Abstract
Recently we showed that nebulized ciprofloxacin and phage PEV20 in combination had a synergistic bactericidal effect against antibiotic-resistant Pseudomonas aeruginosa isolates from patients with cystic fibrosis. Compared to nebulization, dry powders for inhalation may improve patient handling characteristics and compliance. In the present study, we co-spray dried ciprofloxacin and phage PEV20 using L-leucine with or without lactose as excipients. Two formulations were identified for testing in this study. The mass ratios were set at 1:1:1 for ciprofloxacin, lactose and L-leucine (Formulation A) or 2:1 for ciprofloxacin and L-leucine without lactose (Formulation B). Concentrations of PEV20 were set at 108 and 109 PFU/mL for two clinical P. aeruginosa strains FADD1-PA001 and JIP865, respectively. Formulations A and B were characterized as partially crystalline and the powders recrystallized at >40% relative humidity (RH). Both formulations exhibited strong synergistic antimicrobial killing effect on the two strains. Formulations A and B maintained bactericidal synergy after dispersion using both low and high resistance Osmohaler™. Powder aerosol performance was examined by next generation impactor (NGI) in low resistance inhaler at 100 L/min and by multi-stage liquid impinger (MSLI) in high resistance inhaler at 60 L/min. Fine particle fractions (FPF) obtained by NGI were 64.3 ± 2.9% and 59.7 ± 2.1% for A and B, respectively. FPF obtained by MSLI were 71.0 ± 3.4% and 73.3 ± 5.0%, respectively. In conclusion, it is feasible to prepare stable and inhalable combination powder formulations of phage PEV20 and ciprofloxacin for potential treatment of respiratory infections caused by multi-drug resistant (MDR) P. aeruginosa.
Keywords: Ciprofloxacin, bacteriophages, Pseudomonas aeruginosa, Respiratory infection, Spray drying, Cystic fibrosis, Inhalation, L-leucine, Powder formulation
Graphical Abstract
Introduction
Pseudomonas aeruginosa was ranked on the World Health Organization priority list as one of the critical antibiotic-resistant pathogens [1]. This pathogen is a major contributor to morbidity and mortality in patients with cystic fibrosis (CF), chronic obstructive pulmonary disease, non-CF bronchiectasis and sepsis [2–4]. Respiratory infections are difficult to treat because microbes may reside deep in the airways. Repositioning antibiotics for inhalation with higher local drug levels in the lungs could treat refractory pulmonary infections more effectively than oral or parenteral administrations [3].
Ciprofloxacin is a concentration-dependent fluoroquinolone antibiotic, which has been formulated into inhalable liposome [5], metal-complex [6], nanoplex [7] and other dry powders [8, 9] for treating P. aeruginosa respiratory infections. However, P. aeruginosa rapidly acquires antibiotic-resistance to fluoroquinolones and these “superbugs” can readily survive under monotherapy [10, 11]. To prevent emergence of drug resistance, some concomitant formulation of inhaled antimicrobials have been studied [12–15]. Ciprofloxacin was also previously co-spray dried with doxycycline hydrochloride, but no synergy was observed against P. aeruginosa [14].
As an alternative or adjunct to conventional antibiotics, bacteriophage (or phage) therapy has been clinically used to treat multi-drug resistant (MDR) respiratory infections [16–19]. In a recent study, we demonstrated synergistic antimicrobial effect of ciprofloxacin and phage PEV20 combination formulation against MDR P. aeruginosa CF isolates [20]. The synergy was retained after aerosolization using commercial nebulizers. Dry powder formulations provide easy storage, transport, long shelf-life and potentially better patient compliance over liquid formulations [21]. Previously, ciprofloxacin and phage PEV20 were individually formulated into dry powders by spray drying [9, 21]. Spray drying technology is widely used for producing fine particle powders suitable for inhalation delivery using dry powder inhalers (DPIs) [22]. This one-step continuous process is especially suitable for biological and heat-sensitive materials such as phages [23]. In addition, aerosol performance, physic-chemical stability and drug release profiles can be modified using this technology [17].
L-leucine is a dispersion enhancer that can increase the fine particle fraction (FPF) of spray dried ciprofloxacin [24]. Furthermore, enrichment of L-leucine on the particle surface can prevent water absorption, thereby protecting the particles against moisture-induced deterioration of in vitro aerosolization performance [25]. Water is known to act as a plasticizer and can cause recrystallization of amorphous powders [24]. Powder recrystallization could potentially increase phage mobility, causing conformational changes and deactivation [21]. Sugars, including lactose, mannitol, and trehalose, have been studied as phage stabilizers against heat and shear stresses during spray drying [26, 27]. For phage PEV20, lactose in combination with L-leucine showed superior protection over trehalose [21]. Hence, lactose and L-leucine were chosen as excipients for producing powder formulations of ciprofloxacin and PEV20.
To the best of our knowledge, inhaled formulations of phage and antibiotic combination in the form of dry powder has not been explored. The aim of this study was to produce synergistic and inhalable dry powder formulations containing phage PEV20 and ciprofloxacin.
Materials and methods
Bacterial strains
Clinical P. aeruginosa strains (FADD1-PA001 and JIP865) were freshly subcultured from −80 °C stock prior to the experiment. Strain FADD1-PA001 was received from Monash University, Australia and JIP865 from Westmead Hospital, Australia. The two strains were selected based on the antibiotic and phage efficacy screening conducted previously [20].
Phage PEV20 and ciprofloxacin
Phage PEV20 was provided by AmpliPhi Biosciences AU at a titre of 1010 plaque forming unit (PFU)/mL in phosphate buffered saline (PBS, 0.01 M phosphate buffer, 0.0027 M KCl and 0.137 M NaCl) [28]. This phage was formerly isolated from the sewage treatment plant in Olympia (WA, USA) by Kutter lab (Evergreen phage lab). Ciprofloxacin hydrochloride was purchased from Sigma-Aldrich Inc.
Spray drying
A Buchi spray dryer (B-290, Buchi Labortechnik AG, Flawil, Switzerland) was employed to prepare powder formulations of ciprofloxacin and phage PEV20 in combination. Liquid feed for the formulations was made up of 18 mL of ciprofloxacin with excipients in ultra-pure water and 2 mL of PEV20 suspension (109 PFU/mL for strain FADD1-PA001 or 1010 PFU/mL for strain JIP865) in PBS. The solution (18 mL, without phage) of formulation A contained 8 mg/mL of ciprofloxacin, 8 mg/mL of lactose and 8 mg/mL of L-leucine, and formulation B contained 16 mg/mL of ciprofloxacin and 8 mg/mL of L-leucine. Two additional formulations C and D were spray dried to study the crystallinity of combination powder formulations using X-ray diffraction (XRD). Feed solutions (without phage) of formulation C contained 12 mg/mL of ciprofloxacin and 12 mg/mL of L-leucine, and formulation D contained 8 mg/mL of ciprofloxacin and 16 mg/mL of L-leucine (Table 1). A conventional two fluid nozzle with diameter 0.7mm was used for atomization. The suspension was fed at a constant feed rate of 1.8 mL/min and an atomizing airflow of 742 L/h with an aspiration rate of 35 m3/h. The drying inlet air was heated to 60 °C and the outlet temperature range was between 41 and 42 °C. Dried powders were collected in a vial after passing through the cyclone. Powders were stored in a desiccator with silica beads at room temperature.
