SUMMARY
Durable responses and encouraging survival have been demonstrated with immune checkpoint inhibitors in small cell lung cancer (SCLC), but predictive markers are unknown. We used whole exome sequencing to evaluate the impact of tumor mutation burden (TMB) on efficacy of nivolumab monotherapy or combined with ipilimumab in patients with SCLC from the nonrandomized or randomized cohorts of CheckMate 032. Patients received nivolumab (3 mg/kg every 2 weeks) or nivolumab plus ipilimumab (1 mg/kg plus 3 mg/kg every 3 weeks for four cycles, followed by nivolumab 3 mg/kg every 2 weeks). Efficacy of nivolumab ± ipilimumab was enhanced in patients with high TMB. Nivolumab plus ipilimumab appeared to provide a greater clinical benefit than nivolumab monotherapy in the high TMB tertile.
INTRODUCTION
Small cell lung cancer (SCLC) accounts for 10% to 15% of all patients with lung cancer (Alvarado-Luna and Morales-Espinosa, 2016; Herbst et al., 2008), and approximately 75% of patients present with extensive-stage disease. Standard first-line treatment consists of platinum-based chemotherapy, but patients who progress have few effective treatment options and a routinely poor prognosis (Alvarado-Luna and Morales-Espinosa, 2016; National Comprehensive Cancer Network, 2017). In patients with previously treated SCLC, nivolumab, a fully human IgG4 programmed death (PD)-1 immune checkpoint inhibitor antibody, showed durable responses and encouraging survival as monotherapy and in combination with ipilimumab, a fully human IgG1 cytotoxic T lymphocyte antigen-4 (CTLA-4) immune checkpoint inhibitor antibody (Antonia et al., 2016; Hellmann et al., 2016). In a nonrandomized cohort of patients with advanced SCLC treated in CheckMate 032, the estimated 2-year overall survival rate was 14% with nivolumab monotherapy (n = 98) and 26% with nivolumab plus ipilimumab (n = 61) (Hellmann et al., 2017). These results led to the inclusion of nivolumab with or without ipilimumab in the National Comprehensive Cancer Network guidelines as a recommended therapy for second-line or later treatment of SCLC (National Comprehensive Cancer Network, 2017). Additionally, the trial was expanded to include a randomized cohort of patients with SCLC treated with nivolumab with or without ipilimumab (Hellmann et al., 2017).
The identification of predictors of response to immune checkpoint blockade in SCLC has been elusive. Unlike in some other cancers, including non-small cell lung cancer (NSCLC) (Reck et al., 2016), tumor programmed death ligand 1 (PD-L1) expression is uncommon in SCLC (~18%), and responses to nivolumab monotherapy or in combination with ipilimumab have been observed regardless of PD-L1 expression (Antonia et al., 2016). Given the initial signals of benefit with the combination of nivolumab and ipilimumab in SCLC but the potential for greater toxicity compared with nivolumab monotherapy, determining the predictors of response to monotherapy and/or combination therapy is critical (Antonia et al., 2016).
SCLC is characterized by high somatic mutation burden (George et al., 2015; Peifer et al., 2012; Rudin et al., 2012) due to the nearly universal association of SCLC with smoking (Alexandrov et al., 2013; Byers and Rudin, 2015); mutations can affect diverse pathways, including DNA repair mechanisms (Gazdar et al., 2017). Data in other solid tumors, including urothelial carcinoma, melanoma, and NSCLC, have shown an association between high tumor mutation burden and improved efficacy of immune checkpoint blockade monotherapy (Carbone et al., 2017; Galsky et al., 2017; Le et al., 2017; Rizvi et al., 2015; Rosenberg et al., 2016; Snyder et al., 2014); however, this has not previously been examined in patients with SCLC or in patients treated with dual checkpoint blockade. We hypothesized that tumor mutation burden could be associated with efficacy of nivolumab alone and in combination with ipilimumab in previously treated SCLC.
RESULTS
Patients and Tumor Mutation Burden
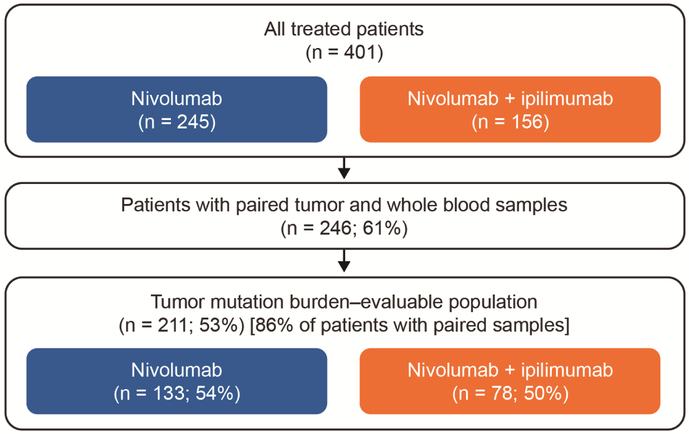
Of all treated patients (n = 401), 245 received nivolumab 3 mg/kg every 2 weeks and 156 received nivolumab plus ipilimumab (1 mg/kg plus 3 mg/kg every 3 weeks for four cycles, followed by nivolumab 3 mg/kg every 2 weeks) in the pooled nonrandomized or randomized SCLC cohorts of CheckMate 032 (Figure 1 and Figure S1). Of all treated patients, 61% had sufficient paired tumor and whole blood samples to attempt whole exome sequencing (WES). WES was successful in 86% of these patients such that overall 211 (53%) of all treated patients were evaluable for efficacy analyses by tumor mutation burden (Figure 1). Baseline patient characteristics were similar between tumor mutation burden–evaluable patients and all treated patients across treatment groups (Table 1). Clinical outcomes, including overall survival, progression-free survival, and objective response rate, also were similar between tumor mutation burden–evaluable and all treated patients (Figure S2 and Table S1). Overall, the tumor mutation burden–evaluable patients were representative of the total trial population.
Figure 1. Flow Diagram of Analyzed Patients.
See also Figures S1-S4, Table S1.
Table 1.
