Abstract
Mycetoma is a devastating neglected tropical disease, caused by various fungal and bacterial pathogens. Correct diagnosis to the species level is mandatory for proper treatment. In endemic areas, various diagnostic tests and techniques are in use to achieve that, and that includes grain culture, surgical biopsy histopathological examination, fine needle aspiration cytological (FNAC) examination and in certain centres molecular diagnosis such as PCR. In this retrospective study, the sensitivity, specificity and diagnostic accuracy of grain culture, surgical biopsy histopathological examination and FNAC to identify the mycetoma causative organisms were determined. The histopathological examination appeared to have better sensitivity and specificity. The histological examination results were correct in 714 (97.5%) out of 750 patients infected with Madurella mycetomatis, in 133 (93.6%) out of 142 patients infected with Streptomyces somaliensis, in 53 (74.6%) out of 71 patients infected with Actinomadura madurae and in 12 (75%) out of 16 patients infected with Actinomadura pelletierii. FNAC results were correct in 604 (80.5%) out of 750 patients with Madurella mycetomatis eumycetoma, in 50 (37.5%) out of 133 Streptomyces somaliensis patients, 43 (60.5%) out of 71 Actinomadura madurae patients and 11 (68.7%) out of 16 Actinomadura pelletierii. The mean time required to obtain the FNAC result was one day, and for the histopathological examinations results it was 3.5 days, and for grain it was a mean of 16 days. In conclusion, histopathological examination and FNAC are more practical techniques for rapid species identification than grain culture in many endemic regions.
Author summary
In mycetoma endemic regions, the medical and health settings are commonly suboptimal, and only a few diagnostic tests and techniques are available. That had badly affected the patients’ proper diagnosis and management and thus the late presentation of patients with advanced disease. In this retrospective study, the experience of the MRC on the common in use diagnostic tests in the period between 1991 and 2018 is presented. In this study, the sensitivity, specificity rates and diagnostic accuracy of grain culture, surgical biopsy histopathological examination and FNAC to identify the mycetoma causative organisms were determined. The histopathological examination appeared to have better sensitivity and specificity. Furthermore, the grain culture identification needs high experience, it is the tedious procedure, and cross-contamination is common hence misdiagnosis is frequent. It can be concluded that histopathological examination and FNAC are more practical techniques for rapid species identification than grain culture in many endemic regions with poor diagnostic setting.
Introduction
Mycetoma is a chronic granulomatous subcutaneous inflammatory infection, endemic in subtropical and tropical regions, but it is reported globally [1, 2]. It is characterised by a painless subcutaneous swelling, multiple sinuses formation and a discharge that contain grains [3, 4]. The clinical presentation can give a clue to the diagnosis, but without further diagnostic testing it will lead to misdiagnosis and inaccurate treatment [5]. Mycetoma can be caused by different bacteria (actinomycetoma) or fungi (eumycetoma) [6, 7]. More than 70 different micro-organisms were reported to cause this infection, and hence it is essential to identifying the causative agents to the highest level of resolution which in turn will contribute to choosing appropriate treatment [8, 9]. In endemic regions, the most commonly used tools are culturing of the grains, surgical biopsy followed by histopathological examination and fine needle aspiration cytological (FNAC) examination [10, 11].
Currently, culturing the grains culture is still considered to be the golden standard for species identification in many centres [12, 13]. However, this technique is tedious, time-consuming due to the slow growth rate and it needs expert microbiologists to identify the causative agents based on the macroscopic appearance of the isolates. Furthermore contamination is common. Patients on medical treatment may have non-viable gains, and hence it is difficult to identify the causative organism [14, 15].
To overcome these difficulties, histological examination is often used complementary to culture. In a histopathological examination, it is easy to discriminate between fungal and bacterial causative agents [16, 17]. However, identification to the species level is more challenging and considered far from reliable [18, 19]. At the Mycetoma Research Centre (MRC), University of Khartoum, Khartoum, Sudan FNAC is a common tool to identify the causative organisms. It is less invasive and time-consuming compared to the histopathological and culture techniques [20, 21]. However, to the best of our knowledge there was no study performed in which the sensitivity and specificity of the two techniques for the identification of the mycetoma causative organisms were compared. With this background, this study was conducted at the Mycetoma Research Centre were 8500 confirmed mycetoma patients were seen and treated. In this retrospective study, the records of these patients were reviewed, and patients who undergone the three diagnostic tests were included.
Materials and methods
Ethics statement
Following the Mycetoma Research Centre Institutional Review Board ethical approval, all the histopathological, cytological and microbiological reports of the patients seen in the Mycetoma Research Centre over a 27-year period (January 1991 to January 2018) were reviewed.
Study cohort
The data were collected in the pre-designed data collection sheet. The patient demographic characteristics, results of the three techniques were collected.
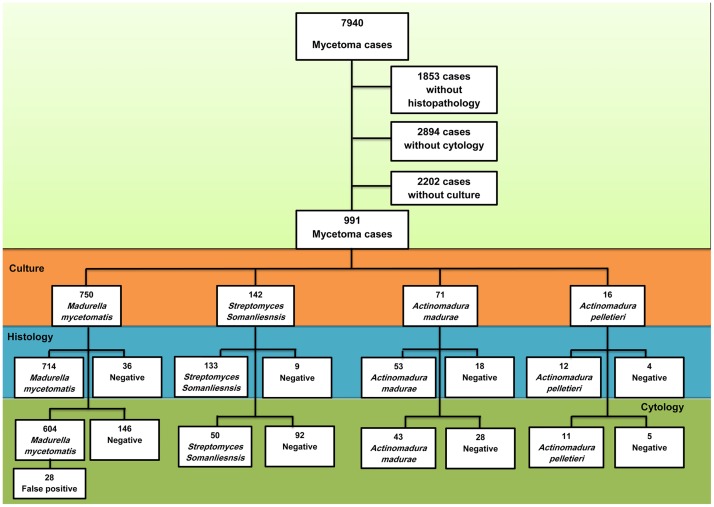
In this study, only patients in whom the causative organisms were identified by culture and had undergone both a fine needle aspirate for cytological examination and deep-seated excisional biopsy for histopathological examination were included. (Fig 1).
