Supplemental Digital Content is available in the text
Keywords: global budgeting, health care expenditures, health service utilization, unexplained fever
Abstract
Unexplained fever is one of the most common and difficult diagnostic problems faced daily by clinicians. This study evaluated the differences in health service utilization, health care expenditures, and quality of care provided to patients with unexplained fever before and after global budget (GB) implementation in Taiwan.
The National Health Insurance Research Database was used for analyzing the health care expenditures and quality of care before and after implementation of the GB system. Patients diagnosed as having unexplained fever during 2000–2001 were recruited; their 2000–2001 and 2004–2005 data were considered baseline and postintervention data, respectively.
Data of 259 patients with unexplained fever were analyzed. The mean lengths of stay (LOSs) before and after GB system implementation were 4.22 ± 0.35 days and 5.29 ± 0.70 days, respectively. The mean costs of different health care expenditures before and after implementation of the GB system were as follows: the mean diagnostic, drug, therapy, and total costs increased respectively from New Taiwan Dollar (NT$) 1440.05 ± NT$97.43, NT$3249.90 ± NT$1108.27, NT$421.03 ± NT$100.03, and NT$13,866.77 ± NT$2,114.95 before GB system implementation to NT$2224.34 ± NT$238.36, NT$4272.31 ± NT$1466.90, NT$2217.03 ± NT$672.20, and NT$22,856.41 ± NT$4,196.28 after implementation. The mean rates of revisiting the emergency department within 3 days and readmission within 14 days increased respectively from 10.5% ± 2.7% and 8.3% ± 2.4% before implementation to 6.3% ± 2.2% and 4.0% ± 1.7% after implementation.
GB significantly increased LOS and incremental total costs for patients with unexplained fever; but improved the quality of care.
1. Introduction
Unexplained fever represents as one of the most common and difficult diagnostic problems encountered daily by clinicians; it is a febrile illness without an initially obvious etiology.[1,2] When unexplained fever prolongs despite intensive evaluation and diagnostic testing, clinicians refer to it as fever of unknown origin (FUO).[3] Earlier, FUO was defined as a fever of ≥38.3°C lasting for ≥3 weeks with undiagnosed etiology even after 1 week of intensive hospital testing.[1] Physicians specializing in infectious diseases have redefined FUO as the fever of ≥38.3°C lasting for ≥3 weeks with undiagnosed etiology after 3 days of in-hospital testing or during ≥2 outpatient visits.[4–6] For pediatric FUO, the generally accepted definition is, a fever lasting 1–3 weeks without positive preliminary investigations or without a diagnosis after three outpatient clinic visits.[7,8]
FUO can be divided into following four general categories based on the etiology of fever: infection, rheumatic-inflammatory, neoplastic, or miscellaneous.[9] Infectious diseases account for approximately one-third of FUO. The most common infections associated with FUO are miliary tuberculosis (TB), Q fever, and brucellosis, followed by human immunodeficiency virus (HIV); cytomegalovirus (CMV); Epstein-Barr virus; intra-abdominal, pelvic, intranephric and perinephric abscess; typhoid or enteric fever; toxoplasmosis; and extrapulmonary TB. Notably, in 75% of the HIV patients, FUO is results from secondary infection, rather than from the HIV infection.[10] Furthermore, rheumatologic and inflammatory disorders, such as rheumatoid arthritis, systemic lupus erythematosus, giant cell/temporal arteritis, adult Still disease, periarteritis nodosa, and microscopic polyangiitis, account for another one-third of FUOs.[10] Moreover, FUOs due to neoplasms and malignancies account for 18% of all FUOs; of them, renal cell carcinoma and lymphoma are the most common neoplasms, followed by acute myeloid leukemia and myeloproliferative disorders.[10] The remaining causes of FUO are miscellaneous disorders, including drug fevers, liver cirrhosis, Crohn disease, and subacute thyroiditis.[10]
FUO, a challenge for physicians to diagnose and manage currently, represents approximately 3% of hospital admissions, with morbidity caused by prolonged hospital stay, and mortality rates accounting 12%–35%. Furthermore, FUO is associated with repeated invasive investigations, presumptive treatment, and a high impact on health care systems due to unnecessary and additional laboratory tests and medications.[10,11] Over 200 causes for FUO have been reported.[10] Relatively few infectious diseases have the potential to cause prolonged fever; therefore, patients with prolonged and perplexing fevers, in whom the infection has been ruled out, pose a diagnostic challenge.[12] Moreover, fever is one of the most common reasons for outpatient visits of children and visits to emergency department (ED).[13] Difficulty in diagnosing FUO also makes its treatment difficult. Empirical antibiotics cannot be indicated unless the patient with FUO is neutropenic. In addition, empiric glucocorticoids cannot be indicated without a strong evidence of rheumatologic disease.[14]
Recurrent FUO, which is a strong independent predictor of unestablished diagnosis, represents 18%–42% of the cases in large series. In addition, a final diagnosis can be established in only 49% of patients with recurrent FUO.[15–21] A comprehensive and careful history-taking as well as examination by physician and exhaustive laboratory testing have are required for a focused diagnostic evaluation of FUO.[4]
The diagnostic approaches for FUO include medical history-taking, physical examination, laboratory tests, and imaging studies. The etiologies of fever may be approached in terms of their height, fever pattern, and duration. Most studies have stressed on the significance of a complete history and comprehensive physical examination. In case of infectious FUO, fever is often accompanied by chills, night sweating, weight loss without loss of appetite, rigors, exudative tonsillitis, or splenomegaly. In general, the longer the FUO remains undiagnosed, the more likely it is to have a noninfectious etiology.[22] A patient presenting with B-symptoms and significant weight loss might have a neoplasm or malignancy as the cause of the FUO. By contrast, joint involvement may indicate rheumatologic disorders.[10,23] Laboratory testing includes complete blood count, three sets of blood cultures, erythrocyte sedimentation rate, complete metabolic panel, urinalysis, urine culture, tuberculin skin test, and tests for biomarkers (such as antinuclear antibodies, rheumatoid factor, CMV immunoglobulin M, HIV antibodies, and heterophile antibodies) in children and young adults. Moreover, imaging techniques include chest radiography, computed tomography (CT), and radionuclide scanning.[24]
FUO is associated with broad differential diagnosis leading to a wide range of potential diagnostic and therapeutic costs. However, little is known regarding the hospitalization costs for patients with FUO. Recent studies have reported high hospitalization charges for FUO (US$25,000–US$180,000).[25,26]
The National Health Insurance (NHI) program, the backbone of the health care system in Taiwan, is the major source of health financing and covers 99% of the population of Taiwan. National health care expenditure in Taiwan increased from 5.3% in 1995 to 6.0% in 2001 of the gross national product. The NHI operated on a fee-for-service (FFS) basis and as a result health care spending increased by approximately 50% from 1995 to 2001. To prevent unlimited and rapid growth of spending on health care, the Bureau of NHI (BNHI) implemented the global budget (GB) system to modify the FFS mechanism in 2002. The GB in Taiwan is an overall spending target, designed to limit the volume of service and its total price.[27–31] Here, we evaluated the differences in health service utilization, health care expenditures, and quality of care among patients with unexplained fever before and after GB system implementation by using NHI Research Database (NHIRD) data.
