Supplemental Digital Content is available in the text
Keywords: intra-aortic balloon pump, left ventricular assist devices, percutaneous coronary intervention, percutaneous mechanical circulatory support devices
Abstract
Background:
Percutaneous mechanical circulatory support devices (pMCSDs) are increasingly used on the assumption (but without solid proof) that their use will improve prognosis. A meta-analysis was undertaken according to the PRISMA guidelines to evaluate the benefits of pMCSDs in patients undergoing high-risk percutaneous coronary intervention (hr-PCI).
Methods:
We searched PubMed, EMbase, Cochrane Library, Clinical Trial.gov, and other databases to identify eligible studies. Relative risks (RRs) and 95% confidence intervals (CIs) were calculated for 30-day and 6-month all-cause mortality rates, reinfarction, and other adverse events using a random effect model.
Results:
Sixteen randomized controlled trials (RCTs) were included in this study. In the pooled analysis, intra-aortic balloon pump (IABP) was not associated with a decrease in 30-day and 6-month all-cause mortality (RR 1.01 95% CI 0.61–1.66; RR 0.88 95% CI 0.66–1.17), reinfarction (RR 0.89 95% CI 0.69–1.14), stroke/transient ischemic attack (TIA) (RR 1.75 95% CI 0.47–6.42), heart failure (HF) (RR 0.54 95% CI 0.11–2.66), repeat revascularization (RR 0.73 95% CI 0.25–2.10), embolization (RR 3.00 95% CI 0.13–71.61), or arrhythmia (RR 2.81 95% CI 0.30–26.11). Compared with IABP, left ventricular assist devices (LVADs) were not associated with a decrease in 30-day and 6-month all-cause mortality (RR 0.96 95% CI 0.71–1.29; RR 1.23 95% CI 0.88–1.72), reinfarction (RR 0.98 95% CI 0.68–1.42), stroke/TIA (RR 0.45 95% CI 0.1–1.95), acute kidney injury (AKI) (RR 0.83 95% CI 0.38–1.80), or arrhythmia (RR 1.52 95% CI 0.71–3.27), but LVADs were associated with a decrease in repeat revascularization (RR 0.26 95% CI 0.08–0.83). However, LVADs significantly increased the risk of bleeding compared with IABP (RR 2.85 95% CI 1.72–4.73).
Conclusions:
Neither LVADs nor IABP improves short or long-term survival in hr-PCI patients. LVADs are more likely to reduce repeat revascularization after PCI, but to increase the risk of bleeding events than IABP.
1. Introduction
According to a report from the American Heart Association, approximately 660,000 Americans will have a new coronary event (defined as first hospitalized myocardial infarction (MI) or coronary heart disease death), and about 305,000 will have a recurrent event per year.[1] Percutaneous coronary intervention (PCI) currently is the preferred method of revascularization according to current guideline.[2] Patients with coronary heart disease undergoing PCI are increasing, especially some high-risk patients due to prohibitively high surgical risk.[3] Briefly, high-risk percutaneous coronary intervention (hr-PCI) mainly includes the following 3 aspects for consideration: patient specific, lesion specific, and clinical presentation. Patient-specific variables include advanced age, underlying diseases, such as peripheral arterial stenosis, severe heart failure (HF) which was defined as left ventricular ejection fraction less than 35%, diabetes, chronic kidney disease (CKD) which meant glomerular filtration rate (GFR) <60 mL/min/1.73 m2 or a urinary albumin-to-creatinine ratio > 30 mg/g for more than 3 months. Lesion-specific variables include unprotected left main stenosis, bifurcation stenosis, triple vessel disease, severe calcification lesion, and chronic total occlusions. Clinical presentation, such as acute coronary syndrome or cardiogenic shock (CS), can increase the risk of PCI.[4] Percutaneous mechanical circulatory devices (pMCSDs) such as intra-aortic balloon pump (IABP) and left ventricular assist devices (LVADs) have been used in hr-PCI.[5] Theoretically, both IABP and LVADs can promote cardiac output (CO) and systemic flow in patients undergoing hr-PCI that may play a positive role in prognosis.[4] However, there are no randomized clinical trials (RCTs) available showing clear benefits from any of the pMCSDs and most of the current guidelines are based on expert consensus.[3] We therefore performed a meta-analysis of pMCSDs in high-risk patients undergoing PCI to provide more clinical evidence.
2. Methods
2.1. Search strategy
PubMed, EMbase, Cochrane Library, Clinical Trial.gov, CNKI, Wanfang, and VIP databases were systematically searched in accordance with the PRISMA guidelines[6] from January 1990 to December 2018 using the following terms: “high-risk percutaneous coronary intervention,” “mechanical circulatory support,” “left ventricular assist devices,” “intra-aortic balloon pump,” “TandemHeart,” “Impella,” “HeartMate,” or “extracorporeal membrane oxygenation (ECMO).” We also analyzed the reference lists of the original studies, review articles, and meta-analyses for potentially eligible studies. Inclusion criteria were the following: randomized trial design, patients undergoing hr-PCI, patients treated with pMCSDs during perioperative period, a report of all-cause mortality and adverse events, and language limited to English and Chinese. Exclusion criteria were cohort studies, cross-sectional surveys, and real-world studies, patients undergoing coronary artery bypass grafting (CABG), and patients treated with systemic thrombolysis. The Human Research Committee of Chongqing Medical University approved this study and waived the need for informed consent. Because this study was a meta-analysis, our data were based on published studies only (see Supplemental Registration Information).
2.2. Outcomes and definitions
The primary end point of this meta-analysis was all-cause mortality. Secondary end points were reinfarction, acute kidney injury (AKI), HF, stroke/transient ischemic attack (TIA), embolization, arrhythmia, repeat revascularization, and bleeding events. Arrhythmias were defined as ventricular tachycardia and ventricular fibrillation.[7,8] HF was defined as congestive heart failure (Killip class 2 to 4).[9] AKI was defined as 2-fold increase in serum creatinine concentration within 48 hours or GFR decreased >50% within 7 days.[10] Embolization was defined as distal embolization or thrombus.[8,11] The definition of major bleeding events was serum hemoglobin level decrease of at least 5 g/dL and needed transfusion therapy or surgery to control the bleeding.[12]
2.3. Quality assessment
The quality of each study was assessed by 2 independent reviewers (K.W. and W.H.) according to the guideline of the Cochrane collaboration's tool which is a domain-based evaluation system composed of 6 principles: selection bias, performance bias, detection bias, attrition bias, reporting bias, other bias. Each item is evaluated by “low risk of bias,” “unclear risk of bias,” or “high risk of bias.”
2.4. Data extraction
Two reviewers (W.S. and W.W.) independently extracted the data from original studies; disagreements were resolved by consultation with a third reviewer. The data we extracted included: first author's name, region and year of each trial, sample size, age distribution, intervention measures, follow-up duration, all-cause mortality and adverse events frequency.
