Abstract
Biomarkers have a key role in Alzheimer’s disease (AD) drug development. Biomarkers can assist in diagnosis, demonstrate target engagement, support disease modification, and monitor for safety. The amyloid (A), tau (T), neurodegeneration (N) Research Framework emphasizes brain imaging and CSF measures relevant to disease diagnosis and staging and can be applied to drug development and clinical trials. Demonstration of target engagement in Phase 2 is critical before advancing a treatment candidate to Phase 3. Trials with biomarker outcomes are shorter and smaller than those required to show clinical benefit and are important to understanding the biological impact of an agent and inform go/no-go decisions. Companion diagnostics are required for safe and effective use of treatments and may emerge in AD drug development programs. Complementary biomarkers inform the use of therapies but are not mandatory for use. Biomarkers promise to de-risk AD drug development, attract sponsors to AD research, and accelerate getting new drugs to those with or at risk for AD.
Keywords: Alzheimer’s disease, Drug development, Clinical trials, Biomarker, Positron-emission tomography, Amyloid, Tau, Neurodegeneration
2.1. The Role of Biomarkers in Alzheimer’s Disease Drug Development
Alzheimer’s disease (AD) is a neurodegenerative disorder that progressively compromises cognition, function, and behavior [1, 2]. AD becomes more common in the elderly and is reaching epidemic proportions with the graying of the global population. The frequency of AD doubles in frequency every 5 years after the age of 60 [3]. An estimated current 35 million victims worldwide will grow to over 130 million by 2050 [4]. The cost of AD to the global economy will increase from its estimated 818 billion US dollars (USD) in 2015 to 2 trillion USD by 2030 [5]. To address this growing public health crisis, it is critical that treatments that defer the onset, slow the progression, or improve the symptoms of AD be identified.
There is high failure rate of AD drug development; there have been no new drug approvals for AD since 2003, and the failure rate in development programs exceeds 99% [6]. To advance new therapies for AD, it is imperative that the vulnerabilities of the drug development process be identified and addressed. The improvement must span target identification, drug screening and optimization, use and interpretation of animal models of AD, and the clinical trial process [7]. The risk of AD drug development is high, and biomarkers represent a promising means of reducing the risk and increasing the likelihood of technical success. Understanding of AD is improving rapidly, and key biological events are being identified. In some cases these events are accompanied by biomarkers measurable by brain imaging or in the cerebrospinal fluid (CSF) or blood. The use of these biomarkers to improve the drug development process can de-risk AD drug development. This contribution describes the increasing role of biomarkers in AD drug development.
Several new advances relevant to biomarkers are included in this review. The amyloid (A), tau (T), neurodegeneration (N) Research Framework uses biomarkers to diagnose AD [8]. These same biomarkers can also serve important roles in drug development including demonstrating target engagement or providing support for disease modification [9]. The US Food and Drug Administration (FDA) developed an AD staging system that facilitates trials in patients with preclinical and prodromal AD and emphasizes the potential role for biomarkers in drug development in early AD [10]. This staging system and the use of biomarkers is described and accelerated approval of new treatments are discussed. The use of biomarkers in both disease-modifying and symptomatic drug development is presented.
2.2. Overview of Biomarkers in AD Drug Development
A biomarker is a characteristic that is objectively measured and evaluated as an indicator of normal biological processes, pathologic processes, or biological responses to a therapeutic intervention [11]. Biomarkers help characterize the baseline state, a disease process, or a response to treatment. Biomarkers include measures of genes, “omics” technologies (genomics, transcriptomics, proteomics, metabolomics, lipidomics), imaging, blood, electrocardiograms, or evaluations of organ function (e.g., liver functions, etc.) [12, 13]. The National Institutes of Health (NIH) developed an extensive glossary of biomarker-related terms—Biomarkers, EndpointS, and other Tools (BEST) resource—to provide a shared vocabulary for biomarker discussions [14].
Table 2.1 presents an overview of the roles played by biomarkers in AD drug development. The principal uses of biomarkers include demonstrating the presence of AD-type pathological changes with CSF measures or amyloid positron-emission tomography (PET) for inclusion in AD trials, demonstrating target engagement by the candidate therapy, generating supportive evidence of disease modification, informing analytic stratification, and monitoring of adverse effects of treatment. Means of scoring biomarkers to increase confidence in their role in drug development have been proposed but not yet widely adopted [15].
Table 2.1.
Roles of biomarkers in AD drug development with examples
| Role in trial | Biology identified | Fluid biomarker | Brain imaging |
|---|---|---|---|
| Diagnosis and participant identification | Presence of AD-type pathological changes | Low CSF Aβ42 or CSF Aβ42/t-tau ratio or Aβ42/p-tau ratio | Positive amyloid imaging |
| Target engagement | Reduction of amyloid production | Reduced Aβ42 production as shown by SILK | |
| Removal of aggregated Aβ | Reduced Aβ aggregation as shown by amyloid imaging | ||
| Support for disease modification | Reduction of measures of neurodegeneration compared to placebo | Reduced CSF t-tau | Drug-placebo difference in favor of active treatment for FDG PET hypometabolism or MRI atrophy |
| Analytic stratification | Identification of ApoE-4 carrier status | ApoE genotype | |
| Analytic stratification | Effects on the liver or blood | Blood liver function tests, complete blood count | |
| Production of ARIA by monoclonal antibodies | MRI monitoring for ARIA |
Aβ42 amyloid beta protein 42 amino acid length fragment, AD Alzheimer’s disease, ApoE apolipoprotein E, ARIA amyloid-related imaging abnormalities, CSF cerebrospinal fluid, FDG fluorodeoxyglucose, MRI magnetic resonance imaging, PET positron-emission tomography, SILK stable isotope labeling kinetics
2.3. A,T,N Framework for Alzheimer’s Disease Diagnosis and Characterization
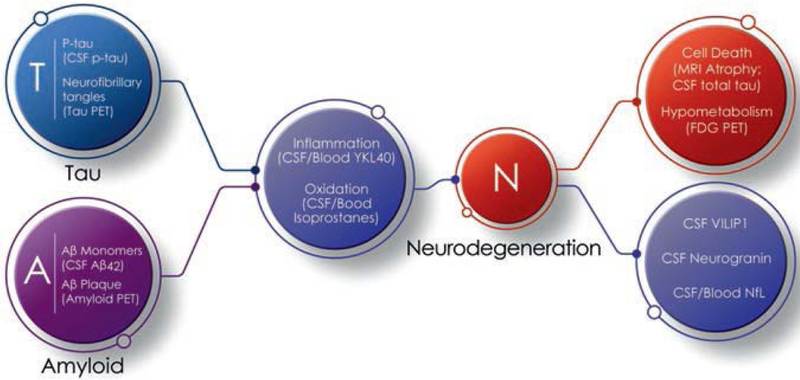
The A,T,N Research Framework uses biomarkers to diagnose and characterize AD [8]. Amyloid measures include amyloid PET (Fig. 2.1) and CSF amyloid beta (Aβ) protein; tau measures include tau PET (Fig. 2.2) and CSF phosphorylated tau (p-tau); neurodegeneration is reflected in atrophy on magnetic resonance imaging (MRI) (Fig. 2.3), CSF levels of total tau (t-tau), or fluorodeoxyglucose (FDG) PET (Fig. 2.4). In this approach, reduced N in the treatment groups compared to the placebo group is the object of disease-modifying therapy (DMT) [16, 17].
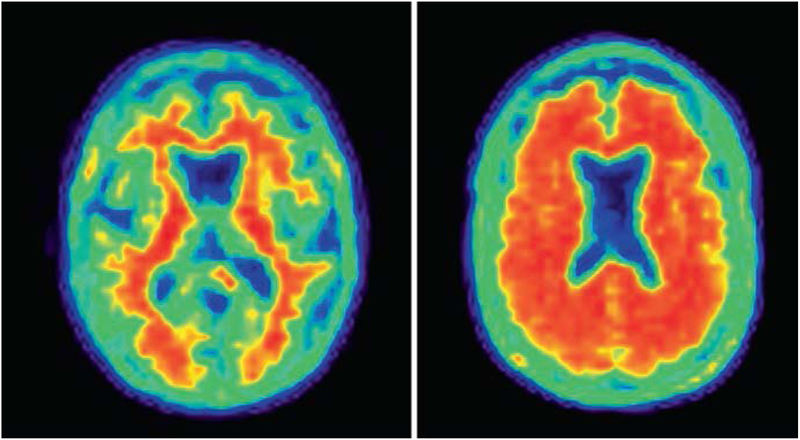
Fig. 2.1.
Normal (left) and abnormal (right) amyloid PET
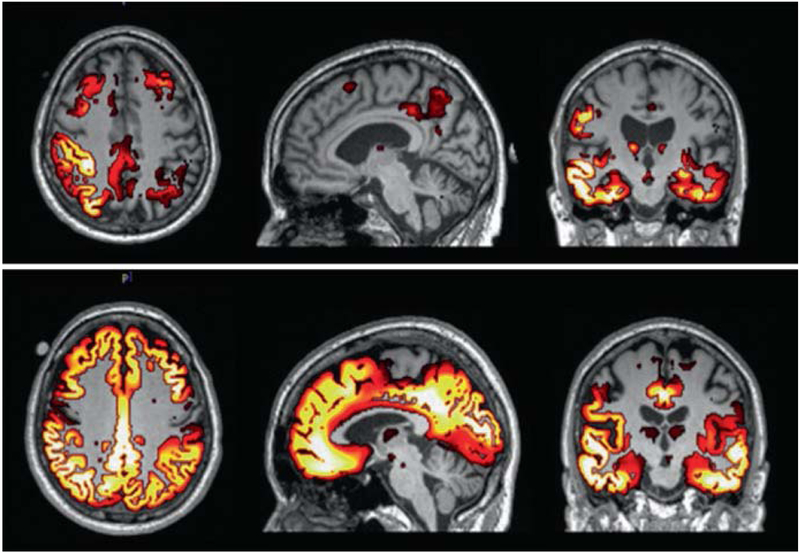
Fig. 2.2.
Low tau (above) and high tau (below) PET aligned with MRI (images courtesy of Dawn Matthews)
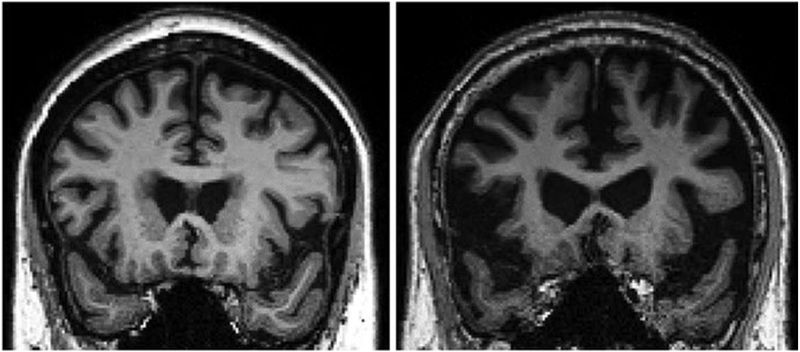
Fig. 2.3.
Early AD (left) and late AD (right) MRI. The scan on the right shows whole brain atrophy and ventricular enlargement (images courtesy of Karthik Sreenivasan)
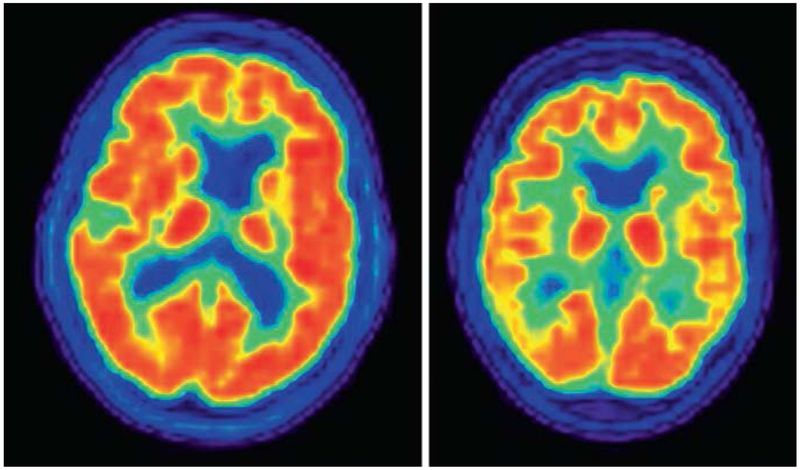
Fig. 2.4.
