other stores such as the endoplasmic reticulum.
There are also cell signaling pathways that oppose the JNK-MAPK and other stress pathways within the HC. Battaglia et al. (2003) identified Ras as a G-protein activated during ototoxin treatment of the organ of Corti, and therefore evaluated the effects of Ras inhibition on HC loss. High levels of the Ras inhibitor FTI-277 were protective, consistent with reducing downstream JNK signaling. However, lower levels of the inhibitor actually enhanced HC loss. This paradoxical finding is related to the differential sensitivity of Ras isoforms to FTI-277. High concentrations inhibit kRas, which leads to JNK activation, while low levels of FTI-277 inhibit another Ras isoform, hRas, which is known to activate an alternative MAPK, extracellular regulated kinase (ERK; Sebti and Der, 2003). ERK is associated with cell survival and proliferation in other tissues (Xia et al., 1995; Xue et al., 2000). This finding suggests that ototoxins activate two distinct and competing cell signaling pathways within HCs. The balance of signaling between these pathways plays a significant role in determining the cellular fate.
ERK signaling is frequently activated by growth factors, which may also explain why several growth factors have been shown to protect HCs from noise damage, including insulin-like growth factor 1 (Iwai et al., 2006), hepatocyte growth factor (Inaoka et al., 2009) and transforming growth factor beta (Murillo-Cuesta et al., 2015). Interestingly, mutations in the insulin-like growth factor 1 (igf1) gene cause syndromic hearing loss in both humans and mice (Cediel et al., 2006), further supporting the role of growth factors in protecting hearing. More direct evidence of the role of hRas and ERK in HC survival is provided by the observation that inhibition of MEK, which links hRas to ERK activation, is toxic to HCs (Chung et al., 2006).
Another well-recognized survival signaling pathway is mediated by phosphatidylinositol 3 kinase (PI3K), protein kinase c (PKC) and protein kinase B (also known as AKT), which can also be activated by growth factors. Chen et al. (2015) found that blocking PI3K/AKT signaling increased sensitivity to NIHL in animals.
4. Implications for pharmacological intervention
Since NIHL is frequently a predictable form of hearing loss, therapeutic intervention for its prevention is feasible. There are at least two potential strategies for therapeutic intervention to reduce cochlear damage. One is to inhibit processes or pathways that lead to the damage of cochlear cells. The other is to enhance processes that enhance cochlear cell survival. Both of these strategies have been attempted with varying degrees of success (see reviews in Oishi and Schacht, 2011; Wong et al., 2013; Mukherjea et al., 2015).
Antioxidants are promising therapeutic interventions, and have been investigated as prophylaxis to enhance cochlear defense against noise-induced ROS. Systemic or locally applied antioxidants have been shown to protect HCs and hearing from noise damage in animals (e.g. Bielefeld et al., 2007; Fetoni et al., 2010, 2011; Le Prell et al., 2011) and mice engineered to overexpress natural antioxidant enzymes such as superoxide dismutase are less sensitive to HC damage (Coling et al., 2003). The elevation of RNS observed in the cochlea after noise was found to be mitigated by treatment with the adenosine A1 receptor agonist adenosine amine congener (ADAC; Vlajkovic et al., 2010). Systemic treatment with ADAC up to 24 h after noise exposure resulted in lower nitro-tyrosine immunore-activity in the cochlea, reduced HC loss, and lower levels of PTS.
Translation of these promising animal antioxidant studies to prevent noise damage in humans has been difficult. For one thing, clinical studies of HC protection are difficult to perform, since stimulation with potentially damaging levels of noise typically cannot be used on human subjects. It is also impossible to examine pathology, so outcome measures are restricted to hearing thresholds. When human protection studies have been performed, the results have been mixed. A trial of the antioxidant N-acetyl cysteine (NAC) in military trainees also showed some degree of protection (Lindblad et al., 2011). In contrast, a recent trial of NAC versus placebo treatment prior to stapedectomy, where drilling noise and surgical trauma can produce SNHL, showed an equivalent level of hearing loss (~10 dB) in both groups, and thus was unable to demonstrate a distinct protective effect (Bagger-Sjöbäck et al., 2015). Kramer et al. (2006) found no effect of NAC on TTS induced by loud music in young adults.
Given the demonstrated role of the MAPK-JNK signaling pathway in acoustic trauma as described above, animal studies have investigated the use of pharmacological blockers to protect HCs and hearing from damage. Pirvola et al. (2000) used an inhibitor of mixed lineage kinases to prevent JNK activation, and observed reduced NIHL and HC loss. A peptide inhibitor of JNK signaling, D-JNKI-1, has also been shown to protect the cochlea against noise-induced HC and hearing loss when delivered directly into the scala tympani or locally to the round window membrane (Wang et al., 2003, 2007; Eshraghi et al., 2007). No clinical trials of JNK inhibition for noise protection have been reported, however.