Baseline Characteristics.
| Nivolumab | Nivolumab Plus Ipilimumab | |||
|---|---|---|---|---|
| Characteristic | All Treated (n = 245) |
Tumor Mutation Burden–Evaluable (n = 133) |
All Treated (n = 156) |
Tumor Mutation Burden–Evaluable (n = 78) |
| Age, median (range) — year | 63 (29–83) | 63 (29–83) | 65 (37–91) | 65 (37–80) |
| Age ≥75 years — no. (%) | 23 (9) | 11 (8) | 17 (11) | 11 (14) |
| Male — no. (%) | 147 (60) | 79 (59) | 95 (61) | 52 (67) |
| Smoking status — no. (%) | ||||
| Current/former smoker | 230 (94) | 126 (95) | 147 (94) | 73 (94) |
| Never smoker | 13 (5) | 6 (5) | 8 (5) | 5 (6) |
| Unknown | 2 (1) | 1 (1) | 1 (1) | 0 |
| ECOGa performance status score — no. (%) | ||||
| 0 | 73 (30) | 42 (32) | 49 (31) | 23 (29) |
| 1 | 171 (70) | 91 (68) | 106 (68) | 54 (69) |
| 2 | 1 (<1) | 0 | 0 | 0 |
| Unknown | 0 | 0 | 1 (0.6) | 1 (1.3) |
| Tumor PD-L1 expression — no. (%) | ||||
| ≥1% | 24 (10) | 17 (13) | 19 (12) | 8 (10) |
| <1% | 149 (61) | 89 (67) | 90 (58) | 51 (65) |
| Unknown | 72 (29) | 27 (20) | 47 (30) | 19 (24) |
| Study cohort — no. (%) | ||||
| Nonrandomized | 98 (40) | 50 (38) | 61 (39) | 25 (32) |
| Randomized | 147 (60) | 83 (62) | 95 (61) | 53 (68) |
ECOG denotes Eastern Cooperative Oncology Group.
Tumor mutation burden was defined as the total number of somatic missense mutations, and patients were divided into tertiles, as done previously (Carbone et al., 2017). Tertile boundaries were defined as: low, 0 to <143 mutations; medium, 143 to 247 mutations, and high, ≥248 mutations. The distribution of patients by tumor mutation burden was similar between those receiving nivolumab or nivolumab plus ipilimumab (Figure S3A), similar to the distribution seen in an independent previous report (George et al., 2015) of patients with SCLC (Figure S3B), and similar to that of patients with NSCLC treated with nivolumab in the CheckMate 026 study (Carbone et al., 2017) but with a narrower range (Figure S3B). Tumor mutation burden estimated by WES closely correlated with the estimate after in silico filtering to the 315 genes in the FoundationOne next-generation sequencing genomic profile (Frampton et al., 2013) (Figure S4).
PD-L1 expression ≥1% was uncommon overall, but was evenly distributed between the mutation burden high and medium/low tertiles. There was no association between PD-L1 expression and tumor mutation burden (Table S2) or between PD-L1 expression and objective response rate in the nonrandomized population (Table S3).
Association of Tumor Mutation Burden and Clinical Outcomes
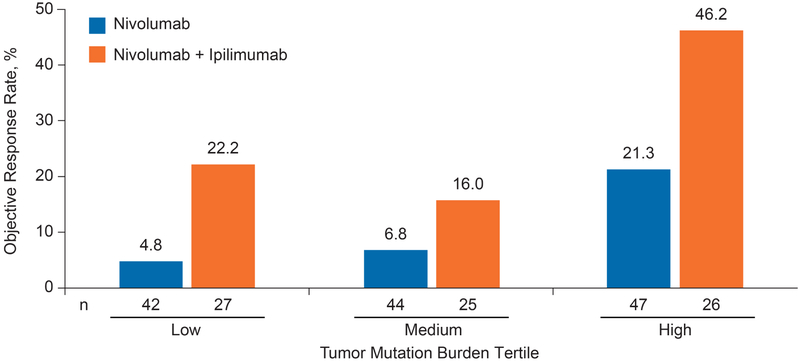
Within both the nivolumab monotherapy and nivolumab plus ipilimumab treatment groups, objective response rates were higher in those patients with high tumor mutation burden (21.3% and 46.2%, respectively) than in patients with low (4.8% and 22.2%, respectively) or medium (6.8% and 16.0%, respectively) tumor mutation burden (Figure 2). Within all tumor mutation burden tertiles, the objective response rates were higher among patients treated with nivolumab plus ipilimumab than those treated with nivolumab monotherapy (Figure 2). Similar associations were seen when tumor mutation burden was grouped by medians or quartile boundaries (Figure S5A and B).
Figure 2. Objective Response Rate by Tumor Mutation Burden Tertile.
We also examined how the association between objective response and tumor mutation burden in SCLC compared with the similar association in NSCLC. The receiver operating characteristics curves of the associations between tumor mutation burden and objective response to nivolumab were similar in patients with SCLC and NSCLC, suggesting that tumor mutation burden is a predictor of response to nivolumab in both patient populations (Figure S5C and D).
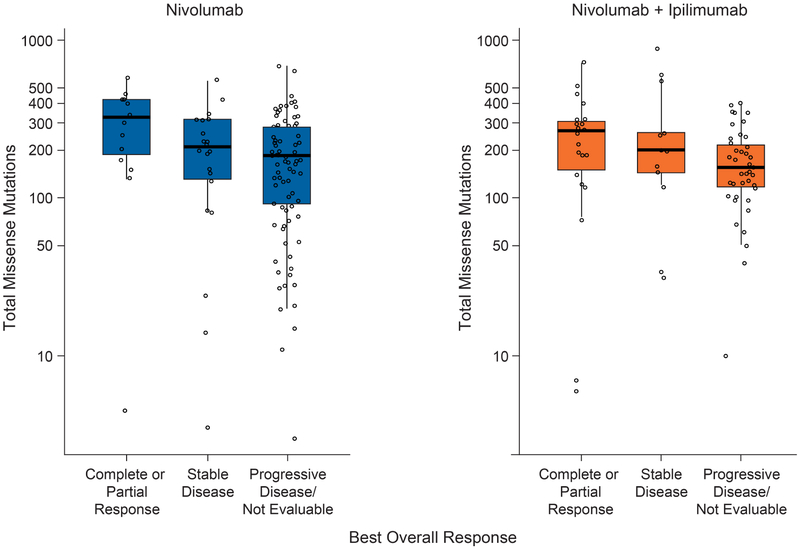
Tumor mutation burden was higher among patients with a complete or partial response to either monotherapy or combination therapy than among those with stable disease or progressive disease (Figure 3).
Figure 3. Tumor Mutation Burden by Best Overall Response in Individual Patients.
Black lines in each box denote the median. The bottom and top of each box denote the first and third quartile, respectively. The lower whisker denotes the value at 1.5 times the interquartile range below the 25th percentile or the minimum value of the dataset, whichever value is larger. The upper whisker denotes the value at 1.5 times the interquartile range above the 75th percentile or the maximum value of the dataset, whichever value is smaller.