2. Materials and methods
This was a pre-post comparison study. The study was based in part on data from NHIRD provided by BNHI, Department of Health and managed by the National Health Research Institutes (NHRI). The database contains the registration files and original claims data for reimbursement. The NHIRD is provided to scientists in Taiwan for research purposes. Each year, the BNHI collects data from the NHI program and sorts it into data files. These data files are de-identified by scrambling the identification codes of both patients and medical facilities and then sent to NHRI to form the original files contained in the NHIRD.[32]
Based on the registration files and original claims data in the NHIRD, specific data subsets can be re-constructed for research purposes. The registration datasets, systemic sampling inpatient expenditures by admissions (DD file) and systemic sampling ambulatory care expenditures by visits (CD file) were used in this study. The interpretation and conclusions contained herein do not represent those of BNHI, the Department of Health, or NHRI.[32]
2.1. Systemic sampling CD and DD files
0.2% of the ambulatory care expenditures, by visit, CD file extracted by systematic sampling method on a monthly basis, together with the related records in details of ambulatory care orders form the Systematic Sampling CD file. 5% of the inpatient expenditures, by admission, DD file extracted by systematic sampling method on a monthly basis, together with the related records in details of inpatient orders form the Systematic Sampling DD file.[32] A total of one million cases of systemic sampling dataset were used in this study.
2.2. Study population
Subjects in Systemic Sampling CD and Systemic Sampling DD files who were diagnosed with unexplained fever, International Classification of Diseases (ICD-9 code) 780.60 excluding 780.64, 778.4, 659.2, 672 in 2000, 2001, 2004, and 2005 were recruited for comparison and analysis.
The GB system in Taiwan was fully implemented in 2002; therefore, data from 2000 and 2001 were used as baseline data (pre-GB). Data from 2004 and 2005 were used as post intervention data (post GB). In 2003, there was an outbreak of Severe Acute Respiratory Syndrome (SARS) in Taiwan. It has been reported that SARS had an impact on health care utilization.[33] Therefore, data from 2003 was not used in this study.
2.3. Charlson comorbidity index
A score of 1 was added when the subjects had co-morbid myocardial infarction, congestive heart failure, peripheral vascular disease, cerebrovascular disease, dementia, chronic pulmonary disease, connective tissue disease, peptic ulcer disease, mild liver disease, or diabetes without end-organ damage. A score of 2 was added when the subjects had co-morbid hemiplegia, moderate or severe renal disease, diabetes with end-organ damage, tumor without metastases, leukemia, or lymphoma. A score of 3 was added when the subjects had co-morbid moderate or severe liver disease. A score of 6 was added when the subjects had co-morbid metastatic solid tumors or acquired immunodeficiency syndrome. The total score was obtained by adding the relative weight of all comorbidities. For each decade > 40 years of age, a score of 1 is added to the sum of the above-mentioned scores.[34–37]
2.4. Income state index
In Taiwan, health insurance premiums are calculated as a percentage of an individual's monthly salary. There were seven income levels in this study, Levels 0∼6[37] (Table S1).
2.5. Ethics Statement
The NHIRD encrypts patient personal information to protect privacy and provides researchers with anonymous identification numbers associated with relevant claims information, including sex, date of birth, medical services received, and prescriptions. Patient consent is not required to access the NHIRD. This study was approved by the Institutional Review Board (IRB) of China Medical University (CMU-REC-101–012). The IRB specifically waived the consent requirement.
2.6. Data Availability Statement
All data and related metadata were deposited in an appropriate public repository. The data on the study population that were obtained from the NHIRD (http://w3.nhri.org.tw/nhird//date_01.html) are maintained in the NHIRD (http://nhird.nhri.org.tw/). The NHRI is a nonprofit foundation established by the government.
2.7. Statistical analysis
Data are described as mean ± standard deviation. The t test was used to compare the differences in mean values. Multilevel and generalized linear model were employed to determine the impact of several independent variables.
Multilevel and generalized linear model were employed to determine the impact of several independent variables on LOS, diagnostic costs, drug costs, therapy costs, total costs, the risk of revisiting the ED within 3 days, and the risk of being readmitted within 14 days after discharge.
There were two nested levels in this study: hospital accreditation levels and regional levels. There are three accreditation hospital levels in Taiwan: medical centers, regional hospitals, and local hospitals. Taiwan is divided into six geographical areas that include Taipei city, northern Taiwan, central Taiwan, southern Taiwan, Kaoshiung, and eastern Taiwan. The independent variables evaluated in this study included pre-post GB, age, gender, income state index, Charlson comorbidity index, the three hospital levels, and the six geographic areas in Taiwan.
All statistical analyses were performed using the statistical package STATA for Windows (version 11.0). A P-value of .05 was considered to represent statistical significance.