Reduction of inflammation also has the potential to protect against NIHL. The TNFá inhibitor etanercept has been shown to reduce noise-induced threshold shifts in animals (Wang et al., 2003). Similarly, Wakabayashi et al. (2010) found that a neutralizing anti-IL-6-receptor antibody protected mice from NIHL. The anti-inflammatory steroid dexamethasone, delivered to the round window membrane, has also been shown to reduce hearing loss after noise (Harrop-Jones et al., 2016). Zhou et al. (2013) treated patients suffering recently (3 days to two weeks) from NIHL with systemic plus intratympanic dexamthasone, and compared them to patients receiving systemic steroid alone. The patients receiving intratympanic treatment showed significantly more improvement in thresholds.
Stimulation of HC survival signaling is another potential means of rescuing HCs and hearing. The animal studies demonstrating protection from NIHL by growth factors, as reviewed above, provide evidence supporting pro-survival compounds as potential interventions. No human trials of growth factors to protect against NIHL have yet been performed.
Of course, there are other issues to be considered in addition to effectiveness when considering pharmacological intervention. Many of the compounds used in animal studies to reduce HC death from noise and other causes could have undesirable effects if delivered systemically. MAPK inhibitors or stimulants, apoptosis inhibitors or growth factors all would be expected to influence cells in other body tissues if delivered systemically. This potential for side effects has led to the adoption by physicians and researchers of local delivery to the inner ear, typically via delivery to the middle ear allowing transit across the round window membrane into the perilymph. This option provides the possibility to utilize a wider variety of potential therapies for inner ear protection from noise.
Acknowledgements
Supported by a Garnett Passe and Rodney William Memorial Foundation Fellowship (AW), a National Health and Medical Research Council (NHMRC) grant APP1089838 (GDH and AFR), and US VA grants BX001205 and RX000977 (AFR).
Footnotes
Disclosure
Dr. Ryan is a co-founder of and consultant to Otonomy, Inc., which develops local delivery of therapies for middle and inner ear disorders.
References
- Agell N, Bachs O, Rocamora N, Villalonga P, 2002. Modulation of the Ras/Raf/MEK/ERK pathway by Ca(2+), and calmodulin. Cell Signal 14, 649–654. [DOI] [PubMed] [Google Scholar]
- Anttonen T, Herranen A, Virkkala J, Kirjavainen A, Elomaa P, Laos M, Liang X, Ylikoski J, Behrens A, Pirvola U, 2016. c-Jun N-terminal phosphorylation: biomarker for cellular stress rather than cell death in the injured cochlea. eNeuro 3, 0047–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagger-Sjöbäck D, Strömbäck K, Hakizimana P, Plue J, Larsson C, Hultcrantz M, Papatziamos G, Smeds H, Danckwardt-Lillieström N, Hellström S, Johansson A, Tideholm B, Fridberger A, 2015. A randomised, double blind trial of NAC for hearing protection during stapes surgery. PLoS One 10, e0115657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batandier C, Leverve X, Fontaine E, 2004. Opening of the mitochondrial permeability transition pore induces reactive oxygen species production at the level of the respiratory chain complex I. J. Biol. Chem 279, 17197–17204. [DOI] [PubMed] [Google Scholar]
- Battaglia A, Pak K, Brors D, Bodmer D, Frangos JA, Ryan AF, 2003. Involvement of Ras activation in toxic hair cell damage of the mammalian cochlea. Neuroscience 122, 1025–1035. [DOI] [PubMed] [Google Scholar]
- Bielefeld EC, 2013. Reduction in impulse noise-induced permanent threshold shift with intracochlear application of an NADPH oxidase inhibitor. J. Am. Acad. Audiol 24, 461–473. [DOI] [PubMed] [Google Scholar]
- Bielefeld EC, Kopke RD, Jackson RL, Coleman JK, Liu J, Henderson D, 2007. Noise protection with N-acetyl-l-cysteine (NAC) using a variety of noise exposures, NAC doses, and routes of administration. Acta Otolaryngol 127, 914–919. [DOI] [PubMed] [Google Scholar]
- Bodmer D, Brors D, Bodmer M, Ryan AF, 2002a. Rescue of auditory hair cells from otoxicity by CEP-11004, an inhibitor of the JNK signaling pathway. Laryngorhinootol 81, 853–856. [DOI] [PubMed] [Google Scholar]
