Abstract
The aim of this article was to present the possibilities of use and application of color-coded Doppler ultrasonography in the diagnosis of various diseases of the eyeball and orbit which result from vascular disorders. Color-coded Doppler ultrasonography is recommended for the assessment of blood flow velocity in the retrobulbar arteries. That is why the article contains current recommendations for Doppler imaging in ophthalmology. The paper provides detailed recommendations for patient’s preparation for the examination, presents the scanning technique and safety of the examination, and lists ophthalmological diseases of vascular origin for which color-coded Doppler ultrasonography can be applied. Furthermore, the article also presents other techniques applied in clinical practice for the assessment of blood flow or imaging of vasculature of a given eyeball structure, inter alia: power Doppler ultrasonography, 3D and 4D ultrasonography, magnetic resonance angiography, spiral computer tomography, transcranial ultrasonography and modern microvascular imaging. The authors emphasize the usefulness of color-coded Doppler ultrasonography in the diagnosis of diseases which result from blood flow disorders within the eyeball, such as amaurosis fugax, ocular ischemic syndrome, insufficiency in vessels supplying the carotid and vertebral arteries, posterior ischemic optic neuropathy, glaucoma, age-related macular degeneration, vascular vision disorders, vascular malformations, such as arteriovenous fistula, orbital varices, systemic connective tissue diseases in retinopathy of prematurity, diabetes, thyroid disorders or strabismus. The application of color-coded Doppler ultrasonography is especially important in the assessment of the vasculature of intrabulbar tumorous lesions and in the differential diagnosis of intrabulbar tumors.
Keywords: color Doppler imaging, technique and safety of color Doppler ultrasonography in ophthalmology, Doppler effect, ophthalmic indications for CDI
Theoretical basics of color Doppler imaging and the Doppler effect
Doppler ultrasonography is recommended for the assessment of blood flow in the retrobulbar and carotid arteries. This examination was introduced in the 1980s. It consists in the determination of the level of artery stenosis on the basis of flow acceleration. An estimated accuracy of this method in the carotid arteries is ca. 50–79% when compared to selective arteriography(1). In the retrobulbar arteries, which are vessels with the smallest diameter, the assessment of the stenosis level is impossible. Also, imaging of mural lesions or occlusions is limited(2). The introduction of the duplex method, i.e. a combination of B-mode with Doppler presentation has been important for the development of non-invasive vascular diagnosis(3). Combining this method with color, so-called color Doppler ultrasonography (CDU) or color Doppler imaging (CDI), allows one to locate lesions and blood flow disorders based on color coding, not only in the carotid arteries, but also in the retrobulbar vessels(4–6). Ultrasound contrast agents are not widely used in the differentiation of changes in the retrobulbar arteries, except for embolism in the ophthalmic artery or central retinal artery, which is in contrast to the carotid and cerebral arteries where these substances may be used(7).The addition of power Doppler (PD) to color Doppler ultrasonography has improved the sensitivity of detection of even small flows but without the determination of their direction and velocity, which has enabled its application in ophthalmology for imaging of intrabulbar pathological (tumorous) lesions often earlier than in a CDI examination, and for differentiation of so-called vascular pseudo-occlusion(8). Owing to computer processing and summarizing ultrasound scans, three- and four-dimensional ultrasound images (3D and 4D US)offer a three-dimensional reconstruction of the vascular structure, enabling vivid representation of the form of intrabulbar lesions and the assessment of the changed area(9,10). Magnetic resonance angiography (MRA) is applied for the imaging of vascular changes through alterations of the electromagnetic field of blood and the adjacent tissues. An advantage of this technique is the possibility to visualize vessels along their entire course, from the aortic arch to the intracranial segment, without the need for contrast. The use of this method in ophthalmology may be significant in the identification of occlusion and advanced stenoses of the internal carotid artery in cases of sudden amaurosis fugax or absolute glaucoma. When compared to arteriography, the reliability of this examination is estimated at ca. 95–100%(11,12). Spiral computer tomography requires contrast agent administration and produces a spatial image of the entire vessel, but it is not used for direct visualization of a retrobulbar vessel(12). A method used for imaging of the ophthalmic artery, central retinal artery and posterior ciliary arteries is transcranial Doppler ultrasonography (TCD-US). Owing to this method, it is possible to evaluate the collateral circulation and determine the autoregulatory capacity of the retinal and cerebral circulation. This is feasible due to transcranial autoregulatory tests with acetazolamide and cerebrovascular reactivity tests with carbon dioxide. This technique also uses color Doppler options (TCD-CD) with power Doppler (PD)(11,12). Superb microvascular imaging (SMI) is a modern technique used for the assessment of blood flow (BF). It has developed in the recent years on the basis of the conventional color Doppler imaging technique (CDIT). SMI may reveal microvascular BF and low-velocity BF. There are two types of SMI: color SMI (cSMI) and monochrome SMI (mSMI). As of this day, the PubMed database does not contain any information about the application of this method in the imaging of ocular vessels. The only available data address the application of cSMI in the examination of microcirculation in the testicles and in lung diseases(13,14).
Doppler effect and blood flow evaluation
The assessment of alterations in the retinal and choroidal circulation has been a subject of interest for many years. Examination methods of arteries and veins are continuously being improved, but to date, apart from CDI ultrasonography, no other methods have emerged. CDI ultrasonography determines circulation in both retinal and uveal vessels in a direct and non-invasive manner, regardless of the opacity of the optical media.
Blood flow velocity in Doppler ultrasonography can be registered owing to the Doppler effect, which consists in a change in the frequency of a reflected ultrasound wave scattered on erythrocytes as this change is proportional to blood flow velocity. The frequency of the reflected wave is presented according to the following formula(15):
2 fn v / c statement is referred to as the Doppler frequency (fd) or the Doppler shift where: fo is the frequency of a reflected wave, fn is the frequency of an emitted wave, v is the velocity of biological structures (here: erythrocytes), and c is the spreading velocity of a reflected wave in a given medium. A simplified version of the formula may be presented as follows:
The application of the Doppler effect allows blood flow evaluation as the Doppler frequency (fd)is proportional to the velocity of moving blood cells. A formula which includes angles between a moving biological structure and a converter that receives ultrasounds is(15):
where α is the angle between the direction of an ultrasound wave and the blood flow velocity vector in a given vessel (the Doppler angle)(15).