2.5. Statistical analyses
We counted frequency of all-cause mortality, survival, reinfarction, bleeding, and other adverse events within 30 days (including in-hospital data) and 6 months. Stata 12.0 (Stata Corporation, College Station, TX) was used for the meta-analysis. Relative risks (RRs) and 95% confidence intervals (CIs) were used to describe the relationship between pMCSDs and the risk of all-cause mortality, reinfarction, bleeding, and other adverse events for the pooled analysis. Heterogeneity was examined using Cochran's Q and the I2 statistic. P values < .05 were considered to indicate significant heterogeneity, and I2 values > 50% were considered to indicate high levels of heterogeneity between studies. We used the random effect models for analyses since all trials were done independently. Publication bias was explained by funnel plot.
3. Results
3.1. Study characteristics
Our search criteria retrieved 1477 articles, including 239 duplicate articles. Twenty-seven articles about interventional clinical trials were screened out by reading the title and abstract. The excluded articles were reviews, clinical guidelines, animal studies, case reports, and cohort studies. After reading the full text, 9 nonrandomized controlled trials were excluded. Finally, 16 RCTs,[7,8,11,13–27] enrolling a total of 3266 patients, remained eligible for meta-analysis, of which 9 used IABP as intervention group[7,8,11,13–20] and 7 used LVADs[21–27] (Fig. 1). All of these articles were published from 1993 to 2018. We did not find any RCT reporting the use of ECMO for hr-PCI. Of the 16 RCTs, 6 were from American clinical centers,[11,13,18,21,25,26] 7 from Europe,[11,14,16,17,19,20,22–25,27] and 3 from Asia.[7,8,15] Patients with advanced age, severe HF, MI, CKD were included. Follow-up period ranged from 28 days to 51 months as Table 1 showed. Table 2 showed all-cause mortality rate and adverse events frequency. The results of literature quality assessment were shown in Figure 2.
Figure 1.
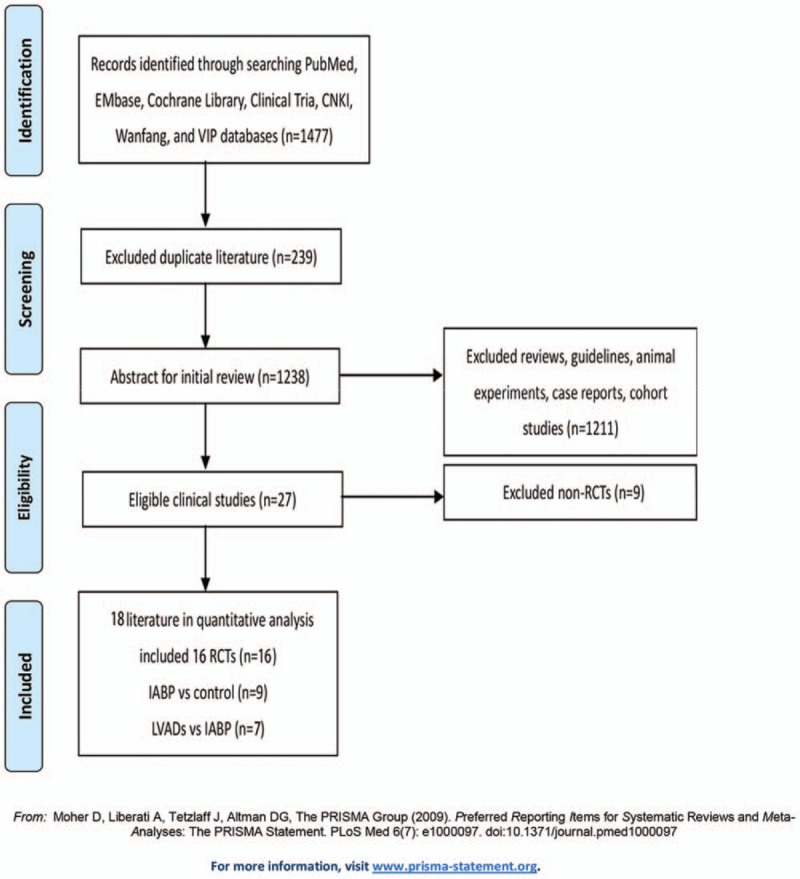
Study selection according to PRISMA principle.
Table 1.
Characteristics of included studies.
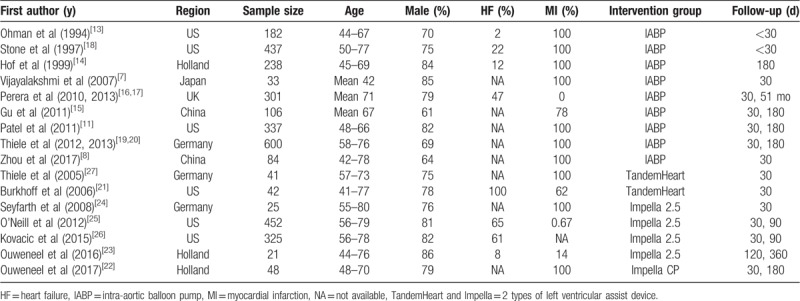
Table 2.
All-cause mortality and adverse events frequency.
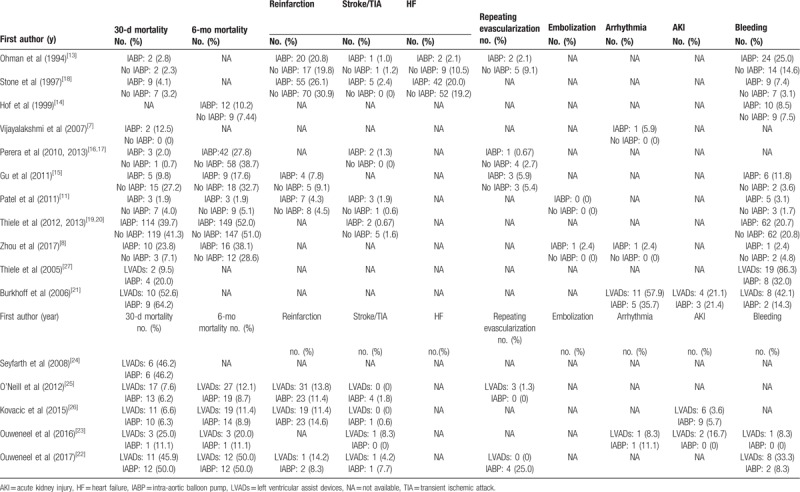
Figure 2.