3. Results
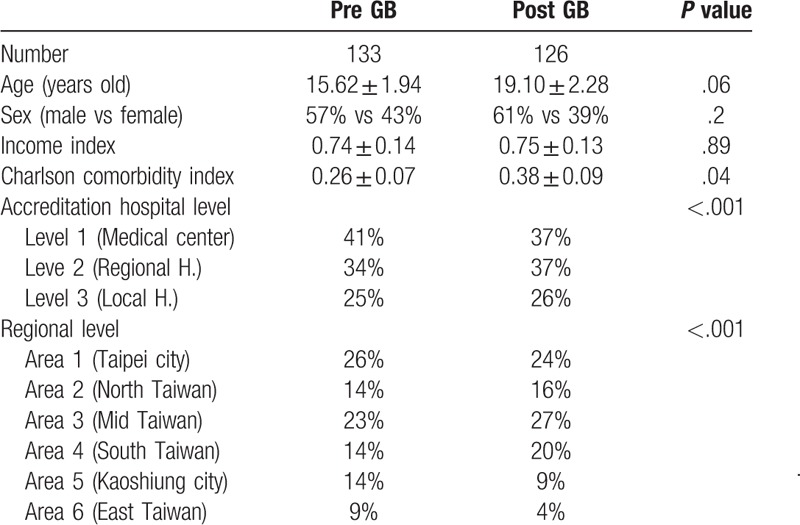
Data on 259 patients with unexplained fever (133 pre- and 126 post-GB) were analyzed in this study. The mean ages of subjects before and after GB were 15.62 ± 1.94 years and 19.10 ± 2.28 years, respectively. In the pre-budget group, 57% of subjects were male and in the post-budget group, 61% of subjects were male. The mean income state indexes before and after GB were 0.74 ± 0.14 and 0.75 ± 0.13, respectively. The mean Charlson comorbidity index before and after GB were 0.26 ± 0.07 and 0.38 ± 0.09, respectively. There were no significant differences in age, male to female ratio, or income state index before and after implementation of the GB system (P = .06, P = .20, P = .89). However, there was a significant difference in Charlson comorbidity index before and after GB (P = .04) (Table 1).
Table 1.
Demographic data of the patients with unexplained fever before and after implementation of GB.
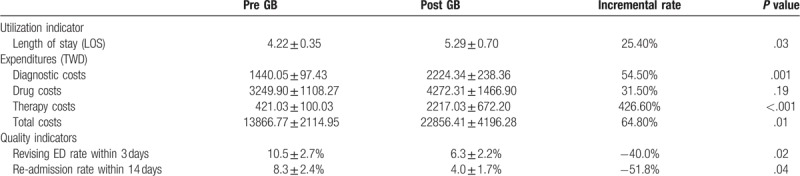
The mean LOS before adoption of the GB was 4.22 ± 0.35 days and the mean LOS after implementation of the system was 5.29 ± 0.70 days. The mean diagnostic costs before and after the GB system went into effect were NT$1,440.05 ± 97.43 and NT$2,224.34 ± 238.36, respectively. The mean drug costs increased from NT$3,249.90 ± 1,108.27 at baseline to NT$4,272.31 ± 1,466.90 after adoption of the GB system. The mean therapy costs before and after GB were NT$421.03 ± 100.03 and NT$2,217.03 ± 672.20, respectively. The mean total costs increased from NT$13,866.77 ± 2,114.95 at baseline to NT$22,856.41 ± 4,196.28 after the system went into effect. The mean 3-day ED revisiting rate decreased from 10.5% ± 2.7% at baseline to 6.3% ± 2.2% after adoption of the GB system. The mean 14-day readmission rates before and after GB were 8.3% ± 2.4% and 4.0% ± 1.7%, respectively. There were significant differences in LOS, diagnostic costs, therapy costs, total costs, 3-day ED revisiting rate, and 14-day readmission rate before and after implementation of the GB system among patients with unexplained fever (P = .03, P = .001, P < .001, P = .01, P = .02, P = .04, respectively). There was no significant difference in drug costs before and after the GB among patients with unexplained fever (P = .19) (Table 2).
Table 2.
Summary of the results for patients with unexplained fever before and after implementation of GB.
3.1. Length of stay (LOS)
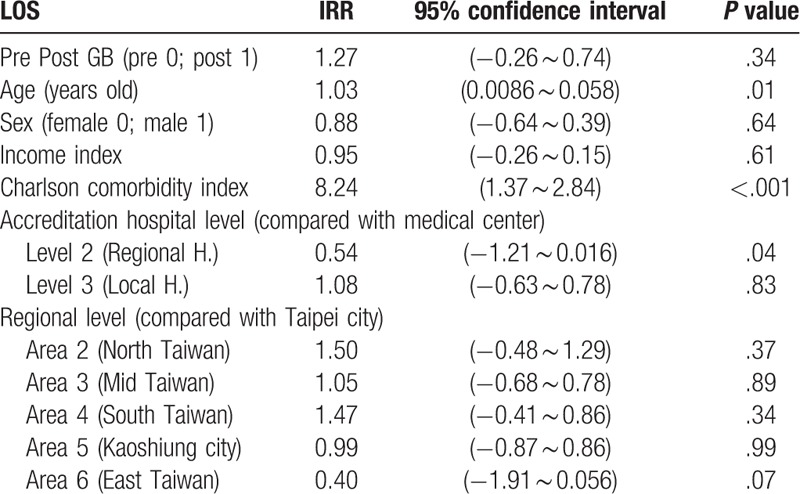
A generalized linear Poisson model was used for clustered count data analysis.[38] The Poisson regression model was fitted using option IRR (incidence-rate ratio) to obtain exponential estimates. The GB system did not have a significant impact on LOS (IRR = 1.27, P = .34). In addition, gender was not a significant predictor of LOS (IRR = 0.88, P = .64). There was a significantly positive correlation between age, Charlson comorbidity index, and LOS (IRR = 1.03, P = .01; IRR = 8.24, P < .001, respectively). There was no significant correlation between income state index and LOS (IRR = 0.95, P = .61). LOS did not differ significantly between patients treated at medical centers and those treated at local hospitals (IRR = 1.08, P = .83). However, patients treated at regional hospitals had a significantly shorter LOS than those treated at medical centers (IRR = 0.54, P = .04). Compared with hospitals in Taipei city, there was no significant difference in LOS among hospitals in northern Taiwan, central Taiwan, southern Taiwan, Kaoshiung city, or eastern Taiwan (IRR = 1.50, P = .37; β = 1.05, P = .89; β = 1.47, P = .34; β = 0.99, P = .99; β = 0.40, P = .07, respectively) (Table 3).