Normal (left) and abnormal (right) fluorodeoxyglucose PET scans
Reductions in aggregated Aβ on amyloid PET or changes in CSF Aβ42 demonstrate impact on A, and drug-placebo differences in aggregated tau on tau PET or CSF p-tau establish effects on T. Amyloid PET measures the aggregated, deposited fibrillar, insoluble form of Aβ, and CSF amyloid is a measure of the soluble monomeric form of the peptide. Similarly, tau PET measures the fibrillar deposited form of the tau protein, and CSF p-tau is the soluble form of the tau protein. Oligomeric Aβ and oligomeric tau may represent the neurotoxic form of these peptides and do not have currently accepted measures that have been shown to be useful in trials. Drug-placebo differences in A and T would represent important effects on AD biology. They are markers of intermediate steps of the biological changes leading to cell death and do not themselves represent evidence of disease modification. Evidence linking these biomarkers to neuronal loss might allow them to function as surrogate markers of N; this evidence is lacking. A and T are currently best regarded as target engagement biomarkers.
2.4. Biomarkers for Participant Selections
Participation in AD treatment trials requires that the patient have AD. The clinical diagnosis of AD dementia is approached using the 1984 criteria of the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association (NINCDS-ADRDA) [18] or the 2011 criteria of the National Institute on Aging-Alzheimer’s Association (NIA-AA) [19]. Recent studies with amyloid imaging show that a substantial portion of individuals diagnosed with these criteria lack biomarker evidence of AD. Using the cohort of the Alzheimer’s Disease Neuroimaging Initiative (ADNI), Landau and colleagues found that 15% of patients diagnosed clinically with AD dementia had amyloid PET and CSF findings incompatible with the diagnosis [20]. Similarly, among patients diagnosed clinically with mild AD dementia, Sevigny and coworkers found that 25% failed to show abnormal amyloid levels on amyloid PET [21]. These findings demonstrate that the clinical diagnosis of AD is insufficient to establish a secure diagnosis or be certain of the associated pathology. Measures of A are critical to supporting the diagnosis of AD and providing the rationale for anti-AD therapy. Patients that are amyloid negative have slower progression than those with AD dementia even if they have evidence of neurodegeneration on MRI; these individuals have been labeled as suspected non-Alzheimer pathology (SNAP) [22]. SNAP patients, if included in trial populations, will decrease the rate of change in the placebo group and compromise the ability to demonstrate a drug-placebo difference in the trial. Thus, amyloid biomarkers are needed to show the presence of the AD pathology substrate and to optimize the rate of decline in the placebo group. These considerations apply to trials of both DMTs and symptomatic cognition enhancers.
A recent drug development program for idalopirdine—a 5-HT6 antagonist targeting cognitive enhancement—recruited patients with mild-to-moderate AD dementia based on clinical diagnostic criteria without a confirmatory biomarker. A subgroup of the patients had known amyloid status, and this group declined significantly faster on the Alzheimer’s Disease Assessment Scale-Cognitive Subscale (ADAS-Cog) than patients with negative or unknown amyloid status [23]. The greater decline in the biomarker-enriched group would have allowed demonstration of a drug-placebo difference with smaller sample sizes if the agent had been efficacious. This is an example of how diagnostic confirmation of an AD diagnosis can be important in development programs for symptomatic as well as disease-modifying agents.
Mild cognitive impairment (MCI) is an etiologically nonspecific syndrome comprised of several entities associated with cognitive impairment including AD, precursor phases of other dementias such as dementia with Lewy bodies (DLB) and frontotemporal dementia (FTD), depression, and other unrecognized states. This heterogeneity is manifest in the longitudinal outcomes of MCI that include progression to AD, progression to other types of dementias, recovery to normal cognition, and continuation in the MCI state [24]. Bangen found that 37% of amnestic MCI patients did not have brain amyloid by PET assessment indicating that they did not have AD as the key associated pathology [25]. Similarly, Wisse and colleagues [22] reported that 36% of the MCI population they assessed lacked positive findings on amyloid imaging. Of MCI patients who progressed to dementia, 29% were found to have non-AD diagnoses as the primary cause of dementia at autopsy [26]. These studies suggest that at least one-third of patients with MCI do not meet biomarker criteria for prodromal AD using the criteria of the International Work Group [27]. As discussed above, the absence of the pathological changes of AD indicates that the substrate of many AD therapies is absent, and the decline in the placebo group on which power calculations and sample sizes are based becomes less predictable. Biomarker confirmation of the presence of AD pathological changes should be pursued in both DMT and symptomatic therapy drug development programs for prodromal AD or MCI due to AD [27, 28].
Preclinical AD participants do not evidence cognitive abnormalities (although they may have decline from past cognitive performance levels) and can be identified only through the use of biomarkers. Primary and secondary prevention trials can target this population. Cognitively normal individuals with normal amyloid PET or normal CSF levels of Aβ are subjects for primary prevention trials; those with normal cognition and evidence of abnormal brain amyloid can be participants in secondary prevention trials. Of 353 ADNI subjects over age 65 with normal cognition, 45% (160) had normal CSF Aβ42 levels and negative amyloid PET, 47% had abnormal CSF Aβ42 and abnormal amyloid PET, and 7% (26) had abnormal CSF Aβ42 and normal amyloid PET [29].
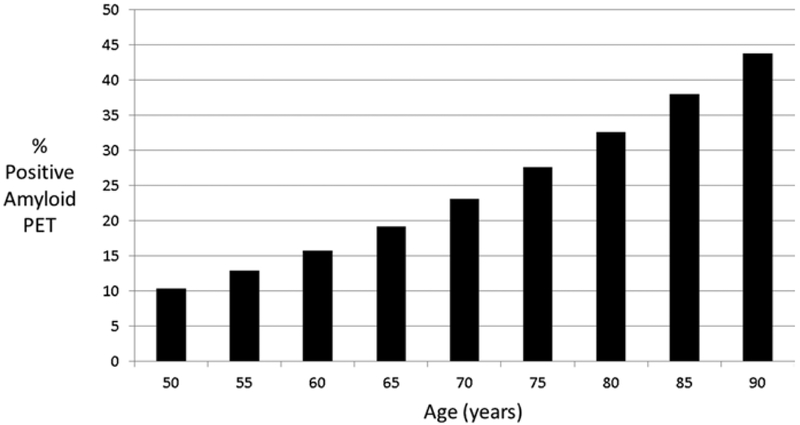
A meta-analysis of studies of amyloid PET in those with normal cognition shows that the rate of amyloid positivity increases from 10.4% in those 50–55 years old to 43.8% among those 90+ years old [30]. Figure 2.5 shows the prevalence of positive amyloid imaging by age and establishes the expected screen fail and screen positive rate for cognitively normal individuals if no other screening criteria are employed. The rate of amyloid positivity is increased twofold in apolipoprotein E (ApoE) 4 gene carriers [31].
Fig. 2.5.
Prevalence of amyloid PET positivity by age (data from Jansen et al. [30])
Together these observations indicate that biomarkers are required for diagnostic confidence in preclinical, prodromal, and dementia trials. Amyloid biomarkers confirm the diagnosis and provide confidence in the predicted decline of the placebo group and the ability to predict effect sizes and sample size requirements. Amyloid biomarkers establish the presence of the target pathology for anti-amyloid trials. Amyloid biomarkers show that the patient has AD and not some other unknown pathology that could create a neuronal environment with an unknown therapeutic response to anti-amyloid treatments. Establishing a firm diagnosis of AD by demonstrating abnormal brain amyloid metabolism or aggregation is important for non-amyloid therapies directed at tau, neuroprotection, inflammation, or other AD-related pathologies. In these trials, amyloid PET or CSF Aβ function as inclusion criteria and not as outcomes. Despite the advantages of confirming the diagnosis of AD with biomarkers, a recent review of current AD trials and the AD pipeline showed that 11 of 25 DMT trials in Phase 3 and 21 of 38 DMT trials in Phase 2 did not require evidence of amyloidosis at baseline [32]. Failure to show a drug-placebo difference in these trials will leave unresolved the question of whether there was lack of efficacy of the test agent or an insufficient number of trial participants with AD.
2.5. Biomarkers of Target Engagement
In drug development programs, Phase 2a is typically used to establish proof of concept (POC) and Phase 2b to determine the doses to be advanced to Phase 3. POC can be based on a clinical response or on a biomarker response or some combination of clinical and biomarker outcomes. Symptomatic agents with detectable clinical responses such as the cholinesterase inhibitors can achieve POC in Phase 2 with clinical measures [33–35]. Symptomatic agents improve cognition above baseline and can show cognitive and global benefit without substantial decline in the placebo group. DMTs target slowing of progression in comparison to the decline observed in the participants in the placebo arm of the study. This typically takes a large trial and long observation period (12 months to 5 years depending on the stage of the participants and the outcomes chosen). Such large long studies are characteristic of Phase 3, not Phase 2. This created the “Phase 2 conundrum” in AD drug development and was resolved by some sponsors by advancing agents from Phase 1 to Phase 3 with little effort to show a drug-placebo efficacy difference or understand the safety issues of the agent in Phase 2 [36]. The Phase 2 conundrum can be addressed by using biomarkers as the principal readout with attention to the directional responses of clinical measures but not requiring demonstration of clinical benefit. Agents with biomarkers showing responses in early phase drug development are more likely to be advanced to later phase of development and to be approved [37, 38]. Drug-placebo differences in biomarker measures can be shown in smaller shorter trials. No biomarker has achieved surrogate status in AD drug development with definite evidence that a change in the biomarker predicts a clinical benefit. Nevertheless, a Phase 3 program can be de-risked by acquiring a repertoire of biomarkers and clinical measures that provide a “weight of evidence” argument supporting drug efficacy and inform the go-no, go decision process at end of Phase 2.
Phase 2 clinical trials should show target engagement and establish the dose(s) to be advanced to Phase 3. Unless target engagement is established, it is impossible to distinguish between a drug that failed to engage the target and a failed trial (usually poorly conducted) as the interpretation of a trial showing no drug-placebo difference [39]. Target engagement can be shown by receptor occupancy or proof of pharmacology. Receptor occupancy is more often used in development programs for symptomatic agents; proof of pharmacology is applicable to both symptomatic and disease-modifying drug development. Symptomatic agents can be advanced on the basis of clinical response, but demonstrating target engagement provides information relevant to brain penetration, dose optimization, and efficacy. DMTs should not be advanced to Phase 3 without evidence of target engagement in Phase 2. There are relatively few well-developed target engagement biomarkers. Sponsors should require development of biomarkers to show target engagement (fluid or imaging) to advance a drug development program.
2.6. Fluid Biomarkers of Target Engagement
Proof of pharmacology for enzyme inhibitors can be shown by stable isotope labeling kinetic (SILK) studies [40]. When used to measure amyloid protein synthesis, this technique involves labeling an amino acid in peripheral blood and then using mass spectrometry to determine when it appears (synthesis rate) in the amyloid protein and disappears (amyloid clearance rate) from the CSF. SILK has been used to show that amyloid clearance is decreased in late-onset AD and that this may be an important contributor to amyloid aggregation in this form of the disease [41]. In the autosomal dominant form of AD (ADAD), production of Aβ40 and Aβ42 was 24% higher in mutation carriers than noncarriers, and the fractional turnover rate of Aβ42 was 65% faster in mutation carriers [42]. These observations show that late-onset AD (LOAD) reflects reduced amyloid clearance; ADAD reflects amyloid over-production.
In drug development programs for inhibitors of enzymes involved in Aβ synthesis, SILK can be used to assess the short-term impact of inhibition of amyloid production. When testing a gamma-secretase inhibitor (semagacestat; LY 450139), patients received an oral dose of the inhibitor at the start of a 9-h infusion of labeled leucine; CSF sampling occurred continuously over the next 36 h. Hours 0–12 were used to calculate Aβ synthesis and hours 24–36 to calculate Aβ clearance. There was a dose-dependent decrease in Aβ production ranging from 47% to 84% with no effect on Aβ clearance [43]. SILK showed proof of pharmacology for inhibition of Aβ production over 36 h. A 16-week study of the agent showed no decrease in CSF Aβ at the end of the exposure period suggesting that short-term inhibition of gamma-secretase may not predict long-term inhibition [44].