Table 1.
Mass concentration of each excipient in 18 mL water
| Concentration (mg/mL) | |||
|---|---|---|---|
| Formulation | Ciprofloxacin | Lactose | L-leucine |
| A | 8 | 8 | 8 |
| B | 16 | 8 | |
| C | 12 | 12 | |
| D | 8 | 16 | |
Aerosol performance and unit dose dispersion
Formulation A (24mg) or formulation B (12mg) loaded in size 3 hydroxypropyl methylcellulose capsules were dispersed in low and high resistance Osmohaler™ to evaluate aerosol performance. Next Generation Impactor (NGI; Copley, Nottingham UK) with a mouthpiece adapter and a NGI induction port (Copley, Nottingham, UK) was operated for low resistance Osmohaler™ at a flow rate of 100 L/min for 2.4 s, while Multi-stage liquid impinger (MSLI; Copley, Nottingham UK) was operated for high resistance Osmohaler™ at a flow rate of 60 L/min for 4 s. Pressure drop across both devices was 4 kPa. The cut-off diameters of the NGI stages 1–7 at 100 L/min were 6.12, 3.42, 2.18, 1.31, 0.72, 0.40 and 0.24 μm, respectively. In stage 8, a Micro-Orifice Collector was used. Silicon grease was used to coat the impactor stages. Particles deposited on the capsule, inhaler, adapter, induction port, stages 1–7 and MOC were dissolved using 10 mL Milli-Q water. The cut-off diameters of the MSLI stages 1–4 at 60 L/min were 13, 6.8, 3.1 and 1.7 μm, respectively. For each stage, 20 mL Milli-Q water was used to dissolve particles. Concentrations of ciprofloxacin were determined using a high performance liquid chromatography (HPLC) system (Model LC-20, Shimazhu, Kyoto, Japan). A Phenosphere-Next C-18 column (5 mm, 4.6×150 mm, Phenomenex, USA) was used at 35 °C. Mobile phase was a mixture of 0.5% triethylamine in water (pH 3.0) and 100% methanol (78: 22 v/v) and isocratic elution was performed at a flow rate of 0.9 mL/min. Ciprofloxacin was assayed at a wavelength of 277 nm. The NGI or MSLI test was conducted in triplicate for each formulation. The cumulative mass of ciprofloxacin at each cut off diameter was calculated and the fine particle dose (FPD) was determined as the cumulative mass of particles ≤ 5 μm. FPF was the percentile of FPD of the total recovered dose. The same dispersion parameters for low and high resistance inhalers were used for unit dose test. Dispersed powders were captured by a 47 mm diameter sample collection tube equipped with a Suregard® filter. The dry powder impacted on the filter paper was collected for bacterial killing effect study. Study was carried out in triplicate.
Time-kill curve assay
Antibacterial activity of the spray dried powder formulations was assessed using the time-kill curve method [29]. A single bacterial colony was inoculated in 20 mL of nutrient broth (NB) for 18 h at 37°C with continuous shaking (150 revolutions per minute, rpm). Overnight culture (10 mL) was mixed with 20 mL of fresh NB and further incubated for 2 h until early-log phase was reached. Concentration of the early-log culture was adjusted to 106 colony-forming units (CFU)/mL. Formulations A and B were reconstituted in PBS to give a final ciprofloxacin concentration of 80 μg/mL against strains FADD1-PA001 and 40 μg/mL against JIP865. Bacterial culture (200 μL) was added to a 96-well plate and then treated with either ciprofloxacin (80 μg/mL or 40 μg/mL) or PEV20 (106 or 109 PFU/mL) alone, or with the reconstituted powder formulations, followed by incubation at 37 °C with continuous shaking. Optical density at 600 nm (OD600) was measured at 24 h using a microplate reader (FLUOstar Optima, BMG Labtechnologies, Offenburg, Germany). The assay was repeated five times.
Particle size distribution
Particle size distributions of the spray dried formulations were analyzed by laser diffraction (Malvern Mastersizer 2000, Malvern Instruments Ltd., UK). Powder samples were dispersed via a Scirocco 2000 dry powder module (Malvern Instruments, UK) with air pressure at 4.0 bar. Refractive indices for formulations A and B were 1.54 and 1.46, respectively. Volumetric diameter (D10, D50, and D90) and span (the difference between D10 and D90 divided by D50) values were reported based on triplicate measurements.
Scanning electron microscopy (SEM)
Scanning electron microscopy (SEM; NeoScope Benchtop JCM-6000, JEOL USA, Inc.) was employed to examine particle morphology of the spray dried powders. Samples were spread on a carbon sticky tape and then mounted on an SEM stub, followed by sputter coating with gold (30 nm thick) using a K550 sputter coater (Quorum Emitech, Kent, UK). The images were captured at 15 kV.
X-ray powder diffraction (XRD)
Powder crystallinity was evaluated by X-ray powder diffraction XRD (Shimadzu XRD-6000, Shimadzu Corporation, Kyoto, Japan). Cu Ka radiation was used at a voltage of 40 kV and a current of 30 mA. Data were recorded from 5° to 45° 20 using a scan speed of 2° per min and a step size of 0.02°.
Differential scanning calorimetry (DSC)
DSC studies were carried out using a Mettler Toledo DSC 1 model with the STARe System (Zurich, Switzerland). Powder formulations A and B (10 mg) were assayed using aluminium pans with a pinhole lid under nitrogen atmosphere (100 mL/min). DSC thermograms were recorded over a temperature range of 25 – 400 °C at a constant rate of 10 °C/min.
Thermogravimetric analysis (TGA)
TGA studies were performed using a Mettler Toledo TGA/DSC 1 model with the STARe System (Zurich, Switzerland). Powder samples (5 – 8 mg) were assayed in a ceramic crucible under nitrogen atmosphere (100 mL/min). Sample weight loss was recorded over a temperature range of 22 – 150 °C at a constant rate of 10 °C/min.
Dynamic vapour sorption (DVS)
Moisture sorption isotherms of powder samples (10 – 20 mg) were assayed using DVS instrument (Surface Management Systems, London, United Kingdom). The chamber was kept at 25 °C with continuous nitrogen flow. Each sample was exposed to one absorption and desorption cycle of 0 – 90% - 0 relative humidity (RH), with 10% RH increments and decrements. The criterion for reaching equilibrium moisture content at each RH step was set at a weight change of ≤ 0.002% per minute.
Light microscopy
Polarized light microscopy was performed on an Olympus CH30/CH40 biological microscope in transmission mode to confirm recrystallization of powder formulation B after the DVS run. Samples were checked before and after conditioning in DVS up to 60% RH. Sprinkled powders immersed in oil were prepared on glass slides. Images were obtained with a digital camera.
