Abstract
The role of cytokines in the systemic inflammatory response (SIR) is now well established. This is in keeping with the role of the SIR in tumorigenesis, malignant spread, and the development of cachexia. However, the relationship between performance status/systemic inflammation frameworks and cytokine profiles is not clear. The aim of the present study was to examine the relationship between the Eastern cooperative oncology group performance status/modified Glasgow prognostic score (ECOG-PS/mGPS) and cooperative oncology group performance status/neutrophil platelet score (ECOG-PS/NPS) frameworks and their cytokine profile in patients with advanced cancer.
This was a retrospective interrogation of data already collected as part of a recent clinical trial (NCT00676936). The relationship between the independent variables (ECOG-PS/mGPS and ECOG-PS/NPS frameworks), and dependent variables (cytokine levels) was examined using independent Mann–Whitney U and Kruskal Wallis tests where appropriate.
Of the 40 patients included in final analysis the majority had evidence of an SIR assessed by mGPS (78%) or NPS (53%). All patients died on follow-up and the median survival was 91 days (4–933 days). With increasing ECOG-PS there was a higher median value of Interleukin 6 (IL-6, P = .016) and C-reactive protein (CRP, P < .01) and lower albumin (P < .01) and poorer survival (P < .001). With increasing mGPS there was a higher median value of IL-6 (P = .016), Macrophage migration inhibitory factor (MIF, P = .010), erythrocyte sedimentation rate (ESR, P < .01) and poorer survival (P < .01). With increasing NPS there was a higher median value of TGF-β (P < .001) and C-reactive protein (P = .020) and poor survival (P = .001). When those patients with an ECOG-PS 0/1 and mGPS0 were compared with those patients with an ECOG-PS 2 and mGPS2 there was a higher median value of IL-6 (P = .017) and poorer survival (P < .001). When those patients with an ECOG-PS 0/1 and NPS0 were compared with those patients with an ECOG-PS 2 and NPS1/2 there was a higher median value of IL-6 (P = .002), TGF-β (P < .001) and poorer survival (P < .01).
In patients with advanced cancer IL-6 was associated with the ECOG-PS/mGPS and ECOG-PS/NPS frameworks and survival in patients with advanced cancer. Therefore, the present work provides supporting evidence that agents targeting IL-6 are worthy of further exploration.
Keywords: advanced inoperable cancer, cancer specific survival, Glasgow prognostic score, inflammatory cytokines, interleukin-6, overall survival, systemic inflammation
1. Introduction
Cancer is responsible for 7.6 million deaths per annum globally.[1] Therefore, while a curative intent is the aim of any surgical or oncological treatment, many patients are likely to develop disseminated disease requiring systemic anti-cancer therapy with the aim of improving quality of life, increasing survival or both. In this setting, measures of Performance Status (PS), such as the Eastern cooperative oncology group (ECOG) criteria guide treatment as this has been consistently shown to predict survival.[2]
Clinical biomarkers of the systemic inflammatory response (SIR) (C-reactive Protein [CRP], albumin, neutrophils, and platelets) have also become established as having prognostic accuracy both in operable and in advanced cancer.[3,4] For example, the modified Glasgow Prognostic Score (mGPS, combining CRP, and Albumin) and the neutrophil platelet score (NPS) have been extensively validated as having prognostic value.[3–6] Furthermore, the mGPS has been combined with ECOG performance status in patients with advanced cancer to reliably stratify quality of life and survival.[2,7,8] These observations consolidate the role of systemic inflammation as the “seventh hallmark of cancer” and the “tip of the iceberg” in terms of cancer biology and treatment.[9–11] Indeed, the activation of the systemic inflammatory response has been strongly implicated in the aggressiveness of the disease and development of cachexia.[11–13]
Beneath the “tip of the iceberg”, cytokine activity plays an important part in the development of a systemic inflammatory response and symptoms of advanced disease.[14] In patients with advanced cancer, pro-inflammatory cytokines become predominant leading to an up-regulation of interleukin (IL) -1, tumour necrosis factor α (TNF-α), IL-6, IL-8, IL-10, IL-18, transforming growth factor β (TGF-β) and macrophage migration inhibitory factor (MIF). However, these cytokines have not been routinely measured in patients with advanced cancer due to the lack of international standardisation of analysis and validation of prognostic value. In contrast, routine measures of the systemic inflammatory response, such as the acute phase proteins CRP and albumin, are well standardised internationally and, combined in the modified Glasgow prognostic score (mGPS), have validated prognostic value.[3,4] Alternatively, neutrophil counts have been combined with various other white cell counts, such as lymphocytes and platelets to improve the prediction of survival.[3,4,15]
To date, the relationship of between cytokines to the ECOG-PS/mGPS and ECOG-PS/NPS frameworks has not been delineated. Understanding which cytokines are related to performance status and systemic inflammation frameworks and survival may inform potential treatment in patients with advanced cancer. It is against this backdrop that we present this retrospective interrogation of the results of a recent “Corticosteroids for Cancer Pain” trial [4,5]. This trial examined the relationship between inflammatory biomarkers and symptoms as well as the effectiveness of steroidal treatment in improving symptoms in patients with advanced cancer [4]. The present study specifically focuses on the relationship between ECOG-PS/mGPS framework and cytokine profiles in patients with advanced cancer.