Verubecestat, a β-site amyloid precursor protein cleaving enzyme 1 (BACE 1) inhibitor, decreased CSF levels of Aβ over a 7-day continuous sampling period [45], and the levels remained 70–80% lower than placebo-treated patients after an 18-month treatment period [46]. In this case, short-term observations were consistent with long-term data. There was no associated cognitive or functional benefit from prolonged Aβ reductions in these patients with mild-to-moderate AD.
Gamma-secretase inhibitors decrease the production of Aβ42 and increase the production of shorter amyloid fragments. A rise in Aβ15–16 is a target engagement biomarker for gamma-secretase inhibitors showing that the enzyme activity has been decreased and pharmacologic consequences are measurable. This can be shown acutely using short-term measures [47].
Labeled leucine has also been used to interrogate tau protein kinetics [48]. No treatment-related effects of tau agents have been reported using this approach. This technique will provide insight into tau therapeutics and impact of drugs on tau dynamics.
CSF p-tau and Aβ1–42 can serve as pharmacodynamic measures of target engagement. P-tau is increased in AD and is hypothesized to reflect either cell death with release of the neurofibrillary tangle-associated p-tau protein into the CSF or leakage of p-tau from the extracellular space into the CSF during the prion-like transfer of p-tau from cell-to-cell [49]. T-tau measures are considered measures of neurodegeneration. Table 2.2 shows the results of trials where p-tau was measured. Patients on active treatment observed with bapineuzumab, gantenerumab, and semagacestat had modest reductions in p-tau in some studies. Most trials showed no effect on p-tau levels.
Table 2.2.
Effects on p-tau and t-tau of recent major clinical trials
| Agent | N in the CSF portion of the study | Percent change p-tau compared to placebo | Percent change T-tau compared to placebo | MOA |
|---|---|---|---|---|
| AN1792 [107] | 21 | Not reported | Reduced in the active treatment group compared to the pbo group (p < 0.001) | Antiamyloid vaccine |
| Antioxidant* trial [108] | 66 | No drug-placebo difference | No drug-placebo difference | Antioxidant |
| Semagacestat [97] | 47 | P-tau increased 16% in the pbo group and declined by 8% and 4%, respectively, in the low- and high-dose groups (p = <0.001) | No drug-pbo difference | GSI |
| Bapineuzumab; ApoE carrier study [109] | 212 | 5.8 pg/ml decrease in treatment group; 0.95 pg/ml increase in the pbo group (p = 0.0.005) | Not reported | mAb |
| Bapineuzumab; ApoE noncarrier study [109] | 188 | No drug-pbo difference in the 5 mg/kg treatment group; for the 10 mg/kg group, there was a 8.18 pg/ml decrease in the treatment group and a 1.98 pg/ml in the placebo group (p = 0.009) | Not reported | |
| Solanezumab; Expedition [51] | 45 | No drug-pbo difference | No drug-pbo difference | mAb |
| Solanezumab; Expedition II [51] | 76 | No drug-pbo difference | No drug-pbo difference | mAb |
| Gantenerumab [69] | 209 | −5.61% change from baseline for 105 mg dose (p = <0.001 compared to pbo); −7.15% change from baseline for 225 mg dose (p = <0.001 compared to pbo) | −1.08% change from baseline for 105 mg dose (p = 0.05 compared to pbo); −2.91% change from baseline for 225 mg dose (p = 0.02 compared to pbo) | mAb |
| IVIG [110] | No drug-placebo difference | No drug-placebo difference | ||
| Solanezumab; Expedition III [52] | 258 | No drug-pbo difference | No drug-pbo difference | mAb |
| Crenezumab [53] | No drug-pbo difference | No drug-pbo difference | mAb | |
| Verubecestat [46] | 111 | Decrease of 0.42% in the pbo group and 5.86% in the 40 mg group (not significant) | Increase of 7.52% in the pbo group and 3.35% in the 40 mg group (not significant) | BACE 1 inhibitor |
| Azeliragon (TTP488) | No drug-placebo difference | No drug-placebo difference | RAGE inhibitor |
BACE β-site amyloid precursor protein cleaving enzyme 1, GSI gamma-secretase inhibitor, IVIG intravenous immunoglobulin, mAb monoclonal antibody, MOA mechanism of action, pbo placebo, RAGE receptor for advance glycation end products
Antioxidant, 3 arm trial comparing placebo to 800 IU/day of vitamin E (α-tocopherol) plus 500 mg/day of vitamin C plus 900 mg/day of α-lipoic acid (E/C/ALA) or 400 mg of coenzyme Q 3 times/day
CSF Aβ1–42 is monitored as a pharmacodynamic outcome in trials of anti-amyloid agents (Table 2.3). Reduction in Aβ1–42 or a decrease in the Aβ42/40 ratio is a diagnostic hallmark of AD and correlates with increased amyloid plaque burden on amyloid PET [50]. Further reduction in CSF levels of Aβ1–42 has been achieved with BACE inhibitors. Verubecestat, for example, produced 80% reduction in Aβ1–42 levels after 18 months of treatment [46]. Solanezumab produced increased levels of CSF Aβ1–42 in Expedition/Expedition II [51] and in Expedition III [52]. Crenezumab was associated with decreased CSF Aβ compared to placebo [53]. The effect of other amyloid-related treatment mechanisms on CSF Aβ1–42 levels is less predictable. For example, it might be anticipated that agents that reduce amyloid aggregation would increase the monomeric form of Aβ1–42 measured in the CSF and decrease the oligomeric form [54]. Resolution of these issues awaits further empirical evidence.
Table 2.3.
CSF amyloid (Aβ42) reduction in clinical trials of anti-amyloid agents
| Agent | Phase | Population | Change compared to placebo | N in the CSF study | Duration of treatment | MOA |
|---|---|---|---|---|---|---|
| AN1792 | 2 | Mild-to-moderate | No drug-placebo difference | 21 | 12 monthsa | Anti-amyloid vaccine |
| Semagacestat [111] | 2 | Mild-to-moderate | No drug-placebo difference | 43 | 14 weeks | Gamma-secretase inhibitor |
| Azeliragon (TTP488) [112] | 2 | Mild-to-moderate | No drug-placebo difference | 65 | 10 weeks | RAGE inhibitor |
| Antioxidantb trial [108] | 2 | Mild-to-moderate | No drug-placebo difference | 66 | 16 weeks | Antioxidant |
| Avagacestat [113] | 2 | Mild-to-moderate | Decrease to 66% of baseline in the high-dose group (p = 0.03 compared to pbo) | 45 | 24 weeks | Gamma-secretase inhibitor |
| Semagacestat [97] | 3 | Mild-to-moderate | No drug-placebo difference | 47 | 76 weeksa | Gamma-secretase inhibitor |
| Solanezumab; Expedition [51] | 3 | Mild-to-moderate | Total Aβ42 increased with no appreciable change in the placebo group (between-group difference, p < 0.001); levels of free Aβ42 did not change significantly | 45 | 80 weeks | mAb |
| Avagacestat [114] | 2 | MCI | No drug-pbo difference | 132 | 104 weeks | Gamma-secretase inhibitor |
| Gantenerumab [69] | 3 | Mild-to-moderate | No drug-pbo difference at either dose group | 209 | 104 weeks | mAb |
| IVIG [110] | 3 | Mild-to-moderate | No drug-placebo difference | 35 | 18 months | Polyclonal antibody |
| Solanezumab; Expedition III [52] | 3 | Mild | Free Aβ42 decreased more in the treatment group compared to the placebo group (−37.3 vs. −9.3; p = 0.005) | 258 | 76 weeks | mAb |
| Crenezumab [53] | 2 | Mild-to-moderate | Aβ1–42 levels declined more in the crenezumab than pbo groups of −120.16 pg/ml (unadjusted p = 0.017) (300 mg SC cohort) and −170.50 pg/ml (unadjusted p = 0.022) (15 mg/kg IV cohort) | 26 | 76 weeks | mAb |
| Verubecestat | 3 | Mild-to-moderate | <10% reductions in pbo group; 62.7–76.4% reductions in active treatment group | 111 | 78 weeks | BACE 1 inhibitor |
BACE β-site amyloid precursor protein cleaving enzyme 1, IVIG intravenous immunoglobulin, mAb monoclonal antibody, MOA mechanism of action, RXR retinoid X receptor
AN1792 and semagacestat trials were interrupted before completion for safety reasons
Antioxidant, 3 arm trial comparing placebo to 800 IU/day of vitamin E (α-tocopherol) plus 500 mg/day of vitamin C plus 900 mg/day of α-lipoic acid (E/C/ALA) or 400 mg of coenzyme Q 3 times/day
Inflammation is increasingly recognized as a critical process of AD neurobiology [55]. Several biomarkers for inflammation have been proposed and may eventually be included as biomarkers in AD clinical trials, especially those where the test agent targets or affects inflammation. In CSF, C-reactive protein (CRP) (decreased) and TREM-2 (increased) differ between normal elderly controls and those with AD dementia [56]. CRP is also reduced in plasma of those with AD dementia compared to those with MCI or normal cognition [57]. Correlations between levels of CRP and cognition or disease progression are weak. Elevated CSF levels of the pro-inflammatory cytokine TNF-alpha and decreased levels of the anti-inflammatory cytokine TGF-β are associated with an increased risk of progression from MCI to AD dementia suggesting that inflammation is playing a role in this early phase of symptomatic disease progression [55]. Chemokines are also altered in AD including elevated levels of monocyte chemoattractant protein-1 [58]. Microglial activation is part of the inflammatory process and stimulates astrocytic expression of YKL-40. Increased levels of YKL-40 are evident in CSF and blood in AD dementia [59]. Microglial activation can be assessed with PET imaging using ligands binding to microglial proteins; elevated microglial activity has been shown in medial temporal, occipital, and parietal lobes in those with AD dementia [60]. Reduction of inflammatory markers may be a means of tracking anti-inflammatory effects of AD therapies.
Isoprostanes are prostaglandin isomers produced from polyunsaturated fatty acids in lipid membranes by free radicals and comprise an index of oxidative injury measurable in the CSF and plasma [61, 62]. Isoprostanes increase in the course of normal aging as well as in AD. F2-isoprostanes have the best measurement performance characteristics of assays of oxidative damage [61] and may be used to reflect decreased membrane injury associated with AD-related treatments that provide neuroprotection [63].
Cyclic guanosine monophosphate (cGMP)-specific phosphodiesterase (PDE) inhibitors are candidates for cognitive enhancement in AD by increasing brain cGMP levels. PDE inhibitors have shown proof of pharmacology by raising CSF levels of cGMP after oral dosing. With BI 409306, a PDE9 inhibitor, maximum CSF cGMP concentrations were achieved within 2–5 h, declining to baseline 10–14 h after dosing in a Phase 1 study with healthy volunteers. This is an example of using a proof-of-pharmacology biomarker in a development program for a symptomatic cognitive enhancing agent. The agent did not produce clinical benefit; other PDE inhibitors are in clinical development [32].
2.7. Imaging Biomarkers of Target Engagement
Receptor occupancy studies are valuable when there is a defined receptor for the test agent; this is most likely in development programs for symptomatic agents—cognitive enhancers or drugs to treat neuropsychiatric syndromes in neurodegenerative disorders. An example of investigation of receptor occupancy in a drug development program for neurodegenerative disease is the PET study of pimavanserin, a 5-HT2A inverse agonist. Ascending doses of pimavanserin were given to healthy volunteers, followed by radio-labeled [11C]N-methylspiperone ([11C]NMSP), a 5-HT2A ligand. Reduced NMSP binding was evident following 1 mg of pimavanserin and reached near maximal displacement with the 10–20 mg doses [64]. The radioligand PET study showed blood-brain barrier penetration, dose-related receptor occupancy, dose of maximal occupancy, and safety. Receptor occupancy studies are a means of showing target engagement in a drug development program. Pimavanserin is approved for hallucinations and delusions in Parkinson’s disease psychosis [65], reduced symptoms of AD-related psychosis [66], and is being studied for dementia-related psychosis [67].
