Abstract
Lung cancer has become the leading cause of cancer-related deaths around the world. In addition to genetic risk factors and smoking, the metabolic risk factors remain to be elusive.
To evaluate the associations between obesity, nonalcoholic fatty liver disease (NAFLD) and pulmonary adenocarcinoma in patients with lung cancer.
Consecutive operation-proven lung cancer patients with assessment of metabolic disorders and liver ultrasound in 2009 and 2013 were retrospectively enrolled. T-test and multivariate logistic regression were applied to evaluate the contribution of individual factors to lung adenocarcinoma, as well as the synergistic effects between these factors.
Among 3664 lung cancer patients with ultrasound examination, 2844 cases were enrolled for further analysis. Of them, 1053 (37.0%) were females, 1242 (43.7%) were cigarette smokers, 1658 (58.3%) were diagnosed as lung adenocarcinoma, 744 (26.2%) had obesity, and 614 (21.6%) had NAFLD. Proportion of female gender, nonsmoker, obesity, NAFLD, and serum lipid levels in patients with adenocarcinoma were significantly higher than those in other subtypes of lung cancer, and in 2013 than in 2009 (all P < .01). NAFLD and obesity were shown as independent factors and positively associated with pulmonary adenocarcinoma, along with female gender and nonsmoking, higher serum levels of cholesterol. NAFLD and other contributing factors exhibited no synergistic effects on adenocarcinoma.
Obesity and NAFLD might increase the risk for pulmonary adenocarcinoma, especially in nonsmoking females, and underscore the need for further study into carcinogenic mechanisms and preventive interventions.
Keywords: hypercholesterolemia, lung cancer, nonalcoholic fatty liver disease, obesity, pulmonary adenocarcinoma
1. Introduction
Primary bronchogenic carcinoma (lung cancer) arises from the cells that line the airways of the respiratory system. Lung cancer is the leading cause of cancer death in the mainland China and around the world.[1] Pulmonary adenocarcinoma is the common type of lung cancer, and is characterized by distinct cellular and molecular features including gland and/or duct formation and/or significant amounts of mucus production. This type of lung cancer is usually found on the periphery of the lungs, as opposed to small cell lung cancer and squamous cell lung cancer, which tend to be located more centrally. Pulmonary adenocarcinoma is also considered as the most frequently diagnosed histological subtype of non-small-cell lung cancer, followed by squamous cell carcinoma.[2] Over the past few decades, the incidence of pulmonary adenocarcinoma has dramatically increased.[3] The prognosis of lung cancer depends on the stage of the respective disease. Though the prognosis acts as a best tool for patients with early stage of the disease, with small tumors, no spread to lymph nodes, and no distant spread (metastasis) to other organs, the overall 5-year survival for all types of lung cancer showed no good results, even with extensive therapeutic intervention. Pulmonary adenocarcinoma has relatively the better prognosis of any type of lung cancer.[4,5]
In addition to the genetic risk associated with lung cancer and pulmonary adenocarcinoma, multiple environmental and host factors are considered to be critical in promoting the carcinogenesis.[6] Based on previous large-scale epidemiological studies, the use of tobacco (most through smoking) is regarded as the leading risk factor for lung cancer, including pulmonary adenocarcinoma.[7] Additionally, the changes in cigarette designs (like cigarette filter ventilation that lowers smoking machine tar yields) have increased the incidence of lung adenocarcinoma.[8] With the dramatic change in diet and human lifestyle during the past decades, obesity has been regarded as one of the major global issues of human health. Obesity has become a well-known risk factor for various cancers, but its potential role in lung cancer has not been thoroughly evaluated. According to a recent report, obesity may contribute to the sex-specific incidence of epidermal growth factor receptor (EGFR) mutation in lung adenocarcinoma in different ways.[9,10] An population-based survey with nearly 40 year follow-up including 35,212 patients indicated that obesity-associated mortality was inversely linear for lung cancer.[11] But further research should aim to confirm this relationship and examine the potentially protective effect of obesity on lung cancer occurrence.