Therefore, the primary aim of this exploratory study was to examine the relationship between ECOG-PS/mGPS and ECOG-PS/NPS frameworks, cytokine profiles and survival in patients with advanced cancer.
2. Patients and methods
This was a retrospective analysis of data already collected as part of a randomised double-blind placebo control trial examining the analgesic effects of corticosteroids in patients with advanced cancer taking opioids.[11,16] The date of inclusion corresponds to the date of diagnosis with advanced disease. Eligible patients met the following criteria: >18 years of age, a diagnosis of advanced cancer where curative treatment was not possible, taking opioids for moderate or severe cancer pain; pain level of 4 (on a 0 ± 10 Numerical Rating Scale (NRS)) at inclusion; expected survival >4 weeks. Exclusion criteria included diabetes mellitus, peptic ulcer disease, and concurrent use of non-steroidal anti-inflammatory drugs (NSAIDs).[11,16] As part of this trial the following inflammatory biomarkers were collected at trial baseline: CRP, albumin, neutrophils, platelets, erythrocyte sedimentation rate (ESR), IL-1β, IL-1ra, TNF-α, IL-2, IL-4, IL-6, IL-8, IL-10, IL-12(p70), IL-18, interferon-γ, TGF-β1, MIF, macrophage inflammatory protein-1α (MIP-1α), monocyte chemoattractant protein-1 (MCP-1) and soluble tumour necrosis factor receptor-1 (sTNF-r1). sTNF-r1 was measured as it reflects TNF-α-activity, since TNF-α is among the most unstable cytokines. The analytical methods are published previously.[11,16] The cytokines were chosen on the basis of previous research on cancer related inflammation.[17–19] The sera underwent one freeze dry cycle.
Overall survival (OS) was measured until the date of death from any cause. Ethical approval for the original study was given by the Regional Committee for Medical Research Ethics Central Norway (4.2007.846) and the Norwegian Directorate of Health, and this included further analysis of biobanked data; Clinical trial information NCT00676936, EudraCT No 2007–005617-19. Procedures were conducted in accordance with the Declaration of Helsinki, as revised in 1983.
2.1. Statistical analysis
Data was presented as medians, ranges, frequencies, and percentages. The mGPS and the neutrophil platelet score (NPS) were calculated according to methods previously described.[6,20] In the present study no formal power calculation was carried out since, to our knowledge, the relationship between ECOG-PS/ systemic inflammation frameworks and cytokine profiles have not been previously examined. The relationship between the independent variables (ECOG-PS/mGPS and ECOG-PS/NPS frameworks), and dependent variables (cytokine levels) was examined using Independent Mann–Whitney U and Kruskal Wallis tests where appropriate. The IL-1ra and IL-6 concentrations below the Lower limit of quantification (LLOQ) are given as ≤21.7 ng/L and ≤2.33 ng/L, respectively. IL-1ra and IL-6 were analysed as continuous and dichotomized variables (IL-1ra: ≤170 ng/L[21] and IL-6: ≤10ng/L[22]) using the commonest thresholds reported in the literature. Correlation between different cytokines and markers of the systemic inflammatory response was assessed using Spearman's rho testing. Given the explorative nature of this study, a significance level of P < .05 was considered significant. The time between the date of inclusion and the date of death of any cause was used to define overall survival (OS). Survival data were analysed using univariate Cox regression analysis. All statistical analysis was performed using SPSS version 22.0 (IBM Corp, Armonk, NY).
