Abstract
The uptake of macromolecules and larger energy-rich particles into the cell is known as phagocytosis. Phagocytosed material is enzymatically degraded in membrane bound vesicles of the endosome/lysosome system (intracellular digestion). Whereas most, if not all, cells of the animal body are equipped with the molecular apparatus for phagocytosis and intracellular digestion, a few cell types are specialized for a highly efficient mode of phagocytosis. These are the (“professional”) macrophages, motile cells that seek out and eliminate pathogenic invaders or damaged cells. Macrophages form the backbone of the innate immune system. Developmentally they derive from specialized compartments within the embryonic mesoderm and early vasculature as part of the process of hematopoiesis. Intensive research has revealed in detail molecular and cellular mechanisms of phagocytosis and intracellular digestion in macrophages. In contrast, little is known about a second type of cell that is “professionally” involved in phagocytosis, namely the “enteric phagocyte”. Next to secretory (zymogenic) cells, enteric phagocytes form one of the two major cell types of the intestine of most invertebrate animals. Unlike vertebrates, these invertebrates only partially digest food material in the intestinal lumen. The resulting food particles are absorbed by phagocytosis or pinocytosis and digested intracellularly. In this review we provide a brief overview of the enteric phagocytes described electron microscopically for diverse invertebrate clades, to then to compare these cells with the “canonical” phagocyte ultrastructure established for macrophages. In addition, we will review observations and speculations associated with the hypothesis that macrophages are evolutionarily derived from enteric phagocytes. This idea was already proposed in the late 19th century by Elias Metschnikoff who pioneered the research of phagocytosis for both macrophages and enteric phagocytes. We presume that modern approaches to better understand phagocytosis will be helped by considering the deep evolutionary relationship between the two cell types.
Keywords: Enteric phagocyte, Macrophage, Phagocytosis, Intracellular digestion, Evolution, Ultrastructure
1. Introduction
All cells devote a substantial fraction of their molecular components towards absorbing and assimilating (“digesting”) energy-rich material from their environment. For obvious reasons, the capability to absorb and digest is absolutely essential for cell survival and must have evolved in single celled organisms, which explains why many of the structural and regulatory molecules involved in these processes are highly conserved (homologous) among present day species.
Different pathways of absorption and digestion can be distinguished (Pollard et al. 2017). Large particles, such as cells and cell fragments, are “engulfed” by membrane protrusions (lamellipodia) in a process called phagocytosis. Smaller particles or macromolecules are taken into the cell through membrane invaginations, a mechanism called pinocytosis. Once inside the cell, phagocytosed or pinocytosed material ends up in a dynamic system of vacuoles where they are enzymatically degraded. This process, termed intracellular digestion, is complemented by extracellular digestion, where secreted enzymes degrade food particles in the extracellular space (e.g., lumen of the digestive tract). Small molecules resulting from extracellular digestion (e.g., amino acids, mono-/disaccharides) are taken up by the cell via diffusion or active transport.
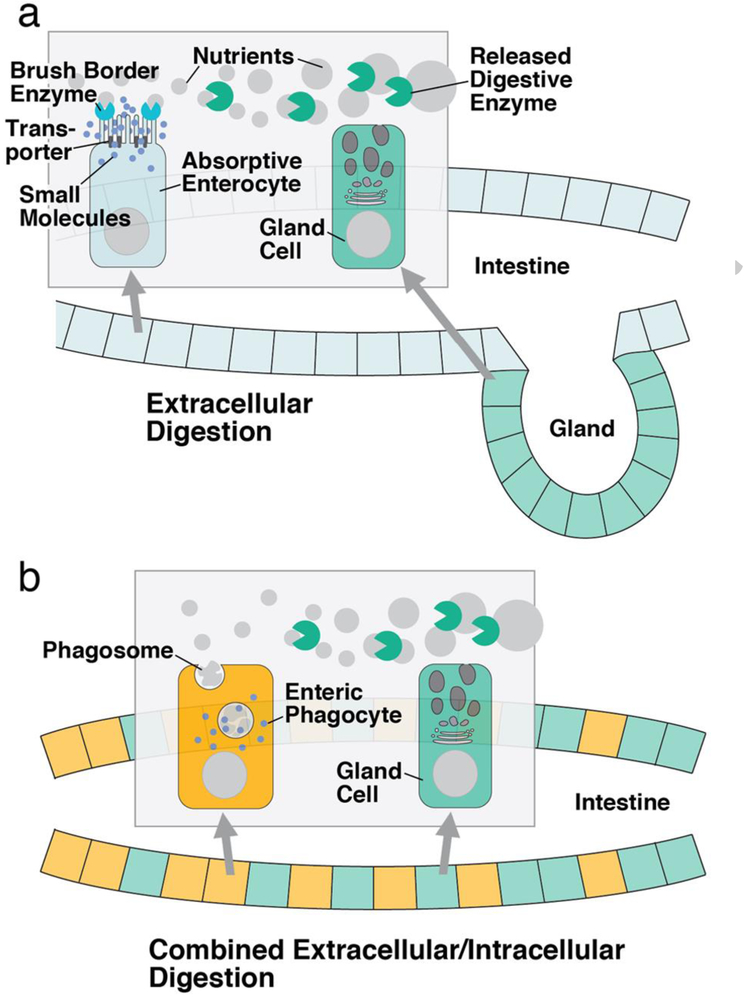
Extracellular digestion is prevalent in multicellular animals, which have evolved complex digestive tracts with diverse cell types. For example, in the vertebrate digestive system, enzymes secreted by specialized glands (e.g., salivary gland, pancreas, intestinal crypts) carry out the first step of the digestive process (Fig. 1a). In a second step, a different set of enzymes associated with the apical membrane of the epithelial cells lining the digestive system (consistently referred to as “enterocytes” in this review) complete digestion, producing small molecules that are subsequently taken up through active transport (Hooton et al. 2015). Phagocytosis or pinocytosis (together called endocytosis), followed by intracellular digestion, plays a less prominent role in the vertebrate digestive system. This is different in most invertebrate taxa, where food is processed by a combination of extracellular and intracellular digestion (Fankboner 2001; Fig. 1b). Typically, the invertebrate digestive system is formed by a mixture of enzyme-secreting gland cells and enterocytes. The enzymes degrade food material to the level of cell fragments and macromolecules. Enterocytes then take up this pre-digested material by endocytosis, after which the digestive process is completed intracellularly. In other words, in many invertebrate phyla, enterocytes of the digestive system operate as effective phagocytes, which we will call “enteric phagocytes” in the following.
Figure 1.
Schematic depiction of extracellular digestion in the vertebrate intestine and mixed extracellular/intracellular digestion observed for most invertebrate clades. a In extracellular digestion, enzymes secreted by specialized glands (e.g., pancreas) predigest food particles into macromolecules. These are then further broken down into small molecules by a different set of enzymes produced by the enterocytes. Small molecules are absorbed by diffusion or active transport. b In combined extracellular/intracellular digestion, gland cells distributed all over the intestine predigest food material into small particles and macromolecules. These are then taken up phagocytotically and digested intracellularly by enteric phagocytes.
In addition to enteric phagocytes, a second class of phagocytic cells found in most animals, invertebrates and vertebrates alike, has become specialized for endocytosis and intracellular digestion: the macrophages of the innate immune system. Macrophages are mesodermally-derived, motile cells that absorb and digest foreign materials (e.g., macromolecules, bacteria, parasites) and cellular detritus that threaten the integrity of the body. As a matter of fact, much of the detailed information on endocytosis and intracellular digestion that is currently available stems from the study of macrophages. One would expect, based on their similar function, that macrophages (“immune phagocytes”) and enteric phagocytes share many common features in terms of ultrastructure and molecular pathways. However, this proposition can’t be confirmed or refuted because relatively little is known about the biology of enteric phagocytes. Part of the problem is that in vertebrates, dedicated enteric phagocytes associated with the digestive tract do not exist, or have not been defined as such. And among the invertebrates, the widely used “model systems” currently used for molecular-genetic studies, Drosophila melanogaster and C. elegans, have a gut in which enteric phagocytes are also not particularly prominent, even though intracellular digestion plays an important role (McGhee 2007; Miguel-Aliaga et al. 2018; see also Maduro, 2019, this issue). It is among the “up-and-coming” model systems, representing basal deuterostomes (ascidians, cephalochordates, hemichordates, echinoderms) and the diverse taxa of lophotrochozoa (e.g., annelids, molluscs, platyhelminths) and cnidaria, that we find enteric phagocytes as the dominant cell type of the digestive tract. To help preparing the way for studies in these animals, the current review attempts to provide a survey of the morphologies, documented electron microscopically, of the enteric phagocytes across the animal kingdom, and to compare these morphologies with the “canonical” phagocyte phenotype exhibited by macrophages. Morphologies are expressed in words, and the terminologies used by microscopists to describe the structural elements of the endocytotic and digestive “machinery” encountered in different enteric phagocytes are inconsistent. Presenting these varied terminologies in the attempt to discover an underlying phagocyte “gestalt” appeared to us as a worthwhile undertaking.
The second objective of the review is to address the possible phylogenetic relationship between enteric phagocytes and macrophages, an idea brought up in a recent hypothesis article (Broderick 2015). Given that nutritive phagocytosis exists in protists it can be surmised that during the evolution of metazoa, enteric phagocytes came first, and mesodermally derived macrophages second. Could the latter have evolved from the former? In the later sections of this review we will discuss findings of studies that address developmental and functional relationships between enteric phagocytes and motile, mesodermally derived macrophages (also called amoebocytes or coelomocytes in several instances) observed in many extant invertebrate species. We will start out by summarizing briefly the current concepts of intracellular digestion: how do the various organelles involved in this process relate to each other, and what is the terminology in place to describe them?
2. Morphological aspects of endocytosis and intracellular digestion: the endosome/lysosome system
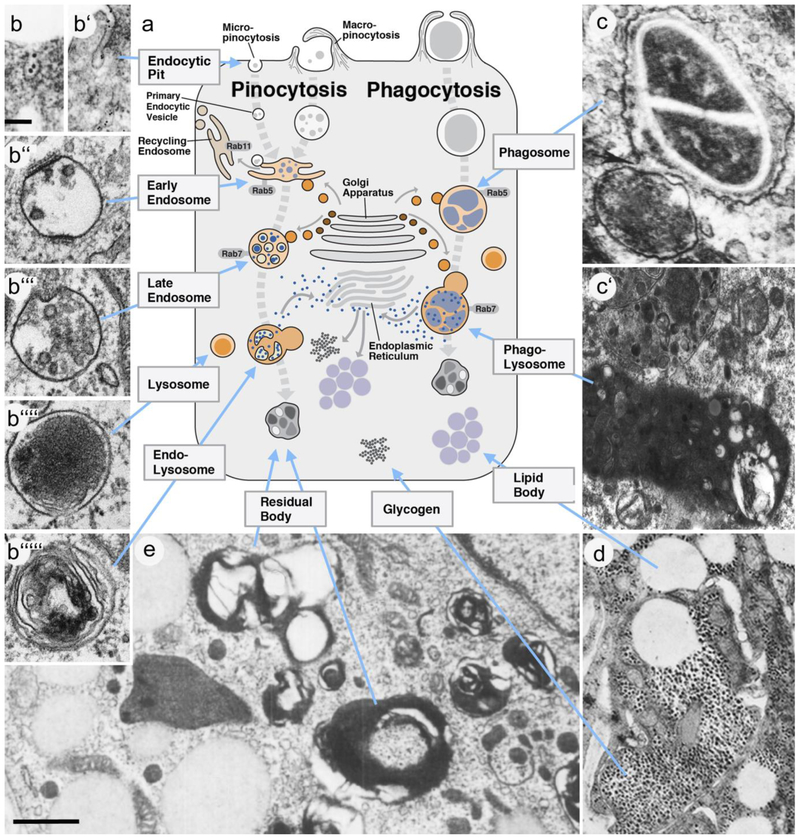
The uptake of material into the cell, termed endocytosis, follows three different pathways, (micro)pinocytosis, phagocytosis, and macropinocytosis (Pollard et al. 2017; Fig.2a, b, b’). Fluids and particles measuring less than 0.5μm are taken up by pinocytosis; the ingestion of larger particles occurs by phagocytosis or macropinocytosis. In pinocytosis the membrane buckles inward in the shape of a small invagination (“pit”). Several different invagination mechanisms, notably clathrin-dependent endocytosis, caveolin-dependent endocytosis and clathrin/caveolin independent endocytosis, have been distinguished thus far (Mayor and Pagano 2007). Endocytotic pits pinch off the membrane to form the primary endocytotic vesicles, which measure 0.1-0.2μm in diameter (Fig.2a, b”). By contrast, in phagocytosis, microfilament-driven lamellipodia extend outward around the large particle (“cargo”; e.g., bacterium, cell, cell fragment) that is to be ingested (Rougerie et al. 2013). This process is orchestrated by the interactions between cargo surface molecules and receptors on the phagocyte which then recruit specific cytoskeletal effector molecules to particular sites of the plasma membrane. Macropinocytosis (Kerr and Teasdale 2009; Bloomfield and Kay 2016) describes a similar process, whereby cytoskeletal movements push out lamellipodia and membrane folds (“ruffles”) which eventually “swallow” relatively big volumes of extracellular fluid, with or without particulate matter. As opposed to phagocytosis, macropinocytosis does not depend on specific ligand-receptor interactions. The vesicles resulting from phagocytosis and macropinocytosis are larger than the primary endocytotic vesicles that follow (micro)pinocytosis, and are termed phagosomes and macropinosomes, respectively (Fig.2a, b, c).
Figure 2.