In accordance with this equation, the Doppler angle α is important for measurement accuracy. In the examination of blood flow velocity, it is often impossible to determine the angle at which the ultrasound beam reaches the examined vessel. Anatomical and radiological data show that at an angle α of 0°cos 0° is 1, and the velocity constituent equals blood flow velocity (v). In the case when blood flow is perpendicular to the beam, the angle α is 90°,and the Doppler effect does not appear as cos 90° is 0. The results are falsified when the angle between the ultrasound beam and blood flow velocity vector increases. In the measurements of the Doppler frequency, a variation from a 60° angle by 0° ± 5° results in a measurement error of ca. 15%,whereas the same deviation for the angle α = 0° changes the result by a practically unimportant value of ca. 0.5%.According to the literature, an angle of 60° (suggested values are 39–54°) is a borderline angle for extracranial arteries at which it is possible to achieve repeatable results. For intracranial arteries, the angle should be between 0° and 30°; in this case, the blood flow velocity measurement error does not exceed 15%(16–19).
In the research on blood flow velocity in the retrobulbar vessels, it has been proven that maintaining the angle α between 20° and 30° allows one to avoid measurement errors, which usually do not exceed10% of the actual value. Maintaining a larger angle between the ultrasound beam and the examined vessel, e.g. over 45°, is a reason for a higher number of incorrect results(16–18). According to the literature, this angle should not exceed 30° in the examination of the posterior ciliary arteries(16–18). In accordance with the recommendations issued in 1986bythe Nomenclature and Standards Committee of the American Society of Echocardiography, blood flow velocity in intracranial and retrobulbar arteries are given in cm/s, and in larger vessels – in m/s. These units are used in order to avoid mistakes when comparing research results obtained with various Doppler devices(19,20). In new generation Doppler equipment, the measurement angle is readily determined by the examiner during the examination, which minimizes the risk of a measurement error. Furthermore, arterial and venous blood can be visualized thanks to color coding based on a difference in colors(red is for flow towards the receiver, i.e. arterial blood, and blue is for flow from the receiver, i.e. venous blood). Within the Doppler frequency range of acoustic wavelengths (<16 000 Hz), an acoustic signal obtained during the examination enables an auditory assessment of the Doppler signal. Exceptions to this are very high blood flow velocities in stenoses e.g. in the carotid arteries. In such cases, Doppler frequencies that exceed the values stated above are inaudible for the examiner(20).
Ultrasonographic methods for blood flow velocity measurement
In clinical practice, blood flow velocity is measured with two methods: the continuous wave method and the pulsation wave method(19,20). Nowadays, a technique which is the most popular in ophthalmological examinations is the duplex method i.e. combination of 2D ultrasonography (two-dimensional real-time ultrasonography, 2D US), impulse method and color-coded Doppler ultrasonography. In this method, imaging of tissue structures is accurate, and the assessment of the blood flow waveform is enabled thanks to precise visualization of the vessel and determination of the measurement capacity in the selected site. The angle between the ultrasound beam and the examined blood vessel may be set automatically. A triplex method involves the simultaneous use of all US techniques, i.e. 2D, impulse Doppler and color Doppler.
A choice of ultrasound frequency is crucial for the assessment of blood flow in examined vessels. An amplitude of the ultrasound wave decreases with the depth of wave penetration, and the attenuation index grows linearly with frequency. This index ranges from 0.5 to 0.7 dB per 1 cm for the frequency of 1 MHz. For the frequency of 4 MHz, attenuation doubles, and for 8 MHz, it increases eightfold. Doubling the frequency decreases the range of the examination twofold. It has been proven that the optimal frequency for the assessment of blood flow in the retrobulbar vessels located at the depth of 1.5–4 cm ranges from 5 to 10 MHz (carotid arteries, peripheral arteries, retrobulbar arteries). Vessels located deeper are examined with transducers of lower frequencies: ca. 2.5–3.5 MHz(18–20).
When passing through tissue, ultrasound waves are reflected, scattered and attenuated. It has been proven that erythrocytes are the main source of ultrasound wave scattering for waves with the frequency of 4 to 16 MHz. They move with various velocities: with the highest velocity in the center of the vessel, and with the lowest velocity near its walls. The power of the Doppler signal is registered in a multilevel scale of greyness, where the lack of the Doppler signal is marked with black, while the strongest power of the signal is marked with white. The Doppler waveform is represented in a three-dimensional presentation, where the horizontal axis stands for time, the vertical axis denotes the Doppler frequency, and the third dimension refers to the amplitude or power of the Doppler signal, which is presented in a multilevel grey scale, and multipoint (128 or 256) Fourier transforms are used in its analysis. In the clinical application, the difference in the waveforms analyzed with this method, is practically invisible(18–20). That is why parabolic flow is characterized by regular distribution of brightness in the whole range of frequencies: a flattened waveform is characteristic of high flow frequencies and a turbulent one is typical of low flow frequencies.
Color coding of the Doppler frequency
In ultrasonographic methods involving color coding, groups of colored pixels are assigned to structures with blood flow. The pixels stand for the value of the momentary frequency measured along the whole ultrasound beam. Blood flow measurement is possible thanks to a digital computer analysis of a large number of echoes from numerous gates located in one measurement volume. Coding of the Doppler signal is visualized on a color scale which depends on a value defined in the legend. Red is assigned to positive Doppler frequencies and denotes flow towards the transducer. Blue is ascribed to negative frequencies and represents reverse blood flow. Organs with no blood flow are presented in the grey scale. Echoes with a high amplitude are viewed as bright points, while dark background represents a decreased value of ultrasound amplitude. The lack of flow is marked black. Doppler waveform amplitude is coded with various brightness of red or yellow as a result of positive and negative Doppler frequencies. No color in the examined area means no flow recorded(15–20).