Nonalcoholic fatty liver disease (NAFLD), which is closely related to obesity and metabolic syndrome, is the leading chronic liver disease in the world. NAFLD is a chronic noninfectious disease affected by environmental and metabolic stresses as well as genetic basis. Ultrasound diagnosis of NAFLD showed abnormal metabolism and so glycolipid toxicity is regarded as more accurate and informative than high body mass index (BMI) or large waist circumference.[12–14] NAFLD is associated with the risk of several cancers, such as hepatocellular carcinoma (HCC) and colorectal carcinomas.[12,15–19] Clinical studies have found that NAFLD patients with HCC were often combined with obesity and metabolic disorders,[20] leading to the speculation that weight control and prevention of NAFLD may help reduce the incidence of HCC and possibly other types of oncogenic lesions like lung adenocarcinoma. Hence, in this study, we conducted a retrospective analysis based on recent clinical-pathological data to explore the association between NAFLD and pulmonary adenocarcinoma in patients with operation-proven lung cancer.
2. Methods
2.1. Study population
The clinical-pathological information of a large cohort of consecutive patients with lung cancer who underwent thoracic surgery in Shanghai Pulmonary Hospital in 2009 and 2013 was retrospective collected. The criteria for patients to be enrolled in the study included: patients
-
(1)
who underwent operation for lung lesion with pathological confirmation of lung cancer,
-
(2)
examined fasting blood biochemical tests and abdominal ultrasound 1 week before the operation,
-
(3)
no alcohol abuse (daily average alcohol consumption less than 30 g in male and 20 g in female),[21]
-
(4)
no chronic liver diseases other than NAFLD.
The study protocol was approved by the Ethics Committee of Shanghai Pulmonary Hospital.
2.2. Data collection
For patients involved in this study, the following information from the EMR system was collected: age, height, body weight, blood pressure, detailed history of smoking, alcohol drinking and diseases, fasting blood biochemical test results (including fasting blood glucose, triglycerides [TG], total cholesterol [TC], high-density-lipoprotein-cholesterol [HDL-C], low-density-lipoprotein-cholesterol [LDL-C], alanine aminotransferase [ALT], aspartate aminotransferase [AST], alkaline phosphatase [AKP], and γ-glutamyltransferase [GGT]), and results of radiographic and pathological examination.
2.3. Diagnostic criteria
The diagnosis of each type of lung cancer (squamous cell carcinoma, pulmonary adenocarcinoma, small cell lung cancer, glandular squamous cell carcinoma, carcinoid, sarcomatoid carcinoma, etc) was based on pathological examination of resection specimens.[22] BMI was calculated by dividing body weight (kg) by the square of height (m2), and obesity was defined as BMI ≥25 kg/m2.[23] The diagnosis of NAFLD is based on the typical changes of abdominal ultrasound (GE Vingmed Ultrasound AS, Horten, Norway).[24]
2.4. Statistical analysis
Data was collected and analyzed by SPSS20 (IFM) software with t test and χ2 test as indicated, and presented the values in means ± standard deviation. The forward multivariate logistic regression was applied to analyze the association between NAFLD and pulmonary adenocarcinoma, and the 2-tailed P-values <.05 were considered to be statistically significant.
