Abstract
Background:
We will investigate the efficacy and safety of weekly cisplatin (WC) for treatment of patients with breast cancer (BC) systematically.
Methods:
This study will describe and critically appraise shared decision approaches used in randomized controlled trials of WC for treatment of patients with BC. We will comprehensively search the following databases: PubMed, EMBASE, Web of Science, Cochrane Library, CINAHL, PsycINFO, Allied and Complementary Medicine Database, Wanfang, and Chinese Biomedical Literature Database from inception through July 1, 2019. We will utilize RevMan V.5.3 software (London, UK) for statistical analysis.
Results:
This study will systematically explore the efficacy and safety of WC for the treatment of patients with BC through evaluating primary outcomes of overall survival, pathological complete response; and secondary outcomes of cancer-specific survival, recurrence-free survival, disease-free survival, quality of life, and toxicities.
Conclusion:
This study will provide latest evidence of WC for the treatment of patients with BC.
Systematic review registration:
PROSPERO CRD42019145358.
Keywords: breast cancer, efficacy, safety, weekly cisplatin
1. Introduction
Breast cancer (BC) is one of the most leading cancers with a high mortality rate.[1–3] Among the 36 most common cancers, BC accounts for 11.6% of all of them.[4–7] It has been reported that BC leads to 6.6% of cancer death in 2018.[8,9] In China, it has been estimated that the rate of BC has increased and associated with the economic status.[10] It has also reported that the incidence rates are 0.034% and 0.017% for urban and rural areas, respectively.[10] In the United States, the cases of BC will reach 268,600 and more than 41,000 patients are predicted to die in 2019.[9] In addition, there are about 3.1 million patients suffering from BC and are receiving treatments.[10]
A variety of clinical studies have reported that weekly cisplatin (WC) has been widely used for the treatment of patients with BC.[11–21] However, its conclusion is still unclear, and no systematic review has assessed the efficacy and safety of WC for BC. Therefore, this study will systematically evaluate the efficacy and safety of WC for the treatment of patients with BC.
2. Methods
2.1. Ethics and dissemination
This study will not inquire ethic approval because we will not use individual patient data. The results of this study are expected to be published at peer-reviewed journals.
2.2. Eligibility criteria for study selection
2.2.1. Type of studies
All randomized controlled trials (RCTs) will be selected if they focus on assessing the efficacy and safety of WC for the treatment of patients with BC. However, we will exclude studies of nonclinical studies, and non-RCTs.
2.2.2. Type of participants
Any patients diagnosed with BC will be included regardless the race, age, gender, and economic status.
2.2.3. Type of interventions
Experimental group: patients received WC monotherapy will be used for inclusion.
Control group: patients received any interventions except any forms of WC will be included.
2.2.4. Type of outcome measurements
Primary outcomes include overall survival, and pathological complete response.
Secondary outcomes consist of cancer-specific survival; recurrence-free survival; disease-free survival; quality of life, as measured by any related scales; and toxicities.