Fig 1. identification of the causative agents: All culture, histopathological and cytological identification reports of patients seen between January 1991 and January 2018 in the Mycetoma Research Centre were retrieved from our electronic database.
Of these patients in 2202 cases no microbiological report was available, of 1853 cases no histopathological report was available and in 2894 cases no cytological report was available leaving 991 mycetoma cases which met our inclusion criteria. The culture identification was considered the golden standard. Of these patients 750 cases were caused by M. mycetomatis, 142 cases by S. somaliensis, 71 were caused by A. madurae and 12 by A. pelletieri. From these cases the histological and cytological reports were compared to the culture results. A histological report or cytological report was either identical to the culture identification or negative. Results for both histology and cytology reports are shown.
The number of true-positive (TP), false-positive (FP), true-negative (TN), and false-negative (FN) test results was calculated for each technique and was compared to the culture which considered as our gold standard method. According to these results, sensitivity, specificity, positive predictive value, negative predictive value, and accuracy were calculated for each test. Accuracy was calculated as the proportion of true results (both true positives and true negatives) among the total number of cases.
Grains isolation
The grains were obtained by surgical biopsy and/or FNAC. For the latter, a 25-gauge needle was inserted into the lesion, and aspirates were taken. The yield of grains was assessed visually by the number and size of grains obtained. If the yield was low, a second aspiration was taken with a 23-gauge needle. When excessive bleeding from the lesions was encountered, a 27-gauge needle was used. The obtained sample usual splited into two parts; one was transported immediately to the microbiology department for culturing, the other part was sent to the histology department for histopathological and cytological.
Culture
The mycetoma grains were washed three times in sterile normal saline. When a fungus was expected based on the clinical data accompanied the request form and the grains color and consistency (fungal grains tend to be hard and are either white, yellow and black in color according to the causatives agent), the grains were cultured on Sabouraud dextrose agar with gentamicin sulphate (400 μg/ml) for fungal grains at 37 °C. When an actinomycete was expected according to the clinical data and ultrasound report as well as the grain color and consistency (actinomycetoma grains tend to be soft and smooth) grains were cultured on Blood agar, Colombia agar, Glucose yeast extract agar, Brain-heart infusion, Löwenstein—Jensen agar and Modified Sabouraud agar supplemented with 0.5% yeast extract. After that the plates were incubated at 37°C for 7 to 14 days.
Identification of the eumycetoma causative agent by culture
When a fungus was grown on the Sabouraud plate, it was identified based on its macroscopic and microscopic morphology. Table 1 demonstrate the characteristic of different eumycetoma causatives agent for macroscopically and microscopically identification.
Table 1. Macroscopical and microscopical characteristic of different Madurella species.
| Organism | Morphology on SDA | |
|---|---|---|
| Morphology | Pigment Production | |
| Madurella mycetomatis | dry, yellowish brown to gray; reverse, yellowish brown to gray. | variable |
| Madurella tropicana | dry, white brown to gray; reverse, white-brown to gray | orange-brown pigment |
| Madurella pseudomycetomatis | dry, brown to gray; reverse, brown to gray. | orange-brown pigment |
| Madurella fahalii | dry, yellowish brown to gray; reverse, yellowish brown to gray. | No pigment |
| Trematosphaeria grisea | greenish grey, becoming faint towards the margin; reverse dark brown to black. | no pigment |
| Medicopsis romeroi | greenish grey, becoming faint towards the margin; reverse dark brown to black. | No pigment |
| Organism | Microscopically appearance | |
| Madurella mycetomatis | Hyphae are septate, thick-walled, pale brown. Conidiophores are absent. Many Chlamydospores are present. | |
| Madurella tropicana | Hyphae are septate, thick-walled, pale brown. Conidiophores are absent. Many Chlamydospores are present. | |
| Madurella pseudomycetomatis | Hyphae are septate, thick-walled, pale brown. Conidiophores are absent. Chlamydospores are present. | |
| Madurella fahalii | Hyphae are septate, thick-walled, pale brown. Conidiophores are absent. Many chlamydospores are present. | |
| Trematosphaeria grisea | Hyphae branched, septate, hyaline or brown, thick-walled, verruculose | |
| Medicopsis romeroi | Hyphae branched, septate, hyaline or brown, thick-walled, verruculose | |
Macroscopically the appearance of M. mycetomatis colonies is quite variable. At the beginning the colonies tend to be white, and upon maturing they change to yellow or brown. Some strains of M. mycetomatis are able to produce a brown pigment in the culture. The texture varies from smooth, flat or heaped. Madurella mycetomatis is differentiated from T. grisea by its ability to grow at temperatures up to 40°C and its inability to assimilate sucrose (Table 2).
Table 2. Carbohydrates Assimilation test for eumycetoma discrimination.
| Organism | Assimilation of Sugar | Growth at 37°c | ||
|---|---|---|---|---|
| Glucose | Lactose | Sucrose | ||
| Madurella mycetomatis | + | + | - | + |
| Madurella pseudomycetomatis | + | - | - | + |
| Trematosphaeria grisea | + | - | + | - |
| Falciformispora senegalensis | + | - | + | + |
| Falciformispora tompkinsii | + | - | + | + |
| Nigrograna mackinnonii | + | - | + | - |
| Medicopsis romeroi | + | - | + | + |
Identification of the actinomycetoma causative agent by culture
When colonies are obtained, presumptive species identification is based on macroscopical and microscopical appearance of the species. Typical colonies of Nocardia spp and Streptomyces spp are dry to chalky in consistency, usually folded. The color will range from yellow to gray white. A. madurae and A. pelletieri strains produce cream- and red-pigmented mycelia respectively and lack aerial filaments on initial isolation. Ziehl-Neelsen staining is used to determine if the isolate is acid fast. Nocardia spp will stain positively and Actinomadura spp will stain negatively.
Different biochemical tests will be performed to identify the causative agent to the species level. These include the degradation of adenine, casein, and hypoxanthine; growth on adonitol; aesculin hydrolysis; glycerol; glycogen; D-raffinose; L-rhamnose; D-turanose; D-xylose; and L-aspartic acid (Tables 3 and 4).