Transmission electron microscopy (TEM)
TEM was performed to study the morphology of PEV20 before and after spray drying. Around 150 mg of combination dry powder formulation was dissolved in 150 mL of PBS using a vortex. A Nanosep centrifugal device with a 10 kDa filter membrane [30] was used for phage enrichment. A final volume of 0.5 mL was obtained. Samples were prepared on carbon copper grids 200 mesh (GSCu200C-50) supplied by ProSciTech Pty Ltd. A drop of 0.1% poly-L-lysine solution was placed on the grid for 1 min and then blotted with a filter paper. A drop of phage sample was loaded onto the grid for 30 min and any excess sample was blotted. The samples were then negatively stained with 1% phosphotungstic acid (pH adjusted to 7.4 with 0.1 M sodium hydroxide) [31] for 20 seconds. Excess stain was blotted and the grids were left to air-dry. A JEOL-JEM 1400 microscope (JEOL, Japan) was used to collect images at ×40 k magnification.
Statistical analysis
Bacterial survival rate at 24 h was calculated by dividing the OD600 value of the treated group by that of the negative control group. The additive survival rate was calculated by multiplying the bacterial survival rates of the phage-treated group with that of antibiotic-treated group. Phage-antibiotic synergy was confirmed if the observed bacterial survival rate of phage-antibiotic combinations was statistically lower than the calculated additive survival rate [32]. Student’s t-test was used to compare the calculated with observed bacterial survival rate for the combination powder. P-value of < 0.05 was considered statistically significant.
Results and Discussion
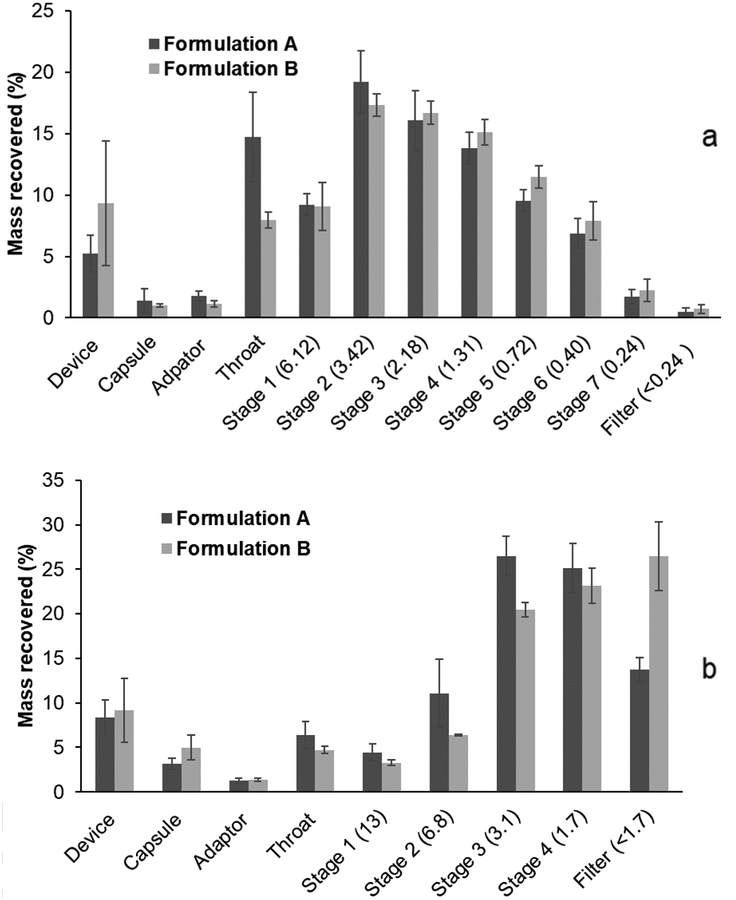
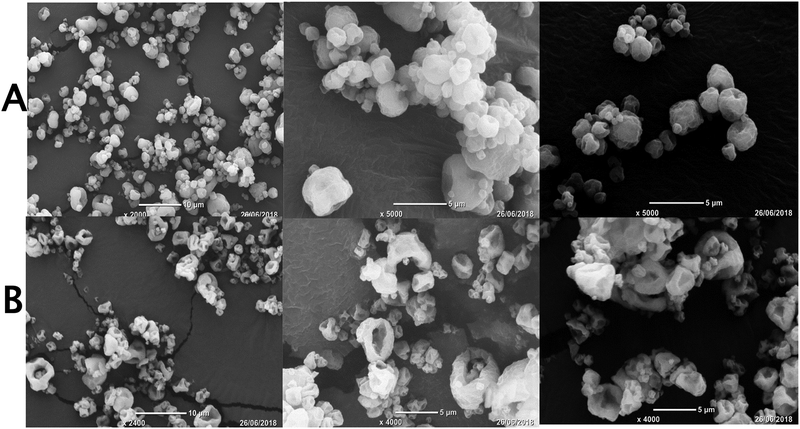
Taking into consideration the possible thermal stress to phage PEV20 during spray drying, the powder formulations were produced at a relatively low inlet temperature of 60 °C in the present study. The production yields of formulations A and B were 66.9% and 73.8%, respectively, indicating the process efficiency at the chosen inlet temperature. Both formulations had a recovery of over 90% (Table 2) from the NGI and MSLI. The FPF values were 59.7 ± 2.1% and 64.3 ± 2.9% for formulations A and B, respectively, obtained from the NGI, and 71.0 ± 3.4% and 73.3 ± 5.0%, respectively, from the MSLI (Fig. 1). The results support the use of either inhaler in generation of a reasonably high FPF, which was likely due to high powder dispersibility of both formulations. In general, a high resistance device with low air flow is expected to reduce oropharyngeal deposition hence minimising variation in the lung dose[33, 34]. However, oropharyngeal deposition does not necessarily improve with increases in device resistance as the air flow field exiting the inhaler mouthpiece will also play an important role[35, 36]. In this study, since the two devices have the same construction except the air inlet dimensions controlling the resistance, the confounding factor due to the mouth-piece does not exist. Therefore, the use of the high-resistance Osmohaler™ is preferred due to the higher FPF and reduced likelihood of oropharyngeal deposition. In addition, the powder dispersion was also measured on the MSLI where particle bounce effect would be minimal, and the FPF results (71–73 % vs 60–64 % in NGI) showed that the NGI dispersion data were not artefacts due to particle bounce. Median particle size values (D50) for both formulations were about 2.5 μm (Table 3), which is considered to be suitable for respiratory delivery [9]. SEM showed formulation A forming nearly spherical and hollow particles with dimpled surfaces (Fig. 2). The particle morphology of formulation B was hollower and more corrugated, and this may have contributed to slightly higher FPF.
Table 2.