Structural aspects of the endosome-lysosome system involved in intracellular digestion. a Schematic diagram of phagocyte with its intracellular membrane bound compartments. Left side of diagram shows the endosomal-lysosomal pathway (thick gray hatched line) that degrades small food particles absorbed by pinocytosis. Compartments are identified by terms generally used in current textbooks and research literature. Right side of diagram depicts the phagosome-lysosome pathway followed by large food particles ingested by phagocytosis. Electron micrographs (b-b’””, c’-c”, d, e) illustrate shape and texture of compartments shown in a. b Clathrin-coated endocytic pit. b’ Non-clathrin-coated endocytic pit. b” Early Endosome. b’” Late Endosome/Multivesicular body. b”” Lysosome. b’”” Endolysosome. c Phagosome containing bacteria. c’ Two phagolysosomes; the one at bottom containg partially digested bacteria. d Lipid bodies and glycogen particles. e Residual bodies. b: from (Mayor and Pagano 2007) with permission; b-b’””: from (Huotari and Helenius 2011) with permission; c from (Hard 1972) with permission; d, e: from (Del Conte 1979) with permission.
Scale bars: 100nm (b-d); 500nm (e).
Once inside the cell, endocytosed material is channeled into the endosome/lysosome system. Endocytotic vesicles, phagosomes or macropinosomes dock at the early endosomes (EE), an elaborate network of interconnected vesicles and tubules located close to the plasma membrane (Huotari and Helenius 2011; Repnik et al. 2013; Pollard et al., 2017; Fig.2a). From here, certain parts of the internalized membrane is continuously recycled outward to the plasma membrane via recycling endosomes (Fig.2a). At the same time, vesicles carrying digestive enzymes and diverse membrane proteins arrive from the trans-Golgi network and merge with the EEs. As a result, large pieces of the EE vesicular network break off and are transported towards more central positions within the cytoplasm via microtubular transport (Bonifacino and Neefjes 2017). These so-called “carrier vesicles”, aside from containing endocytosed food material and digestive enzymes, are characterized structurally by having many small internal vesicles enclosed within their lumen, and are therefore called multivesicular bodies (Gruenberg and Stenmark 2004; Pollard et al. 2017; MVBs in Fig.2a, b’”). MVBs become part of another vesicular network, the late endosome (LE). Like EEs, LEs continuously exchange membrane-bound and intraluminal proteins with the trans-Golgi network. They differ from EEs by a number of specific membrane-bound proteins, such as the GTPase Rab7, which marks LEs, as opposed to Rab5, which is characteristic for EEs (Pollard et al. 2017; Fig.2a). In regard to differentiating between MVBs and LEs the literature is not yet consistent: ultrastructurally, both compartments are characterized by their “multivesicular” appearance. As a result, some authors use the terms LE and MVB interchangeably (Huotari and Helenius 2011); others make the distinction between MVB (carrier vesicle) and LE (compartment targeted by, but separate from, carrier vesicle; Repnik et al. 2013).
The LE system interacts with yet another vesicular intracellular compartment, the lysosome. Lysosomes are round or irregularly shaped vesicles of 0.1-1μm diameter, filled with a homogenous, moderately electron-dense interior surrounded by a narrow, light halo (De Duve and Wattiaux 1966; Huotari and Helenius 2011; Lim and Zoncu 2016; Fig.2a, b””). Lysosomes contain digestive enzymes, like acid phosphatase, and form part of all cells, but occur at greater density in cells that, like macrophages or hepatocytes, digest and store food materials. The fusion of lysosomes and LEs yields a hybrid vesicle, the endo-lysosme. This organelle, also called “secondary lysosome”, is characterized by its heterogenous content that consists of electron-dense droplets of varying size and shape, as well as membraneous vesicles and lamellar structures (Huotari and Helenius 2011; Pollard et al. 2017; Fig.2a, b’””). Like all other components of the endosome/lysosome system, endolysosomes also appear to be dynamic structures that undergo constant fusion-fission events. Parts of their membranes and enzymatic content get removed to reform lysosomes; vesicles derived from the trans-Golgi network carrying cargoes of “fresh” enzymes and membrane proteins fuse with the endolysosomes and lysosomes.
One should note that the structural appearance of EE, MVB, LE and endolysosome appears to be very different depending on the size of the initially endocytosed volume. Primary endocytic vesicles generated through pinocytotic membrane invaginations are similar in size to the vesicular structures of “typical” EEs, MVBs and LEs documented in the literature (Huotari and Helenius 2011; Fig.2b”, b’”). By contrast, phagosomes and macropinosomes (1-5μm) “dwarf” endocytic vesicles (Hard 1972; Buchmeier and Heffron 1991; Tjelle et al. 2000; Haas 2007; Kinchen and Ravichandran 2008; Fig.2a, c, c’). Once moving deeper into the cell, phagosomes remain large, rather than breaking down into smaller vesicles. Most ultrastructural descriptions of phagocytosis imply that phagosomes directly fuse with lysosomes into phagolysosomes (e.g., Kinchen and Ravichandran 2008). Intermediate carrier vesicles resulting from the processing of phagosomes, like MVBs (only much larger), have, to our knowledge, not been defined ultrastructurally. However, experimental studies clearly show that interactions between phagosomes and early/late endosomes via fusion and fission events do take place (Jahraus et al. 1998; Tjelle et al. 2000), suggesting that the intracellular digestion of endocytosed material, independent of size, follows similar pathways.
At the stage of the endolysosome/phagolysosome, food materials are broken down into small molecules that enter the cytoplasm to then exit the cell into the extracellular space, or to become part of anabolic biochemical pathways that synthesize macromolecules utilized in the cell, or deposited in specialized storage organelles. Prominent among these are the lipid droplets, or lipid bodies (LBs). Produced in and budded off from the endoplasmic reticulum, LBs are round, electron-dense vesicles surrounded by a specialized membrane that is formed by a lipid monolayer (Murphy 2001; Melo et al. 2011). In fixation protocols for electron microscopy lipids are often dissolved, leaving “empty spaces” of characteristic size, shape and distribution instead of the dense LBs (Fig.2d).
Not all of the food content of endolysosomes/phagolysomes is broken down into monomeric small molecules. Many documented cases of intracellular digestion result in so called “residual bodies”, also called telolysosomes, which resemble phagolysosomes to a certain extent. Residual bodies also contain highly polymorphic granular and membraneous material (“myelin figures”), but are typically smaller, more irregularly shaped, and higher in electron density than phagolysosomes (Fedorko et al. 1968; Novikoff 1973; Essner and Haimes 1977; Del Conte 1979; Fig.2a, e). Residual bodies can accumulate in the cytoplasm as “lipofuscin granules”, or can be expelled from the cell by exocytosis (Kajihara et al. 1975; Hendriks and Eestermans 1986).
All of the structural components of the intracellular digestive pathway sketched out in the preceding paragraphs are intensively studied and known in considerable detail for vertebrate macrophages in the context of host defense. We will now turn to another cell type that employs the same pathway and, as will be shown further below, exhibits very similar ultrastructural features, but for a seemingly different purpose, namely nutrition. The cell type in question is the enteric phagocyte, which makes up a major fraction of intestinal cells found in most invertebrate phyla. Interestingly, at the time when phagocytosis was first discovered, both mesodermally derived macrophages and endodermal enteric phagocytes were very much considered to be part of the same functional system, and to be closely related ontogenetically and phylogenetically, a theme we will briefly explain in its historical context next, and then later return to towards the end of the review where we address functional and developmental relationships between the two cell types.
3. Enteric phagocytes: a historical introduction
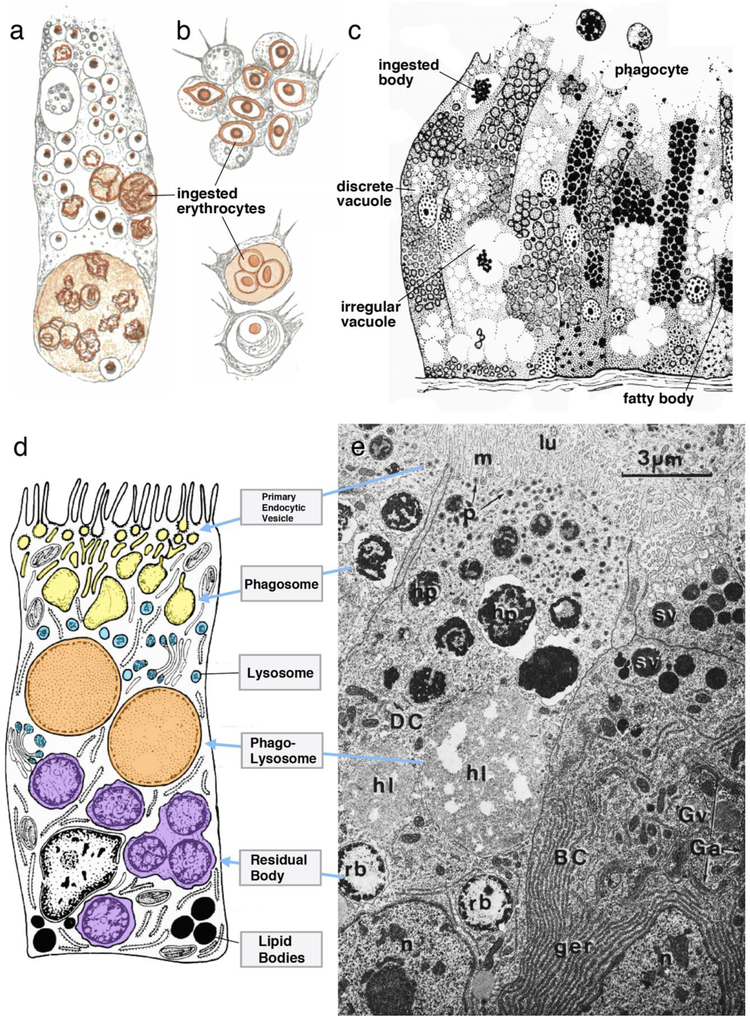
In 1878, Elias Metschnikoff described the intestinal cells (also called gastrodermal cells) of various platyhelminth (flatworm) species as “cylindrical amoeboid cells” capable of ingesting food particles, such as blood cells and liver cells (Metschnikoff 1878). He also surveyed many other invertebrate organisms (Metschnikoff 1884), looking at both intestinal (endodermally derived) cells, as well as stromal (mesodermally derived) cells involved with the uptake and digestion of particles, a process for which he coined the term “phagocytosis” (Fig. 3a, b). Historically, Metschnikoff’s scientific work has to be understood in context of the coming-together of embryology and the (then new) concept of Darwinian evolutionary theory (Tauber 2003). Events shaping the embryonic development of an organism were seen as directly linked to the phylogenetic history of that organism. Furthermore, it was understood that evolution was driven by natural selection, the “survival of the fittest”. Embryologists started to apply this idea for the cells of a (developing) organism, which, they speculated, compete with one another for space and resources. The diverse cell types that originated in a multicellular animal were seen as part of an overall “disharmonious” organism, where the phagocytosis of one cell (of the same organism, or taken in as prey) by another cell was a major cellular activity, directed at regulating proper cell numbers, and at the same time providing energy. Metschnikoff paid much attention to the massive cellular break down that occurs during metamorphosis of many animals, where motile macrophages moving throughout the body cavities and stroma ingest the cellular debris. He also emphasized his findings that in many invertebrates cells of all germ layers, including ectoderm and mesoderm, carry out phagocytosis (Metschnikoff 1884). Macrophages and intestinal phagocytes were seen as being closely related, with stromal macrophages moving in and out of the intestinal epithelium, or (stationary) intestinal cells passing on ingested food particles to (motile) stromal cells, which then further digest and distribute these particles all over the body. Metschnikoff even coined the word “phagocytoblast” for the combined endodermal and mesodermal germ layer.
Figure 3.
Structural aspects of enteric phagocytes. a, b Drawing of planarian enteric phagocyte (a) and invertebrate macrophages (b) phagocytosing erythrocytes (rendered in brown; from Metschnikoff, 1884). (C) Light microscopic appearance of epithelium of mollusk digestive gland, containing enteric phagocytes (left), glandular cells (right; from Millot, 1937, with permission). Terms used by author (“ingested body”, “discrete vacuole”, “irregular vacuole”, “fatty body” likely correspond to phagosome, phagolysosome, and lipid body, respectively. (d, e) Electron micrograph (e) and schematic drawing (d) of molluscan enteric phagocyte (from Owen 1972, with permission). Organelles of the phagosome/lysosome system, depicted in e, are pointed out by blue arrows in d. Abbreviations: BC pyramidal basophilic cell (=zymogenic gland cell); DC digestive cell (=enteric phagocyte); Ga Golgi apparatus; ger granular endoplasmic reticulum; Gv Golgi vesicle; hl heterolysosome (=phagolysosome); hp heterophagosome (=phagosome); lu lumen of tubule; m microvilli; n nucleus; p pinosome (=primary endocytic vesicle); rb residual body; sv secretory vesicle.
Descriptive and experimental studies of many researchers in the early decades of the 20th century focused on intracellular digestion carried out by enteric phagocytes (reviewed in Yonge, 1937). However, before the use of electron microscopy, cellular events involved in phagocytosis were understood only at a very rudimentary level. It was assumed that particles were taken up into the cytoplasm, and were only later (in some cases) incorporated into a fluid-filled vacuole, termed food vacuole, or digestive vacuole (e.g., Hirsch 1925; Millot 1938). Aside from this compartment (nowadays termed phagosome or phagolysosome), all other organelles were too small to be observed light microscopically. On the other hand, secretory granules of secretory (gland) cells could be resolved in the light microscope, and histo-chemical techniques were developed to characterize different types of secretions. It became clear that in most animals, the intestinal epithelium is comprised of a mixture of gland cells and absorptive cells acting as phagocytes; the digestive process was characterized as a hybrid of extracellular and intracellular digestion (Yonge 1937; see Fig.3c). Gland cells secrete enzymes that partially break down the food material; the products are intracellularly digested by enteric phagocytes. It remained an open question (and still is an open question to the present day, for most animals) whether the uptake of small molecules through specialized channels and transporters is a function of the same enteric phagocytes, or whether other cell types dedicated to this function exist.