3. Results
3.1. General characteristics
There were 3664 consecutive nondrinkers with surgical pathology proven lung cancer and hepatic ultrasound findings both in 2009 and in 2013. In total, 2189 (59.7%) cases were lung adenocarcinoma and 807 (22.0%) cases had NAFLD, prevalence of NAFLD in patients with lung adenocarcinoma was 24.9%. 820 cases were rejected from the study as lacking complete medical history or results of serum lipid profile. Therefore, a total of 2844 cases was enrolled for further analysis, the average age was 60.1 ± 9.9 years (range 18–89 years), and 1053 (37.0%) were females, 1242 (43.7%) were cigarette smokers, 1658 (58.3%) were diagnosed as lung adenocarcinoma, 744 (26.2%) had obesity, and 614 (21.6%) had NAFLD (Fig. 1). The proportion of females, adenocarcinoma, and NAFLD in patients with lung cancer in 2013 were significantly higher than those in 2009 (39.5% vs 32.1%, 67.3% vs 40.3%, 23.0% vs18.8%, respectively, all P < .05). After stratification of gender and age, proportion of NAFLD in male patients, especially in younger than 55 years in 2013, were significantly higher than those in 2009 (P < .01). The proportion of adenocarcinoma and NAFLD combined patients in total lung cancer population in 2013 was significantly higher than that in 2009.
Figure 1.
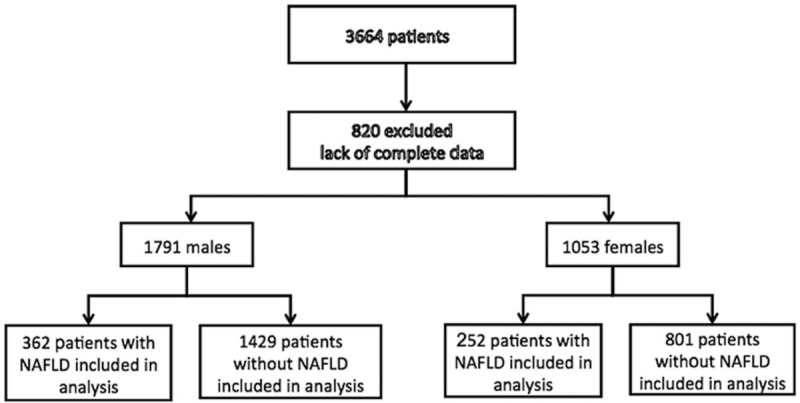
Baseline information of this study.
3.2. Associated factors with pulmonary adenocarcinoma
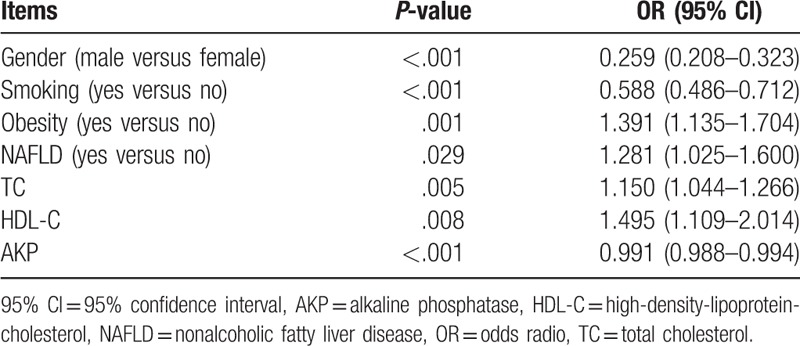
There were significant differences of proportion of gender, smoker, NAFLD, obesity, and serum levels of TC, TG, HDL-C, LDL-C, ALT, AST, AKP, and GGT between patients with adenocarcinoma and other types of lung cancer (Table 1). Therefore, these factors were further analyzed by multivariate logistic regressions to explore the contribution of these factors to the risk for pulmonary adenocarcinoma in 2844 patients with lung cancer. The dependent variable included the occurrence of adenocarcinoma and 12 independent variables included NAFLD, gender, smoking history, obesity, TC, TG, HDL-C, LDL-C, ALT, AST, AKP, and GGT. The forward logistic regression analysis indicated that 7 factors including NAFLD, obesity, smoking, gender, TC, HDL-C, and AKP, significantly contributed to the occurrence of adenocarcinoma in our lung cancer patients (Table 2). The regression equation was as follows: Logit (P) = 1.030 + .247 × NAFLD + .330 × obesity − .530 × smoking − 1.351 × male gender + .139 × TC + .402 × HDL − .01 × AKP.
