Abstract
Human papillomavirus (HPV) infection is a crucial health problem and caused substantial malignancy diseases among female worldwide. We aim to investigate the distribution of HPV subtype and the status of cervical cancer and precancerous lesions caused by HPV infection in North China Plain population. A total of 61,870 samples of outpatients and inpatients from January 2015 to May 2017 at the Affiliated Hospital of Jining Medical University were collected. All of the samples were tested by rapid flow-through hybridization HPV genotyping. Approximately 17,280 of the cases tested positive for HPV, indicating an infection rate of 27.9%. Approximately 7009 cases were compared to the results of cytological diagnosis. The top five HPV genotypes were HPV-16 (4.5%), HPV-52 (2.9%), HPV-58 (2.8%), HPV-53 (1.9%), and HPV-81 (1.9%). The youngest age group (age < 20 years) showed the highest infection rate (59.9%), and then decreased with age. As the degree of cervical lesions worsened gradually, the rate of high-risk HPV infection increased, such as 24.3% (322/1324) in the Cervicitis, 31.30% (560/1785) in the CINI, 54.1% (568/1050) in the CINII, 80.1% (693/865) in the CIN III, and 99.5% (428/430) in the cervical cancer group. These findings were significantly different from the 9.7% (155/1555) observed in the normal medical examination group (P < .05). This is the first study to demonstrate the characteristics of HPV and the association with cervical lesions in North China Plain population.
Keywords: cervical lesions, correlation analysis, high-risk HPV genotypes, HPV
1. Introduction
Recent analyses of the global burden of cancer among women have indicated that cervical cancer ranks second to breast cancer. The number of new cervical cancer cases continuously increases, although this tumor is one of the most preventable malignancies of all relevant human cancers. The genesis of cervical cancer depends essentially on an infection of the uterine cervix with human papillomavirus (HPV) that needs to persist for many years and decades.[1] Currently, the most common method for cervical cancer screening is HPV DNA detection, and combined with liquid-based cytology (TCT) to significantly improve the detection rate of cervical lesions, improves the diagnostic accuracy and reducing the cervix cancer morbidity and mortality.[2] Studies have shown that HPV vaccines can significantly reduce the infection rate of the corresponding type of virus in the genital area. However, HPV vaccines can only produce the corresponding HPV antibody and cannot produce cross-protection against other types of HPV infection. In this study, we analyzed the HR-HPV genotyping results of 61,870 samples from 2015 to 2017, to investigate the prevalence of HR-HPV types regarding age, and to survey HR-HPV related cervical lesions. Therefore, the study of HPV infection characteristics and its subtype distribution in different regions may facilitate in the development of more efficacious HPV vaccines.
2. Materials and methods
2.1. General Information
A total of 61,870 samples of patients with suspected HPV infections from January 2015 to May 2017 at the Affiliated Hospital of Jining Medical University were collected and subjected to HPV genotyping by rapid flow-through hybridization. The age of the patients ranged from 18 to 76 years, with an average of (38.23 ± 6.42) years. Women had a history of having a sexual life, non-pregnancy, no history of pelvic radiation therapy, and chemotherapy. The reasons for seeking medical advice included routine gynecological examination and routine gynecological examination, abnormal vaginal discharge, sexual intercourse, or contact vaginal irregular bleeding. Among these cases, 7009 cases were examined by ThinPrep cytology test (TCT) simultaneously. TCT examination results were abnormal (ASCUS and above) and further classified by histopathology, including 1324 cases of cervical chronic inflammation group, 1785 cases of cervical intraepithelial neoplasia (CIN) group I, 1,050 cases of CINII group, 865 cases of CINIII group, and 430 cases of cervical cancer.
2.2. Instruments and reagents
A gene chip detection system based on rapid flow-through hybridization of nucleic acid molecules for detection of 21 HPVs was provided by Kaipu Biochemical Company in Chaozhou, Guangdong, China.
2.3. Methods
2.3.1. HPV DNA extraction
The 0.5 mL of a cell preservation solution of cervical cells was centrifuged at 14,000 rpm for 1 minute, the supernatant was discarded, and DNA was extracted using a DNA extraction kit and in a biological safety cabinet. All of the samples were processed aseptically. After the extraction, 1 μL of the sample was used for PCR amplification.