3. Results
The clinicopathological characteristics of patients are shown in Table 1. Of the 49 patients previously reported,[11] 9 patients had incomplete data and therefore 40 patients were included in the present analysis. The majority of patients were less than 65 years of age (58%), normal or underweight (73%), had good ECOG-PS (53%) and were not being treated with ongoing oncological treatment (73%). The majority of primary cancers were GI or Lung in origin. Metastatic disease was present in 98% of patients with the most common sites being the liver and bone. The majority of patients had evidence of a systemic inflammatory response whether assessed by the mGPS (78%) or NPS (53%). All patients died on follow-up and the median survival was 91 days (4–933 days).
Table 1.
Clinicopathological characteristics of patients within the “Corticosteroids and Cancer Pain” trial analysed as part of this study.
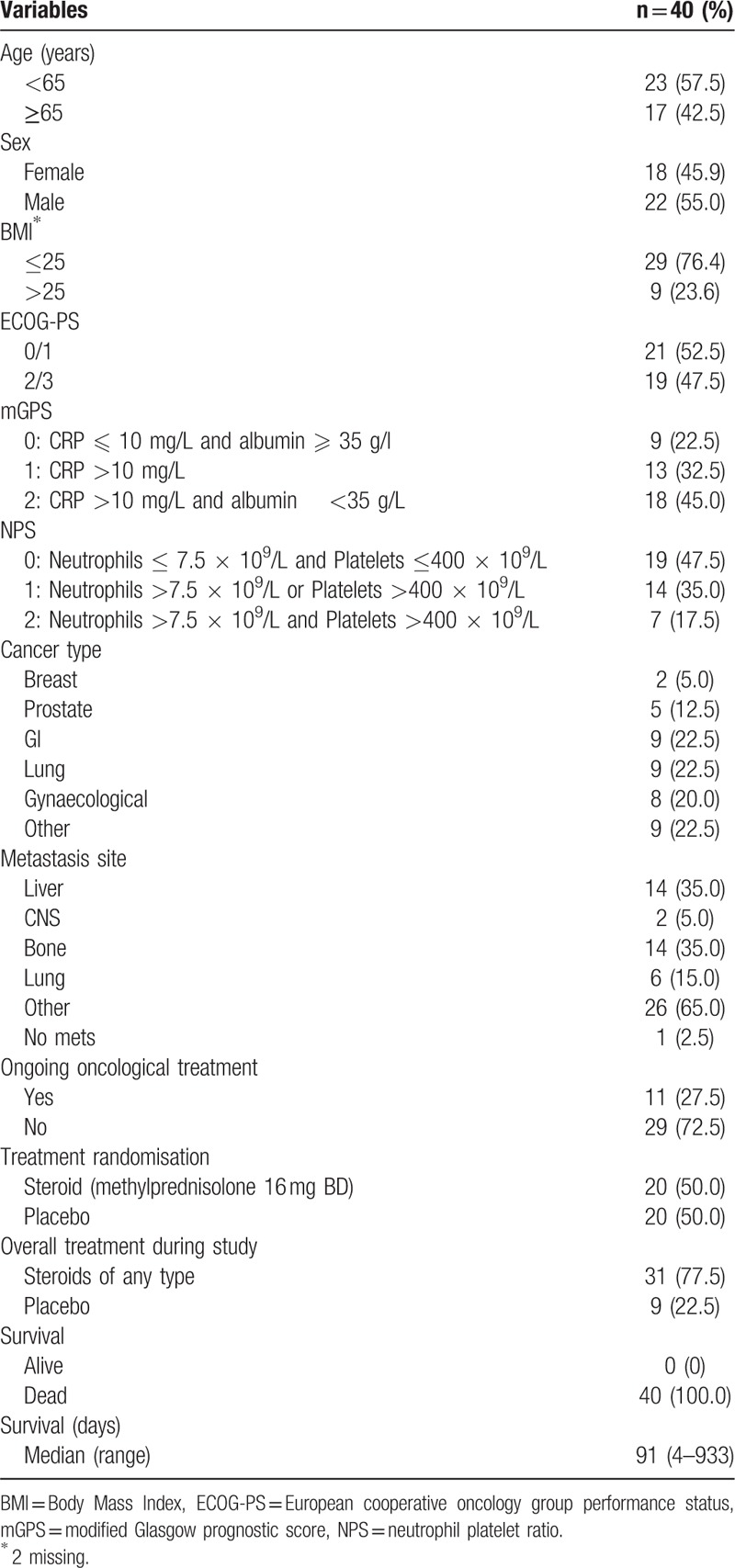
The relationship between cytokine profiles and ECOG-PS, mGPS and NPS are shown in Table 2a, b, and c, respectively. With increasing ECOG-PS (Table 2a, poorer performance status) there was a higher median value of IL-6 (P < .05), ESR (P = .01), CRP (P < .01), albumin (P < .01), neutrophil count (P < .05) and poorer survival (P < .001). With increasing mGPS (Table 2b, increasing systemic inflammation) there was a higher median value of IL-6 (P < .05), MIF (P = .01), ESR (P < .01) and poorer survival (P < .01). With increasing NPS 2 (Table 2c, increasing systemic inflammation) there was a higher median value of TGF-β (P < .001) and ESR (P < .05).
