Abstract
Macular edema (ME) is the most common sight-threatening complication in uveitis. The diagnostic and therapeutic management of the uveitic macular edema (UME) might be challenging due to the complex diagnostic workup and the difficulties physicians face to find the underlying cause, and due to its usually recurrent nature and the fact that it can be refractory to conventional treatment. Some of the mild cases can be treated with topical steroids, which can be combined with non-steroid anti-inflammatory drugs. However, immunomodulators such as methotrexate, tacrolimus, azathioprine, cyclosporine and mycophenolate mofetil together with anti-tumor necrosis factor-α (anti-TNF alpha) monoclonal antibodies such as adalimumab and infliximab, may be required to control the inflammation and the associated ME in refractory cases, or when an underlying disease is present. This review of the literature will focus mostly on the non-infectious UME.
Keywords: non-infectious uveitis, macular edema, NSAIDs, anti-TNF alpha, corticosteroids, immunomodulators
Introduction
Uveitis is the inflammation of the uveal tract, the vascular layer between the sclera and the neuroretina, which can lead to significant visual impairment. Uveal tract consists posteriorly of the choroid, in the middle part of the ciliary body and anteriorly of the iris.
The retina has a double blood supply and each one has a blood-retinal barrier. Choroidal vasculature covers 80% of eye’s blood supply. The inner blood-retina barrier (BRB) is formed by tight junctions between adjacent endothelial cells and the outer BRB by tight junctions between the retinal pigment epithelium (RPE) cells (tight junction proteins include zonula occludens, occludins and VE-cadherins). The outer BRB is essential for maintaining the integrity of the retina and is the one responsible for removal of the metabolic wastes and transportation of nutrients, water and ions. It also separates the neuroretina from the fenestrated choriocapillaris.1
Inflammation to any of the uveal tract structure is called uveitis. Uveitis can be classified further into anterior, intermediate, posterior and panuveitis according to the primary location of the inflammation. The most common form of intraocular inflammation is anterior uveitis (AU), followed by posterior uveitis and panuveitis, while intermediate uveitis is the least common.2,3 Classification of uveitis following the International Uveitis Study Group classification system (SUN)4–6 is depicted in Tables 1 and 2.
Table 1.
Anatomic Classification of uveitis following the International Uveitis Study Group classification system (SUN*).
| Type of uveitis | Primary site of inflammation† | Includes |
| Anterior uveitis | Anterior chamber | Iritis |
| Iridocyclitis | ||
| Anterior cyclitis | ||
| Intermediate uveitis | Vitreous | Pars planitis |
| Posterior cyclitis | ||
| Hyalitis | ||
| Posterior uveitis | Retina or choroid | Focal, multifocal, or diffuse choroiditis |
| Chorioretinitis | ||
| Retinochoroiditis | ||
| Retinitis | ||
| Neuroretinitis | ||
| Panuveitis | Anterior chamber, vitreous and retina or choroid | |
| * SUN = Standardization of uveitis nomenclature. † As determined clinically | ||
Note: Reprinted from the American Journal of Opthalmology, Volume 140, Issue 3, Jabs DA, Nussenblatt RB, Rosenbaum JT, Standardization of Uveitis Nomenclature (SUN) Working Group, Standardization of uveitis nomenclature. Results of the First International Workshop, 509-516, copyright (2005), with permission from Elsevier.5
Table 2.
The SUN* Working Group Descriptors of Uveitis.
| Category | Descriptor | Comment |
|---|---|---|
| Onset | Sudden | |
| Insidious | ||
| Duration | Limited | Uveitis lasting <3 months |
| Persistent | Uveitis lasting >3 months | |
| Course | Acute | Episode characterized by sudden onset and limited duration |
| Chronic | Repeated episodes separated by periods of inactivity without treatment, lasting >3 months | |
| Recurrent | Persistent uveitis with relapse in <3 month·, after discontinuing treatment |
|
| *SUN = Standardization of uveitis nomenclature. | ||
Note: Reprinted from the American Journal of Opthalmology, Volume 140, Issue 3, Jabs DA, Nussenblatt RB, Rosenbaum JT, Standardization of Uveitis Nomenclature (SUN) Working Group, Standardization of uveitis nomenclature. Results of the First International Workshop, 509-516, copyright (2005), with permission from Elsevier.5
Pathophysiology of uveitic macular edema (UME)
The main cause leading to the UME is the breakdown of either inner either outer or both BRBs and is a consequence of chronic inflammation. Extracellular fluid is accumulated either in the intraretinal or the subretinal space.7 A UME might complicate an anterior, intermediate or a posterior uveitis.
The UME can be found in the outer nuclear layer or extend more superficially or deep before resulting to affect all retinal layers, and might even present in the form of a serous retinal detachment due to an RPE dysfunction.8,9 In all cases, it appears to result from the sum-up of cytotoxic and vasogenic effects due to the immunological aggression.10
UME occurs when there is compromised equilibrium of water influx and efflux as a result of the inflammation and the overwhelming of compensatory mechanisms. A breach in the BRB will lead to a vasogenic edema due to the increase of oncotic pressure. Sometimes a dysfunction of the RPE pump and transmembrane ionic channels (Na+, K+, Cl-, HCO3-) and aquaporin 1 (AQP1) might be the cause; in this case no evidential leak is visible on the fluorescein angiography but a serous retinal detachment might exist.11,12 Different therapeutic strategies and pathophysiology implicate in the acute phase of inflammation and chronic stages where atrophy and fibrosis occur.13 The inner BRB breakdown can be triggered by many factors, including vascular endothelial growth factor (VEGF), TNF-a, TGF-β, IL-1, angiotensin II (pro-inflammatory cytokines), adenosine, histamine and glucose. The VEGF is a protein greatly produced by Müller cells and cells that promote neovascularization and causes degradation of tight junction proteins by intracellular phosphorylations.14,15
Müller cells are the most important macroglial cells and their role is to ensure the homeostasis of the retinal extracellular milieu, facilitating the transfer of nutrients and evacuating metabolic by-products.16 They ensure structural integrity and provide a link between neural elements and the vascular network. Müller cells consist also an important source of pigment epithelium-derived factor, contributing in the regulation of retinal angiogenesis. In conditions of stress, Müller cells secrete significant amounts of VEGF, which result in an increase in vascular permeability and neovascularization.16 In the presence of inflammation, Müller cells swell, resulting in the formation of edema. This swelling has also been observed in inflammatory ME after surgery and is presumably derived by the presence of arachidonic acid and prostaglandin E2.17,18 The overall synthesis of potassium-rectifying channels (Kir) decreases also in the presence of inflammation. These functional alterations in Müller cells lead to the formation of cytotoxic ME and favor the formation of intracellular edema and accumulation of subretinal fluid, both characteristics of UME.19 This edema lacks leakage on the angiogram despite manifest edema on the optical coherence tomography (OCT) and is more commonly observed in older individuals, probably because there is a progressive loss of Kir channels with age and Müller cells are less able to excrete water and potassium ions.9
In inflammatory conditions, a vasogenic component coexists. Activated Müller cells and microglial cells synthesize VEGF together with pro-inflammatory cytokines and metalloproteases (such MMP-9) that lead to phosphorylation of occludin and VE-cadherin resulting in losing the integrity of the BRB, as desmosomes between capillary endothelial cells and between the cells of RPE are lost.13
The outer BRB is important for maintaining the adhesion between the RPE and photoreceptors. This is achieved by mechanisms of active transportation from trans-epithelial space to the extraretinal space. Inflammatory conditions that involve the choriocapillaris, choroid and sclera could damage the outer BRB and despite the healthy retinal capillary endothelium, a macular edema (ME) might occur.20,21
In uveitic ocular inflammation despite the inflammatory UME, other causes may increase also the macular thickness, such as:
Inflammatory choroidal neovascularization
Inflammatory epiretinal membrane (ERM) formation with associated vitreomacular traction
Central serous chorioretinopathy exacerbated by steroid therapy
Contiguity with papillary swelling.
Etiology of non-infectious UME
Non-infectious known causes of UME are:
HLA-B27 positive uveitis (HLA-B27 associated diseases, including psoriasis, ankylosing spondylitis, inflammatory bowel disease, and reactive arthritis). A chronic AU, intermediate uveitis, a combination of anterior and intermediate uveitis may occur. AU can be also associated with hypopyon.22
Juvenile idiopathic arthritis (JIA). The most common form of uveitis is chronic AU, which is almost always asymptomatic in the initial stages. However, it can be sight-threatening due to complications, such as glaucoma, cataract, band keratopathy and UME.23
Sarcoidosis. Sarcoidosis-related uveitis is often bilateral and associated with numerous, whitish irregularly scattered granulomatous retinal and choroidal lesions.24
Multiple sclerosis (MS). MS patients have ten times higher prevalence of intermediate uveitis which is often associated with retinal vasculitis.25
Pars-planitis. An idiopathic chronic intermediate uveitis which can be associated with AU and retinal vasculitis.26
Adamantiades–Behçet’s disease. Uveitis is bilateral, often no simultaneous and no granulomatous with coexisting focal or multiple retinal lesions.27
-
Irvine–Gass syndrome and any postoperative ME. Even though the postoperative ME is not considered a typical UME, it should be included in the differential diagnosis of non-infectious UME as most of the time it is related with postoperative inflammation and uveitis.28 Onset is 4–12 weeks with a peak at 4–6 weeks postoperatively. Patients’ typical symptom is deterioration of vision after an initial period of improvement following surgery.29
Uveitis-glaucoma-hyphema syndrome is caused by mechanical trauma due to malpositioned intraocular lens over adjacent structures (iris, ciliary body, iridocorneal angle) and can lead to chronic inflammation, secondary iris neovascularization and ME.30
Drug-induced (or medically induced) uveitis. A number of medications; topical (metipranolol, glucocorticosteroids, brimonidine and prostaglandin analogs), periocular, intraocular (cidofovir, anti-VEGF agents [ranibizumab, bevacizumab, aflibercept] and triamcinolone acetonide), systemic (cidofovir, rifabutin, bisphosphonates, sulfonamides, tumor necrosis factor inhibitors [TNF-a], oral fluoroquinolones and diethylacarbamazine) and vaccines (bacille Calmette–Guérin, measles, mumps and rubella, hepatitis B and varicella) have been associated with uveitis. Mechanisms underlying drug-induced uveitis are unclear but it is suggested that both toxic and inflammatory reactions play a role.31,32
Other collagen diseases including systemic lupus erythematosus (SLE), scleroderma, relapsing polychondritis, necrotizing vasculitis, granulomatosis with polyangiitis (GPA) (formerly known as Wegener’s disease), rheumatoid arthritis, polyarthritis. Non-granulomatous mild AU may occur in SLE patients. However, severe sight-threatening retinal vasculitis with macular involvement is more frequent.33
Birdshot chorioretinopathy (BCR). BCR is strongly associated with HLA-A29 allele and it is believed to be T-cell driven. Typical manifestations include bilateral non- granulomatous uveitis with deep peripapillary or diffuse hypopigmented characteristic multiple cream-colored, irregular choroidal lesions.34
Sympathetic ophthalmia. It is a rare entity, typically presented as a bilateral, granulomatous panuveitis that occurs after surgery or ocular trauma to one eye threatening vision in the other eye.35
Intraocular tumor: primary non-hodgkin oculo-cerebral lymphoma. Typically, it presents as a chronic posterior uveitis with small whitish choroidal lesions, which is the most common masquerade. AU is unusual.36
Vogt–Koyanagi–Harada (VKH) disease. Ocular findings include severe bilateral, chronic granulomatous panuveitis with serous retinal detachment, optic disc swelling and hyalitis.37
Idiopathic uveitis. No cause/extraocular disease is identified.