Power of the ultrasound wave and safety of CDI
The power of ultrasounds which may be safely used in mammalian tissues was defined by the American Institute for Ultrasound in Medicine(21). A paper that is particularly important in Poland is one by Filipczyński et al. who started to examine the possibility of applying ultrasound in ophthalmology and its impact on the eyeball structures in 1967(22). Biological phenomena that occur in response to ultrasounds are determined by intensity (I) in W/cm2. When defining this parameter, it is crucial to obtain information about the correlation of spatial and temporal intensity distribution with parameters of the ultrasound field, i.e.: duration of an impulse, section of an ultrasound beam, as well as length and depth of a focus. Nowadays, it is known that a safe dose of ultrasound intensity, having included a maximal value in space and a mean one in time, is max. 0.1 W/cm2 for anon-focused ultrasound wave and max. 1.0 W/cm2 fora focused beam. In 1992, ultrasonographic devices were standardized, and an obligation was introduced to place a safety note on the screen of the monitor. Values concerning mechanic vibrations and protection against thermal damage to tissues are expressed with mechanical and thermal indices. Maximal intensity of ultrasound devices may reach up to 720 mW/cm2. A range safe for eye tissues is from 17 to 720 mW/cm2 on condition that the mechanical index does not exceed 0.23(22–24).
The mechanical index (MI) informs about the occurrence of possible biological effects and is displayed on the screen during the examination. In ophthalmological examinations, this parameter cannot exceed 0.23 (for other organs, its maximal value may not exceed 1.9). The MI covers a possibility of a cavitation phenomenon in the examined tissues or changes in the gas bubble dynamics in the ultrasound field; they either fall into vibrations near the resonance frequency, or collapse and emit a large amount of energy and induce temperature growth. This phenomenon also results in the emergence of hydroxide and hydrogen free radicals which may cause undesirable biochemical changes, such as changes in the structure of the vitreous body or lens. They are either reversible (when low-frequency but high-intensity transducers are used) or irreversible (in the case of high-frequency and low-intensity ultrasound beam absorption, which results in local temperature growth). These observations are especially important for fetal examinations during pregnancy and in neonatal examinations. The thermal index (TI) is connected with heat production through the absorption of acoustic energy which is concentrated within the region of a focused beam in impulse Doppler ultrasonography. This is in contrast to the B-mode US where energy is distributed over a large area. A phenomenon of energy absorption varies across different tissues in the organism e.g. no energy absorption has been found in blood, amnion and urine. For comparison, bones may absorb 60–80% of acoustic energy. The TI may not exceed 2.0(21–23). What is important for energy accumulation is the attenuation index which depends on tissue properties. A mean value of this index for homogenous tissues is 0.3 dB/cmMHz. A low value of this index at high wave intensity does not result in energy absorption in tissues, whereas low intensity of the acoustic field at a high value of the attenuation index may result in significant temperature growth in the examined tissue. An additional index of local temperature increase is the level of energy absorption in the individual layers of the examined organ. An increase in attenuation when passing through the consecutive tissues decreases energy which changes into heat. The literature provides no data on tissue damage and energy accumulation in in vivo settings when an impulse method is applied for a short period of time. According to the literature, the Doppler assessment should be conducted carefully during long Doppler examinations of blood flow in peripheral vessels (1–10 minutes). This refers to the examinations of the eyeball, umbilical cord and fetus(24,25). The discussed indices do not predict biological effects of tissues damage. However, they provide information about a relative probability of their occurrence. In order to obtain the expected diagnostic information, the MI and TI should be as low as possible(26).
Factors affecting blood flow velocity changes in the retrobulbar vessels in CDI
The basis for correct interpretation of a CDI result is an accurately performed technical part of this examination. Numerous researchers who investigate the implementation of the Doppler technique in ophthalmology emphasize a relationship of examination results with researcher’s experience and their knowledge of a given device(26–32).
Maintaining a proper measurement angle between the axis of the examined vessel and the ultrasound beam reflected from blood cells is important for the correct performance of an ophthalmological examination. It allows the results to be compared as the signal is in this case strong and the measurement error is minor. An important condition that must be met to avoid errors is the use of a proper probe frequency in relation to the depth at which the measurement is taken. For the retrobulbar arteries, the frequency range is 7.5–10 MHz. These parameters of ultrasound transducers are the most favorable at the depth of 4–10 cm(27).
The most commonly encountered problem when recording the Doppler waveform in small vessels in patients with vascular ophthalmological diseases is blood flow turbulence or the impossibility to record blood flow amplitude. The reasons for such phenomena are usually improper power of the Doppler amplification and an improper location of the measurement volume in the examined arteries. Should they occur, the amplification of the measurement beam should be applied to such a value at which the Doppler record will be legible for the examiner, without noises from the Doppler device(15). Depth of measurement is another factor that plays an important role in the interpretation of the results. The distance of a sampling gate should be constant during consecutive measurements of the same vessel in one patient. It has been observed that once the probe position changes even by millimeters, there is also a change in the recorded blood flow velocities in the examined vessel. The access angle should not exceed 30° in CDI examinations of the retrobulbar arteries. Increasing the angle to 80 or 90° makes a correct analysis of blood flood velocity impossible. Also a change in the depth of the measurement volume may result in a measurement error of even 20%(4,15,17,28,29,33,34). The most reliable results of blood flow in the retrobulbar arteries are obtained at angle correction of 0–30°, when the maximal measurement error does not exceed 15%(15,17,28). The larger the distance between the examined vessel and the posterior pole of the eyeball, the higher the parameters of systolic and diastolic blood flow velocity. The Doppler waveform should reach up to 2/3 of the maximal value on a velocity scale, and the zero line should be set as low as possible. However, in order to record a flow velocity waveform from the central retinal artery, the zero line should be set in such a manner to enable the waveform from the central retinal vein to be recorded below it. An outline of the Doppler waveform may be done manually or automatically. The latter method is not always recommended as it includes artefacts in calculations. Manual outline of the waveform may be done continuously or by joining the set points. Depending on the examiner’s experience, the measurement error may reach 20%(27–30), as various authors claim.
