Abstract
Background:
Previous studies associated night-shift work with melatonin disruption, with mixed evidence regarding the modulating effects of chronotype (i.e., diurnal preference).
Methods:
130 active nurses (84 rotating-shift and 46 day-shift workers) in the Nurses’ Health Study II wore a head-mounted light meter and collected spontaneous urine voids over three days. 6-sulfatoxymelatonin (aMT6s), the major urinary metabolite of melatonin, was assessed.
Results:
Rotating-shift workers on night shifts had more light exposure and lower urinary melatonin levels during the night, and urinary melatonin rhythms with smaller peaks (14.83, 95% confidence interval [CI], 11.72–18.75 versus 11.81 ng/mg-creatinine/hour, 95%CI, 9.49–14.71) and later peak onset (4.10, 95%CI, 3.37–4.99 versus 5.71 hours, 95%CI, 4.76–6.85), compared to day-shift workers. Further, evening chronotypes’ melatonin rhythms had later peak onset compared to morning types (4.90 hours, 95%CI, 3.94–6.09 versus 3.64 hours, 95%CI, 2.99–4.43). However, among day-shift workers, morning chronotypes had melatonin rhythms with greater mean levels, larger peaks, and earlier peak onset compared to evening chronotypes; patterns were similar comparing evening versus morning chronotypes among rotating-shift workers on night shifts. The interaction of rotating-shift work and chronotype was significant across all parameters (p<0.05).
Conclusions:
As expected, rotating-shift workers on night shifts had greater light exposure and lower urinary melatonin levels during the night compared to day-shift workers. Intriguingly, melatonin rhythms were dependent on both chronotype and rotating-shift work type, and better alignment of rotating-shift work and chronotype appeared to produce less disrupted melatonin rhythms.
Impact:
The joint effects of shift-work type and chronotype require attention in future studies.
Keywords: Shift Work, Chronotype, Melatonin, Circadian, Light
INTRODUCTION
Shift work is a common aspect of modern life, with nearly 15 million Americans regularly working non-standard shifts (1). Historically, key epidemiologic studies have identified an association between night-shift work and an increased risk of breast cancer, and the World Health Organization classified circadian desynchrony associated with shift work as a probable carcinogen in 2007; however, some epidemiologic studies have indicated no such association (2–4). Night-shift work also has been linked to greater long-term risks of obesity, diabetes, and cardiovascular disease (5–7). Recent studies have corroborated these shift work-chronic disease associations in the Nurses’ Health Studies (8–10).
Melatonin is a pineal hormone with oncostatic, antioxidant, and anti-inflammatory properties; it is entrained to the 24-hour environmental light-dark cycle, and acts as a reliable marker of the circadian system (11). The natural rhythm of melatonin involves peak levels at night, as melatonin is acutely suppressed by light; thus, light at night causing melatonin suppression is one of the hypotheses put forth to explain associations between night-shift work and chronic disease risk (12). A growing number of epidemiologic studies have linked lower levels of melatonin with higher risks of breast cancer, diabetes, and cardiovascular disease (13–16). Other possible mechanisms include sleep, activity, and/or circadian disruption either in conjunction with, or independent of, melatonin suppression (17,18).
Most (19–27), but not all (28,29), epidemiologic studies have suggested that night-shift work is associated with decreased melatonin secretion. However, few studies have explored the potential modulating effects of chronotype, which refers to an individual’s diurnal preference, and these studies have produced mixed results (25–27). Thus, we evaluated the effects of shift work type, chronotype, and their joint effects on 24-hour light exposure and urinary melatonin rhythms in a sub-study of active nurses participating in the Nurses’ Health Study II.
METHODS
Study Population
The Nurses’ Health Study (NHS) II began in 1989, when 116,434 female nurses (aged 25 to 42 years) returned a mailed questionnaire about their health, lifestyle, and medical history (30); thereafter, similar questionnaires were sent to participants every two years. Rotating night-shift work was assessed on the baseline questionnaire and updated on each subsequent questionnaire, where it was defined as at least three shifts per month in addition to day and evening shifts in that month. Chronotype was assessed using question 19 on the Morningness-Eveningness Questionnaire (MEQ) (31): “One hears of morning and evening types of people. Which one of these types do you consider yourself to be?” Five responses were possible: definitely a morning type, rather more a morning than evening type, rather more an evening than a morning type, definitely an evening type, and neither. The present study focused on 148 NHS II participants who were involved in a validation study of the Harvard Light Exposure Assessment (H-LEA) Questionnaire, which included real-life light exposure measurements using head-mounted light meter device called the Daysimeter©. Detailed information regarding inclusion/exclusion criteria and subject recruitment have been published elsewhere (32).
Briefly, 18,044 NHS II participants met inclusion criteria for the H-LEA validation study in 2005 (20% of women who were still alive and participating in the cohort) (32). These women were eligible if they: 1) had answered all shift work questions on the cohort questionnaires; 2) were in the workforce; 3) had no history of cancer (except nonmelanoma skin cancer) or cardiovascular disease; 4) were not taking hormonal preparations; and 5) were not pregnant or lactating. Based on random sampling, two waves of participants were invited to participate in the validation study between September 2006 and April 2008 (both waves were recruited at similar times of the year). This included an initial group of 720 women (equal numbers of women with no rotating-shift work history, women in current rotating-shift work with <15 years of prior rotating-shift work history, and women in current rotating-shift work with ≥15 years of prior rotating-shift work) and an additional group of 242 current rotating-shift workers invited because most initial enrollees were day-shift workers. The second wave of participants were current rotating-shift workers with ≥15 months of night work between 2005 and 2007; they met the original inclusion criteria in 2005, and continued to meet these criteria in 2007.
Recruitment and enrollment were stopped once the target enrollment of approximately 150 participants was achieved, which resulted in a total enrollment of 148 women (32).
Light Exposure Measurement
The degree of melatonin suppression induced by light exposure is wavelength dependent, with shorter wavelengths (visible blue light of ~460–480nm) inducing maximal suppression (33). The Daysimeter©—a small, self-contained, battery-operated headset—was designed specifically for measuring short-wavelength light. This device contained two optical sensors, which were placed near the plane of a participant’s cornea while being worn. The first sensor matched closely with the standard photopic luminous efficiency function (λmax=555nm) and the second sensor responded only to light wavelengths shorter than 570nm, with a λmax=470nm. Light was measured together at regular, 30-second time intervals and stored electronically over a seven-day period. Mean photopic illuminance values (in lux) were derived from the first sensor, and mean spectrally-weighted illuminance values (as a measure of circadian light) were derived from the second sensor. Technical details related to the device and circadian light model have been published previously (33,34).