ME is the main reason for visual loss in patients with uveitis, causing a visual acuity (VA) drop below 20/40 in about 30% of patients with posterior uveitis. VA deteriorates in 45% of the patients with posterior uveitis, in 64% of panuveitis and 28% of intermediate uveitis of which the 28%, 59% and 85%, respectively, were complicated with ME. UME is more frequently found in panuveitis with an incidence of 66%.38
The systemic diseases associated with a poor visual prognosis are juvenile chronic arthritis and sarcoidosis.38
Epidemiology and prevalence of uveitis and UME
Most of the epidemiological data of uveitis have been studied in the developed world.39
The incidence and prevalence of uveitis is between 0.017–0.052% and 0.038–0.714%, respectively, in the population per year.2,3,40,41
Epidemiology changes with geographic location. AU prevalence is low in South Africa, posterior uveitis is more common in Africa, panuveitis is more common in Japan and in India panuveitis is more frequent than posterior uveitis.42–45
The prevalence of non-infectious uveitis has not been thoroughly studied separately from the prevalence of infectious uveitis. A recent original investigation carried out in the US made an effort to study the prevalence of non-infectious uveitis standalone.46 This study reports that non-infectious uveitis affected an estimated 298,801 adults (estimated prevalence 121/100,000) and 21,879 children (estimated prevalence 29/100,000) in the United States in 2015. AU prevalence was 98/100,000 representing the 81% of all non-infectious uveitis cases, followed by non-infectious panuveitis (prevalence 12/100,000), posterior uveitis (prevalence 10/100,000) and intermediate uveitis (prevalence 1/100,000). A smaller study of 927 patients in France studying severe sight-threatening uveitis found that 68% of the cases were non-infectious.47 However, this sample is not representative as it covers only severe cases.
The prevalence mentioned below is the total prevalence of uveitis recorded, unless stated otherwise.
Anterior uveitis
The most common uveitis is AU with prevalence up to 90% of all the cases of uveitis in primary care and 50–60% in tertiary centers. HLA-B27 AU is the most common type of non-infectious uveitis in most of the developed countries (except Japan and Italy).48–50
AU is less frequent in areas with low prevalence of HLA-B27 such as India, South Africa, Japan and Korea.42,51,52
Seronegative spondyloarthropathies (ankylosing spondylitis, psoriatic arthritis, reactive arthritis and Reiter syndrome) are the most usual underlying cause of AU with a prevalence of 5% of all uveitis and 8–12% of acute AU.52–57
Analyzing further, the prevalence of uveitis in systemic autoimmune diseases: 2–9% of patients with inflammatory bowel disease, 7–16% of patients with psoriatic arthritis, 12–37% of patients with reactive arthritis and 20–40% of patients with ankylosing spondylitis will develop AU.48
ME is less common in AU compared to patients with intermediate uveitis, posterior uveitis or panuveitis.15,58,59 Approximately, 11% of patients with isolated AU and 60% of patients with JIA-associated uveitis will develop ME.60,61 The frequency of ME in patients with AU fluctuates between 9% and 28%.38,61–63
Intermediate uveitis
Intermediate uveitis is the least common type of uveitis (15% of all types).64 In most cases of intermediate uveitis, there is no underlying cause identified and they are classified as idiopathic (60–100%). Non-infectious diseases that cause intermediate uveitis include sarcoidosis, MS and intraocular lymphoma (masquerade syndrome).65,66 Despite the fact that intermediate uveitis is the least common type of uveitis, it is the form with the highest frequency of ME, fluctuating between 25% and 70%.38,61–63
Posterior uveitis
It is the second most common uveitis (15–30% of all cases).64 Non-infectious common etiologic factors include sarcoidosis, VKH disease and BCR.43 Sarcoidosis is responsible for 1–13% of uveitis cases in Western World.65,67
According to previous studies, ME rate in posterior uveitis is 19–34%.38,61–63
Panuveitis
The prevalence of panuveitis is greatly variable between geographic locations. It is less common in Europe and the USA and more frequent in Asia, Africa and South America.52,68,69 Japan has a high prevalence of panuveitis due to VKH, Adamantiades–Behçet disease and sarcoidosis.70 VKH panuveitis is rare in Europe with a prevalence of 0–3% and more common in Asia with a prevalence of 11–29%. Adamantiades–Behçet panuveitis has a higher prevalence in Asia and the Mediterranean region (15% in Portugal and 18% in Italy).51
The rate of ME in patients with panuveitis is 18–66%.38,61–63
ME in non-infectious uveitis
In non-infectious uveitis, ME is the most common complication, as it occurs in 8.3% of patients, followed by epiretinal membrane and glaucoma (6.3% and 4.2%, respectively).71
ME in HLA-B27 uveitis ranges between 2% and 32% of cases.72–77 According to a recent study from Turkey, ME in ankylosing spondylitis occurs in about 17.5% of patients and is more frequent in males than in females (18.9% vs 14.3%, respectively),78 while the rate of ME in JIA uveitis is 60%.60,61,79
ME in patients with intermediate uveitis occurs in 60% of cases approximately.61,80–83 The rate of ME in sarcoidosis is 27.3%, while in patients with Admantiades–Behçet’s disease it ranges from 15% to 63%.61,84–86 On the other hand, in BCR uveitis, ME rate is 100%.61
Diagnostic imaging in UME
Diagnosis is usually confirmed using imaging systems, mainly the OCT and fundus fluorescein angiography (FFA).
Usually, a non-infectious UME presents with visual disturbances that are highly variable such as drop in near VA, metamorphopsia, micropsia, blurred vision and positive relative scotomas. In chronic cases where the outer retina has undergone degenerative structural changes, the visual effects can be significant.
The gold standard technique for confirming the diagnosis of UME is the OCT.87,88
Optical coherence tomography (OCT)
OCT provides in vivo near-histological cross-sectional images of the retina. The layers of the retina can be visualized and a detailed analysis of the pathology affecting various structural layers can be done.
Fluid accumulation can be detected in any layer. Furthermore, a quantification of macular thickness can be made, the outer/inner segment line of the photoreceptors can be visualized and examined, intraretinal or subretinal fluid can be seen as well as the presence of ERM or vitreomacular traction. OCT advantages are that it is a non-invasive, reproducible and sensitive imaging system.89,90
Fundus fluorescein angiography (FFA)
FFA despite being more invasive than OCT is a useful tool concerning the inflammatory ME. Dye diffusion can be usually detected in the macular area, which might be associated with pooling in the cystoid spaces. It can help in finding and staging the severity of intraocular inflammation, detecting active choroiditis/retinitis lesions and quantification of retinal vascular (venous, arterial or mixed) leakage. FFA can help to examine the status of macular vasculature which has a direct relationship with the visual morbidity and is the only imaging system that can reveal macular ischemia.87,91
The management of conditions such as posterior uveitis has been revolutionized with the newest ultra-wide field FFA.92,93 Furthermore, with the use of FFA the therapeutic response to a therapeutic intervention can be assessed.
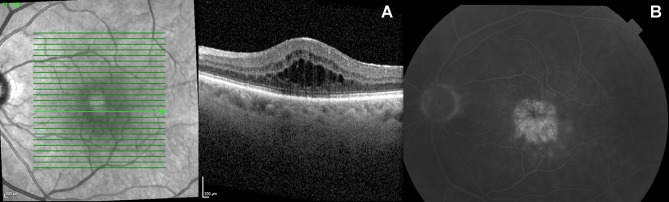
Representative cases with UME on OCT and FFA are depicted in Figures 1 and 2.
Figure 1.
Macular edema secondary to intermediate uveitis in a 58-year-old male patient (left eye). (A) OCT. (B) FFA – typical petaloid pattern.
Abbreviations: OCT ,optical coherence tomography ; FFA, fundus fluorescein angiography.
Figure 2.
Macular edema secondary to UGH syndrome in a 60-year-old male patient (right eye).
Abbreviation: UGH, uveitis-glaucoma-hyphema.
Diagnostic management of UME
When uveitis-related ME is present, physicians need to rule out any infectious or autoimmune underlying disease.
Initial survey should focus on personal medical and surgical history, previous ocular redness, trauma or ocular surgery.
General signs should be researched (fever, sweating and weight loss) and then various organs including skin (herpetic eruption, aphtha, psoriasis or any previous cutaneous eruption or depigmentation), lung (asthma, breathlessness), digestive (abdominal pain, diarrhea, blood stools and hepatitis), joint pain, urinary tract (blood, ulceration) and nervous system (headache, dizziness, sensitive trouble, hypoacusia) should be assessed.
Lifestyle should also be considered, ie alcohol, smoking, toxicomania, and risk of any sexually transmitted disease, presence of pets at home (cat scratch/Lyme disease), risk factors such as raw meat or badly washed salad (toxoplasmosis).
A particular attention should be given to recent traveling and possible administration of immunotherapy, which cause a tremendous increase of ME incidence (ie, Fingolimod, Paclitaxel, Taxane).94
Slit lamp examination should focus on the presence of conjunctival injection, cells in the anterior chamber, the lens status and the presence of cells or floaters in the anterior vitreous. Fundus examination might reveal snowbanking in intermediate uveitis, white cells in the vitreous or inflammatory deposit along the vascular arcade.
During the examination of the fundus, the acuteness or the chronicity of the disease might also be noticed. The presence of exudate will argue for a longstanding edema. The association with an optic nerve swelling could be a sign of worse visual prognosis and therefore should be addressed during the first consultation. Unfortunately, it does not contribute to the etiological orientation.
Bilateral complete peripheral fundus examination is mandatory as it could reveal peripheral ischemia or inflammatory lesions.
As mentioned above, OCT and especially spectral-domain OCT remains the most commonly used imaging technique to assess the patient. It is a useful tool as it allows follow-up. ME should be assessed on focusing on two points: the retinal thickness map and the presence of cyst in the retina. Normal range of central foveal thickness is 182 µm±23. Nevertheless as individual variation might happen, it is always good to have the contralateral eye scanned with OCT to allow comparison, especially in the absence of intraretinal cysts.95
FFA is very useful for the differential diagnosis of ME especially in the young diabetic patient where an ME might be associated with an almost normal appearance of the peripheral fundus, whereas the angiography will reveal extended zone of ischemia and microaneurysms’ leak responsible for the ME.96 Not every retinal leaking is associated with the presence of inflammatory disease and the angiography by its analysis of retinal vascularization is helpful. For example, the scarcity and irregularity of the macular vasculature of a degenerative macular telangiectasia type 2 seen on the FFA would be helpful for the differential diagnosis of other macular cystic degenerations. Finally, FFA could help to exclude a vascular etiology such as vascular occlusion by highlighting tortuous collaterals, delayed filling of vessels or tortuosity of vessels.
Furthermore, FFA might show even late vascular leakage and be very useful in case where there are no intraretinal cysts and only a mild macular thickening is visible on the OCT. On the other hand, the absence of leakage with simultaneous presence of cyst should be suggestive of a different ME cause (ie, X-linked retinoschisis, Goldmann–Favre syndrome, nicotinic retinopathy, Iatrogenic cause such as nab-Paclitaxel, sirolimus).97,98
It is important to mention also that fundus autofluorescence can be helpful in revealing white dot syndromes.99
Once the correct diagnosis of ME is made and no obvious etiology such as Irvine–Gass exists, then blood tests are required.