Table 3.
The impact of several independent variables on LOS in the patients with unexplained fever (Generalized linear Poisson model).
3.2. Diagnostic costs
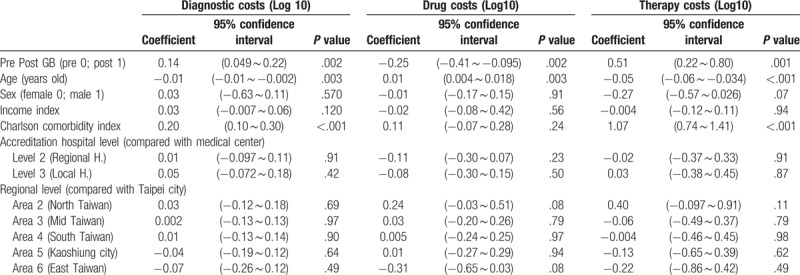
A generalized linear model was used for analysis. Because diagnostic costs were skewed to the right, data were converted to log base 10 for statistical analysis. The GB system had a significantly positive impact on diagnostic costs (β = 0.14, P = .002). Neither gender nor income state index was significantly correlated with diagnostic costs (β = 0.03, P = .57; β = 0.03, P = .12, respectively). There was a significantly negative correlation between age and diagnostic costs (β = −0.01, P = .003), and a significantly positive correlation between Charlson comorbidity index and diagnostic costs (β = 0.20, P < .001). There were no significant differences in diagnostic costs between regional hospitals and medical centers or local hospitals and medical centers (β = 0.01, P = .91; β = 0.05, P = .42, respectively). Compared with hospitals in Taipei city, there were no significant differences in diagnostic costs among hospitals located in northern Taiwan, central Taiwan, southern Taiwan, Kaoshiung city, or eastern Taiwan (β = 0.03, P = .69; β = 0.002, P = .97; β = 0.01, P = .90; β = −0.04, P = .64; β = −0.07, P = .49, respectively) (Table 4).
Table 4.
The impact of several independent variables on diagnostic costs, drug costs and therapy costs in the patients with unexplained fever (Generalized linear model).
3.3. Drug costs
A generalized linear model was used for analysis. Because drug costs were skewed to the right, data were converted to log base 10 for statistical analysis. GB had a significantly negative impact on drug costs (β = −0.25, P = .002). There was a significantly positive correlation between age and drug costs (β = 0.01, P = .003), but there was no significant correlation between gender, income state index or Charlson comorbidity index and drug costs (β = −0.01, P = .91; β = −0.02, P = .56; β = 0.11, P = .24, respectively). There were no significant differences in drug costs between regional hospitals and medical centers or local hospitals and medical centers (β = −0.11, P = .23; β = 0.08, P = .50, respectively). Compared with hospitals in Taipei city, there were no significant differences in drug costs among hospitals in northern Taiwan, central Taiwan, southern Taiwan, Kaoshiung city, or eastern Taiwan (β = 0.24, P = .08; β = 0.03, P = .79; β = 0.005, P = .97; β = 0.01, P = .94; β = −0.31, P = .08, respectively) (Table 4).
3.4. Therapy costs
A generalized linear model was used for analysis. Because the therapy costs were skewed to the right, data were converted to log base 10 for statistical analysis. GB had a significantly positive impact on therapy costs (β = 0.51, P = .001). There was no significant correlation between gender or income state index and therapy costs (β = −0.27, P = .07; β = −0.004, P = .94, respectively). There was a significantly negative correlation between age and therapy costs (β = −0.05, P < .001) and a significantly positive correlation between Charlson comorbidity index and therapy costs (β = 1.07, P < .001). There were no significant differences in therapy costs between regional hospitals and medical centers or local hospitals and medical centers (β = −0.02, P = .91; β = 0.03, P = .87, respectively). Compared with hospitals in Taipei city, there were no significant differences in therapy costs among hospitals in northern Taiwan, central Taiwan, southern Taiwan, Kaoshiung city, or eastern Taiwan (β = 0.40, P = .11; β = −0.06, P = .79; β = −0.004, P = .98; β = −0.13, P = .62; β = −0.22, P = .49, respectively) (Table 4).
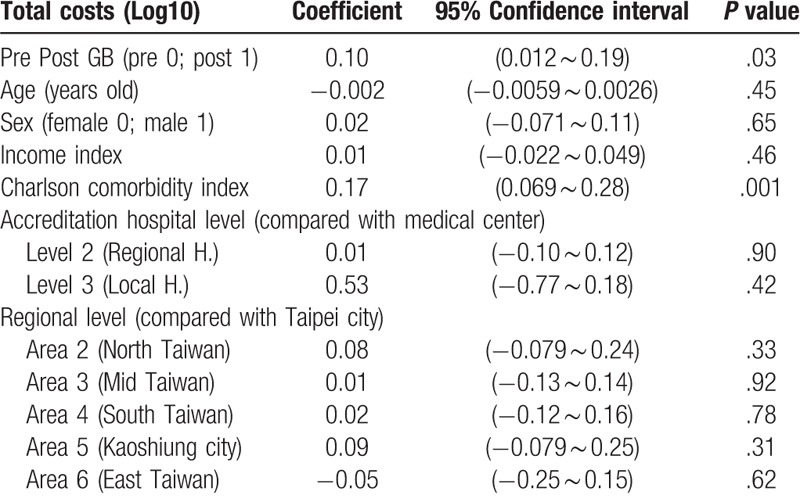
3.5. Total costs
A generalized linear model was used for analysis. Because the total costs were skewed to the right, data were converted to log base 10 for statistical analysis. GB had a significantly positive impact on total costs (β = 0.10, P = .03). There was no significant correlation between gender, age or income state index and total costs (β = 0.02, P = .65; β = −0.002, P = .45; β = 0.01, P = .46, respectively), but there was a significantly positive correlation between Charlson comorbidity index and total costs (β = 0.17, P = .001). There were no significant differences in total costs between regional hospitals and medical centers or local hospitals and medical centers (β = 0.01, P = .90; β = 0.53, P = .42, respectively). Compared with hospitals in Taipei city, there were no significant differences in total costs among hospitals in northern Taiwan, central Taiwan, southern Taiwan, Kaoshiung city, or eastern Taiwan (β = 0.08, P = .33; β = 0.01, P = .92; β = 0.02, P = .78; β = 0.09, P = .31; β = −0.05, P = .62, respectively) (Table 5).