Color imaging of blood flow velocity sometimes generates problems connected with too many colors in the examined site and in the adjacent tissues. When adjusting color, the velocity scale should be set at the level of the expected maximal velocity. However, one should remember that color pixels present average and not the highest values of blood flow velocity. Color enhancement should not exceed 60–70% of velocity adequate for the examination. An image of a multicolor mosaic around the measurement gate may be connected with turbulences in blood flow or depict too high velocities in a given fragment of the vessel. Total color fading in the examined fragment of the vessel is usually a result of slower blood flow. Broadening of the waveform outline or a mirror reflection of the waveform on the other side of the zero line may occur at too high intensity of Doppler signal noises or when the angle between an examined vessel and the ultrasound beam is set improperly.
An unintended pressure of the probe on the eyeball in the assessment of blood flow and secondary growth of intrabulbar pressure may result in a measurement error. When examining the smallest vessels, it is therefore important not to press the transducer on the eyeball in order to obtain a more detailed Doppler signal.
Values of blood flow velocity parameters in the bulbar arteries depend on the patency of the carotid arteries and vessels located distally to these vessels. The formation of collateral vessels (the synonymous anterior carotid artery, anterior and posterior communicating arteries, contralateral internal carotid artery, basilar artery, additional connections in the pia mater, collateral branches of arteries of both cerebral hemispheres) is crucial for blood flow maintenance in internal carotid artery occlusion. The highest pressure reduction in the afferent vessel is observed when an obstacle is located closer to the eyeball. No CDI flow waveform is observed in central retinal artery embolism when collateral circulation cannot be formed. In the case of occlusion in either one or both short posterior ciliary arteries, blood flow is interrupted in the area supplied by these vessels as they are final by function, and CDI reveals turbulent blood flow, without traits of the diastolic phase.
When outflow is hindered as a result of narrowing located peripherally to the examined site, one may observe lowering of the maximal systolic velocity and disturbance of the flow waveform. An unambiguous interpretation of this is, however, difficult due to the fluctuation of velocity values, also when no narrowing is found(15,20). Blood pressure beyond the narrowing depends on the efficiency of the collateral circulation, and abnormalities in these connections may be a reason for flow turbulences and low post-stenotic pressure values. In the case of vascular dilation behind the narrowed fragment, flow is disordered, which results in considerable lowering of blood flow velocity, filling the spectral window and retrograde flow. A so called “back and forth” waveform may be observed directly behind the occlusion, at a dead end of a vessel. Such a flow is observed in occlusion and closure of the central retinal artery or posterior ciliary arteries(15,20).
The Doppler assessment of blood flow in venous vessels of the eyeball and orbit is hindered when compared to the arterial system, mainly because of low perfusion pressure and a specific anatomical structure of these vessels. Furthermore, cardiac efficiency, right atrial pressure and changes in respiratory frequency influence alterations in blood flow capacity, especially in the area of the retrobulbar veins. The Doppler signal of venous blood flow is characterized by flow phasicity depending on the respiration phase, flow acceleration connected with pressure of the ultrasound transducer on the examined fragment of the vessel and no reverse flow. The lack of these traits may be a sign of a difficulty in blood outflow(31,32).
A flow waveform in the retrobulbar arteries in Doppler ultrasonography presents vascular flow velocity alterations in accordance with a cardiac cycle phase. Parameters determined during a Doppler examination are: peak systolic velocity (PSV), end diastolic volume (EDV),mean velocity (Vmean), resistance index (RI), and pulsatility index (PI). Calculations of theses parameters are based on systolic velocity, diastolic velocity and mean velocity of flow. The pulsatility index was introduced by Gosling in 1974, and, in its simplified version, it is expressed as a quotient of a difference between systolic and diastolic velocity, and mean velocity. This index is independent of the frequency of a transmitted ultrasound wave and the angle between the ultrasound beam and the course of a given vessel. This index is very accurate for vessels with a good pulsatility. In the bulbar circulation, it reaches its peak in the ophthalmic artery, and then drops gradually in the short posterior ciliary arteries to reach its lowest value in the central retinal artery. The values of the pulsatility index are highly variable and may increase with age, according to some authors(33–36).
The Pourcelot index, also known as the resistance index (RI), indirectly determines vascular resistance and is based on the analysis of maximal systolic velocity and end-diastolic velocity. It is expressed as a quotient of a difference between systolic velocity and diastolic velocity, and systolic velocity. The value of this index is the highest in the ophthalmic artery and decreases gradually in the short posterior ciliary arteries, just as the pulsatility index, to become the lowest in the central retinal artery. An increase in peripheral resistance in the retrobulbar vessels is connected with the reduction of end-diastolic velocity and may remain unchanged or grow with age in healthy individuals, although this is not confirmed in all publications(34,36,37).
In a CDU examination of the retrobulbar arteries, the assessment of the level of vascular narrowing, determination of its patency and measurement of the vascular cross-section are difficult due to a small diameter of these vessels. An important trait characterizing the narrowing of the vascular diameter is the shape of the flow waveform which depends on several hemodynamic factors, such as: blood inflow and outflow velocity in the examined site, vascular elasticity, cardiac systolic function, direction of ultrasound beam dispersion in relation to flowing blood, and patient’s age. Maximal peak systolic velocity of blood flow in the retrobulbar arteries may remain unchanged in relation to the patient’s age or it may be decreased. Opinions in this subject vary(34–37). Sex has little impact on the character of blood flow in the retrobulbar arteries. Moreover, there are no statistically significant differences between flow velocity in the right and left eye in healthy individuals(32,34).
Scanning technique in retrobulbar vessel examination and patient preparation for CDI
Before a CDI examination, the patient should lie down with their eyes closed, both eyes kept straight. With this eyeball position, one may localize and image all the retrobulbar arteries within the orbit. If small arteries (i.e. the central retinal artery or posterior ciliary arteries) are impossible to find, the patient should be asked to look down. In this eyeball position, the central retinal artery is the easiest to see in diseases connected with blood flow insufficiency in this area(38–43). The most common probe for the assessment of the retrobulbar circulation is a linear probe (or a sector probe) with a frequency of 7.5 MHz. It is placed on the upper eyelid covered with gel, with minimal pressure on the eyeball in order to avoid intrabulbar pressure growth. CDI examination should be conducted after a 5-minute rest and after systemic and intrabulbar blood pressure measurement, the values of which ought to be within referential ranges for a given age(41).