Some authors suggest that clinical examination might be sufficient to make a diagnosis of an underlying disease (lupus, Adamantiades–Behçet’s disease, cytomegalovirus (CMV)-related retinitis, VKH); nevertheless, one should always keep in mind that an accurate diagnosis is of outmost importance as a mistreatment might be harmful.100
The blood test should be tailored following the local incidence of infectious disease, taking into account possible previous patient’s trip and their phenotype (ie, Caucasians and HLA-B27, Mediterraneans and Adamantiades–Behçet disease, Asians and VKH).49
Classically, a blood test might include the following items:
Electrolytes with renal and liver function, blood glucose, red cells count with platelets and inflammatory parameters
Tailored infectious tests: syphilis, Lyme, cat scratch disease, HIV, herpes simplex virus and herpes zoster virus, CMV, Epstein–Barr virus, toxoplasmosis, human herpes virus-6 (HHV-6), tuberculosis (QuantiferonTB) , West Nile virus or other tropical diseases depending on patients trip or demography.
Tailored immunologic tests: lysozyme, angiotensin converting enzyme, plasmatic protein electrophoresis, nuclear antibody (±anti-centromere, anti-dsDNA, anti-histone), ANCA, rheumatoid factor, complement C3, human leukocyte Antigen (HLA) B27, B51, A29.
Radiological exams such as chest computed tomography for sarcoidosis, brain magnetic resonance imaging for lymphoma or positron emission tomography-computed tomography for vasculitis or sinus X-ray for GPA should be done following clinical examination results.
Unfortunately, there are no standard screening tests. It is recommended to exclude the most common infectious causes and especially syphilis as an inadequate immunosuppressive treatment in those patients might have dramatic consequences.101 Also, it is highly recommended to rule out sarcoidosis as it may take various clinical appearances.102
More invasively, in case of unexplained UME, even with a complete clinical and laboratory workup, an anterior chamber tap and a vitreous biopsy might be necessary.103,104
Classically, a high CD4/CD8 ratio will argue for sarcoidosis (sensitivity and specificity up to 100% and 96%, respectively), whereas a high CD8 count and the presence of viral DNA will argue for a viral infection.105 Analysis of CD19 could be helpful to rule out a tumoral etiology.106
Sometimes an intraocular lymphoma might mimic a UME thus ratio of IL-10/IL-6>1 in the aqueous humor will be highly suggestive of this cause.107
Therapeutic management
Infectious UME should be treated with the appropriate etiological treatment. Herein, we will focus on the various UME of non-infectious causes.
An accurate initial diagnosis of ME etiology is of outmost importance, as even with the right treatment, a possible resistance to it might raise doubts over whether the initial investigations were not properly done.
Irvine–Gass syndrome or pseudophakic ME or postoperative ME
The treatment nowadays is mainly based on the prevention of its occurrence. The European Society of Cataract and Refractive Surgeons has largely modified their recommendations following PREMED studies.108,109
Topical steroidal and non-steroidal anti-inflammatory drops have clearly led to a drop of the incidence of ME. Principle of action is mainly related to the decrease of postoperative blood-aqueous barrier breakdown.110
In case of occurrence of ME, despite topical prevention, it is recommended to associate topical steroids and non-steroids anti-inflammatory drops with oral or topical carbonic anhydrase inhibitors usually for a couple of months. Vitrectomy and grid macular laser were used with some success before the introduction of anti-VEGF injections and intravitreal steroid implants.111,112 Periocular steroid injections remain the first-line treatment after failure of topical or oral therapy, followed by intravitreal anti-VEGF agents, like ranibizumab and bevacizumab, or intravitreal steroids as intravitreal administration of triamcinolone was found to be more efficient compared to periocular administration.113,114 One injection might be sufficient with low recurrence rate but for severe cases, repeated injections are often necessary.115,116
Third-line treatment usually consists of intraocular implants. However, physicians should be cautious with this therapeutic option as there is a risk of ocular hypertension and/or migration of the implant in the anterior chamber which can lead to endothelial cells damage.117–119 In a recent study from France, Ozurdex implant improved VA by at least 15 EDTRS letters, while half of the patients did not need a second injection within the first year.120
In some cases, a combination of the above therapies might be considered (ie, intravitreal anti-VEGF and steroids with topical NSAIDs).121
Rarely, in case of refractory ME in presence of an iris-fixated intraocular lens, even with a previous complete vitrectomy, extraction of the lens and replacement with a scleral-fixated lens might be necessary to resolve the ME.122
Over the last decade, some new therapeutical perspectives are coming out such as oral mineralocorticoid-receptor antagonists or subcutaneous interferon-alpha.123,124
Health care professional’s decision about the most appropriate treatment should be made taking into account patient’s health, comfort and safety profile.
ME related to corneal grafts
MΕ following penetrating keratoplasty is a common complication but recently with the development of new endothelial grafting techniques (Descemet’s stripping endothelial automated keratoplasty and Descemet’s membrane endothelial keratoplasty), which are supposed to be less invasive, their incidence has increased.125,126 Keratoplasty-related ME, usually, resolves spontaneously or with topical anti-inflammatory treatment alone, within a few weeks, without affecting the final visual outcome of patients.125–127
ME related to immunological diseases
This kind of ME can be caused by entities, such as Adamantiades–Behçet’s disease, Sarcoidosis, HLA-B27 spondyloarthritis, VKH, JIA and inflammatory bowel disease.
Initial treatment remains oral and topical steroid and non-steroid anti-inflammatory drugs. In case of unilateral ME, periocular use of steroids is indicated, whereas in bilateral form, systemic steroids are usually preferred.
Immunomodulatory treatment such as methotrexate (a folic acid analog which inhibits leukocyte division),128 tacrolimus and sirolimus (macrolides which inhibit T lymphocyte),129 azathioprine (a purine analog which reduces the peripheral T and B lymphocytes and downregulates interleukin-2 synthesis and IgM production),130 mycophenolate mofetil (an inhibitor of the purine synthesis pathway),131 cyclosporine (which is produced from the fungus Tolipocladium inflatum and inhibits T-cells),132 and Type I interferons (cytokines which play an important role in the regulation of innate and adaptive immune response and in the stabilization of the BRB)133,134 were introduced with the hope that they would be more efficient and reduce the side effects of steroids.135–138 Those corticosteroid-sparing agents are of outmost importance for chronic diseases, nevertheless immunomodulatory treatment has also side effects such as nephrotoxicity, neurotoxicity, gastrointestinal disturbances, flu-like syndrome, leucopenia, thrombocytopenia, hypercholesterolemia, hyperglycemia, potentially increased risk of non-hodgkin lymphoma, and requires frequent clinical observation and lab tests (renal and liver function tests, glucose and lipids profile, full blood count).138 Unfortunately, there is no clear evidence for a standardized protocol and, therefore, the choice of the molecule will depend on the physician’s experience and the patient’s state.139,140
In case of failure (due to ineffectiveness or side effects), the more recent anti-TNF alpha treatment can be used as first line or rescue treatment (etanercept, infliximab, adalimumab) as the pro-inflammatory cytokine TNF-alpha was found to be involved in the pathogenesis of non-infectious uveitis.141–149 Infliximab (a mouse-human chimeric IgG1 monoclonal antibody against TNF-alpha, administered intravenously) and adalimumab (a human IgG1 monoclonal antibody against TNF-alpha administered subcutaneously) have proven their efficiency to reduce steroids dependence even in cases refractory to standard immunosuppressive therapy for sarcoidosis, whereas etanercept seems to be less effective than Infliximab for ocular inflammations.150,151 Systemic administration of anti TNF-alpha agents has been linked with serious adverse events, including malignancies, infections (ie tuberculosis) and autoimmune diseases.152
Intravitreal administration of methotrexate can also be considered according to a British study published in 2009, but this was reported prior to the arrival of the new immunomodulatory agents.153
In cases of persisting UME to conventional pharmacological treatment, pars plana vitrectomy (with or without internal limiting membrane peeling) may be indicated.154 Although the mechanism of UME regression following surgical intervention is not fully understood, there is some evidence that reduction of inflammatory mediators in the vitreous body leads to reduction of antigen presentation.155
ME related to ocular diseases
Retinitis pigmentosa might be associated with uveitis and ME at any stage of the disease.156,157 The pathophysiology of this edema is poorly understood, it might be related to inflammatory reaction due to autoantibodies and abnormal vascular permeability.158 Topical or systemic carbonic anhydrase inhibitors are used as first-line treatment and in case of resistance intravitreal triamcinolone acetonide has shown good results.159,160
Birdshot retinopathy usually responds to systemic steroids, but sometimes resistance even to immunosuppressive agents might threaten the visual outcome.161 More recent anti-TNF alpha agents can be good alternative therapeutic options in those situations.162 However, even these anti -TNF alpha agents might fail to achieve a resolution of ME. Recently, Leclercq et al reported the effectiveness of tocilizumab in refractory birdshot UME cases.163
Medically induced ME
Taxane-induced ME often needs withdrawal of treatment as topical dorzolamide has little effectiveness and anti-VEGF agents do not seem to have better results.164 ME in this condition is probably related to aquaporin interaction rather than inflammatory reactions.165 This might explain the reason that only the first injection of subconjunctival triamcinolone is effective, whereas the second one does not seem to be beneficial.166
Fingolimod, commonly used nowadays in MS, has the particularity to induce ME usually a few months after initiation in approximately 0.5% of patients.167,168 Withdrawal of treatment is not always necessary, as steroid or non-steroid treatment can be effective with continued Fingolimod use.169,170
Patients with UME should be monitored closely initially. It is important to examine them 4–6 weeks after steroid treatment initiation to check intraocular pressure and the effectiveness of the treatment. A collaborative follow-up with a rheumatologist or immunologist is recommended in case of auto-immune disease. OCT monitoring and angiography should be repeated in conjunction with a regular VA assessment and complete slit lamp examination.
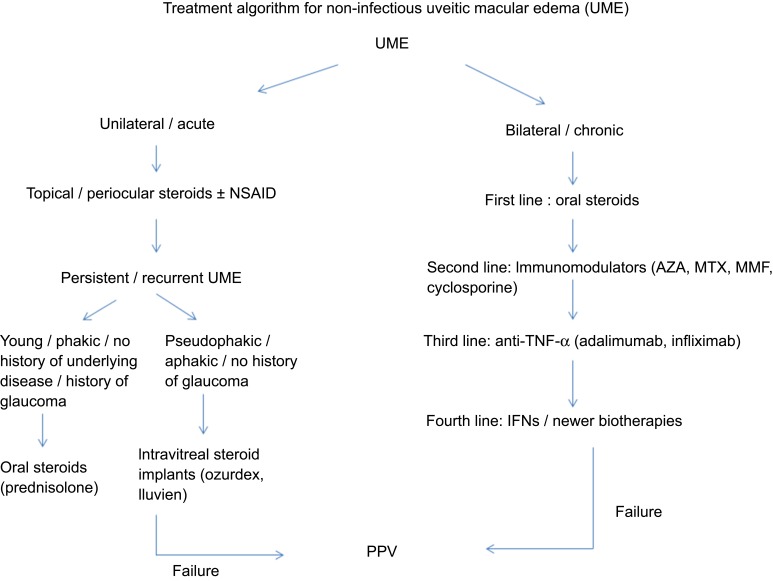
A summary of the most important studies and the algorithm involving the treatment of non-infectious UME is depicted in Table 3 and Figure 3, respectively.
Table 3.