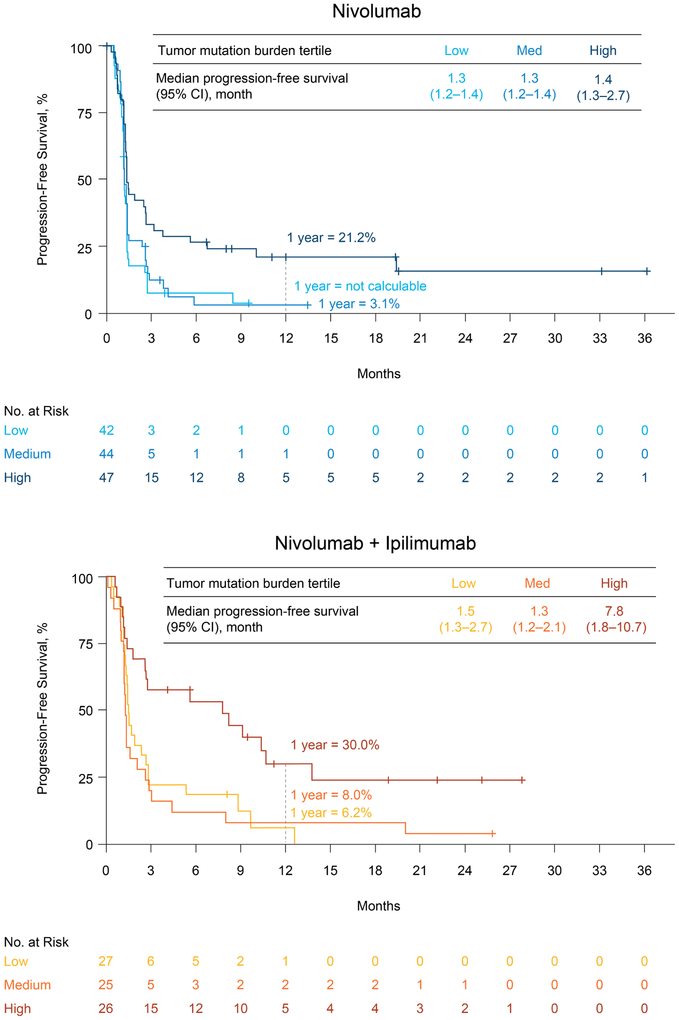
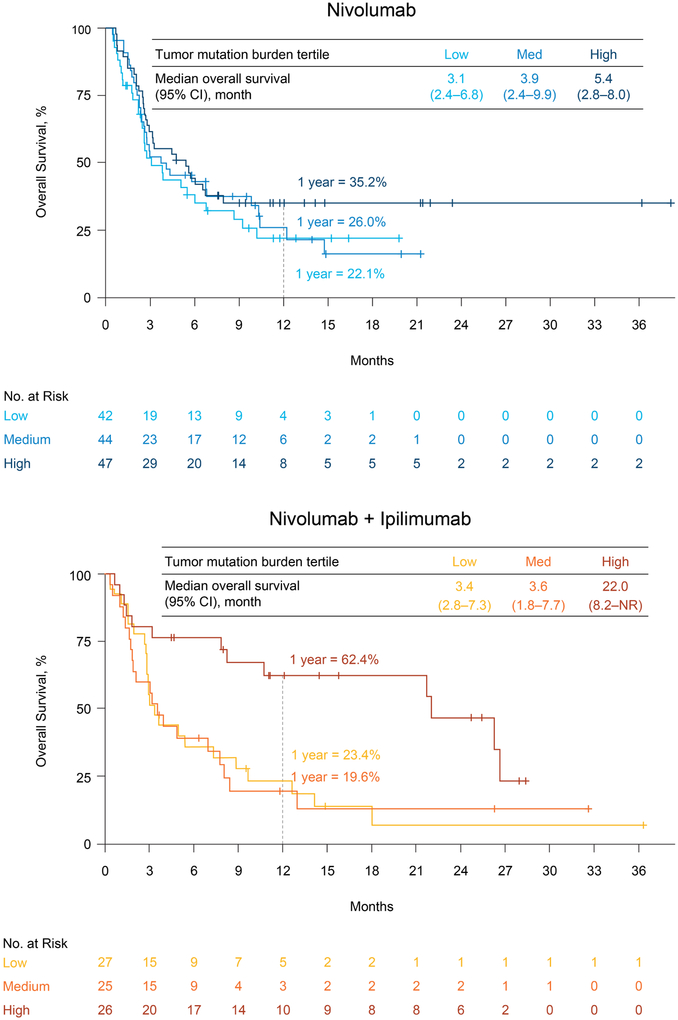
In both treatment groups, estimated 1-year progression-free survival rates were higher in the high tumor mutation burden group (21.2% and 30.0% for nivolumab monotherapy and nivolumab plus ipilimumab, respectively) compared with the low (not calculable and 6.2%, respectively) or medium (3.1% and 8.0%, respectively) tumor mutation burden groups (Figure 4). Similar trends were observed for overall survival (Figure 5). Within each treatment group, the estimated 1-year overall survival rate was higher in the high tumor mutation burden group (35.2% and 62.4% for nivolumab monotherapy and nivolumab plus ipilimumab, respectively) than in the low (22.1% and 23.4%, respectively) or medium (26.0% and 19.6%, respectively) tumor mutation burden groups. In patients with high tumor mutation burden, 1-year progression-free survival and 1-year overall survival rates were higher with combination therapy than with nivolumab monotherapy; no substantial differences in progression-free and overall survival between treatment groups were observed among patients with lower tumor mutation burden (Figures 4 and 5).
Figure 4. Progression-Free Survival by Treatment and Tumor Mutation Burden Tertile.
CI denotes confidence interval.
Figure 5. Overall Survival by Treatment and Tumor Mutation Burden Tertile.
CI denotes confidence interval. See also Figure S6.
Lastly, we investigated whether there was a prognostic association between tumor mutation burden and overall survival in an independent cohort of patients with SCLC who did not receive immunotherapy (George et al., 2015). We found that there was no prognostic difference in survival based on tumor mutation burden tertile (Figure S6). These data suggest that tumor mutation burden is predictive of improved outcomes specifically in the context of immunotherapy, rather than more generally prognostic in patients with SCLC.
DISCUSSION
This report evaluated the role of tumor mutation burden in SCLC, and its association with outcomes with dual immune checkpoint blockade. We found that patients with high tumor mutation burden treated with either nivolumab monotherapy or nivolumab plus ipilimumab had improved efficacy compared with those with medium or low tumor mutation burden. Similar to what has been seen in previous reports of nivolumab in NSCLC and urothelial carcinoma (Carbone et al., 2017; Galsky et al., 2017) and ipilimumab in melanoma (Snyder et al., 2014), tumor mutation burden may be a relevant biomarker in SCLC.