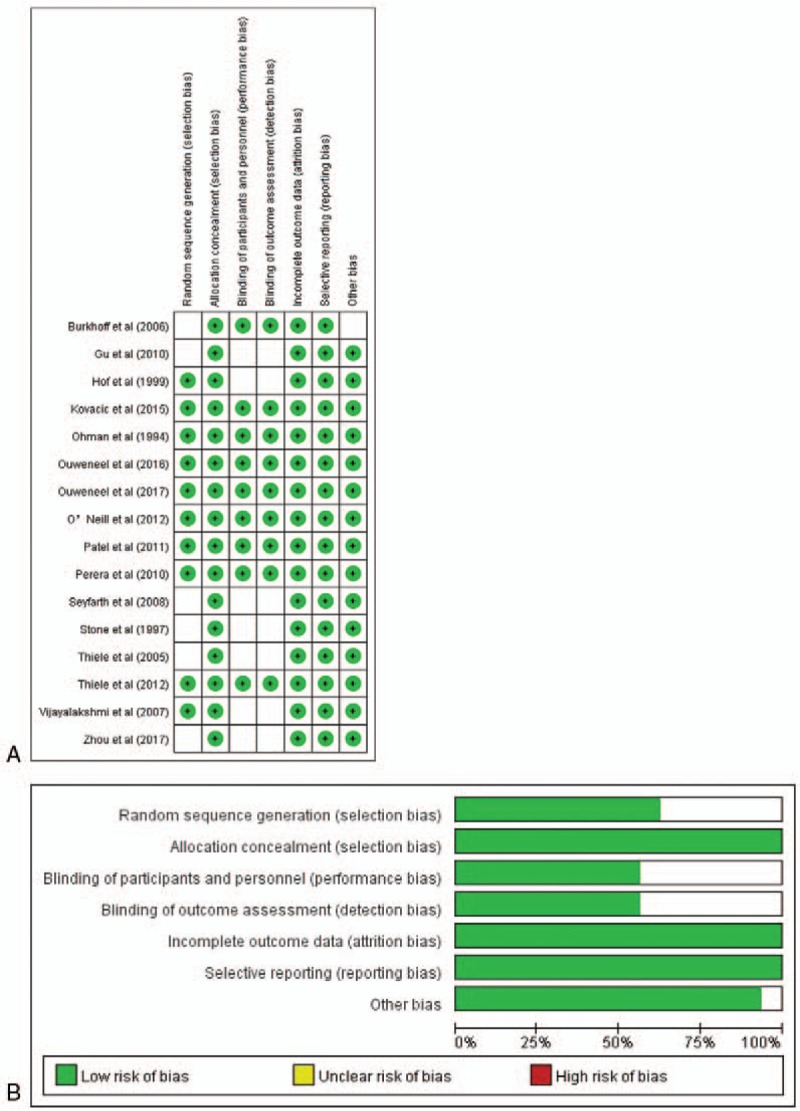
Risk of bias. A, Risk of bias summary. B, Risk of bias graph.
3.2. The primary end point
3.2.1. All-cause mortality
Eight RCTs reported IABP use and short-term all-cause mortality.[7,8,11,13,15,17–19] It is noteworthy that in the IABP-SHOCK II study,[19] the proportion of patients undergoing PCI was 95.8%, so we excluded 3 patients with CABG. The heterogeneity of the 8 RCTs was moderate (P = .103, I2 = 41.3%). The pooled analysis revealed that compared with the blank control group, IABP was not associated with a decrease in all-cause mortality within 30 days (RR 1.01 95% CI 0.61–1.66) (Fig. 3A). No obvious publication bias was found in funnel plot (see Supplemental Figure S1). Considering that CS might have a negative effect on prognosis, we did subgroup analyses on patients with and without shock. No significant difference was observed in patients with or without CS (RR 1.54 95% CI 0.47–5.10; RR 0.82 95% CI 0.40–1.67) (Fig. 3A).
Figure 3.
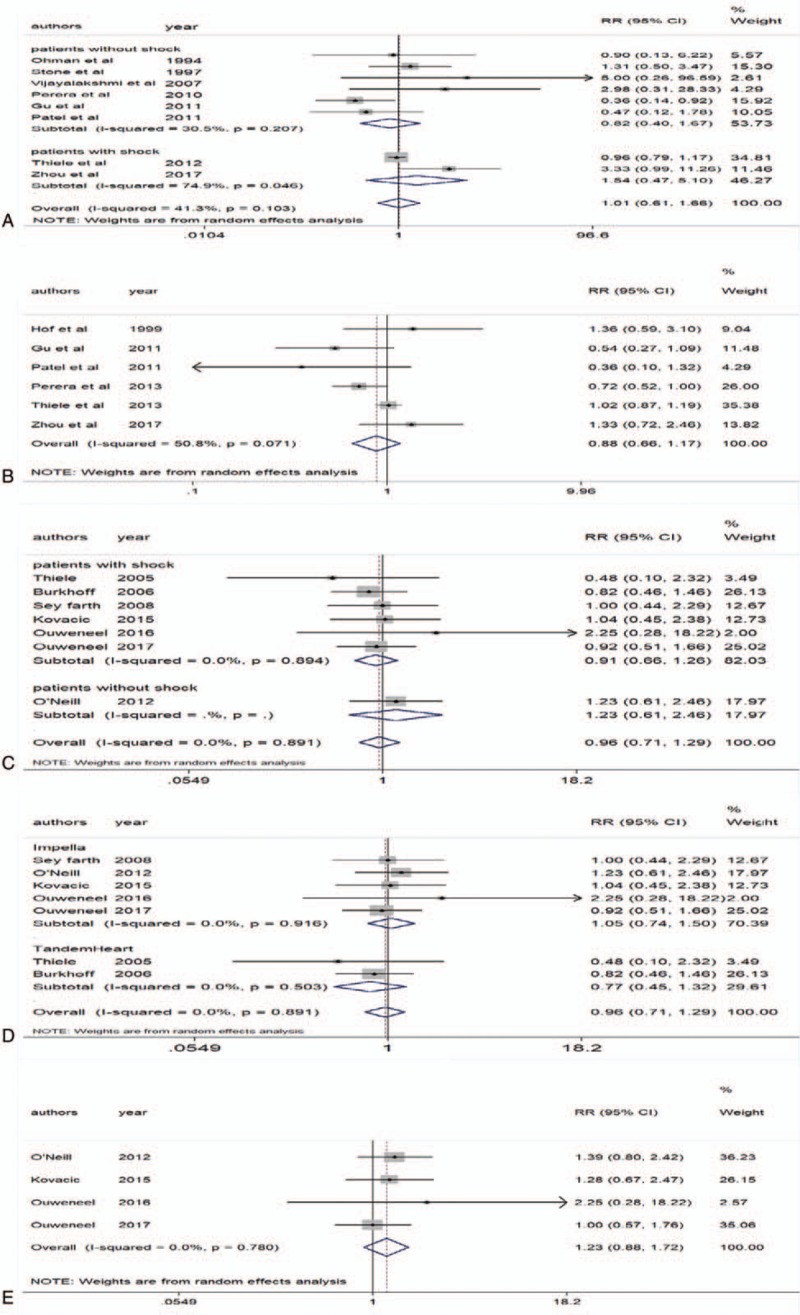
All-cause mortality. A, Intra-aortic balloon pump (IABP) and 30-d all-cause mortality. B, IABP and 6-mo all-cause mortality. C, Left ventricular assist devices (LVADs) for patients with or without shock and 30-d all-cause mortality. D, Two types of LVADs and 30-d all-cause mortality. E, LVADs and 6-mo all-cause mortality.
