Endothelial cells, as the innermost layer in all vessels, play an essential role in regulating tissue homeostasis by controlling the extravasation of circulating cells into tissues. By altering their production of cytokines, chemokines and adhesion molecules endothelial cells control the traffic of immune cells into sites of injury1. Injury or dysfunction of the endothelial layer is known to contribute to many pathologies, including atherosclerosis. Atherosclerosis is a chronic inflammatory disease in which recruitment and trapping of immune cells, especially monocytes, is a hallmark2, 3. The recent CANTOS trial (Canakinumab Antiinflammatory Thrombosis Outcome Study) has highlighted the importance of inflammation; specifically, inflammasome-derived inflammation in cardiovascular disease4. Macrophages are thought to be the main producers of inflammasome-derived mediators and less is known about inflammasome activation in endothelial cells and how that contributes to disease.
In this issue of Circulation Research, Zhuang and co-workers uncover that forkhead box protein P1 (FOXP1) is a key regulator of endothelial cell inflammation and atherosclerosis (Figure)5. FOXP1 is a large transcriptional repressor that binds highly conserved regions of the DNA6 and previous work has indicated that endothelial FOXP1 is critical for an organism to develop7. Consistent with the idea that FOXP1 is important in endothelial cells; the same group recently reported that endothelial FOXP1 is a key regulator of pathological myocardial fibrosis8, but the role of FOXP1 in atherosclerosis is unknown.
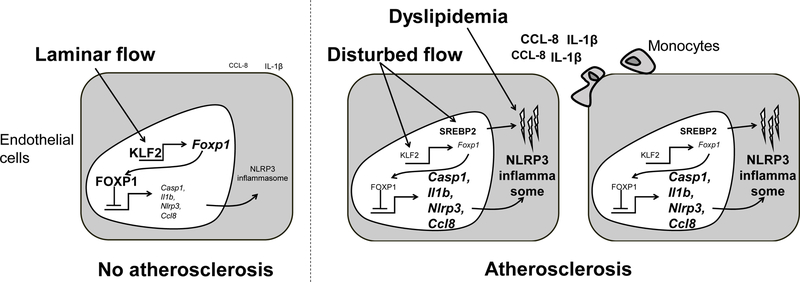
Figure.
FOXP1 acts as a gatekeeper of endothelial inflammation. In areas where laminar flow is present the Krüppel-like factor 2 (KLF2) is expressed which in turn stimulates expression of FOXP1. FOXP1 suppresses the expression of components of the NLRP3-inflammasome and Ccl8. In areas of disturbed flow, which also develops atherosclerosis, FOXP1 levels are reduced allowing for increased expression of inflammasome components and chemokines. Disturbed flow triggers expression of SREBP2 (sterol regulatory element binding protein 2) which increases cholesterol accumulation which might act as the second signal to activate the NLRP3 inflammasome. Also, dyslipidemia induces cholesterol crystal formation which might further activate the inflammasome in endothelial cells. All of which results in increased monocyte recruitment and acceleration of atherosclerosis.
The first clue that FOXP1 might be involved in endothelial cell dysfunction and atherosclerosis came when the authors demonstrated that FOXP1 is downregulated in areas prone to atherosclerosis and areas that already exhibit atherosclerosis, in both mice and humans5. Targeted deletion of FOXP1 in adult mice, selectively in endothelial cells, resulted in increased atherosclerosis at two different time points in mice that were also deficient in APOE5. The increase in atherosclerosis was associated with greater macrophage accumulation within the lesion. FOXP1 deletion augmented monocyte adhesion and conditioned media from endothelial cells without FOXP1 stimulated monocyte migration, clearly indicating that FOXP1 is critical for regulating endothelial-monocyte interactions and highlights the importance of endothelial cells in regulating monocyte trafficking in atherogenesis. Importantly, Zhuang et al. could show that overexpression of FOXP1 had the opposite effect to that of FOXP1 deletion.