Table 1.
Characteristics of pulmonary adenocarcinoma and nonadenocarcinoma patients involved in this study 5656.
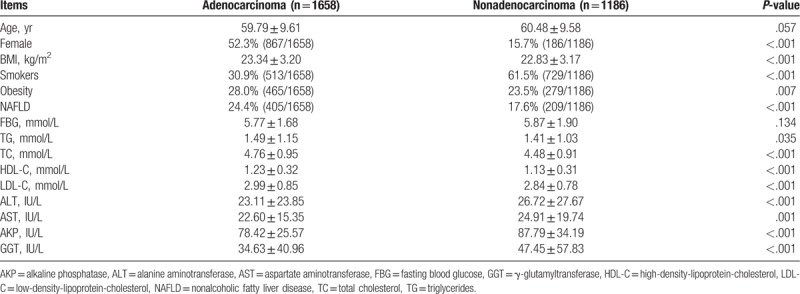
Table 2.
Multivariate logistic regression analysis on the risk factors of pulmonary adenocarcinoma.
3.3. Association between NAFLD and pathological types of lung cancer
Of the 2844 cases with lung cancer, the prevalence of NAFLD in patients with pulmonary adenocarcinoma, squamous cell carcinoma, small cell lung cancer, and other pathological types of lung cancer was 24.4% (405/1658), 16.1% (117/727), 18.1% (13/72), and 20.4% (79/387), respectively. The association of NAFLD with different pathological types of lung cancer showed significant differences (P < .01), (Fig. 2).
Figure 2.
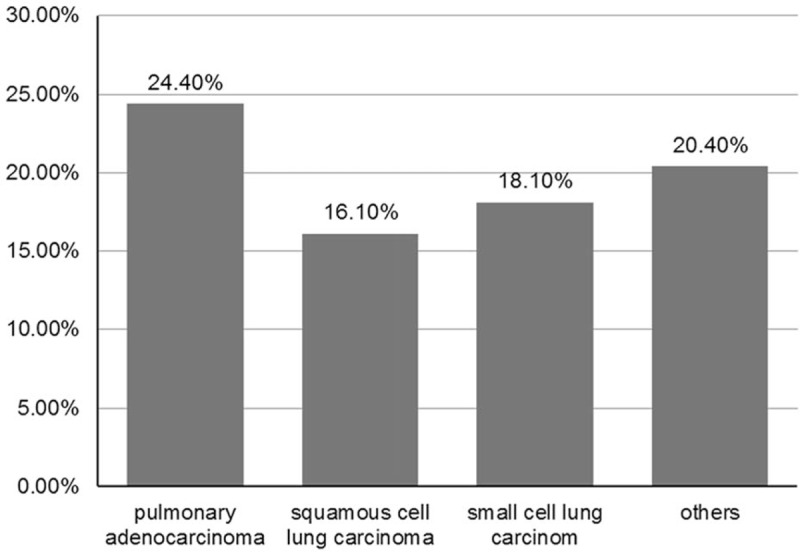
Association of NAFLD with different pathological types of lung cancer. All the cases with combination of NAFLD and pulmonary adenocarcinoma, squamous cell lung carcinoma, small cell lung carcinoma, and other types of lung cancers were analyzed to reveal different association rates. NAFLD = nonalcoholic fatty liver disease.