The arrival of electron microscopy in the 1950ies made it possible to resolve the architecture of cytoplasmic organelles, including the interconnected tubules and vesicles that make up the “vacuome” (endoplasmic reticulum, Golgi apparatus, endosomes, lysosomes) of the cell. Lysosomes as carriers of intracellular digestive enzymes were isolated from hepatocytes for the first time in 1955 (De Duve et al. 1955) and soon shown to be part of other cell types in all organisms (De Duve and Wattiaux 1966). Electron microscopic investigations of the intestinal tract of many animal taxa confirmed the earlier established notion of a combined extracellular/intracellular digestive system carried out by an admixture of gland cells and enteric phagocytes, and documented the diversity of membrane-bound organelles associated with intracellular digestion (Fig.3d, e). Several researchers, aware of the ongoing biochemical-ultrastructural work addressing the dynamic system of membrane-bound intracellular compartments in vertebrate cells, employed the “modern” nomenclature [e.g., endocytic vesicles/phagosomes, primary lysosomes, secondary lysosome (=phagolysosome)] to describe what they observed in invertebrate enteric phagocytes. For example, Riley (1973) investigating nematodes, and Owen (1972) in molluscs, explicitly referred in their work to the system of terms and functional relationships introduced by cell biologists that studied vertebrate cells at a molecular level (Fig.3d, e). However, overall, the literature describing the ultrastructure of enteric phagocytes employs a nomenclature that is somewhat varied and often vague. Given the fact that experimental studies addressing the function and interrelationships of the different organelles observed are difficult or impossible to perform, this lack in terminological clarity and/or precision is of course understandable. Table 1 provides an overview of the terminology used to describe the organelles of intracellular digestion in electron microscopic investigations of the digestive system of different invertebrate clades.
Table 1. Terminology used for enteric phagocytes and their organelles.
Information tabulated here is taken from a comprehensive, yet non-exhaustive survey of published electron microscopic studies of the ultrastructure of invertebrate digestive systems. Columns A and B list animal clades. Column C indicates terms used for enteric phagocyte in corresponding animal clade. The term used for enteric phagocytes varies, often simply referring to the function (e.g., absorptive cell, digestive cell) or position (e.g., stomach cell, midgut cell) of the cell. Columns D-F show terms used for organelles associated with enteric phagocytes. The three columns reflect the position of the corresponding organelles along the apical-basal axis, with those referring to the apical membrane and its immediate vicinity placed in column D (yellow), the ones naming organelles in the supra/perinuclear space in column E (orange), and the ones referring to more basal structures in column F (purple). Based on this topology, structures referred to in column D would most likely correspond to pinocytotic/phagocytotic membrane specializations, primary endocytic vesicles/phagosomes and early endosomes; structures of column E to late endosomes, lysosomes and endolysosomes/phagolysosomes, and structures in column F to residual bodies and storage organelles. Column G indicates types of particles or cells used experimentally to ascertain phagocytosis. Literature is provided in column H.
Abbreviations: Ap. Apical; Cil. Ciliated; Col. Columnar; Endocyt. Endocytotic; Glyc. Glycogen particle; Incl. Inclusion; Intest. Intestinal; Multives. Multivesicular; Pinocyt. Pinocytotic; Prim. Primary; Resid. Residual; Sec. Secondary; Spher. Spherical; Vac. Vacuole; Ves. Vesicle
| Clade | Subclade | Term for Cell |
Membrane/Apical | Supranuclear/Perinuclear | Basal | Exp. Feeding |
Reference |
|---|---|---|---|---|---|---|---|
| Porifera | Demospongiae | Choanocyte | Lamellipodia | Phagosome, Food Vac., Lysosome | Bacteria | 1, 2 | |
| Demospongiae | Choanocyte | Small Ves. | Phagosome, Lysosome-like Ves. | Microvesicles | 3 | ||
| Calcarea | Choanocyte | Lamellipodia | Phagosome | Bead 1μm | 4 | ||
| Homoscleromorpha | Choanocyte | Phagosome | 5 | ||||
| Ctenophora | Digestive Cell | Ves. | Phagosome, Lysosome, Lysosome-like Body | Thorotrast | 6, 7 | ||
| Cnidaria | Anthozoa | Phagocytic Cell | Pinocytic Ves. | Phagosome, Postphagosome, Lysosome | Wax Droplets, Glyc. | 8 | |
| Cubozoa | Absorptive Cell | Apical Discoideal Coated Ves. | Watery Vac., Phagosome, Lysosome | Lipid Droplets | 9 | ||
| Hydrozoa | Gastrodermal Cell | Food Vac. | 10 | ||||
| Hydrozoa | Absorptive Cell | Discoideal Flattened Ves. | Ferritin | 11 | |||
| Hydrozoa | Gastrodermal Cell | Flatttened Coated Ves. | Large Vac. | Carbon part. | 12 | ||
| Hydrozoa | Nutritive Phagocyte | Pinocyt. Ves., Microves. | Phagosome, Sec. Lysosome | Carbon part. | 13, 14 | ||
| Echinodermata | Asteroidea | Enterocyte | Pinocyt. Vac. | Vac. | 15, 16 | ||
| Asteroidea | Choanocyte-like Cell | Coated Ves. | Phagosome, Multives. Body, Sec. Lysosome | 17 | |||
| Ophiuroidea | Enterocyte | Pinocyt. Invagination, Ves. | Lipid Droplets, Glyc. | 18 | |||
| Hemichordata | Absorptive Phase Cell | Apical Coated Pit, Ves. | Heteromorphic Incl. Body, Digestive Vac. | 19, 20 | |||
| Pterobranchia | Epithelial Cell | Apical Coated Pit, Coated Ves. | Multives. Body, Heteromorphic Vac. Body | 21 | |||
| Urochordata | Absorptive Cell | Coated Pit, Small Ves., Pinosome | Multives. Body, Vac., Sec. Lysosome | Lipid Droplet | 22, 23, 24 25 | ||
| Vacuolated Cell | Micropinocyt. Ves. | Vac. | 26 | ||||
| Cephalochordata | Epithelial Cell | Coated Pit, Ves. | Lysosome, Granule | Lipid Droplet | 27, 28 | ||
| Phagocyte | Lysosome, Phagosome | Algae | 29 | ||||
| Chaetognatha | Absorptive Cell | Pinocyt. Ves. | Lysosome-like Body | 30, 31 | |||
| Absorptive Vac. Cell | Coated Pit, Endocyt. Ves. | Vac., Endolysosomal Compartment | 32 | ||||
| Gastrotricha | Absorptive Cell | Pinocyt. Ves., Small Vac. | Lysosome-like Body, Sec. Lososome | Glyc. | 33, 34 | ||
| Rotifera | Stomach Cell | Caveolae, Coated Ves. | Food Vac. | Thorotrast | 35, 36 | ||
| Stomach Cell | Food Vac., Incl. Body (=Lysosome) | 37 | |||||
| Platyhelminthes | Col. Phagocytic Cell | Pinocyt. Ves. | Phagosome, Lysosome, Sec. Lysosome | Lipid Droplet, Glyc. | 38 | ||
| Tricladida | Phagocytic Cell | Pseudopodia, Food Vac. | Lysosome, Digestive Vac. | Resid. Body, Lipid Droplet, Glyc. | 39, 40, 41 | ||
| Dalytyphloplanida | Gastrodermal Cell | Micropinocyt. Ves. | Phagosome, Vac. | Lipid Globule | 42 | ||
| Catenulida | Phagocyte | Coated Endocyt. Ves. | Vac., Lysosome | Resid. Body, Lipid Incl. | Latex Bead | 43 | |
| Macrostomida | Phagocytic Cell | Food Vac. | Food Vac., Lysosome | Lipid Droplet | 44, 45 | ||
| Bryozoa | Absorpt. Acid. Cell | Endocyt. Ves., Prim. Lysosome | Digestive Vac. (Sec. Lysosome) | 46 | |||
| Phoronida | Cil. Epithelial Cell | Pinocyt. Ves., Ap. Spher. Granule | Digestive Vac., Ves. Containing Nutrient | Lipid Body | 47, 48 | ||
| Nemertinea | Cil. Gastrodermal Cell | Oval Body, Food Vac. | Lysosome | 49, 50 | |||
| Annelida | Polychaeta | Absorptive Cell | Dense Body | Lysosome | Lipid Droplet, Glyc. | 51, 52 | |
| Hirudinea | Intest. Epithelial Cell | Caveolae, Apical Dense Body | Vac. | Lipid Inclusion | 53, 54 | ||
| Mollusca | Gastropoda | Digestive Cell | Endocyt. Ves., Multives. Body | Lysosome, Heterolysosome, Large Vac. | Resid. Body, Lipid Droplet, Glyc. | 55, 56, 57 | |
| Gastropoda | Digestive Cell | Small Coated Ves. | Large Vac. | Thorotrast | 58 | ||
| Bivalvia | Digestive Cell | Microves., Pinosome, Phagosome | Macroves. (Phagolysosome) | Resid. Body, Lipid-like Droplet | Graphite | 59, 60, 61 | |
| Bivalvia | Absorptive Cell | Pino/Macropinosome, Phagosome | Lysosome, Heterolysosome | Resid. Body | Nanoparticle | 62 | |
| Cephalopoda | Digestive Cell | Pinocytotic Ves. | Digestive Vac. | Brown-Body Vac., Lipid Droplet | 63 | ||
| Priapulida | Epithelial Cell | Ves., Lysosome | Digestive Vac. | Lipid Droplet, Glyc. Particles | 64, 65 | ||
| Kinorhyncha | Epithelial Cell | Digestive Vac. | 66, 67 | ||||
| Nematoda | Intest. Epithelial Cell | Pinocyt. Ves., Phagosome | Prim. Lysosome, Phagolysosome | Pigment Gran., Lipid Droplet, Glyc. | Hemoglobin | 68, 69, 70 | |
| Intest. Cell | Endosome, Phagosome | Lysosome, Sec. Lysosome | Lipofuscin Granule, Telolysosome | 71 | |||
| Tardigrada | Digestive Cell | Coated Pit, Ves., Multives. Body | Digestive Vac., Lysosome, Sec. Lysosome | Spher. Incl., Lipid Droplet, Glyc. | 72, 73, 74 | ||
| Onychophora | Absorptive Cell | Ves. | Membrane-bound Incl. | Lipid Droplet, Glyc. | 75 | ||
| Crustacea | Copepoda | Midgut Cell | Pinocyt. Vesicle | Lysosome, Heterophagosome | 76 | ||
| Isopoda | Digestive Cell | Pinocyt. Vesicle | Lysosome-like Body, Vac. | Lipid Droplet, Glyc. | 77 | ||
| Malacostraca | Digestive Cell | Vesicle | Phagosome, Lysosome-like Granule | Lipid Droplet | 78 | ||
| Decapoda | Digestive (B) Cell | Pinocyt. Vesicle | Subapical Vac., Supranuclear Vac. | 79, 80 | |||
| Chelicerata | Scorpiones | Digestive Cell | Pinocyt. Vesicle, Channel Vesicle | Lysosome-like Organelle, Brown Body Vac. | Glyc., Lipidic Inclusions | Colloid. Gold | 81 |
| Acariformes | Digestive Cell | Pinocyt. Vesicle, Phagosome | Lysosome, Sec. Lysosome | Resid. Body, Lipid Droplet | 82, 83, 84 |
4. Structure and distribution of enteric phagocytes
Enteric phagocytes are polarized epithelial cells joined together by an apical junctional complex that, in most cases, is described as a combined zonula adherens and septate junction. The apical membrane facing the gut lumen bears conspicuous cilia and/or microvilli, as summarized for the different invertebrate clades in Fig.4 (for references, see Table 1). If microvilli are present, they often form a dense array of evenly spaced elements that, based on its light microscopic appearance, is called brush border. Non-bilaterian clades (ctenophores, cnidarians) typically have ciliated (or flagellated) gastrodermal cells with more sparse microvilli. The same is the case for the choanocytes and ventral epithelial cells of sponges and placozoans, respectively; these cells may functionally be considered to represent enteric phagocytes, without necessarily implying homology. For the enteric phagocytes of deuterostomes, with the exception of ascidians and vertebrates, a dense brush border is accompanied by cilia. The same applies to most lophotrochozoan phyla. In some phyla (e.g., nemertines), microvilli appear to be missing, whereas in others, (e.g., gastrotrichs, bryozoans) cilia may be absent. Ecdysozoa also appear to lack cilia in their gastrodermal cells.