Table 3. Different Biochemical test that used in discrimination between the actinomycetoma causatives agent.
| Organism | Acid Fast | Urease | Gelatin Hydrolysis | Decomposition of | |||
|---|---|---|---|---|---|---|---|
| Casein | Tyrosine | Xanthine | Hypoxanthine | ||||
| Nocardia asteroides | + | + | - | - | - | - | - |
| Nocardia brasiliensis | + | + | + | + | + | - | + |
| Nocardia otitidiscaviarum | + | + | - | - | - | + | + |
| Nocardia farcinica | + | + | - | - | - | - | - |
| Actinomadura madurae | - | - | + | + | + | - | + |
| Actinomadura pelletieri | - | - | + | + | + | - | + |
| Streptomyces somaliensis | - | - | + | + | + | - | - |
Table 4. Carbohydrates fermentation test for actinomycetoma discrimination.
| Organism | Utilization as a sole source of carbon | ||||||
|---|---|---|---|---|---|---|---|
| D- Galactose | D- glucose | I -myoinositol | D- manitol | L-ramnose | D-sorbitol | D-trehalose | |
| Nocardia asteroides | V | + | - | - | V | - | V |
| Nocardia brasiliensis | + | + | + | + | - | - | + |
| Nocardia otitidiscaviarum | - | + | + | V | - | - | V |
| Nocardia farcinica | - | + | - | - | + | - | - |
| Actinomadura madurae | NA | NA | + | + | + | + | + |
| Actinomadura Pelletieri | NA | NA | + | - | - | - | + |
| Streptomyces somaliensis | NA | NA | + | - | - | - | - |
V, variable; NA, not available
Cytological examination
The aspirate was allowed to air dry and was stained using Diff-Quick stain. The stained aspirates were examined by an expert histopathologist for the presence of the following cytomorphological features: smears cellularity, the host inflammatory tissue reaction, the presence and types of the causative organisms’ grains. Species identification was based on species-specific criteria. In general, M. mycetomatis grains can be either small or large, are light to dark brown in colour and have irregular outlines and a crushing artefact when stained with hematoxylin and eosin (H&E) (Fig 2A).
Fig 2. Cytology of mycetoma causative agents.
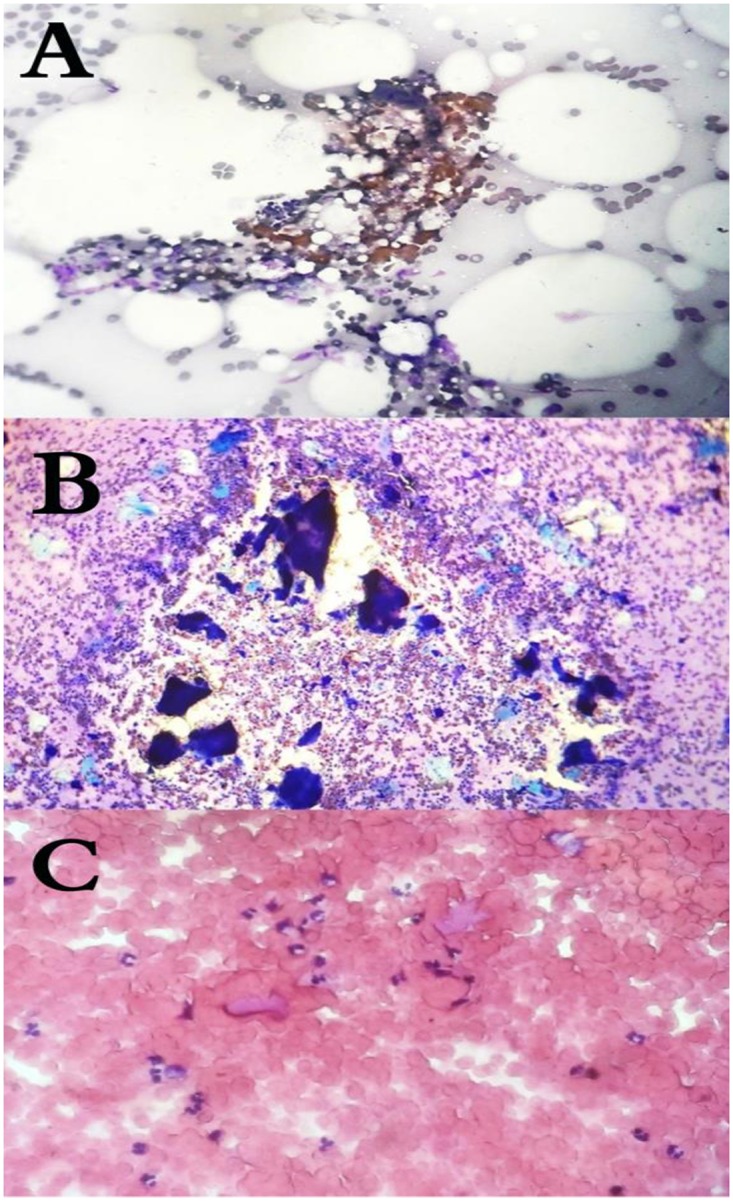
(A): The cytological appearance of M. mycetomatis, (B): A. pelletierii, (C): S. somaliensis in fine needle aspirates stained with HE (magnification 10 times).
S. somaliensis grains are difficult to see in H&E stained sections, they stain bright pink to hazy pink in colour, are often oval to irregular shaped and can be as aggregates (Fig 2C).
A. madurae grains are small oval shaped, and it stained pink to red colour in H&E and tend to be as one mass without any fractures. A. pelletierii grains are small rounded to oval shaped, and they stained deep blue in H&E stained sections and tend to be fractured.