The device also had an accelerometer and a thermometer, and data from these sensors was used to assess participants’ compliance with wearing the device.
Urinary Melatonin Measurements
Urine Collection
Nurses were instructed to collect urine samples for three sequential days following at least three days without night-shift work; this approach was designed to assess each participant’s baseline urinary melatonin rhythm. If feasible, rotating-shift workers were asked to start the collection on a day shift followed by a night shift, and day workers were asked to collect their samples on three day shifts (or days off). Women collected each spontaneous urine void in plastic containers for 72 hours, starting after their first morning void on the first day of collection. Women merged all voids within five predetermined time periods (7:00–10:59, 11:00–14:59, 15:00–18:59, 19:00–22:59, and 23:00–6:59), and reported the total urine volume for each period. The timing of the sample collections were not recorded and therefore all samples provided within each fixed time window were assigned that arbitrary time (the midpoint of the collection time period) and duration in the analysis. A 4.5-ml sample from each period was either refrigerated or frozen (the rest was discarded), resulting in 15 samples per participant. At the end of the 72-hour collection period, nurses sent all samples under cool conditions via overnight courier to Channing Laboratory (Boston, MA), where samples were immediately aliquoted into 1.5-ml vials and frozen at −80°F until shipment to the laboratory for analysis.
Laboratory Analyses
Urinary 6-sulfatoxymelatonin (aMT6s) concentration was assessed in urine samples using commercially available ELISA kits (Immuno-Biological Laboratories International, Hamburg, Germany). Urinary creatinine was assessed by buffered kinetic Jaffe reaction using the COBAS Integra 400 (Roche Diagnostics, Indianapolis, Indiana). Urine samples were analyzed in two batches, with intra-batch coefficients of variation of 12.6% and 14.3% for urinary aMT6s and 5.2% and 7.0% for urinary creatinine, respectively.
Statistical Analysis
Of 148 nurse-participants in the validation study, 16 were excluded from analysis due to technical issues or noncompliance with light meter recordings (defined as extended episodes of inactivity based on temperature sensor and accelerometer data). Two additional participants were excluded because they did not provide urine samples, leaving 130 women (46 day-shift workers and 84 rotating-shift workers) with complete data for these analyses.
Urinary aMT6s levels below the detection limit of the aMT6s assay were set to this limit (0.4 ng/ml). We calculated mean urinary aMT6s concentrations for each of the five predetermined urine collection time periods. We standardized aMT6s measurements (ng/ml) by creatinine level (mg/ml) of each urine sample to account for differences arising from variations in urine concentrations. We calculated total 24-hour aMT6s production by summing the total aMT6s production for each void (ng/ml × total ml) and expressing it in ng/24 hours.
This study evaluated day-shift workers and current rotating-shift workers over three days. Day-shift workers might be day shift or off shift during this period, and current rotating-shift workers might be night shift, day shift, or off shift. Given that light exposure and melatonin patterns were similar for day versus off shifts, these data were analyzed together within each shift work type. Therefore, we had three main groups for comparison: day-shift workers, rotating-shift workers on day/off shifts, and rotating-shift workers on night shifts.
In statistical models, we evaluated shift work categories in relation to light exposure and urinary aMT6s levels using mixed models for repeated measurements with time as a categorical variable. This method takes into account intra-individual and inter-individual variation of the repeated measures of the outcome variable. Covariance structures were selected based on model fit statistics and visualization of changes in covariance and correlation among residuals on the same individual over lag times.
To evaluate 24-hour light exposure and urinary melatonin rhythms, we used harmonic mixed models and cosinor analysis (35), and estimated circadian mean (mesor), difference between the peak and circadian mean (amplitude), and time of peak onset (acrophase) for urinary melatonin across categories of shift work and chronotype, and across categories of chronotype stratifying by shift work. (As described in the Introduction, melatonin is acutely suppressed by light, and therefore its natural rhythm involves peak levels at night). A multiplicative interaction term was added to the model for shift work and chronotype. Initial models were adjusted for age, and additional models were also adjusted for body-mass index, melatonin batch, region (time zone), and month of melatonin collection. Mutual adjustment for shift work and chronotype was considered, but did not change model estimates materially and was not included in final models.
In all analyses, we log-transformed light exposure and urinary aMT6s levels and reported their geometric means and 95% confidence intervals (CI). Statistical tests were two sided and Bonferroni correction was applied as indicated to adjust for multiple testing and to determine the threshold for statistical significance. All analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC, USA).
RESULTS
In this randomly-selected sample of NHS II participants, women had characteristics that were largely similar to the overall NHS II cohort (Table 1). Within this sample, rotating-shift workers were slightly younger and heavier, on average, compared to day-shift workers. In addition, a higher percentage of rotating-shift workers exercised multiple times weekly, and reported use of sleeping pills and NSAIDs. As expected, rotating-shift workers were more likely to have a short-term and long-term history of rotating-shift work compared to day-shift workers.
Table 1.
Characteristics of 46 day-shift workers and 84 rotating-shift workers randomly selected from the Nurses’ Health Study II cohort
| Day-shift workers | Rotating-shift workers | |
|---|---|---|
| Age, in years, mean (SD) | 53.7 (4.4) | 52.1 (4.9) |
| Body-mass index, in kg/m2, mean (SD) | 26.3 (6.3) | 27.9 (6.8) |
| History of rotating-shift work during 1989–2005, in years, N (%) | ||
| None | 24 (52) | 16 (19) |
| 1–4 | 6 (13) | 9 (11) |
| 5–9 | 4 (9) | 16 (19) |
| ≥10 | 9 (20) | 29 (34) |
| Missing | 3 (6) | 14 (17) |
| History of night-shift work in the past 30 days, in number of nights, N (%) | ||
| None | 37 (81) | 2 (2) |
| 1–4 | 6 (13) | 15 (18) |
| 5–9 | 2 (4) | 31 (37) |
| ≥10 | 0 (0) | 35 (42) |
| Missing | 1 (2) | 1 (1) |
| Alcohol intake, ≥2 drinks/week, N (%) | 16 (35) | 26 (31) |
| Smoking history, N (%) | ||
| Never | 43 (94) | 81 (97) |
| Ever | 2 (4) | 1 (1) |
| Missing | 1 (2) | 2 (2) |
| Exercise, in hours/week, N (%) | ||
| 0–1 | 16 (34) | 20 (24) |
| 1–3 | 12 (26) | 28 (33) |
| 3–6 | 9 (20) | 22 (26) |
| ≥6 | 9 (20) | 14 (17) |
| Currently pregnant or breast feeding, N (%) | 1 (2) | 3 (4) |
| HRT use, N (%) | 4 (9) | 4 (5) |
| Sleeping pill use, N (%) | 14 (30) | 39 (46) |
| NSAID use, N (%) | 13 (28) | 35 (42) |
| Beta-blocker use, N (%) | 4 (9) | 6 (7) |
| Benzodiazepene use, N (%) | 1 (2) | 1 (1) |
| Antidepressant use, N (%) | 4 (9) | 10 (12) |
HRT=hormone replacement therapy; NSAID=non-steroidal anti-inflammatory drug; SD=standard deviation
The spectral responses of the Daysimeter’s first and second sensors were highly correlated, and results were similar when we evaluated measurements from the first versus second sensor. Therefore, we present only the results for the photopic sensor in lux.