The current study suggests that tumor mutation burden may help inform the benefit/risk of nivolumab monotherapy or nivolumab plus ipilimumab for a given patient. Given the initial observation of increased benefit of combination therapy with nivolumab and ipilimumab in SCLC, but also of potentially greater toxicity compared with nivolumab alone, identifying the differential predictors of response to monotherapy or combination therapy is critical (Antonia et al., 2016). Specifically, patients with SCLC and high tumor mutation burden treated with nivolumab plus ipilimumab had outcomes that surpass historical survival expectations for patients with previously treated SCLC. Conversely, although patients with low or medium tumor mutation burden had increased objective response rates with nivolumab plus ipilimumab compared to nivolumab monotherapy, there were no substantial differences between the treatment groups in progression-free or overall survival. Together, these observations suggest a benefit of the combination over nivolumab monotherapy for patients with high tumor mutation burden, while nivolumab alone may be a favorable option for those with medium or low tumor mutation burden. However, this was an exploratory analysis and these findings require further evaluation in larger randomized studies.
It was not a foregone conclusion that tumor mutation burden would be informative in SCLC. Given the nearly universal association with smoking in patients with SCLC, tumor mutation burden is high but the range is relatively narrow (Alexandrov et al., 2013). It was not known whether there would be sufficient molecular diversity to identify subgroups with distinct clinical responses to immunotherapy; nevertheless, clinical benefit with immunotherapy was greatest among those with the highest mutation burden. Furthermore, it was speculated that tumor mutation burden analysis may not be feasible given the small biopsies used and necrotic tissue found in SCLC tumors. In this retrospective analysis, 61% of patients had sufficient tumor biopsy material and whole blood sample for WES. As the WES analysis was not pre-planned and tissue requirements for eligibility were relatively minimal, this success rate is perhaps better than expected. Additionally, in the vast majority (86%) of these patients, WES and subsequent determination of tumor mutation burden was successful. Overall, this analysis demonstrates that molecular analysis of SCLC for testing of tumor mutation burden is feasible and we would expect that prospective efforts may achieve even higher success rates.
It was also unknown how tumor mutation burden would associate with outcomes in patients treated with nivolumab plus ipilimumab. One hypothesis was that the addition of ipilimumab would broaden the repertoire of anti-tumor T-cell clones and diminish the predictive relevance of tumor mutation burden. That does not appear to be the case in SCLC, as tumor mutation burden appears to be a strong biomarker for nivolumab plus ipilimumab. These data may provide insight into molecular determinants of response to combination therapy that may have implications for use in other cancers. Of note, and consistent with the observation in this report, improvement of response to nivolumab plus ipilimumab with high TMB also has been observed in patients with NSCLC (Hellmann et al., submitted). Further work is needed to fully understand the precise immunobiologic mechanisms underlying synergy between PD-1 and CTLA-4 blockade (Wei et al., 2017).
This study is an exploratory analysis and the results presented here will need to be confirmed in larger, prospective datasets. Although WES was performed retrospectively, the clinical and outcome features of the tumor mutation burden–evaluable cohort were similar to those of all treated patients and generally typical of patients with SCLC. Inclusion of a control arm would further confirm the predictive (rather than prognostic) nature of tumor mutation burden, although no association of tumor mutation burden and survival was seen in previously published data of SCLC not treated with immunotherapy (George et al., 2015). The association of improved outcomes with higher tumor mutation burden was consistently seen when patients were divided by median, tertile, or quartile. This observation highlights the stability of the association between mutation burden and benefit with immunotherapy in SCLC, but also suggests that there is not a singular cutpoint to enrich for benefit. Further validation and optimization of tumor mutation burden cutoff are warranted, in addition to efforts to refine our understanding of the somatic molecular features that most contribute to immunogenicity (Turajlic et al., 2017). Two ongoing phase 3 trials evaluating the efficacy of nivolumab or nivolumab plus ipilimumab in SCLC (CheckMate 331, and CheckMate 451, ) will assess the association between tumor mutation burden and treatment outcomes in larger datasets.
In conclusion, in patients with SCLC treated with either nivolumab monotherapy or nivolumab plus ipilimumab, efficacy was enhanced among patients with high tumor mutation burden. In the high tumor mutation burden subgroup, the combination of nivolumab and ipilimumab appeared to provide a greater clinical benefit compared with nivolumab monotherapy, with near doubling of estimated 1-year survival rates for the combination versus monotherapy. The progression-free and overall survival seen among patients with high tumor mutation burden treated with nivolumab plus ipilimumab was particularly remarkable for patients with previously treated SCLC. These data, along with existing data in NSCLC (Carbone et al., 2017), suggest that tumor mutation burden has a potential role as a biomarker for immunotherapy across lung cancers.
STAR METHODS
CONTACT FOR REAGENT AND RESOURCE SHARING
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Matthew D. Hellmann, (hellmanm@mskcc.org). Human sequencing data (WES; from patients who provided full written informed consent to share these data) were deposited into the European Variation Archive (Accession numbers: PRJEB25808 for Strelka and PRJEB25807 for TNsnv).
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Patients
The SCLC cohort of this phase 1/2 multicenter, multi-arm, open-label trial included patients with histologically or cytologically confirmed, limited- or extensive-stage SCLC with progression after at least one platinum-based chemotherapy regimen (Figure S1) (Antonia et al., 2016). Inclusion and exclusion criteria have been previously described. Briefly, eligible patients were aged 18 years or older and had an Eastern Cooperative Oncology Group performance status of 0 or 1. Patients were eligible irrespective of PD-L1 expression and platinum sensitivity (relapse ≥90 days after chemotherapy) or resistance (relapse <90 days after or during chemotherapy). Baseline or on-study tumor biopsy was required for biomarker analyses; four patients included in this analysis did not have baseline tissue but had on-study biopsy that was used for tumor mutation burden testing. Written informed consent was collected from all patients prior to enrollment.
Trial Design and Treatment
Patients analyzed here include those treated as part of both the initial nonrandomized and subsequent randomized SCLC cohorts. Patients received nivolumab (3 mg/kg intravenously every 2 weeks until disease progression or unacceptable toxicity) or nivolumab plus ipilimumab (1 mg/kg plus 3 mg/kg intravenously every 3 weeks for four cycles, followed by nivolumab 3 mg/kg every 2 weeks until disease progression or unacceptable toxicity). Patients were permitted to receive therapy beyond Response Evaluation Criteria In Solid Tumors (RECIST) v1.1–defined progression (Eisenhauer et al., 2009) if protocol-defined criteria were met.
Here, we report an exploratory analysis of the SCLC cohort based on a database lock of March 30, 2017. Median follow-up times in the nonrandomized cohort were 23.3 months for nivolumab (n = 98) and 28.6 months for nivolumab plus ipilimumab (n = 61); median follow-up times in the randomized cohort were 10.8 months for nivolumab (n = 147) and 11.2 months for nivolumab plus ipilimumab (n = 95). Patients in the nonrandomized cohort who were treated with nivolumab 3 mg/kg plus ipilimumab 1 mg/kg (n = 54) were not included in the March 2017 database lock (because this regimen was not chosen for subsequent development) and therefore were not available for this analysis.