Histopathological examination
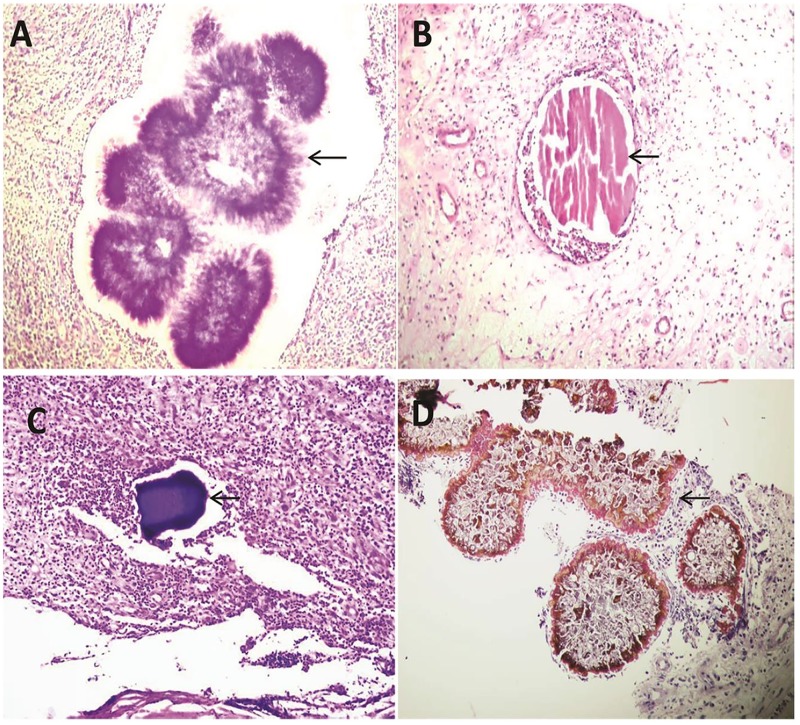
All patients underwent surgical biopsy under anaesthesia, which was fixed in 10% formalin and processed further into paraffin blocks. 3-5-μm sections were obtained and stained with H&E. In our Histopathological laboratory the histopathologist issued the report with the species name according to the following criteria i.e. species specific criteria which have been used by all of them. M. mycetomatis grains tend to be large, light to dark brown in colour with irregular outlines. They tend to fracture when sections are cut. M. mycetomatis has two different types of grains, and these are the filamentous and vesicular. The filamentous type, is the most common type and consists of brown septated and branched hyphae that may be slightly more swollen towards the edges (Fig 3D).
Fig 3. Histopathological appearance of mycetoma causative agents.
The histopathological appearance of A. madurae (A), S. somaliensis (B), A. pelletierii (C) and M. mycetomatis (D) in HE stained sections (Magnification 10x).
S. somaliensis grains are rounded to oval in shape, with homogenous appearance in tissue sections. They appear faint yellow in unstained sections, and the grains are not well stained with H&E. Moreover, as a result of sectioning they may show longitudinal cracks, the filaments are fine (measured between 0.5–2 μm in diameter), closely packaged and embedded in cement matrix (Fig 3B).
A. pelletierii grains are small, round to oval in shape and semicircular and sickle like shapes have been observed as well. The filamentous structures are pretty difficult to be detected. However, a careful and meticulous examination of the periphery of the grains may show some of them. A. pelletierii grains stain deep violet with H&E, which is very characteristic and allows the definitive diagnosis without a need for culturing techniques (Fig 3C).
A. madurae grains ranged from yellow to white. Therefore, it can be difficult to discriminate them from the surrounding fat. Histologically the grain size ranges from small to large. The large grains have a characteristic variegated pattern. The periphery of the grain is opaque, homogenous and deep purple when stained with H&E stain, while the centre is less densely stained. Additionally, the periphery of the grains shows an eosinophilic material (Fig 3A). Smaller grains are more homogeneous and are difficult to distinguish from A. pelletierii. However, even the small grains of A. madurae have a more deeply stained purple fringe, which is not seen in A. pelletierii.
Results
In this study, 991 patients out of 7940 patients were eligible and were included in the analysis. Their ages ranged between 5 and 75 years old. The majority were males 737 (74.3%), and most of them were students 327 (32.9%) and farmers 167 (16.8%). The majority of the patients (837 out of 991), gave a history of discharge that contained grains and the majority of these grains were black (565; 57%)) followed by yellow (104;10.5%), white (60; 6.1%) and red grains (14; 1.4%). In this cohort, the majority of patients, (72.6%) had no history of local trauma, only 191 (19.3%) patients did recall a local trauma and the remaining 73 (7.4%) patients were not certain.
Based on the culture reports of the grains, in 750/991 (75.6%) of the patients the mycetoma was caused by M. mycetomatis, in 142/991 (14.4%) it was caused by S. somaliensis, in 71/991 (7.16%) it was caused by A. madurae and in 16/991 (1.6%) it was caused by A. pelletieri. In 11 patients no growth was reported from the grains obtained during the sample collection. The time to growth differed case by case and ranged between 5 and 28 days.
In this study, out of the 991 mycetoma cases, the correct species identification was obtained for 912 cases using histopathological examination. Using FNAC, the correct diagnosis was obtained in 708 cases. The histopathological examination confirmed the diagnosis of M. mycetomatis in 714 of 750 cases with 95.2% sensitivity, 95.4% specificity and diagnostic accuracy of 95.3%. For FNAC only 604 out of 750 M. mycetomatis cases were identified, resulting in a sensitivity of 80.5%, a specificity of 88.4% and a diagnostic accuracy of 82.4%.
Out of 142 S. somaliensis cases, 133 were also identified with histopathological examination with 93.7% sensitivity, 98.9% specificity and diagnostic accuracy of 98.2%. With FNAC only 50 out of 133 S. somaliensis cases were identified, resulting in a sensitivity of 35.2%, a specificity of 99.3% and a diagnostic accuracy of 90.1%.
53 out of 71 cases with A. madurae identification were identifuied by histopathological examination, with a sensitivity of 74.7%, 99.5% specificity and diagnostic accuracy of 97.7%. FNAC identified 43 out of 71 cases with a sensitivity of 60.6%, specificity of 94.4% and diagnostic accuracy of 91.9%.
For A. pelletierii out of 16 cases; 12 were also identified with histological examination with 75.0% sensitivity, 100% specificity, and diagnostic accuracy of 99.6%. For FNAC a sensitivity of 68.8%, a specificity of 99.7% and a diagnostic accuracy of 99.2% were obtained.