Table 5.
The impact of several independent variables on total costs in the patients with unexplained fever (Generalized linear model).
3.6. Risk of revisiting the ED within 3 days
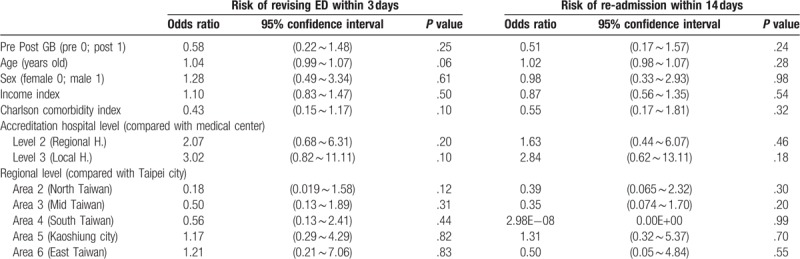
A generalized linear binary regression model was used for analysis. GB did not have a significant impact on the risk of revisiting the ED within 3 days (OR = 0.58; P = .25). There was no significant correlation between age, gender, income state index or Charlson comorbidity index and the risk of revisiting the ED within 3 days (OR = 1.04, P = .06; OR = 1.28, P = .61; OR = 1.10, P = .50; OR = 0.43, P = .10, respectively). In addition, there were no significant differences in the risk of revisiting the ED within 3 days between regional hospitals and medical centers or between local hospitals and medical centers (OR = 2.07, P = .20; OR = 3.02, P = .10, respectively). Compared with hospitals in Taipei city, there were no significant differences in the risk of revisiting the ED within 3 days among hospitals in northern Taiwan, central Taiwan, southern Taiwan, Kaoshiung city, or eastern Taiwan (OR = 0.18, P = .12; OR = 0.50, P = .31; OR = 0.56, P = .44; OR = 1.17, P = .82; OR = 1.21, P = .83, respectively) (Table 6).
Table 6.
The impact of several independent variables on the risk of revisiting ED within 3 days and the risk of re-admission within 14 days among the patients with unexplained fever (Generalized binary linear model).
3.7. Risk of readmission within 14 days
A generalized linear binary regression model was used for analysis. GB did not have a significant impact on the risk of being readmitted within 14 days (OR = 0.51; P = .24). There were no significant correlations between age, gender, income state index, or Charlson comorbidity index and the risk of readmission within 14 days (OR = 1.02, P = .28; OR = 0.98, P = .98; OR = 0.87, P = .54; OR = 0.55, P = .32, respectively). No significant differences in the risk of readmission within 14 days were noted between regional hospitals and medical centers or between local hospitals and medical centers (OR = 1.63, P = .46; OR = 2.84, P = .18, respectively). Compared with hospitals in Taipei city, there were no significant differences in the risk of being readmitted within 14 days among hospitals in northern Taiwan, central Taiwan, southern Taiwan, Kaoshiung city, or eastern Taiwan (OR = 0.39, P = .30; OR = 0.35, P = .20; OR = 2.98E−08, P = .99; OR = 1.31, P = .70; OR = 0.50, P = .55, respectively) (Table 6).
4. Discussion
Unexplained fever imposes additional burden on both the NHI system and patients. However, studies on hospitalization costs for patients with unexplained fever are limited. In this study, we investigated the impact of the GB system for patients with unexplained fever in Taiwan. The GB system was associated with significantly longer LOS of the patients with unexplained fever. After adjustments for other covariates, age and Charlson comorbidity index were independent LOS predictors. GB did not significantly affect LOS in patients with unexplained fever. Patients with unexplained fever in regional hospitals had a significantly shorter LOS than patients in medical centers. FUO is diagnosed less frequently in adults than in children; however, adult patients are more likely to be admitted and have longer LOSs.[39] The mean age of patients with unexplained fever in medical centers was 21.53 ± 2.50 years, whereas that of patients with idiopathic fever in regional hospitals was 16.63 ± 2.44 years. The mean Charlson comorbidity indexes in medical centers and regional hospitals were 0.37 ± 0.095 and 0.30 ± 0.093, respectively. Thus, the younger the age and the fewer the comorbidities, the shorter may be the LOSs in patients with unexplained fever in the regional hospitals.
Infections are the main causes of FUO in children,[8,25,26,40] including the children in Taiwan.[41,42] Notably, a significant proportion of pediatric patients, accounting approximately 25%–30%, remain undiagnosed.[8,25,26,40] However, in adults, the number of undiagnosed cases has been demonstrated to be more common than that of the infectious etiology cases.[15,43]
As fluorodeoxyglucose positron emission tomography (FDG-PET)/CT emerged in the end of 20th century,[44] it was applied for the diagnosis of FUO. Compared to conventional scintigraphic techniques, FDG-PET/CT has following advantages: higher resolution and higher sensitivity for anatomic localization of infectious, inflammatory, or neoplastic processes.[45] The work-up of patients with FUO demonstrated that the diagnostic performance of 18F-FDG-PET/CT is superior to 67Ga single-photon emission computed tomography (SPECT)/CT.[46] Furthermore, previous studies have suggested that using FDG-PET/CT shortened the diagnostic work-up for FUO.[15,47] A meta-analysis reported that PET and PET/CT had successfully localized the source of fever in 44% and 58% of the patients with classic FUO, respectively, after a series of unsuccessful investigations.[48] The major drawbacks are cost and accessibility; FDG-PET/CT costed £800, compared with £250 for a chest–abdomen–pelvis CT. However, this could be offset by earlier definitive treatment due to higher diagnostic sensitivity, which could reduce LOS with an average of £400 per day.[49] Therefore, FDG-PET/CT can aid in diagnosing FUO, particularly when diagnostic clues are absent.