Overall, day-shift workers recorded significantly greater light exposure over 24 hours compared to rotating-shift workers, and rotating-shift workers on nights shifts recorded significantly greater 24-hour average light exposure compared to rotating-shift workers on off shifts (Table 2). When light exposure was examined during five pre-determined time periods across the 24-hour day, day-shift workers and rotating-shift workers on day/off shifts generally recorded greater light exposure during the day compared to the night; however, light exposure appeared to be more similar during the day and night for rotating-shift workers on night shifts. Across shift work categories, day-shift workers had significantly higher recorded light exposure during the day (7:00 to 18:59) compared to rotating-shift workers on night shifts, with intermediate light exposure recorded by rotating-shift workers on day/off shifts. During the night (23:00–6:59), rotating-shift workers on night shifts had significantly greater light exposure compared to day-shift workers, and rotating-shift workers on day/off shifts had significantly lower light exposure compared to the other groups (Table 2). When the number of hours workers were exposed to >20 lux light was compared across shift worker categories, day-shift workers and rotating-shift workers on off shifts generally recorded significantly longer exposure during the day (7:00 to 18:59), whereas rotating-shift workers on night shifts had significantly longer exposure at night, particularly from 23:00 to 6:59 (Table 2).
Table 2.
| Day-shift workers | Rotating-shift workers |
||
|---|---|---|---|
| Day/off shifts | Night shifts | ||
| Geometric means (95% CI) | Geometric means (95% CI) | Geometric means (95% CI) | |
| Light exposure | |||
| Average light exposure over 24 hours (in lux) | 128.1 (87.1–188.3)a,b | 50.5 (24.0–106.6)a,c | 84.7 (55.5–129.3)b,c |
| Average light exposure over 24 hours, by time period (in lux) | |||
| 7:00–10:59 | 171.5 (133.6–220.1)a,b | 72.1 (45.3–114.8)a | 68.0 (48.4–95.5)b |
| 11:00–14:59 | 272.7 (208.6–356.6)a,b | 123.0 (74.5–203.2)a,c | 30.2 (17.9–51.0)b,c |
| 15:00–18:59 | 204.0 (152.5–272.8)b | 109.6 (68.6–175.1) | 64.5 (45.4–91.6)b |
| 19:00–22:59 | 28.7 (22.3–36.8)a | 14.6 (10.4–20.6)a,c | 36.8 (27.6–48.9)c |
| 23:00–6:59 | 3.0 (2.2–4.0)a,b | 1.6 (1.0–2.4)a,c | 42.9 (31.8–58.0)b,c |
| Duration of light exposure at intensity >20 lux (in hours) | |||
| 7:00–10:59 | 3.6 (3.5–3.8)a,b | 2.9 (2.6–3.1)a,c | 2.4 (2.2–2.6)b,c |
| 11:00–14:59 | 3.8 (3.7–4.0)a,b | 3.3 (3.0–3.5)a,c | 2.3 (2.0–2.5)b,c |
| 15:00–18:59 | 3.4 (3.2–3.6)a,b | 3.0 (2.7–3.3)a | 2.7 (2.5–3.0)b |
| 19:00–22:59 | 1.9 (1.7–2.2)b | 1.6 (1.3–1.9)c | 2.9 (2.6–3.1)b,c |
| 23:00–6:59 | 0.8 (0.6–0.9)b | 1.1 (0.7–1.5)c | 6.1 (5.6–6.6)b,c |
| Melatonin levels | |||
| Total urinary melatonin excretion over 24 hours (in ng) | 22,822 (20,267–25,698) | 20,618 (17,699–24,018) | 20,938 (18,500–23,697) |
| Urinary melatonin concentration over 24 hours by time period (in ng/mg-creatinine) | |||
| 7:00–10:59 | 18.6 (16.5–21.1) | 20.7 (17.6–24.2) | 16.5 (14.2–19.0) |
| 11:00–14:59 | 8.4 (7.4–9.5) | 7.6 (6.1–9.5) | 9.1 (7.7–10.7) |
| 15:00–18:59 | 6.7 (5.8–7.6) | 6.3 (5.4–7.4) | 7.0 (5.9–8.1) |
| 19:00–22:59 | 9.4 (8.4–10.6) | 9.3 (7.8–11.2) | 8.9 (7.7–10.3) |
| 23:00–6:59 | 32.9 (29.3–37.0)b | 30.0 (25.6–35.3)c | 20.9 (18.4–23.7)b,c |
CI=confidence interval
Data from rotating-shift workers was divided into categories of “night shifts” or “day/off shifts” based on whether participants were working (or not working) night shifts during each day of the study.
Results were obtained using ANOVA with Bonferroni correction.
Statistically significant differences for group comparisons are indicated as follows:
for day-shift workers versus rotating-shift workers on day/off shifts
for day-shift workers versus rotating-shift workers on night shifts
for rotating-shift workers on day/off shifts versus rotating-shift workers on night shifts.
Total urinary melatonin concentrations calculated across 24 hours were similar across shift work categories (Table 2). However, these concentrations varied over the course of the day: they were highest during the night (23:00 to 6:59), decreasing in the morning hours (7:00 to 10:59), and were lowest during the day (11:00 to 22:59) across all shift work categories. Notably, melatonin concentrations were significantly lower at night (23:00 to 6:59) in rotating-shift workers on night shifts compared to both day-shift workers and rotating-shift workers on day/off shifts.