Amyloid and tau PET are biomarkers of target engagement but are not direct measures of cell loss and neurodegeneration. An example of reduction of fibrillar amyloid in a clinical trial emerged from the trial of the monoclonal antibody aducanumab [68]. There was a dose-dependent decrease in Aβ plaque evident at 6 and 12 months of exposure. There was a corresponding amelioration in progression at the highest doses as measured by the Clinical Dementia Rating Sum of Boxes (CDR-sb) and the Mini-Mental State Examination (MMSE) but not on the Neuropsychological Test Battery (NTB) or the Free and Cued Selective Reminding Test (FCSRT). Dose- and genotype-dependent amyloid-related imaging abnormalities (ARIA) were evident. This agent is now in Phase 3 clinical trials. Reduced plaque amyloid has been observed with the monoclonal antibodies gantenerumab and BAN-2401 [69] and with the small molecules verubecestat [46] and bexarotene [70].
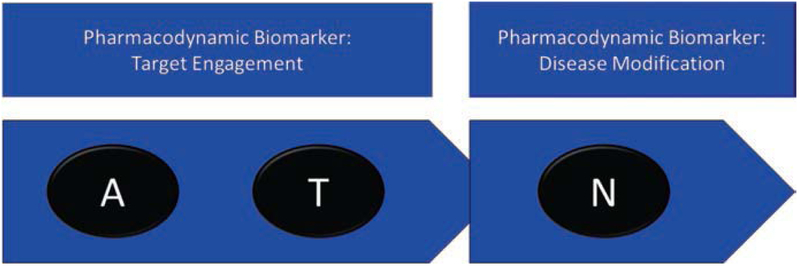
Trials of a few other agents have shown reductions in brain plaque burden on amyloid PET but with no corresponding benefit for cognition or function (Table 2.4). These studies show that demonstration of target engagement in Phase 2 may not predict cognitive benefit in Phase 3. Figure 2.6 shows the relationship of A and T imaging to impact on N; ATN biomarkers are pharmacodynamic measures.
Table 2.4.
Amyloid reduction in clinical trials of anti-amyloid agents as measured by amyloid PET standardized uptake ratio values (SUVR)
| Agent | Phase | Population | Percent Reduction compared to placebo | N in the study | Duration of treatment | MOA |
|---|---|---|---|---|---|---|
| Bapineuzumab; ApoE carrier study [115] | 3 | Mild-to-moderate | Increase in pbo; no change in treatment group (p = 0.004) | 75 | 71 weeks | mAb |
| Bapineuzumab; ApoE noncarrier study [115] | 3 | Mild-to-moderate | No drug-pbo difference | 39 | 71 weeks | mAb |
| Gantenerumab [69] | 3 | Mild-to-moderate | 4.8% reduction from baseline in the 225 mg dose group (p = 0.01 compared to pbo); no drug-pbo difference in the 105 mg dose group | 55 | 100 weeks | mAb |
| Solanezumab; Expedition [51] | 3 | Mild-to-moderate | No drug-pbo difference | 169 | 80 weeks | mAb |
| Solanezumab; Expedition II [51] | 3 | Mild-to-moderate | No drug-pbo difference | 97 | 80 weeks | mAb |
| Solanezumab; Expedition III [52] | 3 | Mild | No drug-pbo difference | Not reported | 76 weeks | mAb |
| Aducanumab | 1 | Mild | SUVR decreased significantly in the 3, 6, 10 mg/kg doses (p = <0.001); 20% reduction (mean score 1.44 at baseline to 1.16 at week 54) in the 10 mg/kg group | 125 | 54 weeks | mAb |
| Semagacestat [97] | 3 | Mild-to-moderate | No drug-placebo difference | 59 | 76 weeksa | Gamma-secretase inhibitor |
| Verubecestat | 3 | Mild-to-moderate | BACE 1 inhibitor | |||
| Bexarotene | 2 | Mild-to-moderate | 7% reduction in ApoE-4 noncarriers (p = 0.012); no significant reduction in ApoE-4 carriers | 20 | 4 weeks | RXR antagonist |
| IVIG [110] | 3 | Mild-to-moderate | 20 drug-placebo difference | 61 | 18 months | Polyclonal antibody |
BACE β-site amyloid precursor protein cleaving enzyme 1, IVIG intravenous immunoglobulin, mAb monoclonal antibody, MOA mechanism of action, MCI mild cognitive impairment, RAGE receptor for advanced glycation end products, RXR retinoid X receptor
The semagacestat study was terminated by the Data Safety Monitoring Board, and completion rates were 23–38%
Fig. 2.6.
Pharmacodynamic biomarkers. Tau (T) and amyloid (A) biomarkers function as target engagement biomarkers showing that an agent affects the brain protein; N biomarkers support disease modification if a drug-placebo difference is demonstrated
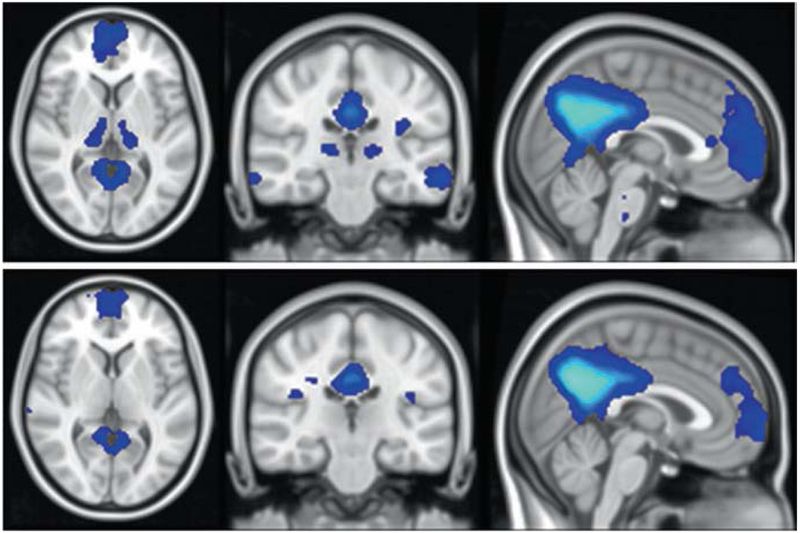
An underutilized opportunity to show target engagement and garner information supportive of proof of pharmacology is the use of functional MRI (fMRI) to explore circuit-level effects of Phase 2 interventions [71]. A,T,N are cellular- and tissue-level measures that reflect the core pathologic involvement of the brain in AD. Cognition and behavior are supported by complex brain circuits that are comprised of integrated nodes and connections [72] (Fig. 2.7). Aspects of these circuits are active at rest and differ from circuits that are dynamically engaged with specific cognitive activities. The activity pattern of resting state fMRI includes a posterior default mode network (DMN) and an anterior salience network (SN). The posterior DMN is comprised of a temporal-parietal network associated with memory and visuospatial function; the anterior SN includes frontal structures relevant to social-emotional function. In AD, the posterior DMN connectivity to posterior hippocampus and medial cingulo-parieto-occipital regions is diminished in contrast to intensified activity in the anterior SN [73, 74] (Fig. 2.8). Circuit function deteriorates in the preclinical phases of AD and continues to decline in prodromal and dementia phases [75]. Activated fMRI wherein patients perform cognitive tasks while in the MRI scanner show task-related regional activation and can also be used to investigate characteristic changes in AD [76]. Treatments that affect core A,T,N biology but do not impact circuit function are unlikely to produce a cognitive benefit compared to placebo. fMRI has had only limited application in multisite trials, but preliminary reliability studies support its implementation in Phase 2 trials [77, 78]. Treatment benefits demonstrated with fMRI would support circuit-level effects and increase confidence that the treatment will have cognitive benefit [71]. FDG PET circuit analyses and EEG may provide similar insights into circuit-level function but have not been explored in this context in multicenter studies [79].
Fig. 2.7.
Relationship of ATN (amyloid, tau, neurodegeneration) pathology to circuits that underlie human cognition and emotion is comprised in AD
Fig. 2.8.
Area of difference in default mode network (DMN) activation on functional MRI (fMRI) between cognitively normal amyloid-negative older adults and amyloid-positive individuals with mild cognitive impairment (MCI) from the AD Neuroimaging Initiative (ADNI) (figure courtesy of Zhengshi Yang)
2.8. Biomarkers Evidence of Disease Modification
A major role for biomarkers in current drug development programs is to provide evidence in support of disease modification for clinical trials of DMTs. Disease modification is defined as ameliorating the basic processes leading to cell death with a corresponding clinical benefit [16, 17]. To show that disease modification has occurred requires an impact on N (neurodegeneration) of the A,T,N Research Framework [8]. Recognized assessments of N include atrophy on MRI, hypometabolism on FDG PET, or increases in total tau in the CSF. Measures that are promising candidate biomarkers of N include neurofilament light (NfL), neurogranin, and visinin-like protein-1 (VILIP-1) (Fig. 2.9). NfL is an axonal protein appearing in CSF and plasma with neurodegeneration [80]. VILIP-1 is a neuronal calcium sensor protein previously used as a marker of acute ischemic stroke and found to be elevated in CSF of AD patients [81]. Neurogranin is a synaptic protein that is shed into CSF under circumstances of synaptic degeneration in AD [82]. NfL, total tau, and neurogranin have all been shown to correlate with regional cerebral atrophy, although the strength of correlations may vary [83].
Fig. 2.9.
The amyloid (A), tau (T), and neurodegeneration (N) framework of AD with consensus and emerging biomarkers (figure courtesy of Mike de la Flor)
MRI shows progressive atrophy in the course of AD with decline of whole brain and hippocampal volume and increasing ventricular size [84] (Fig. 2.3). Atrophy is correlated with cell loss [85] suggesting that interfering with neurodegeneration and cell loss should slow the rate of atrophy and create a drug-placebo difference at end of trials in favor of active treatment. Review of studies reporting MRI results and listed in Table 2.5 shows that this anticipated result has rarely been achieved. In most trials reporting MRI findings, there has been no drug-placebo difference at trial termination; in a few, the active treatment group has shown greater atrophy. It is uncertain whether the apparently greater shrinkage or “pseudo-atrophy” in the treatment group reflects amyloid removal or reduction of inflammation or if, at least with some treatment mechanisms, neurotoxicity and true increase in atrophy has occurred.
Table 2.5.