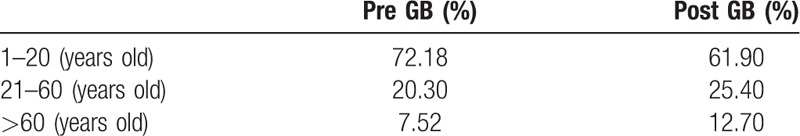
Diagnostic, therapy, and total costs incurred for treating patients with unexplained fever significantly increased after GB system implementation. After adjustments for other covariates, the GB system and Charlson comorbidity index was found to be positively correlated with increased diagnostic, therapy, and total costs. The increase in health care expenditures from 25.4% to 426.6% significantly exceeded the 2.2% growth in consumer price index for the same period in Taiwan (Table S2). The possible reasons for the higher costs include longer LOSs, additional comorbidities, and higher proportion of older individuals in the study population after GB system implementation (Table 7). Moreover, salaries of physicians in Taiwan are calculated more or less on the basis of FFS. Therefore, physicians demonstrate a natural proclivity to maximize medical services to maximize their income; and the GB system does not provide an incentive for physicians to reduce the volume of medical care. Health care expenditures incurred by patients with unexplained fever did not differ between hospitals with different accreditation levels or in different geographic areas in Taiwan.
Table 7.
Age stratification of unexplained fever patients before and after implementation of global budget (GB).
The risks of revisiting the ED within 3 days and readmission within 14 days significantly decreased after implementation of the GB system, which suggested that the quality of care was improved. However, after controlling other covariates, the GB was not associated with reduced risks of revisiting the ED within 3 days or readmission within 14 days. Furthermore, none of the tested variables were independent predictors of revisiting the ED within 3 days or being readmitted within 14 days for patients with unexplained fever. A possible reason this observation are the regulations for evaluating the quality of care in hospitals in Taiwan. BNHI has developed a series of plans structured to improve the quality of care while keeping costs under control. The plans offer incentives to health care providers by paying them based on clinical outcomes to care for overall wellbeing of patients. The risks of revisiting the ED within 3 days and readmission within 14 days are the two indicators used by BNHI to measure the quality of care. These two indicators are linked to the reimbursement rate at each hospital. The amount of reimbursement is reduced if the quality of care is determined to be poor. To safeguard against being penalized, all hospitals in Taiwan have made concerted efforts to reduce the 3-day ED revisiting and 14-day readmission rates. In this study, no difference in the risks of revisiting the ED within 3 days or readmission within 14 days between hospitals with different accreditation levels or between different geographic areas of Taiwan was noted.
This research has some limitations. First, the NHIRD data did not provide detailed information of patients regarding factors, such as their lifestyle, habits, socioeconomic status, and family history; all of which are possible confounding factors in this study. Second, the NHI claims registries are primarily used for administrative billing alone and thus are not verified for scientific purposes. The laboratory data and information regarding symptoms of patients were lacking, and therefore, discussion of these factors in relation to the GB system is not possible. Furthermore, because of the anonymity of the identification numbers, obtaining additional information by directly contacting the patients was not possible. The accuracy of medical coding in the claims data may affect the data validity. However, the diagnosis data in the NHIRD are highly reliable. The insurance system has mechanisms to monitor the insurance claims. Third, the conclusions derived from a cohort study are generally of lower methodological quality than those from randomized trials because a cohort study is subject to several biases and the necessary adjustments for confounding factors are required. Despite the meticulous design of this study and adequate control of confounding factors, biases associated with possibly unmeasured or unknown confounding factors can occur.
5. Conclusions
GB significantly increased LOS and incremental diagnostic, therapeutic, and total costs; however, the quality of care improved for patients with unexplained fever. A similar health policy can be applied in other countries to benefit more patients with unexplained fever.
Author contributions
Conceptualization: Chun-Yi Lin.
Data curation: Keh-Sen Liu, Chun-Yi Lin.
Formal analysis: Keh-Sen Liu, Tsung-Fu Yu, Hsing-Ju Wu, Chun-Yi Lin.
Funding acquisition: Chun-Yi Lin.
Methodology: Chun-Yi Lin.
Project administration: Hsing-Ju Wu.
Supervision: Chun-Yi Lin.
Validation: Chun-Yi Lin.
Writing – original draft: Keh-Sen Liu, Tsung-Fu Yu, Chun-Yi Lin.
Writing – review & editing: Hsing-Ju Wu, Chun-Yi Lin.
Supplementary Material
Footnotes
Abbreviations: BNHI = Bureau of National Health Insurance, CMV = cytomegalovirus, CT = computed tomography, ED = emergency department, FDG-PET = fluorodeoxyglucose positron emission tomography, FFS = fee-for-service, FUO = fever of unknown origin, GB = global budget, HIV = human immunodeficiency virus, IRB = Institutional Review Board, IRR = incidence-rate ratio, LOS = length of stay, NHI = National Health Insurance, NHIRD = National Health Insurance Research Database, NHRI = National Health Research Institutes, SARS = severe acute respiratory syndrome, SPECT = single-photon emission computed tomography, TB = tuberculosis.
How to cite this article: Liu KS, Yu TF, Wu HJ, Lin CY. The impact of global budgeting in Taiwan on inpatients with unexplained fever. Medicine. 2019;98:37(e17131).