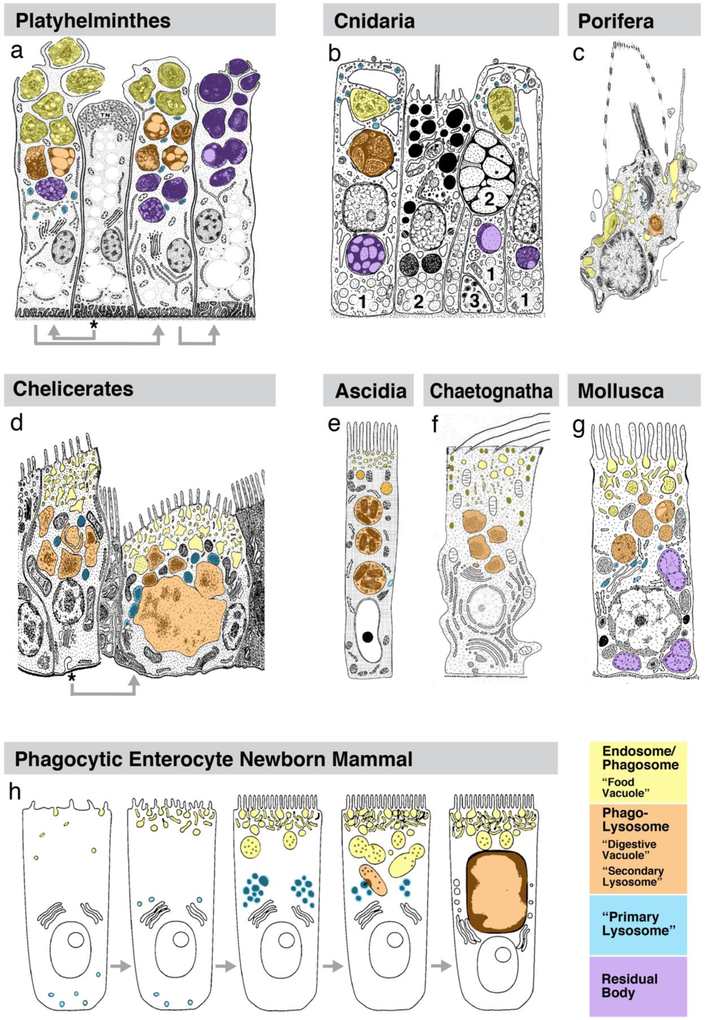
Figure 4.
Digestive cell types in animals. Representative phyla are named (blue boxes) and ordered according to currently widely used phylogenetic trees (Peterson and Eernisse 2016; Kocot et al. 2017; Giribet and Edgecombe 2017) as indicated by blue lines. For each phylum, colored icons in right gray box symbolize major cell types forming the epithelium of the mid-intestine, as explained in framed box at upper right of figure. Nutrient absorption by enteric phagocytes described in the literature can be tentatively assigned to two types: Type A (orange) represents “canonical” phagocytosis, where entire cells or large particles are taken up; type B (yellow) describes a process where mostly smaller particles are taken up into endocytic vesicles that secondarily fuse into large phagosomes. Left grey boxes show different types of apical specializations (cilia, microvilli, dense microvilli organized as brushborder) of enteric phagocytes. For references of publications documenting digestive cell types, see Table 1.
Enteric phagocytes and secretory gland cells (as well as some other cell types, including endocrine cells, stem cells, and neurons, which will not be further considered in the following) typically occur intermingled in the middle segment (midgut) of the digestive tract (Fankboner 2001). In most bilaterian animals, the digestive tract is divided into an anterior foregut, middle midgut and posterior hindgut (Brusca et al. 2016). The foregut (pharynx, esophagus) is often specialized for functions of food comminution (e.g., milling, crushing, shredding) and food storage; it also has sometimes glands secreting digestive enzymes or mucus. The midgut is the location of food digestion and absorption, and the hindgut subserves the resorption of water and excretion of wastes. The digestive system of all animals, excepting the sponges and placozoans, forms an elongated cylinder (“gut” or “intestine”) with a single opening (cnidarians, xenacoelomorphs, platyhelminths, gnathostomulids) or a separate mouth and anal opening (all other phyla). In many clades, blind-ending sacs (“diverticula” or “caeca”) branch off the midgut (Brusca et al. 2016). These usually represent compartments that are particularly rich in phagocytes dedicated to food absorption and digestion (intestinal caeca in echinoderms, bryozoans, chelicerates and hexapods; intestinal diverticula in nemertines; pyloric gland in ascidians; hepatic sacculations or hepatic caecum in hemichordates and cephalochordates, respectively; hepatopancreas in molluscs and crustaceans). In some cnidarians, notably the Anthozoa, the different cell types involved in digestion (glandular cells, phagocytes, purely resorptive cells) are spatially separated in longitudinal “tracts” that extend along the edges of the mesenteries and mesenterial filaments (Van-Praët 1985; see also Steinmetz, 2019, this issue).
For some animal groups a single cell type which appears to carry out the function of both (enzyme) secretion and absorption/digestion dominates the intestinal epithelium. This has been observed for a number of ecdysozoan phyla, notably priapulids (Storch et al. 1989; Storch 1991), nematodes (Lee 1969; Riley 1973; Wright 1991), and most hexapods (see Holthof et al. 2019 and Caccia et al 2019 this issue). In priapulids and most nematodes, hallmarks of intracellular digestion (e.g., phagosomes) were seen in these cells. In the same cells, secretory vesicles originating within the ER and fusing with the apical membrane to release their content were observed (Riley 1973). The characteristic electron dense secretory granules characteristic for gland cells in general are rare or absent from intestinal cells of nematodes and insects. It has been speculated that the process of enzyme concentration, which results in the dense secretory granules, is shortened or omitted in these animals (De Priester 1971; Richards and Richards 1977; Bignell et al. 1982) and secretions are discharged continuously.
A characteristic shared by macrophages and enteric phagocytes in many animals is their tendency to fuse into multinucleate syncytia (or, terminologically more correct, plasmodia). This was already emphasized by Metschnikoff (1884), and confirmed in several consecutive studies, notably in members of the clades Platyhelminthes (Holt and Mettrick 1975), Phoronida (Herrmann, 1997) and Cnidaria (for medusa of Clytia hemispherica: V.H., unpublished observation). It is possible that large multinucleate cells are more effective in phagocytosing large food particles. The tendency towards forming a digestive syncytium is taken to an extreme in most members of the Acoela (members of the Xenacoelomorpha; see Gavilan et al., 2019, this issue). Here, the entire “central parenchyma” consists of a mass of merged phagocytic cells, and is able to “swallow” entire prey animals, such as nematodes or copepods, into gigantic phagosomes. We would argue that researching the ultrastructural and biochemical details of intracellular digestion in acoels will reveal many novel and interesting aspects of this process.
5. The diversity of organelles of intracellular digestion in enteric phagocytes
The uptake of food particles by canonical phagocytosis, as defined by the extension of lamelliopodia around the particles, followed by their engulfment and sequestrations into large subapical phagosomes, has been described for sponges, ctenophores, cnidarians, acoels, platyhelminths, nemertines, and cephalochordates (Fig.4, type “B”; Fig.5a-c; see also Tab.1 and references therein). In planarians (platyhelminths) fed with liver, entire liver cells are ingested. After only minutes post-feeding, phagosomes, often called “food vacuoles” based on the older term introduced in the light microscopic literature, appear in the subapical cytoplasm. They measure in excess of 5üm and consist of a double membrane tightly enclosing the engulfed cell (Garcia-Corrales and Gamo 1988; Fig.5a). Approximately one hour post-feeding, phagosomes move deeper into the cell where they fuse with multiple, small lysosomes to become phagolysosomes, also termed “digestive vacuoles” or “secondary lysosomes” by various authors (Tab.1 and references therein). Phagolysosomes are similar in size to phagosomes, but differ in the appearance of their content. Thus, the integrity of the ingested cell has broken down, giving rise to irregular clumps of electron-dense granular and membraneous material. Several hours later, the content of phagolysosomes further condenses to morph into a more or less heterogeneous matrix of electron-dense material. The resulting organelle constitutes the residual body. In planarians and some other species, residual bodies were observed to move apically and then being released into the gut lumen exocytotically, similar to what has been observed for sponges and unicellular organisms (Willenz and Van de Vyver 1982; Garcia-Corrales and Gamo 1988; Fig.5c). This exocytotic “cellular defecation” process occurring in enteric phagocytes is also known for vertebrate cells (e.g., hepatocytes: Kerr 1970; Munnell and Cork 1980; macrophages: Song and Hanayama 2016).
Figure 5.
Schematic drawings of enteric phagocytes in representative phyla. Organelles of she phagosome/lysosome system are differentially rendered in yellow (apically located pits, endocytic vesicles, phagosomes), orange (phagolysosomes), blue (lysosomes), and violet (residual bodies; see bottom right of figure). a Phagocytes in platyhelminths (Dugesia gonocephala; from Garcia-Corrales and Gamo 1988, with permission). b Gastrodermal cell types in cnidarians (Tripedalia cystophora; from Chapman 1978, with permission). Numbers refer to phagocyte (1), secretory (spumous) cell (2), neurosecretory cell (3). c Choanocyte in sponges (Ephydatia fluviatilis; from Weissenfels 1976, with permission). Enteric phagocytes in (a-c) perform “canonical” phagocytosis, whereby large food particles are surrounded by lamellipodia and drawn into large apical phagosomes. Panels of middle row (d-g) show different scenario, where food particles and macromolecules are endocytosed into small endocytic vesicles which, together with lysosomes, merge into larger phagolysosomes. This has been described, among others, for chelicerates (d; Hyalomma asiaticum; from Coons and Alberti 1999, with permission), ascidians (e; generic; from Burighel and Cloney 1997, with permission), chaetognaths (f; Tenuisagitta setosa; from Shinn 1997, with permission) and molluscs (g; Mya arenaria; from Pal 1972, with permission). h A similar type of endocytosis has also been described and illustrated for absorptive enterocytes of newborn mammals by (Rattus norvegicus; Wilson et al. 1991, with permission). Asterisks and grey arrows in panels (a) and (d) indicate time sequence, with cell marked by asterisk presenting morphology prior to food uptake, followed by later stages.
Nutritive phagocytosis often follows a mechanism (called “B” in Fig.4; Fig.5d-g) that deviates, at least in its beginning stages, from the canonical pathway described above. Thus, food particles that are smaller in size than whole cells, are taken up through membrane invaginations into small subapical endocytic vesicles to which a plethora of different terms have been applied (e.g., microvesicle, pinocytotic vesicle, coated vesicle, discoideal flattened vesicle, pinosome, among others; see Table 1 and literature therein). As a next step, these endocytic vesicles fuse with each other into larger vacuoles, variably termed macrovesicles, food vacuoles, phagosomes, large endosomes, among others (Owen 1970; Owen 1972; Riley 1973; Filimonova 2008). The merging of lysosomes to these phagosomes results in phagolysosomes (“digestive vacuoles”), which move deeper into the cell and are processed further as described above. Experimental studies, using application of small particles like graphite, thorotrast, or nanoparticles (see Table 1), confirmed the initial uptake of these materials via membrane invaginations into small vesicles, which then transferred them to large endosomes. This type of “pino-phagocytosis” is less frequently discussed in studies of macrophages or other phagocytotic cell types in vertebrates, where a “microvesicular pathway” (pinocytosis > pinosome > endosome > endolysosome) appears to exist separately from a “macrovesicular” pathway (phagocytosis > phagosome > phagolysosome). However, the merging of pinocytotic vesicles into larger phagosomes is known (e.g., Straus 1964, for kidney cells), and has been documented in detail for a peculiar absorptive cell type that exists in the intestine of newborn mammals (Wilson et al. 1991; Fig.5h). These absorptive cells ingest protein complexes by pinocytotic vesicles that become part of the early endosome. Maturing to late endosomes (“multivesicular bodies”), the vesicles fuse with each other, and with lysosomes, into a “giant lysosome” (phagolysosome) located in the supranuclear cytoplasm. It is tempting to speculate that this endocytotically active mammalian enterocyte, which only exists in the perinatal period, is an evolutionary vestige of the enteric phagocyte, which dominates the digestive system of invertebrates, including that of ascidians and cephalochordates, the closest vertebrate relatives.
Residual bodies have been described for enteric phagocytes of both the “canonical” type (A) and the pino-phagocytotic type (B; see Table 1 and references therein). Residual bodies can adopt different shapes and, accordingly, received different names, such as (among others) brown body vacuole, pigment granule, and spherical inclusion. Aside from residual bodies, energy storage granules, notably lipid bodies and glycogen granules, exist in most enteric phagocytes documented in the literature. Glycogen granules are arrays of densely packed, electron-dense “grains” of 15-40nm diameter (Revel et al. 1960). Lipid bodies are larger spherical inclusions, occurring singly or merged into tightly packed “grapes”. With lipids still in place they are electron-dense; more often, lipids are dissolved by the embedding process, and the lipid bodies appear as clear spherical spaces. Lipid bodies were sometimes interpreted as directly emerging out of the phago-lysosomal digestive process. For example, in the detailed analysis of enteric phagocytes in the cnidarian Tripedalia, Chapman (1978) concludes that “ingested material is progressively transformed into free basal droplets”. The author’s interpretation is based on the occurrence of lipid inclusions, within phagolysosomes, of similar size and texture as the free lipid bodies. However, given our current knowledge of lipid synthesis and the Golgi/Endoplasmic Reticulum/Lysosome (GERL) sytem in general, this interpretation is unlikely to be correct; rather, lipid bodies arise from the ER where fatty acids released from phagolysosomes are used to synthesize triglycerides (Murphy 2001; Melo et al. 2011).
6. Interrelationship between enteric phagocytes and macrophages
We discussed in the above historical introduction how at a very early stage in the research on phagocytosis, Metschnikoff (1884) and other observers emphasized the close functional and ontogenetic/phylogenetic relationship between enteric phagocytes and macrophages. In sponges, considered to be among the most basal metazoan clades, macrophages, called archaeocytes, are directly involved in the uptake of algae and other single-celled food organisms. In several other invertebrate phyla, motile macrophages in the stroma or coelom take up cellular debris resulting from dying cells (e.g., during metamorphosis) as well as particles injected into the body cavity. Aside from this direct participation in food uptake, macrophages, which actively move throughout the body, were thought to play an important role in distributing digested food stuffs, receiving this material from stationary phagocytes lining the gut, and transporting it to other tissues.
Electron-microscopic studies have largely confirmed these early observations and speculations. In sponges, stationary choanocytes and pinacocytes and motile archaeocytes all interact during the process of food uptake and intracellular digestion (Weissenfels 1976; Imsiecke 1993; Fig.6, left). Bacteria or single-celled algae enter the sponge body through ingestive channels, and are taken up into large phagosomes within pinacocytes (the cells lining ingestive channels), choanocytes, and archaeocytes. Archaeocytes move in between choanocyte chambers and canals and exchange phagosomes (or phagolysosomes) with choanocytes/pinacocytes, whereby plasma membranes of two cells come into close contact, the “donor cell” exocytoses the phagosome, and the “recipient cell” endocytosis the content of the phagosome. In the same manner, residual bodies (“digestive remnants”; Imsiecke 1993) are exchanged between cells, and eventually exocytosed into the excurrent canals. Aside from their involvement in absorption and digestion of nutrients, archaeocytes also give rise to cell types forming spicules, and providing immunity (Funayama et al. 2005).