Table 2.
a–c: The relationship between ECOG-PS (2a), mGPS (2b), and NPS (2c) and the cytokine profile.
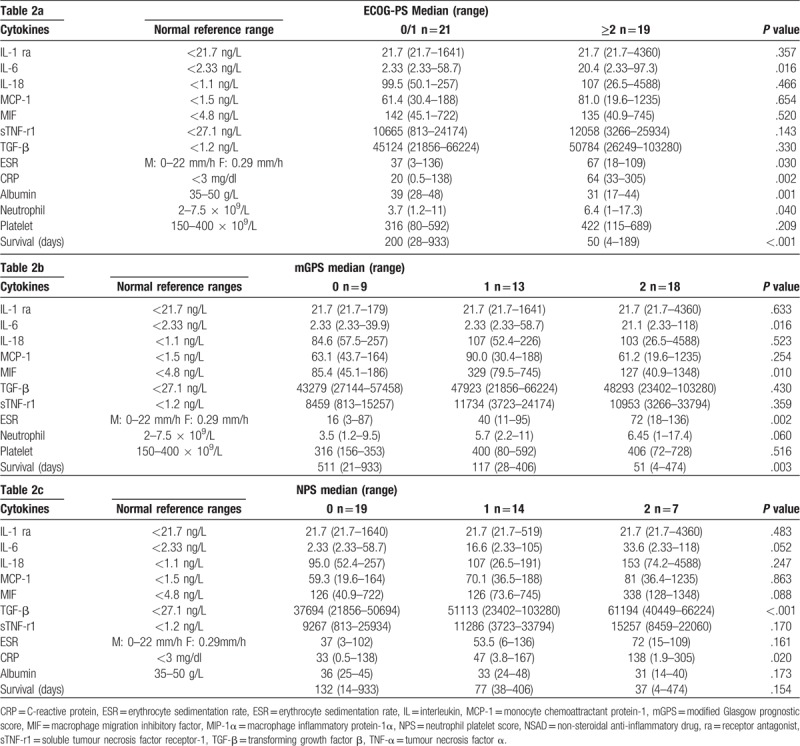
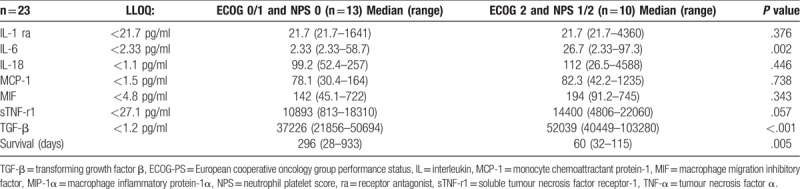
The relationship between ECOG-PS and mGPS framework and the cytokine profile is shown in Table 3. When those patients with an ECOG-PS 0/1 and mGPS0 were compared with those patients with an ECOG-PS 2 and mGPS2 there was a higher median value of IL-6 (P = .017) and poorer survival (P < .001). The relationship between ECOG-PS and NPS framework and the cytokine profile is shown in Table 4. When those patients with an ECOG-PS 0/1 and NPS0 were compared with those patients with an ECOG-PS 2 and NPS1/2 there was a higher median value of IL-6 (P = .002) and TGF-β (P < .001) and poorer survival (P < .01). The majority of IL-1ra and IL-6 and concentrations were below the limit of detection. There was an increase in IL-6 concentrations between ECOG-PS 0/1 (2.33 ng/L) and ECOG_PS 2 (20.4 ng/L). In addition, there was an increase in median IL-6 concentrations between mGPS 0 (2.33 ng/L) and mGPS 2 (21.1 ng/L). There was also a more progressive increase in IL-6 concentrations between NPS 1 (2.33 ng/L), NPS 2 (16.6 ng/L) and NPS 3 (33.6 ng/L).