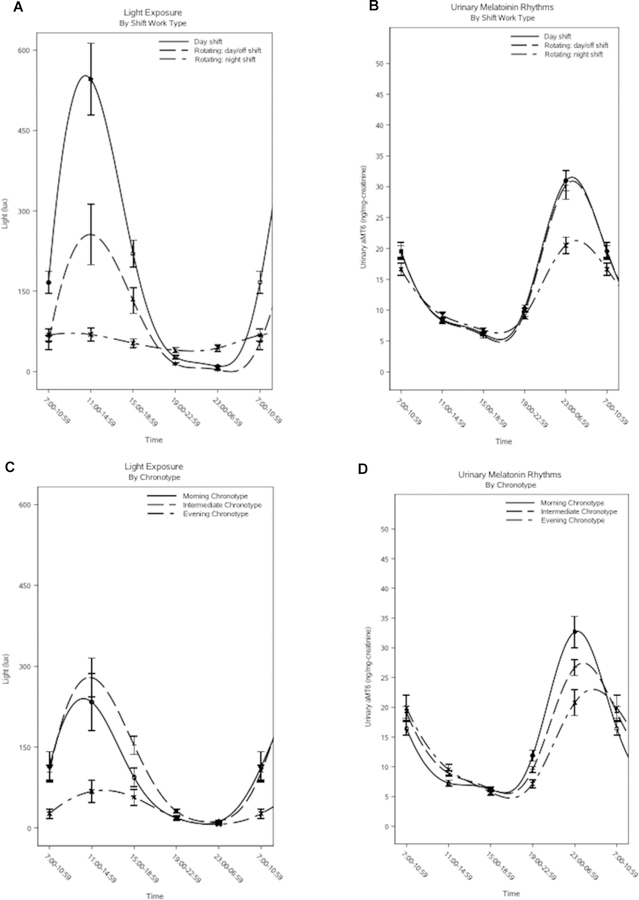
When urinary melatonin circadian rhythm parameters were evaluated (Table 3), rotating-shift workers on night shifts had a significantly later peak onset (multivariable-adjusted geometric means: 5.71 hours [95% CI, 4.76–6.85] versus 4.10 hours [95% CI, 3.37–4.99]) and smaller peaks (11.81 [95% CI, 9.49–14.71] versus 14.83 [95% CI, 11.72–18.75]) compared to day-shift workers, and a significantly smaller mean levels (multivariable-adjusted geometric means: 16.51 [95% CI, 13.97–19.50] versus 20.60 [95% CI, 17.33–24.48]) and smaller peaks (11.81 [95% CI, 9.49–14.71] versus 16.26 [95% CI, 12.95–20.40]) compared to rotating-shift workers on day/off shifts. Across chronotypes, analyses of urinary melatonin rhythms identified significant differences in time of peak onset only, such that intermediate types and evening types had later peak onsets compared to morning types (multivariable-adjusted geometric means: 5.24 hours [95% CI, 4.48–6.11] and 4.90 hours [95% CI, 3.94–6.09] versus 3.64 hours [95% CI, 2.99–4.43], respectively). However, there was a significant interaction between shift work category and chronotype for all circadian parameters related to urinary melatonin (all p-values for interaction<0.05). Curves for light exposure and urinary melatonin across shift work and chronotype categories are depicted in Figure 1 (A: light exposure by shift work; B: urinary melatonin by shift work; C: light exposure by chronotype; D: urinary melatonin by chronotype).
Table 3.
Geometric means for urinary melatonin circadian rhythm parameters across shift work and chronotype categories1,2
| Shift work | Chronotype | ||||||
|---|---|---|---|---|---|---|---|
| Day-shift workers | Rotating-shift workers |
Morning type | Intermediate type | Evening type | P-value for interaction3 | ||
| Day/off shifts | Night shifts | ||||||
| Time of Peak Onset (in hours) Geometric mean (95% CI) | |||||||
| Model 14 | 4.26b (3.82–4.76) |
4.66 (4.10–5.30) |
5.46b (4.90–6.09) |
3.50a,b (3.05–4.02) |
5.15a (4.75–5.59) |
5.33b (4.52–6.30) |
|
| Model 25 | 4.10b (3.37–4.99) |
4.82 (3.99–5.82) |
5.71b (4.76–6.85) |
3.64a,b (2.99–4.43) |
5.24a (4.48–6.11) |
4.90b (3.94–6.09) |
0.04 |
| Mean Levels (in ng/mg-creatinine/hour) Geometric mean (95% CI) | |||||||
| Model 14 | 18.79 (16.96–20.82) |
19.44c (17.25–21.92) |
15.88c (14.35–17.59) |
18.24 (15.99–20.81) |
18.01 (16.67–19.47) |
16.00 (13.66–18.74) |
|
| Model 25 | 18.23 (15.25–21.80) |
20.60c (17.33–24.48) |
16.51c (13.97–19.50) |
17.94 (14.94–21.55) |
18.44 (15.97–21.29) |
17.61 (14.39–21.55) |
0.04 |
| Peak Size (in ng/mg-creatinine) Geometric mean (95% CI) | |||||||
| Model 14 | 14.63b (12.77–16.76) |
15.52c (13.24–18.19) |
11.39b, c (9.95–13.03) |
14.61 (12.24–17.43) |
13.41 (12.09–14.88) |
12.64 (10.22–15.64) |
|
| Model 25 | 14.83 (11.72–18.75) |
16.26c (12.95–20.40) |
11.81c (9.49–14.71) |
14.19 (11.11–18.12) |
13.65 (11.27–16.53) |
15.38 (11.75–20.13) |
0.002 |
CI=confidence interval
Bonferroni correction was applied to address multiple comparisons.
Statistically significant differences for group comparisons are indicated as follows:
for day-shift workers versus rotating-shift workers on day/off shifts,
for day-shift workers versus rotating-shift workers on night shifts,
for rotating-shift workers on day/off shifts versus rotating-shift workers on night shifts,
for morning types versus intermediate types,
for morning types versus evening types,
for intermediate types versus evening types.
The p-value for interaction refers to the multiplicative interaction between shift work and chronotype.
Model 1 is adjusted for age only.
Figure 1. Plots of light exposure and urinary melatonin level across shift-work and chronotype categories.
Figure 1A contains a plot of light exposure (in lux) and Figure 1B contains a plot of urinary melatonin rhythms (in ng/mg-creatinine) for day-shift workers (solid line), rotating-shift workers on day/off shifts (evenly broken line), and rotating-shift workers on night shifts (unevenly broken line). Figure 1C contains a plot of light exposure (in lux) and Figure 1D contains a plot of urinary melatonin rhythms (in ng/mg-creatinine) for morning chronotypes (solid line), intermediate chronotypes (evenly broken line), and evening chronotypes (unevenly broken line).