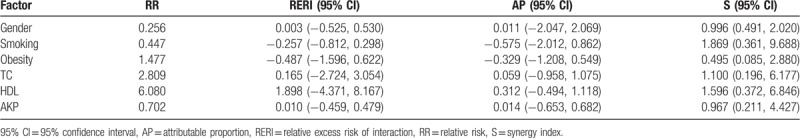
3.4. Synergistic effects of NAFLD with other factors on pulmonary adenocarcinoma
As shown in Table 2, male gender, smoking, obesity, TC, HDL-C, and AKP showed significant differences between lung patients with adenocarcinoma and nonadenocarcinoma (P < .05). It would be intriguing to explore the potential interactions between NAFLD and the above-mentioned factors in predicting the existence of pulmonary adenocarcinoma in patients with lung cancer. Hence, additional interaction analysis was performed. The results showed that the independent variable factor interacted with NAFLD in the occurrence of pulmonary adenocarcinoma. Among 2448 patients with lung cancer, the groups of obese patients, regardless of combination of NAFLD or not, displayed a higher rate of pulmonary adenocarcinoma than other groups of non-obese patients, and the proportion of NAFLD and obesity in lung cancer patients with pulmonary adenocarcinoma were significantly higher than that in patients with non-adenocarcinoma lung cancer. However, NAFLD and obesity showed no synergistic or antagonistic effects to each other (Table 3).
Table 3.
Analysis of interactions between NAFLD and other factors on pulmonary adenocarcinoma.
4. Discussion
This is a single-center retrospective study using a large cohort of consecutive operation-proven lung cancer patients with abdominal ultrasound examination within 1 week. We have demonstrated that the proportion of pulmonary adenocarcinoma and the prevalence of obesity and NAFLD in 2844 patients with lung cancer was 58.3%, 26.2%, and 21.6%, respectively, and all reported rates significantly increased from 2009 to 2013. Percent of female gender, nonsmoker, and prevalence of obesity, and NAFLD in patients with pulmonary adenocarcinoma were significantly higher than those in patients with other subtypes of lung cancer. After adjustment of age, gender, smoking, obesity, and NAFLD were found to be independent predictive factors of pulmonary adenocarcinoma in patients with lung cancer. However, there was no synergistic effect between NAFLD and other associated factors in pulmonary adenocarcinoma.
Tobacco smoking is accepted as a major risk factor for lung cancer, especially for squamous cell carcinoma.[7,8] According to 1985 Global estimation, 85% of the 676,000 cases of lung cancer in men are attributable to tobacco smoking. With the tobacco-control efforts strengthened in some countries and areas, proportion of cigarette smoking-related cancer cases have produced a comparable reduction. In Taiwan, greater than 50% of patients with lung cancer had never smoked. At the present study, only 43.7% of patients had smoking history, and the proportion of smoking in lung adenocarcinoma cases was significantly lower than that in other type of lung cancer (30.9% vs 61.5%, P < .001). Therefore, other risk factors for lung cancer, especially for increased incident lung adenocarcinoma should be explored.
The association between BMI and the incidence of lung cancer still remained controversial. In a Swedish study, obese men demonstrated a significantly increased risk of all cancers combined when compared to men with normal BMI.[25] According to a meta-analysis, overweight (BMI ≥25 kg/m2) were inversely associated with lung cancer incidence compared with normal weight (BMI = 18.5–24.9 kg/m2) in current and former smokers.[26] In contrast, Kabat et al used data from the Canadian National Breast Screening study and found that BMI was negatively correlated with the occurrence of lung cancer in female patients with smoking history, but the BMI and lung cancer was positively correlated in nonsmokers.[27] Recently, Kim et al reported that age and obesity may contribute to the sex-specific incidence of EGFR mutation in lung adenocarcinoma in different manners. In this cohort study, prevalence of obesity was higher in adenocarcinoma cases than other type lung cancer cases, and hypercholestemia was common in patients with lung adenocarcinoma. Further, multivariate regression analysis showed that obesity is independently associated with adenocarcinoma in patients with lung cancer. These results suggested that obesity might contribute to increasing rates of lung adenocarcinoma, especially in nonsmokers. Excessive consumption of diets rich in total fat, saturated fat, cholesterol, and well-done read meat, might be the common mechanism of obesity and related lung adenocarcinoma.[28–34]