Table 3.
The relationship between combined ECOG-PS 0/1 and mGPS 0 and combined ECOG-PS 2 and mGPS 2 and cytokine levels.
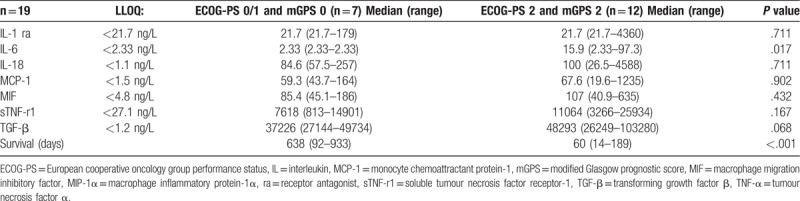
Table 4.
The relationship between combined ECOG 0/1 and NPS 0 and combined ECOG 2 and NPS 1/2 and cytokine levels.
There was no significant association between either cancer type or treatment and IL-6 concentrations (P = .939 and P = .171, respectively).
4. Discussion
The results of the present study show, for the first time, that only IL-6 was consistently associated with ECOG-PS/mGPS and ECOG-PS/NPS frameworks in patients with advanced cancer. These prognostic stratification frameworks identify IL-6 as a key pro-inflammatory cytokine in the functional decline and elaboration of the SIR, important determinants of survival in patients with cancer and therefore a potential therapeutic target.
Although the present study was carried out in a relatively small number of patients it does provide pilot data within the context of an established framework (ECOG-PS/mGPS) that is known to effectively stratify quality of life and survival in patients with advanced cancer. For example, the mGPS enables reliable comparison between studies of different tumour types and stages of disease. Indeed, Kantola, and colleagues in primary operable CRC (n = 148) reported that the mGPS was significantly associated with IL1-ra and IL-6 thus confirming the validity of the present results.[23,24]
The present results are consistent with a recent systematic review by Saligan and co-workers including 6266 patients that showed serum IL-6 levels were consistently associated with increased fatigue and poorer performance.[25] In addition, Lippitz and co-workers, including 11,583 patients reported that serum IL-6 levels were consistently associated with survival in 23 types of cancer.[22] The average IL-6 threshold was approximately 10 ng/l and this is above the top of the recognised reference range (in the present analysis < 2.33 ng/l). In the present study when this threshold was applied IL-6 was significantly associated with ECOG-PS, mGPS, and survival. Therefore, the results of the present study are consistent with the literature and define IL-6 as a tumour type independent factor for the progressive functional decline (ECOG-PS), elaboration of the systemic inflammatory response (mGPS) and poorer survival in patients with advanced cancer.
It has long been recognised that interleukin-6 was associated with pain,[26] weight loss,[27] and inflammatory responses in patients with cancer.[28,29] However, it is only in recent years that the systemic inflammatory response, in particular as measured by the mGPS, has become central to the symptoms associated with advanced cancer[2,13] and there has been a repertoire of agents targeting IL-6, directly or indirectly, to clinically test these associations in a robust manner.