Six RCTs reported IABP use and long-term all-cause mortality.[8,11,14–16,20] The heterogeneity was slightly higher (P = .071, I2 = 50.8%). The pooled analysis revealed that compared with the blank control group, IABP was not associated with a decrease in all-cause mortality over 6 months (RR 0.88 95% CI 0.66–1.17) (Fig. 3B).
Seven RCTs reported LVADs use and short-term all-cause mortality.[21–27] It is noteworthy that in the PROTECT II study,[25] we extracted the outcomes for the intention-to-treat population. The heterogeneity was moderate (P = .891, I2 = 0%). The pooled analysis revealed that compared with IABP, LVADs were not associated with a decrease in all-cause mortality over 30 days (RR 0.96 95% CI 0.71–1.29) (Fig. 3C). Also no significant difference was observed in patients with or without CS (RR 0.91 95% CI 0.66–1.26; RR 1.23 95% CI 0.61–2.46) (Fig. 3C). Considering that 2 trials used Tandem Heart,[21,27] and the other 5 trials used Impella,[22–26] which had different procedures and hemodynamics, we did subgroup analyses showed that neither Impella2.5/CP nor TandemHeart was associated with a decrease in all-cause mortality for 30 days (RR 1.05 95% CI 0.74–1.50; RR 0.77 95% CI 0.45–1.32) (Fig. 3D). Additionally, no obvious publication bias was found (see Supplemental Figure S2).
Four RCTs reported LVADs use and long-term all-cause mortality.[22,23,25,26] The heterogeneity was moderate (P = .78, I2 = 0%). The pooled analysis revealed that compared with IABP, LVADs were not associated with a decrease in all-cause mortality over 6 months (RR 1.23 95% CI 0.88–1.72) (Fig. 3E).
3.3. Secondary end points
3.3.1. Reinfarction
Reinfarction mainly included an acute myocardial infarction that occurred within 28 days of an incident or recurrent MI. A total of 4 studies provided the data on IABP[11,13,15,18] and 3 studies provided data on LVADs.[22,24,25] As shown in Figure 4A, IABP versus no IABP was not associated with a decrease in reinfarction within 30 days (RR 0.89 95% CI 0.69–1.14; P = .922, I2 = 0%). Similarly, we found that LVADs compared with IABP were not associated with a decrease in reinfarction over 6 months (RR 0.98 95% CI 0.68–1.42; P = .449, I2 = 0%) (Fig. 4B).
Figure 4.
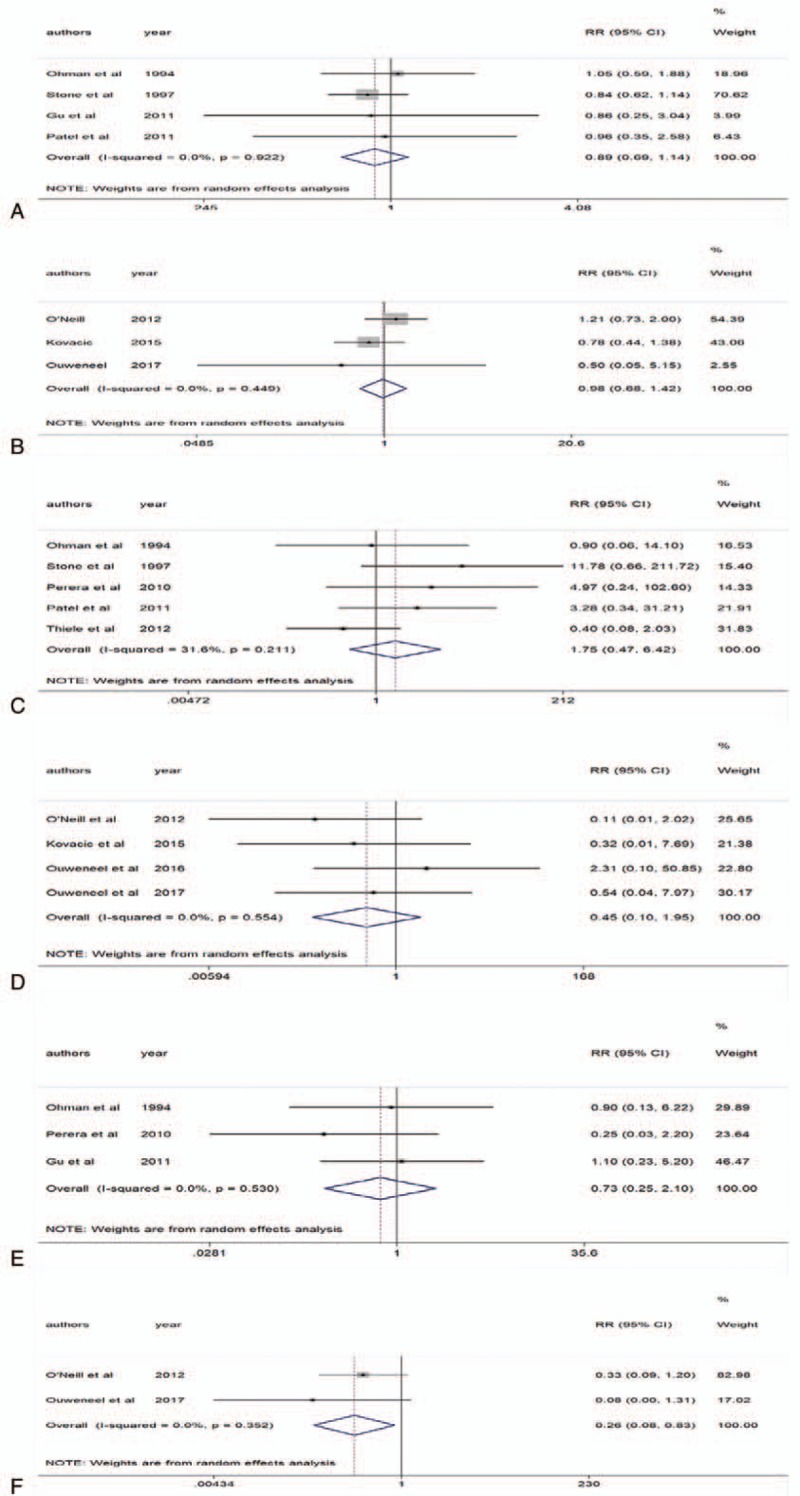
Part one of adverse events. A, Intra-aortic balloon pump (IABP) and 30-d reinfarction. B, Left ventricular assist devices (LVADs) and 6-mo reinfarction. C, IABP and stroke/transient ischemic attack (TIA). D, LVADs and stroke/TIA. E, IABP and repeat revascularization. F, LVADs and repeat revascularization.