Volumetric MRI results in major recent clinical trials
| Agent | Drug-placebo difference at the end of study | Duration of study |
|---|---|---|
| AN1792 [116] | Greater atrophy in the active treatment group (significant for whole brain and ventricular volume; not the hippocampal volume) | 12 monthsa |
| Vitamin E or donepezil [117] | No drug-placebo difference in whole brain, ventricular, entorhinal cortex, or hippocampal volume with either treatment | 36 months |
| Tramiprosate [118] | Dose-dependent preservation of hippocampal volume (post hoc analysis) | 70 weeks |
| DHA [119] | No drug-placebo difference in whole brain volume, ventricular volume, or hippocampal volume | 18 months |
| Valproate [111] | Greater atrophy in the treatment group compared to the placebo group (whole brain, ventricular, hippocampal) | 24 months |
| Avagacestat [113] | No drug-pbo difference in whole brain, ventricular, or hippocampal volume | 24 weeks |
| Semagacestat [97] | No drug-pbo difference in entorhinal cortex or hippocampal volume | 76 weeksa |
| Bapineuzumab; ApoE carrier study [109] | No drug-pbo difference in whole brain volume | 71 weeks |
| Bapineuzumab; ApoE noncarrier study [109] | No drug-pbo difference in whole brain volume | 71 weeks |
| Solanezumab; Expedition [51] | No drug-pbo difference in whole brain or hippocampal volume | 80 weeks |
| Solanezumab Expedition II [51] | No drug-pbo difference in whole brain or hippocampal volume | 80 weeks |
| Azeliragon (TTP488) [120] | No drug-placebo difference in whole brain volume or hippocampal volume | 18 months |
| Resveratrol [121] | Whole brain volume decreased and ventricular volume increased significantly in the treatment group compared to the placebo group | 52 weeks |
| Avagacestat [114] | Greater atrophy rates were observed in the active treatment group for ventricular and whole brain volumes; differences were significant at weeks 24 and 56 but not at 104 (possibly due to small the number of patients remaining the study) | 104 weeks |
| Avagacestat [114] | No drug-placebo difference in whole brain, ventricular, or hippocampal volume at study end; greater atrophy I the treatment group at weeks 24 and 56 | 104 weeks |
| Gantenerumab [69] | No drug-pbo difference in either dose group for whole brain, ventricular, or left-right hippocampal volume | 100 weeks |
| IVIG [110] | No drug-placebo difference in whole brain volume, ventricular volume, or hippocampal volume | 18 months |
| Solanezumab; Expedition III [52] | No drug-pbo difference in whole brain or ventricular volume | 76 weeks |
| Crenezumab [52] | No drug-pbo difference in ventricular volume or whole brain volume | 73 weeks |
| Verubecestat [46] | No significant drug-placebo difference in hippocampal volumes; numerically greater in the active treatment groups | 78 weeks |
BACE β-site amyloid precursor protein cleaving enzyme 1, DHA docosahexaenoic acid, GSI gamma-secretase inhibitor, GSK glycogen synthase kinase
IVIG intravenous immunoglobulin, mAb monoclonal antibody, MOA mechanism of action, pbo placebo
AN1792 and semagacestat trials were stopped before planned completion
CSF total tau is included in the A,T,N Framework as a marker of N [8]. Tau protein is a microtubule-associated protein that has a critical role in intracellular transportation. It may become hyper-phosphorylated to p-tau in the process of forming neurofibrillary tangles where it becomes a marker of T, or it may appear directly in the CSF presumably as a product of cell death and reflecting N. CSF measures of p-tau and total tau have been collected in several major trials (Table 2.2). In a few trials, relatively small magnitude changes in either tau species have been observed. The interpretation of these changes is uncertain. Until recently, measurement of tau across or within laboratories produced inconsistent results, and the evolution of new techniques in tau measures will assist in using tau as an outcome in drug development [86].
Fluorodeoxyglucose PET is among the N measures of the A,T,N Framework (Figs. 2.4 and 2.9). The metabolic activity measured with FDG PET is largely reflective of synaptic activity and neuronal activating [8, 87], and hypometabolism is regarded as a reflection of synaptic compromise in the course of cell death. Relatively few AD clinical trials have included FDG PET as an outcome. Methodologies have evolved to suggest that it can be performed reliably as part of a multisite trial and have the ability to detect treatment effects with relatively small sample sizes [88, 89]. Brain metabolism can be increased with symptomatic treatments, and this potential confound must be considered in FDG studies [90]. Novel imaging biomarkers such as the synaptic vesicle glycoprotein 2A (SV2A) PET ligand, indicative of synaptic density, may present novel opportunities to document neurodegeneration [91, 92].
2.9. Biomarkers for Safety in AD Drug Development
Liver functions, blood counts, muscle enzymes, and electrocardiograms are key biomarkers for drug toxicity [93], and toxicity accounts for the termination of approximately 30% of drug development programs [94]. These measures are included in AD drug development programs. Liver toxicity has been observed with some BACE inhibitors and some 5-HT6 antagonists such as the Phase 2 studies of idalopirdine [95], and QTc prolongation was observed in a trial of citalopram for agitation in AD [96].
Off-target adverse events occurred in the course of gamma-secretase drug development with hypopigmentation, skin cancers, and cognitive and functional impairment relative to placebo [97]. The dermatologic changes are attributed to inhibition of NOTCH proteases; the cognitive and functional toxicity is of uncertain origin. Amyloid-related imaging abnormalities (ARIA) occur with some monoclonal antibodies, and monitoring these with MRI in the course of trials is critical to insuring the safety of these treatments [98].
2.10. FDA Classification of Biomarkers and Integration into Stages of Alzheimer’s Disease
The FDA has proposed a staging system for AD beginning with biomarker-positive asymptomatic individuals (Stage 1); those with cognitive impairment measurable only with sensitive neuropsychological instruments and no functional impairment (Stage 2); those with mild cognitive impairment and functional compromise measurable with sensitive instruments but not sufficient to meet criteria for dementia (Stage 3); and those with mild, moderate, and severe AD dementia (Stages 4, 5, 6) (Table 2.6) [10]. Biomarkers play an important role in this staging system particularly in the Stage 1 where there are no cognitive or functional changes, but biomarker changes indicative of AD pathological changes are present. Biomarkers that are abnormal in this early preclinical phase of the disease include positive amyloid imaging and low CSF Aβ42. The FDA Guidance provides for accelerated approval for a treatment at this stage based on a biomarker thought to be reasonably likely to predict clinical benefit and coupled with a post-approval plan to gather evidence on clinical outcomes. The FDA Guidance noted that no current AD biomarker can be regarded as a surrogate that reliably predicts clinical measure. Full approval would require demonstrating a drug-placebo difference on a clinical outcome. The FDA Guidance [10] indicates that in Stage 2, approval could be based on persuasive effects on neuropsychological measures supported by effects on the characteristic pathophysiological (biomarker) changes of AD. These approval discussions are independent of discussions of acceptable labeling of a new treatment where the designation of being a DMT will likely require robust effects on N-type biomarkers or effects on biomarkers known to predict N or to predict sustained cognitive and functional benefit.
Table 2.6.
Staging system for AD proposed by the FDA with means of achieving full approval or accelerated approval of drugs developed for early AD (FDA Guidance [10])
| Stage 1 | Stage 2 | Stage 3 | Stage 4, 5, and 6 | |
|---|---|---|---|---|
| Biomarkers reflecting underlying AD pathophysiological changes | Positive | Positive | Positive | Positive |
| Cognition | Truly asymptomatic with no subjective complaint or detectable abnormalities on sensitive neuropsychological measures | Subtle detectable abnormalities on sensitive neuropsychological measures | Subtle or more apparent detectable abnormalities on sensitive neuropsychological measures | Mild, moderate, and severe AD dementia with worsening cognitive impairment |
| Function | No functional impairment | No functional impairment. The emergence of subtle functional impairment signals a transition to Stage 3 | Mild but detectable functional impairment. The functional impairment in this stage is not severe enough to warrant a diagnosis of overt dementia | Mild, moderate, and severe AD dementia with worsening functional impairment |
| Clinical endpoints | A clinically meaningful benefit cannot be measured in these patients because there is no clinical impairment to assess (assuming that the duration of a trial is not sufficient to observe and assess the development of clinical impairment during the conduct of the trial) | Persuasive effect on sensitive measures of neuropsychological performance may provide adequate support for a marketing approval. A pattern of putatively beneficial effects demonstrated across multiple individual tests would increase the persuasiveness of the finding. A large magnitude of effect on sensitive measures of neuropsychological performance may also increase their persuasiveness | Favorable effect on cognitive and functional deficits. An integrated scale that adequately and meaningfully assesses both daily function and cognitive effects in early AD patients is acceptable as a single primary efficacy outcome measure | Co-primary approach to assessment of cognitive and functional (or global) measures |
| Alternate approach to clinical endpoints | Conduct a study of sufficient duration to allow the evaluation of the measures indicated for Stage 2 patients | A possible approach is to conduct a study of sufficient duration to allow the evaluation of the measures discussed for Stage 3 patients | Not specified in the guidance | Not specified in the guidance |
| Biomarker endpoints | An effect on the characteristic pathophysiologic changes of AD. A pattern of treatment effects seen across multiple individual biomarker measures would increase the persuasiveness of the putative effect | Supported by similarly persuasive effects on the characteristic pathophysiologic changes of AD | Not specified in the guidance | Not specified in the guidance |
| Full approval | An effect on the characteristic pathophysiologic changes of AD when the fundamental understanding of AD evolves sufficiently to establish surrogacy (no AD biomarkers currently meet the criteria for surrogacy) | The cognitive effects were found to be inherently clinically meaningful, either on face or because they reliably and inevitably are associated with functional benefit later in the course of the disease | Not specified in the guidance | Not specified in the guidance |
| Accelerated approval | An effect on the characteristic pathophysiologic changes of AD analyzed as a primary efficacy measure, may, serve as the basis for an accelerated approval (i.e., the biomarker effects would be found to be reasonably likely to predict clinical benefit, with a post-approval requirement for a study to confirm the predicted clinical benefit) | The cognitive effects were found to be reasonably likely to predict clinical benefit, with a post-approval requirement for a study to confirm the predicted clinical benefit required | Not specified in the guidance | Not specified in the guidance |
The FDA divides biomarkers into categories of diagnostic, prognostic, predictive, response, and safety [99] (Table 2.7). Diagnostic biomarkers insure accurate diagnosis and allow categorization of a condition by the presence or absence of a specific pathophysiological state. Prognostic biomarkers indicate disease course and can be used to enrich populations to optimize establishing a drug-placebo difference. Predictive biomarkers assist in forecasting the response to treatment. Pharmacodynamic or activity biomarkers show that a biological response has occurred in an individual who received the therapeutic intervention. Pharmacodynamic biomarkers are used in Phase 2 studies to improve understanding of how to use a drug and to guide dose or regimen decisions for Phase 3. Evidence of disease modification also depends on pharmacodynamic biomarkers. Safety bio-markers are used to capture adverse events.
Table 2.7.
FDA terminology for biomarkers and identification of biomarkers in each category for AD drug development (adapted from Amur et al. [103])
| FDA biomarker type | Examples for drug development | Examples from AD drug development |
|---|---|---|
| Diagnostic biomarkers | Patient selection | Positive amyloid imaging Low CSF Aβ42 or change in Aβ/tau or Aβ/p-tau ratio |
| Prognostic biomarkers | Stratify patients or enrich trials with patients likely to have disease | Tau PET to identify AD patients likely to have more rapid cognitive progression ApoE-4 carriers as a prognostic marker for ARIA in immunotherapy programs |
| Predictive biomarkers | Stratification Enrichment/inclusion criteria Enrichment/companion diagnostic | Use of tau PET to identify AD patients more likely to respond to anti-tau therapies |
| Response biomarkers | Pharmacodynamic biomarker as an indicator of intended drug activity Efficacy response biomarker as a surrogate for a clinical endpoint | Target engagement biomarkers (e.g., reduction in amyloid plaque in anti-amyloid programs) Markers of disease modification (e.g., drug-placebo differences in CSF total tau, FDG PET hypometabolism, or MRI atrophy) |
| Safety biomarkers | Biomarkers to detect adverse and off-target drug responses | MRI to monitor for ARIA in immunotherapy programs |
ApoE apolipoprotein E, ARIA amyloid-related imaging abnormalities, CSF cerebrospinal fluid, FDG fluorodeoxyglucose, MRI magnetic resonance imaging, PET positron-emission tomography
In AD drug development, evidence of amyloidosis is considered diagnostic of the AD pathological process; tau PET or MRI atrophy might serve a prognostic biomarkers of participants who will decline more rapidly; inflammatory markers might serve as a predictive biomarker for patients most likely to respond to anti-inflammatory therapies; both target engagement biomarkers and biomarkers of disease modification are pharmacodynamic biomarkers (Fig. 2.6); and use of MRI to monitor of ARIA is an example of a safety biomarker (Table 2.7).
2.11. Biomarker Qualification and Context of Use
Qualification refers to the FDA-defined process of reviewing drug development tools (DDTs) intended for use in multiple development programs [99]. DDTs include biomarkers, clinical outcome assessments, and animal models of drug development. Once qualified, drug developers can use the biomarker within the specific context of use (COU) for the qualified purpose as long as no new information that conflicts with the original basis for qualification has evolved. The qualification process is intended to expedite drug development by making publically available DDTs that can be widely employed. A qualified DDT can be relied on to have a specific interpretation in drug development and regulatory review. Qualification is a complex process that begins with an initiation request and DDT letter of intent. This stage (Stage 1) is followed by consultation and advice with an FDA Qualification Review Team (QRT) regarding the submitter’s goals and the COU of the DDT, current understanding of the available data, identification of information gaps, discussion of additional information that may be needed, and construction of a plan for the qualification process (Stage 2). Stage 3 is comprised of review of the full qualification package [99]. The DDT COU proposal is reviewed by individual disciplines, and a final combined executive summary and recommendation are issued by the QRT. The qualification process is typically pursued by organizations such as the Coalition Against Major Disease (CAMD) of the Critical Path Institute [100]. No AD-related biomarkers have been advanced to qualification by the FDA. The European Medicines Agency (EMA) approved low CSF Aβ1–42 and high t-tau as qualified for identification of prodromal AD; CSF Aβ1–42, t-tau, and amyloid PET to enrich for subjects in trials of mild-to-moderate AD; and hippocampal volume for enrichment of trials in predementia stages of AD [101, 102].