NAFLD is usually the manifestation of obesity in the liver and NAFLD is regarded as visceral obesity as well. NAFLD has been linked with increased risk for cancers of the liver, colon, and breast. However, the relationship of NAFLD with lung cancer is not reported as yet. The present study demonstrated, for the first time, that NAFLD is close associated with pulmonary adenocarcinoma, regardless of age, gender, smoking, obesity, and serum cholesterol level in patients with lung cancer. Lung adenocarcinoma is associated with distinct genomic alterations compared with other lung cancer subtypes and the EGFR mutation is one of the most important factors in the development and treatment of lung adenocarcinoma. Scheving et al reported that EGFR plays a role in the regulation of liver and plasma lipid levels in adult male mice. Recently, Lou et al found that the combination of the liver X receptors agonist T0901317 and gefitinib (epidermal growth factor tyrosine kinase inhibitor) can inhibit the migration and invasion of lung cancer both in vivo and in vitro.[35] These results suggest that regulation of cholesterol, fatty acid metabolism, inflammatory responses, and glucose homestatsis in patients with NAFLD might play a role in the development of lung adenocarcinoma. Therefore, obesity, hypercholesterolemia, and NAFLD might be independent risk factors for pulmonary adenocarcinoma in both nonsmokers and smokers. Lung adenocarcinoma might be screened in older patients with obesity and NAFLD. Since unhealthy lifestyle plays a role in the development of lung adenocarcinoma, patients suspected for lung cancer should be investigated for smoking history and their dietary and exercise habits as well. Unless integrated measures for tobacco-control and intensive lifestyle modification are strengthened in China, the massive rise in cigarette consumption and prevalence of obesity and NAFLD over the past decades will produce a continued rise in lung cancer within the next 10 to 30 years.
However, the present study has some limitations. This was a retrospective study conducted in a single-center without dada between 2010 and 2012. Regarding this, the EMR system was used for data collection. However, the EMR of our hospital was established and tested in 2009, and then was re-designed from 2010 to 2012. It was officially put into use in 2013. This study began in 2014, and so we had data only in 2009 and in 2013. In addition, occupations of the patients (whether they were exposure to radon, asbestos, arsenic, or other carcinogens) and dietary habit (whether they favor foods of Vitamin A or beta-carotene) are also important in etiology of lung cancer, but most of the cases we got from EMR were lack of these detail information. These results should be further confirmed in large sample multi-center prospective cohort studies with long-term follow-up in the future.
In summary, our study found that obesity and NAFLD are more common in patients with pulmonary adenocarcinoma than other subtypes of lung cancer. Obesity, hypercholesterolemia, and NAFLD might contribute to the increased risk for pulmonary adenocarcinoma, especially in female gender and nonsmokers. These findings underscore the need for further study into carcinogenic mechanisms and preventive interventions.
Acknowledgments
We wish to thank all patients who participated in our study. This work was supported by National Key R&D Program of China (2017YFC0908903), and Key Specialty of Pudong New Area of Shanghai Municipal Commission of Health and Family Planning (PWZzk2017-11).
Author contributions
Conceptualization: Jian-gGao Fan.
Investigation: Chan-Yan Zhu, Ji-Chen Qu, Hai-Xia Cao, Guang-Yu Chen, Yi-Hai Shi.
Methodology: Chan-Yan Zhu, Ji-Chen Qu, Hai-Xia Cao, Guang-Yu Chen, Yi-Hai Shi.
Writing – original draft: Chan-Yan Zhu, Ji-Chen Qu.
Writing – review and editing: Jian-Gao Fan.
Footnotes
Abbreviations: AKP = alkaline phosphatase, ALT = alanine aminotransferase, AST = aspartate aminotransferase, EGFR = epidermal growth factor receptor, GGT = γ-glutamyltransferase, HCC = hepatocellular carcinoma, HDL-C = high-density-lipoprotein-cholesterol, LDL-C = low-density-lipoprotein-cholesterol, NAFLD = nonalcoholic fatty liver disease, TC = total cholesterol, TG = triglycerides.