Therapeutic strategies have been suggested to target IL-6 directly, upstream or downstream. Currently inhibitors that target IL-6 directly, IL-6R or selectively block IL-6 trans-signalling are in clinical trial development and may be useful in managing immune mediated adverse events associated with PD-1 inhibitors.[23] Furthermore, Clazakizumab, which targets IL-6, has also been examined in phase II trials and showed attenuation of muscle loss and improvements in anaemia.[30]
In terms of upstream signalling IL-1 plays a significant role.[31] Both IL-1α and IL-1β have pro-inflammatory properties through the IL-1R1 receptor. IL-1α shows a strong association with monocytes and lymphocytes while IL-1β shows as similar association with neutrophils.[32] These results are consistent with the recent report that targeting IL-1α was associated with beneficial effects on muscle mass and quality of life.[33] In the present study it was of interest that of the cytokines measured only IL-1ra was significantly associated with IL-6 (rs 0.537, P < .001), CRP (rs 0.320, P < .05) and neutrophil count (rs 0.353, P < .05) (results not tabulated). There are also a number of approaches to down regulate IL-1 signalling that look promising in patients with advanced cancer and worthy of clinical investigation.[34]
In terms of downstream signalling IL-6 activates the JAK/ STAT3 pathway.[35] Ruxolitinib is a potent JAK1/JAK2 inhibitor which is effective in treating patients with myelofibrosis, myeloproliferative neoplasm associated with cachexia, weight loss, elevated proinflammatory cytokines, and dysregulated JAK/STAT signalling.[36] Ruxolitinib acts by reducing the proinflammatory cytokine levels which leads to improved myelofibrosis-related symptoms, weight gain, and improved overall survival (OS).[36]
Therefore, although there are agents that can target IL-6 upstream and downstream, such complexity, many studies carried out have been pre-clinical and the data makes it difficult to predict the likely benefits of any particular agent in patients with advanced cancer. In this context, the results of the present study would suggest that such agents may be useful in patients with poor performance status and elevated systemic inflammatory response, that is, ECOG-PS 2 and mGPS 2 for moderation of symptoms.
Although assays have been available for the measurement of IL-6 in the plasma for approximately 30 years there remain a number of obstacles to be overcome before IL-6 will become a routinely available in clinical practice. Until such time the ECOG-PS/mGPS framework will continue to offer reliable risk stratification for patients with advanced cancer. Indeed, given the extensively validated prognostic value of the ECOG-PS/ mGPS framework, it is clear that of the cytokines measured, IL-6 may represent a potentially useful therapeutic target to improve patient status in the context of this framework. Furthermore, as clinical trials continue IL-1 and IL-6 or their surrogate markers could be used to assess the effectiveness of an intervention to dampen cancer associated inflammation. This is particularly true for CRP as a singular marker and the mGPS as a combined prognostic score.[3,4]
The present study had some limitations. There were relatively small numbers of patient observations in some of the subgroup analysis. This may explain why markers previously associated with cancer inflammation in the literature, such as TGF-β and MIF approached but did not reach significance. A large number of cytokines were analysed as part of this study however these were all previously associated with cancer related inflammation. Given the exploratory nature of this study and the small number of patients, no correction for multiple testing was performed. Also, the present results are a retrospective analysis of trial data obtained from a study examining the relationship between cytokine concentrations and symptoms in patients with advanced cancer.[11] As a result, prospective confirmation of the results obtained and measurement of key cytokines would be important in future studies.
In summary, the present work provides supporting evidence that agents targetting IL-6 are worthy of further exploration. However, it will be important to include some stratification for the systemic inflammatory response, for example, the ECOG-PS/mGPS framework, into trial designs, to enable the effect of these agents to be optimally assessed. Such an approach has been advocated recently[37] and demonstrated as being efficacious in similar settings.[36]
Author contributions
Conceptualization: Ross D. Dolan, Barry J.A. Laird, Pål Klepstad, Ørnulf Paulsen, Donald C. McMillan.
Data curation: Barry J.A. Laird, Pål Klepstad, Ørnulf Paulsen.
Formal analysis: Ross D. Dolan, Barry J.A. Laird, Donald C. McMillan.
Funding acquisition: Ross D. Dolan.
Methodology: Ross D. Dolan.
Project administration: Ross D. Dolan, Stein Kaasa.
Resources: Ross D. Dolan.
Supervision: Stein Kaasa, Paul G. Horgan.
Writing – original draft: Ross D. Dolan, Donald C. McMillan.
Writing – review & editing: Ross D. Dolan, Barry J.A. Laird, Pål Klepstad, Stein Kaasa, Paul G. Horgan, Ørnulf Paulsen, Donald C. McMillan.
Ross D. Dolan orcid: 0000-0002-5272-5906.
Footnotes
Abbreviations: TGF-β = transforming growth factor β, BMI = Body Mass Index, CRP = C-reactive protein, ECOG-PS = Eastern cooperative oncological group performance status, ESR = erythrocyte sedimentation rate, IL = interleukin, LLOQ = lower limit of quantification, MCP-1 = monocyte chemoattractant protein-1, mGPS = modified Glasgow prognostic score, MIF = macrophage migration inhibitory factor, MIP-1α = macrophage inflammatory protein-1α, NPS = neutrophil platelet score, NSAD = non-steroidal anti-inflammatory drug, OS = overall survival, ra = receptor antagonist, SIR = systemic inflammatory response, sTNF-r1 = soluble tumour necrosis factor receptor-1, TNF-α = tumour necrosis factor α.