Figure 6.
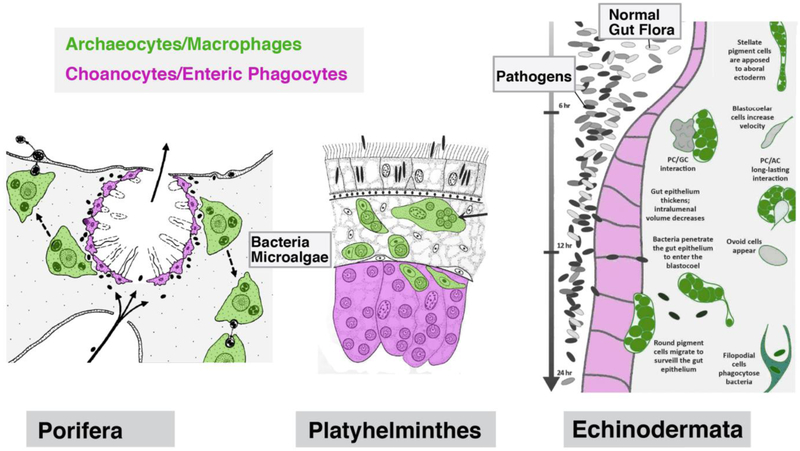
Functional interactions between enteric phagocytes and macrophages. Left: In sponges, food particles (bacteria, microalgae) are taken up by, and “swapped in between, stationary choanocytes (enteric phagocytes) and motile archaeocytes (macrophages; from Weissenfels 1976, with permission). Center: Even though not investigated at any length, transfer of algae taken up by enteric phagocytes to motile stromal cells has been documented for some flatworms (from Rieger 1991, with permission). Right: Coelomocytes (macrophages) in echinoderms are recruited towards the gastrodermis when pathogenic bacteria have entered the lumen of the gut (from Buckley and Rast 2017, with permission).
Stromal cells with attributes of phagocytes, containing lysosomes, phagolysosomes and nutrient storage organelles, have also been observed in several bilaterian clades, notably the platyhelminths (flatworms). In these animals, the stroma (paradoxically called parenchyma in the classical literature) is formed by large cells with abundant processes that interdigitate with and surround other cells (e.g., neurons, muscles, glands). Like gastrodermal, enteric phagocytes, these stromal cells also show phagocytotic activity when challenged with experimentally applied particles, and are thought to be involved in the uptake and distribution of intracellularly digested food material or symbiotic microalgae (Haffner 1925; Jennings 1957; Pedersen 1961; Morita and Best 1984; Rieger 1991; Fig.6, center). In acoels, formerly counted as members of the Platyhelminthes but now considered part of a separate superphylum, Xenacoelomorpha (see Gavilan et al. 2019, this issue) the role of absorbing gastrodermal phagocytes and distributing stromal macrophages has been combined in a single multinucleated cell type, the digestive syncytium. Here, endodermal cells merge into a syncytial mass that takes up whole prey animals into large phagosomes. At the same time, the digestive syncytium forms abundant lamellar processes that reach virtually every cell of the body, and therefore is in a position to distribute the nutrients widely among all tissues.
In most bilaterian animals the mesoderm forms epithelial layers surrounding inner cavities, called coelomata (singular: coelom). Spaces in between coelomata become the vascular system (Ruppert and Carle 1983; Hartenstein and Mandal 2006). Specialized motile, mesodermally derived cells populate the coelomata (“coelomocytes”) and the vessels (“hemocytes” or blood cells). Cells with the characteristics of macrophages are prominent among both hemocytes and coelomocytes (Hartenstein 2006). Macrophages move throughout the body cavity and are able to invade the stroma of all organs. Invertebrate macrophages have been studied extensively in the context of development and innate immunity (Cooper 2010; Vlisidou and Wood 2015; Abnave et al. 2017). During development, they represent the “professional macrophages” that eliminate apoptotic cells. In addition, they cooperate with humoral factors to battle invading parasites and microbes. Since many of these invaders enter through the digestive tract they typically first encounter gastrodermal cells. The complex interactions between macrophages and gastrodermal cells that occurs as part of the immune response has been studied in some detail in echinoderms. In sea urchin larvae, pathogenic bacteria introduced into the gut cavity evoke the release of cytokines from gastrodermal cells, which in turn recruit certain types of coelomocytes (pigment cells) towards the gut epithelium (Buckley and Rast 2017; 2019, this issue; Fig.6, right). According to a number of studies (e.g., Chia and Koss 1991), coelomocytes appear to become integrated into the gut epithelium. Their possible participation in nutritive phagocytosis, aside from immune-related processes, seems likely and has been reported in the classical literature (van der Heyde 1922; Kindred 1924), but has not been experimentally assessed in the recent literature. Phagocytic amoebocytes shuttling between the coelomic cavity and the intestine (where they become transiently integrated into the epithelium) have also been observed in several annelid species (Kermack 1955; Marsden 1963, 1966).
7. Ontogeny and phylogeny of phagocytes
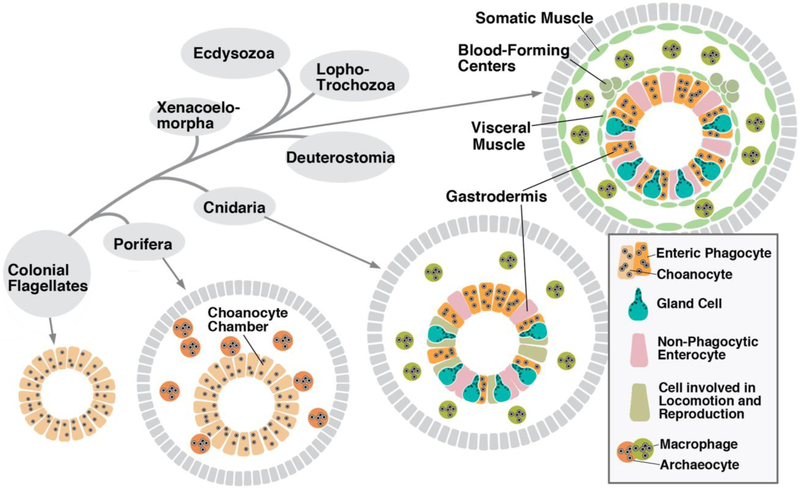
Coming towards the end of our review we would like to ask whether the close structural and functional relationships between enteric phagocytes and macrophages are also reflected in the ontogeny and phylogeny of these cell types. In animals that branched off close to the base of the metazoa, the two types of phagocytes may simply represent different developmental stages of the same cells. This can still be observed in extant porifera, in which the archaeocytes of the mesohyl (the mass of extracellular matrix and cellular elements filling the space in between pinacoderm and choanoderm) can convert into choanocytes (enteric phagocytes), and vice versa (Nakanishi et al. 2014; Sogabe et al. 2016; Fig.7, bottom center). Note that, while it appears clear that archaeocytes are involved in immunity (Funayama et al. 2005), molecular evidence for the homology between these cells and bilaterian macrophages has not yet been provided. In cnidarians, such as the soft corals (octocorallia), phagocytic amoebocytes with important immune functions populate the mesoglea, the extracellular matrix in between epidermis and gastrodermis (Menzel et al. 2015; Fig.7, bottom right). It is unknown whether these cells are derivatives of the gastrodermis, or could even develop directly from enteric phagocytes.
Figure 7.
Distribution of of enteric phagocytes and macrophages among animals. Simplistic phylogenetic tree is presented at upper left. Schematic cross sections of sponge (bottom center), cnidarian (bottom right) and coelomate bilaterian (top right) are grouped around the phylogenetic tree. Different cell types of endodermal digestive epithelium are represented in different shapes and colors, as explained in box at bottom right of figure. In sponges, motile macrophages and stationary phagocytes (choanocytes) may represent different stages in the same cell lineage; one stage is able to “transdifferentiate” into the other (Nakanishi et al. 2014; Sogabe et al. 2016). In cnidarians, the endodermal gastrodermis contains several differentiated cell types, among them enteric phagocytes. Motile macrophages (amoebocytes) with immune functions have been described (Menzel et al. 2015); it is possible that they are derived from the endoderm. In bilateria, mesodermal and endodermal cell lineages are separated early in development. Enteric phagocytes continue to develop from the endoderm; macrophages, along with other motile blood cells (hemocytes) are generated in specific hematopoietic centers within the mesoderm.
In bilaterian animals where macrophage development has been studied, these cells form part of mesodermally derived lineages. Their development is embedded in the process of hematopoiesis, which starts out in pockets of cells that are typically associated with the mesoderm flanking the gut [visceral mesoderm, or splanchopleura; Ruppert and Carle 1983; Hartenstein and Mandal 2006; Fig.7, top right). In the most simple scenario found, for example, in polychaete annelids, specialized domains within the splanchnopleura (and to a lesser extent, the somatopleura) exhibit higher rates of proliferation and extrude coelomocytes and blood cells into the lumen of the coelom or blood vessels, respectively (Eckelbarger 1976; Dales and Dixon 1981). More typically, one observes a hematopoietic mechanism whereby stem cells delaminate from the splanchnopleural epithelium and aggregate to form compact hematopoietic organs, called lymphoid organs or lymph glands. These structures are attached to the coelomic wall or large blood vessels, as seen, for example, in representatives of annelids, molluscs, and arthropods (Hoffmann et al. 1979; Hartenstein 2006; Grigorian and Hartenstein 2013). In vertebrates, macrophages (among other blood cell types) are initially formed from the embryonic endothelia (“hemogenic endothelia”) associated with the yolk sac and the large blood vessels (e.g., aorta; McGrath et al. 2015). In addition, the hemogenic endothelia produce hematopoietic stem cells (HSCs) that settle in the liver and bone marrow and form a permanent, self renewing source of monocytes and other blood cells. Another group of immune-related macrophages are the tissue resident macrophages found in the brain (microglia) and other organs (e.g., Kupffer cells in the liver). These cells derive from early embryonic hematopoietic cells (other than HSCs) that settle in the organ primordia where they replicate in situ.
Despite of the fact that, at the time when they are specified as distinct cell types, enteric phagocytes and macrophages of bilaterians reside in different tissue layers (i.e., endoderm-derived digestive epithelium and mesoderm-derived stroma), one may still recognize genetic and phylogenetic affiliations between these cells. Phylogenetically, mesoderm is thought to have evolved from within the endoderm (Rodaway and Patient 2001; Grapin-Botton and Constam 2007). In cnidarians, such as the sea anemone Nematostella vectensis, the endodermal layer that gives rise to the gastrodermis expresses many genes that, in bilaterian animals, occur in mesodermal derivatives. Specifically, the so-called mesenteries of Nematostella, which contain cells functioning in reproduction (somatic gonadal cells) and locomotion (parietal and circular muscle fibers), express many mesodermal determinants, such as mef2, six4/5, tbx1/10, foxC, nkx3/bagpipe, and others (Steinmetz et al. 2017; Steinmetz, this issue). It is possible that during the early evolution of the bilateria, certain sets of cells with functions like locomotion, reproduction and immunity separated from the endoderm to be better able to perform their functions as contractile fibers or motile macrophages. Eventually this separation was brought forward in development, resulting in the establishment of a distinct middle germ layer, the mesoderm, in the early embryo. From that time onward, enteric macrophages and macrophages formed as ontogenetically separate cell lineages (Fig.7, top right). Note also a more far-reaching version of this hypothesis (Steinmetz 2019, this issue) according to which the entire endoderm, including the enteric phagocytes, of the cnidarian/bilaterian ancestor became the mesoderm, and the (ectodermal) pharyngeal epithelium gave rise to the bilaterian endoderm. In this scenario, enteric phagocytes found in the bilaterian gastrodermis would have to evolve convergently from other cell types of the gastrodermis, and/or descend from mesodermal macrophages resettling the gut.
Assessing the homology of cell types across different clades relied, traditionally, on the comparison of their ultrastructure (i.e. from TEM). The introduction of sequencing methodologies applied to single cells offers a new possibility for classifying cells using their transcriptional profiles (Sebé-Pedros et al. 2018). The assumption is that similar profiles should be good indicators of their “homology” (Moroz 2018; Arendt et al 2019). Does this approach add to clarify the relationship between enteric phagocytes (such as those existing in planarians), and vertebrate macrophages? In the two systems where extensive transcriptomic analysis has been done to characterize the diversity of macrophage/phagocyte cell types , the flatworm Schmidtea mediterranea (Plass et al. 2018 and Fincher et al. 2018)) and the mice lung tissue (Aran et al. 2019) the datasets are mostly identifying highly expressed, structural or metabolic, components (and thus, mostly inconclusive for homology assessments); a fact that highlights the difficulties in comparing cell types across widely divergent clades. In fact, it is becoming clear that homologous cell types often don’t show highest transcriptome similarity (even when comparing cell types of known homology between different vertebrates; see: Liang et al, 2018). The fact that transcription factors are absent or rarely present from these datasets (a consequence of the low levels of expression) points to the urgent need of using alternative methodologies directly targeted to the identification of transcriptional regulators and their functional interactions (Gene Regulatory Networks; GRNs) which should provide more solid proofs of homology. Needless to say, a framework of embryological origin should be taken into account, since they can inform us of putative convergence effects. The use of concepts such as “character identity networks” (Wagner, 2007), “synexpression groups” (Gawantka et al., 1998) or “network kernels” have been suggested as better representatives of the cells or tissue “deep homologies” (Davidson and Erwin, 2006). One relevant example here is the modeling effort carried out by Collombet and collaborators (Collombet et al. 2017) aiming at understanding the specification of different hematopoietic lineages, in particular the differentiation of macrophages (monocytes) and B-lymphocytes from a common MB progenitor. These authors identified 23 factors, most of them transcription factors, but also a few cytokine receptors, that were preferentially expressed in the progenitors, macrophages, or B cells. Experimentally tested expression patterns and genetic interactions place the factors into a complex web that represents a “macrophage identity network”. The large majority of the factors is conserved in Drosophila, and, in part has retained a role in the specification of fly macrophages. Most factors are also found in other bilaterians; for example, about half of the factors were identified in the transcriptome of the acoel Symsagittifera roscoffensis (Philippe et al 2019). Expression data and functional studies will help to discern whether the “macrophage identify network” also plays a role in the specification of enteric phagocytes, which would further confirm a common evolutionary origin of the two cell types.