Smokers should be asked to avoid smoking and drinking alcohol and caffeine for ca. 12 hours before the CDI examination. Factors that must always be taken into consideration during the assessment of blood flow in the retrobulbar vessels are cardiovascular diseases, optic nerve disorders, ophthalmological disorders with a vascular background, especially in diabetes mellitus and proliferative retinopathy, diseases connected with the carotid and vertebral artery dysfunction as well as eyeball and orbital injuries. Furthermore, a person performing and interpreting the examination should pay special attention to the morphology of the vascular wall and efficiency of the carotid and vertebral supply arteries. An additional factor that influences the retrobulbar circulation is the application of local medications in the form of eye drops. This should always be noted during the flow analysis, especially in patients with glaucoma.
The knowledge of the anatomical course and diameters of the arteries and veins in the retrobulbar area is significant for the correct performance of a color Doppler examination. A reference point for the location of the retrobulbar vessels is the hypoechogenic shadow of the optic nerve (US B). In order to detect an individual vessel, one needs to be acquainted with the anatomy of the retrobulbar area and the characteristic flow waveforms in the examined arteries. The results of retrobulbar artery examination with CDI are characterized by high repeatability, but the measurements are observed to vary across examiners(1–3).
Ophthalmic artery
The first assessed artery that supplies blood to the eyeball is the ophthalmic artery (OA), which is the first branch of the internal carotid artery. It separates from the internal carotid artery inside the dural sac. The OA enters the optic canal in the orbital apex by piercing the dural sheath of the optic nerve. The OA changes its course in the orbit; it is first located laterally on the dural sheath of the optic nerve, next it moves onto its upper and medial parts and runs between the rectus, medial rectus and superior oblique muscles. The OA vascularizes the area supplied by the first branch of the trigeminal nerve and gives rise to the following branches: the central retinal artery, the long posterior ciliary arteries and the short posterior ciliary arteries. Next, the OA gives rise to muscular branches to the eyeball muscles and the lacrimal artery to the lacrimal gland and eyelids. It also gives rise to vessels running to the nasal cavity and paranasal sinuses: the anterior and posterior ethmoidal arteries. The posterior ethmoidal artery reaches the sphenoidal sinus, while the anterior ethmoidal artery gives rise to the anterior meningeal branch to the anterior cranial fossa. Its further branches are nasal cavity arteries: the anterior nasal arteries, collateral nasal arteries and nasal septum arteries. The OA’s final branches are the supratrochlear artery and the supraorbital artery. The supratrochlear artery runs on the inner side of the orbital vault. Having left the orbit, it gives rise to the dorsal nasal artery that communicates with the angular artery, which is the terminal branch of the facial artery. The supraorbital artery supplies the superior rectus muscle and levator palpebrae superioris. According to numerous investigations, the OA usually runs laterally to the optic nerve, although it has also been found inferior and medially to the optic nerve. Some researchers have reported the location of the OA under the optic nerve(35,36). In the cases of circulatory insufficiency in the retina, blood is supplied to the eyeball through anastomoses formed between the OA and other arteries, which include the connections between the medial meningeal artery and the lacrimal artery, between the angular artery branching off from the facial artery and the dorsal nasal artery separating from the OA, between the infraorbital artery of the maxillary artery and muscular branches of the OA, between the temporal artery and lacrimal and muscular branches from OA, between terminal branches of the internal carotid artery and similar branches from the OA, which connect with each other in the superior orbital fissure. The OA lumen is from 0.7 to 1.4 mm(36). The OA has a high-resistance flow waveform with a rapid increase of the systole and a shortened diastolic phase (Fig. 1). The measurement angle for this vessel should not exceed 60°. Flow velocity parameters in the OA are usually taken at the intersection of the OA with the optic nerve, i.e. 20–25 mm behind the eyeball in the nasal part of the orbit.
Fig. 1.
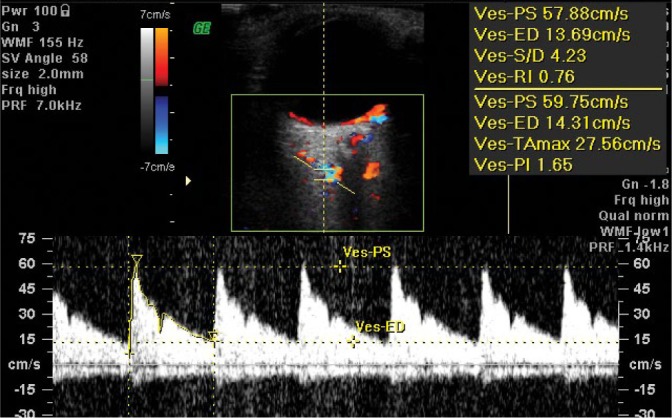
Normal blood flow velocity waveform in the ophthalmic artery (OA) in CDI
Central retinal artery and vein
The retinal and ciliary arteries are two separate systems that vascularize the eyeball. These systems differ in terms of anatomy and functions. A diameter of the central retinal artery is ca. 0.2 mm(37,38).
The central retinal artery (CRA) is the terminal vessel branching off from the OA. The outer diameter of the CRA is 0.6 mm and does not significantly differ between the right and left side in the same individual(39–41). A diameter of the central artery is ca. 0.2 mm and its branches are anatomically terminal. In a layer of nerve fibers, there are primary and secondary CRA arterial and venous branches. Directly behind the lamina cribrosa sclerae in the optic nerve canal, one can observe double red and blue color coding in a CDI image, which depicts the presence of the central retinal artery and vein in the same area. The Doppler examination presents arterial and venous flow velocity waveforms which correspond with the central retinal artery (CRA) and the central retinal vein(CRV) running side by side (Fig. 2). For these arteries, velocity is measured at a distance of 1–3 mm beyond the posterior pole of the eyeball. The flow velocity waveform in the CRA is characteristic of arterial flow with a low amplitude and typical peaks for systolic and diastolic velocities visible above the zero line. The flow velocity waveform in the CRV presents even flow below the zero line(39).
Fig. 2.