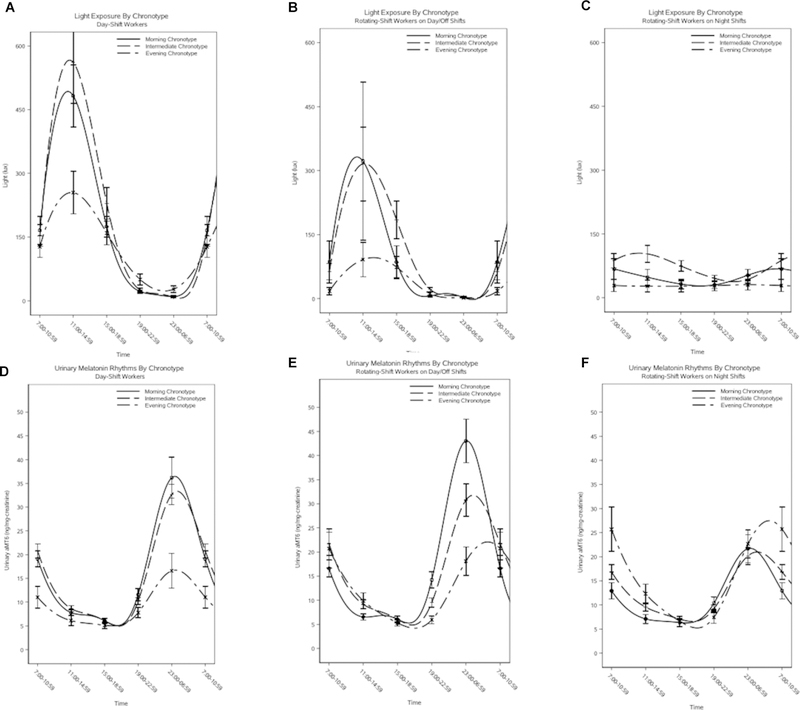
Stratified analyses of chronotype and urinary melatonin parameters by shift work category showed notable differences (Table 4). Across all shift-work categories, there was an earlier peak for morning types compared to intermediate and evening types, with differences reaching statistical significance for the morning type versus intermediate type comparison among rotating-shift workers on day/off shifts and rotating-shift workers on night shifts (multivariable-adjusted geometric means: 3.48 hours [95% CI, 2.62–4.62] versus 6.01 hours [95% CI, 4.88–7.42] for rotating-shift workers on day/off shifts; 3.56 hours [95% CI, 2.39–5.30] versus 6.23 hours [95% CI, 4.69–8.26] for rotating-shift workers on night shifts). For day-shift workers and rotating-shift workers on day/off shifts, there were qualitative trends of decreasing mean levels and peak sizes across morning, intermediate, and evening types; however, the only comparisons reaching statistical significance were for evening types compared to morning and intermediate types among day-shift workers (multivariable-adjusted geometric means: 9.76 [95% CI, 6.58–14.49] versus 18.53 [95% CI, 13.32–25.78] and 14.62 [95% CI, 11.06–19.33], respectively). Among rotating-shift workers on night shifts, the opposite qualitative trends were observed: mean levels and peak sizes increased across categories of morning, intermediate, and evening types, although most comparisons did not reach statistical significance. Curves for urinary melatonin levels and light exposure across chronotype categories, stratified by shift work, are depicted in Figure 2 (A-C: light exposure in day-shift workers, rotating-shift workers on day/off shifts, and rotating-shift workers on night shifts; D-F: urinary melatonin in day-shift workers, rotating shift-workers on day/off shifts, and rotating-shift workers on night shifts).
Table 4.
Geometric means for urinary melatonin circadian rhythm parameters across chronotype categories, stratified by shift work1,2
| Day-shift workers | Rotating-shift workers | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Day/off shifts | Day/off shifts | Night shifts | |||||||
| Morning type | Intermediate type | Evening type | Morning type | Intermediate type | Evening type | Morning type | Intermediate type | Evening type | |
|
Time of Peak Onset (in
hours) Geometric mean (95% CI) | |||||||||
| Model 13 | 4.02 (3.36–4.82) |
4.39 (3.97–4.86) |
4.14 (3.22–5.31) |
2.95a,b (2.36–3.69) |
4.99a (4.37–5.71) |
5.82b (4.57–7.41) |
3.43a,b (2.59–4.54) |
6.26a (5.28–7.43) |
6.21b (4.39–8.76) |
| Model 24 | 4.96 (3.58–6.86) |
5.48 (4.16–7.22) |
5.60 (3.79–8.27) |
3.48a (2.62–4.62) |
6.01a (4.88–7.42) |
5.22 (3.85–7.09) |
3.56a (2.39–5.30) |
6.23a (4.69–8.26) |
5.51 (3.57–8.50) |
|
Mean Levels (in
ng/mg-creatinine/hour) Geometric mean (95% CI) | |||||||||
| Model 13 | 21.29b (17.43–26.01) |
19.06c (17.05–21.31) |
13.22b,c (10.03–17.43) |
21.54 (16.34–28.39) |
19.23 (16.28–22.71) |
15.27 (11.33–20.59) |
14.03 (11.26–17.49) |
16.30 (14.25–18.64) |
17.37 (13.25–22.78) |
| Model 24 | 18.53b (13.32–25.78) |
14.62c (11.06–19.33) |
9.76b,c (6.58–14.49) |
21.57 (15.27–30.48) |
17.61 (13.64–22.73) |
17.58 (12.12–25.48) |
12.03 (8.81–16.45) |
14.57 (11.67–18.20) |
16.43 (11.68–23.12) |
|
Peak Size (in
ng/mg-creatinine) Geometric mean (95% CI) | |||||||||
| Model 13 | 19.30b (14.63–25.46) |
13.98 (11.98–16.32) |
9.22b (6.29–13.52) |
19.78 (13.89–28.16) |
15.12 (12.23–18.71) |
11.87 (8.11–17.40) |
9.14 (6.84–12.21) |
11.84 (9.93–14.13) |
14.85 (10.39–21.21) |
| Model 24 | 14.42b (9.13–22.80) |
9.96 (6.77–14.67) |
6.39b (3.69–11.05) |
18.25 (11.57–28.81) |
12.92 (9.22–18.10) |
12.87 (7.88–21.02) |
7.42b (4.96–11.09) |
9.81 (7.37–13.07) |
17.15b (11.04–26.64) |
CI=confidence interval
Bonferroni correction was applied to address multiple comparisons.
Statistically significant differences for group comparisons are indicated as follows:
for morning types versus intermediate types,
for morning types versus evening types,
for intermediate types versus evening types.