- Bodmer D, Brors D, Pak K, Gloddek B, Ryan AF, 2002b. Rescue of auditory hair cells from aminoglycoside toxicity by C difficile toxin B, an inhibitor of small GTPases Rho/Rac/Cdc42. Hear Res 172, 81–86. [DOI] [PubMed] [Google Scholar]
- Bohne BA, Harding GW, Lee SC, 2007. Death pathways in noise-damaged outer hair cells. Hear Res 223, 61–70. [DOI] [PubMed] [Google Scholar]
- Bramble W, 2009. A Modern Approach to Noise-induced Hearing Loss from Military Operations Ministry of Defense Report, London, UK. [Google Scholar]
- Cediel R, Riquelme R, Contreras J, Díaz A, Varela-Nieto I, 2006. Sensorineural hearing loss in insulin-like growth factor I-null mice: a new model of human deafness. Eur. J. Neurosci 23, 587–590. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control, 2011. Severe hearing impairment among military veterans –-United States. Morb. Mortal. Wkly. Rep 60, 955–958. [PubMed] [Google Scholar]
- Chen J, Yuan H, Talaska AE, Hill K, Sha SH, 2015. Increased sensitivity to noise-induced hearing loss by blockade of endogenous PI3K/Akt signaling. J. Assoc. Res. Otolaryngol 16, 347–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng PW, Liu SH, Young YH, Hsu CJ, Lin-Shiau SY, 2008. Protection from noise-induced temporary threshold shift by D-methionine is associated with preservation of ATPase activities. Ear Hear 29, 65–75. [DOI] [PubMed] [Google Scholar]
- Choung YH, Taura A, Pak K, Choi SJ, Masuda M, Ryan AF, 2009. Generation of highly-reactive oxygen species is closely related to hair cell damage in rat organ of Corti treated with gentamicin. Neuroscience 161, 214–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung WH, Pak K, Lin B, Webster N, Ryan AF, 2006. A PI3K pathway mediates hair cell survival and opposes gentamicin toxicity in neonatal rat organ of Corti. J. Assoc. Res. Otolaryngol 7, 373–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coling DE, Yu KC, Somand D, Satar B, Bai U, Huang TT, Seidman MD, Epstein CJ, Mhatre AN, Lalwani AK, 2003. Effect of SOD1 overexpression on age- and noise-related hearing loss. Free Radic. Biol. Med 34, 873–880. [DOI] [PubMed] [Google Scholar]
- Dröge W, 2002. Free radicals in the physiological control of cell function. Physiol. Rev 82, 47–95. [DOI] [PubMed] [Google Scholar]
- Duan M, Agerman K, Ernfors P, Canlon B, 2000. Complementary roles of neurotrophin 3 and a N-methyl-D-aspartate antagonist in the protection of noise and aminoglycoside-induced ototoxicity. Proc. Natl. Acad. Sci. U. S. A 97, 7597–7602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eshraghi AA, Wang J, Adil E, He J, Zine A, Bublik M, Bonny C, Puel JL, Balkany TJ, Van De Water TR, 2007. Blocking c-Jun-N-terminal kinase signaling can prevent hearing loss induced by both electrode insertion trauma and neomycin ototoxicity. Hear Res 226, 168–177. [DOI] [PubMed] [Google Scholar]
- Esterberg R, Hailey DW, Coffin AB, Raible DW, Rubel EW, 2013. Disruption of intracellular calcium regulation is integral to aminoglycoside-induced hair cell death. J. Neurosci 33, 7513–7525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esterberg R, Hailey DW, Rubel EW, Raible DW, 2014. ER-mitochondrial calcium flow underlies vulnerability of mechanosensory hair cells to damage. J. Neurosci 34, 9703–9719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fausti S, Wilmington DJ, Gallun FJ, Myers PH, Henry JA, 2009. Auditory and vestibular dysfunction associated with blast-related traumatic brain injury. J. Rehabil. Res. Dev 46, 797–810. [DOI] [PubMed] [Google Scholar]
- Fetoni AR, De Bartolo P, Eramo SL, Rolesi R, Paciello F, Bergamini C, Fato R, Paludetti G, Petrosini L, Troiani D, 2013. Noise-induced hearing loss (NIHL) as a target of oxidative stress-mediated damage: cochlear and cortical responses after an increase in antioxidant defense. J. Neurosci 33, 4011–4023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fetoni AR, Eramo S, Troiani D, Paludetti G, 2011. Therapeutic window for ferulic acid protection against noise-induced hearing loss in the Guinea pig. Acta Otolaryngol 131, 419–427. [DOI] [PubMed] [Google Scholar]