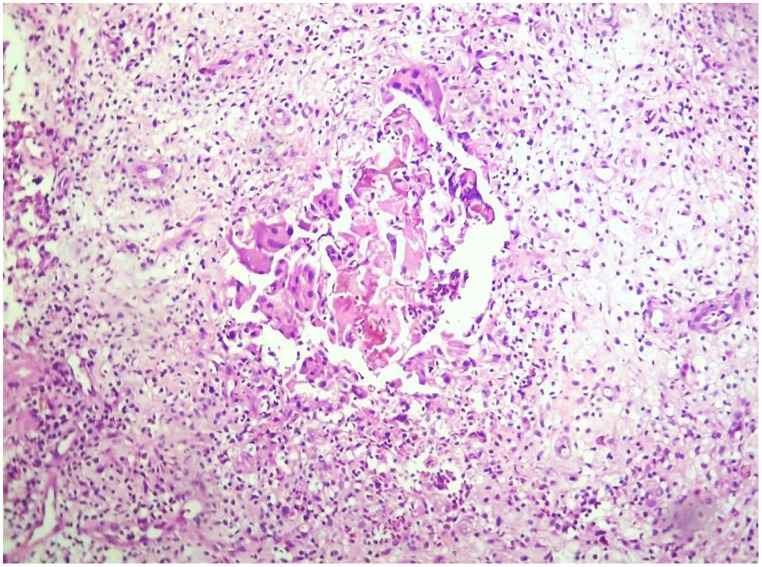
With the histopathological examination, false negative result was reported in 36/750 M. mycetomatis cases, 9/142 S. somaliensis cases, 18/71 A. madurae cases and 4/16 A. pelletieri cases. To determine the false negative results reasons, the histopathological slides were re-examined. There were various reasons for the false negative, and that included the absence of mycetoma histopathological architecture resulted in overlooking the causative agent (Fig 4).
Fig 4. Histopathological section from patient diagnosed as M. mycetomatis based on the culture results; Histopathology report was been negative for fungus and when we retrieved the slide we can see adherence of histiocytes at the center with sparse amount of the grains (H and E, X 10).
Furthermore, in some blocks, the grains were absent; either because the tissue was not homogenously infected by the causative agent and that the part which was taken for histology or the section contained no grains. This latter might be overcome by examining multiple sections at different depths of the histology blocks especially when inflammation and necrosis are noted.
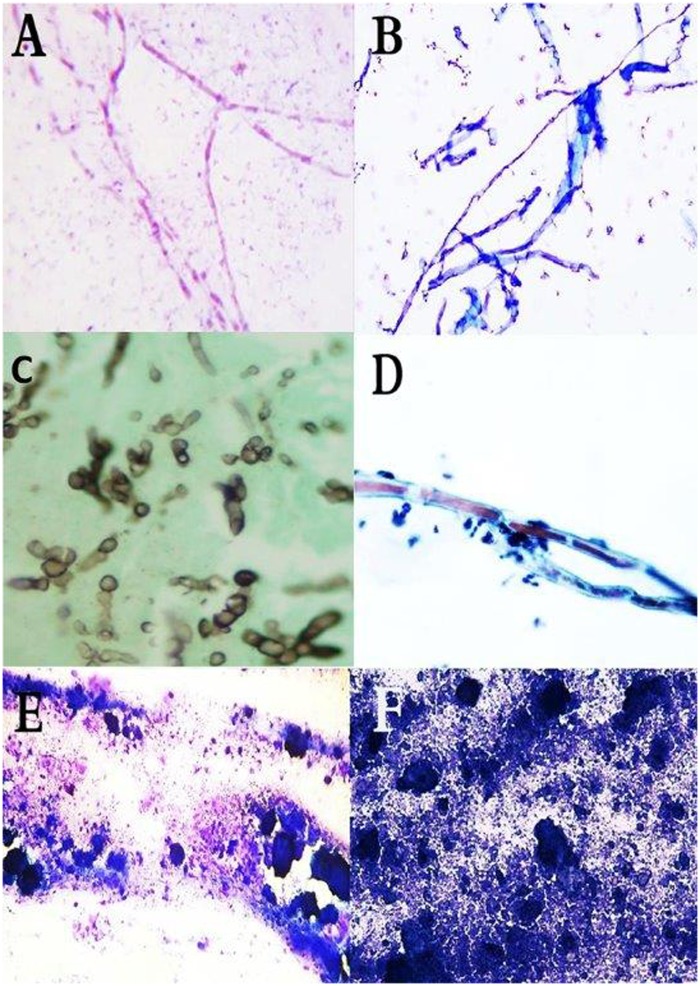
False positive results were obtained in 28 of the cases. This was attributed to the presences of numerous structures that can mimic the appearance of M. mycetomatis and that included vegetables, synthetic fibres and algae which can resemble fungal hyphae and calcification (Fig 5).
Fig 5. False positive identifications in cytology.
Showing cytological smears of (A) M. mycetomatis hyphae stained with Giemsa stain, (B) Synthetic Fibers stained Wright-Giemsa stain. (Giemsa stain, X40). (C) Smear showing hyphae of M. mycetomatis. (D) Smear showing elongated structures of the Oedogoniales order. The chloroplasts form a chain interrupted by clear zones (X40). (E) Smear showing M. mycetomatis grains after being crushed on the smear. (F) smear with abundant calcific debris without intact cells taken from patient with tumoral calcinosis (Diff Quick, X10).
In overall, using histology correct species identification was obtained in the majority of cases. The mean time to identify the culture isolates was 16 days (range 5 to 28 days), for histology it was 3.5 days (range 2 to 5 days), and for cytology, it was one day (range 1 to 2 days). This demonstrated that reliable species identification using histology was obtained in 92.0% of cases within an average time reduction of 13.5 days, for cytology this was 71.4% of cases with time reduction of 15 days, indicating that adding histology or cytology to the diagnostic techniques used for species identification resulted in an earlier start of treatment.
Discussion
The accurate identification of mycetoma causative agents is considered the cornerstone for the initiation of appropriate therapy. Hence a rapid and accurate diagnostic tool to achieve the definitive species identification is considered a critical part in patient treatment and management [21–23]. Different laboratory techniques for species identification are in use, including culture, histopathology, [7, 25], FNAC [8, 24], serological assays and imaging [26–29] as well as different molecular diagnostic tools [30–35]. However, not all these assays are available in endemic regions. In the Mycetoma Research Centre, culturing of the grains, histopathology and FNAC are routinely performed and have been used for the past 27 years. In this communication we have used the data collected for the last 27 years to assess the sensitivity, specificity and diagnostic accuracy of histopathology and cytology in the identification of mycetoma causative agents in comparison to the current golden standard: culturing.