Non-infectious UME. Summary of the most important studies
| Study | Design | Number of patients/eyes | Key results |
|---|---|---|---|
| Vallet et al145 (2016) | Multicenter, observational, infliximab vs adalimumab |
160 patients | 1. Mean response to treatment: 90% 2. Mean complete response: 27% 3. Median time to response: 2 months 4. Rate of serious adverse events: 13% 5. Both treatment are equivalent |
| Diaz-Llopis et al146 (2012) | Prospective case series adalimumab in refractory uveitis 39 JIA, 16 pars planitis, 13 Adamantiades –Behçet |
131 patients | 1. Mean visual acuity at baseline: 0.38±0.44 logMAR improved to 0.26±0.39 logMAR at month 6. 2. Mean macular thickness reduction from 296.95 μm±102 to 240.11 μm±36.1 3. 40 patients had clear visible cystic changes initially 4. 28 of these 40 patient had complete regression of Cystoid ME 5. Improvement of macular edema was significantly correlated with visual acuity |
| Arida et al144 (2011) | Meta-analysis infliximab and Adamantiades–Behçet’s disease |
369 patients | 1. 89% response to infliximab vs 60% etanercept (ten patients) 2. 65% complete response 3.Combination of cyclosporine A and/or azathioprine seem to have better outcome than Infliximab alone |
| Calvo-Rio et al147 (2011) | Multicenter, anti-TNF-alpha and Adamantiades–Behçet disease |
124 patients (221 eyes) |
|
| Mesquida et al148 (2018) | Retrospective: tocilizumab for refractory uveitic macular edema (non-infectious) |
16 eyes of 12 patients |
|
| Calvo-Rio et al149 (2016) | Open-label, multicenter study. Golimumab for spondyloarthritis-related uveitis |
15 patients (18 eyes) |
|
| Jaffe et al162 (2016) | Multicenter, multinational Phase III study, adalimumab in non-infectious uveitis | 110 patients adalimumab group vs 107 placebo group |
|
| Thorne et al for the MUST Research Group114 (2019) |
Multicenter, randomized clinical trial | 192 patients (235 eyes with UME) |
|
Abbreviation: JIA, juvenile idiopathic arthritis.
Figure 3.
Treatment algorithm for non-infectious uveitic macular edema.
Abbreviations: UME, uveitic macular edema; AZA, azathioprine; MTX, methotrexate; MMF, mycophenolate mofetil; IFN, interferon-alpha; PPV, pars plana vitrectomy.
Prognosis
Uveitis-related ME is considered as a risk factor for severe vision loss.171 The prognosis depends on the etiology of the uveitis and also the severity of the ocular inflammation and the activity of the potentially coexisting systemic disease,172 for example a UME secondary to Adamantiades–Behçet disease will have a poorer prognosis compared to a sarcoidosis related UME. The location of the inflammation and the type of the lesion are also important prognostic factors; for example, coexisting vitreoretinal interface alterations and posterior location of the uveitis are bad prognostic factors.173
The prognosis of pediatric UME has drastically improved over the last decades despite its chronicity and legal blindness decreased by more than 50% with a strict control of the inflammation.174,175 These data highlight the significance and the need for aggressive treatment such as long-lasting intravitreal steroids.176
Impact of UME on VA and quality of life
Regardless of its cause, ME leads to reduced VA which can affect patients’ quality of life.177 Lardenoye et al in a cross-sectional study reported that 43% of patients with UME presented significant visual loss (≤20/60). Factors associated with poor vision were advanced age of the patients, chronic inflammation, and specific uveitis entities with intraocular lymphoma and BCR having the worse visual prognosis among the non-infectious causes, while HLA-B27-related uveitis, sarcoidosis and Adamantiades–Behçet disease seem to have lower proportions of impaired vision secondary to ME.61 The same group had previously demonstrated that 35% of patients with uveitis experienced significant visual reduction.38 In a large retrospective study conducted by Durrani et al, VA <6/18 was found in 47% of patients with UME (27% due to UME alone and 20% UME combined with cataract).178 Taylor et al, in a retrospective study, observed that UME was associated with reduced overall visual field sensitivity, while eyes with cystoid UME had VA almost four lines worse compared to eyes without cystoid UME.179 The proportion of eyes with vision <20/40 was 70% when cystoid UME was present vs 30% in eyes without cystoid UME. In a more recent retrospective study in a pediatric population with JIA, it was found that the impact on vision was more significant when both macular thickening and cysts were present, and that the central macular thickness was correlated with VA, but not with disease activity.60
Systemic treatment for UME is associated with systemic adverse events, such as diabetes, osteoporosis and hypertension affecting the quality of life of these patients. Systemic immunomodulatory treatment is also associated with significant adverse events, such as skin reaction, renal and liver dysfunction.137 On the other hand, local ocular administration of steroids has a high risk of inducing ocular complications. Up to 40% of patients might need surgery to control IOP whereas this rate remains at <10% in case of systemic steroids.180
Conclusion
ME is a common, sight-threatening complication of non-infectious uveitis and can persist or recur despite improvement or resolution of the ocular inflammation. The diagnostic and therapeutic management of non-infectious UME remains one of the biggest challenges in ophthalmology. New pharmaceutical agents such as ACTHAR gel (a repository adrenocorticotropic hormone injection for the treatment of sarcoidosis),181 the selective janus kinase 1 inhibitor filgotinib (for the treatment of rheumatoid arthritis and possibly for active non-infectious uveitis)182,183 and ustekinumab (a monoclonal antibody targeting the p40 subunit of interleukin-12 and interleukin-23 which can be a safe therapeutic option for psoriatic arthritis and Crohn’s disease)184,185 are expected with great interest. Moreover, the role of vitrectomy with or without peeling of the internal limiting membrane should be studied deeper, as the mechanism of UME improvement after vitrectomy is still unclear.183
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Evans M. Uveitis In: Yanoff M, Duker J, editors. Ophthalmology. 4th ed. Philadelphia: Elsevier; 2014:687. [Google Scholar]
- 2.Tran VT, Auer C, Guex-Crosier Y, Pittet N, Herbort CP. Epidemiology of uveitis in Switzerland. Ocul Immunol Inflamm. 1994;2(3):169–176. doi: 10.3109/09273949409057073 [DOI] [PubMed] [Google Scholar]
- 3.Paivonsalo-Hietanen T, Tuominen J, Vaahtoranta-Lehtonen H, Saari KM. Incidence and prevalence of different uveitis entities in Finland. Acta Ophthalmol Scand. 1997;75(1):76–81. [DOI] [PubMed] [Google Scholar]
- 4.Bloch-Michel E, Nussenblatt RB. International Uveitis Study Group recommendations for the evaluation of intraocular inflammatory disease. Am J Ophthalmol. 1987;103(2):234–235. doi: 10.1016/s0002-9394(14)74235-7 [DOI] [PubMed] [Google Scholar]
- 5.Jabs DA, Nussenblatt RB, Rosenbaum JT, Standardization of Uveitis Nomenclature (SUN) Working Group. Standardization of uveitis nomenclature for reporting clinical data. Results of the First International Workshop. Am J Ophthalmol. 2005;140(3):509–516. doi: 10.1016/j.ajo.2005.03.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deschenes J, Murray PI, Rao NA, Nussenblatt RB; International Uveitis Study Group. International Uveitis Study Group (IUSG): clinical classification of uveitis. Ocul Immunol Inflamm. 2008;16(1):1–2. doi: 10.1080/09273940801899822 [DOI] [PubMed] [Google Scholar]
- 7.Yhuel Y, Weber M. Physiopathologie de l’oedème maculaire inflammatoire In: Cohen S, Gaudric A, editors. Rétine. Les Ulis: Médecine Sciences Publications; 2012:184–189. [Google Scholar]
- 8.Munk MR, Ram R, Rademaker A, et al. Influence of the vitreomacular interface on the efficacy of intravitreal therapy for uveitis-associated cystoid macular oedema. Acta Ophthalmol. 2015;93(7):e561–567. doi: 10.1111/aos.12699 [DOI] [PubMed] [Google Scholar]
- 9.Tran TH, de Smet MD, Bodaghi B, Fardeau C, Cassoux N, Lehoang P. Uveitic macular oedema: correlation between optical coherence tomography patterns with visual acuity and fluorescein angiography. Br J Ophthalmol. 2008;92(7):922–927. doi: 10.1136/bjo.2007.136846 [DOI] [PubMed] [Google Scholar]
- 10.Valentincic NV, de Groot-Mijnes JD, Kraut A, Korosec P, Hawlina M, Rothova A. Intraocular and serum cytokine profiles in patients with intermediate uveitis. Mol Vis. 2011;17:2003–2010. [PMC free article] [PubMed] [Google Scholar]
- 11.Schafer DP, Lehrman EK, Kautzman AG, et al. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron. 2012;74(4):691–705. doi: 10.1016/j.neuron.2012.03.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stamer WD, Bok D, Hu J, Jaffe GJ, McKay BS. Aquaporin-1 channels in human retinal pigment epithelium: role in transepithelial water movement. Invest Ophthalmol Vis Sci. 2003;44(6):2803–2808. doi: 10.1167/iovs.03-0001 [DOI] [PubMed] [Google Scholar]
- 13.de Smet MD. Insights into the physiopathology of inflammatory macular edema. Dev Ophthalmol. 2017;58:168–177. doi: 10.1159/000455279 [DOI] [PubMed] [Google Scholar]
- 14.Omri S, Behar-Cohen F, de Kozak Y, et al. Microglia/macrophages migrate through retinal epithelium barrier by a transcellular route in diabetic retinopathy: role of PKCzeta in the Goto Kakizaki rat model. Am J Pathol. 2011;179(2):942–953. doi: 10.1016/j.ajpath.2011.04.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fardeau C, Champion E, Massamba N, LeHoang P. Uveitic macular edema. Eye (Lond). 2016;30(10):1277–1292. doi: 10.1038/eye.2016.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bringmann A, Pannicke T, Grosche J, et al. Muller cells in the healthy and diseased retina. Prog Retin Eye Res. 2006;25(4):397–424. doi: 10.1016/j.preteyeres.2006.05.003 [DOI] [PubMed] [Google Scholar]