Model 1 is adjusted for age only.
Figure 2. Plots of light exposure and urinary melatonin level across chronotype categories, stratified by shift work.
Figures 2A-2C contain plots of light exposure (in lux) for morning chronotypes (solid line), intermediate chronotypes (evenly broken line), and evening chronotypes (unevenly broken line) among day-shift workers (Figure 2A), rotating-shift workers on day/off shifts (Figure 2B), and rotating-shift workers on night shifts (Figure 2C). Figures 2D-2F contain plots of urinary melatonin rhythms (in ng/mg-creatinine) for morning chronotypes (solid line), intermediate chronotypes (evenly broken line), and evening chronotypes (unevenly broken line) among day-shift workers (Figure 2D), rotating-shift workers on day/off shifts (Figure 2E), and rotating-shift workers on night shifts (Figure 2F).
DISCUSSION
This study has two main findings. First, rotating-shift workers on night shifts had higher levels of light exposure and lower levels of urinary melatonin production during the night compared to day-shift workers. These results were generally consistent with previous studies that have measured light exposure and melatonin levels in night-shift workers, and may support the proposition that night-shift workers experience more nocturnal melatonin suppression due to higher levels of light exposure at night compared to day workers—one of the hypotheses underlying the potential association of night-shift work and an increased risk of breast cancer. Other known risk factors for breast cancer (e.g., age, family history of breast cancer, and multiple reproductive factors) are less likely to mediate the association between night-shift work and breast cancer because these factors do not appear to differ according to night-shift work status (10,36).
Second, analyses that modeled 24-hour urinary melatonin rhythms indicated certain differences in circadian parameters across shift work and chronotype, which were largely consistent with prior literature describing these associations. Furthermore, a significant interaction of shift work and chronotype was identified for all parameters of the melatonin rhythm, suggesting that interpretation of these results should focus on the joint effects of these exposures. In particular, a distinct pattern of greater mean levels, larger peaks, and earlier peaks was observed for morning types compared to all other chronotypes among day-shift workers, and a similar pattern was apparent for evening types compared to the other chronotypes among rotating-shift workers on night shifts. Together, these results suggest that better alignment of shift-work type and chronotype may produce melatonin rhythms more appropriately aligned with the imposed sleep-wake and dark-light schedules.
Three prior epidemiologic studies have examined joint effects of shift work and chronotype in relation to urinary melatonin (25–27). In Spain, a study of fixed-shift workers (75 night-shift workers, 42 day-shift workers) collected urine over 24 hours on a working day, and found that the circadian mean for urinary aMT6s was higher for evening compared to morning types among night-shift workers (27). The authors reported that the mean levels were highly correlated with peak size in the Spanish study (and therefore results were not presented separately for peak size), suggesting that these results were similar to ours, which identified a larger peak among evening compared to morning types among rotating-shift workers on night shifts. However, this study did not find differential effects of chronotype on circadian phase among night-shift workers. In the United States, another study conducted among fixed-shift workers (354 night-shift workers and 310 day-shift workers) evaluated urinary aMT6s levels during relevant time periods (i.e., during night sleep for both groups, and during day sleep and night work for night-shift workers), and identified that evening chronotypes had higher melatonin levels compared to morning chronotypes during the first day sleep following a night shift (25). The authors interpreted these results to mean that morning chronotypes were better able to maintain “normal” melatonin rhythms because their melatonin levels were closer to those of day-shift workers; however, such an interpretation does not acknowledge that, in their comparison, the early chronotypes were sleeping during their biological day, whereas the evening chronotypes were sleeping during their biological night (a point that we have previously addressed (37)). If this point were acknowledged, the observed difference may indicate that evening types have more adaptability to night-shift work (i.e., higher levels of melatonin during their biological night), which is consistent with the notion that alignment of shift work type and chronotype is important for optimal melatonin rhythms (38).
Finally, in Canada, a study assessed urinary aMT6s rhythms based on 48 hours of urine collection, and compared data from 114 rotating-shift workers (including data on one night shift and one day shift) versus 147 fixed-day workers (26). The authors reported that, among rotating-shift workers, aMT6s curves of evening chronotypes had lower mean levels and earlier peak onset compared to morning chronotypes. These findings seem to contradict the results of our study, although it is difficult to compare results in detail because the definitions of rotating-shift work, as well as recent and long-term history of such work, are not entirely clear in the Canadian study. For example, participants in our study were instructed to begin urine collection following ≥3 days without night-shift work (to assess participants’ baseline urinary melatonin rhythms), which was not a requirement in the Canadian study and could lead to less straightforward interpretation of results because their participants’ urinary melatonin rhythms could have been influenced by carryover effects of night-shift work performed in the days preceding urine collection. More research is clearly needed to understand the joint effects of different night-shift work schedules and chronotype on 24-hour melatonin rhythms.
Limitations of note include that women who volunteered to participate in our study appeared more health conscious, as reflected in the lower rate of smoking and alcohol consumption among rotating-shift workers in the current study compared to the overall NHS II cohort participants (32). Further adjustment for these covariates did not change our results, however. Also, there was potential for noncompliance with wearing the light meter in our study; however, a thermometer and accelerometer built into the device allowed us to detect noncompliance patterns, and participants with such patterns were excluded from our analyses. Another limitation of note is that even though plasma aMT6s levels are suppressed within 15 minutes of plasma melatonin suppression, it is less clear how quickly these changes can also be detected in urine (39–43). Even though frequency and timing of urine samples will affect the time of measurable urinary effect of light, any delay is likely only in the order of one hour (44,45), hence we expect it to have had minimal and only non-differential impact on our data analyses. Finally, in this study, the rotating night-shift workers on off shifts were probably a mixed group of individuals with variable sleep/activity patterns on their days off; thus, it is difficult to interpret the data from this group and compare it to other groups of interest.
In summary, we found that rotating-shift workers on night shifts had greater light exposure and lower urinary melatonin levels during the night compared to day-shift workers, as expected. Moreover, our results provide strong evidence, based on in-field measurements, that circadian rhythm parameters for the major urinary melatonin metabolite are dependent on both chronotype and shift work type. Future studies of shift workers should focus on the joint effects of these parameters, as better alignment of shift-work type and chronotype may produce less disrupted melatonin rhythms.
ACKNOWLEDGMENTS
We would like to thank the Nurses’ Health Study II participants for their dedication to this research, particularly those who provided additional information for this validation study. We would also like to thank Mark Rea, PhD (Lighting Research Center, Rensselaer Polytechnic Institute) for his insightful comments.