FPF of formulations A and B dispersed in NGI at 100 L/min for 2.4 s or MSLI at 60 L/min for 4 s, respectively using the low or high resistance Osmohaler™. Data presented as mean ± standard deviation (n=3).
| NGI | MSLI | |||
|---|---|---|---|---|
| A | B | A | B | |
| Nominala (mg) | 8.1 ± 0.5 | 10.0 ± 0.3 | 7.7 ± 1.2 | 8.8 ± 0.8 |
| Recoveredb (mg) | 7.6 ± 0.1 | 9.0 ± 0.6 | 7.5 ± 1.0 | 8.3 ± 0.4 |
| Recovery % | 94.5 ± 4.4 | 90.2 ± 7.0 | 97.4 ± 4.1 | 94.4 ± 4.4 |
| FPD (mg) | 4.5 ± 0.2 | 5.8 ± 0.4 | 5.3 ± 0.5 | 6.1 ± 0.6 |
| FPF% (Nominal) | 56.5 ± 4.5 | 58.0 ± 3.7 | 69.3 ± 5.5 | 68.3 ± 3.8 |
| FPF% (Recovered) | 59.7 ± 2.1 | 64.3 ± 2.9 | 71.0 ± 3.4 | 73.3 ± 5.0 |
| Emittedc (mg) | 7.1 ± 0.2 | 8.1 ± 0.6 | 6.6 ± 0.9 | 7.1 ± 0.6 |
| FPF% (Emitted) | 64.0 ± 3.2 | 71.5 ± 1.4 | 80.3 ± 4.3 | 85.4 ± 1.1 |
Nominal: Ciprofloxacin mass content in powder loaded in the capsule
Recovered: Ciprofloxacin mass recovered from capsule, inhaler, adaptor and NGI or MSLI
Emitted: Ciprofloxacin mass exited the capsule and inhaler
Fig. 1.
The NGI (a) and MSLI (b) deposition profiles of formulations A and B (n=3). The aerodynamic cut-off diameter (μm) of each stage is quoted in parentheses. Powders were dispersed in NGI at 100 L/min for 2.4 s using low resistance Osmohaler™ and in MSLI at 60 L/min for 4 s using high resistance Osmohaler™.
Table 3.
Primary particle size distribution of formulations A and B. Data presented as mean ± standard deviation (n=3).
| Formulation | D10 (μm) | D50 (μm) | D90 (μm) | Span |
|---|---|---|---|---|
| A | 1.0 ± 0.1 | 2.5 ± 0.2 | 5.1 ± 0.3 | 1.6 ± 0.0 |
| B | 1.2 ± 0.1 | 2.4 ± 0.0 | 5.1 ± 0.6 | 1.6 ± 0.3 |
Fig. 2.
SEM images of formulations A and B.
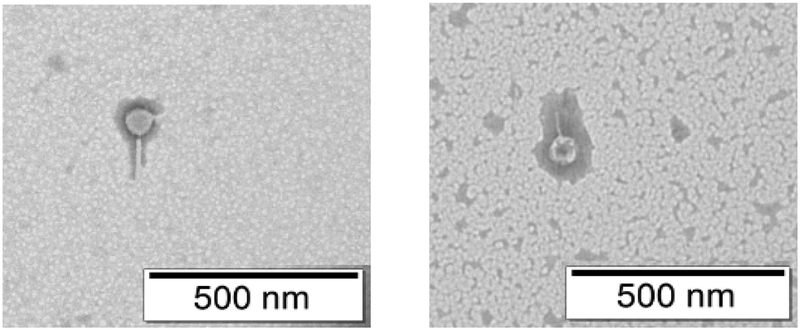
Intact phage particles were observed in TEM after reconstituting the spray dried powder in PBS (Fig. 3), supporting the in vitro release of phage particle in a neutral pH environment. However, powder dissolution profile in the airways and alveolar space are complex. Hence, further in vivo evaluation is necessary to study the release profile of PEV20 and ciprofloxacin in combination powder formulations in lung epithelial lining fluid.
Fig. 3.
TEM images of phage PEV20 before and after spray drying.
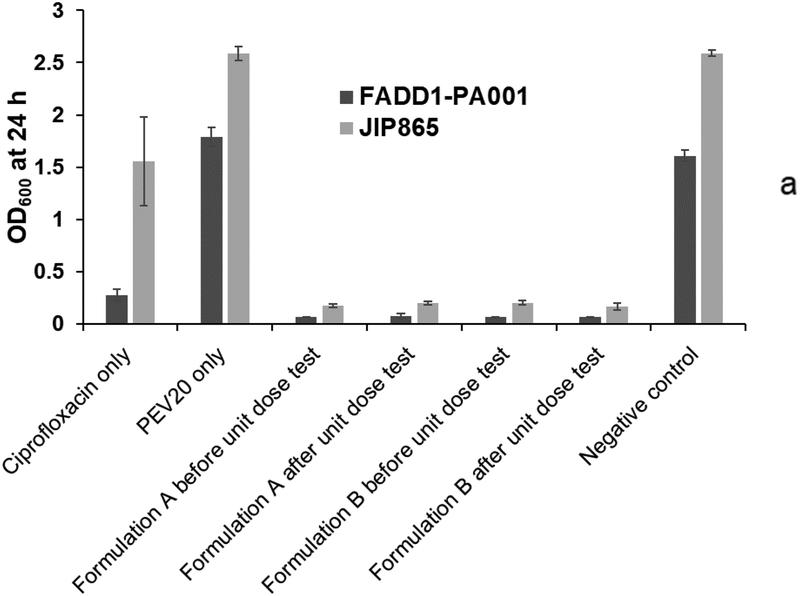
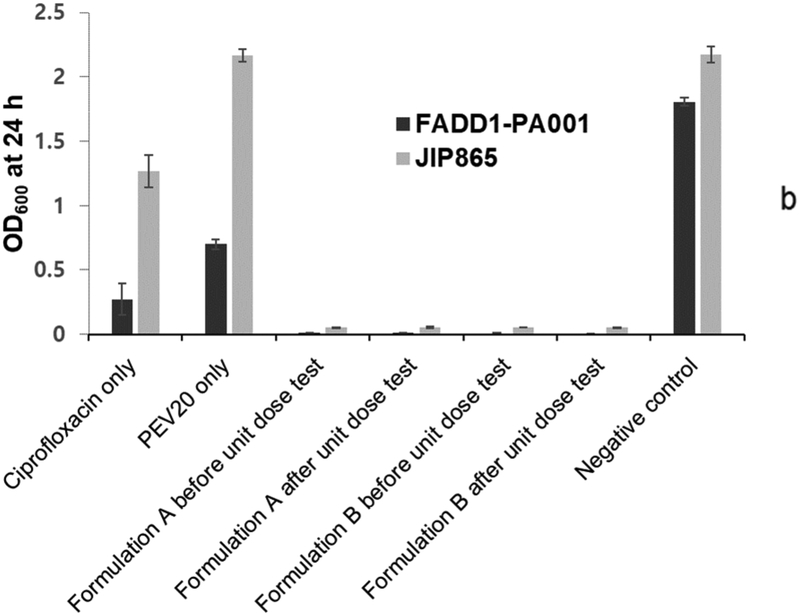
The observed bacterial survival rates for formulations A and B before and after powder dispersion were statistically lower (p < 0.05) (Table 4) than the calculated values from the individual treatment with PEV20 or ciprofloxacin (Fig 4). In our previous study, nebulized phage PEV20 with sub-inhibitory ciprofloxacin concentration was shown to produce synergy in vitro against two clinical MDR P. aeruginosa strains FADD1-PA001 and JIP865 [20]. To induce synergy, it is crucial to understand the minimum concentrations required between selected antimicrobials. Co-spray drying PEV20 with ciprofloxacin would allow the intended ratio, so that ciprofloxacin concentrations on infection site are known to be effective together with phage after lung delivery. The in vitro synergistic antimicrobial effect of our two inhaled powder formulations was comparable to the effect observed in our previous study with the nebulized aerosols. The powder formulations suppressed bacterial regrowth even after 24 h, whereas significant regrowth was observed in groups treated with either ciprofloxacin or PEV20.