- 17.Pannicke T, Uckermann O, Iandiev I, Wiedemann P, Reichenbach A, Bringmann A. Ocular inflammation alters swelling and membrane characteristics of rat Muller glial cells. J Neuroimmunol. 2005;161(1–2):145–154. doi: 10.1016/j.jneuroim.2005.01.003 [DOI] [PubMed] [Google Scholar]
- 18.Miyake K, Ibaraki N. Prostaglandins and cystoid macular edema. Surv Ophthalmol. 2002;47(Suppl 1):S203–S218. [DOI] [PubMed] [Google Scholar]
- 19.Liu XQ, Kobayashi H, Jin ZB, Wada A, Nao IN. Differential expression of Kir4.1 and aquaporin 4 in the retina from endotoxin-induced uveitis rat. Mol Vis. 2007;13:309–317. [PMC free article] [PubMed] [Google Scholar]
- 20.Gaudric A, Privat E. Ischémie choroïdienne aigue In: CJ P, editor. Pathologies vasculaires oculaires. Paris (France): Société Française d’Ophtalmologie; 2008:555–571. [Google Scholar]
- 21.Pavesio CE, Meier FM. Systemic disorders associated with episcleritis and scleritis. Curr Opin Ophthalmol. 2001;12(6):471–478. [DOI] [PubMed] [Google Scholar]
- 22.Sharma SM, Jackson D. Uveitis and spondyloarthropathies. Best Pract Res Clin Rheumatol. 2017;31(6):846–862. doi: 10.1016/j.berh.2018.08.002 [DOI] [PubMed] [Google Scholar]
- 23.Sen ES, Ramanan AV. Juvenile idiopathic arthritis-associated uveitis. Best Pract Res Clin Rheumatol. 2017;31(4):517–534. doi: 10.1016/j.berh.2018.01.002 [DOI] [PubMed] [Google Scholar]
- 24.Salah S, Abad S, Monnet D, Brezin AP. Sarcoidosis. J Fr Ophtalmol. 2018;41(10):e451–e467. doi: 10.1016/j.jfo.2018.10.002 [DOI] [PubMed] [Google Scholar]
- 25.Stubiger N, Ruprecht K, Pleyer U. [Intraocular inflammation in multiple sclerosis]. Ophthalmologe. 2018;115(6):531–542. doi: 10.1007/s00347-018-0673-5 [DOI] [PubMed] [Google Scholar]
- 26.Chauhan K, Tripathy K. Pars planitis In: StatPearls. Treasure Island (FL); 2018. [PubMed] [Google Scholar]
- 27.Bazvand F, Zarei M, Ebrahimiadib N, et al. Ocular manifestations, conventional fundus fluorescein angiographic findings, and relationship between angiographic findings and visual acuity in Behcet’s disease. Semin Ophthalmol. 2017;32(6):764–771. doi: 10.1080/08820538.2016.1178310 [DOI] [PubMed] [Google Scholar]
- 28.Cho H, Madu A. Etiology and treatment of the inflammatory causes of cystoid macular edema. J Inflamm Res. 2009;2:37–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zur D, Loewenstein A. Postsurgical cystoid macular edema. Dev Ophthalmol. 2017;58:178–190. doi: 10.1159/000455280 [DOI] [PubMed] [Google Scholar]
- 30.Zemba M, Camburu G. Uveitis-glaucoma-hyphaema syndrome. General review. Rom J Ophthalmol. 2017;61(1):11–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moorthy RS, Moorthy MS, Cunningham ET Jr. Drug-induced uveitis. Curr Opin Ophthalmol. 2018;29(6):588–603. doi: 10.1097/ICU.0000000000000530 [DOI] [PubMed] [Google Scholar]
- 32.London NJ, Garg SJ, Moorthy RS, Cunningham ET. Drug-induced uveitis. J Ophthalmic Inflamm Infect. 2013;3(1):43. doi: 10.1186/1869-5760-3-43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shoughy SS, Tabbara KF. Ocular findings in systemic lupus erythematosus. Saudi J Ophthalmol. 2016;30(2):117–121. doi: 10.1016/j.sjopt.2016.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shao EH, Menezo V, Taylor SR. Birdshot chorioretinopathy. Curr Opin Ophthalmol. 2014;25(6):488–494. doi: 10.1097/ICU.0000000000000101 [DOI] [PubMed] [Google Scholar]
- 35.Castiblanco CP, Adelman RA. Sympathetic ophthalmia. Graefes Arch Clin Exp Ophthalmol. 2009;247(3):289–302. doi: 10.1007/s00417-008-0939-8 [DOI] [PubMed] [Google Scholar]
- 36.Choi JY, Kafkala C, Foster CS. Primary intraocular lymphoma: A review. Semin Ophthalmol. 2006;21(3):125–133. doi: 10.1080/08820530500350498 [DOI] [PubMed] [Google Scholar]
- 37.Bonnet C, Daudin JB, Monnet D, Brezin A. [Vogt-Koyanagi-Harada disease]. J Fr Ophtalmol. 2017;40(6):512–519. doi: 10.1016/j.jfo.2017.02.006 [DOI] [PubMed] [Google Scholar]
- 38.Rothova A, Suttorp-van Schulten MS, Frits Treffers W, Kijlstra A. Causes and frequency of blindness in patients with intraocular inflammatory disease. Br J Ophthalmol. 1996;80(4):332–336. doi: 10.1136/bjo.80.4.332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miserocchi E, Fogliato G, Modorati G, Bandello F. Review on the worldwide epidemiology of uveitis. Eur J Ophthalmol. 2013;23(5):705–717. doi: 10.5301/ejo.5000278 [DOI] [PubMed] [Google Scholar]
- 40.Gritz DC, Wong IG. Incidence and prevalence of uveitis in Northern California; the Northern California Epidemiology of Uveitis Study. Ophthalmology. 2004;111(3):491–500; discussion 500. doi: 10.1016/j.ophtha.2003.06.014 [DOI] [PubMed] [Google Scholar]
- 41.Dandona L, Dandona R, John RK, McCarty CA, Rao GN. Population based assessment of uveitis in an urban population in southern India. Br J Ophthalmol. 2000;84(7):706–709. doi: 10.1136/bjo.84.7.706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ayanru JO. The problem of uveitis in Bendel State of Nigeria: experience in Benin City. Br J Ophthalmol. 1977;61(10):655–659. doi: 10.1136/bjo.61.10.655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang P, Zhang Z, Zhou H, et al. Clinical patterns and characteristics of uveitis in a tertiary center for uveitis in China. Curr Eye Res. 2005;30(11):943–948. doi: 10.1080/02713680500263606 [DOI] [PubMed] [Google Scholar]
- 44.Wakabayashi T, Morimura Y, Miyamoto Y, Okada AA. Changing patterns of intraocular inflammatory disease in Japan. Ocul Immunol Inflamm. 2003;11(4):277–286. [DOI] [PubMed] [Google Scholar]
- 45.Khairallah M, Yahia SB, Ladjimi A, et al. Pattern of uveitis in a referral centre in Tunisia, North Africa. Eye (Lond). 2007;21(1):33–39. doi: 10.1038/sj.eye.6702111 [DOI] [PubMed] [Google Scholar]
- 46.Thorne JE, Suhler E, Skup M, et al. Prevalence of noninfectious uveitis in the United States: a claims-based analysis. JAMA Ophthalmol. 2016;134(11):1237–1245. doi: 10.1001/jamaophthalmol.2016.3229 [DOI] [PubMed] [Google Scholar]
- 47.Bodaghi B, Cassoux N, Wechsler B, et al. Chronic severe uveitis: etiology and visual outcome in 927 patients from a single center. Medicine (Baltimore). 2001;80(4):263–270. doi: 10.1097/00005792-200107000-00005 [DOI] [PubMed] [Google Scholar]
- 48.Tsirouki T, Dastiridou A, Symeonidis C, et al. A focus on the epidemiology of uveitis. Ocul Immunol Inflamm. 2018;26(1):2–16. doi: 10.1080/09273948.2016.1196713 [DOI] [PubMed] [Google Scholar]
- 49.Smit RL, Baarsma GS, de Vries J. Classification of 750 consecutive uveitis patients in the Rotterdam Eye Hospital. Int Ophthalmol. 1993;17(2):71–76. [DOI] [PubMed] [Google Scholar]
- 50.Mercanti A, Parolini B, Bonora A, Lequaglie Q, Tomazzoli L. Epidemiology of endogenous uveitis in north-eastern Italy. Analysis of 655 new cases. Acta Ophthalmol Scand. 2001;79(1):64–68. [DOI] [PubMed] [Google Scholar]
- 51.Chang JH, Wakefield D. Uveitis: a global perspective. Ocul Immunol Inflamm. 2002;10(4):263–279. [DOI] [PubMed] [Google Scholar]
- 52.Biswas J, Narain S, Das D, Ganesh SK. Pattern of uveitis in a referral uveitis clinic in India. Int Ophthalmol. 1996;20(4):223–228. [DOI] [PubMed] [Google Scholar]
- 53.Perkins ES, Folk J. Uveitis in London and Iowa. Ophthalmologica. 1984;189(1–2):36–40. doi: 10.1159/000309382 [DOI] [PubMed] [Google Scholar]
- 54.Henderly DE, Genstler AJ, Smith RE, Rao NA. Changing patterns of uveitis. Am J Ophthalmol. 1987;103(2):131–136. doi: 10.1016/s0002-9394(14)74217-5 [DOI] [PubMed] [Google Scholar]
- 55.McCannel CA, Holland GN, Helm CJ, Cornell PJ, Winston JV, Rimmer TG; UCLA Community-Based Uveitis Study Group. Causes of uveitis in the general practice of ophthalmology. Am J Ophthalmol. 1996;121(1):35–46. doi: 10.1016/s0002-9394(14)70532-x [DOI] [PubMed] [Google Scholar]
- 56.Thean LH, Thompson J, Rosenthal AR. A uveitis register at the Leicester Royal Infirmary. Ophthalmic Epidemiol. 1996;3(3):151–158. [DOI] [PubMed] [Google Scholar]
- 57.Munoz-Fernandez S, Martin-Mola E. Uveitis. Best Pract Res Clin Rheumatol. 2006;20(3):487–505. doi: 10.1016/j.berh.2006.03.008 [DOI] [PubMed] [Google Scholar]
- 58.Grajewski RS, Boelke AC, Adler W, et al. Spectral-domain optical coherence tomography findings of the macula in 500 consecutive patients with uveitis. Eye (Lond). 2016;30(11):1415–1423. doi: 10.1038/eye.2016.133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.de Smet MD, Okada AA. Cystoid macular edema in uveitis. Dev Ophthalmol. 2010;47:136–147. doi: 10.1159/000320077 [DOI] [PubMed] [Google Scholar]
- 60.de Boer J, Steijaert A, van den Bor R, Stellato R, Ossewaarde-van Norel J. Development of macular edema and impact on visual acuity in uveitis associated with juvenile idiopathic arthritis. Ocul Immunol Inflamm. 2015;23(1):67–73. doi: 10.3109/09273948.2013.871566 [DOI] [PubMed] [Google Scholar]
- 61.Lardenoye CW, van Kooij B, Rothova A. Impact of macular edema on visual acuity in uveitis. Ophthalmology. 2006;113(8):1446–1449. doi: 10.1016/j.ophtha.2006.03.027 [DOI] [PubMed] [Google Scholar]
- 62.Levin MH, Pistilli M, Daniel E, et al. Incidence of visual improvement in uveitis cases with visual impairment caused by macular edema. Ophthalmology. 2014;121(2):588–595 e581. doi: 10.1016/j.ophtha.2013.09.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pivetti-Pezzi P, Accorinti M, La Cava M, Colabelli Gisoldi RA, Abdulaziz MA. Endogenous uveitis: an analysis of 1,417 cases. Ophthalmologica. 1996;210(4):234–238. doi: 10.1159/000310715 [DOI] [PubMed] [Google Scholar]
- 64.Bajwa A, Osmanzada D, Osmanzada S, et al. Epidemiology of uveitis in the mid-Atlantic United States. Clin Ophthalmol. 2015;9:889–901. doi: 10.2147/OPTH.S80972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Birnbaum AD, French DD, Mirsaeidi M, Wehrli S. Sarcoidosis in the national veteran population: association of ocular inflammation and mortality. Ophthalmology. 2015;122(5):934–938. doi: 10.1016/j.ophtha.2015.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Merle H, Cabre P, Olindo S, Merle S, Smadja D. Ocular lesions in 200 patients infected by the human T-cell lymphotropic virus type 1 in martinique (French West Indies). Am J Ophthalmol. 2002;134(2):190–195. doi: 10.1016/s0002-9394(02)01521-0 [DOI] [PubMed] [Google Scholar]