Endpoints and Assessments
The primary endpoint of the trial was the objective response rate, defined as the proportion of patients with a best overall response of complete response or partial response with nivolumab plus ipilimumab versus nivolumab monotherapy, per RECIST v1.1, as assessed by a blinded, independent central review. Secondary endpoints included overall survival and progression-free survival.
Tumor assessments by radiographic imaging (computed tomography and magnetic resonance imaging) were performed at baseline, every 6 weeks for the first 24 weeks, and every 12 weeks thereafter until disease progression (investigator-assessed per RECIST v1.1) or treatment discontinuation. Survival was monitored continuously while patients were on treatment and every 3 months after treatment discontinuation until death or conclusion of study.
Study Oversight
The study was designed by the academic authors in collaboration with the sponsor (Bristol-Myers Squibb); the sponsor worked jointly with the investigators to collect and analyze data. The study protocol was approved by an institutional review board or ethics committee at each of the participating centers (all participating centers listed in the Acknowledgements). The study was conducted in accordance with the provisions of the Declaration of Helsinki and Good Clinical Practice guidelines as defined by the International Conference on Harmonisation. An independent data and safety monitoring committee provided oversight of safety and efficacy. This study is registered with ClinicalTrials.gov, number .
All authors attest that the study was conducted in accordance with the protocol and vouch for the accuracy and completeness of the data and analyses. All authors signed a confidentiality agreement with the sponsor. Medical writing support, including writing of the first draft, was provided by Beth Burke, Ph.D., CMPP, and Stefanie Puglielli, Ph.D., of Evidence Scientific Solutions, with funding from the sponsor.
METHOD DETAILS
PD-L1 Expression
Tumor PD-L1 expression was retrospectively assessed in pretreatment (archival or fresh) tumor biopsy specimens using an automated immunohistochemical assay (Dako North America, Carpinteria, CA) and a rabbit anti-human PD-L1 antibody (clone 28-8; Epitomics Inc, Burlingame, CA).
Exploratory Biomarker Analysis of Tumor Mutation Burden
Whole exome capture and sequencing
DNA was isolated from fresh or archival formalin-fixed, paraffin-embedded tumor tissue using the Allprep DNA/RNA kit (Qiagen, Hilden, Germany). Germline DNA from whole blood was isolated using the QIAamp DNA Blood Midi Kit (Qiagen, Hilden, Germany) following the manufacturer’s instructions.
Genomic DNA (150 ng) was used for library preparation. Genomic DNA was fragmented to approximately 150 bp using the Covaris instrument (Covaris, Woburn, MA), and the fragmented DNA was then purified with Agencourt AMPure XP beads (Beckman Coulter, Indianapolis, IN). The library was prepared using the Agilent SureSelectXT reagent kit (Agilent Technologies, Santa Clara, CA) with the on-bead modifications of Fisher et al, 2011 (Fisher et al., 2011). Briefly, the DNA was blunted and a single “A-tail” was added to each fragment. Truncated PE P5 and P7 adaptors were ligated to the DNA fragments and the fragments were purified with AMPure beads. The purified DNA fragments were amplified by polymerase chain reaction (PCR; 6 cycles).
A total of 500 ng of enriched library was used in the hybridization and captured with the SureSelect All Exon v5 (Agilent Technologies) bait. Following hybridization, the captured libraries were purified according to the manufacturer's recommendations and amplified by PCR for 11 cycles using a universal primer and a unique index primer specific to each library. The amplified product was checked for quality using the Tapestation (Agilent Technologies) and quantified by qPCR (Kapa Biosystems). Normalized libraries were pooled and DNA was sequenced on the Illumina HiSeq 2500 using 2 × 100-bp paired-end reads at a plex-level appropriate to the coverage required for the particular application. An average of 93 million reads were sequenced per tumor sample (average of 103 × the mean tumor target coverage), and an average of 94 million reads were sequenced per germline sample (average of 104 × the mean germline target coverage).
Alignment and assembly
Exome sequence data processing was performed using Sentieon’s somatic mutation pipeline, version 201704.01 (Weber et al., 2016), a proprietary reimplementation of The Broad Institute’s best practices for somatic mutation calling with MuTect1 (Cibulskis et al., 2013). Briefly, BWA (version 0.7.15) (Li, 2013) was used to align FASTQ files to the publicly available human genome reference hg19, followed by removal of duplicates, realignment around insertions and deletions identified by Mills (Mills et al., 2006), and recalibration of base quality scores. Finally, a co-realignment step was run for patients who had matching tumor and blood sequencing data to increase the quality of the somatic mutations identified.
Sequencing quality control
The quality of the aligned sequencing data (mean base quality for each flowcell cycle, the base quality score distribution, GC bias metrics, alignment metrics, and insert size metrics) was calculated for each sample with Sentieon's quality control algorithms based on Picard's various alignment metrics. Additional targeted metrics were collected with Picard’s CollectHsMetrics (http://broadinstitute.github.io/picard/) based on the Agilent SureSelect v5 target bed file. CollectHsMetrics collected the key metrics of the aligned reads, the average coverage, the percentage of bases >20× as well as other metrics. Finally, BMS cohort-matcher tool (https://github.com/golharam/cohort-matcher), which utilizes BAM-matcher (Wang et al., 2016), compared the tumor and blood BAMs to ensure that they came from the same patient, in addition to checking for potential sample swaps within the cohort. If any sample (tumor or blood) of a tumor-blood patient pair failed quality control (total reads <45,000,000, mean target coverage <50×, or percentage of bases >20× <80%) or genotype match between tumor and blood samples was <0.85, then the pair was rejected from the final analysis.
Mutation calling
The somatic mutations were called from the co-realigned BAM file, which contains the tumor and blood alignments from the same patient. Somatic single-nucleotide mutations were called by Sentieon’s TNsnv (reimplementation of Mutect1) (Cibulskis et al., 2013; Weber et al., 2016). In addition, both single-nucleotide mutations and insertions/deletions were called with Strelka (v1.0.15) (Saunders et al., 2012). Both these callers have different approaches to identifying the specific variants present in the tumor but not the blood sample.
Annotation and filtering
Variant Call Formats (VCFs) generated from TNsnv and Strelka were filtered to retain only variants that passed all internal filters and met a minimum quality standard. These variants were then annotated with snpEff (v4.1c) (Cingolani et al., 2012). Single nucleotide mutations generated by TNsnv and Strelka were filtered to exclude common single nucleotide polymorphisms (SNPs) by comparison to 1000 Genomes phase 1 snps and indels (http://www.internationalgenome.org/), Mills insertions and deletions (Mills et al., 2006), ExAC (r3.0, http://exac.broadinstitute.org/), and dbsnp (release 138, https://www.ncbi.nlm.nih.gov/projects/SNP/). If variants found in SNP databases, but also in COSMIC (v67, http://cancer.sanger.ac.uk/cosmic) (Bamford et al., 2004), then they were retained.