Acknowledgments
Funding: NIH R01 NS054814
Footnotes
This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Conflict of Interest: none
Informed consent: N/A
Ethical approval: This article does not contain any studies with human participants or animals performed by any of the authors.
References
- Abnave P, Muracciole X, Ghigo E (2017) Macrophages in Invertebrates: From Insects and Crustaceans to Marine Bivalves. Results Probl Cell Differ 62:147–158. doi: 10.1007/978-3-319-54090-0_6 [DOI] [PubMed] [Google Scholar]
- Afzelius BA, Rosén B (1965) Nutritive phagocytosis in animal cells. An electron microscopical study of the gastroderm of the hydroid Clava squamata Müll. Z Zellforsch Mikrosk Anat 67:24–33. [PubMed] [Google Scholar]
- Al-Mohanna SY, Nott JA (1987) R-cells and the digestive cycle in Penaeus semisulcatus (Crustacea: Decapoda). Marine Biology 95:129–137. doi: 10.1007/BF00447494 [DOI] [Google Scholar]
- Al-Mohanna SY, Nott JA (1989) Functional cytology of the hepatopancreas of Penaeus semisulcatus (Crustacea: Decapoda) during the moult cycle. Marine Biology 101:535–544. doi: 10.1007/BF00541656 [DOI] [Google Scholar]
- Antoniazzi MM, Silveira M (1996) Studies on Stenostomum grandeChild, 1902 (Platyhelminthes, Catenulida): fine structure of the digestive tract and the endocytotic activity of the gastrodermis. Acta Zoologica 77:25–32. doi: 10.1111/j.1463-6395.1996.tb01250.x [DOI] [Google Scholar]
- Aran D, Looney AP, Liu L, et al. (2019) Reference-based analysis of lung single-cell sequencing reveals a transitional profibrotic macrophage. Nat Immunol 20:163–172. doi: 10.1038/S41590-018-0276-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arendt D, Bertucci PY, Achim K, Musser JM (2019) Evolution of neuronal types and families. Curr Opin Neurobiol 56:144–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnaud J, Brunet M, Casanova J-P, et al. (1996) Morphology and ultrastructure of the gut in Spadella cephaloptera (chaetognatha). Journal of Morphology 228:27–44. doi: [DOI] [PubMed] [Google Scholar]
- Arnaud J, Brunet M, Mazza J (1978) Studies on the midgut of Centropages typicus (copepod, calanoid). I. Structural and Ultrastructural Data. Cell Tissue Res 187:333–353. [DOI] [PubMed] [Google Scholar]
- Benito J, Pardos F (1997) Hemichordata In Harrison FW (ed) Microscopic anatomy of invertebrates, vol 15 John Wiley and Sons, New York, pp 15–101. [Google Scholar]
- Bignell DE, Oskarsson H, Anderson JM (1982) Formation of membrane-bounded secretory granules in the midgut epithelium of a termite, Cubitermes severus, and a possible intercellular route of discharge. Cell Tissue Res. doi: 10.1007/BF00218299 [DOI] [PubMed] [Google Scholar]
- Biserova NM, Mustafina AR (2015) Comparative midgut ultrastructure in three species of Tardigrada. Arthropoda Selecta 24:373–385. [Google Scholar]
- Bloomfield G, Kay RR (2016) Uses and abuses of macropinocytosis. J Cell Sci 129:2697–2705. doi: 10.1242/jcs.176149 [DOI] [PubMed] [Google Scholar]
- Bonifacino JS, Neefjes J (2017) Moving and positioning the endolysosomal system. Curr Opin Cell Biol 47:1–8. doi: 10.1016/j.ceb.2017.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucaud-Camou E, Yim M (2009) Fine structure and function of the digestive cell of Sepia officinalis (Mollusca: Cephalopoda). Journal of Zoology 191:89–105. doi: 10.1111/j.1469-7998.1980.tb01451.x [DOI] [Google Scholar]
- Boury-Esnault N, De Vos L, Donadey C, Vacelet J (1984) Comparative study of the choanosome of Porifera: 1. The Homoscleromorpha. Journal of Morphology 180:3–17. doi: 10.1002/jmor.1051800103 [DOI] [PubMed] [Google Scholar]
- Bowen ID, Ryder TA, Thompson JA (1974) The fine structure of the planarian Polycelis tenuis Iijima. II. The intestine and gastrodermal phagocytosis. Protoplasma 79:1–17. [DOI] [PubMed] [Google Scholar]
- Broderick NA (2015) A common origin for immunity and digestion. Front Immunol 6:72. doi: 10.3389/fimmu.2015.00072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brusca R, Moore W, Shuster SM, Haver N (2016) Invertebrates. Oxford University Press [Google Scholar]
- Buchmeier NA, Heffron F (1991) Inhibition of macrophage phagosome-lysosome fusion by Salmonella typhimurium. Infect Immun 59:2232–2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley KM, Rast JP (2017) An Organismal Model for Gene Regulatory Networks in the Gut-Associated Immune Response. Front Immunol 8:1297. doi: 10.3389/fimmu.2017.01297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burighel P, Milanesi C (1973) Fine structure of the gastric epithelium of the ascidian Botryllus schlosseri. Vacuolated and zymogenic cells. Z Zellforsch Mikrosk Anat 145:541–555. [DOI] [PubMed] [Google Scholar]
- Burighel P, Milanesi C (1975) Fine structure of the gastric epithelium of the ascidian Botryllus schlosseri. Mucous, endocrine and plicated cells. Cell Tissue Res 158:481–496. [DOI] [PubMed] [Google Scholar]
- Burighel P, Cloney RA (1997) Urochordata: Ascidiacea In: Harrison FW, Ruppert EE (eds) Microscopic anatomy of invertebrates vol. 15, John Wiley and Sons, New York: 1997, pp 221–347. [Google Scholar]
- Byrne M (1994) Ophiuroidea In: Harison FW, Chia F-S (eds) Microscopic anatomy of invertebrates, vol 14 John Wiley and Sons, New York, pp 247–343. [Google Scholar]
- Chapman DM (1978) Microanatomy of the cubopolyp,Tripedalia cystophora (Class Cubozoa). Helgoländer Wissenschaftliche Meeresuntersuchungen 31:128–168. doi: 10.1007/BF02296994 [DOI] [Google Scholar]
- Chia FS, Koss R (1991) Asteroidea In: Harrison FW, Chia TS (eds). Microscopic anatomy of invertebrates, vol 14 John Wiley and Sons, New York, pp 169–246. [Google Scholar]
- Clément P, Wurdak ES (1991) Rotifera In: Harrison FW, Ruppert EE (eds) Microscopic anatomy of invertebrates, vol 4 John Wiley and Sons, New York, pp 219–297. [Google Scholar]
- Clokey GV, Jacobson LA (1986) The autofluorescent “lipofuscin granules” in the intestinal cells of Caenorhabditis elegans are secondary lysosomes. Mech Ageing Dev 35:79–94. doi: 10.1016/0047-6374(86)90068-0 [DOI] [PubMed] [Google Scholar]
- Collombet S, van Oevelen C, Sardina Ortega JL, et al. (2017) Logical modeling of lymphoid and myeloid cell specification and transdifferentiation. Proc Natl Acad Sci USA 114:5792–5799. doi: 10.1073/pnas.1610622114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coons LB, Alberti G (1999) Acari: Ticks In: Harrison FW (ed) Microscopic anatomy of invertebrates, vol 8b John Wiley and Sons, New York, pp 267–514. [Google Scholar]
- Cooper EL (2010) Evolution of immune systems from self/not self to danger to artificial immune systems (AIS). Phys Life Rev 7:55–78. doi: 10.1016/j.plrev.2009.12.001 [DOI] [PubMed] [Google Scholar]
- Dales RP, Dixon LRJ. 1981. Polychaetes In: Ratcliffe NA, Rowley AF (eds) Invertebrate Blood Cells, vol 1 Academic Press, pp 35–75. [Google Scholar]
- Davidson EH, Erwin DH (2006) Gene regulatory networks and the evolution of animal body plans. Science 311:796–800. doi: 10.1126/science.1113832 [DOI] [PubMed] [Google Scholar]
- De Duve C, Pressman BC, Gianetto R, et al. (1955) Tissue fractionation studies. 6. Intracellular distribution patterns of enzymes in rat-liver tissue. Biochem J 60:604–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Duve C, Wattiaux R (1966) Functions of lysosomes. Annu Rev Physiol 28:435–492. doi: 10.1146/annurev.ph.28.030166.002251 [DOI] [PubMed] [Google Scholar]
- De Priester W (1971) Ultrastructure of the midgut epithelial cells in the fly Calliphora erythrocephala. Journal of Ultrastructure Research 36:783–805. [DOI] [PubMed] [Google Scholar]
- Del Conte E (1979) Ultrastructural aspects of degradation and necrosis of Leydig cells in lizards by effect of metyrapone. Gen Comp Endocrinol 37:101–110. [DOI] [PubMed] [Google Scholar]
- Dewel RA, Nelson DR, Dewel WC (1993) Tardigrada In: Harrison FW, Rice EM (eds) Microscopic anatomy of invertebrates, vol 12 John Wiley and Sons, New York, pp143–183. [Google Scholar]
- Dilly PN, Welsch U, Rehkämper G (1986) On the Fine Structure of the Alimentary Tract of Cephalodiscus gracilis(Pterobranchia, Hemichordata). Acta Zoologica 67:87–95. doi: 10.1111/j.1463-6395.1986.tb00852.x [DOI] [Google Scholar]
- Doe DA (1981) Comparative ultrastructure of the pharynx simplex in turbellaria. Zoomorphology 97:133–193. doi: 10.1007/BF00310107 [DOI] [Google Scholar]
- Eckelbarger KJ (1976) Origin and development of the amoebocytes of Nicolea zostericola (Polychaeta: Terebellidae) with a discussion of their possible role in oogenesis. Marine Biology 36:169–182. doi: 10.1007/BF00388440 [DOI] [Google Scholar]
- Essner E, Haimes H (1977) Ultrastructural study of GERL in beige mouse alveolar macrophages. J Cell Biol 75:381–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fankboner PV (2001) Digestive System of Invertebrates. John Wiley & Sons, Ltd, Chichester [Google Scholar]
- Fedorko ME, Hirsch JG, Cohn ZA (1968) Autophagic vacuoles produced in vitro. I. Studies on cultured macrophages exposed to chloroquine. J Cell Biol 38:377–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez J, Tellez V, Olea N (1992) Hirudinea In: Harrison FW, Gardiner SL (eds) Microscopic anatomy of invertebrates, vol 7 John Wiley and Sons, New York, pp 323–394. [Google Scholar]
- Filimonova SA (2013) Morphological aspects of blood digestion in a parasitic mite Bakericheyla chanayi. Arthropod Struct Dev 42:265–276. doi: 10.1016/j.asd.2013.02.006 [DOI] [PubMed] [Google Scholar]
- Filimonova SA (2008) The fine structure of the midgut in the mite Anystis baccarum (L.) (Acari, Actinedida: Anystidae). Arthropod Struct Dev 37:299–309. doi: 10.1016/j.asd.2007.11.005 [DOI] [PubMed] [Google Scholar]
- Fincher CT, Wurtzel O, de Hoog T, et al. (2018) Cell type transcriptome atlas for the planarian Schmidtea mediterranea. Science 360:eaaq1736. doi: 10.1126/science.aaq1736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitt WK, Trench RK (1983) Endocytosis of the symbiotic dinoflagellate Symbiodinium microadriaticum Freudenthal by endodermal cells of the scyphistomae of Cassiopeia xamachana and resistance of the algae to host digestion. J Cell Sci 64:195–212. [DOI] [PubMed] [Google Scholar]
- Franc JM (1972) [Activity and ultrastructure of the ctenophore cell rosettes]. Z Zellforsch Mikrosk Anat 130:527–544. [PubMed] [Google Scholar]
- Funayama N, Nakatsukasa M, Kuraku S, Takechi K, Dohi M, Iwabe N, Miyata T, Agata K (2005) Isolation of Ef silicatein and Ef lectin as molecular markers for sclerocytes and cells involved in innate immunity in the freshwater sponge Ephydatia fluviatilis. Zoolog Sci 22:1113–22. [DOI] [PubMed] [Google Scholar]
- Garcia-Corrales P, Gamo J (1988) Ultrastructural changes in the gastrodermal phagocytic cells of the planarian Dugesia gonocephala s.l. during food digestion (Plathelminthes). Zoomorphology 108:109–117. doi: 10.1007/BF00539786 [DOI] [Google Scholar]
- Gawantka V, Pollet N, Delius H, et al. (1998) Gene expression screening in Xenopus identifies molecular pathways, predicts gene function and provides a global view of embryonic patterning. Mech Dev 77:95–141. [DOI] [PubMed] [Google Scholar]
- Giribet G, Edgecombe GD (2017) Current Understanding of Ecdysozoa and its Internal Phylogenetic Relationships. Integr Comp Biol 57:455–466. doi: 10.1093/icb/icx072 [DOI] [PubMed] [Google Scholar]
- Gonobobleva E, Maldonado M (2009) Choanocyte ultrastructure in Halisarca dujardini (Demospongiae, Halisarcida). Journal of Morphology 270:615–627. doi: 10.1002/jmor.10709 [DOI] [PubMed] [Google Scholar]