3.3.2. Stroke/TIA
Stroke included hemorrhage and ischemia. There were 5 studies that provided the data on IABP[11,13,16,18,19] and 4 studies provided data on LVADs.[22,23,25,26] The pooled analysis revealed that IABP versus no IABP was not associated with a decrease in stroke/TIA (RR 1.75 95% CI 0.47–6.42; P = .211, I2 = 31.6%) (Fig. 4C). Similarly, we found that LVADs compared with IABP were not associated with a decrease in stroke/TIA (RR 0.45 95% CI 0.10–1.95; P = .554, I2 = 0%) (Fig. 4D).
3.4. Repeat revascularization
There were 3 studies that provided the data on IABP[13,15,16] and 2 studies provided data on LVADs.[22,25] The pooled analysis revealed that IABP versus no IABP was not associated with a decrease in repeat revascularization (RR 0.73 95% CI 0.25–2.10; P = .53, I2 = 0%) (Fig. 4E). However, we found that LVADs were significantly associated with a decrease in repeat revascularization compared with IABP (RR 0.26 95% CI 0.08–0.83; P = .352, I2 = 0%) (Fig. 4F).
3.5. Arrhythmia
There were 2 studies provided the data on IABP[7,8] and LVADs.[21,23] The pooled analysis revealed that IABP versus no IABP was not associated with a decrease in arrhythmia (RR 2.81 95% CI 0.30–26.11; P = .995, I2 = 0%) (Fig. 5A). LVADs were not associated with a decrease in arrhythmia compared with IABP (RR 1.52 95% CI 0.71–3.27; P = .58, I2 = 0%) (Fig. 5B).
Figure 5.
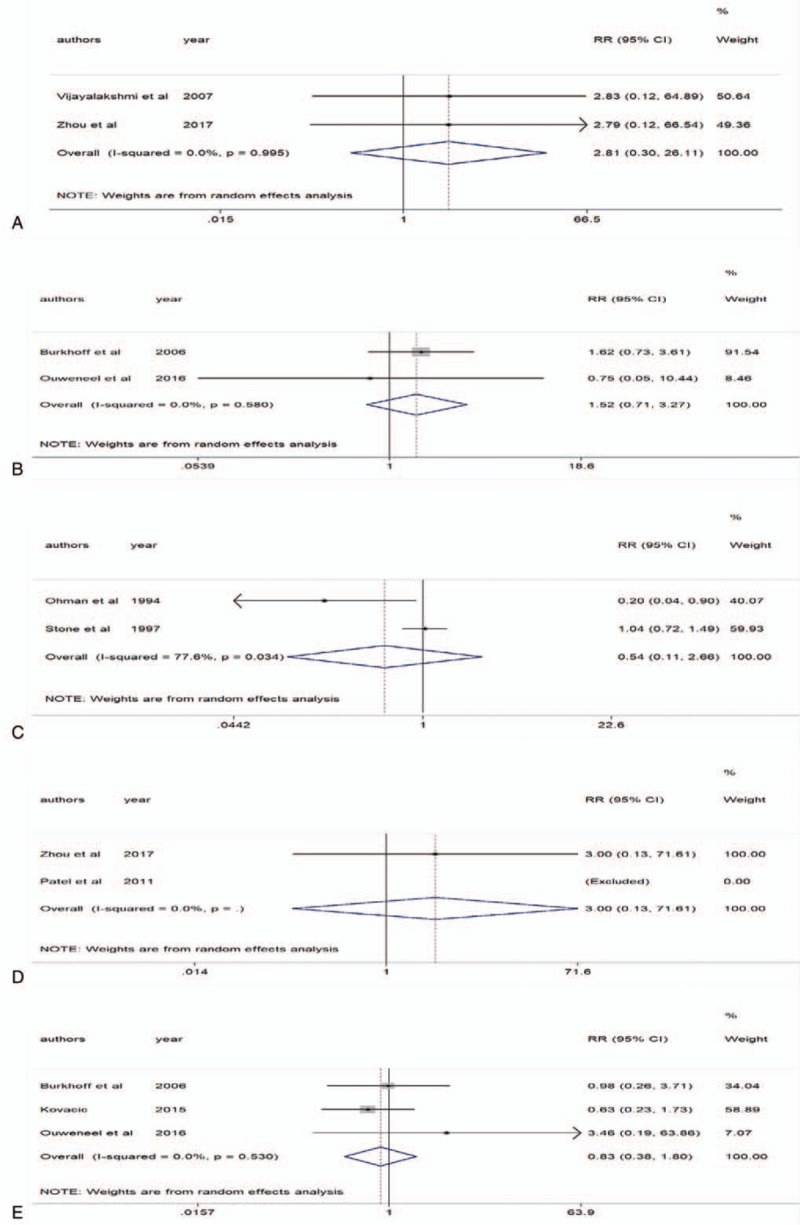
Part 2 of adverse events. A, Intra-aortic balloon pump (IABP) and arrhythmia. B, Left ventricular assist devices (LVADs) and arrhythmia. C, IABP and heart failure (HF). D, IABP and embolization. E, LVADs and acute kidney injury (AKI).
3.6. HF
Two studies provided the data on IABP.[13,18] The heterogeneity was moderate (P = .034, I2 = 77.6%). The pooled analysis revealed that IABP versus no IABP was not associated with a decrease in HF (RR 0.54 95% CI 0.11–2.66) (Fig. 5C). Only 1 trial reported LVADs use and postoperative HF, therefore we did not have enough data for meta-analysis.
3.7. Embolization
Two studies provided the data on IABP,[8,11] but the trial by Patel et al was excluded by Stata12.0 due to no thromboembolic events occurred in IABP group or no IABP group. The pooled analysis revealed that IABP versus no IABP was not associated with a decrease in embolization (RR 3.00 95% CI 0.13–71.61) (Fig. 5D). We did not analyze the association between LVADs use and embolization due to limited data.
3.8. AKI
Three RCTs reported LVADs use and AKI.[21,23,26] The heterogeneity was moderate (P = .53, I2 = 0%). The pooled analysis revealed that LVADs versus IABP were not associated with a decrease in AKI (RR 0.83 95% CI 0.38–1.80) (Fig. 5E). No clinical trial reported the association between IABP use and AKI.