Financial Support: This research was supported by the Centers for Disease Control and Prevention (CDC) and the National Institutes of Health (R01 OH009803, R01 OH008171, E. Schernhammer). The Nurses’ Health Study II infrastructure is supported by the National Cancer Institute (UM1 CA176726).
Footnotes
Conflict of Interest Statement: The authors report no conflicts of interest. EED has received consulting fees from Epi Excellence and Bohn Epidemiology. SLW has financial disclosures that are listed in an attachment to this manuscript.
REFERENCES
- 1.Bureau of Labor Statistics. Workers on Flexible and Shift Schedules in 2004. Volume USDL 05–1198 Washington, D.C.: U.S. Department of Labor; 2005. [Google Scholar]
- 2.Straif K, Baan R, Grosse Y, Secretan B, Ghissassi FE, Bouvard V, et al. Carcinogenicity of shift-work, painting, and fire-fighting. The Lancet Oncology 2007;8(12):1065–6. [DOI] [PubMed] [Google Scholar]
- 3.Travis RC, Balkwill A, Fensom GK, Appleby PN, Reeves GK, Wang X-S, et al. Night Shift Work and Breast Cancer Incidence: Three Prospective Studies and Meta-analysis of 5 Published Studies. J Natl Cancer Inst 2016;108(12):in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pahwa M, Labreche F, Demers PA. Night shift work and breast cancer risk: what do the meta-analyses tell us? Scand J Work Environ Health 2018;44(4):432–5 doi 10.5271/sjweh.3738. [DOI] [PubMed] [Google Scholar]
- 5.Gan Y, Yang C, Tong X, Sun H, Cong Y, Yin X, et al. Shift work and diabetes mellitus: a meta-analysis of observational studies. Occup Environ Med 2015;72(1):72–8 doi 10.1136/oemed-2014-102150. [DOI] [PubMed] [Google Scholar]
- 6.Liu Q, Shi J, Duan P, Liu B, Li T, Wang C, et al. Is shift work associated with a higher risk of overweight or obesity? A systematic review of observational studies with meta-analysis. Int J Epidemiol 2018. doi 10.1093/ije/dyy079. [DOI] [PubMed] [Google Scholar]
- 7.Vyas MV, Garg AX, Iansavichus AV, Costella J, Donner A, Laugsand LE, et al. Shift work and vascular events: systematic review and meta-analysis. BMJ 2012;345:e4800 doi 10.1136/bmj.e4800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pan A, Schernhammer ES, Sun Q, Hu FB. Rotating night shift work and risk of type 2 diabetes: two prospective cohort studies in women. PLoS Med 2011;8(12):e1001141 doi 10.1371/journal.pmed.1001141PMEDICINE-D-11-01447 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vetter C, Devore EE, Wegrzyn LR, Massa J, Speizer FE, Kawachi I, et al. Association Between Rotating Night Shift Work and Risk of Coronary Heart Disease Among Women. JAMA 2016;315(16):1726–34 doi 10.1001/jama.2016.4454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wegrzyn LR, Tamimi RM, Rosner BA, Brown SB, Stevens RG, Eliassen AH, et al. Rotating Night-Shift Work and the Risk of Breast Cancer in the Nurses’ Health Studies. Am J Epidemiol 2017;186(5):532–40 doi 10.1093/aje/kwx140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hardeland R, Cardinali DP, Srinivasan V, Spence DW, Brown GM, Pandi-Perumal SR. Melatonin--a pleiotropic, orchestrating regulator molecule. Prog Neurobiol 2011;93(3):350–84 doi 10.1016/j.pneurobio.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 12.Hunter CM, Figueiro MG. Measuring Light at Night and Melatonin Levels in Shift Workers: A Review of the Literature. Biol Res Nurs 2017;19(4):365–74 doi 10.1177/1099800417714069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang WS, Deng Q, Fan WY, Wang WY, Wang X. Light exposure at night, sleep duration, melatonin, and breast cancer: a dose-response analysis of observational studies. Eur J Cancer Prev 2014;23(4):269–76 doi 10.1097/CEJ.0000000000000030. [DOI] [PubMed] [Google Scholar]
- 14.McMullan CJ, Schernhammer ES, Rimm EB, Hu FB, Forman JP. Melatonin secretion and the incidence of type 2 diabetes. JAMA 2013;309(13):1388–96 doi 10.1001/jama.2013.2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Forman JP, Curhan GC, Schernhammer ES. Urinary melatonin and risk of incident hypertension among young women. J Hypertens 2010;28(3):446–51 doi 10.1097/HJH.0b013e3283340c16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McMullan CJ, Rimm EB, Schernhammer ES, Forman JP. A nested case-control study of the association between melatonin secretion and incident myocardial infarction. Heart 2017;103(9):694–701 doi 10.1136/heartjnl-2016-310098. [DOI] [PubMed] [Google Scholar]
- 17.Rahman SA, St Hilaire MA, Gronfier C, Chang AM, Santhi N, Czeisler CA, et al. Functional decoupling of melatonin suppression and circadian phase resetting in humans. J Physiol 2018;596(11):2147–57 doi 10.1113/JP275501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kecklund G, Axelsson J. Health consequences of shift work and insufficient sleep. BMJ 2016;355:i5210 doi 10.1136/bmj.i5210. [DOI] [PubMed] [Google Scholar]
- 19.Schernhammer ES, Rosner B, Willett WC, Laden F, Colditz GA, Hankinson SE. Epidemiology of urinary melatonin in women and its relation to other hormones and night work. Cancer Epidemiol Biomarkers Prev 2004;13(6):936–43 doi 13/6/936 [pii]. [PubMed] [Google Scholar]
- 20.Burch JB, Yost MG, Johnson W, Allen E. Melatonin, sleep, and shift work adaptation. J Occup Environ Med 2005;47(9):893–901. [DOI] [PubMed] [Google Scholar]
- 21.Marie Hansen A, Helene Garde A, Hansen J. Diurnal urinary 6-sulfatoxymelatonin levels among healthy Danish nurses during work and leisure time. Chronobiol Int 2006;23(6):1203–15. [DOI] [PubMed] [Google Scholar]
- 22.Davis S, Mirick DK, Chen C, Stanczyk FZ. Night Shift Work and Hormone Levels in Women. Cancer Epidemiol Biomarkers Prev 2012. doi 10.1158/1055-9965.EPI-11-1128. [DOI] [PubMed] [Google Scholar]