2.3.2. PCR amplification
HPV universal primers were used for PCR amplification, and the primer sequence was labeled with biotin at the 5’ end, then the PCR products were also labeled with biotin. The 23.25 μL PCR-MIX, 0.75 μL Taq enzyme, and 1 μL of the DNA template were blended. At the same time, negative and positive controls were set up to control false positives or false negatives caused by operation. The PCR conditions of the ABI 2720 amplifier were as follows: UNG (Uracil DNA Glycosylase) enzyme was treated at 20°C for 10 minutes, pre-denatured at 95°C for 9 minutes, denatured at 95°C for 20 seconds, annealed at 55°C for 30 seconds, extended at 72°C for 30 seconds for 40 cycles, and then extended at 72°C for 5 minutes. The distribution of HPV typing hybridization membrane probe was used to detect the 21 HPV subtypes, which accounted for 95% of the HPV infections in the Chinese population, among which the 13 high-risk subtypes are HPV 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 68; the 5 low-risk subtypes are HPV 6, 11, 42, 43, and 44; and 3 common subtypes of Chinese population are HPV53, 66, and CP8304.
2.3.3. Flow-through hybridization
According to the manufacturer's instruction, before conduction hybridization, the PCR products were denatured at 95°C for 5 minutes and then placed in a frozen water bath for more than 2 minutes. The hybrids were detected by the addition of streptavidin-horse-radish peroxidase conjugate which bound to the biotinylated PCR products and incubated at 25°C for 3.5 minutes. After 4 times washing, the substrate NBT/BICP (nitroblue tetrozolium and 5-bromo-4-chloro-3-indoylphosphate) was added at 37°C for 5 minutes, followed by 4 times washing. The final results could be detected under direct visualization.
2.3.4. Assessment of results
By visual assessment or using Cap's special result analysis software, the positive point is a clearly visible blue-violet dot. According to the HPV classification map of the film strip, the HPV virus type is determined as the positive point. The biotin control point reflects the enzyme and the color reaction solution should be positive in the detection, and the internal control point (IC) is the quality control template DNA probe. If there is no inhibitory factor in the amplification reaction system, then the IC spot appears.
2.3.5. Statistical analysis
Data processing was performed using the SPSS 18.0 software. Any differences in prevalence, odds ratio (OR), together with 95% confidence interval (CI), were assessed by Chi-squared tests. All statistical tests were two-sided, P < .05 was considered statistically significant.
3. Results
3.1. The overall prevalence of HPV infections
The statistical results show that (Table 1) the 21 subtypes of HPV gene of the Affiliated Hospital of Jining Medical University were as follows: 16, 52, 58, 53, CP8304, 39, 51, 6, 11, 18, 66, 31, 33, 68, 56, 59, 35, 45, 44, 42, and 43. Among these, there were 15 high-risk genotypes, 6 low-risk genotypes, and 3 common genotypes in Chinese population (53, 66, CP8304). The overall HPV prevalence was 27.9% (17,280/61,870) among Jining outpatient or inpatient person aged 18 to 76 years from 2015 to 2017. The top 5 HPV genotypes were HPV-16 (4.5%), HPV-52 (2.9%), HPV-58 (2.8%), HPV-53 (1.9%), and HPV-81 (1.9%). The top 4 are high-risk genotypes, which were also HPV high-risk genotypes that cause a high incidence of cervical cancer. Since HPV is a sexually transmitted disease, not only women need to prevent HPV infection, but also men need timely examination and treatment to avoid cross infection and cancer.
Table 1.
The Prevalence of HPV genetypes of 61,870 people among outpatient or inpatient.

3.2. Correlation analysis of HPV infection status and age
Based on the results of HPV and TCT analyses of female patients, the infection rate of HPV is related to age. The positive rates of infection varied among age groups. Our results showed that subjects aged <20 years had the highest risk of HPV infection, followed by the 21- to 30-year-old group, which was also higher than other age groups (Table 2 and Fig. 1). Only one 5-month-old infant was found to be infected with HPV-16 39 58 (not included in the data of this study), and the cause was related to the mother's infection with HPV. Comparison of the results of the 31 to 50 year age group with other age groups indicated a χ2 = 4.308 (P = .044<.05), and the difference was statistically significant.