- Fetoni AR, Mancuso C, Eramo SL, Ralli M, Piacentini R, Barone E, Paludetti G, Troiani D, 2010. In vivo protective effect of ferulic acid against noise-induced hearing loss in the Guinea-pig. Neurosci 169, 1575–1588. [DOI] [PubMed] [Google Scholar]
- Fridberger A, Flock A, Ulfendahl M, Flock B, 1998. Acoustic overstimulation increases outer hair cell Ca2+ concentrations and causes dynamic contractions of the hearing organ. Proc. Natl. Acad. Sci. U. S. A 95, 7127–7132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujioka M, Kanzaki S, Okano HJ, Masuda M, Ogawa K, Okano H, 2006. Proinflammatory cytokines expression in noise-induced damaged cochlea. J. Neurosci. Res 83, 575–583. [DOI] [PubMed] [Google Scholar]
- Furuta H, Luo L, Hepler K, Ryan AF, 1998. Evidence for differential regulation of calcium by outer versus inner hair cells: plasma membrane Ca-ATPase gene expression. Hear Res 123, 10–26. [DOI] [PubMed] [Google Scholar]
- Furutate S, Iwasaki S, Nishio SY, Moteki H, Usami S, 2011. Clinical profile of hearing loss in children with congenital cytomegalovirus (CMV) infection: CMV DNA diagnosis using preserved umbilical cord. Acta Otolaryngol 131, 976–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackney CM, Mahendrasingam S, Penn A, Fettiplace R, 2005. The concentrations of calcium buffering proteins in mammalian cochlear hair cells. J. Neurosci 25, 7867–7875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harr MW, Distelhorst CW, 2010. Apoptosis and autophagy: decoding calcium signals that mediate life or death. Cold Spring Harb. Perspect. Biol 2, a005579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrop-Jones A, Wang X, Fernandez R, Dellamary L, Ryan AF, LeBel C, Piu F, 2016. The sustained-exposure dexamethasone formulation OTO-104 offers effective protection against noise-induced hearing loss. Audiol. Neurotol 21, 12–21. [DOI] [PubMed] [Google Scholar]
- Henderson D, Bielefeld E, Harris KC, Hu BH, 2006. The role of oxidative stress in noise induced hearing loss. Ear Hear 27, 1–19. [DOI] [PubMed] [Google Scholar]
- Hirose K, Hockenbery DM, Rubel EW, 1997. Reactive oxygen species in chick hair cells after gentamicin exposure in vitro. Hear Res 104, 1–14. [DOI] [PubMed] [Google Scholar]
- Housley GD, Morton-Jones R, Vlajkovic SM, Telang RS, Paramananthasivam V, Tadros SF, Wong AC, Froud KE, Cederholm JM, Sivakumaran Y, Snguanwongchai P, Khakh BS, Cockayne DA, Thorne PR, Ryan AF, 2013. ATP-gated ion channels mediate adaptation to elevated sound levels. Proc. Natl. Acad. Sci. U. S. A 110, 7494–7499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu BH, Guo W, Wang PY, Henderson D, Jiang SC, 2000. Intense noise-induced apoptosis in hair cells of Guinea pig cochleae. Acta Otolaryngol 120, 19–24. [PubMed] [Google Scholar]
- Hu BH, Henderson D, Nicotera TM, 2002a. F-actin cleavage in apoptotic outer hair cells in chinchilla cochleas exposed to intense noise. Hear Res 172, 1–9. [DOI] [PubMed] [Google Scholar]
- Hu BH, Henderson D, Nicotera TM, 2002b. Involvement of apoptosis in progression of cochlear lesion following exposure to intense noise. Hear Res 166, 62–71. [DOI] [PubMed] [Google Scholar]
- Hu BH, Henderson D, Nicotera TM, 2006. Extremely rapid induction of outer hair cell apoptosis in the chinchilla cochlea following exposure to impulse noise. Hear Res 211, 16–25. [DOI] [PubMed] [Google Scholar]
- Inaoka T, Nakagawa T, Kikkawa YS, Tabata Y, Ono K, Yoshida M, Tsubouchi H, Ido A, Ito J, 2009. Local application of hepatocyte growth factor using gelatin hydrogels attenuates noise-induced hearing loss in Guinea pigs. Acta Otolaryngol 129, 453–457. [DOI] [PubMed] [Google Scholar]
- Infante EB, Channer GA, Telischi FF, Gupta C, Dinh JT, Vu L, Eshraghi AA, Van De Water TR, 2012. Mannitol protects hair cells against tumor necrosis factor α-induced loss. Otol. Neurotol 33, 1656–1663. [DOI] [PubMed] [Google Scholar]
- Iwai K, Nakagawa T, Endo T, Matsuoka Y, Kita T, Kim TS, Tabata Y, Ito J, 2006. Cochlear protection by local insulin-like growth factor-1 application using biodegradable hydrogel. Laryngoscope 116, 529–533. [DOI] [PubMed] [Google Scholar]
- Jacono AA, Hu B, Kopke RD, Henderson D, Van De Water TR, Steinman HM, 1998. Changes in cochlear antioxidant enzyme activity after sound conditioning and noise exposure in the chinchilla. Hear Res 117, 31–38. [DOI] [PubMed] [Google Scholar]