How to cite this article: Dolan RD, Laird BJ, Klepstad P, Kaasa S, Horgan PG, Paulsen Ø, McMillan DC. An exploratory study examining the relationship between performance status and systemic inflammation frameworks and cytokine profiles in patients with advanced cancer. Medicine 2019;98:37(e17019).
Ørnulf Paulsen and Donald C. McMillan are joint senior authors.
University of Glasgow Clinical Research Fellow Fund.
The authors have no conflicts of interests to disclose.
References
- [1].WHO. World Health Organization Cancer Fact Sheet. WHO Media Centre Cancer Fact Sheet Web site. Available at: http://www.who.int/mediacentre/factsheets/fs297/en/ Published 2018. Updated 01/02/2018. Accessed March 20, 2018. [Google Scholar]
- [2].Laird BJ, Fallon M, Hjermstad MJ, et al. Quality of life in patients with advanced cancer: differential association with performance status and systemic inflammatory response. J Clin Oncol 2016;34:2769–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Dolan RD, Lim J, McSorley ST, et al. The role of the systemic inflammatory response in predicting outcomes in patients with operable cancer: systematic review and meta-analysis. Sci Rep 2017;7:16717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Dolan RD, McSorley ST, Horgan PG, et al. The role of the systemic inflammatory response in predicting outcomes in patients with advanced inoperable cancer: systematic review and meta-analysis. Crit Rev Oncol Hematol 2017;116:134–46. [DOI] [PubMed] [Google Scholar]
- [5].Dupre A, Malik HZ. Inflammation and cancer: what a surgical oncologist should know. Eur J Surg Oncol 2018;44:566–70. [DOI] [PubMed] [Google Scholar]
- [6].Watt DG, Proctor MJ, Park JH, et al. The neutrophil-platelet score (NPS) predicts survival in primary operable colorectal cancer and a variety of common cancers. PloS One 2015;10:e0142159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Laird BJ, Kaasa S, McMillan DC, et al. Prognostic factors in patients with advanced cancer: a comparison of clinicopathological factors and the development of an inflammation-based prognostic system. Clin Cancer Res 2013;19:5456–64. [DOI] [PubMed] [Google Scholar]
- [8].Simmons C, McMillan DC, Tuck S, et al. How long have I got?”– A prospective cohort study comparing validated prognostic factors for use in patients with advanced cancer. The Oncol 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].McAllister SS, Weinberg RA. The tumour-induced systemic environment as a critical regulator of cancer progression and metastasis. Nat Cell Biol 2014;16:717–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Mantovani A, Romero P, Palucka AK, et al. Tumour immunity: effector response to tumour and role of the microenvironment. Lancet 2008;371:771–83. [DOI] [PubMed] [Google Scholar]
- [11].Paulsen O, Laird B, Aass N, et al. The relationship between pro-inflammatory cytokines and pain, appetite and fatigue in patients with advanced cancer. PloS One 2017;12:e0177620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Park JH, Watt DG, Roxburgh CS, et al. Colorectal cancer, systemic inflammation, and outcome: staging the tumor and staging the host. Ann Surg 2016;263:326–36. [DOI] [PubMed] [Google Scholar]
- [13].Arends J, Bachmann P, Baracos V, et al. ESPEN guidelines on nutrition in cancer patients. Clin Nutr 2017;36:11–48. [DOI] [PubMed] [Google Scholar]
- [14].Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med 1999;340:448–54. [DOI] [PubMed] [Google Scholar]
- [15].Dolan RD, McSorley ST, Park JH, et al. The prognostic value of systemic inflammation in patients undergoing surgery for colon cancer: comparison of composite ratios and cumulative scores. Br J Cancer 2018;119:40–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Paulsen O, Klepstad P, Rosland JH, et al. Efficacy of methylprednisolone on pain, fatigue, and appetite loss in patients with advanced cancer using opioids: a randomized, placebo-controlled, double-blind trial, Journal of clinical oncology: official. J Am Soc Clin Oncol 2014;32:3221–8. [DOI] [PubMed] [Google Scholar]
- [17].Makimura C, Arao T, Matsuoka H, et al. Prospective study evaluating the plasma concentrations of twenty-six cytokines and response to morphine treatment in cancer patients. Anticancer Res 2011;31:4561–8. [PubMed] [Google Scholar]