Table 2.
HPV infection in different age groups.
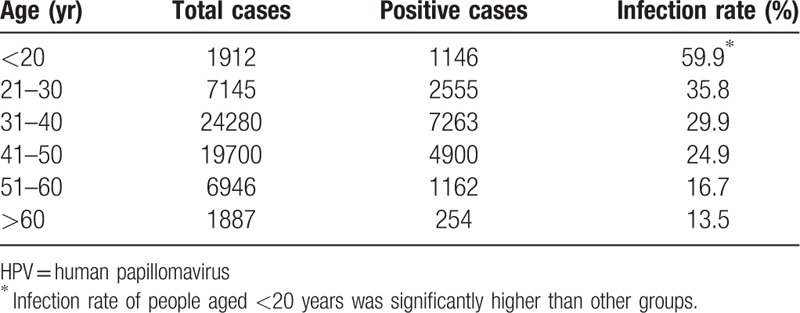
Figure 1.

The age-specific prevalence of HPV infection among the whole population in North China Plain. HPV = human papillomavirus.
3.3. Correlation analysis of HPV infection status and cervical lesions
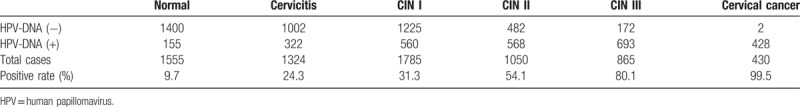
In this study, we divided into 6 groups according to the results of cytology histopathology, such as normal, cervicitis, CIN (I, II, and III) and cervical cancer. The data showed that the positive rate of HPV significantly differed among these groups (Table 3). With the increase of cervical lesions, HPV positive rate is higher, indicating that high-risk HPV infection more likely leads to cervical precancerous lesions and cervical cancer. The detection rate of HPV infection in cervicitis group (24.3%) was higher than that in normal group (9.7%), so cervical inflammation may be associated with cervical HPV infection and intraepithelial neoplasia. The infection rate of HPV-DNA (+) genotype in cervical cancer group was 99.5%, which was significantly higher than that the other groups. Therefore, the risk of HPV infection could be predicted according to the type of HPV infection. The positive rate was significantly different from that of the normal group (χ2 = 32.35, P < .005).
Table 3.
The HPV infection status in different cervical lesions.
3.4. The 4 most frequent HR-HPV genotypes and cervical lesions
The 4 most common high-risk genotypes of cervical cancer at the Affiliated Hospital of Jining Medical University were HPV-16, HPV-52, HPV-58, and HPV-53 and there were significant differences among the four genotypes (P < .05) (Table 4). HPV-16 showed the highest infection rate (62.6%), which was significantly correlated with the severity of cervical cancer (P < .005).
Table 4.
The infection rates of the top four HPV high-risk genotypes in different cervical lesions.

3.5. Correlation between multiple infection and cervical lesions
Multiple infections of HPV were detected by modified flow-through hybridization in different groups of patients: single infection rate was 17.9%, double infection rate was 6.9%, triple infection rate was 2.0%, and quadruple infection rate was 0.5%. There was a significant difference in the infection rate among the 3 groups (χ2 = 26.75, P < .05). The infection rate of cervical cancer group (35.8%) was significantly higher than that of the inflammation group (2.3%). There was a significant difference between the 2 groups (P < .05) (Table 5), suggesting that cervical cancer may have more HPV double infections.
Table 5.
Comparison of multiple HPV infection cases in different groups.