- 23.Dumont M, Lanctot V, Cadieux-Viau R, Paquet J. Melatonin production and light exposure of rotating night workers. Chronobiol Int 2012;29(2):203–10 doi 10.3109/07420528.2011.647177. [DOI] [PubMed] [Google Scholar]
- 24.Dumont M, Paquet J. Progressive decrease of melatonin production over consecutive days of simulated night work. Chronobiol Int 2014;31(10):1231–8 doi 10.3109/07420528.2014.957304. [DOI] [PubMed] [Google Scholar]
- 25.Bhatti P, Mirick DK, Davis S. The impact of chronotype on melatonin levels among shift workers. Occupational and environmental medicine 2014;71(3):195–200 doi 10.1136/oemed-2013-101730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leung M, Tranmer J, Hung E, Korsiak J, Day AG, Aronson KJ. Shift Work, Chronotype, and Melatonin Patterns among Female Hospital Employees on Day and Night Shifts. Cancer Epidemiol Biomarkers Prev 2016;25(5):830–8 doi 10.1158/1055-9965.EPI-15-1178. [DOI] [PubMed] [Google Scholar]
- 27.Papantoniou K, Pozo OJ, Espinosa A, Marcos J, Castano-Vinyals G, Basagana X, et al. Circadian variation of melatonin, light exposure, and diurnal preference in day and night shift workers of both sexes. Cancer Epidemiol Biomarkers Prev 2014;23(7):1176–86 doi 10.1158/1055-9965.EPI-13-1271. [DOI] [PubMed] [Google Scholar]
- 28.Grundy A, Tranmer J, Richardson H, Graham CH, Aronson KJ. The influence of light at night exposure on melatonin levels among Canadian rotating shift nurses. Cancer Epidemiol Biomarkers Prev 2011;20(11):2404–12 doi 10.1158/1055-9965.EPI-11-0427. [DOI] [PubMed] [Google Scholar]
- 29.Grundy A, Sanchez M, Richardson H, Tranmer J, Borugian M, Graham CH, et al. Light intensity exposure, sleep duration, physical activity, and biomarkers of melatonin among rotating shift nurses. Chronobiol Int 2009;26(7):1443–61 doi 10.3109/07420520903399987 [pii] 10.3109/07420520903399987. [DOI] [PubMed] [Google Scholar]
- 30.Colditz GA, Manson JE, Hankinson SE. The Nurses’ Health Study: 20-year contribution to the understanding of health among women. J Womens Health 1997;6(1):49–62. [DOI] [PubMed] [Google Scholar]
- 31.Horne JA, Østberg O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int J Chronobiol 1976;4(2):97–110. [PubMed] [Google Scholar]
- 32.Bajaj A, Rosner B, Lockley SW, Schernhammer ES. Validation of a light questionnaire with real-life photopic illuminance measurements: the Harvard Light Exposure Assessment questionnaire. Cancer Epidemiol Biomarkers Prev 2011;20(7):1341–9 doi 10.1158/1055-9965.EPI-11-0204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lockley SW, Brainard GC, Czeisler CA. High sensitivity of the human circadian melatonin rhythm to resetting by short wavelength light. J Clin Endocrinol Metab 2003;88(9):4502–5 doi 10.1210/jc.2003-030570. [DOI] [PubMed] [Google Scholar]
- 34.Rea MS, Figueiro MG, Bierman A, Bullough JD. Circadian light. J Circadian Rhythms 2010;8(1):2 doi 10.1186/1740-3391-8-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Albert PS, Hunsberger S. On analyzing circadian rhythms data using nonlinear mixed models with harmonic terms. Biometrics 2005;61(4):1115–20; discussion 20–2 doi BIOM464_1 [pii] 10.1111/j.0006-341X.2005.464_1.x. [DOI] [PubMed] [Google Scholar]
- 36.Tamimi RM, Spiegelman D, Smith-Warner SA, Wang M, Pazaris M, Willett WC, et al. Population Attributable Risk of Modifiable and Nonmodifiable Breast Cancer Risk Factors in Postmenopausal Breast Cancer. Am J Epidemiol 2016;184(12):884–93 doi 10.1093/aje/kww145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vetter C, Schernhammer ES. Early, but not late chronotypes, are up during their biological night when working the night shift. Occupational and environmental medicine 2015;72(3):235 doi 10.1136/oemed-2014-102572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stone JE, Sletten TL, Magee M, Ganesan S, Mulhall MD, Collins A, et al. Temporal dynamics of circadian phase shifting response to consecutive night shifts in healthcare workers: role of light-dark exposure. J Physiol 2018;596(12):2381–95 doi 10.1113/JP275589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bojkowski CJ, Aldhous ME, English J, Franey C, Poulton AL, Skene DJ, et al. Suppression of nocturnal plasma melatonin and 6-sulphatoxymelatonin by bright and dim light in man. Hormone and metabolic research = Hormon- und Stoffwechselforschung = Hormones et metabolisme 1987;19(9):437–40 doi 10.1055/s-2007-1011846. [DOI] [PubMed] [Google Scholar]
- 40.Bojkowski CJ, Arendt J. Factors influencing urinary 6-sulphatoxymelatonin, a major melatonin metabolite, in normal human subjects. Clin Endocrinol (Oxf) 1990;33(4):435–44. [DOI] [PubMed] [Google Scholar]
- 41.Bojkowski CJ, Arendt J, Shih MC, Markey SP. Melatonin secretion in humans assessed by measuring its metabolite, 6-sulfatoxymelatonin. Clinical chemistry 1987;33(8):1343–8. [PubMed] [Google Scholar]
- 42.St Hilaire MA, Gronfier C, Zeitzer JM, Klerman EB. A physiologically based mathematical model of melatonin including ocular light suppression and interactions with the circadian pacemaker. J Pineal Res 2007;43(3):294–304 doi 10.1111/j.1600-079X.2007.00477.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Abeysuriya RG, Lockley SW, Robinson PA, Postnova S. A unified model of melatonin, 6-sulfatoxymelatonin, and sleep dynamics. J Pineal Res 2018;64(4):e12474 doi 10.1111/jpi.12474. [DOI] [PubMed] [Google Scholar]
- 44.Naidoo R Investigation of rhythmic endocrine function in intensive care with emphasis on melatonin Surrey: University of Surrey; 1999. [Google Scholar]
- 45.Le Bars D, Thivolle P, Vitte PA, Bojkowski C, Chazot G, Arendt J, et al. PET and plasma pharmacokinetic studies after bolus intravenous administration of [11C]melatonin in humans. Int J Rad Appl Instrum B 1991;18(3):357–62. [DOI] [PubMed] [Google Scholar]