3.9. Major bleeding events
Bleeding mainly occurred at the puncture site, gastrointestinal tract, and intracranial vessels in identified studies. Disseminated intravascular coagulation (DIC) also was grouped with hemorrhagic events. There were 7 RCTs with low levels of heterogeneity (P = .367, I2 = 8.1%) which provided the data on IABP.[8,11,13–15,18,19] IABP showed a strong trend to increase bleeding events, but without statistical significance (RR 1.25 95% CI 0.94–1.66) (Fig. 6A). However, analysis of 4 RCTs[21–23,27] suggested that LVADs were significantly associated with an increase in bleeding events compared with IABP (RR 2.85 95% CI 1.72–4.73, heterogeneity P = .964, I2 = 0%) (Fig. 6B). For LVADs, subgroup analyses showed that patients with or without CS had similar bleeding events rate (RR 2.74 95% CI 1.58–4.73; RR 3.63 95% CI 0.98–13.40) (Fig. 6B).
Figure 6.
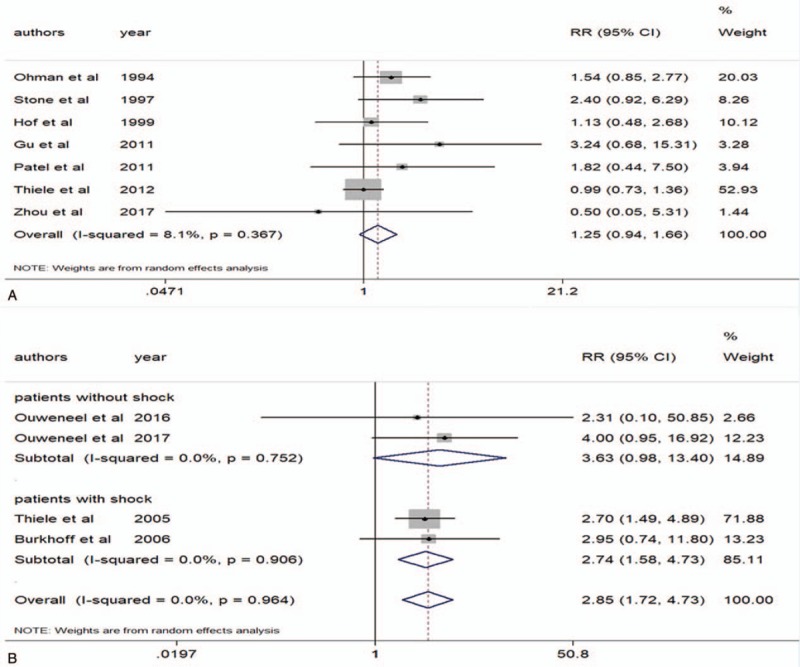
Bleeding events. A, Intra-aortic balloon pump (IABP) and bleeding events. B, Left ventricular assist devices (LVADs) and bleeding events.
4. Discussion
PMCSDs include IABP, LVADs (Impella, TandemHeart, HeartMate), and ECMO.[28] Existing clinical trials have confirmed that pMCSDs maintain vital organ perfusion by mechanically improving CO in patients undergoing hr-PCI.[29] Additionally, pMCSDs have been shown to improve left ventricular unloading, and reduce myocardial oxygen consumption and left ventricular wall tension, and thus alleviate pulmonary congestion.[30] By contrast, some patients with complex medical comorbidities are not candidates for surgical revascularization, thus PCI is the only acceptable treatment.[31]
Our meta-analysis showed that neither IABP nor LVADs could improve the survival of patients with PCI treatment over 30 days and 6 months, besides they had a neutral effect on preventing reinfarction, stroke/TIA, AKI, and arrhythmia. Because both IABP and LVADs improve the hemodynamic stability of the coronary artery, they may play a role in reducing the rupture of unstable plaques.[32] However, MI, HF, CS, and implantation of IABP and LVADs can induce systemic inflammatory response syndrome (SIRS).[33] SIRS might be associated with ischemia-reperfusion injury after PCI, infection, and overreaction of immune system to the catheter of IABP or LVADs.[34] It may stimulate the production of toxic NO and superoxide ions, resulting in sustained myocardial cell injury.[35] Therefore, high-risk patients with severe HF, CKD, and diabetes might be vulnerable to multiple organ dysfunction syndrome (MODS), which cannot be relieved by pMCSDs.[22] Hypoperfusion of organs, disorders of internal environment, and coagulation system may further increase the risk of reinfarction, stroke/TIA, arrhythmia, embolization, and AKI.[36] Recent studies suggested that PCI, rather than pMCSDs, have positive effects on long-term prognosis by salvaging myocardium.[20] The result of our analysis that LVADs were more effective than IABP in reducing repeat revascularization rate might be due to their powerful hemodynamic support.[37]
On the other hand, our meta-analysis suggested that LVADs have a higher risk of bleeding compared with IABP. There are several possible explanations. Compared with IABP, LVADs have more complex operations and larger sheaths, thus increasing the risk of bleeding at the puncture site. The routine use of dual antiplatelet therapy in addition to anticoagulation after PCI may also increase the risk of bleeding. LVADs implantation may be more likely to activate exogenous coagulation pathways, even to induce DIC.[38]
Compared with the clinical trials, patients in the USpella registry suffered from more underlying diseases (CKD, prior MI) and extensive coronary artery disease.[39] In this real-world study, 637 patients were enrolled, 339 of whom met enrollment criteria for the PROTECT II trial.[25,39] Despite the higher risk of registry patients, the 30-day incidence of mortality and adverse events were not different for patients with IABP or Impella2.5, which is similar to the outcomes in our meta-analysis and PROTECT II trial.[39] Since less rigorous follow-up medical records in registry studies were incomparable in RCTs, it was inaccurate to guide clinical treatment based on the results of registry studies. Therefore, more powerful RCTs are required to assess benefits from pMCSDs in patients with hr-PCI.
Compared with previous literature,[40,41] our meta-analysis has several innovations. First, we included the most recent clinical trials. Second, we conducted a subgroup analysis that evaluated the efficacy of Impella and TandemHeart respectively. In a meta-analysis including 4 RCTs and 2 observational studies,[40] significant differences were observed between LVADs and IABP group in the composite, in-hospital, nonmajor adverse cardiac and cerebrovascular events rate (RR 1.30 95% CI 1.01–1.68). In the present analysis we did not include observational studies because they differed from RCTs in aspects of population selection and study design. Rios et al[41] included 5 RCTs and 1 nonrandomized controlled trial and found that LVADs increased the whole adverse events rate (AKI, limb ischemia, infection, major bleeding, and vascular injury) compared with IABP (RR 1.65, 95% CI 1.14–2.39). Our study had analyzed the incidence rate of AKI, embolization, stroke, and major bleeding events respectively but only found out that LVADs increased the risk of bleeding compared with IABP. The possible explanation was that bleeding events accounted for a high weight (31.2%) of the total adverse events, leading to an increase in overall RR value in Rios et al's study.