Table 4.
Calculated and observed bacterial survival rate of P. aeruginosa strains FADD1-PA001 or JIP 865 of co-spray dried PEV20-ciprofloxacin formulation A and B before and after unit dose test using low and high resistance Osmohaler™ (n=6).
| Low resistance | High resistance | |||||
|---|---|---|---|---|---|---|
| Strains | Formulations | Unit dose test | Calculated | Observed | Calculated | Observed |
| FADD1-PA001 | 0.2±0.06 | 0.06±0.03 | ||||
| A | Before | 0.04±0.001* | 0.005±0.0008* | |||
| After | 0.05±0.002* | 0.006±0.0003* | ||||
| B | Before | 0.04±0.002* | 0.004±0.0022* | |||
| After | 0.04±0.002* | 0.0004±0.0002* | ||||
| JIP 865 | 0.6±0.2 | 0.6±0.08 | ||||
| A | Before | 0.07±0.006* | 0.02±0.002* | |||
| After | 0.08±0.007* | 0.02±0.005* | ||||
| B | Before | 0.08±0.009* | 0.02±0.001* | |||
| After | 0.07±0.01* | 0.02±0.002* | ||||
Statistically significant (p<0.05) according to Student’s t-test.
Fig. 4.
Antibacterial activities of ciprofloxacin (80 μg/mL for strain FADD1-PA001 and 40 μg/mL for JIP865), PEV20 (105 PFU/mL for strain FADD1-PA001 and 106 PFU/mL for JIP865), and spray dried combinations before and after unit dose test using low (a) and high (b) resistance Osmohaler™ (n=6).
During the powder dispersion, phages may lose activity due to shear and impaction forces [27]. Inhalers with low and high resistance cause different impaction on powders (and phages) during dispersion. Our results showed that in vitro bactericidal synergy of the powder formulations remained intact when dispersed by both low and high resistance Osmohaler™. This indicates the suitability of this low cost, passive inhalation device for the present combination powder delivery. It is unknown whether the phage and/or the synergy remains using other inhaler devices such as ciprofloxacin DPI device T-326[37, 38] having different shear forces generated with different fluid dynamic design. Formulation of high-dose antimicrobials for DPI delivery is challenging due to bulk volume of dose, the filled weight in the capsules, and the number of capsules and inhalations required by the patients. In this study, 8 mg of ciprofloxacin was contained in 24 mg and 12 mg of formulations A and B per capsule, respectively. Our formulation design was based on considerations from clinical trials of inhaled ciprofloxacin. Bronchiectasis patients were given 32.5 mg of inhaled ciprofloxacin twice daily for treatment of respiratory infections[9, 37]. The Phase I study predicted that 7.2 mg ciprofloxacin will be deposited in the trachea/bronchi and 5.6 mg in the alveolar space when 32.5 mg was administered. In the Phase II study, a trend for improvement was demonstrated with this dose, but this was not statistically significant compared with the placebo. In our previous study [20], phage PEV20 in combination with ciprofloxacin at 1/4 MIC and 1/2 MIC showed synergy on strain FADD1-PA001 and JIP865, respectively. Therefore, a single dose of inhaled ciprofloxacin in the present study was proportionally reduced to roughly 8 and 16 mg (i.e., 25 or 50% of the 32.5 mg 2 doses) in our powder formulations.
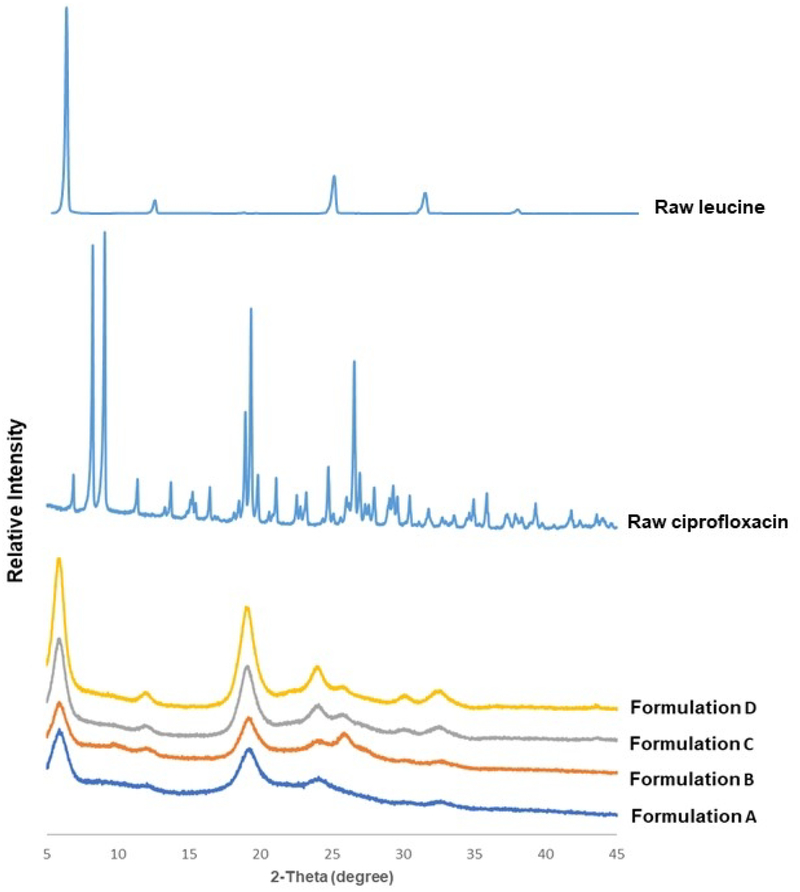
Formulations A and B were both partially crystalline, exhibiting diffraction peaks at similar positions around 6°, 19°, 24°, 26°, 31° and 33° (Fig. 5). To identify the origin of these peaks, we prepared two additional formulations (C and D) which contained less ciprofloxacin and more leucine. The first three peaks and those at 31° and 33° are readily attributed to L-leucine as the peak intensity scaled with the leucine concentration (Fig. 5). These peaks are also consistent with those reported for co-spray dried formulations of leucine and ciprofloxacin, in which ciprofloxacin was identified as amorphous [24]. In contrast, the peak at 26° was ascribed to ciprofloxacin in formulation B as the peak became shaper with increasing ciprofloxacin concentration. This discrepancy was likely due to use of different inlet temperature (120 °C) with different mass ratio of ciprofloxacin and leucine (9:1) and/or total solute concentration (16 mg/mL) of feed solution than the literature. Higher drying temperature and lower feed concentration may affect the time to supersaturation and supersaturation level, nucleation and subsequent crystal growth and packing, which could lead to the change of polymorphic form of ciprofloxacin.
Fig. 5.