How to cite this article: Zhu CY, Qu JC, Cao HX, Chen GY, Shi YH, Fan JG. Obesity and nonalcoholic fatty liver disease associated with adenocarcinoma in patients with lung cancer. Medicine. 2019;98:37(e17098).
C-YZ and J-CQ contributed equally to this work.
The authors have no conflicts of interest to disclose.
References
- [1].Devarakonda S, Morgensztern D, Govindan R. Genomic alterations in lung adenocarcinoma. Lancet Oncol 2015;16:e342–51. [DOI] [PubMed] [Google Scholar]
- [2].Travis WD. Pathology of lung cancer. Clin Chest Med 2011;32:669–92. [DOI] [PubMed] [Google Scholar]
- [3].Dela Cruz CS, Tanoue LT, Matthay RA. Lung cancer: epidemiology, etiology, and prevention. Clin Chest Med 2011;32:605–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Motono N, Funasaki A, Sekimura A, et al. Prognostic value of epidermal growth factor receptor mutations and histologic subtypes with lung adenocarcinoma. Med Oncol 2018;35:22. [DOI] [PubMed] [Google Scholar]
- [5].Tseng CH, Chiang CJ, Tseng JS, et al. EGFR mutation, smoking, and gender in advanced lung adenocarcinoma. Oncotarget 2017;8:98384–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Tseng CH, Tsuang BJ, Chiang CJ, et al. The relationship between air pollution and lung cancer in nonsmokers in Taiwan. J Thorac Oncol 2019;14:784–92. [DOI] [PubMed] [Google Scholar]
- [7].Parkin DM, Pisani P, Lopez AD, et al. At least one in seven cases of cancer is caused by smoking. Global estimates for 1985. Int J Cancer 1994;59:494–504. [DOI] [PubMed] [Google Scholar]
- [8].Song MA, Benowitz NL, Berman M, et al. Cigarette filter ventilation and its relationship to increasing rates of lung adenocarcinoma. J Natl Cancer Inst 2017;109:djx075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Kim HR, Kim SY, Kim CH, et al. Sex-specific incidence of EGFR mutation and its association with age and obesity in lung adenocarcinomas: a retrospective analysis. J Cancer Res Clin Oncol 2017;143:2283–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Scheving LA, Zhang X, Garcia OA, et al. Epidermal growth factor receptor plays a role in the regulation of liver and plasma lipid levels in adult male mice. Am J Physiol Gastrointest Liver Physiol 2014;306:G370–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Faeh D, Kaufmann M, Haile SR, et al. BMI-mortality association: shape independent of smoking status but different for chronic lung disease and lung cancer. Int J Chron Obstruct Pulmon Dis 2018;13:1851–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Duan XY, Zhang L, Fan JG, et al. NAFLD leads to liver cancer: do we have sufficient evidence? Cancer Lett 2014;345:230–4. [DOI] [PubMed] [Google Scholar]
- [13].Diehl AM, Day C. Cause, pathogenesis, and treatment of nonalcoholic steatohepatitis. N Engl J Med 2017;377:2063–73. [DOI] [PubMed] [Google Scholar]
- [14].Fan JG, Kim SU, Wong Vincent WS. New trends on obesity and NAFLD in Asia. J Hepatol 2017;67:862–73. [DOI] [PubMed] [Google Scholar]
- [15].Bugianesi E. Non-alcoholic steatohepatitis and cancer. Clin Liver Dis 2007;11:191–207. [DOI] [PubMed] [Google Scholar]
- [16].Stadlmayr A, Aigner E, Steger B, et al. Nonalcoholic fatty liver disease: an independent risk factor for colorectal neoplasia. J Intern Med 2011;270:41–9. [DOI] [PubMed] [Google Scholar]
- [17].Page JM, Harrison SA. NASH and HCC. Clin Liver Dis 2009;13:631–47. [DOI] [PubMed] [Google Scholar]