- Jaumann M, Dettling J, Gubelt M, Zimmermann U, Gerling A, Paquet-Durand F, Feil S, Wolpert S, Franz C, Varakina K, Xiong H, Brandt N, Kuhn S, Geisler HS, Rohbock K, Ruth P, Schlossmann J, Hütter J, Sandner P, Feil R, Engel J, Knipper M, Rüttiger L, 2012. cGMP-Prkg1 signaling and Pde5 inhibition shelter cochlear hair cells and hearing function. Nat. Med 18, 252–259. [DOI] [PubMed] [Google Scholar]
- Johansson M, Arlinger S, 2004. Reference data for evaluation of occupationally noise-induced hearing loss. Noise Health 6, 35–41. [PubMed] [Google Scholar]
- Keithley EM, Wang X, Barkdull GC, 2008. Tumor necrosis factor alpha can induce recruitment of inflammatory cells to the cochlea. Otol. Neurotol 29, 854–859. [DOI] [PubMed] [Google Scholar]
- Kennedy HJ, 2002. Intracellular calcium regulation in inner hair cells from neonatal mice. Cell Calcium 31, 127–136. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Lee JH, Kim SJ, Oh GS, Moon HD, Kwon KB, Park C, Park BH, Lee HK, Chung SY, Park R, So HS, 2010. Roles of NADPH oxidases in cisplatin-induced reactive oxygen species generation and ototoxicity. J. Neurosci 30, 3933–3946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer S, Dreisbach L, Lockwood J, Baldwin K, Kopke R, Scranton S, O’Leary M, 2006. Efficacy of the antioxidant N-acetylcysteine (NAC) in protecting ears exposed to loud music. J. Am. Acad. Audiol 17, 265–278. [DOI] [PubMed] [Google Scholar]
- Kujawa SG, Liberman MC, 2006. Acceleration of age-related hearing loss by early noise exposure: evidence of a misspent youth. J. Neurosci 26, 2115–2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa SG, Liberman MC, 2009. Adding insult to injury: cochlear nerve degeneration after “temporary” noise-induced hearing loss. J. Neurosci 29, 14077–14085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Prell CG, Gagnon PM, Bennett DC, Ohlemiller KK, 2011. Nutrient-enhanced diet reduces noise-induced damage to the inner ear and hearing loss. Transl. Res 158, 38–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberman MC, Beil DG, 1979. Hair cell condition and auditory nerve response in normal and noise-damaged cochleas. Acta Otolaryngol 88, 161–176. [DOI] [PubMed] [Google Scholar]
- Lindblad AC, Rosenhall U, Olofsson A, Hagerman B, 2011. The efficacy of N-acetylcysteine to protect the human cochlea from subclinical hearing loss caused by impulse noise: a controlled trial. Noise Health 13, 392–401. [DOI] [PubMed] [Google Scholar]
- Liu YM, Li XD, Guo X, Liu B, Lin AH, Rao SQ, 2010. Association between polymorphisms in SOD1 and noise-induced hearing loss in Chinese workers. Acta Otolaryngol 130, 477–486. [DOI] [PubMed] [Google Scholar]
- Maeda Y, Fukushima K, Omichi R, Kariya S, Nishizaki K, 2013. Time courses of changes in phospho- and total- MAP kinases in the cochlea after intense noise exposure. PLoS One 8, e58775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda M, Nagashima R, Kanzaki S, Fujioka M, Ogita K, Ogawa K, 2006. Nuclear factor-kappa B nuclear translocation in the cochlea of mice following acoustic overstimulation. Brain Res 1068, 237–247. [DOI] [PubMed] [Google Scholar]
- McFadden SL, Ding D, Reaume AG, Flood DG, Salvi RJ, 1999. Age-related cochlear hair cell loss is enhanced in mice lacking copper/zinc superoxide dismutase. Neurobiol. Aging 20, 1–8. [DOI] [PubMed] [Google Scholar]
- Mori Y., Watanabe M., Inui T., Nimura Y., Araki M., Miyamoto M., Takenaka H., Kubota T., 2009. Ca(2+) regulation of endocochlear potential in marginal cells. J. Physiol. Sci 59, 355–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton-Jones RT, Vlajkovic SM, Thorne PR, Cockayne DA, Ryan AF, Housley GD, 2015. Properties of ATP-gated ion channels assembled from P2X2 subunits in mouse cochlear Reissner’s membrane epithelial cells. Purinergic Signal 11, 551–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhr P, Rosenhall U, 2011. The influence of military service on auditory health and the efficacy of a Hearing Conservation Program. Noise Health 13, 320–327. [DOI] [PubMed] [Google Scholar]