Determination of tumor mutation burden
The data processing pipeline used to determine tumor mutation burden has been published previously (Carbone et al., 2017). Tumor mutation burden was evaluated in patients with matched tumor and blood samples who had sufficient whole exome sequencing to pass quality control. Tumor mutation burden was defined as the total number of somatic missense mutations identified by either (or both) somatic variant callers after filtering. For patients with more than one tumor sample whose matched pair passed quality control, the average value from all samples was used to calculate tumor mutation burden. Tumor mutation burden testing was conducted in a research laboratory, and the methodology is not a Clinical Laboratory Improvement Amendments–approved clinical diagnostic test.
For most analyses of treatment outcomes by tumor mutation burden, patients were grouped in thirds according to tumor mutation burden (analysis by tumor mutation burden tertiles); additional analyses were based on median or quartiles. A receiver operating characteristic analysis of best overall response by tumor mutation burden as a continuous variable was also performed.
Molecular Features of Previously Published Small Cell Lung Cancers
Called mutations and clinical outcomes were collected from a cohort of SCLCs examined by whole genome sequencing as described (George et al., 2015). A total of 100 patients had paired tumor and normal sequencing, available molecular data, and known overall survival. To align with methods applied here, only missense mutations were used for quantifying tumor mutation burden.
QUANTIFICATION AND STATISTICAL ANALYSIS
All analyses were performed on the basis of the original treatment assignment, regardless of crossover status. The Kaplan–Meier method was used to estimate survival. The Clopper–Pearson method was used to estimate response rates and their exact 95% confidence intervals. Statistical analyses were performed using R version 3.4.1.
Supplementary Material
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Rabbit anti-human PD-L1 antibody | Epitomics Inc, Burlingame, CA | Clone 28-8 |
| Critical Commercial Assays | ||
| Agilent/Dako PD-L1 IHC 28-8 pharmDx | Agilent Technologies, Santa Clara, CA | Code SK005 |
| Allprep DNA/RNA kit | Qiagen, Hilden, Germany | Cat# 80234 |
| QIAamp DNA Blood Midi Kit | Qiagen, Hilden, Germany | Cat# 51185 |
| Agilent SureSelectXT reagent kit | Agilent Technologies, Santa Clara, CA | Cat# 5500-0133 |
| Tapestation | Agilent Technologies, Santa Clara, CA | |
| FoundationOne comprehensive genomic profile | Foundation Medicine, Inc, Cambridge, MA | |
| Deposited Data | ||
| VCF files containing somatic snvs per Tumor-Normal pair | Sentieon’s TNsnv software | European Variation Archive Accession Number: PRJEB25807 |
| VCF files containing somatic snvs per Tumor-Normal pair | Strelka software | European Variation Archive Accession Number: PRJEB25808 |
| Oligonucleotides | ||
| SureSelect All Exon v5 bait | Agilent Technologies, Santa Clara, CA | Cat# 5190-6210 |
| Software and Algorithms | ||
| Sentieon’s somatic mutation pipeline, version 201704.01 | Sentieon, Inc., Mountain View, CA | |
| The Broad Institute’s best practices for somatic mutation calling with MuTect1.3 | https://software.broadinstitute.org/gatk/best-practices/ | |
| BWA (version 0.7.15) | Sentieon, Inc., Mountain View, CA | |
| Sentieon’s TNsnv (reimplementation of Mutect1) | Sentieon, Inc., Mountain View, CA | |
| Strelka (v1.0.15) | https://sites.google.com/site/strelkasomaticvariantcaller/home | |
| snpEff (v4.1c) | http://snpeff.sourceforge.net/index.html | |
| Mills’ insertions and deletions | https://software.broadinstitute.org/gatk/download/bundle | |
| Other | ||
| Agencourt AMPure XP beads | Beckman Coulter, Indianapolis, IN | Cat# A63882 |
Highlights.
This study evaluated the role of tumor mutation burden (TMB) in SCLC
Efficacy of nivolumab with or without ipilimumab increases with higher TMB in SCLC
The benefit of nivolumab plus ipilimumab is greatest in patients with high-TMB SCLC
TMB has a potential role as a biomarker for immunotherapy across lung cancers
Hellmann et al. evaluate the impact of tumor mutation burden (TMB) on the efficacy of nivolumab monotherapy or combination with ipilimumab in patients with small cell lung cancer (SCLC). They show that treatment efficacy and the increased benefit of the combination are most substantial in SCLC with high TMB.
SIGNIFICANCE.
The identification of predictors of response to immune checkpoint blockade in SCLC has been elusive. Expression of tumor programmed death ligand 1, a common biomarker in non-small cell lung cancer, is uncommon and not predictive in SCLC. We show that efficacy of nivolumab with or without ipilimumab was increased in SCLCs that have high tumor mutation burden. The differential benefit of combination immunotherapy was most substantial in patients with high tumor mutation burden. Our results suggest that tumor mutation burden has a role as a biomarker in SCLC and may help identify patients most likely to benefit from combination immunotherapy. Our findings are a critical contribution in moving towards greater precision in patient selection for treatments in lung cancer.