2.3. Data sources and search strategy
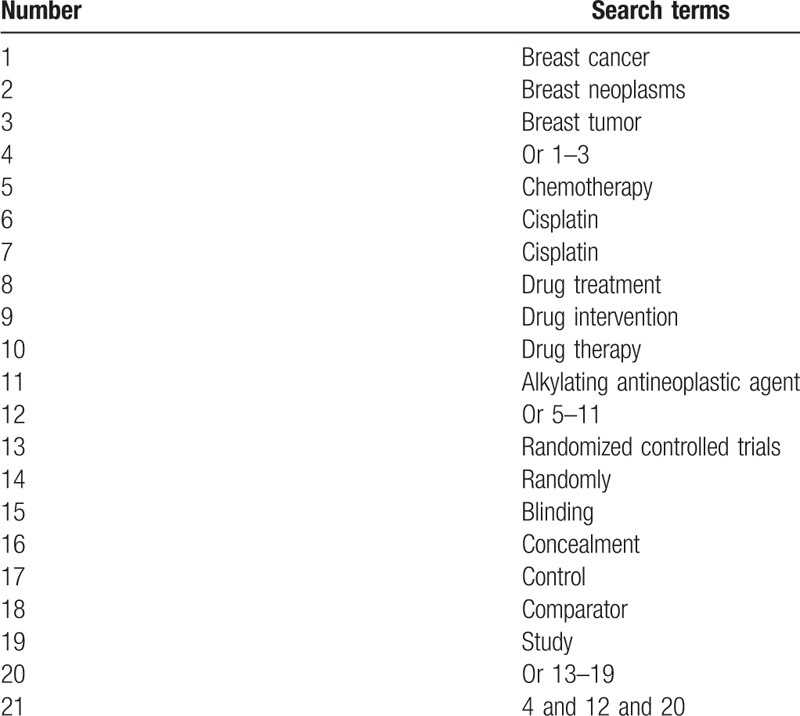
The plan is based on a comprehensively approach to RCTs identification using the following databases: PubMed, EMBASE, Web of Science, Cochrane Library, CINAHL, PsycINFO, Allied and Complementary Medicine Database, Wanfang, and Chinese Biomedical Literature Database from inception through July 1, 2019. Additionally, we will also search dissertations, conference proceedings, and reference lists of included studies. The search strategy for PubMed is showed in Table 1.
Table 1.
Search strategy for PubMed.
2.4. Data collection and management
2.4.1. Study selection
Two authors will independently scan all titles and abstracts identified from the search strategy for all databases. All duplicated and irrelevant studies will be excluded. Any discrepancies between 2 authors will be solved by a third author via discussion. Full reports will be further obtained for all remaining studies to judge whether they meet the final eligibility criteria. The results of study selection will be presented in the flowchart.
2.4.2. Data extraction
Two authors will carry out data extraction using a standardized data extraction table to collect relevant data from each eligible trial. The data comprises of publication details, study characteristics, study setting, study design, sample size, patient characteristics, diagnostic criteria, eligibility criteria, experimental and control details, outcome measurements, safety, and follow-up information. Any divergences regarding data extraction between 2 authors will be solved by a third author via discussion. If there is insufficient or missing information, we will contact corresponding authors of primary RCTs. If we cannot receive those data, we will just analyze available data. Moreover, we will discuss its possible impacts in the text.
2.5. Risk of bias assessment for eligible studies
In this study, risk of bias for all eligible studies will be assessed using Cochrane risk of bias tool. This tool has 7 aspects and each aspect is judged as 3 levels: high risk of bias, unclear risk of bias, and low risk of bias. Two authors will independently perform risk of bias for all included studies. All different opinions between 2 authors will be solved by a third author through discussion.
2.6. Treatment effect measurements
For continuous outcomes, mean differences or standardized mean differences with 95% confidence intervals will be exerted. For dichotomous outcomes, risk ratios or odds ratios with 95% confidence intervals will be calculated.
2.7. Statistical analysis
We will use RevMan V.5.3 software (London, UK) for statistical analysis in this study. We will use I2 statistic test to identify heterogeneity among eligible studies. Values of I2 ≤ 50% exert low heterogeneity, and a fixed-effect model will be used. In addition, meta-analysis will be carried out. On the other hand, values of I2 > 50% mean significant heterogeneity, and a random-effect model to will be applied. Under such situation, we will perform subgroup analysis to explore any factors of such high heterogeneity. We will report outcome results as a narrative summary if substantial heterogeneity still exerts after subgroup analysis.
Subgroup analysis will be performed based on the different treatments, controls, and outcomes. Sensitivity analysis will also be carried out by removing studies with high risk of bias. Finally, we will investigate the reporting bias if sufficient trials are entered using funnel plots and Egger linear regression test.