- Mukherjea D, Ghosh S, Bhatta P, Sheth S, Tupal S, Borse V, Brozoski T, Sheehan KE, Rybak LP, Ramkumar V, 2015. Early investigational drugs for hearing loss. Expert Opin. Investig. Drugs 24, 201–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murai N, Kirkegaard M, Järlebark L, Risling M, Suneson A, Ulfendahl M, 2008. Activation of JNK in the inner ear following impulse noise exposure. Neurotrauma 25, 72–77. [DOI] [PubMed] [Google Scholar]
- Murillo-Cuesta S, Rodríguez-de la Rosa L, Contreras J, Celaya AM, Camarero G, Rivera T, Varela-Nieto I, 2015. Transforming growth factor β1 inhibition protects from noise-induced hearing loss. Front. Aging Neurosci 7, 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicotera T, Hu B, Henderson D, 2004. The caspase pathway in noise-induced apoptosis of the chinchilla cochlea. JARO 4, 466–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordmann AS, Bohne BA, Harding GW, 2000. Histopathological differences between temporary and permanent threshold shift. Hear Res 139, 13–30. [DOI] [PubMed] [Google Scholar]
- Ohinata Y, Miller JM, Altschuler RA, Schacht J, 2000. Intense noise induces formation of vasoactive lipid peroxidation products in the cochlea. Brain Res 878, 163–173. [DOI] [PubMed] [Google Scholar]
- Oishi N, Schacht J, 2011. Emerging treatments for noise-induced hearing loss. Expert Opin. Emerg. Drugs 16, 235–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orrenius S, Zhivotovsky B, Nicotera P, 2003. Regulation of cell death: the calcium-apoptosis link. Nat. Rev. Mol. Cell Biol 4, 552–565. [DOI] [PubMed] [Google Scholar]
- Patuzzi RB, Yates GK, Johnstone BM, 1989. Outer hair cell receptor current and sensorineural hearing loss. Hear Res 42, 47–72. [DOI] [PubMed] [Google Scholar]
- Pearson C, 2009. The Extent of Operational NIHL Ministry of Defense Report, London, UK. [Google Scholar]
- Pirvola U, Xing-Qun L, Virkkala J, Saarma M, Murakata C, 2000. Rescue of hearing, auditory hair cells, and neurons by CEP- 1347/KT7515, an inhibitor of JNK activation. J. Neurosci 20, 43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puel JL, Ruel J, Gervais d’Aldin C, Pujol R, 1998. Excitotoxicity and repair of cochlear synapses after noise-trauma induced hearing loss. Neuroreport 9, 2109–2114. [DOI] [PubMed] [Google Scholar]
- Riva C, Donadieu E, Magnan J, Lavieille JP, 2007. Age-related hearing loss in CD/1 mice is associated to ROS formation and HIF target proteins up-regulation in the cochlea. Exp. Gerontol 42, 327–336. [DOI] [PubMed] [Google Scholar]
- Roland PS, Rutka JA, 2004. Ototoxicity BC Decker, London, UK. [Google Scholar]
- Ryan A, Bone RC, 1978. NIHL and cochlear pathology in the gerbil. J. Acoust. Soc. Am 63, 1145–1151. [DOI] [PubMed] [Google Scholar]
- Ryan AF, 2000. Protection of auditory receptors and neurons: evidence for interactive damage. Proc. Natl. Acad. Sci. U. S. A 97, 6939–6940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacht J, 1986. Molecular mechanisms of drug-induced hearing loss. Hear Res 22, 297–304. [DOI] [PubMed] [Google Scholar]
- Schuknecht HF, 1993. Pathology of the Ear Lea & Febiger, Philadelphia, PA. [Google Scholar]
- Sebti SM, Der CJ, 2003. Opinion: searching for the elusive targets of farnesyl-transferase inhibitors. Nat. Rev. Cancer 3, 945–951. [DOI] [PubMed] [Google Scholar]
- Seidman MD, Quirk WS, Shirwany NA, 1999. Mechanisms of alterations in the microcirculation of the cochlea. Ann. N. Y. Acad. Sci 884, 226–232. [DOI] [PubMed] [Google Scholar]
- Sha SH, Taylor R, Forge A, Schacht J, 2001. Differential vulnerability of basal and apical hair cells is based on intrinsic susceptibility to free radicals. Hear Res 155, 1–8. [DOI] [PubMed] [Google Scholar]
- Shi X, Nuttall AL, 2003. Upregulated iNOS and oxidative damage to the cochlear stria vascularis due to noise stress. Brain Res 967, 1–10. [DOI] [PubMed] [Google Scholar]
- Siddiqui WA, Ahad A, Ahsan H, 2015. The mystery of BCL2 family: Bcl-2 proteins and apoptosis: an update. Arch. Toxicol 89, 289–317. [DOI] [PubMed] [Google Scholar]
- Slepecky N, 1986. Overview of mechanical damage to the inner ear: noise as a tool to probe cochlear function. Hear Res 22, 307–321. [DOI] [PubMed] [Google Scholar]