DDT qualification is not necessary for use of a DDT within an individual drug development program, and use of a DDT in a program does not automatically qualify the DDT for the general COU. When qualified biomarkers are not available, the pharmaceutical developer engages with FDA to reach agreement on the use of a particular biomarker in the drug development program [103]. In the case of fluid biomarkers, the sponsor must present information on specified reagents, analytical validation, rigorous process standardization, procedures for sample collection and handling, measurement stability, environmental change tolerance, lot-to-lot variability, computational procedures, and validated, reliable, and accurate interpretation [13, 104]. The specific use of the biomarker in the development program must be specified with a program-specific COU. These discussions are confidential and do not become available to other sponsors.
2.12. Companion and Complementary Diagnostics
The reliance on biomarkers in the drug development process lends itself to the development of companion or complementary diagnostics. A companion diagnostic is an in vitro diagnostic device (IVD) required for the safe and effective use of a corresponding therapeutic. The companion test may identify those most likely to respond or to have side effects, monitor therapy, or identify those in whom the therapeutic product has been adequately studied and found to be safe and effective [99, 105]. When approved as a companion diagnostic, use of the IVD is mandatory before prescribing the drug or biologic. Extensive analytical and clinical validation is required for IVDs used as companion diagnostics [13]. A critical part of the companion diagnostic development is determining the cutoff level which determines that the test is abnormal and dichotomizes the population into those with normal or abnormal status [105]. If an anti-amyloid therapy is approved based on a clinical trial in which participants were defined by abnormal amyloid imaging, then amyloid PET may be identified as a companion diagnostic required for the safe and effective use of the therapeutic. Determining the standard uptake value ratio (SUVR) defining the participant as having brain amyloidosis will be required if the SUVR was used in the pivotal trial. Visual reads of amyloid PET would be required if that is the approach used in the trial.
Complementary diagnostic tests are not required for prescribing a therapeutic agent but can identify a biomarker-defined subset of patients that responds particularly well or aids in the risk/benefit assessments for individual patients [105, 106]. For example, an anti-amyloid monoclonal antibody might benefit both ApoE-4 carriers and noncarriers, but a higher rate of ARIA in carriers would play a role in the risk/benefit discussion with the potential treatment recipient. In this case, the ApoE genotype test would function as a complementary biomarker.
2.13. Summary
Biomarkers play a central role in AD drug development and are likely to become increasingly important as the biology of AD is more understood and the repertoire of biomarkers is expanded. Biomarkers can be used to assist in diagnosis, demonstrate target engagement, provide support for disease modification, and monitor for safety (Table 2.8). Diagnostic, predictive, prognostic, pharmacodynamic, and safety biomarkers have been identified. The A,T,N Research Framework integrates bio-markers into the process of AD diagnosis and can be applied to drug development and clinical trials. The FDA staging of AD facilitates drug development for predementia stages of AD and integrates biomarkers into the staging system. Companion and complementary biomarkers may be developed in concert with new therapeutics in drug development programs. Informed use of biomarkers promises to accelerate AD drug development and assist in bringing new therapies to those with or at risk for AD.
Table 2.8.
Examples of biomarkers for each phase of AD drug development
| Type of biomarker | Phase 1 | Phase 2 | Phase 3 |
|---|---|---|---|
| Diagnostic biomarker (e.g., amyloid PET or CSF Aβ or Aβ/tau ratio) | If AD-spectrum patients are included in the Phase 1 program | All patients | All patients |
| Target engagement biomarker | Critical outcome of Phase 2 to allow progression to Phase 3 | ||
| Marker of disease modification | Critical outcome to allow labeling of the intervention as a DMT | ||
| Prognostic biomarker (e.g., ApoE-4 carrier status) | Important for analysis of outcomes and prediction of ARIA in immunotherapy programs | Important for analysis of outcomes and prediction of ARIA in immunotherapy programs | |
| Safety biomarkers | Liver function and other laboratory tests, ECG, MRI to monitor for ARIA in immunotherapy programs | Liver function and other laboratory tests, ECG, MRI to monitor for ARIA in immunotherapy programs | Liver function and other laboratory tests, ECG, MRI to monitor for ARIA in immunotherapy programs |
ApoE apolipoprotein, ARIA amyloid-related imaging abnormalities, ECG electrocardiography, CSF cerebrospinal fluid, MRI magnetic resonance imaging, PET positron-emission tomography
Acknowledgment
J.C. acknowledges funding from the National Institute of General Medical Sciences (Grant: P20GM109025) and support from Keep Memory Alive.
Declaration of Interest: J.C. has provided consultation to Acadia, Avanir, BiOasis Technologies, Biogen, Boehringer-Ingelheim, Bracket, Eisai, Genentech, Grifols, Intracellular Therapies, Kyowa, Lilly, Lundbeck, Medavante, Merck, Nutricia, Otsuka, Pfizer, QR, Resverlogix, Samus, Servier, Suven, Takeda, Toyama, and United Neuroscience companies. J.C. acknowledges the funding from the National Institute of General Medical Sciences (Grant: P20GM109025) and support from Keep Memory Alive.
References
- 1.Masters CL, Bateman R, Blennow K, Rowe CC, Sperling RA, Cummings JL (2015) Alzheimer’s disease. Nat Rev Dis Primers 1:15056 10.1038/nrdp.2015.56 [DOI] [PubMed] [Google Scholar]
- 2.Scheltens P, Blennow K, Breteler MM, de Strooper B, Frisoni GB, Salloway S et al. (2016) Alzheimer’s disease. Lancet 388:505–517 [DOI] [PubMed] [Google Scholar]
- 3.Alzheimer’s Association (2016) 2016 Alzheimer’s disease facts and figures. Alzheimers Dement 12:459–509 [DOI] [PubMed] [Google Scholar]
- 4.Prince M, Wimo A, Guerchet M, Ali GC, Wu YT, Prina M et al. (2015) Alzheimer’s Disease International World Alzheimer Report 2015: the global impact of dementia, an analysis of prevalence, incidence, cost and trends. London, 2015. https://www.alz.co.uk/research/world-report-2015 [Google Scholar]
- 5.Wimo A, Guerchet M, Ali GC, Wu YT, Prina AM, Winblad B et al. (2017) The worldwide costs of dementia 2015 and comparisons with 2010. Alzheimers Dement 13:1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cummings JL, Morstorf T, Zhong K (2014) Alzheimer’s disease drug-development pipeline: few candidates, frequent failures. Alzheimers Res Ther 6:37–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cummings J, Ritter A, Zhong K (2018) Clinical trials for disease-modifying therapies in Alzheimer’s disease: a primer, lessons learned, and a blueprint for the future. J Alzheimers Dis 64:S3–S22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jack CR Jr, Bennett DA, Blennow K, Carrillo MC, Dunn B, Haeberlein SB et al. (2018) NIA-AA Research framework: toward a biological definition of Alzheimer’s disease. Alzheimers Dement 14:535–562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cummings J (2018) The National Institute on Aging-Alzheimer’s Association framework on Alzheimer’s disease: application to clinical trials. Alzheimers Dement June 21 10.1016/j.jalz.2018.05.006. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.U.S. Food and Drug Administration (2018) Early Alzheimer’s disease: developing drugs for treatment, guidance for industry. Office of Communications, Division of Drug Information, Center for Drug Evaluation and Research, Food and Drug Administration, Silver Spring, MD: https://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM596728.pdf [Google Scholar]
- 11.Biomarkers Definitions Working Group (2001) Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin Pharmacol Ther 69:89–95 [DOI] [PubMed] [Google Scholar]
- 12.U.S. Food and Drug Administration (2014) In vitro companion diagnostic devices: guidance for industry and Food and Drug Administration staff. Center for Drug Evaluation and Research, Division of Drug Information, Silver Spring, MD: https://www.fda.gov/downloads/MedicalDevices/DeviceRegulationandGuidance/GuidanceDocuments/UCM262327.pdf [Google Scholar]
- 13.Kraus VB (2018) Biomarkers as drug development tools: discovery, validation, qualification and use. Nat Rev Rheumatol 14:354–362 [DOI] [PubMed] [Google Scholar]
- 14.FDA-NIH Biomarker Working Group (2016) BEST (Biomarkers, EndpointS, and other Tools) resource. Food and Drug Administration and National Institutes of Health, Silver Spring, MD: [PubMed] [Google Scholar]
- 15.Day M, Rutkowski JL, Feuerstein GZ (2009) Translational medicine--a paradigm shift in modern drug discovery and development: the role of biomarkers. Adv Exp Med Biol 655:1–12 [DOI] [PubMed] [Google Scholar]
- 16.Cummings J (2017) Disease modification and neuroprotection in neurodegenerative disorders. Transl Neurodegener 6:25 10.1186/s40035-017-0096-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cummings JL, Fox N (2017) Defining disease modification for Alzheimer’s disease clinical trials. J Prev Alzheimers Dis 4:109–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM (1984) Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 34:939–944 [DOI] [PubMed] [Google Scholar]
- 19.McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR Jr, Kawas CH et al. (2011) The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7:263–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Landau SM, Horng A, Fero A, Jagust WJ, Alzheimer’s Disease Neuroimaging Initiative (2016) Amyloid negativity in patients with clinically diagnosed Alzheimer disease and MCI. Neurology 86:1377–1385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sevigny J, Suhy J, Chiao P, Chen T, Klein G, Purcell D et al. (2016) Amyloid PET screening for enrichment of early-stage Alzheimer disease clinical trials: experience in a phase 1b clinical trial. Alzheimer Dis Assoc Disord 30:1–7 [DOI] [PubMed] [Google Scholar]
- 22.Wisse LEM, Butala N, Das SR, Davatzikos C, Dickerson BC, Vaishnavi SN et al. (2015) Suspected non-AD pathology in mild cognitive impairment. Neurobiol Aging 36:3152–3162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ballard C, Atri A, Boneva N, Cummings JL, Frolich L, Molinuevo JL et al. (2018) Predictors of Alzheimer disease progression for enrichment of clinical trials: analysis of placebo data from a phase 3 program (submitted)
- 24.Ellendt S, Vobeta B, Kohn N, Wagels L, Goerlich KS, Drexler E et al. (2017) Predicting stability of mild cognitive impairment (MCI): findings of a community based sample. Curr Alzheimer Res 14:608–619 [DOI] [PubMed] [Google Scholar]
- 25.Bangen KJ, Clark AL, Werhane M, Edmonds EC, Nation DA, Evangelista N et al. (2016) Cortical amyloid burden differences across empirically-derived mild cognitive impairment subtypes and interaction with APOE varepsilon4 genotype. J Alzheimers Dis 52:849–861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jicha GA, Parisi JE, Dickson DW, Johnson K, Cha R, Ivnik RJ et al. (2006) Neuropathologic outcome of mild cognitive impairment following progression to clinical dementia. Arch Neurol 63:674–681 [DOI] [PubMed] [Google Scholar]