4. Discussion
Cervical cancer is a common gynecological genital tract malignancy, the incidence of which is second only to breast cancer and is the only gynecological malignancy with a clear etiology. The incidence of cervical cancer is mainly related to the persistent infection of high-risk HPV. In recent years, the incidence of cervical cancer has rapidly increased.[3] HPV infection is the main cause of cervical cancer and its precancerous lesions. Therefore, HPV typing can be used as a screening tool for cervical cancer. HPV is a double-stranded circular DNA of various subtypes. Based on its relationship with cervical cancer, HPV can be divided into high-risk type and low-risk type, among which high-risk types include 16, 18, 31, 33, 35, 39, 45, 51, 52, 58, 59, and 68. The low-risk types include 6, 11, 42, 43, and 44, while types 53, 66, and CP8304 are common subtypes in China. In this study, 61,870 outpatient and inpatient samples of Jining were tested for HPV typing. The results showed that the HPV infection rate was 27.9% (17,280/61,870), which was consistent with the research results.[4] However, some women with autoimmunity increased within 8 to 10 months of HPV infection can be automatically cleared, and some patients with persistent infection. This study compared 7009 cases with cytological diagnosis results. The results showed that 5454 patients had abnormal cytology. The positive rate of HPV in patients with normal cytology was significantly lower than that in patients with chronic inflammation, CIN and cervical cancer. The statistical data of this study showed that the positive rate of HPV in cervical cancer patients was obvious. Higher than chronic inflammation (or) normal patients, and with the increase of cervical lesions, the positive rate of HPV detection increases, so HPV is closely related to the occurrence and development of cervical cancer. Especially in patients with CINII, CINIII or cervical cancer, the infection rate of HPV was as high as 54.1%, 80.1%, and 99.5%, respectively, which confirmed the correlation between the severity of HPV infection and the degree of cervical lesion.[5]
This study showed that the HPV infection rate in the cervical cancer group was significantly higher than that in the inflammation group and/or the normal group, and was also associated with the genotype of infection (Table 1). The distribution of HPV subtypes and the positive rates of each subtype were found in Affiliated Hospital of Jining Medical University. The most common 5 genotypes were 16 (4.5%), 52 (2.9%), 58 (2.8%), 53 (1.9%), and CP8304 (1.9%). The highest infection rate of HPV-16 was the subtype most closely related to the severity of cervical cancer lesions, and the infection types and their proportions are different in different regions. While Xiayan et al[6] reported HPV52, HPV16, HPV53, HPV58, and HPV51 in Shanghai, China. Jing et al.[7] reported HPV-16, HPV-52, HPV-58, HPV-18, and HPV-6 in the Pearl River Delta region of Guangdong province, which was basically the same types as those reported in this study, but CP8304 infection was not reported in Sanya and Nanchong. The CP8304 infection in Jining occupies the top 5, which is quite different from other areas and may be related to regional differences. In addition, the results of the 2015 survey in Beijing and Shanghai showed that HPV58 and HPV52 were the highest HPV subtypes, even higher than HPV-16 and HPV-18.[8] Therefore, different regions have various types of infection and their respective proportions. The distribution of the 4 common subtypes in this region is shown in Table 3. The data show that HPV-16 (62.6%), which has the highest infection rate, is significantly associated with the severity of cervical cancer. According to the design of the common types of screening in this area, this plays an important role in the prevention and treatment of cervical cancer. This study found that HPV infection in cervical lesions involved double and multiple infections. With an increase in HPV typing site infections, the severity of cervical cancer gradually increases. Table 4 statistically analyzed the number of HPV multiple infections in different groups. Among the 430 patients with cervical cancer, 207 (48.2%) had single infection, 154 (35.8%) had double infection, 50 (11.6%) had triple infection, and 18 (4.2%) had quadruple infection. The risk and severity of cervical cancer can be predicted based on the HPV genotype of infection or the presence or absence of double or more infections to determine the treatment options after screening. If the most common high-risk genotype such as HPV-16 is detected, then the risk of cervical cancer is higher in those with more than two infections. In addition, genotyping of HPV is of great significance in judging the type and treatment of diseases, and provides basic data for the development of HPV vaccine. Therefore, clinical HPV genotype plays an important role in early prevention, early detection, and early treatment of cervical cancer.
A large number of epidemiological data show that high-risk HPV infection is a necessary condition for the pathogenesis of cervical cancer and precancerous lesions,[9] and studies have reported that HPV infection is the cause of cervical cancer.[10] Detection of HPV-DNA typing can screen women with cervical cancer or precancerous lesions, as well as women at potential risk. Therefore, HPV-DNA typing detection as a screening method for female precancerous lesions, combined with cervical cytology detection is more diagnostic value for the clinic. This study investigated cervical cytology and HPV-DNA-typing-positive patients, cytology-negative, and high-risk HPV-positive patients. The incidence of cervical cancer is as high as 99.5%, and the risk of the latter is relatively high. In recent years, more and more attention has been paid to HPV as a screening method for cervical cancer in developed countries such as European and American. A large number of studies have proposed that HPV typing test is more advantageous than cytological test in screening cervical cancer.[11] In this study, 3700 cases of cervical CINI-CINIII lesions, with the level increases, the infection rate also increases, 31.3%, 54.1%, and 80.1% respectively. Among the 430 patients with cervical cancer diagnosed, only 2 cases generated negative HPV-DNA typing results, the false negative rate was 0.46% (2/430), and the infection rate was 99.5%. There were several reasons for HPV-DNA genotyping to be false negative:
-
1.