- Goyffon M, Martoja R (1983) Cytophysiological aspects of digestion and storage in the liver of a scorpion, Androctonus australis (Arachnida). Cell Tissue Res 228:661–675. doi: 10.1007/BF00211482 [DOI] [PubMed] [Google Scholar]
- Grapin-Botton A, Constam D (2007) Evolution of the mechanisms and molecular control of endoderm formation. Mech Dev 124:253–278. [DOI] [PubMed] [Google Scholar]
- Greven H (1976) Some ultrastructural observations on the midgut epithelium of Isohypsibius augusti (Murray, 1907) (Eutardigrada). Cell Tissue Res 166:339–351. [DOI] [PubMed] [Google Scholar]
- Grigorian M, Hartenstein V (2013) Hematopoiesis and hematopoietic organs in arthropods. Dev Genes Evol 223:103–115. doi: 10.1007/s00427-012-0428-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruenberg J, Stenmark H (2004) The biogenesis of multivesicular endosomes. Nature Reviews Molecular Cell Biology 2011 12:45:317–323. doi: 10.1038/nrm1360 [DOI] [PubMed] [Google Scholar]
- Haas A (2007) The Phagosome: Compartment with a License to Kill. Traffic 8:311–330. [DOI] [PubMed] [Google Scholar]
- Haffner K (1925) Untersuchungen über die Symbiose von Dalyellia viridis und Chlorohydra viridissima mit Chlorellen. Z Wiss Zool 126: 1–69. [Google Scholar]
- Hammersen F, Pokahr A (1972) [Electron microscopic studies of epithelial structure of the gastrointestinal tract of Hirudo medicinalis L. I. Epithelium of the diverticulum]. Z Zellforsch Mikrosk Anat 125:378–403. [PubMed] [Google Scholar]
- Hard GC (1972) Examination by electron microscopy of the interaction between peritoneal phagocytes and Corynebacterium ovis. J Med Microbiol 5:483–491. doi: 10.1099/00222615-5-4-483 [DOI] [PubMed] [Google Scholar]
- Hartenstein V (2006) Blood Cells and Blood Cell Development in the Animal Kingdom. Annu Rev Cell Dev Biol 22:677–712. doi: 10.1146/annurev.cellbio.22.010605.093317 [DOI] [PubMed] [Google Scholar]
- Hartenstein V, Mandal L (2006) The blood/vascular system in a phylogenetic perspective. Bioessays 28:1203–1210. doi: 10.1002/bies.20497 [DOI] [PubMed] [Google Scholar]
- He C, Han T, Liao X, et al. (2018) Phagocytic intracellular digestion in amphioxus (Branchiostoma). Proc Biol Sci 285:20180438. doi: 10.1098/rspb.2018.0438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendriks HR, Eestermans IL (1986) Phagocytosis and lipofuscin accumulation in lymph node macrophages. Mech Ageing Dev 35:161–167 [DOI] [PubMed] [Google Scholar]
- Hernandez-Nicaise ML (1991) Ctenophora In: Harrison FW, Westfall JA (eds) Microscopic anatomy of invertebrates vol. 2 John Wiley and Sons, New York, pp 359–418. [Google Scholar]
- Herold RC, Meadow ND (1970) Age-related changes in ultrastructure and histochemistry of rotiferan organs. Journal of Ultrastructure Research 33:203–218. doi: 10.1016/S0022-5320(70)90016-X [DOI] [PubMed] [Google Scholar]
- Herrmann K (1997) Phoronida In: Harrison FW and Woollacott RM(Eds.) Microscopic Anatomy of Invertebrates, vol 13 John Wiley and Sons, New York, pp 207–235. [Google Scholar]
- Hirsch GC (1925) Probleme der intraplasmatischen Verdauung. Ihre Beziehungen zur Resorption, Diffusion, Nahrungsaufnahme, Darmbau und Nahrungswahl bei den Metazoen. Zeitschrift für Vergleichende Physiologie 3:183–208. doi: 10.1007/BF00343742 [DOI] [Google Scholar]
- Hoffmann JA, Zachary D, Hoffmann D, Brehelin M, Porte A (1979) Postembryonic development and differentiation: hemopoietic tissues and their functions in some insects In: Gupta AP (ed) Insect Hemocytes. Cambridge University Press; pp 29–66. [Google Scholar]
- Holt PA, Mettrick DF (1975) Ultrastructural studies of the epidermis and gastrodermis of Syndesmis franciscana(Turbellaria: Rhabdocoela). Canadian Journal of Zoology 53:536–549. doi: 10.1139/z75-068 [DOI] [Google Scholar]
- Hooton D, Lentle R, Monro J, et al. (2015) The Secretion and Action of Brush Border Enzymes in the Mammalian Small Intestine. Rev Physiol Biochem Pharmacol 168:59–118. doi: 10.1007/112_2015_24 [DOI] [PubMed] [Google Scholar]
- Huotari J, Helenius A (2011) Endosome maturation. EMBO J 30:3481–3500. doi: 10.1038/emboj.2011.286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imsiecke G (1993) Ingestion, digestion, and egestion in Spongilla lacustris (Porifera, Spongillidae) after pulse feeding with Chlamydomonas reinhardtii (Volvocales). Zoomorphology 113:233–244. doi: 10.1007/BF00403314 [DOI] [Google Scholar]
- Jahraus A, Tjelle TE, Berg T, et al. (1998) In vitro fusion of phagosomes with different endocytic organelles from J774 macrophages. J Biol Chem 273:30379–30390. [DOI] [PubMed] [Google Scholar]
- Jangoux M (1972) [The fine structure of the rectal caeca of two Asteriidae: Marthasterias glacialis (L) and Coscinasterias tenuispina (Lam) (echinodermata asteroidea)]. Z Zellforsch Mikrosk Anat 128:366–384. [DOI] [PubMed] [Google Scholar]
- Jennings JB (1957) Studies on feeding, digestion, and food storage in free-living flatworms (Platyhelminthes: Turbellaria). The Biological Bulletin 112:63–80. doi: 10.2307/1538879 [DOI] [Google Scholar]
- Jennings JB (1969) Ultrastructural observations on the phagocytic uptake of food materials by the ciliated cells of the rhynchocoelan intestine. The Biological Bulletin 137:476–485. doi: 10.2307/1540169 [DOI] [PubMed] [Google Scholar]
- Kajihara H, Totović V, Gedigk P (1975) [Ultrastructure and morphogenesis of ceroid pigment. I. Phagocytosis and formation of lipid-containing lysosomes in Kupffer Cells after intravenous injection of unsaturated lipids (author's transl)]. Virchows Arch B Cell Pathol 19:221–237. [PubMed] [Google Scholar]
- Kermack DM (1955) The anatomy and physiology of the gut of the polychaete Arenicola marina (L.). Proc Zool Soc Lond 125:347–381 [Google Scholar]
- Kerr JFR (1970) Liver cell defaecation: An electron-microscope study of the discharge of lysosomal residual bodies into the intercellular space. The Journal of Pathology 100:99–103. doi: 10.1002/path.1711000204 [DOI] [PubMed] [Google Scholar]
- Kerr MC, Teasdale RD (2009) Defining macropinocytosis. Traffic 10:364–371. doi: 10.1111/j.1600-0854.2009.00878.x [DOI] [PubMed] [Google Scholar]
- Kinchen JM, Ravichandran KS (2008) Phagosome maturation: going through the acid test. Nature Reviews Molecular Cell Biology 2011 12:4 9:781–795. doi: 10.1038/nrm2515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindred JE (1924) The cellular elements in the perivisceral fluid of echinoderms. Biol Bull 46: 228–251. [Google Scholar]
- Kocot KM, Struck TH, Merkel J, et al. (2017) Phylogenomics of Lophotrochozoa with Consideration of Systematic Error. Syst Biol 66:256–282. doi: 10.1093/sysbio/syw079 [DOI] [PubMed] [Google Scholar]
- Kristensen RM, Higgins RP (1991) Kinorhyncha In: Harrison FW, Ruppert EE (eds) Microscopic anatomy of invertebrates, vol 4 John Wiley and Sons, New York, pp 377–404 [Google Scholar]
- Lee CC (1969) Ancylostoma caninum: Fine structure of intestinal epithelium. Exp Parasitol 24: 336–347. [DOI] [PubMed] [Google Scholar]
- Leys SP, Eerkes-Medrano DI (2006) Feeding in a Calcareous Sponge: Particle Uptake by Pseudopodia. The Biological Bulletin 211:157–171. doi: 10.2307/4134590 [DOI] [PubMed] [Google Scholar]
- Liang C, Musser JM, Cloutier A, Prum RO, Wagner GP. Pervasive Correlated Evolution in Gene Expression Shapes Cell and Tissue Type Transcriptomes. (2018) Genome Biol Evol. 10(2):538–552. doi: 10.1093/gbe/evy016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim C-Y, Zoncu R (2016) The lysosome as a command-and-control center for cellular metabolism. J Cell Biol 214:653–664. doi: 10.1083/jcb.201607005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobo-da-Cunha A (2000) The digestive cells of the hepatopancreas in Aplysia depilans (Mollusca, Opisthobranchia): ultrastructural and cytochemical study. Tissue and Cell 32:49–57. doi: 10.1054/tice.1999.0082 [DOI] [PubMed] [Google Scholar]
- Lobo-da-Cunha A, Batista-Pinto C (2003) Stomach ofAplysia depilans (Mollusca, Opisthobranchia): A histochemical, ultrastructural, and cytochemical study. Journal of Morphology 256:360–370. doi: 10.1002/jmor.10099 [DOI] [PubMed] [Google Scholar]
- Lobo-da-Cunha A, Batista-Pinto C (2007) Ultrastructural, histochemical and cytochemical characterization of intestinal epithelial cells in Aplysia depilans (Gastropoda, Opisthobranchia). Acta Zoologica 88:211–221. doi: 10.1111/j.1463-6395.2007.00268.x [DOI] [Google Scholar]
- Loizzi RF (1971) Interpretation of crayfish hepatopancreatic function based on fine structural analysis of epithelial cell lines and muscle network. Cell Tissue Res 113:420–440. doi: 10.1007/BF00968548 [DOI] [PubMed] [Google Scholar]
- Lunger PD (1963) Fine-structural aspects of digestion in a colonial hydroid. Journal of Ultrastructure Research 9:362–380. doi: 10.1016/S0022-5320(63)80012-X [DOI] [Google Scholar]
- Marsden JR (1963) The digestive tract of Hermodice carunculata (Pallas), Polychaeta: Amphinomidae. Can J Zool 41:165–183. [Google Scholar]
- Marsden JR (1966) The coelomocytes of Hermodice carunculata (Polychaeta: Amphinomidae). Can J Zool 44:377–389. [DOI] [PubMed] [Google Scholar]
- Martinez A, Lopez J, Villaro AC, Sesma P (1991) Choanocyte-like cells in the digestive system of the starfishMarthasterias glacialis (Echinodermata). Journal of Morphology 208:215–225. doi: 10.1002/jmor.1052080207 [DOI] [PubMed] [Google Scholar]
- Mayor S, Pagano RE (2007) Pathways of clathrin-independent endocytosis. Nature Reviews Molecular Cell Biology 8:603–612. doi: 10.1038/nrm2216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGhee J (2007) The C. elegans intestine. WormBook. doi: 10.1895/wormbook.1.133.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath KE, Frame JM, Palis J (2015) Early hematopoiesis and macrophage development. Semin Immunol 27:379–87. doi: 10.1016/j.smim.2016.03.013. PMID:27021646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeil PL (1981) Mechanisms of nutritive endocytosis. I. Phagocytic versatility and cellular recognition in Chlorohydra digestive cells, a scanning electron microscope study. J Cell Sci 49:311–339. [DOI] [PubMed] [Google Scholar]
- Melo RCN, D'Avila H, Wan H-C, et al. (2011) Lipid bodies in inflammatory cells: structure, function, and current imaging techniques. J Histochem Cytochem 59:540–556. doi: 10.1369/0022155411404073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menzel LP, Tondo C, Stein B, Bigger CH (2015) Histology and ultrastructure of the coenenchyme of the octocoral Swiftia exserta, a model organism for innate immunity/graft rejection. Zoology 118:115–124. doi: 10.1016/j.zool.2014.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metschnikoff E (1878) Über die Verdauungsorgane einiger Süsswasserturbellarien. Zool Anz, Bd. 1, 387–390. [Google Scholar]
- Metschnikoff E (1884) Memoirs: Researches on the Intracellular Digestion of Invertebrates. J Cell Sci 93: 89–111. [Google Scholar]
- Michel C, Bhaud M, Boumati P, Halpern S (1984) Physiology of the digestive tract of the sedentary polychaete Terebellides stroemi. Marine Biology 83:17–31. doi: 10.1007/BF00393082 [DOI] [Google Scholar]
- Miguel-Aliaga I, Jasper H, Lemaitre B (2018) Anatomy and Physiology of the Digestive Tract of Drosophila melanogaster. Genetics 210:357–396. doi: 10.1534/genetics.118.300224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millot N (1938) On the morphology of the alimentary canal, process of feeding, and physiology of digestion of the nudibranch mollusc Jorunna tomentosa (Cuvier). Phil TransRoy Soc Lond Ser B 228: 173–218. [Google Scholar]
- Morita M, Best JB (1984) Electron microscopic studies of planarian regeneration. III. Degeneration and differentiation of muscles. Journal of Experimental Zoology 229:413–424. doi: 10.1002/jez.1402290309 [DOI] [PubMed] [Google Scholar]