This study showed that the histopathology was more accurate to FNAC in terms of species identification. Our results are in line with that reported previously by Yousif and colleagues [36]. They reported 90.9% agreement when histopathology was compared to FNAC for the diagnosis of M. mycetomatis (90.9%) while for actinomycetoma causative agents it was only 60%. The lower diagnostic agreement of actinomycetoma causative agents could have been caused by morphological similarities of these microorganisms. Furthermore, both techniques are operator dependent and need intensive training and experience which could have its reflections on the accuracy.
Mycetoma can be caused by more than 70 different causative agents [37], but the distribution of these species is not everywhere the same which could cause differences in diagnostic accuracy in different regions. In some of the mycetoma endemic regions, mycetoma is caused by closely related species. Morphologically these organisms may look similar which could cause a challenge in the identification of these organisms. In Mexico, the most common causative agents are Nocardia brasiliensis and Nocardia asteroides [37], two closely related species which are difficult to differentiate from each other based on histopathology [38, 39]. In Senegal, the most common causative agents of eumycetoma are M. mycetomatis and Falciformispora senegalensis which both can cause black grain mycetoma [37, 40]. In the black grains of F. senegalensis, the centre is non-pigmented, and the cement is absent, whereas at the peripheries the grains are dark coloured and brown cement is present. However, this is also seen in black grains of Trematosphaeria grisea and certain grains of M. mycetomatis. Hence an expert pathologist is needed to differentiate between these organisms [41].
The study performed here was a retrospective study, looking back at the records of the Mycetoma Research Centre for the past 27 years. During that time molecular identification of the causative agents was not performed and culture was considered the golden standard. Recently in the study conducted by Borman and colleagues demonstrated that using morphological identification, misidentifications occurred in many cases [42]. Out of 28 previously identified Trematosphaeria grisea isolates, 22 were, other fungal species [42]. For actinomycetoma causative organisms, misidentifications also have been described. In 2008, Quintana and associates demonstrated that half of the S. somaliensis isolates obtained from Sudan appeared to be Streptomyces sudanensis [43]. Furthermore, next to A. madurae and A. pelletieri also Actinomadura latina was described [44]. Therefore a current ongoing study is including molecular diagnosis to determine the true etiology and predictive value of culture, FNAC and histology. With the introduction of molecular diagnosis in our centre we already made the first step in this respect.
In this study, the sensitivity of histopathological technique was superior to that FNAC for all species tested. In that study they studied the performance of FNAC in comparison to histology in 19 different mycetoma patients. Out of these 19 patients, five patients had to be excluded due to inadequate aspirated materials. From the 14 remaining patients, 10 were diagnosed as M. mycetomatis with histopathology, and 4 were actinomycetoma. With this limited number of patients they could conclude that FNAC could identify the causative agent in 9 out of 10 M. mycetomatis patients. One patient identified by histology could not be identified with cytology, again confirming that histology was superior to FNAC in respect to species identification [15]. A result confirmed in our current study, as in our study 146 patients with M. mycetomatis mycetoma were missed with FNAC. However, of the three different identification methods used, FNAC was the most rapid and resulted in species identification within 1 day, instead of 3.5 days for histology or 16 days for culture. FNAC is a simple and rapid diagnostic technique which can be used at the one-stop diagnosis clinic and in epidemiological and field surveys. However, it has many limitations: it is an operator dependent technique can be painful and can lead to deep-seated bacterial infections. FNAC is less invasive than a deep-seated biopsy, as only a small puncture hole is obtained. With a deep-seated biopsy a larger area of the lesion is removed thereby also exposing a larger part of the lesion to secondary bacterial infections and creating a bigger risk for dissemination of the infection. Currently, deep-seated biopsies are only performed to obtain a diagnostic sample, not to reduce the burden of infection at the site of the lesion. This, because the lesion can be extensive, even when on the outside only a small lesion is seen. At the moment the fine needle aspirate is often taken blindly without guidance of ultrasound imaging which creates a risk that the operator might miss the pockets which contains grains. With the use of the ultrasound-guided aspiration, the diagnostic yield of the technique will improve which in its turn could enhance the number of cases in which positive species identification might be obtained.
The grains culture remains in many centres the cornerstone for the diagnosis of mycetoma. moreover, morphological identification of mycetoma causatives agent may be some times be difficult to achieved due to the overlapping and similarities encountered between different species as demonestrated in Table 1, However culture is a time-consuming procedure and an experienced microbiologist is needed to identify the organisms to the species level [45]. Cross-contamination is a common problem.
Recently an identification scheme of eumycetoma causative agents has been published which was based on the pysiological properties of the causative agent, indicating that it is possible to use physiological properties to identify eumycetoma causative agents more reliably [1]. These include culturing at 37°C and growth on actidione, L-sorbose, glycetol, potassium 2-keto-gluconate, methyl-D-glucopyranoside, inositol and D-sorbitol. However, these tests are more time consurming and delay the identification of the causative agent which will potentially leed to delay in patient treatment. However, by complementing culturing with histology or FNAC a preliminary identification might be obtained earlier. Especially, since the treatment of mycetoma infections is dependent on the causative agent. Actinomycetoma is treated differently than eumycetoma. [22].
Correct identification to the species level will influence clinical decision making. The first diagnostic discrimination needed is the distinction between actinomycetoma causative agents and eumycetoma causative agents, since this would implicate either antibacterial treatment or a treatment based on surgery and antifungal treatment. In this situtation fine needle aspiaration cytology and histopathological examination were found to be highly sensitive in the discrimination between actinomycetoma from eumycetoma based on the morphological characteristics; therefore, and based on our experience we recommended the used of FNAC in a low resources centers like in the rural centers were there is no Histopathology laboratories.
For the actinomycetoma causative agents, it is currently not known if the choice of antibacterial agent is dependent on the causative agents and studies are needed to evaluate if differrent treatment regimens are needed for each of the different bacterial causative agents. When this appears to be the case, identification to the species level becomes essential. For eumycetoma causative agents, surgery is always combined with itraconazole. However, Medicopsis romeroi and Madurella fahalii, were not susceptibile towards itraconazole in vitro. This would indicate that discrimination between these species and the susceptible species would be mandatory and could potentially effect clinical management. Clinical evaluation of such cases and the effect on the therapeutic success rates are needed. However, before such a study can be performed proper species identification needs to be obtained.