To mechanistically understand how FOXP1 in endothelial cells could control monocyte recruitment RNA-sequencing was carried out. Endothelial cell FOXP1 deletion resulted in upregulation of genes associated with inflammasome activation such as Nlrp3, Casp1 and Il1b. The NLRP3-inflammasome is a multimeric complex that processes pro-IL-1β into mature IL-1β9. Full activation of the NLRP3-inflammasome normally requires 2 signals; a priming and an activating signal. In atherosclerotic lesions, cholesterol crystals have been proposed to be the main activator9, 10. To test if FOXP1 deficiency turns on the NLRP3 inflammasome in vivo, the authors blocked NLRP3 activation using 3 different approaches: endothelial cell-specific NLRP3 deficient mice (crossed into their FOXP1-deficient mice), the NLRP3 inhibitor MCC950 and the Caspase-1/IL-1β inhibitor Diacerein. Little is known about activation of the NLRP3-inflammasome in endothelial cells in vivo and whole-body inhibition has generated some contradictory results11–13. Many investigators would have predicted that the majority of the inflammasome activation would be derived from the hematopoietic compartment9 and thus one would not have expected such a profound effect of the endothelial-selective deficiency in NLRP3. Therefore it was somewhat surprising that all three approaches including the targeted deletion of NLRP3 in endothelial cells reduced atherosclerosis and monocyte recruitment in APOE-deficient mice5, arguing that endothelial NLRP3-derived inflammation is more important than previously appreciated. Furthermore, the decrease in atherosclerotic burden was greater in mice where FOXP1 had been deleted. From this, the authors concluded that FOXP1 downregulation accelerates atherogenesis via enhanced inflammasome activation. As stated above, the inflammasome typically requires 2 signals. The deletion of FOXP1 serves as the priming signaling as evident by increased expression of the genes involved, but what is the activating signal in endothelial cells? Hyperlipidemia can induce cholesterol crystal formation in endothelial cells14 and disturbed flow induces sterol regulatory element binding protein 2 in endothelial cells resulting in increased cholesterol accumulation15, potentially suggesting that altered flow might serve as both primer and activator of the inflammasome which is then potentiated by hyperlipidemia (Figure).
To complicate things, in addition to inflammasome activation, the RNA-sequencing data indicted the FOXP1 deletion was associated with an abundance of inflammatory changes, such as elevated chemokine expression. For example, one of the most highly upregulated genes in FOXP1-deficient endothelial cells was the chemokine Ccl8, which was also demonstrated to be a direct target of FOXP1. Thus, part of the effect of FOXP1 deletion could potentially be independent of NLRP3-inflammasome activation and might be driven by increased chemokine expression. But either way, the data demonstrate that FOXP1 is a critical player in regulating endothelial cell behavior, although the target(s) appears to be context depend8.
Why and how is FOXP1 downregulated in atherosclerotic lesions? Zhuang and colleagues went on to show that FOXP1 is a target of the laminar flow sensor Krüppel-like factor 2 (KLF2), explaining why the expression of FOXP1 is lower in areas of disturbed flow. Furthermore, the authors demonstrate that at least some of the pro-inflammatory effects of reduced KLF2 are mediated via FOXP15. In summary, Zhuang and colleagues have highlighted the importance of endothelial cell inflammatory status in atherosclerosis and demonstrated that FOXP1 is a key regulator of endothelial inflammation. In future research, it would be of interest to expand these studies to conditions associated with exaggerated endothelial cell dysfunction such as diabetes-associated complications.
Acknowledgments
Sources of Funding
Research in the author’s laboratory is funded in part by the American Diabetes Association grant 1-16-IBS-15 and a Pilot and Feasibility grant from NIDDK Diabetic Complications Consortium (grants DK076169 and DK115255).