- 67.Wakefield D, Chang JH. Epidemiology of uveitis. Int Ophthalmol Clin. 2005;45(2):1–13. [DOI] [PubMed] [Google Scholar]
- 68.Merrill PT, Kim J, Cox TA, Betor CC, McCallum RM, Jaffe GJ. Uveitis in the southeastern United States. Curr Eye Res. 1997;16(9):865–874. [DOI] [PubMed] [Google Scholar]
- 69.Aydin T, Taspinar O, Guneser M, Keskin Y. Association of Vogt Koyanagi Harada syndrome and seronegative rheumatoid arthritis. Ethiop J Health Sci. 2016;26(2):193–196. doi: 10.4314/ejhs.v26i2.14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nashtaei EM, Soheilian M, Herbort CP, Yaseri M. Patterns of uveitis in the middle East and europe. J Ophthalmic Vis Res. 2011;6(4):233–240. [PMC free article] [PubMed] [Google Scholar]
- 71.Lee JH, Mi H, Lim R, et al. Ocular autoimmune systemic inflammatory infectious study - report 3: posterior and panuveitis. Ocul Immunol Inflamm. 2019;27(1):89–98. doi: 10.1080/09273948.2017.1358377 [DOI] [PubMed] [Google Scholar]
- 72.Power WJ, Rodriguez A, Pedroza-Seres M, Foster CS. Outcomes in anterior uveitis associated with the HLA-B27 haplotype. Ophthalmology. 1998;105(9):1646–1651. doi: 10.1016/S0161-6420(98)99033-9 [DOI] [PubMed] [Google Scholar]
- 73.Linssen A, Meenken C. Outcomes of HLA-B27-positive and HLA-B27-negative acute anterior uveitis. Am J Ophthalmol. 1995;120(3):351–361. doi: 10.1016/s0002-9394(14)72165-8 [DOI] [PubMed] [Google Scholar]
- 74.Braakenburg AM, de Valk HW, de Boer J, Rothova A. Human leukocyte antigen-B27-associated uveitis: long-term follow-up and gender differences. Am J Ophthalmol. 2008;145(3):472–479. doi: 10.1016/j.ajo.2007.11.009 [DOI] [PubMed] [Google Scholar]
- 75.Hoeksema L, Los LI. Visual prognosis and ocular complications in herpetic versus HLA-B27- or ankylosing spondylitis-associated anterior uveitis. Ocul Immunol Inflamm. 2016;24(3):302–312. doi: 10.3109/09273948.2015.1005237 [DOI] [PubMed] [Google Scholar]
- 76.Accorinti M, Iannetti L, Liverani M, Caggiano C, Gilardi M. Clinical features and prognosis of HLA B27-associated acute anterior uveitis in an Italian patient population. Ocul Immunol Inflamm. 2010;18(2):91–96. doi: 10.3109/09273941003597268 [DOI] [PubMed] [Google Scholar]
- 77.Pathanapitoon K, Suksomboon S, Kunavisarut P, et al. HLA-B27-associated acute anterior uveitis in the University Referral Centre in North Thailand: clinical presentation and visual prognosis. Br J Ophthalmol. 2006;90(12):1448–1450. doi: 10.1136/bjo.2006.099788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sungur G, Yakin M, Uzman S, Balta O, Orman G, Ornek F. Clinical features and prognosis of uveitis in a Turkish patient population with ankylosing spondylitis: incidence and management of ocular complications. Ocul Immunol Inflamm 2019;27(4):551–559. doi: 10.1080/09273948.2018.1431290 [DOI] [PubMed] [Google Scholar]
- 79.Angeles-Han ST, McCracken C, Yeh S, et al. Characteristics of a cohort of children with juvenile idiopathic arthritis and JIA-associated uveitis. Pediatr Rheumatol Online J. 2015;13:19. doi: 10.1186/s12969-015-0018-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Heinz C, Schoonbrood S, Heiligenhaus A. Intermediate uveitis in children and young adults: differences in clinical course, associations and visual outcome. Br J Ophthalmol. 2014;98(8):1107–1111. doi: 10.1136/bjophthalmol-2013-304589 [DOI] [PubMed] [Google Scholar]
- 81.Paroli MP, Spinucci G, Monte R, Pesci FR, Abicca I, Pivetti Pezzi P. Intermediate uveitis in a pediatric Italian population. Ocul Immunol Inflamm. 2011;19(5):321–326. doi: 10.3109/09273948.2011.603878 [DOI] [PubMed] [Google Scholar]
- 82.de Boer J, Berendschot TT, van der Does P, Rothova A. Long-term follow-up of intermediate uveitis in children. Am J Ophthalmol. 2006;141(4):616–621. doi: 10.1016/j.ajo.2005.09.035 [DOI] [PubMed] [Google Scholar]
- 83.Arellanes-Garcia L, Navarro-Lopez L, Recillas-Gispert C. Pars planitis in the Mexican Mestizo population: ocular findings, treatment, and visual outcome. Ocul Immunol Inflamm. 2003;11(1):53–60. [DOI] [PubMed] [Google Scholar]
- 84.Accorinti M, Pesci FR, Pirraglia MP, Abicca I, Pivetti-Pezzi P. Ocular Behcet’s disease: changing patterns over time, complications and long-term visual prognosis. Ocul Immunol Inflamm. 2017;25(1):29–36. doi: 10.3109/09273948.2015.1094095 [DOI] [PubMed] [Google Scholar]
- 85.Tugal-Tutkun I, Onal S, Altan-Yaycioglu R, Huseyin Altunbas H, Urgancioglu M. Uveitis in Behcet disease: an analysis of 880 patients. Am J Ophthalmol. 2004;138(3):373–380. doi: 10.1016/j.ajo.2004.03.022 [DOI] [PubMed] [Google Scholar]
- 86.Accorinti M, Okada AA, Smith JR, Gilardi M. Epidemiology of macular edema in uveitis. Ocul Immunol Inflamm 2019;27(2):169–180. doi: 10.1080/09273948.2019.1576910 [DOI] [PubMed] [Google Scholar]
- 87.Gupta V, Al-Dhibi HA, Arevalo JF. Retinal imaging in uveitis. Saudi J Ophthalmol. 2014;28(2):95–103. doi: 10.1016/j.sjopt.2014.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gaucher D, Tadayoni R, Erginay A, Haouchine B, Gaudric A, Massin P. Optical coherence tomography assessment of the vitreoretinal relationship in diabetic macular edema. Am J Ophthalmol. 2005;139(5):807–813. doi: 10.1016/j.ajo.2004.12.084 [DOI] [PubMed] [Google Scholar]
- 89.Grewal DS, O’Sullivan ML, Kron M, Jaffe GJ. Association of disorganization of retinal inner layers with visual acuity in eyes with uveitic cystoid macular edema. Am J Ophthalmol. 2017;177:116–125. doi: 10.1016/j.ajo.2017.02.017 [DOI] [PubMed] [Google Scholar]
- 90.Roesel M, Henschel A, Heinz C, Dietzel M, Spital G, Heiligenhaus A. Fundus autofluorescence and spectral domain optical coherence tomography in uveitic macular edema. Graefes Arch Clin Exp Ophthalmol. 2009;247(12):1685–1689. doi: 10.1007/s00417-009-1149-8 [DOI] [PubMed] [Google Scholar]
- 91.Thomas AS, Redd T, Campbell JP, et al. The impact and implication of peripheral vascular leakage on ultra-widefield fluorescein angiography in uveitis. Ocul Immunol Inflamm 2019;27(3):349–355. doi: 10.1080/09273948.2017.1367406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Karampelas M, Sim DA, Chu C, et al. Quantitative analysis of peripheral vasculitis, ischemia, and vascular leakage in uveitis using ultra-widefield fluorescein angiography. Am J Ophthalmol. 2015;159(6):1161–1168 e1161. doi: 10.1016/j.ajo.2015.02.009 [DOI] [PubMed] [Google Scholar]
- 93.Campbell JP, Leder HA, Sepah YJ, et al. Wide-field retinal imaging in the management of noninfectious posterior uveitis. Am J Ophthalmol. 2012;154(5):908–911 e902. doi: 10.1016/j.ajo.2012.05.019 [DOI] [PubMed] [Google Scholar]
- 94.Willis MA, Cohen JA. Fingolimod therapy for multiple sclerosis. Semin Neurol. 2013;33(1):37–44. doi: 10.1055/s-0033-1343794 [DOI] [PubMed] [Google Scholar]
- 95.Chan A, Duker JS, Ko TH, Fujimoto JG, Schuman JS. Normal macular thickness measurements in healthy eyes using Stratus optical coherence tomography. Arch Ophthalmol. 2006;124(2):193–198. doi: 10.1001/archopht.124.2.193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kozak I, Morrison VL, Clark TM, et al. Discrepancy between fluorescein angiography and optical coherence tomography in detection of macular disease. Retina. 2008;28(4):538–544. doi: 10.1097/IAE.0b013e318167270b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rahman HT, Yeh S, Bergstrom CS. Cystoid macular edema without leakage secondary to nab-Paclitaxel (Abraxane): clinical experience with intravitreal bevacizumab. J Ocul Pharmacol Ther. 2013;29(3):360–362. doi: 10.1089/jop.2011.0178 [DOI] [PubMed] [Google Scholar]
- 98.Nayak SK, Jeloka TK, Sreepada SV. Sirolimus-induced pneumonitis, sinusitis and macular oedema. Nephrol Dial Transplant. 2004;19(11):2931. doi: 10.1093/ndt/gfh465 [DOI] [PubMed] [Google Scholar]
- 99.Yeh S, Forooghian F, Wong WT, et al. Fundus autofluorescence imaging of the white dot syndromes. Arch Ophthalmol. 2010;128(1):46–56. doi: 10.1001/archophthalmol.2009.368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.de-la-Torre A, Valdes-Camacho J, de Mesa CL, et al. Coinfections and differential diagnosis in immunocompetent patients with uveitis of infectious origin. BMC Infect Dis. 2019;19(1):91. doi: 10.1186/s12879-018-3613-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Shaikh SI, Biswas J, Rishi P. Nodular syphilitic scleritis masquerading as an ocular tumor. J Ophthalmic Inflamm Infect. 2015;5:8. doi: 10.1186/s12348-015-0040-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Thorne JE, Brucker AJ. Choroidal white lesions as an early manifestation of sarcoidosis. Retina. 2000;20(1):8–15. [DOI] [PubMed] [Google Scholar]
- 103.Margolis R. Diagnostic vitrectomy for the diagnosis and management of posterior uveitis of unknown etiology. Curr Opin Ophthalmol. 2008;19(3):218–224. doi: 10.1097/ICU.0b013e3282fc261d [DOI] [PubMed] [Google Scholar]
- 104.Rothova A, de Boer JH, Ten Dam-van Loon NH, et al. Usefulness of aqueous humor analysis for the diagnosis of posterior uveitis. Ophthalmology. 2008;115(2):306–311. doi: 10.1016/j.ophtha.2007.05.014 [DOI] [PubMed] [Google Scholar]
- 105.Kojima K, Maruyama K, Inaba T, et al. The CD4/CD8 ratio in vitreous fluid is of high diagnostic value in sarcoidosis. Ophthalmology. 2012;119(11):2386–2392. doi: 10.1016/j.ophtha.2012.05.033 [DOI] [PubMed] [Google Scholar]