This study was supported in part by Taiwan Ministry of Health and Welfare Clinical Trial and Research Center of Excellence (Grant number MOHW104-TDU-B-212–113002); China Medical University Hospital, Academia Sinica Taiwan Biobank, Stroke Biosignature Project (Grant number BM104010092); NRPB Stroke Clinical Trial Consortium (Grant number MOST 103–2325-B-039–006); Tseng-Lien Lin Foundation, Taichung, Taiwan; Taiwan Brain Disease Foundation, Taipei, Taiwan; Katsuzo and Kiyo Aoshima Memorial Funds, Japan; and Health and welfare surcharge of tobacco products, China Medical University Hospital Cancer Research Center of Excellence (Grant number MOHW104-TDU-B-212–124–002, Taiwan). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. No additional external funding received for this study.
The authors declare no conflicts of interest.
Supplemental Digital Content is available for this article.
References
- [1].Petersdorf RG, Beeson PB. Fever of unexplained origin: report on 100 cases. Medicine (Baltimore) 1961;40:1–30. [DOI] [PubMed] [Google Scholar]
- [2].Camus F, Henzel D, Janowski M, et al. Unexplained fever and chronic fatigue: abnormal circadian temperature pattern. Eur J Med 1992;1:30–6. [PubMed] [Google Scholar]
- [3].Hayakawa K, Ramasamy B, Chandrasekar PH. Fever of unknown origin: an evidence-based review. Am J Med Sci 2012;344:307–16. [DOI] [PubMed] [Google Scholar]
- [4].Cunha BA. Fever of unknown origin: focused diagnostic approach based on clinical clues from the history, physical examination, and laboratory tests. Infect Dis Clin North Am 2007;21:1137–87. [DOI] [PubMed] [Google Scholar]
- [5].Durack DT, Street AC. Fever of unknown origin—reexamined and redefined. Curr Clin Top Infect Dis 1991;11:35–51. [PubMed] [Google Scholar]
- [6].Bryan CS. Fever of unknown origin: the evolving definition. Arch Intern Med 2003;163:1003–4. [DOI] [PubMed] [Google Scholar]
- [7].Seashore CJ, Lohr JA. Fever of unknown origin in children. Pediatr Ann 2011;40:26–30. [DOI] [PubMed] [Google Scholar]
- [8].Chow A, Robinson JL. Fever of unknown origin in children: a systematic review. World J Pediatr 2011;7:5–10. [DOI] [PubMed] [Google Scholar]
- [9].Cunha BA. Fever of unknown origin: clinical overview of classic and current concepts. Infect Dis Clin North Am 2007;21:867–915. [DOI] [PubMed] [Google Scholar]
- [10].Bleeker-Rovers CP, Vos FJ, Corstens FH, et al. Imaging of infectious diseases using [18F] fluorodeoxyglucose PET. Q J Nucl Med Mol Imaging 2008;52:17–29. [PubMed] [Google Scholar]
- [11].Mourad O, Palda V, Detsky AS. A comprehensive evidence-based approach to fever of unknown origin. Arch Intern Med 2003;163:545–51. [DOI] [PubMed] [Google Scholar]
- [12].Barclay WR. Fever of unknown origin. JAMA 1977;238:2404. [PubMed] [Google Scholar]
- [13].Esposito S, Rinaldi VE, Argentiero A, et al. Approach to neonates and young infants with fever without a source who are at risk for severe bacterial infection. Mediators Inflamm 2018;2018:4869329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Brown I, Finnigan NA. Fever of Unknown Origin (FUO). Treasure Island (FL): StatPearls; 2019. [PubMed] [Google Scholar]
- [15].Bleeker-Rovers CP, Vos FJ, de Kleijn EM, et al. A prospective multicenter study on fever of unknown origin: the yield of a structured diagnostic protocol. Medicine (Baltimore) 2007;86:26–38. [DOI] [PubMed] [Google Scholar]
- [16].Knockaert DC, Vanneste LJ, Bobbaers HJ. Recurrent or episodic fever of unknown origin. Review of 45 cases and survey of the literature. Medicine (Baltimore) 1993;72:184–96. [PubMed] [Google Scholar]
- [17].Doffoël-Hantz V, Fauchais AL, Sparsa A, et al. Abrupt onset of papulovesicular lesions: diagnostic features and outcome. Rev Med Interne 2007;28:127–30. [DOI] [PubMed] [Google Scholar]
- [18].de Kleijn EM, Vandenbroucke JP, van der Meer JW. Fever of unknown origin (FUO). I A. prospective multicenter study of 167 patients with FUO, using fixed epidemiologic entry criteria. The Netherlands FUO Study Group. Medicine (Baltimore) 1997;76:392–400. [DOI] [PubMed] [Google Scholar]
- [19].de Kleijn EM, van der Meer JW. Fever of unknown origin (FUO): report on 53 patients in a Dutch university hospital. Neth J Med 1995;47:54–60. [DOI] [PubMed] [Google Scholar]
- [20].Knockaert DC, Vanneste LJ, Vanneste SB, et al. Fever of unknown origin in the 1980s. An update of the diagnostic spectrum. Arch Intern Med 1992;152:51–5. [PubMed] [Google Scholar]
- [21].Vanderschueren S, Knockaert D, Adriaenssens T, et al. From prolonged febrile illness to fever of unknown origin: the challenge continues. Arch Intern Med 2003;163:1033–41. [DOI] [PubMed] [Google Scholar]
- [22].Vickery DM, Quinnell RK. Fever of unknown origin. An algorithmic approach. JAMA 1977;238:2183–8. [PubMed] [Google Scholar]
- [23].Statler VA, Marshall GS. Evaluation of prolonged and recurrent unexplained fevers. Pediatr Ann 2018;47:e347–50. [DOI] [PubMed] [Google Scholar]
- [24].Arnow PM, Flaherty JP. Fever of unknown origin. Lancet 1997;350:575–80. [DOI] [PubMed] [Google Scholar]
- [25].Antoon JW, Peritz DC, Parsons MR, et al. Etiology and resource use of fever of unknown origin in hospitalized children. Hosp Pediatr 2018;8:135–40. [DOI] [PubMed] [Google Scholar]