- [18].Seruga B, Zhang H, Bernstein LJ, et al. Cytokines and their relationship to the symptoms and outcome of cancer. Nat Rev Cancer 2008;8:887–99. [DOI] [PubMed] [Google Scholar]
- [19].Wang XS, Shi Q, Williams LA, et al. Inflammatory cytokines are associated with the development of symptom burden in patients with NSCLC undergoing concurrent chemoradiation therapy. Brain Behav Immun 2010;24:968–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].McMillan DC. The systemic inflammation-based Glasgow Prognostic Score: a decade of experience in patients with cancer. Cancer Treat Rev 2013;39:534–40. [DOI] [PubMed] [Google Scholar]
- [21].Kleiner G, Marcuzzi A, Zanin V, et al. Cytokine levels in the serum of healthy subjects. Mediators Inflamm 2013;2013:434010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Lippitz BE, Harris RA. Cytokine patterns in cancer patients: a review of the correlation between interleukin 6 and prognosis. Oncoimmunology 2016;5:e1093722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Kantola T, Klintrup K, Vayrynen JP, et al. Stage-dependent alterations of the serum cytokine pattern in colorectal carcinoma. Br J Cancer 2012;107:1729–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Guthrie G, McMillan DC. Comment on 'stage-dependent alterations of the serum cytokine pattern in colorectal carcinoma’. Br J Cancer 2013;108:1915–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Saligan LN, Kim HS. A systematic review of the association between immunogenomic markers and cancer-related fatigue. Brain Behav Immun 2012;26:830–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Laird BJ, Scott AC, Colvin LA, et al. Cancer pain and its relationship to systemic inflammation: an exploratory study. Pain 2011;152:460–3. [DOI] [PubMed] [Google Scholar]
- [27].Scott HR, McMillan DC, Crilly A, et al. The relationship between weight loss and interleukin 6 in non-small-cell lung cancer. Br J Cancer 1996;73:1560–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Fearon KC, McMillan DC, Preston T, et al. Elevated circulating interleukin-6 is associated with an acute-phase response but reduced fixed hepatic protein synthesis in patients with cancer. Ann Surg 1991;213:26–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Guthrie GJ, Roxburgh CS, Horgan PG, et al. Does interleukin-6 link explain the link between tumour necrosis, local and systemic inflammatory responses and outcome in patients with colorectal cancer? Cancer Treat Rev 2013;39:89–96. [DOI] [PubMed] [Google Scholar]
- [30].Bayliss TJ, Smith JT, Schuster M, et al. A humanized anti-IL-6 antibody (ALD518) in non-small cell lung cancer. Expert Opin Biol Ther 2011;11:1663–8. [DOI] [PubMed] [Google Scholar]
- [31].Dinarello CA. Overview of the IL-1 family in innate inflammation and acquired immunity. Immunol Rev 2018;281:8–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].McDonald JJ, McMillan DC, Laird BJA. Targeting IL-1alpha in cancer cachexia: a narrative review. Curr Opin Support Palliat Care 2018;12:453–9. [DOI] [PubMed] [Google Scholar]
- [33].Hickish T, Andre T, Wyrwicz L, et al. MABp1 as a novel antibody treatment for advanced colorectal cancer: a randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol 2017;18:192–201. [DOI] [PubMed] [Google Scholar]
- [34].Mantovani A, Barajon I, Garlanda C. IL-1 and IL-1 regulatory pathways in cancer progression and therapy. Immunol Rev 2018;281:57–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Johnson DE, O’Keefe RA, Grandis JR. Targeting the IL-6/JAK/STAT3 signalling axis in cancer. Nat Rev Clin Oncol 2018;15:234–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Hurwitz HI, Uppal N, Wagner SA, et al. Randomized, double-blind, phase II study of ruxolitinib or placebo in combination with capecitabine in patients with metastatic pancreatic cancer for whom therapy with gemcitabine has failed. J Clin Oncol 2015;33:4039–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Dolan RD, Laird BJA, Horgan PG, et al. The prognostic value of the systemic inflammatory response in randomised clinical trials in cancer: a systematic review. Crit Rev Oncol Hematol 2018;132:130–7. [DOI] [PubMed] [Google Scholar]