- 27.Dubois B, Feldman HH, Jacova C, Hampel H, Molinuevo JL, Blennow K et al. (2014) Advancing research diagnostic criteria for Alzheimer’s disease: the IWG-2 criteria. Lancet Neurol 13:614–629 [DOI] [PubMed] [Google Scholar]
- 28.Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC et al. (2011) The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7:270–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Palmqvist S, Mattsson N, Hansson O, Alzheimer’s Disease Neuroimaging Initiative (2016) Cerebrospinal fluid analysis detects cerebral amyloid-beta accumulation earlier than positron emission tomography. Brain 139:1226–1236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jansen WJ, Ossenkoppele R, Knol DL, Tijms BM, Scheltens P, Verhey FR et al. (2015) Prevalence of cerebral amyloid pathology in persons without dementia: a meta-analysis. JAMA 313:1924–1938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Insel PS, Palmqvist S, Mackin RS, Nosheny RL, Hansson O, Weiner MW et al. (2016) Assessing risk for preclinical beta-amyloid pathology with APOE, cognitive, and demographic information. Alzheimers Dement (Amst) 4:76–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cummings J, Lee G, Ritter A, Zhong K (2018) Alzheimer’s disease drug development pipeline: 2018. Alzheimers Dement (N Y) 4:195–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Farlow M, Gracon SI, Hershey LA, Lewis KW, Sadowsky CH, Dolan-Ureno J (1992) A controlled trial of tacrine in Alzheimer’s disease. The Tacrine Study Group. JAMA 268:2523–2529 [PubMed] [Google Scholar]
- 34.Rogers SL, Farlow MR, Doody RS, Mohs R, Friedhoff LT (1998) A 24-week, double-blind, placebo-controlled trial of donepezil in patients with Alzheimer’s disease. Donepezil Study Group. Neurology 50:136–145 [DOI] [PubMed] [Google Scholar]
- 35.Tan CC, Yu JT, Wang HF, Tan MS, Meng XF, Wang C et al. (2014) Efficacy and safety of donepezil, galantamine, rivastigmine, and memantine for the treatment of Alzheimer’s disease: a systematic review and meta-analysis. J Alzheimers Dis 41:615–631 [DOI] [PubMed] [Google Scholar]
- 36.Cummings JL (2008) Optimizing phase II of drug development for disease-modifying compounds. Alzheimers Dement 4:S15–S20 [DOI] [PubMed] [Google Scholar]
- 37.Cook D, Brown D, Alexander R, March R, Morgan P, Satterthwaite G et al. (2014) Lessons learned from the fate of AstraZeneca’s drug pipeline: a five-dimensional framework. Nat Rev Drug Discov 13:419–431 [DOI] [PubMed] [Google Scholar]
- 38.Morgan P, Brown DG, Lennard S, Anderton MJ, Barrett JC, Eriksson U et al. (2018) Impact of a five-dimensional framework on R&D productivity at AstraZeneca. Nat Rev Drug Discov 17:167–181 [DOI] [PubMed] [Google Scholar]
- 39.Simon GM, Niphakis MJ, Cravatt BF (2013) Determining target engagement in living systems. Nat Chem Biol 9:200–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bateman RJ, Munsell LY, Morris JC, Swarm R, Yarasheski KE, Holtzman DM (2006) Human amyloid-beta synthesis and clearance rates as measured in cerebrospinal fluid in vivo. Nat Med 12:856–861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mawuenyega KG, Sigurdson W, Ovod V, Munsell L, Kasten T, Morris JC et al. (2010) Decreased clearance of CNS beta-amyloid in Alzheimer’s disease. Science 330:1774 10.1126/science.1197623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Potter R, Patterson BW, Elbert DL, Ovod V, Kasten T, Sigurdson W et al. (2013) Increased in vivo amyloid-beta42 production, exchange, and loss in presenilin mutation carriers. Sci Transl Med 5:189ra77 10.1126/scitranslmed.3005615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bateman RJ, Siemers ER, Mawuenyega KG, Wen G, Browning KR, Sigurdson WC et al. (2009) A gamma-secretase inhibitor decreases amyloid-beta production in the central nervous system. Ann Neurol 66:48–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fleisher AS, Raman R, Siemers ER, Becerra L, Clark CM, Dean RA et al. (2008) Phase 2 safety trial targeting amyloid beta production with a gamma-secretase inhibitor in Alzheimer disease. Arch Neurol 65:1031–1038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kennedy ME, Stamford AW, Chen X, Cox K, Cumming JN, Dockendorf MF et al. (2016) The BACE1 inhibitor verubecestat (MK-8931) reduces CNS beta-amyloid in animal models and in Alzheimer’s disease patients. Sci Transl Med 8:363ra150 10.1126/scitranslmed.aad9704 [DOI] [PubMed] [Google Scholar]
- 46.Egan MF, Kost J, Tariot PN, Aisen PS, Cummings JL, Vellas B et al. (2018) Randomized trial of verubecestat for mild-to-moderate Alzheimer’s disease. N Engl J Med 378:1691–1703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Portelius E, Zetterberg H, Dean RA, Marcil A, Bourgeois P, Nutu M et al. (2012) Amyloid-beta(1–15/16) as a marker for gamma-secretase inhibition in Alzheimer’s disease. J Alzheimers Dis 31:335–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sato C, Barthelemy NR, Mawuenyega KG, Patterson BW, Gordon BA, Jockel-Balsarotti J et al. (2018) Tau kinetics in neurons and the human central nervous system. Neuron 97:1284–1298.e7. 10.1016/j.neuron.2018.04.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Coughlin D, Irwin DJ (2017) Emerging diagnostic and therapeutic strategies for tauopathies. Curr Neurol Neurosci Rep 17:72 10.1007/s11910-017-0779-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lewczuk P, Matzen A, Blennow K, Parnetti L, Molinuevo JL, Eusebi P et al. (2017) Cerebrospinal fluid Abeta42/40 corresponds better than Abeta42 to amyloid PET in Alzheimer’s disease. J Alzheimers Dis 55:813–822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Doody RS, Thomas RG, Farlow M, Iwatsubo T, Vellas B, Joffe S et al. (2014) Phase 3 trials of solanezumab for mild-to-moderate Alzheimer’s disease. N Engl J Med 370:311–321 [DOI] [PubMed] [Google Scholar]
- 52.Honig LS, Vellas B, Woodward M, Boada M, Bullock R, Borrie M et al. (2018) Trial of solanezumab for mild dementia due to Alzheimer’s disease. N Engl J Med 378:321–330 [DOI] [PubMed] [Google Scholar]
- 53.Cummings JL, Cohen S, van Dyck CH, Brody M, Curtis C, Cho W et al. (2018) ABBY: a phase 2 randomized trial of crenezumab in mild to moderate Alzheimer disease. Neurology 90:e1889–e1e97. 10.1212/WNL.0000000000005550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee SJ, Nam E, Lee HJ, Savelieff MG, Lim MH (2017) Towards an understanding of amyloid-beta oligomers: characterization, toxicity mechanisms, and inhibitors. Chem Soc Rev 46:310–323 [DOI] [PubMed] [Google Scholar]
- 55.Heneka MT, Carson MJ, El Khoury J, Landreth GE, Brosseron F, Feinstein DL et al. (2015) Neuroinflammation in Alzheimer’s disease. Lancet Neurol 14:388–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brosseron F, Traschutz A, Widmann CN, Kummer MP, Tacik P, Santarelli F et al. (2018) Characterization and clinical use of inflammatory cerebrospinal fluid protein markers in Alzheimer’s disease. Alzheimers Res Ther 10:25 10.1186/s13195-018-0353-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yarchoan M, Louneva N, Xie SX, Swenson FJ, Hu W, Soares H et al. (2013) Association of plasma C-reactive protein levels with the diagnosis of Alzheimer’s disease. J Neurol Sci 333:9–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Serpente M, Bonsi R, Scarpini E, Galimberti D (2014) Innate immune system and inflammation in Alzheimer’s disease: from pathogenesis to treatment. Neuroimmunomodulation 21:79–87 [DOI] [PubMed] [Google Scholar]
- 59.Muszynski P, Groblewska M, Kulczynska-Przybik A, Kulakowska A, Mroczko B (2017) YKL-40 as a potential biomarker and a possible target in therapeutic strategies of Alzheimer’s disease. Curr Neuropharmacol 15:906–917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Passamonti L, Rodriguez PV, Hong YT, Allinson KSJ, Bevan-Jones WR, Williamson D et al. (2018) [(11)C]PK11195 binding in Alzheimer disease and progressive supranuclear palsy. Neurology 90:e1989–e1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Galasko D, Montine TJ (2010) Biomarkers of oxidative damage and inflammation in Alzheimer’s disease. Biomark Med 4:27–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Montine TJ, Peskind ER, Quinn JF, Wilson AM, Montine KS, Galasko D (2011) Increased cerebrospinal fluid F2-isoprostanes are associated with aging and latent Alzheimer’s disease as identified by biomarkers. NeuroMolecular Med 13:37–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kester MI, Scheffer PG, Koel-Simmelink MJ, Twaalfhoven H, Verwey NA, Veerhuis R et al. (2012) Serial CSF sampling in Alzheimer’s disease: specific versus non-specific markers. Neurobiol Aging 33:1591–1598 [DOI] [PubMed] [Google Scholar]
- 64.Nordstrom AL, Mansson M, Jovanovic H, Karlsson P, Halldin C, Farde L et al. (2008) PET analysis of the 5-HT2A receptor inverse agonist ACP-103 in human brain. Int J Neuropsychopharmacol 11:163–171 [DOI] [PubMed] [Google Scholar]
- 65.Cummings J, Isaacson S, Mills R, Williams H, Chi-Burris K, Corbett A et al. (2014) Pimavanserin for patients with Parkinson’s disease psychosis: a randomised, placebo-controlled phase 3 trial. Lancet 383:533–540 [DOI] [PubMed] [Google Scholar]
- 66.Ballard C, Banister C, Khan Z, Cummings J, Demos G, Coate B et al. (2018) Evaluation of the safety, tolerability, and efficacy of pimavanserin versus placebo in patients with Alzheimer’s disease psychosis: a phase 2, randomised, placebo-controlled, double-blind study. Lancet Neurol 17:213–222 [DOI] [PubMed] [Google Scholar]
- 67.Cummings J, Ballard C, Tariot P, Owen R, Foff E, Youakim J et al. (2018) Pimavanserin: potential treatment for dementia-related psychosis. J Prev Alzheimer Dis 5(4):253–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sevigny J, Chiao P, Bussiere T, Weinreb PH, Williams L, Maier M et al. (2016) The antibody aducanumab reduces Abeta plaques in Alzheimer’s disease. Nature 537:50–56 [DOI] [PubMed] [Google Scholar]
- 69.Ostrowitzki S, Lasser RA, Dorflinger E, Scheltens P, Barkhof F, Nikolcheva T et al. (2017) A phase III randomized trial of gantenerumab in prodromal Alzheimer’s disease. Alzheimers Res Ther 9:95 10.1186/s13195-017-0318-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cummings JL, Zhong K, Kinney JW, Heaney C, Moll-Tudla J, Joshi A et al. (2016) Double-blind, placebo-controlled, proof-of-concept trial of bexarotene in moderate Alzheimer’s disease. Alzheimers Res Ther 8:4 10.1186/s13195-016-0173-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cummings J, Zhong K, Cordes D (2017) Drug development in Alzheimer’s disease: the role of default mode network assessment in phase II. US Neurol 13:67–69 [Google Scholar]
- 72.Canter RG, Penney J, Tsai LH (2016) The road to restoring neural circuits for the treatment of Alzheimer’s disease. Nature 539:187–196 [DOI] [PubMed] [Google Scholar]
- 73.Sorg C, Riedl V, Muhlau M, Calhoun VD, Eichele T, Laer L et al. (2007) Selective changes of resting-state networks in individuals at risk for Alzheimer’s disease. Proc Natl Acad Sci USA 104:18760–18765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhou J, Greicius MD, Gennatas ED, Growdon ME, Jang JY, Rabinovici GD et al. (2010) Divergent network connectivity changes in behavioural variant frontotemporal dementia and Alzheimer’s disease. Brain 133:1352–1367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.McLaren DG, Sreenivasan A, Diamond EL, Mitchell MB, Van Dijk KR, Deluca AN et al. (2012) Tracking cognitive change over 24 weeks with longitudinal functional magnetic resonance imaging in Alzheimer’s disease. Neurodegener Dis 9:176–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dickerson BC, Sperling RA (2009) Large-scale functional brain network abnormalities in Alzheimer’s disease: insights from functional neuroimaging. Behav Neurol 21:63–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Atri A, O’Brien JL, Sreenivasan A, Rastegar S, Salisbury S, DeLuca AN et al. (2011) Test-retest reliability of memory task functional magnetic resonance imaging in Alzheimer disease clinical trials. Arch Neurol 68:599–606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sutton BP, Goh J, Hebrank A, Welsh RC, Chee MW, Park DC (2008) Investigation and validation of intersite fMRI studies using the same imaging hardware. J Magn Reson Imaging 28:21–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Soares HD (2010) The use of mechanistic biomarkers for evaluating investigational CNS compounds in early drug development. Curr Opin Investig Drugs 11:795–801 [PubMed] [Google Scholar]