- [26].Szymanski AM, Clifford H, Ronis T. Fever of unknown origin: a retrospective review of pediatric patients from an urban, tertiary care center in Washington, DC. World J Pediatr 2019;Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- [27].Lu JF, Hsiao WC. Does universal health insurance make health care unaffordable? Lessons from Taiwan. Health Aff (Millwood) 2003;22:77–88. [DOI] [PubMed] [Google Scholar]
- [28].Wen CP, Tsai SP, Chung WS. A 10-year experience with universal health insurance in Taiwan: measuring changes in health and health disparity. Ann Intern Med 2008;148:258–67. [DOI] [PubMed] [Google Scholar]
- [29].Chen FJ, Laditka JN, Laditka SB, et al. Providers’ responses to global budgeting in Taiwan: what were the initial effects? Health Serv Manage Res 2007;20:113–20. [DOI] [PubMed] [Google Scholar]
- [30].Burea of National Health Insurance DoH, Executive Yuan. 2010. About National Health Insurance, Global Budget Payment Scheme. [Cited June 20 2010]. Available at: http://www.nhi.gov.tw/resource/Webdata/20774_1_NHI%20IN%20TAIWAN%202011%20ANNUAL%20REPORT.pdf, page 20–22, Accessed March 20, 2018. [Google Scholar]
- [31].Chang L, Hung JH. The effects of the global budget system on cost containment and the quality of care: experience in Taiwan. Health Serv Manage Res 2008;21:106–16. [DOI] [PubMed] [Google Scholar]
- [32].Introduction to the National Health Insurance Research Database (NHIRD), Taiwan. 2010. [Cited June 18 2010]. Available at: https://nhird.nhri.org.tw/en/ Accessed March 12, 2018. [Google Scholar]
- [33].Lu TH, Chou YJ, Liou CS. Impact of SARS on healthcare utilization by disease categories: implications for delivery of healthcare services. Health Policy 2007;83:375–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Fernández-Ruiz M, Guerra-Vales JM, Colina-Ruizdelgado F. Comorbidity negatively influences prognosis in patients with extrahepatic cholangiocarcinoma. World J Gastroenterol 2009;15:5279–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Jang SH, Chea JW, Lee KB. Charlson comorbidity index using administrative database in incident PD patients. Clin Nephrol 2010;73:204–9. [DOI] [PubMed] [Google Scholar]
- [36].Kyung MH, Yoon SJ, Ahn HS, et al. Prognostic impact of Charlson comorbidity index obtained from medical records and claims data on 1-year mortality and length of stay in gastric cancer patients. J Prev Med Public Health 2009;42:117–22. [DOI] [PubMed] [Google Scholar]
- [37].Introduction of National Health Insurance Premium Level. 2010. [Cited June, 23 2010]. Available at: http://www.nhi.gov.tw/resource/Webdata/20774_1_NHI%20IN%20TAIWAN%202011%20ANNUAL%20REPORT.pdf, page 14–16 Accessed March 20, 2018. [Google Scholar]
- [38].Rabe-Hesketh S, Skrondal A. Multilevel and Longitudinal Modeling Using STATA. 2nd edTexas: Stata Press; 2008. [Google Scholar]
- [39].Ingarfield SL, Celenza A, Jacobs IG, et al. Outcomes in patients with an emergency department diagnosis of fever of unknown origin. Emerg Med Australas 2007;19:105–12. [DOI] [PubMed] [Google Scholar]
- [40].Antoon JW, Potisek NM, Lohr JA. Pediatric fever of unknown origin. Pediatr Rev 2015;36:380–90. [DOI] [PubMed] [Google Scholar]
- [41].Chien YL, Huang FL, Huang CM, et al. Clinical approach to fever of unknown origin in children. J Microbiol Immunol Infect 2017;50:893–8. [DOI] [PubMed] [Google Scholar]
- [42].Cho CY, Lai CC, Lee ML, et al. Clinical analysis of fever of unknown origin in children: a 10-year experience in a northern Taiwan medical center. J Microbiol Immunol Infect 2017;50:40–5. [DOI] [PubMed] [Google Scholar]
- [43].Horowitz HW. Fever of unknown origin or fever of too many origins? N Engl J Med 2013;368:197–9. [DOI] [PubMed] [Google Scholar]
- [44].Rennen H, Bleeker-Rovers C, Oyen W. Baert A, Sartor K, Schiepers C. Imaging infection and inflammation. Springer, Diagnostic Nuclear Medicine, 113–126. Berlin Heidelberg: 2006. [Google Scholar]
- [45].Bleeker-Rovers CP, Boerman OC, Rennen HJ, et al. Radiolabeled compounds in diagnosis of infectious and inflammatory disease. Curr Pharm Des 2004;10:2935–50. [DOI] [PubMed] [Google Scholar]
- [46].Hung BT, Wang PW, Su YJ, et al. The efficacy of (18)F-FDG PET/CT and (67)Ga SPECT/CT in diagnosing fever of unknown origin. Int J Infect Dis 2017;62:10–7. [DOI] [PubMed] [Google Scholar]
- [47].Kouijzer IJE, Mulders-Manders CM, Bleeker-Rovers CP, et al. Fever of unknown origin: the value of FDG-PET/CT. Semin Nucl Med 2018;48:100–7. [DOI] [PubMed] [Google Scholar]
- [48].Takeuchi M, Dahabreh IJ, Nihashi T, et al. Nuclear imaging for classic fever of unknown origin: meta-analysis. J Nucl Med 2016;57:1913–9. [DOI] [PubMed] [Google Scholar]
- [49].Bharucha T, Rutherford A, Skeoch S, et al. Diagnostic yield of FDG-PET/CT in fever of unknown origin: a systematic review, meta-analysis, and Delphi exercise. Clin Radiol 2017;72:764–71. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data and related metadata were deposited in an appropriate public repository. The data on the study population that were obtained from the NHIRD (http://w3.nhri.org.tw/nhird//date_01.html) are maintained in the NHIRD (http://nhird.nhri.org.tw/). The NHRI is a nonprofit foundation established by the government.