- Tabuchi K, Pak K, Chavez E, Ryan AF, 2007. Role of inhibitor of apoptosis protein in gentamicin-induced cochlear hair cell damage. Neuroscience 149, 213–222. [DOI] [PubMed] [Google Scholar]
- Tadros SF, D’Souza M, Zhu X, Frisina RD, 2008. Apoptosis-related genes change their expression with age and hearing loss in the mouse cochlea. Apoptosis 13, 1303–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tait SW, Green DR, 2008. Caspase-independent cell death: leaving the set without the final cut. Oncogene 27, 6452–6461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan WJ, Thorne PR, Vlajkovic SM, 2016. Characterization of cochlear inflammation in mice following acute and chronic noise exposure. Histochem. Cell Biol 146, 219–230. [DOI] [PubMed] [Google Scholar]
- Telang RS, Paramananthasivam V, Vlajkovic SM, Munoz DJ, Housley GD, Thorne PR, 2010. Reduced P2x(2) receptor-mediated regulation of endocochlear potential in the ageing mouse cochlea. Purinergic Signal 6, 263–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theodoroff SM, Lewis MS, Folmer RL, Henry JA, Carlson KF, 2015. Hearing impairment and tinnitus: prevalence, risk factors, and outcomes in US service members and veterans deployed to the Iraq and Afghanistan wars. Epidemiol. Rev 37, 71–85. [DOI] [PubMed] [Google Scholar]
- Thorne PR, Muñoz DJB, Housley GD, 2004. Purinergic modulation of cochlear partition resistance and its effect on the endocochlear potential in the Guinea-pig. JARO J. Assoc. Res. Otolaryngol 5, 58–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorne PR, Nuttall AL, Scheibe F, Miller JM, 1987. Sound-induced artifact in cochlear blood flow measurements using the laser Doppler flowmeter. Hear Res 31, 229–234. [DOI] [PubMed] [Google Scholar]
- Tornabene SV, Sato K, Pham L, Billings P, Keithley EM, 2006. Immune cell recruitment following acoustic trauma. Hear. Res 222, 115–124. [DOI] [PubMed] [Google Scholar]
- Torres M, 2003. Mitogen-activated protein kinase pathways in redox signaling. Front. Biosci 8 d369–91. [DOI] [PubMed] [Google Scholar]
- Vethanayagam RR, Yang W, Dong Y, Hu BH, 2016. Toll-like receptor 4 modulates the cochlear immune response to acoustic injury. Cell Death Dis 7, e2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlajkovic SM, Lee K-H, Wong ACY, Guo CX, Gupta R, Housley GD, Thorne PR, 2010. Adenosine amine congener mitigates noise-induced cochlear injury. Purinergic Signal 6, 273–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vona B, Haaf T, 2016. Genetics of deafness. In: Monographs in Human Genetics Karger, Basel, Switzerland. [Google Scholar]
- Wakabayashi K, Fujioka M, Kanzaki S, Okano HJ, Shibata S, Yamashita D, Masuda M, Mihara M, Ohsugi Y, Ogawa K, Okano H, 2010. Blockade of interleukin-6 signaling suppressed cochlear inflammatory response and improved hearing impairment in noise-damaged mice cochlea. Neurosci. Res 66, 345–352. [DOI] [PubMed] [Google Scholar]
- Wang J, Ruel J, Ladrech S, Bonny C, van de Water TR, Puel JL, 2007. Inhibition of the c-Jun N-terminal kinase-mediated mitochondrial cell death pathway restores auditory function in sound-exposed animals. Mol. Pharmacol 71, 654–666. [DOI] [PubMed] [Google Scholar]
- Wang X, Truong T, Billings PB, Harris JP, Keithley EM, 2003. Blockage of immune-mediated inner ear damage by etanercept. Otol. Neurotol 24, 52–57. [DOI] [PubMed] [Google Scholar]
- Webster M, Webster DB, 1981. Spiral ganglion neuron loss following organ of Corti loss: a quantitative study. Brain Res 212, 17–30. [DOI] [PubMed] [Google Scholar]
- Wells TS, Seelig AD, Ryan MA, Jones JM, Hooper TI, Jacobson IG, Boyko EJ, 2015. Hearing loss associated with US military combat deployment. Noise Health 17, 34–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong AC, Ryan AF, 2015. Mechanisms of sensorineural cell damage, death and survival in the cochlea. Front. Aging Neurosci 7, 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong AC-Y, Froud KE, Hsieh YS-Y, 2013. Noise-induced hearing loss in the 21st century: a research and translational update. World J. Otorhinolaryngol 3, 58–70. [Google Scholar]
- World Health Organization, 2015. Deafness and Hearing Loss Fact Sheet N° 300 http://www.who.int/mediacentre/factsheets/fs300/en/ (Accessed 26 June 2016).