- 80.Mattsson N, Andreasson U, Zetterberg H, Blennow K, Alzheimer’s Disease Neuroimaging Initiative (2017) Association of plasma neurofilament light with neurodegeneration in patients with Alzheimer disease. JAMA Neurol 74:557–566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Babic Leko M, Borovecki F, Dejanovic N, Hof PR, Simic G (2016) Predictive value of cerebrospinal fluid visinin-like protein-1 levels for Alzheimer’s disease early detection and differential diagnosis in patients with mild cognitive impairment. J Alzheimers Dis 50:765–778 [DOI] [PubMed] [Google Scholar]
- 82.Tarawneh R, D’Angelo G, Crimmins D, Herries E, Griest T, Fagan AM et al. (2016) Diagnostic and prognostic utility of the synaptic marker neurogranin in Alzheimer disease. JAMA Neurol 73:561–571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pereira JB, Westman E, Hansson O, Alzheimer’s Disease Neuroimaging Initiative (2017) Association between cerebrospinal fluid and plasma neurodegeneration biomarkers with brain atrophy in Alzheimer’s disease. Neurobiol Aging 58:14–29 [DOI] [PubMed] [Google Scholar]
- 84.Jack CR Jr, Shiung MM, Weigand SD, O’Brien PC, Gunter JL, Boeve BF et al. (2005) Brain atrophy rates predict subsequent clinical conversion in normal elderly and amnestic MCI. Neurology 65:1227–1231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Whitwell JL, Josephs KA, Murray ME, Kantarci K, Przybelski SA, Weigand SD et al. (2008) MRI correlates of neurofibrillary tangle pathology at autopsy: a voxel-based morphometry study. Neurology 71:743–749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Molinuevo JL, Blennow K, Dubois B, Engelborghs S, Lewczuk P, Perret-Liaudet A et al. (2014) The clinical use of cerebrospinal fluid biomarker testing for Alzheimer’s disease diagnosis: a consensus paper from the Alzheimer’s Biomarkers Standardization Initiative. Alzheimers Dement 10:808–817 [DOI] [PubMed] [Google Scholar]
- 87.Dupont AC, Largeau B, Guilloteau D, Santiago Ribeiro MJ, Arlicot N (2018) The place of PET to assess new therapeutic effectiveness in neurodegenerative diseases. Contrast Media Mol Imaging 2018:7043578 10.1155/2018/7043578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Alexander GE, Chen K, Pietrini P, Rapoport SI, Reiman EM (2002) Longitudinal PET evaluation of cerebral metabolic decline in dementia: a potential outcome measure in Alzheimer’s disease treatment studies. Am J Psychiatry 159:738–745 [DOI] [PubMed] [Google Scholar]
- 89.Chen K, Langbaum JB, Fleisher AS, Ayutyanont N, Reschke C, Lee W et al. (2010) Twelve-month metabolic declines in probable Alzheimer’s disease and amnestic mild cognitive impairment assessed using an empirically pre-defined statistical region-of-interest: findings from the Alzheimer’s Disease Neuroimaging Initiative. NeuroImage 51:654–664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mega MS, Dinov ID, Porter V, Chow G, Reback E, Davoodi P et al. (2005) Metabolic patterns associated with the clinical response to galantamine therapy: a fludeoxyglucose f 18 positron emission tomographic study. Arch Neurol 62:721–728 [DOI] [PubMed] [Google Scholar]
- 91.Mercier J, Provins L, Valade A (2017) Discovery and development of SV2A PET tracers: potential for imaging synaptic density and clinical applications. Drug Discov Today Technol 25:45–52 [DOI] [PubMed] [Google Scholar]
- 92.Chen MK, Mecca AP, Naganawa M, Finnema SJ, Toyonaga T, Lin SF et al. (2018) Assessing synaptic density in Alzheimer disease with synaptic vesicle glycoprotein 2A positron emission tomographic imaging. JAMA Neurol 75(10):1215–1224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kleinstreuer NC, Sullivan K, Allen D, Edwards S, Mendrick DL, Embry M et al. (2016) Adverse outcome pathways: from research to regulation scientific workshop report. Regul Toxicol Pharmacol 76:39–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Arrowsmith J, Miller P (2013) Trial watch: phase II and phase III attrition rates 2011–2012. Nat Rev Drug Discov 12:569 10.1038/nrd4090 [DOI] [PubMed] [Google Scholar]
- 95.Wilkinson D, Windfeld K, Colding-Jorgensen E (2014) Safety and efficacy of idalopirdine, a 5-HT6 receptor antagonist, in patients with moderate Alzheimer’s disease (LADDER): a randomised, double-blind, placebo-controlled phase 2 trial. Lancet Neurol 13:1092–1099 [DOI] [PubMed] [Google Scholar]
- 96.Porsteinsson AP, Drye LT, Pollock BG, Devanand DP, Frangakis C, Ismail Z et al. (2014) Effect of citalopram on agitation in Alzheimer disease: the CitAD randomized clinical trial. JAMA 311:682–691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Doody RS, Raman R, Farlow M, Iwatsubo T, Vellas B, Joffe S et al. (2013) A phase 3 trial of semagacestat for treatment of Alzheimer’s disease. N Engl J Med 369:341–350 [DOI] [PubMed] [Google Scholar]
- 98.Sperling RA, Jack CR Jr, Black SE, Frosch MP, Greenberg SM, Hyman BT et al. (2011) Amyloid-related imaging abnormalities in amyloid-modifying therapeutic trials: recommendations from the Alzheimer’s Association Research Roundtable Workgroup. Alzheimers Dement 7:367–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.U.S. Food and Drug Administration (2014) Guidance for industry and FDA staff: qualification process for drug development tools. Division of Drug Information, Center for Drug Evaluation and Research, Food and Drug Administration, Silver Spring, MD: https://www.fda.gov/downloads/drugs/guidances/ucm230597.pdf [Google Scholar]
- 100.Romero K, de MM, Frank D, Anthony M, Neville J, Kirby L et al. (2009) The Coalition Against Major Diseases: developing tools for an integrated drug development process for Alzheimer’s and Parkinson’s diseases. Clin Pharmacol Ther 86:365–367 [DOI] [PubMed] [Google Scholar]
- 101.Hill DL, Schwarz AJ, Isaac M, Pani L, Vamvakas S, Hemmings R et al. (2014) Coalition Against Major Diseases/European Medicines Agency biomarker qualification of hippocampal volume for enrichment of clinical trials in predementia stages of Alzheimer’s disease. Alzheimers Dement 10:421–429.e3. 10.1016/j.jalz.2013.07.003 [DOI] [PubMed] [Google Scholar]
- 102.Arneric SP, Batrla-Utermann R, Beckett L, Bittner T, Blennow K, Carter L et al. (2017) Cerebrospinal fluid biomarkers for Alzheimer’s disease: a view of the regulatory science qualification landscape from the Coalition Against Major Diseases CSF Biomarker Team. J Alzheimers Dis 55:19–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Amur S, LaVange L, Zineh I, Buckman-Garner S, Woodcock J (2015) Biomarker qualification: toward a multiple stakeholder framework for biomarker development, regulatory acceptance, and utilization. Clin Pharmacol Ther 98:34–46 [DOI] [PubMed] [Google Scholar]
- 104.Gerlach CV, Derzi M, Ramaiah SK, Vaidya VS (2018) Industry perspective on biomarker development and qualification. Clin Pharmacol Ther 103:27–31 [DOI] [PubMed] [Google Scholar]
- 105.Jorgensen JT, Hersom M (2018) Clinical and regulatory aspects of companion diagnostic development in oncology. Clin Pharmacol Ther 103:999–1008 [DOI] [PubMed] [Google Scholar]
- 106.Scheerens H, Malong A, Bassett K, Boyd Z, Gupta V, Harris J et al. (2017) Current status of companion and complementary diagnostics: strategic considerations for development and launch. Clin Transl Sci 10:84–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gilman S, Koller M, Black RS, Jenkins L, Griffith SG, Fox NC et al. (2005) Clinical effects of Abeta immunization (AN1792) in patients with AD in an interrupted trial. Neurology 64:1553–1562 [DOI] [PubMed] [Google Scholar]
- 108.Galasko DR, Peskind E, Clark CM, Quinn JF, Ringman JM, Jicha GA et al. (2012) Antioxidants for Alzheimer disease: a randomized clinical trial with cerebrospinal fluid biomarker measures. Arch Neurol 69:836–841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Salloway S, Sperling R, Fox NC, Blennow K, Klunk W, Raskind M et al. (2014) Two phase 3 trials of bapineuzumab in mild-to-moderate Alzheimer’s disease. N Engl J Med 370:322–333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Relkin NR, Thomas RG, Rissman RA, Brewer JB, Rafii MS, van Dyck CH et al. (2017) A phase 3 trial of IV immunoglobulin for Alzheimer disease. Neurology 88:1768–1775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Fleisher AS, Truran D, Mai JT, Langbaum JB, Aisen PS, Cummings JL et al. (2011) Chronic divalproex sodium use and brain atrophy in Alzheimer disease. Neurology 77:1263–1271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sabbagh MN, Agro A, Bell J, Aisen PS, Schweizer E, Galasko D (2011) PF-04494700, an oral inhibitor of receptor for advanced glycation end products (RAGE), in Alzheimer disease. Alzheimer Dis Assoc Disord 25:206–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Coric V, van Dyck CH, Salloway S, Andreasen N, Brody M, Richter RW et al. (2012) Safety and tolerability of the gamma-secretase inhibitor avagacestat in a phase 2 study of mild to moderate Alzheimer disease. Arch Neurol 69:1430–1440 [DOI] [PubMed] [Google Scholar]
- 114.Coric V, Salloway S, van Dyck CH, Dubois B, Andreasen N, Brody M et al. (2015) Targeting prodromal Alzheimer disease with avagacestat: a randomized clinical trial. JAMA Neurol 72:1324–1333 [DOI] [PubMed] [Google Scholar]
- 115.Liu E, Schmidt ME, Margolin R, Sperling R, Koeppe R, Mason NS et al. (2015) Amyloid-beta 11C-PiB-PET imaging results from 2 randomized bapineuzumab phase 3 AD trials. Neurology 85:692–700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Fox NC, Black RS, Gilman S, Rossor MN, Griffith SG, Jenkins L et al. (2005) Effects of Abeta immunization (AN1792) on MRI measures of cerebral volume in Alzheimer disease. Neurology 64:1563–1572 [DOI] [PubMed] [Google Scholar]
- 117.Petersen RC, Thomas RG, Grundman M, Bennett D, Doody R, Ferris S et al. (2005) Vitamin E and donepezil for the treatment of mild cognitive impairment. N Engl J Med 352:2379–2388 [DOI] [PubMed] [Google Scholar]
- 118.Gauthier S, Aisen PS, Ferris SH, Saumier D, Duong A, Haine D et al. (2009) Effect of tramiprosate in patients with mild-to-moderate Alzheimer’s disease: exploratory analyses of the MRI sub-group of the Alphase study. J Nutr Health Aging 13:550–557 [DOI] [PubMed] [Google Scholar]
- 119.Quinn JF, Raman R, Thomas RG, Yurko-Mauro K, Nelson EB, Van Dyck C et al. (2010) Docosahexaenoic acid supplementation and cognitive decline in Alzheimer disease: a randomized trial. JAMA 304:1903–1911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Galasko D, Bell J, Mancuso JY, Kupiec JW, Sabbagh MN, van DC et al. (2014) Clinical trial of an inhibitor of RAGE-Abeta interactions in Alzheimer disease. Neurology 82:1536–1542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Turner RS, Thomas RG, Craft S, van Dyck CH, Mintzer J, Reynolds BA et al. (2015) A randomized, double-blind, placebo-controlled trial of resveratrol for Alzheimer disease. Neurology 85:1383–1391 [DOI] [PMC free article] [PubMed] [Google Scholar]