severe lesions such as micro-bias adenocarcinoma, gastric, and intestinal cancer, and renal tubular carcinoma and clear cell carcinoma[12] were undetectable due to severe cervical lesions and severe tissue damage;
-
2.
there are unknown HPV-DNA subtypes; and
-
3.
unsatisfactory material extraction, low HPV titers and copy number, and limited detection methods; and 2 false negative samples in this study.
The clinical cases were all high-risk patients with HPV infection. The reason for the analysis may be drug inhibition. Therefore, it is recommended to retake the specimen after stopping the drug. The results were positive after the repeated collection of specimens.
The results of this study showed that the infection rate of HPV was the highest in women under 20 years of age. With the increase in age, the infection rate of HPV gradually decreased. Consistent with Dickson et al, HPV infection rate decreased significantly with age.[13] Some studies have shown that the age distribution of HPV infection in Chinese women is characterized by “double peaks”: the first peak is “17 to 24 years old” and the second peak is “40 to 44 years old”. However, here had some difference. The high rate of HPV infection in young women in China may be related to the early age of first sexual activity, an excessive number of sexual partners, and low immune function or immunodeficiency.[14] The second peak in this area did not appear, which may be related to Confucianism in adult, the couple was more specific to each other and the number of sexual activity be reduced, etc. Therefore, more attention should be paid to the high incidence of HPV infection in young women. HPV vaccination for women aged 13 to 15 may help prevent HPV infection and cervical cancer in China, especially the 9vHPV vaccine. Because of the results of the study had told us that the high HPV infection types in this area were so different from other regions.
The results of this study indicate that HPV typing is more sensitive than TCT in cervical disease screening. HPV typing is higher than TCT detection rate, but the operation is more complicated, and there are a small number of false negative cases. Therefore, HPV typing test combined with pathological cytology examination is an important means of screening for cervical cancer.
In summary, high-risk HPV infection is the main outpatient and inpatients at the Affiliated Hospital of Jining Medical University, among which HPV-16 is the most common, followed by 52 and 58. In the Jining area, CP8304 infection accounted for the top five, which was quite different from other areas. In this study, HPV16, 52, and 58 are common subtypes, which are closely related to cervical lesions, of which HPV-16 is the most common. With the severity of cervical lesions, the infection rate is on the rise. This study identified the major subtypes of HPV in the region and its relationship with the extent of cervical lesions. At the same time, the detection and screening of the above three HPV genotypes should be strengthened to provide favorable evidence for the screening and prevention of cervical lesions in this area.
Acknowledgments
We thank LetPub for its linguistic assistance during the preparation of this manuscript.
Author contributions
Conceptualization: Haixin Dong, Chengqiang Jin, Linqing Yang, Yunfei Wang.
Data curation: Lihua Liu, Dexue Wang, Chengqiang Jin, Liqing Jiang, Hui Song, Chao Jin, Tong Wang, Cuiming Shi, Yunfei Wang.
Formal analysis: Chengqiang Jin, Liqing Jiang, Hui Song, Chao Jin, Tong Wang, Cuiming Shi, Yunfei Wang.
Funding acquisition: Linqing Yang, Yunfei Wang.
Investigation: Lihua Liu, Dexue Wang, Chengqiang Jin, Liqing Jiang, Hui Song, Chao Jin, Tong Wang, Cuiming Shi, Linqing Yang, Yunfei Wang.
Methodology: Haixin Dong, Linqing Yang, Yunfei Wang.
Resources: Lihua Liu, Haixin Dong, Linqing Yang, Yunfei Wang.
Supervision: Haixin Dong, Linqing Yang, Yunfei Wang.