3. Discussion
BC is one of most common cancers in female population. WC has been widely used clinically to treat this disorder. Currently, there is limited evidence to determine whether WC has similar effect on patients with BC. Therefore, the comparisons of the efficacy and safety will be investigated between WC and other interventions in the experimental group and control group. This study will provide high-quality evidence-based medicine to determine whether WC is an effective and safety treatment for patients with BC.
Author contributions
Conceptualization: Ying Ma, Ning An, Yan-cui Liu.
Data curation: Ying Ma, Nai-peng Zhang, Wen-yuan Li, Wei Zhao, Yan-cui Liu.
Formal analysis: Ying Ma, Nai-peng Zhang, Ning An, Wen-yuan Li, Wei Zhao.
Funding acquisition: Yan-cui Liu.
Investigation: Yan-cui Liu.
Methodology: Nai-peng Zhang, Ning An, Wen-yuan Li, Wei Zhao.
Project administration: Ning An, Yan-cui Liu.
Resources: Nai-peng Zhang, Ning An, Wen-yuan Li, Wei Zhao, Yan-cui Liu.
Software: Ying Ma, Nai-peng Zhang, Ning An, Wen-yuan Li, Wei Zhao.
Supervision: Ying Ma, Yan-cui Liu.
Validation: Ying Ma, Ning An, Wei Zhao.
Visualization: Nai-peng Zhang, Wen-yuan Li, Wei Zhao.
Writing – original draft: Ying Ma, Nai-peng Zhang, Ning An, Wen-yuan Li, Wei Zhao, Yan-cui Liu.
Writing – review & editing: Ying Ma, Nai-peng Zhang, Ning An, Wen-yuan Li, Wei Zhao, Yan-cui Liu.
Footnotes
Abbreviations: BC = breast cancer, RCTs = randomized controlled trials, WC = weekly cisplatin.
How to cite this article: Ma Y, Zhang Np, An N, Li Wy, Zhao W, Liu Yc. Clinical efficacy of weekly cisplatin for treatment of patients with breast cancer. Medicine. 2019;98:37(e17114).
This study is supported by National Natural Science Foundation of China (81870977); Heilongjiang Provincial Health and Family Planning Research Project (2017–314); Heilongjiang Province Provincial Higher Education Fund Research Project (2018-KYYWFMY-0026); Mudanjiang Science and Technology Bureau Research Project (G2018d2524). The funder does not have any roles in this study.
The authors have no conflicts of interest to disclose.
References
- [1].Armstrong N, Ryder S, Forbes C, et al. A systematic review of the international prevalence of BRCA mutation in breast cancer. Clin Epidemiol 2019;11:543–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Strober JW, Brady MJ. Dietary fructose consumption and triple-negative breast cancer incidence. Front Endocrinol (Lausanne) 2019;10:367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Pilevarzadeh M, Amirshahi M, Afsargharehbagh R, et al. Global prevalence of depression among breast cancer patients: a systematic review and meta-analysis. Breast Cancer Res Treat 2019;176:519–33. [DOI] [PubMed] [Google Scholar]
- [4].Momenimovahed Z, Salehiniya H. Epidemiological characteristics of and risk factors for breast cancer in the world. Breast Cancer (Dove Med Press) 2019;11:151–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].DeSantis CE, Ma J, Sauer AG, et al. Breast cancer statistics, 2017, racial disparity in mortality by state. CA Cancer J Clin 2017;67:439–48. [DOI] [PubMed] [Google Scholar]
- [6].Wendt C, Margolin S. Identifying breast cancer susceptibility genes: a review of the genetic background in familial breast cancer. Acta Oncol 2019;58:135–46. [DOI] [PubMed] [Google Scholar]
- [7].Husain M, Nolan TS, Foy K, et al. An overview of the unique challenges facing African-American breast cancer survivors. Support Care Cancer 2019;27:729–43. [DOI] [PubMed] [Google Scholar]