ACKNOWLEDGMENTS
We thank the patients and their families, as well as the participating study teams: Denmark: Ulrik Lassen (Rigshospitalet); Finland: Katriina Peltola, Petri Bono (Helsinki University Hospital); Germany: Akin Atmaca (Krankenhaus Nordwest Gmbh, Frankfurt), Peter Brossart (Universitätsklinikum Bonn), Dirk Jäger (Nationales Centrum für Tumorerkrankungen Heidelberg; Italy: Andrea Ardizzoni (Azienda Ospedaliera S. Orsola-Malpighi), Paolo Antonio Ascierto (Istituto Nazionale Tumori Fondazione G. Pascale), Filippo De-Braud (Istituto Nazionale Tumori), Valentina Guarneri (Istituto Oncologico Veneto IOV-IRCCS); Spain: Emiliano Calvo (Hospital De Madrid Norte Sanchinarro), José López-Martín (Hospital Universitario 12 De Octubre), Victor Moreno García (Fundación Jiménez Díaz), Noemi Reguart (Hospital Clinic I Provincial); United Kingdom: Ian Chau (Royal Marsden Hospital), Jeff Evans (The Beatson West of Scotland Cancer Centre); United States: Asim Amim (Levine Cancer Institute), Johanna Bendell (Tennessee Oncology PLLC), Scott Antonia (H. Lee Moffitt Cancer Center & Research Institute, Inc.), Margaret Callahan (Memorial Sloan Kettering Cancer Center), David Chism (Vanderbilt-Ingram Cancer Center), Joseph Eder (Yale University), Jennifer Diamond (University of Colorado Hospital), Ralph Hauke (Nebraska Cancer Specialists), Frederic Kaye (University of Florida), Dung Le (Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins), Michael Morse (Duke Cancer Institute), Rathi Pillai (Emory University – Winship Cancer Institute), Patrick Ott (Dana-Farber Cancer Institute, Massachusetts General Hospital, Brigham & Women’s Hospital), Jeffrey Schneider (New York University Winthrop Hospital), Padmani Sharma (The University of Texas MD Anderson Cancer Center), and Mathew Taylor (Oregon Health & Science University), for making this study possible; Dako for collaborative development of the PD-L1 IHC 28-8 pharmDx assay; Andrea Renninger, Rupa Guduru, Marsha Smith, George Green, and Steve Lane for their contributions to experimental design, downstream analysis, and interpretation of the tumor mutation burden data; Peter Szabo for validating data analysis; and Ana Moreno and Soumaya Bendahmane for serving as the protocol managers. Medical writing and editorial assistance was provided by Stefanie Puglielli, Ph.D., and Beth Burke, Ph.D., CMPP, of Evidence Scientific Solutions, with funding from Bristol-Myers Squibb.
Funding
This study was funded by Bristol-Myers Squibb (Princeton, NJ) and ONO Pharmaceutical Company, Ltd. (Osaka, Japan).
Footnotes
DECLARATION OF INTERESTS
Matthew D. Hellmann reports paid consultancy from AstraZeneca/MedImmune, Bristol-Myers Squibb, Genentech, Janssen, Merck, Mirati Therapeutics, Novartis, Shattuck Labs, research funding from Bristol-Myers Squibb, and patent filed by Memorial Sloan Kettering related to the use of tumor mutation burden to predict response to immunotherapy (PCT/US2015/062208); Margaret K. Callahan reports grants from and employment of a family member by Bristol-Myers Squibb; personal fees for advisory board participation from AstraZeneca and Incyte; Mark M. Awad reports consulting or advisory role for AbbVie, AstraZeneca, Boehringer Ingelheim, Genentech, Merck, and Pfizer; Paolo A. Ascierto reports grants for research funding and personal fees for advisory/consultant role from Array, Bristol-Myers Squibb, and Roche-Genentech, and personal fees for advisory/consultant role from Amgen, Genmab, Incyte, Medimmune, Merck Serono, NewLink Genetics, Novartis, and Pierre Fabre; Akin Atmaca reports travel grants and honoraria for advisory board participation from Bristol-Myers Squibb, Merck Sharp & Dohme, and Roche; Naiyer A. Rizvi reports a leadership role with ARMO Biosciences, stock or other ownership from ARMO Biosciences and Gritstone Oncology, consulting or advisory role for AstraZeneca, Bristol-Myers Squibb, Eli Lilly, Genentech, MedImmune, Merck Sharp & Dohme, Novartis, Pfizer, and Roche; Giovanni Selvaggi reports employment by Bristol-Myers Squibb; Joseph D. Szustakowski reports employment and stock ownership from Bristol-Myers Squibb and previous employment and stock ownership from Novartis; Ariella Sasson reports employment by Bristol-Myers Squibb; Ryan Golhar reports employment by Bristol-Myers Squibb; Patrik Vitazka reports employment by Bristol-Myers Squibb; Han Chang reports employment by Bristol-Myers Squibb; William J. Geese reports employment and stock ownership from Bristol-Myers Squibb; Scott J. Antonia reports stock or other ownership from Cellular Biomedicine Group, and honoraria, consulting or advisory role, travel, accommodations, and expenses from AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, and Merck; all other authors report nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
ADDITIONAL RESOURCES
Clinical trial registry: https://clinicaltrials.gov/ct2/show/NCT01928394
1000 Genomes phase 1 snps and indels: http://www.internationalgenome.org/
COSMIC (v67): http://cancer.sanger.ac.uk/cosmic
ExAC (r3.0): http://exac.broadinstitute.org/
dbsnp (release 138): https://www.ncbi.nlm.nih.gov/projects/SNP/
Picard’s CollectHsMetrics: http://broadinstitute.github.io/picard/
BMS cohort-matcher tool: https://github.com/golharam/cohort-matcher
REFERENCES
- Alexandrov LB, Nik-Zainal S, Wedge DC, Aparicio SA, Behjati S, Biankin AV, Bignell GR, Bolli N, Borg A, Børresen-Dale AL, et al. (2013). Signatures of mutational processes in human cancer. Nature 500, 415–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarado-Luna G, and Morales-Espinosa D (2016). Treatment for small cell lung cancer, where are we now?—a review. Transl. Lung Cancer Res. 5, 26–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonia SJ, López-Martin JA, Bendell J, Ott PA, Taylor M, Eder JP, Jäger D, Pietanza MC, Le DT, de Braud F, et al. (2016). Nivolumab alone and nivolumab plus ipilimumab in recurrent small-cell lung cancer (CheckMate 032): a multicentre, open-label, phase 1/2 trial. The Lancet. Oncology 17, 883–895. [DOI] [PubMed] [Google Scholar]