- 106.Maruyama K, Inaba T, Sugita S, et al. Comprehensive analysis of vitreous specimens for uveitis classification: a prospective multicentre observational study. BMJ Open. 2017;7(11):e014549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Pochat-Cotilloux C, Bienvenu J, Nguyen AM, et al. Use of a threshold of interleukin-10 and Il-10/Il-6 ratio in ocular samples for the screening of vitreoretinal lymphoma. Retina. 2018;38(4):773–781. doi: 10.1097/IAE.0000000000001922 [DOI] [PubMed] [Google Scholar]
- 108.Wielders LHP, Schouten J, Winkens B, et al. European multicenter trial of the prevention of cystoid macular edema after cataract surgery in nondiabetics: ESCRS PREMED study report 1. J Cataract Refract Surg. 2018;44(4):429–439. doi: 10.1016/j.jcrs.2018.01.029 [DOI] [PubMed] [Google Scholar]
- 109.Wielders LHP, Schouten J, Winkens B, et al. Randomized controlled European multicenter trial on the prevention of cystoid macular edema after cataract surgery in diabetics: ESCRS PREMED Study Report 2. J Cataract Refract Surg. 2018;44(7):836–847. doi: 10.1016/j.jcrs.2018.05.015 [DOI] [PubMed] [Google Scholar]
- 110.Sanders DR, Kraff M. Steroidal and nonsteroidal anti-inflammatory agents. Effect on postsurgical inflammation and blood-aqueous humor barrier breakdown. Arch Ophthalmol. 1984;102(10):1453–1456. doi: 10.1001/archopht.1984.01040031173012 [DOI] [PubMed] [Google Scholar]
- 111.Young PW, Shea M. Pars plasma vitrectomy in the management of the Irvine-Gass syndrome. Can J Ophthalmol. 1980;15(4):172–175. [PubMed] [Google Scholar]
- 112.Lardenoye CW, van Schooneveld MJ, Frits Treffers W, Rothova A. Grid laser photocoagulation for macular oedema in uveitis or the Irvine-Gass syndrome. Br J Ophthalmol. 1998;82(9):1013–1016. doi: 10.1136/bjo.82.9.1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Loewenstein A, Zur D. Postsurgical cystoid macular edema. Dev Ophthalmol. 2010;47:148–159. doi: 10.1159/000320078 [DOI] [PubMed] [Google Scholar]
- 114.Thorne JE, Sugar EA, Holbrook JT, et al. Periocular triamcinolone vs. intravitreal triamcinolone vs. intravitreal dexamethasone implant for the treatment of uveitic macular edema: the PeriOcular vs. INTravitreal corticosteroids for uveitic macular edema (POINT) trial. Ophthalmology. 2019;126(2):283–295. doi: 10.1016/j.ophtha.2018.08.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Mitropoulos PG, Chatziralli IP, Peponis VG, Drakos E, Parikakis EA. Intravitreal ranibizumab for the treatment of Irvine-Gass syndrome. Ocul Immunol Inflamm. 2015;23(3):225–231. doi: 10.3109/09273948.2014.898775 [DOI] [PubMed] [Google Scholar]
- 116.Arevalo JF, Maia M, Garcia-Amaris RA, et al. Intravitreal bevacizumab for refractory pseudophakic cystoid macular edema: the Pan-American Collaborative Retina Study Group results. Ophthalmology. 2009;116(8):1481–1487, 1487 e1481. doi: 10.1016/j.ophtha.2009.04.006 [DOI] [PubMed] [Google Scholar]
- 117.Malcles A, Janin-Manificat H, Yhuel Y, et al. [Anterior chamber migration of intravitreal dexamethasone implant (Ozurdex(R)) in pseudophakic eyes: report of three cases]. J Fr Ophtalmol. 2013;36(4):362–367. doi: 10.1016/j.jfo.2012.11.003 [DOI] [PubMed] [Google Scholar]
- 118.Bahadorani S, Krambeer C, Wannamaker K, et al. The effects of repeated Ozurdex injections on ocular hypertension. Clin Ophthalmol. 2018;12:639–642. doi: 10.2147/OPTH.S148990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Massa H, Georgoudis P, Panos GD. Dexamethasone intravitreal implant (OZURDEX((R))) for macular edema secondary to noninfectious uveitis: a review of the literature. Ther Deliv. 2019;10(6):343–351. doi: 10.4155/tde-2019-0024 [DOI] [PubMed] [Google Scholar]
- 120.Bellocq D, Korobelnik JF, Burillon C, et al. Effectiveness and safety of dexamethasone implants for post-surgical macular oedema including Irvine-Gass syndrome: the EPISODIC study. Br J Ophthalmol. 2015;99(7):979–983. doi: 10.1136/bjophthalmol-2014-306159 [DOI] [PubMed] [Google Scholar]
- 121.Warren KA, Bahrani H, Fox JE. NSAIDs in combination therapy for the treatment of chronic pseudophakic cystoid macular edema. Retina. 2010;30(2):260–266. [DOI] [PubMed] [Google Scholar]
- 122.Massa HF, Gobej I, Jacquier P, Jonescu-Cuypers C, Le Quoy O. Cystoid macular oedema and iris-fixated intraocular lens treated with intraocular lens exchange: a case series and review. J Int Med Res. 2019;47(1):188–195. doi: 10.1177/0300060518799004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Matet A, Daruich A, Behar-Cohen F. Irvine-Gass macular edema responding to the combination of oral mineralocorticoid-receptor antagonist with dexamethasone drops. Ophthalmic Surg Lasers Imaging Retina. 2017;48(11):936–942. doi: 10.3928/23258160-20171030-11 [DOI] [PubMed] [Google Scholar]
- 124.Deuter CM, Gelisken F, Stubiger N, Zierhut M, Doycheva D. Successful treatment of chronic pseudophakic macular edema (Irvine-Gass syndrome) with interferon alpha: a report of three cases. Ocul Immunol Inflamm. 2011;19(3):216–218. doi: 10.3109/09273948.2011.562341 [DOI] [PubMed] [Google Scholar]
- 125.Nirankari VS, Karesh JW. Cystoid macular edema following penetrating keratoplasty: incidence and prognosis. Ophthalmic Surg. 1986;17(7):404–407. [PubMed] [Google Scholar]
- 126.Kocaba V, Mouchel R, Fleury J, et al. Incidence of cystoid macular edema after descemet membrane endothelial keratoplasty. Cornea. 2018;37(3):277–282. doi: 10.1097/ICO.0000000000001501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kitazawa K, Kayukawa K, Wakimasu K, et al. Topical non-steroidal anti-inflammatory drugs for the treatment of cystoid macular edema post Descemet’s stripping automated endothelial keratoplasty. Jpn J Ophthalmol. 2018;62(6):615–620. doi: 10.1007/s10384-018-0621-6 [DOI] [PubMed] [Google Scholar]
- 128.Jabs DA, Rosenbaum JT, Foster CS, et al. Guidelines for the use of immunosuppressive drugs in patients with ocular inflammatory disorders: recommendations of an expert panel. Am J Ophthalmol. 2000;130(4):492–513. doi: 10.1016/s0002-9394(00)00659-0 [DOI] [PubMed] [Google Scholar]
- 129.Hogan AC, McAvoy CE, Dick AD, Lee RW. Long-term efficacy and tolerance of tacrolimus for the treatment of uveitis. Ophthalmology. 2007;114(5):1000–1006. doi: 10.1016/j.ophtha.2007.01.026 [DOI] [PubMed] [Google Scholar]
- 130.Bacon PA, Salmon M. Modes of action of second-line agents. Scand J Rheumatol Suppl. 1987;64:17–24. [DOI] [PubMed] [Google Scholar]
- 131.Thorne JE, Jabs DA, Qazi FA, Nguyen QD, Kempen JH, Dunn JP. Mycophenolate mofetil therapy for inflammatory eye disease. Ophthalmology. 2005;112(8):1472–1477. doi: 10.1016/j.ophtha.2005.02.020 [DOI] [PubMed] [Google Scholar]
- 132.Nussenblatt RB, Palestine AG, Chan CC. Cyclosporin A therapy in the treatment of intraocular inflammatory disease resistant to systemic corticosteroids and cytotoxic agents. Am J Ophthalmol. 1983;96(3):275–282. doi: 10.1016/s0002-9394(14)77814-6 [DOI] [PubMed] [Google Scholar]
- 133.Gillies MC, Su T. Interferon-alpha 2b enhances barrier function of bovine retinal microvascular endothelium in vitro. Microvasc Res. 1995;49(3):277–288. doi: 10.1006/mvre.1995.1024 [DOI] [PubMed] [Google Scholar]
- 134.Feron EJ, Rothova A, van Hagen PM, Baarsma GS, Suttorp-Schulten MS. Interferon-alpha 2b for refractory ocular Behcet’s disease. Lancet. 1994;343(8910):1428. doi: 10.1016/S0140-6736(94)92549-6 [DOI] [PubMed] [Google Scholar]
- 135.Neri P, Mariotti C, Cimino L, Mercanti L, Giovannini A. Long-term control of cystoid macular oedema in noninfectious uveitis with Mycophenolate Mofetil. Int Ophthalmol. 2009;29(3):127–133. doi: 10.1007/s10792-008-9200-z [DOI] [PubMed] [Google Scholar]
- 136.Ramanan AV, Dick AD, Jones AP, et al. Adalimumab plus methotrexate for uveitis in juvenile idiopathic arthritis. N Engl J Med. 2017;376(17):1637–1646. doi: 10.1056/NEJMoa1614160 [DOI] [PubMed] [Google Scholar]
- 137.Trivedi A, Katelaris C. The use of biologic agents in the management of uveitis. Intern Med J. 2018. doi: 10.1111/imj.14215 [DOI] [PubMed] [Google Scholar]
- 138.Yanai R, Takeda A, Yoshimura T, Sonoda KH. [Pathophysiology and new treatment of uveitis]. Nihon Rinsho Meneki Gakkai Kaishi. 2014;37(2):74–82. [DOI] [PubMed] [Google Scholar]
- 139.Tallouzi MO, Mathers JM, Moore DJ, et al. COSUMO: study protocol for the development of a core outcome set for efficacy and effectiveness trials in posterior segment-involving uveitis. Trials. 2017;18(1):576. doi: 10.1186/s13063-017-2294-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Tallouzi MO, Moore DJ, Calvert M, Murray PI, Bucknall N, Denniston AK. The effectiveness of pharmacological agents for the treatment of uveitic macular oedema (UMO): a systematic review protocol. Syst Rev. 2016;5:29. doi: 10.1186/s13643-016-0289-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Sharma SM, Nestel AR, Lee RW, Dick AD. Clinical review: anti-TNFalpha therapies in uveitis: perspective on 5 years of clinical experience. Ocul Immunol Inflamm. 2009;17(6):403–414. doi: 10.3109/09273940903072443 [DOI] [PubMed] [Google Scholar]
- 142.Markomichelakis NN, Theodossiadis PG, Pantelia E, Papaefthimiou S, Theodossiadis GP, Sfikakis PP. Infliximab for chronic cystoid macular edema associated with uveitis. Am J Ophthalmol. 2004;138(4):648–650. doi: 10.1016/j.ajo.2004.04.066 [DOI] [PubMed] [Google Scholar]
- 143.Schaap-Fogler M, Amer R, Friling R, Priel E, Kramer M. Anti-TNF-alpha agents for refractory cystoid macular edema associated with noninfectious uveitis. Graefes Arch Clin Exp Ophthalmol. 2014;252(4):633–640. doi: 10.1007/s00417-013-2552-8 [DOI] [PubMed] [Google Scholar]
- 144.Arida A, Fragiadaki K, Giavri E, Sfikakis PP. Anti-TNF agents for Behcet’s disease: analysis of published data on 369 patients. Semin Arthritis Rheum. 2011;41(1):61–70. doi: 10.1016/j.semarthrit.2010.09.002 [DOI] [PubMed] [Google Scholar]
- 145.Vallet H, Seve P, Biard L, et al. Infliximab versus adalimumab in the treatment of refractory inflammatory uveitis: a multicenter study from the french uveitis network. Arthritis Rheumatol. 2016;68(6):1522–1530. doi: 10.1002/art.39667 [DOI] [PubMed] [Google Scholar]