In summary, the Cytopathologist/Histopathologist need to be aware of the many mimics that can look like the mycetoma causatives agent, and we highly recommended that the pathologists after issuing the diagnosis should recommend correlation with microbiology or provide a cautionary statement to advise clinicians of the limitations of identifying organisms with histopathologic/cytopathologic examination.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Reis CMS, Reis-Filho EGM. Mycetomas: an epidemiological, etiological, clinical, laboratory and therapeutic review. An Bras Dermatol. 2018; 93 (1):8–18. 10.1590/abd1806-4841.20187075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fahal AH. Mycetoma: A global medical and socio-economic dilemma. PLoS Negl Trop Dis. 2017; 11(4): e0005509 10.1371/journal.pntd.0005509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zijlstra EE, van de Sande WWJ, Fahal AH. Mycetoma: A Long Journey from Neglect. PLoS Negl Trop Dis. 2016; 10(1): e0004244 10.1371/journal.pntd.0004244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Suleiman SH, Wadaella ES, Fahal AH. The Surgical Treatment of Mycetoma. PLoS Negl Trop Dis. 2016; 10(6): e0004690 10.1371/journal.pntd.0004690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van de Sande Wendy, Fahal Ahmed, Ahmed Sarah Abdalla, Serrano Julian Alberto, Bonifaz Alexandro, Zijlstra Ed, on behalf of the eumycetoma working group; Closing the mycetoma knowledge gap, Medical Mycology, 2018; 56(supp 1):153–164. [DOI] [PubMed] [Google Scholar]
- 6.Fahal A, Mahgoub ES, Hassan AME, Abdel-Rahman ME. Mycetoma in the Sudan: An Update from the Mycetoma Research Centre, University of Khartoum, Sudan. PLoS Negl Trop Dis. 2015; 9(3): e0003679 10.1371/journal.pntd.0003679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Siddig EE, Fahal AH. Histopathological Approach in Diagnosis of Mycetoma Causative Agents: A Mini Review. J Cytol Histol. 2017; 8: 466. [Google Scholar]
- 8.Siddig EE, Yousif BM, Edris AM, Fahal AH. The Touch Imprint Cytology: A simple method for the diagnosis of mycetoma causative agents. Khartoum Medical Journal. 2016; 9(1):1211–1216. [Google Scholar]
- 9.Ahmed AA, van de Sande W, Fahal AH. Mycetoma laboratory diagnosis: Review article. PLoS Negl Trop Dis. 2017; 11(8): e0005638 10.1371/journal.pntd.0005638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Verghese A, Klokke AH. Histologic diagnosis of species of fungus causing mycetoma. Indian J Med Res. 1966; 54: 524–530. [PubMed] [Google Scholar]
- 11.Alam K, Maheshwari V, Bhargava S, Jain A, Fatima U, Haq E. Histological Diagnosis of Madura Foot (Mycetoma): A Must for Definitive Treatment. Journal of Global Infectious Diseases. 2009; 1(1), 64–67. 10.4103/0974-777X.52985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chufal SS, Thapliyal NC, Gupta MK. An approach to histology-based diagnosis and treatment of Madura foot. J Infect Dev Ctries. 2012; 6(9):684–8. 10.3855/jidc.2387 [DOI] [PubMed] [Google Scholar]
- 13.Ibrahim AI, El Hassan AM, Fahal A, van de Sande WW. A histopathological exploration of the Madurella mycetomatis grain. PLoS One. 2013;8(3):e57774 10.1371/journal.pone.0057774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Efared B, Tahiri L, Boubacar MS, Atsam-Ebang G, Hammas N, Hinde EF, et al. Mycetoma in a non-endemic area: a diagnostic challenge. BMC Clinical Pathology. 2017; 17, 1 10.1186/s12907-017-0040-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.EL Hag IA, Fahal AH, Khalil EAG. Fine needle aspiration cytology of Mycetoma. Acta Cytologica. 1996; 40(3): 461–46. 10.1159/000333899 [DOI] [PubMed] [Google Scholar]
- 16.Fahal AH. Mycetoma thorn on the flesh Review article. Trans R Soc Trop Med Hyg. 2004; 98(1):3–11. Review. [DOI] [PubMed] [Google Scholar]
- 17.Zein HAM, Fahal AH, Mahgoub ES, EL Hassan T, Abdel Rahman ME. The Predictors of Cure, Amputation & Follow-up dropout among Mycetoma Patients as seen at The Mycetoma Research Centre, University of Khartoum. Trans R Soc Trop Med Hyg. 2012; 106(11):639– 10.1016/j.trstmh.2012.07.003 [DOI] [PubMed] [Google Scholar]
- 18.van de Sande WWJ, Maghoub ES, Fahal AH, Goodfellow M, Welsh O, Zijlstra E. The Mycetoma Knowledge Gap: Identification of Research Priorities. PLoS Negl Trop Dis. 2014; 8(3): e2667 10.1371/journal.pntd.0002667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mitjà O, Marks M, Bertran L, Kollie K, Argaw D, Fahal AH, et al. Integrated Control and Management of Neglected Tropical Skin Diseases. PLoS Negl Trop Dis. 2017; 11(1): e0005136 10.1371/journal.pntd.0005136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van de Sande WWJ, Fahal AH, Goodfellow M, Mahgoub ES, Welsh O, Zijlstra EE. Merits and Pitfalls of Currently Used Diagnostic Tools in Mycetoma. PLoS Negl Trop Dis. 2014; 8(7): e2918 10.1371/journal.pntd.0002918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hay RJ, Fahal AH. Mycetoma: an old and still neglected tropical disease. Trans R Soc Trop Med Hyg. 2015;109(3):169–70. 10.1093/trstmh/trv003 [DOI] [PubMed] [Google Scholar]