- [18].Tilg H, Diehl AM. NAFLD and extrahepatic cancers: have a look at the colon. Gut 2011;60:745–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Qian Y, Fan JG. Obesity, fatty liver and liver cancer. Hepatobiliary Pancreat Dis Int 2005;4:173–7. [PubMed] [Google Scholar]
- [20].Duan XY, Qiao L, Fan JG. Clinical features of nonalcoholic fatty liver disease-associated hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int 2012;11:18–27. [DOI] [PubMed] [Google Scholar]
- [21].Wong VW, Chan WK, Chitturi S, et al. The Asia-Pacific working party on nonalcoholic fatty liver disease guidelines 2017 part 1: definition, risk factors and assessment. J Gastroenterol Hepatol 2018;33:70–85. [DOI] [PubMed] [Google Scholar]
- [22].Lee HJ, Lee CH, Jeong YJ, et al. IASLC/ATS/ERS international multidisciplinary classification of lung adenocarcinoma: novel concepts and radiologic implications. J Thorac Imaging 2012;27:340–53. [DOI] [PubMed] [Google Scholar]
- [23].WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 2004;363:157–63. [DOI] [PubMed] [Google Scholar]
- [24].Fan JG, Jia JD, Li YM, et al. Guidelines for the diagnosis and management of nonalcoholic fatty liver disease: update 2010: (published in Chinese on Chinese Journal of Hepatology 2010; 18:163-166). J Dig Dis 2011;12:38–44. [DOI] [PubMed] [Google Scholar]
- [25].Samanic C, Chow WH, Gridley G, et al. Relation of body mass index to cancer risk in 362,552 Swedish men. Cancer Causes Control 2006;17:901–9. [DOI] [PubMed] [Google Scholar]
- [26].Yang Y, Dong J, Sun K, et al. Obesity and incidence of lung cancer: a meta-analysis. Int J Cancer 2013;132:1162–9. [DOI] [PubMed] [Google Scholar]
- [27].Kabat GC, Miller AB, Rohan TE. Body mass index and lung cancer risk in women. Epidemiology 2007;18:607–12. [DOI] [PubMed] [Google Scholar]
- [28].Alavanja MC, Brown CC, Swanson C, et al. Saturated fat intake and lung cancer risk among nonsmoking women in Missouri. J Natl Cancer Inst 1993;85:1906–16. [DOI] [PubMed] [Google Scholar]
- [29].Ooi EM, Ng TW, Watts GF, et al. Dietary fatty acids and lipoprotein metabolism: new insights and updates. Curr Opin Lipidol 2013;24:192–7. [DOI] [PubMed] [Google Scholar]
- [30].Fan JG, Li F, Cai XB, et al. Effects of nonalcoholic fatty liver disease on the development of metabolic disorders. J Gastroenterol Hepatol 2007;22:1086–91. [DOI] [PubMed] [Google Scholar]
- [31].Fan JG, Zhu J, Li XJ, et al. Fatty liver and the metabolic syndrome among Shanghai adults. J Gastroenterol Hepatol 2005;20:1825–32. [DOI] [PubMed] [Google Scholar]
- [32].Fan JG. Impact of non-alcoholic fatty liver disease on accelerated metabolic complications. J Dig Dis 2008;9:63–7. [DOI] [PubMed] [Google Scholar]
- [33].Lin X, Liu L, Fu Y, et al. Dietary cholesterol intake and risk of lung cancer: a meta-analysis. Nutrients 2018;10:E185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Swanson CA, Brown CC, Sinha R, et al. Dietary fats and lung cancer risk among women: the Missouri Women's Health Study (United States) Cancer Causes Control 1997;8:883–93. [DOI] [PubMed] [Google Scholar]
- [35].Lou R, Cao H, Dong S, et al. Liver X receptor agonist T0901317 inhibits the migration and invasion of non-small-cell lung cancer cells in vivo and in vitro. Anticancer Drugs 2019;30:495–500. [DOI] [PMC free article] [PubMed] [Google Scholar]