Our analyses have some limitations. Amalgamation of aggregate patient data in meta-analyses has well-known limitations. Due to the limitation of original data, we did not analyze cardiac mortality and noncardiac mortality separately. The small number of trials reporting association between LVADs and repeat revascularization rate could possibly lead to a type II error of the heterogeneity test.
5. Conclusions
For high-risk patients undergoing PCI, pMCSDs do not reduce in short or long-term all-cause mortality. LVADs seem to reduce repeat revascularization rate compared with IABP. However, the use of LVADs increases the risk of major bleeding events.
Author contributions
Conceptualization: Wenhai Shi.
Data curation: Wenhai Shi, Wuwan Wang.
Formal analysis: Kechun Wang.
Funding acquisition: Wei Huang.
Methodology: Wenhai Shi, Wuwan Wang, Kechun Wang, Wei Huang.
Resources: Wenhai Shi, Wuwan Wang.
Software: Wuwan Wang.
Supervision: Wei Huang.
Writing – original draft: Wenhai Shi.
Writing – review & editing: Wenhai Shi, Wuwan Wang, Kechun Wang, Wei Huang.
Wenhai Shi orcid: 0000-0002-3060-7191.
Supplementary Material
Supplementary Material
Footnotes
Abbreviations: AKI = acute kidney injury, CABG = coronary artery bypass grafting, CI = confidence interval, CKD = chronic kidney disease, CO = cardiac output, CS = cardiogenic shock, DIC = disseminated intravascular coagulation, ECMO = extracorporeal membrane oxygenation, GFR = glomerular filtration rate, HF = heart failure, hr-PCI = high-risk percutaneous coronary intervention, IABP = intra-aortic balloon pump, LVADs = left ventricular assist devices, MI = myocardial infarction, MODS = multiple organ dysfunction syndrome, PCI = percutaneous coronary intervention, pMCSDs = percutaneous mechanical circulatory devices, RCTs = randomized clinical trials, RR =relative risk, SIRS = systemic inflammatory response syndrome, TIA = transient ischemic attack.
How to cite this article: Shi W, Wang W, Wang K, Huang W. Percutaneous mechanical circulatory support devices in high-risk patients undergoing percutaneous coronary intervention. Medicine. 2019;98:37(e17107).
The work was supported by the National Natural Science Foundation of China [grant numbers 81170188 and 30971212].
The authors declare that there is no conflicts of interest.
Supplemental Digital Content is available for this article.
References
- [1].Writing Group M, Mozaffarian D, Benjamin EJ, et al. Heart Disease and Stroke Statistics-2016 Update: a report from the American Heart Association. Circulation 2016;133:e38–60. [DOI] [PubMed] [Google Scholar]
- [2].O’Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2013;127:e362-eL 425. [DOI] [PubMed] [Google Scholar]
- [3].Rihal CS, Naidu SS, Givertz MM, et al. 2015 SCAI/ACC/HFSA/STS Clinical Expert Consensus Statement on the use of percutaneous mechanical circulatory support devices in cardiovascular care. J Am Coll Cardiol 2015;65:e7–26. [DOI] [PubMed] [Google Scholar]
- [4].Myat A, Patel N, Tehrani S, et al. Percutaneous circulatory assist devices for high-risk coronary intervention. JACC Cardiovasc Interv 2015;8:229–44. [DOI] [PubMed] [Google Scholar]
- [5].Naidu SS. Novel percutaneous cardiac assist devices: the science of and indications for hemodynamic support. Circulation 2011;123:533–43. [DOI] [PubMed] [Google Scholar]
- [6].Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol 2009;62:1006–12. [DOI] [PubMed] [Google Scholar]
- [7].Vijayalakshmi K, Kunadian B, Whittaker VJ, et al. Intra-aortic counterpulsation does not improve coronary flow early after PCI in a high-risk group of patients: observations fr. Invasive Cardiol 2007;19:1–2. [PubMed] [Google Scholar]
- [8].Zhou M, Yu K, Wang XH, et al. Analysis on application timing of IABP in emergency PCI treatment of patients with combined acute myocardial infarction and cardiac shock. Eur Rev Med Pharmacol Sci 2017;21:2934–9. [PubMed] [Google Scholar]
- [9].Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur J Heart Fail 2016;18:891–975. [DOI] [PubMed] [Google Scholar]
- [10].Bellomo R, Ronco C, Kellum JA, et al. Acute renal failure—definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care 2004;8:R204–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Patel MR, Smalling RW, Thiele H, et al. Intra-aortic balloon counterpulsation and infarct size in patients with acute anterior myocardial infarction without shock: the CRISP AMI randomized trial. JAMA 2011;306:1329–37. [DOI] [PubMed] [Google Scholar]
- [12].Kinnaird TD, Stabile E, Mintz GS, et al. Incidence, predictors, and prognostic implications of bleeding and blood transfusion following percutaneous coronary interventions. Am J Cardiol 2003;92:930–5. [DOI] [PubMed] [Google Scholar]
- [13].Ohman EM, George BS, White CJ, et al. Use of aortic counterpulsation to improve sustained coronary artery patency during acute myocardial infarction. Circulation 1994;90:792–8. [DOI] [PubMed] [Google Scholar]
- [14].Hof AW, Liem A, Boer MJ, et al. A randomized comparison of intra-aortic balloon pumping after primary coronary angioplasty in high risk patients with acute myocardial infarction. Eur Heart J 1999;20:659–65. [DOI] [PubMed] [Google Scholar]
- [15].Jun G, Wei H, Hongbing X, et al. Prophylactic intra-aortic balloon pump reduces C-reactive protein levels and early mortality in high-risk patients undergoing percutaneous coronary intervention. Acta Cardiol 2011;66:499–504. [DOI] [PubMed] [Google Scholar]
- [16].Perera D, Stables R, Thomas M, et al. Elective intra-aortic balloon counterpulsation during high-risk percutaneous coronary intervention: a randomized controlled trial. JAMA 2010;304:867–74. [DOI] [PubMed] [Google Scholar]
- [17].Perera D, Stables R, Clayton T, et al. Long-term mortality data from the balloon pump-assisted coronary intervention study (BCIS-1): clinical perspective. Circulation 2013;127:207–12. [DOI] [PubMed] [Google Scholar]