- 22.Welsh O, Al-Abdely HM, Salinas-Carmona MC, Fahal AH. Mycetoma Medical Therapy. PLoS Negl Trop Dis. 2014; 8(10): e3218 10.1371/journal.pntd.0003218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van de Sande WWJ, Maghoub ES, Fahal AH, Goodfellow M, Welsh O, Zijlstra E. The Mycetoma Knowledge Gap: Identification of Research Priorities. PLoS Negl Trop Dis. 2014; 8(3): e2667 10.1371/journal.pntd.0002667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.EL Hag IA, Fahal AH, Gasim ET. Fine needle aspiration cytology of mycetoma. Acta Cytol. 1996; 40(3):461–4. 10.1159/000333899 [DOI] [PubMed] [Google Scholar]
- 25.Ibrahim AI, El Hassan AM, Fahal A, van de Sande WW. A Histopathological Exploration of the Madurella mycetomatis Grain. PLoS ONE. 2013; 8(3): e57774 10.1371/journal.pone.0057774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fahal AH. Mycetoma, Clinicopathological Monograph. Khartoum: Khartoum University Press, 2006. [Google Scholar]
- 27.Abd Bagi ME, Fahal AH, Sheik HE, Abdul Wahab O, Taifoor MK, et al. Pathological fractures in mycetoma. Trans R Soc Trop Med Hyg. 2003; 97: 582–584. 10.1016/s0035-9203(03)80036-6 [DOI] [PubMed] [Google Scholar]
- 28.Fahal AH, Sheik HE, Homeida MM, Arabi YE, Mahgoub ES.Ultrasonographic imaging of mycetoma. Br J Surg. 1997; 84: 1120–1122. [PubMed] [Google Scholar]
- 29.El Shamy ME, Fahal AH, Shakir MY, Homeida MM.New MRI grading system for the diagnosis and management of mycetoma. Trans R Soc Trop Med Hyg. 2012; 106: 738–742. 10.1016/j.trstmh.2012.08.009 [DOI] [PubMed] [Google Scholar]
- 30.Ahmed AO, Mukhtar MM, Kools-Sijmons M, Fahal AH, de Hoog S, van den Ende BG, et al. Development of a species-specific PCR-restriction fragment length polymorphism analysis procedure for identification of Madurella mycetomatis. J Clin Microbiol. 1999;37: 3175–3178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ahmed A, van de Sande W, Verbrugh H, Fahal A, van Belkum A. Madurella mycetomatis strains from mycetoma lesions in Sudanese patients are clonal. J Clin Microbiol. 2003; 41(10):4537–41. 10.1128/JCM.41.10.4537-4541.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Desnos-Ollivier M, Bretagne S, Dromer F, Lortholary O, Dannaoui E. (2006) Molecular identification of black-grain mycetoma agents. J Clin Microbiol. 44(10):3517–23. 10.1128/JCM.00862-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ahmed SA, van den Ende BHGG, Fahal AH, van de Sande WWJ, de Hoog GS. Rapid Identification of Black Grain Eumycetoma Causative Agents Using Rolling Circle Amplification. PLoS Negl Trop Dis. 2014; 8(12): e3368 10.1371/journal.pntd.0003368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ahmed SA, van de Sande WW, Desnos-Ollivier M, Fahal AH, Mahmoud NA, de Hoog GS. Application of Isothermal Amplification Techniques for Identification of Madurella mycetomatis, the Prevalent Agent of Human Mycetoma. J Clin Microbiol. 2015; 53(10):3280–5. 10.1128/JCM.01544-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van de Sande WW, Gorkink R, Simons G, Ott A, Ahmed AO, Verbrugh H, et al. (2005) Genotyping of Madurella mycetomatis by selective amplification of restriction fragments (amplified fragment length polymorphism) and subtype correlation with geographical origin and lesion size. J Clin Microbiol. 43(9):4349–56. 10.1128/JCM.43.9.4349-4356.2005 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yousif BM, Fahal AH, Shakir MY. A new technique for the diagnosis of mycetoma using fixed blocks of aspirated material. Trans R Soc Trop Med Hyg. 2010; 104(1):6–9. 10.1016/j.trstmh.2009.06.015 [DOI] [PubMed] [Google Scholar]
- 37.van de Sande WWJ.Global Burden of Human Mycetoma: A Systematic Review and Meta-analysis. PLoS Negl Trop Dis. 2013; 7(11): e2550 10.1371/journal.pntd.0002550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Destombes P, Mariat F, Rosati L, Segretain G. [Mycetoma in Somalia—results of a survey done from 1959 to 1964]. Acta Trop. 1977; 34: 355–373. [PubMed] [Google Scholar]
- 39.Destombes P. [Histologic Structure of Mycetomas]. Ann Soc Belges Med Trop Parasitol Mycol. 1964; 44: 897–908. [PubMed] [Google Scholar]
- 40.Ndiaye B, Develoux M, Dieng MT, Kane A, Ndir O, Raphenon G, et al. Current report of mycetoma in Senegal: report of 109 cases. Journal de Mycologie Médicale 2000; 10(3): 140–144. [Google Scholar]
- 41.Verghese A, Klokke AH. Histologic diagnosis of species of fungus causing mycetoma. Indian J Med Res. 1966;54(6):524–30. [PubMed] [Google Scholar]
- 42.Fraser M, Borman AM, Johnson EM. Rapid and Robust Identification of the Agents of Black-Grain Mycetoma by Matrix-Assisted Laser Desorption Ionization–Time of Flight Mass Spectrometry. Diekema DJ, ed. Journal of Clinical Microbiology. 2017;55(8):2521–2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Quintana ET, Wierzbicka K, Mackiewicz P, Osman A, Fahal AH, Hamid ME, et al. Streptomyces sudanensis sp. nov., a new pathogen isolated from patients with actinomycetoma. Antonie Van Leeuwenhoek. 2008;93(3):305–13. 10.1007/s10482-007-9205-z [DOI] [PubMed] [Google Scholar]
- 44.Trujillo ME, Goodfellow M. Polyphasic taxonomic study of clinically significant actinomadurae including the description of Actinomadura latina sp.nov. Zentralbl Bakteriol. 1997;285(2):212–33. [DOI] [PubMed] [Google Scholar]
- 45.de Hoog GS, van Diepeningen AD, Mahgoub el-S, van de Sande WW. New species of Madurella, causative agents of black-grain mycetoma. J Clin Microbiol. 2012;50(3):988–94. 10.1128/JCM.05477-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.