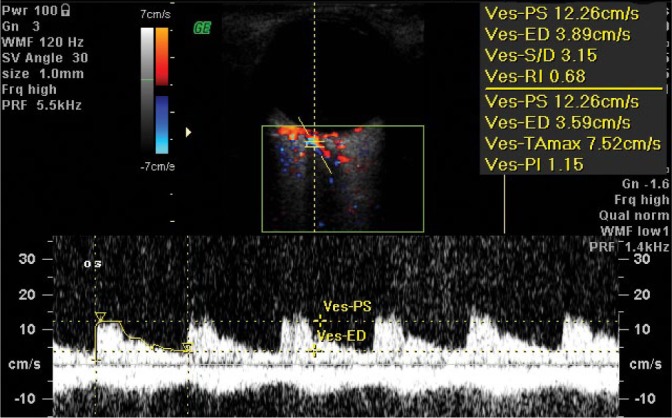
Normal blood flow velocity waveform in the central retinal artery (CRA). Note the amplitude of the flow waveform characteristic of the central retinal vein, visible below the isoelectric line
Posterior ciliary arteries
The lateral posterior ciliary artery (LPCA) and the medial posterior ciliary artery (MPCA) give rise to numerus branches (10–20) of the short posterior ciliary arterioles and two long posterior ciliary arterioles. A diameter of short posterior ciliary arteries (SPCAs) is ca. 0.1–0.2 mm. These arteries penetrate the sclera in the posterior pole and form one layer of capillary vessels, adherent to Bruch’s membrane and the retinal pigment epithelium (RPE). The measurement of flow parameters in the PCAs is possible at a distance of ca. 3–5 mm from the posterior pole of the eyeball (Fig. 3 and Fig. 4).
Fig. 3.
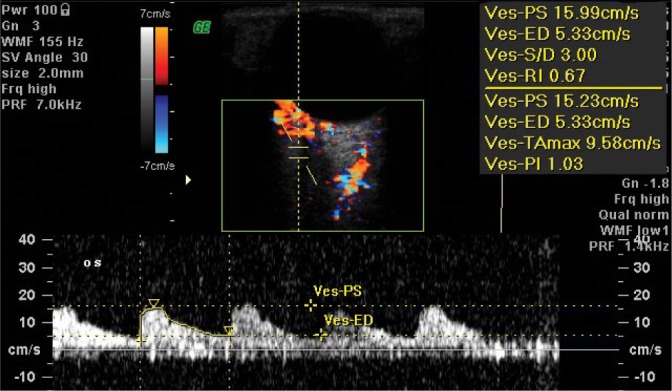
Normal blood flow velocity waveform in the posterior ciliary artery: the medial/nasal branch (MPCA/NPCA)
Fig. 4.
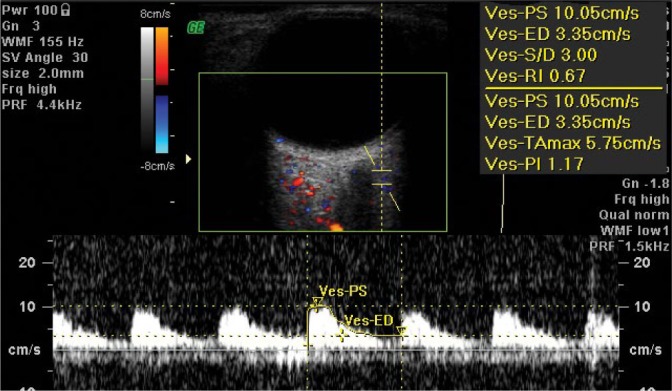
Normal blood flow velocity waveform in the lateral/temporal posterior ciliary artery (LPCA / TPCA)
CDI in ophthalmological practice
A CDI examination has been used for the identification of ophthalmological disorders with a vascular background for many years. It is a method of choice in the diagnosis of bulbar circulation disorders, such as: amaurosis fugax, ocular ischemic syndrome, insufficiency in vessels supplying the carotid and vertebral arteries, posterior ischemic optic neuropathy, glaucoma, age-related macular degeneration, vascular vision disorders, vascular malformations, such as arteriovenous fistula, orbital varices, and systemic connective tissue diseases(42–47). What is more, the diagnostic application of the CDI method has been used in the assessment of flow hemodynamics in retinopathy of prematurity, diabetes mellitus, thyroid disorders, and strabismus(48–50). On the basis of long-term research, it seems that diagnostic CDI is particularly valuable in the assessment of vascularization of intrabulbar tumorous changes and in differential diagnosis of intrabulbar tumors. The assessment of intrabulbar tumor vasculature combined with a US B examination and other ultrasound traits determined with CDI enable the differentiation between a benign tumor and a malignant one with sensitivity of 83.7% and specificity of 75.7%(51). It also seems that the introduction of the modern SMI technique into Doppler diagnostic imaging, enabling detection of very slow blood flow, may increase the detectability of intrabulbar tumors at a very early stage(52). Owing to the availability of CDI, its safety and non-invasiveness, this method can have multiple applications as an adjunct to the differential diagnosis in numerous ophthalmological disorders with a vascular background. In order to reduce the variability of results, connected with the use of devices by various producers, one should remember about the necessity to conduct CDI with particular attention to the highest standards discussed above.
Footnotes
Conflict of interests
Author does not declare any financial or personal relations with other people or organizations which may negatively influence the content of this publication or claim the right to this publication.