- [18].Stone GW, Marsalese D, Brodie BR, et al. A prospective, randomized evaluation of prophylactic intraaortic balloon counterpulsation in high risk patients with acute myocardial infarction treated with primary angioplasty fn1fn1 funding for this study was provided in part by unrestricted grants from Advanced Cardiovascular Systems, Inc., Santa Clara, California; Mallinkrodt Medical, Inc., Saint Louis, Missouri; Datascope Corporation, Montvale, New Jersey; St. Jude Medical, Chelmsford, Massachusetts; and Siemens Corporation, Iselin, New Jersey. J Am Coll Cardiol 1997;29:1459–67. [DOI] [PubMed] [Google Scholar]
- [19].Thiele H, Zeymer U, Neumann FJ, et al. Intraaortic balloon support for myocardial infarction with cardiogenic shock. N Engl J Med 2012;367:1287–96. [DOI] [PubMed] [Google Scholar]
- [20].Thiele H, Zeymer U, Neumann F-J, et al. Intra-aortic balloon counterpulsation in acute myocardial infarction complicated by cardiogenic shock (IABP-SHOCK II): final 12 month results of a randomised, open-label trial. Lancet 2013;382:1638–45. [DOI] [PubMed] [Google Scholar]
- [21].Burkhoff D, Cohen H, Brunckhorst C, et al. A randomized multicenter clinical study to evaluate the safety and efficacy of the TandemHeart percutaneous ventricular assist device versus conventional therapy with intraaortic balloon pumping for treatment of cardiogenic shock. Am Heart J 2006;152: 469.e1–469.e8. [DOI] [PubMed] [Google Scholar]
- [22].Ouweneel DM, Eriksen E, Sjauw KD, et al. Percutaneous mechanical circulatory support versus intra-aortic balloon pump in cardiogenic shock after acute myocardial infarction. J Am Coll Cardiol 2017;69:278–87. [DOI] [PubMed] [Google Scholar]
- [23].Ouweneel DM, Engstrom E, Sjauw KD, et al. Experience from a randomized controlled trial with Impella 2.5 versus IABP in STEMI patients with cardiogenic pre-shock Lessons learned from the IMPRESS in STEMI trial. Int J Cardiol 2016;202:894–6. [DOI] [PubMed] [Google Scholar]
- [24].Seyfarth M, Sibbing D, Bauer I, et al. A randomized clinical trial to evaluate the safety and efficacy of a percutaneous left ventricular assist device versus intra-aortic balloon pumping for treatment of cardiogenic shock caused by myocardial infarction. J Am Coll Cardiol 2008;52:1584–8. [DOI] [PubMed] [Google Scholar]
- [25].O’Neill WW, Kleiman NS, Moses J, et al. A prospective, randomized clinical trial of hemodynamic support with Impella 2.5 versus intra-aortic balloon pump in patients undergoing high-risk percutaneous coronary intervention: the PROTECT II study. Circulation 2012;126:1717–27. [DOI] [PubMed] [Google Scholar]
- [26].Kovacic JC, Kini A, Banerjee S, et al. Patients with 3-vessel coronary artery disease and impaired ventricular function undergoing PCI with Impella 2.5 hemodynamic support have improved 90-day outcomes compared to intra-aortic balloon pump: a sub-study of the PROTECT II trial. J Interv Cardiol 2015;28:32–40. [DOI] [PubMed] [Google Scholar]
- [27].Thiele H, Sick P, Boudriot E, et al. Randomized comparison of intra-aortic balloon support with a percutaneous left ventricular assist device in patients with revascularized acute myocardial infarction complicated by cardiogenic shock. Eur Heart J 2005;26:1276–83. [DOI] [PubMed] [Google Scholar]
- [28].Aggarwal B, Aman W, Jeroudi O, et al. Mechanical circulatory support in high-risk percutaneous coronary intervention. Methodist Debakey Cardiovasc J 2018;14:23–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Dixon SR, Henriques JP, Mauri L, et al. A prospective feasibility trial investigating the use of the Impella 2.5 system in patients undergoing high-risk percutaneous coronary intervention (The PROTECT I Trial): initial U.S experience. JACC Cardiovasc Interv 2009;2:91–6. [DOI] [PubMed] [Google Scholar]
- [30].Alli OO, Singh IM, Holmes DR, Jr, et al. Percutaneous left ventricular assist device with TandemHeart for high-risk percutaneous coronary intervention: the Mayo Clinic experience. Catheter Cardiovasc Interv 2012;80:728–34. [DOI] [PubMed] [Google Scholar]
- [31].Kar S. Percutaneous mechanical circulatory support devices for high-risk percutaneous coronary intervention. Curr Cardiol Rep 2018;20:2. [DOI] [PubMed] [Google Scholar]
- [32].Remmelink M, Sjauw KD, Henriques JP, et al. Effects of left ventricular unloading by Impella Recover LP2.5 on coronary hemodynamics. Cathet Cardiovasc Interv 2007;70:532–7. [DOI] [PubMed] [Google Scholar]
- [33].Neumann FJ, Ott I, Gawaz M, et al. Cardiac release of cytokines and inflammatory responses in acute myocardial infarction. Circulation 1995;92:748–55. [DOI] [PubMed] [Google Scholar]
- [34].Davies MG, Hagen PO. Systemic inflammatory response syndrome. Br J Surg 1997;84:920–35. [DOI] [PubMed] [Google Scholar]
- [35].Hochman JS. Cardiogenic shock complicating acute myocardial infarction: expanding the paradigm. Circulation 2003;107:2998–3002. [DOI] [PubMed] [Google Scholar]
- [36].Harjola V-P, Mullens W, Banaszewski M, et al. Organ dysfunction, injury and failure in acute heart failure: from pathophysiology to diagnosis and management. A review on behalf of the Acute Heart Failure Committee of the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur J Heart Fail 2017;19:821–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Vetrovec GW. Hemodynamic support devices for shock and high-risk PCI: when and which one. Curr Cardiol Rep 2017;19:100. [DOI] [PubMed] [Google Scholar]
- [38].Gando S, Kameue T, Nanzaki S, et al. Disseminated intravascular coagulation is a frequent complication of systemic inflammatory response syndrome. Thromb Haemost 1996;75:224–8. [PubMed] [Google Scholar]
- [39].Cohen MG, Matthews R, Maini B, et al. Percutaneous left ventricular assist device for high-risk percutaneous coronary interventions: real-world versus clinical trial experience. Am Heart J 2015;170:872–9. [DOI] [PubMed] [Google Scholar]
- [40].Hu F-B, Cui L-Q. Percutaneous left ventricular assist device vs. intra-aortic balloon pump in patients with severe left ventricular dysfunction undergoing cardiovascular intervention: a meta-analysis. Chronic Dis Transl Med 2018;4:260–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Rios SA, Bravo CA, Weinreich M, et al. Meta-analysis and trial sequential analysis comparing percutaneous ventricular assist devices versus intra-aortic balloon pump during high-risk percutaneous coronary intervention or cardiogenic shock. Am J Cardiol 2018;122:1330–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