Validation: Lihua Liu, Yunfei Wang.
Writing – original draft: Lihua Liu.
Writing – review & editing: Lihua Liu, Yunfei Wang.
Footnotes
Abbreviations: CIN = cervical intraepithelial neoplasia, HPV = human papillomavirus, HR = high risk, TCT = liquid-based cytology.
How to cite this article: Liu L, Wang D, Dong H, Jin C, Jiang L, Song H, Jin C, Wang T, Shi C, Yang L, Wang Y. Characteristics of carcinogenic HPV genotypes in North China Plain and the association with cervical lesions. Medicine 2019;98:37(e17087).
We gratefully acknowledge the financial support from the National Natural Science Foundation of China (No. 81502255); Medical Science and Technology Development Plans Foundation of Shandong Province (No. 2017WS336); Science and Technology Development Plan Foundation of Jining (No. 2014jnjc09); The “Nursery” Program of Affiliated Hospital of Jining Medical University (No. MP-2014–001); Young Teacher Research Support Fund of Jining Medical University (No. JY2017FS004 and JYFC2018FKJ091) and Program for Scientific research innovation team in Precision medicine of Gynecologic Oncology.
The authors have no conflicts of interest to disclose.
References
- [1].Petry KU. HPV and cervical cancer. Scand J Clin Lab Invest Suppl 2014;74:59–62. [DOI] [PubMed] [Google Scholar]
- [2].Vorsters A, Micalessi I, Bilcke J, et al. Detection of human papillomavirus DNA in urine. A review of the literature. Eur J Clin Microbiol Infect Dis 2012;31:627–40. [DOI] [PubMed] [Google Scholar]
- [3].Tribius S, Hoffmann M. Human papilloma virus infection in head and neck cancer. Dtsch Arztebl Int 2013;110:184–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Kececioglu M, Seckin B, Baser E, et al. Cost and effectiveness comparison of immediate colposcopy versus human papillomavirus DNA testing in management of atypical squamous cells of undetermined significance in Turkish women. Asian Pac J Cancer Prev 2013;14:511–4. [DOI] [PubMed] [Google Scholar]
- [5].Maranga IO, Hampson L, Oliver AW, et al. HIV infection alters the spectrum of HPV subtypes found in cervical smears and carcinomas from kenyan women. Open Virol J 2013;7:19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Xia Y, Jin ZJ, Yun-Xiang NI, et al. Investigation of human papilloma virus infection and virus genotyping in patients with cervical lesions in Shanghai. Acad J Sec Milit Med Univ 2017;38:1526–31. [Google Scholar]
- [7].Miao Z, Zhang X, Zou J, et al. HPV genotypes and associated cervical cytological abnormalities in women from the Pearl River Delta region of Guangdong province, China: a cross-sectional study. BMC Infect Dis 2014;14:388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Singh S, Zhou Q, Yu Y, et al. Distribution of HPV genotypes in Shanghai women. Int J Clin Exp Pathol 2015;8:11901–8. [PMC free article] [PubMed] [Google Scholar]
- [9].Muñoz N, Bosch FX, De SS, et al. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med 2003;348:518–27. [DOI] [PubMed] [Google Scholar]
- [10].Walboomers JM, Jacobs MV, Manos MM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol 1999;189:12–9. [DOI] [PubMed] [Google Scholar]
- [11].Li N, Franceschi S, Howelljones R, et al. Human papillomavirus type distribution in 30,848 invasive cervical cancers worldwide: Variation by geographical region, histological type and year of publication. Int J Cancer 2011;128:927–35. [DOI] [PubMed] [Google Scholar]
- [12].McCluggage WG. New developments in endocervical glandular lesions. Histopathology 2013;62:138–60. [DOI] [PubMed] [Google Scholar]
- [13].Dickson EL, Vogel RI, Luo X, et al. Recent trends in type-specific HPV infection rates in the United States. Epidemiol Infect 2015;143:1042–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Zhao FH, Tiggelaar SM, Hu SY, et al. A multi-center survey of age of sexual debut and sexual behavior in Chinese women: suggestions for optimal age of human papillomavirus vaccination in China. Cancer Epidemiol 2012;36:384–90. [DOI] [PMC free article] [PubMed] [Google Scholar]