References
- 1.Riley WA, Barnes RW, Bond MG, Evans G, Chambless LE, Heiss G: High-resolution B-mode ultrasound scanning method in the atherosclerosis risk in communities (ARIC) cohort. The ARIC Study Group J Neuroimag 1991; 1: 168–172. [PubMed] [Google Scholar]
- 2.Saionen R, Salonen JT: Progression of carotid atherosclerosis and its determinants: A population-based ultrasonography study. Atherosclerosis 1990; 81: 33–40. [DOI] [PubMed] [Google Scholar]
- 3.Daigle RJ, Stavros AT, Platou RP, Anderst D, Nurre P: Velocity criteria for differentiation of 60–79% carotid stenosis from 80% or greater stenosis. J Vasc Technol 1988; 12: 177–183. [Google Scholar]
- 4.Erickson SJ, Hendrix LE, Massaro BC, Harris GJ, Lewandowski MF, Foley WD et al.: Color Doppler flow imaging of the normal and abnormal orbit. Radiology 1989; 173: 511–516. [DOI] [PubMed] [Google Scholar]
- 5.Görtler M, Niethammer R, Widder B: Differentiating subtotal carotid artery stenoses from occlusions by colour-coded duplex sonography. J Neurol 1994; 241: 301–305. [DOI] [PubMed] [Google Scholar]
- 6.Modrzejewska M: Zastosowanie kolorowej ultrasonografii dopple-rowskiej w okulistyce In: Kęcik T, Lewandowski P, Kęcik D (eds.): Metody obrazowania w okulistyce. Alcon, Warszawa: 2001: 81–100. [Google Scholar]
- 7.Sitzer M, Fürst G, Siebler M, Steinmetz H: Usefulness of an intravenous contrast medium in the characterization of high-grade internal carotid stenosis with color Doppler-assisted duplex imaging. Stroke 1994; 25: 385–389. [DOI] [PubMed] [Google Scholar]
- 8.Griewing B, Morgenstern C, Driesner F, Kallwellis G, Walker ML, Kessler C: Cerebrovascular disease assessed by color-flow and power Doppler ultrasonography. Comparison with digital subtraction angiography in internal carotid artery stenosis. Stroke 1996; 27: 95–100. [DOI] [PubMed] [Google Scholar]
- 9.Bucek RA, Reiter M, Dirisamer A, Haumer M, Fritz A, Minar E et al.: Three-dimensional color Doppler sonography in carotid artery stenosis. AJNR Am J Neuroradiol 2003; 24: 1294–1299. [PMC free article] [PubMed] [Google Scholar]
- 10.Meairs S, Röther J, Neff W, Hennerici M: New and future developments in cerebrovascular ultrasound, magnetic resonance angiography, and related techniques. J Clin Ultrasound 1995; 23: 139–149. [DOI] [PubMed] [Google Scholar]
- 11.Polak JF, Kalina P, Donaldson MC, O’Leary DH, Whittemore AD, Mannick JA: Carotid endarterectomy: preoperative evaluation of candidates with combined Doppler sonography and MR angiography. Work in progress. Radiology 1993; 186: 333–338. [DOI] [PubMed] [Google Scholar]
- 12.Masaryk AM, Ross JS, DiCello MC Modic MT, Paranandi L, Masaryk TJ: 3DFT MR angiography of the carotid bifurcation: potential and limitations as a screening examination. Radiology 1991; 179: 797–804. [DOI] [PubMed] [Google Scholar]
- 13.Durmaz MS, Sivri M: Comparison of superb micro-vascular imaging (SMI) and conventional Doppler imaging techniques for evaluating testicular blood flow. J Med Ultrason (2001) 2018; 45: 443–452. [DOI] [PubMed] [Google Scholar]
- 14.Xiao XY, Chen X, Guan XF, Wu H, Qin W, Luo BM: Superb microvascular imaging in diagnosis of breast lesions: a comparative study with contrast-enhanced ultrasonographic microvascular imaging. Br J Radiol 2016; 89: 20160546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Małek G: Postępy w ultrasonografii naczyń. Ultrasonografia 2004; 15: 20. [Google Scholar]
- 16.Guthoff RF, Berger RW, Winkler P, Helmke K, Chumbley LC: Doppler ultrasonography of the ophthalmic and central retinal vessels. Arch Ophthalmol 1991; 109: 532–536. [DOI] [PubMed] [Google Scholar]
- 17.Rojanapongpun P, Drance SM: Velocity of ophthalmic arterial flow recorded by Doppler ultrasound in normal subjects. Am J Ophthalmol 1993; 115: 174–180. [DOI] [PubMed] [Google Scholar]
- 18.Williamson TH, Harris A: Color Doppler ultrasound imaging of the eye and orbit. Surv Ophthalmol 1996; 40: 255–267. [DOI] [PubMed] [Google Scholar]
- 19.Nowicki A: Echografia dopplerowska. Instytut Podstawowych Problemów Techniki PAN, Warszawa: 1985. [Google Scholar]
- 20.Nowicki A: Podstawy obrazowania przepływów metodą dopplerowską. Ultrason Pol 1991; 1: 163–183. [Google Scholar]
- 21.American Institute of Ultrasound in Medicine: Bioeffects and Safety of Diagnostic Ultrasound. AIUM, Laurel, Maryland: 1993. [Google Scholar]
- 22.Filipczyński L, Etienne J, Łypacewicz G, Sałkowski J: Visualizing internal structures of the eye by means of ultrasonics. Proc Vibr Probl 1967; 4: 357–368. [Google Scholar]
- 23.Nowicki A, Lewin PA, Łypacewicz G: Dopuszczalne dawki mocy akustycznych w ultradźwiękowych urządzeniach diagnostycznych. Ultrasonografia 2000; 4: 17–25. [Google Scholar]
- 24.King RL, Liu Y, Harris GR: Quantification of temperature rise within the lens of the porcine eye caused by ultrasound insonation. Ultrasound Med Biol 2017; 43: 476–481. [DOI] [PubMed] [Google Scholar]
- 25.Barnett SB, ter Haar GR, Ziskin MC, Nyborg WL, Maeda K, Bang J: Current status of research on biophysical effects of ultrasound. Ultrasound Med Biol 1994; 20: 205–218. [DOI] [PubMed] [Google Scholar]
- 26.Modrzejewska M: The power of ultrasonic beam and safety of its use in examination of the eye ball. Ultrasonografia 2011; 11: 70–72. [Google Scholar]
- 27.Baxter GM, Williamson TH: Color Doppler imaging of the eye: normal ranges; reproducibility, and observer variation. J Ultrasound Med 1995; 14: 91–96. [DOI] [PubMed] [Google Scholar]