- [8].Beckwitt CH, Brufsky A, Oltvai ZN, Wells A. Statin drugs to reduce breast cancer recurrence and mortality. Breast Cancer Res 2018;20:144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J Clin 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- [10].Fan L, Strasser-Weippl K, Li JJ, et al. Breast cancer in China. Lancet Oncol 2014;15:e279–89. [DOI] [PubMed] [Google Scholar]
- [11].Frasci G, D’Aiuto G, Comella P, et al. A 2-month cisplatin-epirubicin-paclitaxel (PET) weekly combination as primary systemic therapy for large operable breast cancer: a phase II study. Ann Oncol 2005;16:1268–75. [DOI] [PubMed] [Google Scholar]
- [12].Sánchez-Escribano Morcuende R, Alés-Martínez JE, Aramburo González PM. Low dose gemcitabine plus cisplatin in a weekly-based regimen as salvage therapy for relapsed breast cancer after taxane-anthracycline-containing regimens. Clin Transl Oncol 2007;9:459–64. [DOI] [PubMed] [Google Scholar]
- [13].Torrisi R, Balduzzi A, Ghisini R, et al. Tailored preoperative treatment of locally advanced triple negative (hormone receptor negative and HER2 negative) breast cancer with epirubicin, cisplatin, and infusional fluorouracil followed by weekly paclitaxel. Cancer Chemother Pharmacol 2008;62:667–72. [DOI] [PubMed] [Google Scholar]
- [14].Huang XE, Li CG, Li Y, et al. Weekly TP regimen as a postoperative adjuvant chemotherapy for completely resected breast cancer in China: final result of a phase II trial. Asian Pac J Cancer Prev 2011;12:2797–800. [PubMed] [Google Scholar]
- [15].Tang LC, Wang BY, Sun S, et al. Higher rate of skin rash in a phase II trial with weekly nanoparticle albumin-bound paclitaxel and cisplatin combination in Chinese breast cancer patients. BMC Cancer 2013;13:232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Sun S, Tang L, Zhang J, et al. Cisplatin improves antitumor activity of weekly nab-paclitaxel in patients with metastatic breast cancer. Int J Nanomedicine 2014;9:1443–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Cancello G, Bagnardi V, Sangalli C, et al. Phase II study with epirubicin, cisplatin, and infusional fluorouracil followed by weekly paclitaxel with metronomic cyclophosphamide as a preoperative treatment of triple-negative breast cancer. Clin Breast Cancer 2015;15:259–65. [DOI] [PubMed] [Google Scholar]
- [18].Frasci G, D’Aiuto G, Comella P, et al. Southern Italy Cooperative Oncology Group, Italy. Preoperative weekly cisplatin, epirubicin, and paclitaxel (PET) improves prognosis in locally advanced breast cancer patients: an update of the Southern Italy Cooperative Oncology Group (SICOG) randomised trial 9908. Ann Oncol 2010;21:707–16. [DOI] [PubMed] [Google Scholar]
- [19].Frasci G, Comella P, Rinaldo M, et al. Preoperative weekly cisplatin-epirubicin-paclitaxel with G-CSF support in triple-negative large operable breast cancer. Ann Oncol 2009;20:1185–92. [DOI] [PubMed] [Google Scholar]
- [20].Frasci G, D’Aiuto G, Comella P, et al. Southern Italy Cooperative Oncology Group (SICOG). Weekly cisplatin, epirubicin, and paclitaxel with granulocyte colony-stimulating factor support vs triweekly epirubicin and paclitaxel in locally advanced breast cancer: final analysis of a sicog phase III study. Br J Cancer 2006;95:1005–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Donadio M, Ardine M, Berruti A, et al. Weekly cisplatin plus capecitabine in metastatic breast cancer patients heavily pretreated with both anthracycline and taxanes. Oncology 2005;69:408–13. [DOI] [PubMed] [Google Scholar]