Footnotes
Conflicts of interest
None
References
- [1].Gerhardt T, Ley K: Monocyte trafficking across the vessel wall. Cardiovasc Res 2015, 107:321–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Ross R: Atherosclerosis--an inflammatory disease. N Engl J Med 1999, 340:115–26. [DOI] [PubMed] [Google Scholar]
- [3].Tabas I, Bornfeldt KE: Macrophage Phenotype and Function in Different Stages of Atherosclerosis. Circ Res 2016, 118:653–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W, Anker SD,K et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N Engl J Med 2017, 377:1119–31. [DOI] [PubMed] [Google Scholar]
- [5].Zhuang T, Liu J, Chen X, Zhang L, Pi J, Sun H, Li L, Bauer RC, Wang H, Yu Z, et al. Endothelial Foxp1 Suppresses Atherosclerosis via Modulation of Nlrp3 Inflammasome Activation. Circ Res 2019: doi: 10.1161/CIRCULATIONAHA.119.039767. [DOI] [PubMed]
- [6].Shu W, Yang H, Zhang L, Lu MM, Morrisey EE: Characterization of a new subfamily of winged-helix/forkhead (Fox) genes that are expressed in the lung and act as transcriptional repressors. J Biol Chem 2001, 276:27488–97. [DOI] [PubMed] [Google Scholar]
- [7].Zhang Y, Li S, Yuan L, Tian Y, Weidenfeld J, Yang J, Liu F, Chokas AL, Morrisey EE: Foxp1 coordinates cardiomyocyte proliferation through both cell-autonomous and nonautonomous mechanisms. Genes Dev 2010, 24:1746–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Liu J, Zhuang T, Pi J, Chen X, Zhang Q, Li Y, Wang H, Shen Y, Tomlinson B, Chan P, et al. : Endothelial Foxp1 Regulates Pathological Cardiac Remodeling Through TGF-beta1-Endothelin-1 Signal Pathway. Circulation 2019. [DOI] [PubMed]
- [9].Grebe A, Hoss F, Latz E: NLRP3 Inflammasome and the IL-1 Pathway in Atherosclerosis. Circ Res 2018, 122:1722–40. [DOI] [PubMed] [Google Scholar]
- [10].Duewell P, Kono H, Rayner KJ, Sirois CM, Vladimer G, Bauernfeind FG, Abela GS, Franchi L, Nunez G, Schnurr M, et al. : NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature 2010, 464:1357–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Menu P, Pellegrin M, Aubert JF, Bouzourene K, Tardivel A, Mazzolai L, Tschopp J: Atherosclerosis in ApoE-deficient mice progresses independently of the NLRP3 inflammasome. Cell Death Dis 2011, 2:e137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Usui F, Shirasuna K, Kimura H, Tatsumi K, Kawashima A, Karasawa T, Hida S, Sagara J, Taniguchi S, Takahashi M: Critical role of caspase-1 in vascular inflammation and development of atherosclerosis in Western diet-fed apolipoprotein E-deficient mice. Biochem Biophys Res Commun 2012, 425:162–8. [DOI] [PubMed] [Google Scholar]
- [13].Gage J, Hasu M, Thabet M, Whitman SC: Caspase-1 deficiency decreases atherosclerosis in apolipoprotein E-null mice. Can J Cardiol 2012, 28:222–9. [DOI] [PubMed] [Google Scholar]
- [14].Baumer Y, McCurdy S, Weatherby TM, Mehta NN, Halbherr S, Halbherr P, Yamazaki N, Boisvert WA: Hyperlipidemia-induced cholesterol crystal production by endothelial cells promotes atherogenesis. Nat Commun 2017, 8:1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Xiao H, Lu M, Lin TY, Chen Z, Chen G, Wang WC, Marin T, Shentu TP, Wen L, Gongol B, et al. : Sterol regulatory element binding protein 2 activation of NLRP3 inflammasome in endothelium mediates hemodynamic-induced atherosclerosis susceptibility. Circulation 2013, 128:632–42. [DOI] [PMC free article] [PubMed] [Google Scholar]