X-ray powder diffraction patterns of formulations A, B, C, D, raw leucine and ciprofloxacin
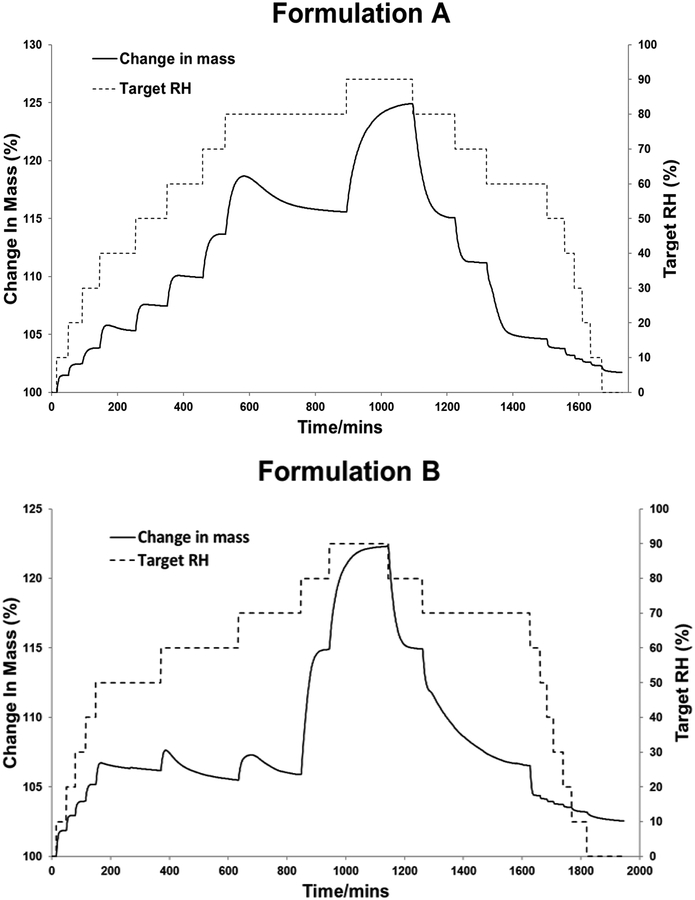
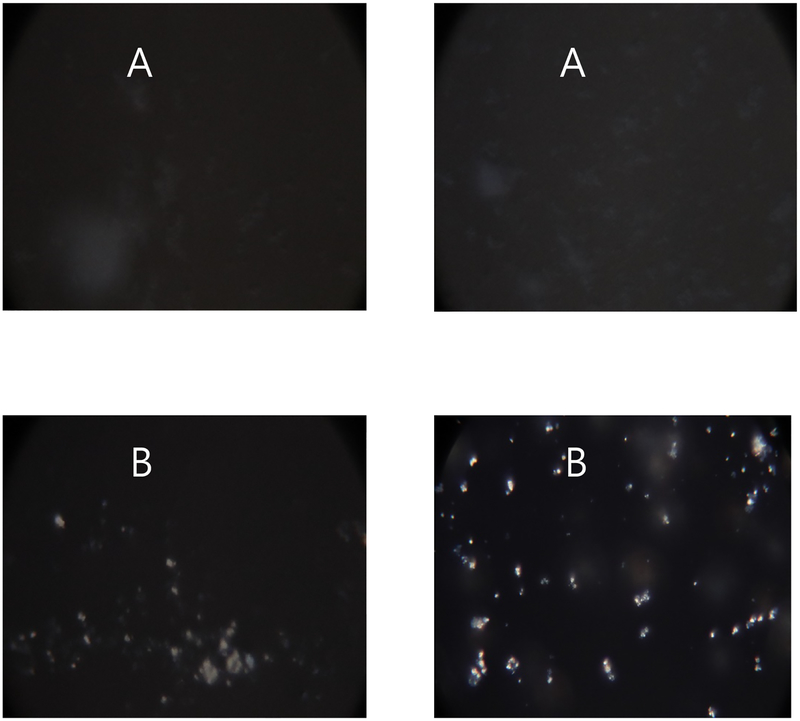
Both formulations A and B showed a sorption profile with the characteristic of a recrystallization event. Powders initially absorbed moisture with increase in mass. Then between 40 – 80% RH, formulation A recrystallized, which caused decrease in mass as water was released from the crystal lattice (Fig. 6). Formulation B recrystallized at 50, 60 and 70% RH. Moisture-induced recrystallization was confirmed by polarized light microscopy showing increased transmitted light (optical birefringence) (Fig. 7). Our data are consistent with previous studies reporting recrystallization of spray dried ciprofloxacin alone [39] or with leucine [24] at ≥55% RH. Hence, spray dried formulations A and B, being partially amorphous, should be handled and stored at low RH [40].
Fig. 6.
Moisture sorption kinetic profiles of formulations A and B.
Fig. 7.
Polarized light microscope images of formulation A and B before and after exposure to moisture from 0–60% RH
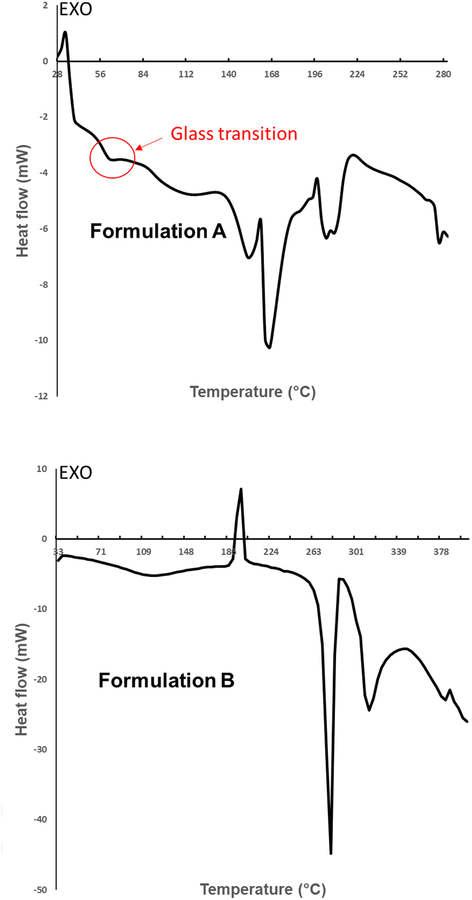
Besides humidity, storage temperature could also cause recrystallization if the temperature is higher than the glass transition temperature (Tg) of the powder formulation [41]. Formulation A showed a Tg at 54°C (Fig. 8), which was comparable to the value of 63°C for a PEV20 powder formulation containing 70% lactose and 30% leucine [42]. The difference was possibly caused by the decreased lactose content (33%) in formulation A. No Tg was observed for formulation B. The exothermic peak at 198 °C represents crystallization of amorphous ciprofloxacin, and this is in agreement with a previous study [39]. The moisture contents measured by TGA were 2.0 ± 0.4% and 2.7 ± 1.0% for formulations A and B, respectively.
Fig. 8.
DSC thermograms of formulations A and B.