- 28.Kaiser HJ, Schötzau A, Flammer J: Blood-flow velocities in the extraocular vessels in normal volunteers. Am J Ophthalmol 1996; 122: 364–370. [DOI] [PubMed] [Google Scholar]
- 29.Baxter GM, Williamson TW, McKillop G, Dutton GN: Color Doppler ultrasound of orbital and optic nerve blood flow: effects of posture and timolol 0.5%. Invest Ophthalmol Vis Sci 1992; 33: 604–610. [PubMed] [Google Scholar]
- 30.Modrzejewska M, Karczewicz D, Pieńkowska-Machoy E: Wpływ miejscowo podawanego inhibitora anhydrazy węglanowej na przepływ krwi w naczyniach gałki ocznej i na funkcję czynnościową siatkówki u osób z jaskrą – doniesienie wstępne. Ultrasonografia 2006; 26: 64–69. [Google Scholar]
- 31.Galassi F, Sodi A, Renieri G, Ucci F, Pieri B, Harris A et al.: Effects of timolol and dorzolamide on retrobulbar hemodynamics in patients with newly diagnosed primary open-angle glaucoma. Ophthalmologica 2002; 216: 123–128. [DOI] [PubMed] [Google Scholar]
- 32.Williamson TH, Harris A: Ocular blood flow measurement. Br J Ophthalmol 1994; 78: 939–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Erdogmus S, Govsa F: Topography of the posterior arteries supplying the eye and relations to the optic nerve. Acta Ophthalmol Scand 2006; 84: 642–649. [DOI] [PubMed] [Google Scholar]
- 34.Harris A, Harris M, Biller J, Garzozi H, Zarfty D, Ciulla TA et al.: Aging affects the retrobulbar circulation differently in woman and men. Arch Ophthalmol 2000; 118: 1076–1080. [DOI] [PubMed] [Google Scholar]
- 35.Gillies WE, Brooks AM, Scott M, Ryan L: Comparison of colour Doppler imaging of orbital vessels in elderly compared with young adult patients. Aust N Z J Ophthalmol 1999; 27: 173−175. [DOI] [PubMed] [Google Scholar]
- 36.Modrzejewska M, Siesky B, Amireskandari A, Holland S, Grzesiak W, Zaborski D et al.: Parameters characterizing age-dependent retrobulbar circulation in healthy subjects measured by color Doppler ultrasonography. Curr Eye Res 2015; 40: 729–736. [DOI] [PubMed] [Google Scholar]
- 37.Stalmans I, Vandewalle E, Anderson DR, Costa VP, Frenkel RE, Garhofer G et al.: Use of colour Doppler imaging in ocular blood flow research. Acta Ophthalmol 2011; 89: e609–630. [DOI] [PubMed] [Google Scholar]
- 38.Hayreh SS, Dass R: The ophthalmic artery: I. Origin and intra-cranial and intra-canalicular course. Br J Ophthalmol 1962; 46: 65–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.François J, Fryczkowski A: Functional importance of central retinal artery anastomoses in the anterior part of the optic nerve. Ophthalmologica 1982; 185: 15–25. [DOI] [PubMed] [Google Scholar]
- 40.Hayreh SS: Blood supply of the optic nerve head and its role in optic atrophy, glaucoma and oedema of the optic disc. Br J Ophthalmol 1969; 53: 721–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Modrzejewska M: Ultrasonographic measurements of blood flow parameters in eyeball and retrobulbar vessels. Ultrasonografia 2011; 11: 73–77. [Google Scholar]
- 42.Harris A, Evans D, Martin B, Zalish M, Kagemann L, McCranor L et al.: Nocturnal blood pressure reduction: effect on retrobulbar hemodynamics in glaucoma. Graefes Arch Clin Exp Ophthalmol 2002; 240: 372−378. [DOI] [PubMed] [Google Scholar]
- 43.Modrzejewska M, Ostanek L, Bobrowska-Snarska D, Karczewicz D, Wilk G, Brzosko M et al.: Ocular circulation in systemic lupus erythematosus. Med Sci Monit 2009; 15: CR573–578. [PubMed] [Google Scholar]
- 44.Modrzejewska M: Characteristics of changes in blood flow velocity parameters in Doppler ultrasonography examination in some of the ophthalmologic ailments of vascular origin. Ultrasonografia 2006; 26: 21–28. [Google Scholar]
- 45.Hussain RM, Harris A, Siesky, Yung CW, Ehrlich R, Prall R: The effect of pegaptanib (Macugen) injection on retinal and retrobulbar blood flow in retinal Ischaemic diseases. Acta Ophthalmol 2015; 93: e399–400. [DOI] [PubMed] [Google Scholar]
- 46.Tobe LA, Harris A, Hussain RM, Eckert G, Huck A, Park J et al.: The role of retrobulbar and retinal circulation on optic nerve head and retinal nerve fibre layer structure in patients with open-angle glaucoma over an 18-month period. Br J Ophthalmol 2015; 99: 609–612. [DOI] [PubMed] [Google Scholar]
- 47.Dimitrova G, Kato S: Color Doppler imaging of retinal diseases. Surv Ophthalmol 2010; 55: 193–214. [DOI] [PubMed] [Google Scholar]
- 48.Modrzejewska M, Pieńkowska-Machoy E, Grzesiak W, Karczewicz D, Wilk G: Predictive value of color Doppler imaging in an evaluation of retrobulbar blood flow perturbation in young type-1 diabetic patients with regard to dyslipidemia. Med Sci Monit 2008; 14: MT47–52. [PubMed] [Google Scholar]
- 49.Ozcan PY, Dogan F, Sonmez K, Con R, Dokumaci DS, Seyhanli ES: Assessment of orbital blood flow velocities in retinopathy of prematurity. Int Ophthalmol 2017; 37: 795–799. [DOI] [PubMed] [Google Scholar]
- 50.Safina ER, Gabdrakhmanova AF, Verzakova IV: [Value of complex ultrasonography in children with concomitant strabismus]. Vestn Oftalmol 2011; 127: 16–19. [PubMed] [Google Scholar]
- 51.Modrzejewska M, Wiącek MP: A novel approach to the differentiation of intrabulbar tumors in color Doppler imaging. Curr Eye Res 2017; 42: 1482–1490. [DOI] [PubMed] [Google Scholar]
- 52.Grand-Perret V, Jacquet JR, Leguerney I, Benatsou B, Grégoire JM, Willoquet G et al.: A novel microflow phantom dedicated to ultrasound microvascular measurements. Ultrason Imaging 2018; 40: 325–338. [DOI] [PubMed] [Google Scholar]