- 146.Diaz-Llopis M, Salom D, Garcia-de-Vicuna C, et al. Treatment of refractory uveitis with adalimumab: a prospective multicenter study of 131 patients. Ophthalmology. 2012;119(8):1575–1581. doi: 10.1016/j.ophtha.2012.02.018 [DOI] [PubMed] [Google Scholar]
- 147.Calvo-Rio V, Blanco R, Beltran E, et al. Anti-TNF-alpha therapy in patients with refractory uveitis due to Behcet’s disease: a 1-year follow-up study of 124 patients. Rheumatology (Oxford). 2014;53(12):2223–2231. doi: 10.1093/rheumatology/keu266 [DOI] [PubMed] [Google Scholar]
- 148.Mesquida M, Molins B, Llorenc V, et al. Twenty-four month follow-up of tocilizumab therapy for refractory uveitis-related macular edema. Retina. 2018;38(7):1361–1370. doi: 10.1097/IAE.0000000000001690 [DOI] [PubMed] [Google Scholar]
- 149.Calvo-Rio V, Blanco R, Santos-Gomez M, et al. Golimumab in refractory uveitis related to spondyloarthritis. Multicenter study of 15 patients. Semin Arthritis Rheum. 2016;46(1):95–101. doi: 10.1016/j.semarthrit.2016.03.002 [DOI] [PubMed] [Google Scholar]
- 150.Riancho-Zarrabeitia L, Calvo-Rio V, Blanco R, et al. Anti-TNF-alpha therapy in refractory uveitis associated with sarcoidosis: multicenter study of 17 patients. Semin Arthritis Rheum. 2015;45(3):361–368. doi: 10.1016/j.semarthrit.2015.05.010 [DOI] [PubMed] [Google Scholar]
- 151.Galor A, Perez VL, Hammel JP, Lowder CY. Differential effectiveness of etanercept and infliximab in the treatment of ocular inflammation. Ophthalmology. 2006;113(12):2317–2323. doi: 10.1016/j.ophtha.2006.04.038 [DOI] [PubMed] [Google Scholar]
- 152.Imrie FR, Dick AD. Biologics in the treatment of uveitis. Curr Opin Ophthalmol. 2007;18(6):481–486. doi: 10.1097/ICU.0b013e3282f03d42 [DOI] [PubMed] [Google Scholar]
- 153.Taylor SR, Habot-Wilner Z, Pacheco P, Lightman SL. Intraocular methotrexate in the treatment of uveitis and uveitic cystoid macular edema. Ophthalmology. 2009;116(4):797–801. doi: 10.1016/j.ophtha.2008.10.033 [DOI] [PubMed] [Google Scholar]
- 154.Golan S, Loewenstein A. Surgical treatment for macular edema. Semin Ophthalmol. 2014;29(4):242–256. doi: 10.3109/08820538.2013.796394 [DOI] [PubMed] [Google Scholar]
- 155.Muhaya M, Calder VL, Towler HM, Jolly G, McLauchlan M, Lightman S. Characterization of phenotype and cytokine profiles of T cell lines derived from vitreous humour in ocular inflammation in man. Clin Exp Immunol. 1999;116(3):410–414. doi: 10.1046/j.1365-2249.1999.00921.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Liew G, Strong S, Bradley P, et al. Prevalence of cystoid macular oedema, epiretinal membrane and cataract in retinitis pigmentosa. Br J Ophthalmol. 2018. doi: 10.1136/bjophthalmol-2018-311964 [DOI] [PubMed] [Google Scholar]
- 157.Dutta Majumder P, Menia N, Roy R, et al. Uveitis in patients with retinitis pigmentosa: 30 years’ consecutive data. Ocul Immunol Inflamm. 2018;26(8):1283–1288. doi: 10.1080/09273948.2017.1348527 [DOI] [PubMed] [Google Scholar]
- 158.Moldow B, Sander B, Larsen M, et al. The effect of acetazolamide on passive and active transport of fluorescein across the blood-retina barrier in retinitis pigmentosa complicated by macular oedema. Graefes Arch Clin Exp Ophthalmol. 1998;236(12):881–889. [DOI] [PubMed] [Google Scholar]
- 159.Grover S, Fishman GA, Fiscella RG, Adelman AE. Efficacy of dorzolamide hydrochloride in the management of chronic cystoid macular edema in patients with retinitis pigmentosa. Retina. 1997;17(3):222–231. [DOI] [PubMed] [Google Scholar]
- 160.Saraiva VS, Sallum JM, Farah ME. Treatment of cystoid macular edema related to retinitis pigmentosa with intravitreal triamcinolone acetonide. Ophthalmic Surg Lasers Imaging. 2003;34(5):398–400. [PubMed] [Google Scholar]
- 161.Menezo V, Taylor SR. Birdshot uveitis: current and emerging treatment options. Clin Ophthalmol. 2014;8:73–81. doi: 10.2147/OPTH.S54832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Jaffe GJ, Dick AD, Brezin AP, et al. Adalimumab in patients with active noninfectious uveitis. N Engl J Med. 2016;375(10):932–943. doi: 10.1056/NEJMoa1509852 [DOI] [PubMed] [Google Scholar]
- 163.Leclercq M, Le Besnerais M, Langlois V, et al. Tocilizumab for the treatment of birdshot uveitis that failed interferon alpha and anti-tumor necrosis factor-alpha therapy: two cases report and literature review. Clin Rheumatol. 2018;37(3):849–853. doi: 10.1007/s10067-018-4007-4 [DOI] [PubMed] [Google Scholar]
- 164.Hassall MM, Andrew NH. Single-eye trial of a topical carbonic anhydrase inhibitor versus intravitreal bevacizumab for the treatment of taxane drug-induced cystoid macula oedema. BMJ Case Rep. 2016;2016. doi: 10.1136/bcr-2015-212733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Kanakis M, Georgalas I, Makatsoris T, Pharmakakis N. Taxane induced cystoid macular edema: case report and integrated pathogenic theory. Curr Drug Saf. 2019;14(1):43–47. doi: 10.2174/1574886313666180828163016 [DOI] [PubMed] [Google Scholar]
- 166.Matsuoka N, Hasebe H, Mayama T, Fukuchi T. Sub-tenon injections of triamcinolone acetonide had limited effect on cystoid macular edema secondary to nanoparticle albumin-bound-paclitaxel (Abraxane). Case Rep Ophthalmol Med. 2015;2015:181269. doi: 10.1155/2015/181269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Turaka K, Bryan JS. Does fingolimod in multiple sclerosis patients cause macular edema? J Neurol. 2012;259(2):386–388. doi: 10.1007/s00415-011-6367-4 [DOI] [PubMed] [Google Scholar]
- 168.Jain N, Bhatti MT. Fingolimod-associated macular edema: incidence, detection, and management. Neurology. 2012;78(9):672–680. doi: 10.1212/WNL.0b013e318248deea [DOI] [PubMed] [Google Scholar]
- 169.Chui J, Herkes GK, Chang A. Management of fingolimod-associated macular edema. JAMA Ophthalmol. 2013;131(5):694–696. doi: 10.1001/jamaophthalmol.2013.47 [DOI] [PubMed] [Google Scholar]
- 170.Minuk A, Belliveau MJ, Almeida DR, Dorrepaal SJ, Gale JS. Fingolimod-associated macular edema: resolution by sub-tenon injection of triamcinolone with continued fingolimod use. JAMA Ophthalmol. 2013;131(6):802–804. doi: 10.1001/jamaophthalmol.2013.2465 [DOI] [PubMed] [Google Scholar]
- 171.Albaroudi N, Tijani M, Boutimzine N, Cherkaoui O, Laghmari M. [Prognostic factors in uveitis]. J Fr Ophtalmol. 2017;40(9):751–757. doi: 10.1016/j.jfo.2017.04.005 [DOI] [PubMed] [Google Scholar]
- 172.Takeuchi M, Kanda T, Kaburaki T, et al. Real-world evidence of treatment for relapse of noninfectious uveitis in tertiary centers in Japan: a multicenter study. Medicine (Baltimore). 2019;98(9):e14668. doi: 10.1097/MD.0000000000014668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Matas J, Llorenc V, Fonollosa A, et al. Predictors for functional and anatomic outcomes in macular edema secondary to non-infectious uveitis. PLoS One. 2019;14(1):e0210799. doi: 10.1371/journal.pone.0210799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Eiger-Moscovich M, Tomkins-Netzer O, Amer R, et al. Visual and clinical outcome of macular edema complicating pediatric noninfectious uveitis. Am J Ophthalmol. 2019;202:72–78. doi: 10.1016/j.ajo.2019.02.011 [DOI] [PubMed] [Google Scholar]
- 175.Garweg JG. Macular edema in childhood uveitis. Klin Monbl Augenheilkd. 2018;235(4):373–376. doi: 10.1055/s-0043-123650 [DOI] [PubMed] [Google Scholar]
- 176.Leinonen S, Immonen I, Kotaniemi K. Fluocinolone acetonide intravitreal implant (Retisert((R))) in the treatment of sight threatening macular oedema of juvenile idiopathic arthritis-related uveitis. Acta Ophthalmol. 2018;96(6):648–651. doi: 10.1111/aos.13744 [DOI] [PubMed] [Google Scholar]
- 177.Kaleemunnisha S, Sudharshan S, Biswas J. Quality of life in non-infectious uveitis patients on immunosuppressive therapy. Middle East Afr J Ophthalmol. 2014;21(3):225–231. doi: 10.4103/0974-9233.134675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Durrani OM, Tehrani NN, Marr JE, Moradi P, Stavrou P, Murray PI. Degree, duration, and causes of visual loss in uveitis. Br J Ophthalmol. 2004;88(9):1159–1162. doi: 10.1136/bjo.2003.037226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Taylor SR, Lightman SL, Sugar EA, et al. The impact of macular edema on visual function in intermediate, posterior, and panuveitis. Ocul Immunol Inflamm. 2012;20(3):171–181. doi: 10.3109/09273948.2012.658467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Multicenter Uveitis Steroid Treatment Trial Follow-up Study Research G. Quality of life and risks associated with systemic anti-inflammatory therapy versus fluocinolone acetonide intraocular implant for intermediate uveitis, posterior uveitis, or panuveitis: fifty-four-month results of the multicenter uveitis steroid treatment trial and follow-up study. Ophthalmology. 2015;122(10):1976–1986. doi: 10.1016/j.ophtha.2015.06.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Baughman RP, Barney JB, O’Hare L, Lower EE. A retrospective pilot study examining the use of Acthar gel in sarcoidosis patients. Respir Med. 2016;110:66–72. doi: 10.1016/j.rmed.2015.11.007 [DOI] [PubMed] [Google Scholar]
- 182.Taylor PC, Abdul Azeez M, Kiriakidis S. Filgotinib for the treatment of rheumatoid arthritis. Expert Opin Investig Drugs. 2017;26(10):1181–1187. doi: 10.1080/13543784.2017.1372422 [DOI] [PubMed] [Google Scholar]
- 183.Koronis S, Stavrakas P, Balidis M, Kozeis N, Tranos PG. Update in treatment of uveitic macular edema. Drug Des Devel Ther. 2019;13:667–680. doi: 10.2147/DDDT.S166092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Feagan BG, Sandborn WJ, Gasink C, et al. Ustekinumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med. 2016;375(20):1946–1960. doi: 10.1056/NEJMoa1602773 [DOI] [PubMed] [Google Scholar]
- 185.Lopez-Ferrer A, Laiz A, Puig L. The safety of ustekinumab for the treatment of psoriatic arthritis. Expert Opin Drug Saf. 2017;16(6):733–742. doi: 10.1080/14740338.2017.1323864 [DOI] [PubMed] [Google Scholar]