Previous results showed that the effect of temperature and humidity on phage stability in powders are both phage- and formulation-dependent. Trehalose and L-leucine were able to maintain the viability of phage PEV2 with no significant titre reduction when stored at 4°C under vacuum for a year. However, at 20°C the formulation containing 40% leucine reduced 0.9 log10 pfu/mL after 1 year storage [43]. Lactose provide better phage PEV20 protection over trehalose in the spray drying process [21]. Spray dried PEV20 powder containing lactose and L-leucine remained stable for 1 year at 20°C when stored inside an aluminium pouch – heat-sealed at <15% RH [21]. Lactose with leucine also provided biological and physical stability for spray dried PEV phage powders at ambient temperature [42]. Although lactose is a reducing sugar and potentially react with proteins, our data to date showed lactose does not impact the biological activity of the phage. These findings may also be applicable to our current phage PEV20 and ciprofloxacin formulations. Hence, stability studies of the powder formulations at different storage conditions are warranted.
Conclusion
Two inhalable, co-spray dried phage PEV20 and ciprofloxacin combination dry powder formulations showed strong synergistic antimicrobial effect on two clinical MDR P. aeruginosa strains. Dispersion of the two formulations using a low-resistance Osmohaler™ (connected to an NGI operating at 100 L/min) resulted in an FPF of 60–64%, while a high-resistance Osmohaler™ produced FPF of 71–73% (MSLI at 60 L/min). The antimicrobial synergy was maintained after powder dispersion for both formulations using the two inhalers. Both formulations were characterized as partially crystalline and the powders recrystallized at >40% RH. Our work confirm the feasibility of preparing inhalable and biochemically stable combination formulations of phage PEV20 and ciprofloxacin for treatment of respiratory infection caused by MDR P. aeruginosa. The best combination powder formulations for different phages and antibiotics necessitate further investigations.
Acknowledgement
This study was supported by National Institute of Allergy and Infectious Disease of the National Institutes of Health under award number R33 AI121627 (H.-K.C and J.L). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allery and Infectious Diseases or the National Institute of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].WHO, Global priority list of antibiotic-resistant bacteria to guide research, discovery, and development of new antibiotics, 2017. https://www.who.int/medicines/publications/WHO-PPL-Short_Summary_25Feb-ET_NM_WHO.pdf?ua=1,(accessed on 24th Febraray 2019).
- [2].Parkins MD, Somayaji R, Waters VJ, Epidemiology, Biology, and Impact of Clonal Pseudomonas aeruginosa Infections in Cystic Fibrosis, Clin. Microbiol. Rev, 31 (2018) e00019–00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Zhou QT, Leung SSY, Tang P, Parumasivam T, Loh ZH, Chan H-K, Inhaled formulations and pulmonary drug delivery systems for respiratory infections, Adv. Drug Deliv. Rev, 85 (2015) 83–99. [DOI] [PubMed] [Google Scholar]
- [4].Britt NS, Ritchie DJ, Kollef MH, Burnham C-AD, Durkin MJ, Hampton NB, Micek ST, Importance of site of infection and antibiotic selection in the treatment of carbapenem-resistant Pseudomonas aeruginosa sepsis, Antimicrob. Agents Chemother, (2018) AAC. 02400–02417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Finlay W, Wong J, Regional lung deposition of nebulized liposome-encapsulated ciprofloxacin, Int. J. Pharm, 167 (1998) 121–127. [Google Scholar]
- [6].Lamy B, Tewes F, Serrano DR, Lamarche I, Gobin P, Couet W, Healy AM, Marchand S, New aerosol formulation to control ciprofloxacin pulmonary concentration, J. Control. Release, 271 (2018) 118–126. [DOI] [PubMed] [Google Scholar]
- [7].Tran T-T, Vidaillac C, Yu H, Yong VF, Roizman D, Chandrasekaran R, Lim AY, Low TB, Tan GL, Abisheganaden JA, A new therapeutic avenue for bronchiectasis: Dry powder inhaler of ciprofloxacin nanoplex exhibits superior ex vivo mucus permeability and antibacterial efficacy to its native ciprofloxacin counterpart, Int. J. Pharm, 547 (2018) 368–376. [DOI] [PubMed] [Google Scholar]
- [8].Adi H, Young PM, Chan H-K, Agus H, Traini D, Co-spray-dried mannitol–ciprofloxacin dry powder inhaler formulation for cystic fibrosis and chronic obstructive pulmonary disease, Eur. J. Pharm. Sci, 40 (2010) 239–247. [DOI] [PubMed] [Google Scholar]
- [9].McShane PJ, Weers JG, Tarara TE, Haynes A, Durbha P, Miller DP, Mundry T, Operschall E, Elborn JS, Ciprofloxacin Dry Powder for Inhalation (ciprofloxacin DPI): Technical design and features of an efficient drug–device combination, Pulm. Pharmacol. Ther, (2018). [DOI] [PubMed] [Google Scholar]
- [10].Cartlidge MK, Hill AT, Inhaled or nebulised ciprofloxacin for the maintenance treatment of bronchiectasis, Expert. Opin. Investig. Drugs, 26 (2017) 1091–1097. [DOI] [PubMed] [Google Scholar]
- [11].Zhou Q, Sun S-P, Chan JGY, Wang P, Barraud N, Rice SA, Wang J, Li J, Chan H-K, Novel inhaled combination powder containing amorphous colistin and crystalline rifapentine with enhanced antimicrobial activities against planktonic cells and biofilm of Pseudomonas aeruginosa for respiratory infections, Mol Pharm, 12 (2014) 2594–2603. [DOI] [PubMed] [Google Scholar]
- [12].MacLeod DL, Barker LM, Sutherland JL, Moss SC, Gurgel JL, Kenney TF, Burns JL, Baker WR, Antibacterial activities of a fosfomycin/tobramycin combination: a novel inhaled antibiotic for bronchiectasis, J. Antimicrob. Chemother, 64 (2009) 829–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Zhou QT, Gengenbach T, Denman JA, Heidi HY, Li J, Chan HK, Synergistic antibiotic combination powders of colistin and rifampicin provide high aerosolization efficiency and moisture protection, AAPS J, 16 (2014) 37–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Gupta PV, Nirwane AM, Belubbi T, Nagarsenker MS, Pulmonary delivery of synergistic combination of fluoroquinolone antibiotic complemented with proteolytic enzyme: A novel antimicrobial and antibiofilm strategy, Nanomedicine, 13 (2017) 2371–2384. [DOI] [PubMed] [Google Scholar]
- [15].Mangal S, Park H, Zeng L, Heidi HY, Lin Y.-w., Velkov T, Denman JA, Zemlyanov D, Li J, Zhou QT, Composite particle formulations of colistin and meropenem with improved in-vitro bacterial killing and aerosolization for inhalation, Int. J. Pharm, 548 (2018) 443–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Czaplewski L, Bax R, Clokie M, Dawson M, Fairhead H, Fischetti VA, Foster S, Gilmore BF, Hancock RE, Harper D, Alternatives to antibiotics—a pipeline portfolio review, Lancet. Infect. Dis, 16 (2016) 239–251. [DOI] [PubMed] [Google Scholar]