- Wortzel I, Seger R, 2011. The ERK cascade: distinct functions within various subcellular organelles. Genes Cancer 2, 195–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Z, Dickens M, Raingeaud J, Davis RJ, Greenberg ME, 1995. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science 270, 1326–1331. [DOI] [PubMed] [Google Scholar]
- Xue L, Murray JH, Tolkovsky AM, 2000. The Ras/phosphatidylinositol 3-kinase and Ras/ERK pathways function as independent survival modules each of which inhibits a distinct apoptotic signaling pathway in sympathetic neurons. J. Biol. Chem 275, 8817–8824. [DOI] [PubMed] [Google Scholar]
- Yakovlev AG, Faden AI, 2001. Caspase-dependent apoptotic pathways in CNS injury. Mol. Neurobiol A24, 131–144. [DOI] [PubMed] [Google Scholar]
- Yamamoto H, Omelchenko I, Shi X, Nuttall AL, 2009. The influence of NF-κB signal-transduction pathways on the murine inner ear by acoustic overstimulation. J. Neurosci. Res 87, 1832–1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamane H, Nakai Y, Takayama M, Iguchi H, Nakagawa T, Kojima A, 1995. Appearance of free radicals in the Guinea pig inner ear after noise-induced acoustic trauma. Eur. Arch. Otorhinolaryngol 252, 504–508. [DOI] [PubMed] [Google Scholar]
- Yamashita D, Jiang HY, Schacht J, Miller JM, 2004. Delayed production of free radicals following noise exposure. Brain Res 1019, 201–209. [DOI] [PubMed] [Google Scholar]
- Yamashita D, Minami SB, Kanzaki S, Ogawa K, Miller JM, 2008. Bcl-2 genes regulate noise-induced hearing loss. J. Neurosci. Res 86, 920–928. [DOI] [PubMed] [Google Scholar]
- Yan D, Zhu Y, Walsh T, Xie D, Yuan H, Sirmaci A, Fujikawa T, Wong AC, Loh TL, Du L, Grati M, Vlajkovic SM, Blanton S, Ryan AF, Chen ZY, Thorne PR, Kachar B, Tekin M, Zhao HB, Housley GD, King MC, Liu XZ, 2013. Mutation of the ATP-gated P2X(2) receptor leads to progressive hearing loss and increased susceptibility to noise. Proc. Natl. Acad. Sci. U. S. A 110, 2228–2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang WP, Henderson D, Hu BH, Nicotera TM, 2004. Quantitative analysis of apoptotic and necrotic outer hair cells after exposure to different levels of continuous noise. Hear Res 196, 69–76. [DOI] [PubMed] [Google Scholar]
- Yong JS, Wang DY, 2015. Impact of noise on hearing in the military. Mil. Med. Res 2, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Liu H, McGee J, Walsh EJ, Soukup GA, He DZ, 2013. Identifying microRNAs involved in degeneration of the organ of corti during age-related hearing loss. PLoS One 8 e62786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng HW, Chen J, Sha SH, 2014. Receptor-interacting protein kinases modulate noise-induced sensory hair cell death. Cell Death Dis 5, e1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Zheng G, Zheng H, Zhou R, Zhu X, Zhang Q, 2013. Primary observation of early transtympanic steroid injection in patients with delayed treatment of noise-induced hearing loss. Audiol. Neurootol 18, 89–94. [DOI] [PubMed] [Google Scholar]