- Bamford S, Dawson E, Forbes S, Clements J, Pettett R, Dogan A, Flanagan A, Teague J, Futreal PA, Stratton MR, and Wooster R (2004). The COSMIC (Catalogue of Somatic Mutations in Cancer) database and website. Br. J. Cancer 91, 355–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byers LA, and Rudin CM (2015). Small cell lung cancer: where do we go from here? Cancer 121, 664–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbone DP, Reck M, Paz-Ares L, Creelan B, Horn L, Steins M, Felip E, van den Heuvel MM, Ciuleanu TE, Badin F, et al. (2017). First-line nivolumab in stage IV or recurrent non–small-cell lung cancer. N. Engl. J. Med 376, 2415–2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cibulskis K, Lawrence MS, Carter SL, Sivachenko A, Jaffe D, Sougnez C, Gabriel S, Meyerson M, Lander ES, and Getz G (2013). Sensitive detection of somatic point mutations in impure and heterogeneous cancer samples. Nat. Biotechnol 31, 213–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cingolani P, Platts A, Wang le L, Coon M, Nguyen T, Wang L, Land SJ, Lu X, and Ruden DM (2012). A program for annotating and predicting the effects of single nucleotide polymorphisms, SnpEff: SNPs in the genome of Drosophila melanogaster strain w1118; iso-2; iso-3. Fly 6, 80–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, et al. (2009). New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur. J. Cancer 45, 228–247. [DOI] [PubMed] [Google Scholar]
- Fisher S, Barry A, Abreu J, Minie B, Nolan J, Delorey TM, Young G, Fennell TJ, Allen A, Ambrogio L, et al. (2011). A scalable, fully automated process for construction of sequence-ready human exome targeted capture libraries. Genome Biol. 12, R1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frampton GM, Fichtenholtz A, Otto GA, Wang K, Downing SR, He J, Schnall-Levin M, White J, Sanford EM, An P, et al. (2013). Development and validation of a clinical cancer genomic profiling test based on massively parallel DNA sequencing. Nat. Biotechnol 31, 1023–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galsky MD, Saci A, Szabo PM, Azrilevich A, Horak C, Lambert A, Siefker-Radtke A, Necchi A, and Sharma P (2017). Impact of tumor mutation burden on nivolumab efficacy in second-line urothelial carcinoma patients: exploratory analysis of the phase II CheckMate 275 study [abstract 848PD]. Ann. Oncol 28 (suppl 5), v295–v329. [Google Scholar]
- Gazdar AF, Bunn PA, and Minna JD (2017). Small-cell lung cancer: what we know, what we need to know and the path forward. Nat. Rev. Cancer 17, 725–737. [DOI] [PubMed] [Google Scholar]
- George J, Lim JS, Jang SJ, Cun Y, Ozretić L, Kong G, Leenders F, Lu X, Fernández-Cuesta L, Bosco G, et al. (2015). Comprehensive genomic profiles of small cell lung cancer. Nature 524, 47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellmann MD, Antonia SJ, Ponce S, Ott PA, Calvo E, Taylor M, Ready N, Hann CL, De Braud F, Eder JP, et al. (2016). Nivolumab alone or with ipilimumab in recurrent small cell lung cancer (SCLC): 2-year survival and updated analyses from the CheckMate 032 trial [abstract MA09.05]. J. Thorac. Oncol 12, S393–S394. [Google Scholar]
- Hellmann MD, Nathanson T, Rizvi H, Creelan BC, Sanchez-Vega F, Ahuja A, Ni A, and Novik JB (submitted). Genomic features of response to combination immunotherapy in patients with advanced non-small cell lung cancer. (2018) Cancer Cell. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellmann MD, Ott PA, Zugazagoitia J, Ready NE, Hann CL, De Braud FG, Antonia SJ, Ascierto PA, Moreno V, Atmaca A, et al. (2017). Nivolumab (nivo) ± ipilimumab (ipi) in advanced small-cell lung cancer (SCLC): first report of a randomized expansion cohort from CheckMate 032 [abstract 8503]. J. Clin. Oncol 35 (suppl 15), 8503. [Google Scholar]
- Herbst RS, Heymach JV, and Lippman SM (2008). Lung cancer. N. Engl. J. Med 359, 1367–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le DT, Durham JN, Smith KN, Wang H, Bartlett BR, Aulakh LK, Lu S, Kemberling H, Wilt C, Luber BS, et al. (2017). Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 357, 409–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H (2013). Aligning sequence reads, clone sequences and assembly contigs with BWA-MEM. arXiv:1303.3997v1 [q-bio.GN]. [Google Scholar]
- Mills RE, Luttig CT, Larkins CE, Beauchamp A, Tsui C, Pittard WS, and Devine SE (2006). An initial map of insertion and deletion (INDEL) variation in the human genome. Genome Res 16, 1182–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Comprehensive Cancer Network (2017). Clinical Practice Guidelines in Oncology. Small Cell Lung Cancer. Version ****1.2018. [Google Scholar]
- Peifer M, Fernández-Cuesta L, Sos ML, George J, Seidel D, Kasper LH, Plenker D, Leenders F, Sun R, Zander T, et al. (2012). Integrative genome analyses identify key somatic driver mutations of small-cell lung cancer. Nat. Genet 44, 1104–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reck M, Rodriguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, Gottfried M, Peled N, Tafreshi A, Cuffe S, et al. (2016). Pembrolizumab versus chemotherapy for PD-L1–positive non–small-cell lung cancer. N. Engl. J. Med 375, 1823–1833. [DOI] [PubMed] [Google Scholar]
- Rizvi NA, Hellmann MD, Snyder A, Kvistborg P, Makarov V, Havel JJ, Lee W, Yuan J, Wong P, Ho TS, et al. (2015). Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 348, 124–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg JE, Hoffman-Censits J, Powles T, van der Heijden MS, Balar AV, Necchi A, Dawson N, O'Donnell PH, Balmanoukian A, Loriot Y, et al. (2016). Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet 387, 1909–1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudin CM, Durinck S, Stawiski EW, Poirier JT, Modrusan Z, Shames DS, Bergbower EA, Guan Y, Shin J, Guillory J, et al. (2012). Comprehensive genomic analysis identifies SOX2 as a frequently amplified gene in small-cell lung cancer. Nat. Genet 44, 1111–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders CT, Wong WS, Swamy S, Becq J, Murray LJ, and Cheetham RK (2012). Strelka: accurate somatic small-variant calling from sequenced tumor–normal sample pairs. Bioinformatics 28, 1811–1817. [DOI] [PubMed] [Google Scholar]
- Snyder A, Makarov V, Merghoub T, Yuan J, Zaretsky JM, Desrichard A, Walsh LA, Postow MA, Wong P, Ho TS, et al. (2014). Genetic basis for clinical response to CTLA-4 blockade in melanoma. N. Engl. J. Med 371, 2189–2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turajlic S, Litchfield K, Xu H, Rosenthal R, McGranahan N, Reading JL, Wong YNS, Rowan A, Kanu N, Al Bakir M, et al. (2017). Insertion-and-deletion-derived tumour-specific neoantigens and the immunogenic phenotype: a pan-cancer analysis. The Lancet. Oncology 18, 1009–1021. [DOI] [PubMed] [Google Scholar]
- Wang PP, Parker WT, Branford S, and Schreiber AW (2016). BAM-matcher: a tool for rapid NGS sample matching. Bioinformatics 32, 2699–2701. [DOI] [PubMed] [Google Scholar]
- Weber JA, Aldana R, Gallagher BD, and Edwards JS (2016). Sentieon DNA pipeline for variant detection - software-only solution, over 20× faster than GATK 3.3 with identical results. PeerJ PrePrints 4, e1672. [Google Scholar]
- Wei SC, Levine JH, Cogdill AP, Zhao Y, Anang NAS, Andrews MC, Sharma P, Wang J, Wargo JA, Pe'er D, and Allison JP (2017). Distinct cellular mechanisms underlie anti-CTLA-4 and anti-PD-1 checkpoint blockade. Cell 170, 1120–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.