- [17].Chang RYK, Wallin M, Lin Y, Leung SSY, Wang H, Morales S, Chan H-K, Phage therapy for respiratory infections, Adv. Drug Deliv. Rev, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Kutter E, De Vos D, Gvasalia G, Alavidze Z, Gogokhia L, Kuhl S, Abedon ST, Phage therapy in clinical practice: treatment of human infections, Curr. Pharm. Biotechnol, 11 (2010) 69–86. [DOI] [PubMed] [Google Scholar]
- [19].Hoyle N, Zhvaniya P, Balarjishvili N, Bolkvadze D, Nadareishvili L, Nizharadze D, Wittmann J, Rohde C, Kutateladze M, Phage therapy against Achromobacter xylosoxidans lung infection in a patient with cystic fibrosis: a case report, Res. Microbiol, (2018). [DOI] [PubMed] [Google Scholar]
- [20].Lin Y, Chang RYK, Britton WJ, Morales S, Kutter E, Chan H-K, Synergy of nebulized phage PEV20 and ciprofloxacin combination against Pseudomonas aeruginosa, Int. J. Pharm, 551 (2018) 158–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Chang RY, Wong J, Mathai A, Morales S, Kutter E, Britton W, Li J, Chan HK, Production of highly stable spray dried phage formulations for treatment of Pseudomonas aeruginosa lung infection, Eur. J. Pharm. Biopharm, 121 (2017) 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Seville PC, Li HY, Learoyd TP, Spray-dried powders for pulmonary drug delivery, Critical reviews in therapeutic drug carrier systems, 24 (2007) 307–360. [DOI] [PubMed] [Google Scholar]
- [23].Wisniewski R, Spray Drying Technology Review, in, 45th International Conference on Environmental Systems, 2015. [Google Scholar]
- [24].Shetty N, Park H, Zemlyanov D, Mangal S, Bhujbal S, Zhou QT, Influence of excipients on physical and aerosolization stability of spray dried high-dose powder formulations for inhalation, Int. J. Pharm, 544 (2018) 222–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Li L, Sun S, Parumasivam T, Denman JA, Gengenbach T, Tang P, Mao S, Chan H-K, l-Leucine as an excipient against moisture on in vitro aerosolization performances of highly hygroscopic spray-dried powders, Eur. J. Pharm. Biopharm, 102 (2016) 132–141. [DOI] [PubMed] [Google Scholar]
- [26].Matinkhoo S, Lynch KH, Dennis JJ, Finlay WH, Vehring R, Spray-dried respirable powders containing bacteriophages for the treatment of pulmonary infections, J. Pharm. Sci, 100 (2011) 5197–5205. [DOI] [PubMed] [Google Scholar]
- [27].Leung SS, Parumasivam T, Gao FG, Carrigy NB, Vehring R, Finlay WH, Morales S, Britton WJ, Kutter E, Chan H-K, Production of inhalation phage powders using spray freeze drying and spray drying techniques for treatment of respiratory infections, Pharm. Res, 33 (2016) 1486–1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Chang RYK, Chen K, Wang J, Wallin M, Britton W, Morales S, Kutter E, Li J, Chan H-K, Anti-Pseudomonal activity of phage PEV20 in a dry powder formulation—A proof-of-principle study in a murine lung infection model, Antimicrob. Agents Chemother, (2017) AAC. 01714–01717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Chan BK, Sistrom M, Wertz JE, Kortright KE, Narayan D, Turner PE, Phage selection restores antibiotic sensitivity in MDR Pseudomonas aeruginosa, Sci. Rep, 6 (2016) 26717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Cipolla D, Wu H, Eastman S, Redelmeier T, Gonda I, Chan HK, Development and characterization of an in vitro release assay for liposomal ciprofloxacin for inhalation, J. Pharm. Sci, 103 (2014) 314–327. [Google Scholar]
- [31].Astudillo A, Leung SSY, Kutter E, Morales S, Chan H-K, Nebulization effects on structural stability of bacteriophage PEV 44, Eur J Pharm Biopharm, 125 (2018) 124–130. [DOI] [PubMed] [Google Scholar]
- [32].Chaudhry WN, Concepción-Acevedo J, Park T, Andleeb S, Bull JJ, Levin BR, Synergy and order effects of antibiotics and phages in killing Pseudomonas aeruginosa biofilms, PLoS One, 12 (2017) e0168615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].de Boer AH, Hagedoorn P, Hoppentocht M, Buttini F, Grasmeijer F, Frijlink HW, Dry powder inhalation: past, present and future, Expert Opin. Drug Deliv, 14 (2017) 499–512. [DOI] [PubMed] [Google Scholar]
- [34].GOOD WIHR, THE ADVANTAGES OF DESIGNING HIGH-RESISTANCE SWIRL CHAMBERS FOR USE IN DRY-POWDER INHALERS.
- [35].Weers JG, Son Y-J, Glusker M, Haynes A, Huang D, Kadrichu N, Le J, Li X, Malcolmson R, Miller DP, Idealhalers Versus Realhalers: Is It Possible to Bypass Deposition in the Upper Respiratory Tract?, J. Aeroso.l Med. Pulm. Drug. Deliv, 32 (2019) 55–69. [DOI] [PubMed] [Google Scholar]
- [36].Coates MS, Chan H-K, Fletcher DF, Chiou H, Influence of mouthpiece geometry on the aerosol delivery performance of a dry powder inhaler, Pharm. Res, 24 (2007) 1450–1456. [DOI] [PubMed] [Google Scholar]
- [37].Stass H, Nagelschmitz J, Willmann S, Delesen H, Gupta A, Baumann S, Inhalation of a dry powder ciprofloxacin formulation in healthy subjects: a phase I study, Clin. Drug. Investig, 33 (2013) 419–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Heijerman H, Westerman E, Conway S, Touw D, G.D.f.t.c.w. group, Inhaled medication and inhalation devices for lung disease in patients with cystic fibrosis: a European consensus, J. Cyst. Fibros, 8 (2009) 295–315. [DOI] [PubMed] [Google Scholar]
- [39].Shetty N, Zeng L, Mangal S, Nie H, Rowles MR, Guo R, Han Y, Park JH, Zhou QT, Effects of Moisture-Induced Crystallization on the Aerosol Performance of Spray Dried Amorphous Ciprofloxacin Powder Formulations, Pharm. Res, 35 (2018) 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Chen L, Okuda T, Lu X-Y, Chan H-K, Amorphous powders for inhalation drug delivery, Adv. Drug Deliv. Rev, 100 (2016) 102–115. [DOI] [PubMed] [Google Scholar]
- [41].Saluja V, Amorij J, Kapteyn J, De Boer A, Frijlink H, Hinrichs W, A comparison between spray drying and spray freeze drying to produce an influenza subunit vaccine powder for inhalation, J. Control. Release, 144 (2010) 127–133. [DOI] [PubMed] [Google Scholar]
- [42].Chang RYK, Wallin M, Kutter E, Morales S, Britton W, Li J, Chan H-K, Storage stability of inhalable phage powders containing lactose at ambient conditions, Int. J. Pharm, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Leung SS, Parumasivam T, Nguyen A, Gengenbach T, Carter EA, Carrigy NB, Wang H, Vehring R, Finlay WH, Morales S, Effect of storage temperature on the stability of spray dried bacteriophage powders, Eur. J. Pharm. Biopharm, 127 (2018) 213–222. [DOI] [PMC free article] [PubMed] [Google Scholar]