- Moroz LL (2018) NeuroSystematics and Periodic System of Neurons: Model vs Reference Species at Single-Cell Resolution. ACS Chem Neurosci 9:1884–1903. doi: 10.1021/acschemneuro.8b00100 [DOI] [PubMed] [Google Scholar]
- Mukai H, Terakado K, Reed CG (1997) Bryozoa In: Harrison FW, Woolacott RM (eds) Microscopic anatomy of invertebrates, vol 13 John Wiley and Sons, New York, pp 45–206. [Google Scholar]
- Munnell JF, Cork LC (1980) Exocytosis of residual bodies in a lysosomal storage disease. Am J Pathol 98:385–394. [PMC free article] [PubMed] [Google Scholar]
- Murphy D (2001) The biogenesis and functions of lipid bodies in animals, plants and microorganisms. Progress in Lipid Research 40:325–438. doi: 10.1016/S0163-7827(01)00013-3 [DOI] [PubMed] [Google Scholar]
- Nakanishi N, Sogabe S, Degnan BM (2014) Evolutionary origin of gastrulation: insights from sponge development. BMC Biology 12:26. doi: 10.1186/1741-7007-12-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuhaus B, Higgins RP (2002) Ultrastructure, biology, and phylogenetic relationships of kinorhyncha. Integr Comp Biol 42:619–632. doi: 10.1093/icb/42.3.619 [DOI] [PubMed] [Google Scholar]
- Noventa S, Hacker C, Correia A, et al. (2018) Gold nanoparticles ingested by oyster larvae are internalized by cells through an alimentary endocytic pathway. Nanotoxicology 12:1–13. doi: 10.1080/17435390.2018.1487601 [DOI] [PubMed] [Google Scholar]
- Novikoff AB (1973) Lysosomes: a personal account In Lysosomes and Storage Diseases. Hers HG and van Hoof F, editors. Academic Press Inc, N.Y. pp.1–41. [Google Scholar]
- Owen G (1970) The fine structure of the digestive tubules of the Marine Bivalve Cardium edule. Philos Trans R Soc Lond, B, Biol Sci 258:245–260. doi: 10.1098/rstb.1970.0035 [DOI] [PubMed] [Google Scholar]
- Owen G (1972) Lysosomes, peroxisomes and bivalves. Sci Prog 60:299–318. [PubMed] [Google Scholar]
- Pal SG (1972) The fine structure of the digestive tubules of Mya arenaria L. II. Digestive cell. Proc malac Soc Lond, 40:161–170. [Google Scholar]
- Pascolini R, Gargiulo AM (1975) Ultrastructural Modifications of Cells in the Intestine of Dugesia Lugubris. Bolletino di zoologia 42:123–131. doi: 10.1080/11250007509431420 [DOI] [Google Scholar]
- Pedersen KJR (1961) Studies on the nature of planarian connective tissue. Cell Tissue Res 53:569–608. doi: 10.1007/BF00339508 [DOI] [Google Scholar]
- Perez Y, Casanova JP, Mazza J (2001) Degrees of vacuolation of the absorptive intestinal cells of five Sagitta (Chaetognatha) species: possible ecophysiological implications. Marine Biology 138:125–133. doi: 10.1007/s002270000438 [DOI] [Google Scholar]
- Peterson KJ, Eernisse DJ (2016) The phylogeny, evolutionary developmental biology, and paleobiology of the Deuterostomia: 25 years of new techniques, new discoveries, and new ideas. Org Divers Evol 16:401–418. doi: 10.1007/s13127-016-0270-x [DOI] [Google Scholar]
- Philippe H, Poustka AJ, Chiodin M, Hoff KJ, Dessimoz C, Tomiczek B, Schiffer PH, Müller S, Domman D, Horn M, Kuhl H, Timmermann B, Satoh N, Hikosaka-Katayama T, Nakano H, Rowe ML, Elphick MR, Thomas-Chollier M, Hankeln T, Mertes F, Wallberg A, Copley RR, Martinez P, and Telford MJ (2019) Mitigating anticipated effects of systematic errors supports sister-group relationship between Xenacoelomorpha and Ambulacraria Current Biology. In press. [DOI] [PubMed] [Google Scholar]
- Plass M, Solana J, Wolf FA, et al. (2018) Cell type atlas and lineage tree of a whole complex animal by single-cell transcriptomics. Science 360:eaaq1723. doi: 10.1126/science.aaq1723 [DOI] [PubMed] [Google Scholar]
- Pollard TD, Earnshaw WC, Lippincott-Schwartz J, Johnson GT (2017) Cell Biology, 3rd Edition. Elsevier. [Google Scholar]
- Repnik U, Česen MH, Turk B (2013) The endolysosomal system in cell death and survival. Cold Spring Harb Perspect Biol 5:a008755–a008755. doi: 10.1101/cshperspect.a008755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter M, Palmberg I (1987) An Ultrastructural and Immunocytochemical Study of Gastrodermal Cell Types in Microstomum lineare(Turbellaria, Macrostomida). Acta Zoologica 68:153–163. doi: 10.1111/j.1463-6395.1987.tb00886.x [DOI] [Google Scholar]
- Revel JP, Napolitano L, Fawcett DW (1960) Identification of glycogen in electron micrographs of thin tissue sections. J Biophys Biochem Cytol 8:575–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards AG, Richards PA (1977) The peritrophic membranes of insects. Annu Rev Entomol 22:219–240. doi: 10.1146/annurev.en.22.010177.001251 [DOI] [PubMed] [Google Scholar]
- Rieger R (1991) Turbellaria In: Harrison FW, Bogitsh J (eds) Microscopic anatomy of invertebrates, vol 2 John Wiley and Sons, New York, pp 1–140. [Google Scholar]
- Riley J (1973) Histochemical and ultrastructural observations on digestion in Tetrameres fissispina Diesing, 1861 (Nematoda : Spiruridea) with special reference to intracellular digestion. International Journal for Parasitology 3:157–164. doi: 10.1016/0020-7519(73)90021-0 [DOI] [PubMed] [Google Scholar]
- Rodaway A, Patient R (2001) Mesendoderm: an ancient germ layer? Cell 105:169–172. [DOI] [PubMed] [Google Scholar]
- Rougerie P, Miskolci V, Cox D (2013) Generation of membrane structures during phagocytosis and chemotaxis of macrophages: role and regulation of the actin cytoskeleton. Immunological Reviews 256:222–239. doi: 10.1111/imr.12118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruppert EE, Carle KJ (1983) Morphology of metazoan circulatory systems. Zoomorphology 103:193–208. doi: 10.1007/BF00310477 [DOI] [Google Scholar]
- Ruppert EE (1991) Gastrotricha In: Harrison FW, Ruppert EE (eds) Microscopic anatomy of invertebrates, vol 4 John Wiley and Sons, New York, pp 41–109. [Google Scholar]
- Ruppert EE (1997) Cephalochordata (Acrania) In: Harrison FW, Ruppert EE (eds) Microscopic anatomy of invertebrates vol. 15 John Wiley and Sons, New York, pp 349–504. [Google Scholar]
- Saulnier-Michel C (1992) Polychaeta: digestive system In: Harrison FW, Gardiner SL (eds) Microscopic anatomy of invertebrates, vol 7 John Wiley and Sons, New York, pp 53–69. [Google Scholar]
- Sebé-Pedrós A, Chomsky E, Pang K, et al. (2018) Early metazoan cell type diversity and the evolution of multicellular gene regulation. Nature Ecology & Evolution 2:1176–1188. doi: 10.1038/s41559-018-0575-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinn G (1997) Chaetognatha In: Harrison FW, Woollacott RM (eds). Microscopic anatomy of invertebrates, vol. 15 John Wiley and Sons, New York: pp 103–220. [Google Scholar]
- Slautterback DB (1967) Coated vesicles in absorptive cells of Hydra. J Cell Sci 2:563–572. [DOI] [PubMed] [Google Scholar]
- Sogabe S, Nakanishi N, Degnan BM (2016) The ontogeny of choanocyte chambers during metamorphosis in the demosponge Amphimedon queenslandica. Evodevo 7:6. doi: 10.1186/s13227-016-0042-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J-H, Hanayama R (2016) Mechanisms of Lysosomal Exocytosis by Immune Cells In: Chronic Inflammation. Springer; Japan, Tokyo, pp 369–378 [Google Scholar]
- Steinmetz PRH, Aman A, Kraus JEM, Technau U (2017) Gut-like ectodermal tissue in a sea anemone challenges germ layer homology. Nature Ecology & Evolution 1:1535–1542. doi: 10.1038/s41559-017-0285-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storch V (1991) Priapulida In: Harrison FW, Ruppert EE (eds) Microscopic anatomy of invertebrates, vol 4 John Wiley and Sons, New York, pp 333–350. [Google Scholar]
- Storch V, Higgins RP, Morse MP (1989) Internal Anatomy of Meiopriapulus fijiensis (Priapulida). Transactions of the American Microscopical Society 108:245. doi: 10.2307/3226343 [DOI] [Google Scholar]
- Storch V, Ruhberg H (1993) Onychophora In: Harrison FW, Rice EM (eds) Microscopic anatomy of invertebrates, vol 12 John Wiley and Sons, New York, pp 11–56. [Google Scholar]
- Straus W (1964) Occurrence of phagosomes and phago-lysosomes in different segments of the nephron in relation to the reabsorption, transport, digestion, and extrusion of intravenously injected horseradish-peroxidase. J Cell Biol 21:295–308. doi: 10.1083/jcb.21.3.295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- AI Tauber (2003) Metchnikoff and the phagocytosis theory. Nature Reviews Molecular Cell Biology 2011 12:4 4:897–901. doi: 10.1038/nrm1244 [DOI] [PubMed] [Google Scholar]
- Teuchert G (1977) The ultrastructure of the marine gastrotrichTurbanella cornuta Remane (Macrodasyoidea) and its functional and phylogenetical importance. Zoomorphologie 88:189–246. doi: 10.1007/BF00995474 [DOI] [Google Scholar]
- Thomas NW (1970) Morphology of the cell types from the gastric epithelium of Ciona intestinalis. J Mar Biol Ass UK 50:737–746. [Google Scholar]
- Thorndyke MC (1977) Observations on the gastric epithelium of ascidians with special reference to Styela clava. Cell Tissue Res 184:539–550. doi: 10.1007/BF00220977 [DOI] [PubMed] [Google Scholar]
- Tjelle TE, Lovdal T, Berg T (2000) Phagosome dynamics and function. Bioessays 22:255–263. doi: [DOI] [PubMed] [Google Scholar]
- Turbeville JM (1991) Nemertinea In: Harrison FW, Bogitsh (eds) Microscopic anatomy of invertebrates, vol 3 John Wiley and Sons, New York, pp 285–328. [Google Scholar]
- Van der Heyde HC (1922) On the Physiology of Digestion, Respiration and Excretion in Echinoderms. C de Boer Publ, Amsterdam, pp 119. [Google Scholar]
- Van-Praët M (1985) Nutrition of Sea Anemones In: Advances in Marine Biology Volume 22 Elsevier, pp 65–99. [Google Scholar]
- Vandermeulen JH (1970) Functional morphology of the digestive tract epithelium inPhoronis vancouverensis (Pixell): An ultrastructural and histochemical study. Journal of Morphology 130:271–285. doi: 10.1002/jmor.1051300302 [DOI] [PubMed] [Google Scholar]
- Vlisidou I, Wood W (2015) Drosophila blood cells and their role in immune responses. FEBS J 282:1368–1382. doi: 10.1111/febs.13235 [DOI] [PubMed] [Google Scholar]
- Wägele J-W, Welsch U, Müller W (1981) Fine structure and function of the digestive tract of Cyathura carinata (Crustacea, Isopoda). Zoomorphology 98:69–88. doi: 10.1007/BF00310321 [DOI] [Google Scholar]
- Wagner GP (2007) The developmental genetics of homology. Nat Rev Genet 8:473–479. doi: 10.1038/nrg2099 [DOI] [PubMed] [Google Scholar]
- Walker G (1972) The digestive system of the slug, Agriolimax reticulatus (Muller): Experiments on phagocytosis and nutrient absorption. Proc malac Soc Lond, 40:33–43. [Google Scholar]
- Weissenfels N (1976) Bau und Funktion des Süwasserschwamms Ephydatia fluviatilis L. (Porifera). Zoomorphologie 85:73–88. doi: 10.1007/BF00995405 [DOI] [Google Scholar]
- Welsch U, Dilly PN (1980) Elektronenmikroskopische Beobachtungen am Epithel des Verdauungstraktes der Hemichordaten. Ein Beitrag zur Evolution des Darmtraktes niederer Deuterostomier (Hemi- und Protochordaten) und seiner nervoesen und hormonalen Steuerung. Zool Jb (Anat) 104:25–39. [Google Scholar]
- Welsch U, Storch V (1969) Zur Feinstruktur und Histochemie des Kiemendarmes und der ,,Leber" von Branchiostoma lanceolatum (Pallas). Z Zellforsch 102:432–446. [PubMed] [Google Scholar]
- Willenz P, Van de Vyver G (1982) Endocytosis of latex beads by the exopinacoderm in the fresh water sponge Ephydatia fluviatilis: An in vitro and in situ study in SEM and TEM. Journal of Ultrastructure Research 79:294–306. doi: 10.1016/S0022-5320(82)90005-3 [DOI] [PubMed] [Google Scholar]
- Wilson JM, Whitney JA, Neutra MR (1991) Biogenesis of the apical endosome-lysosome complex during differentiation of absorptive epithelial cells in rat ileum. J Cell Sci 100(Pt 1): 133–143. [DOI] [PubMed] [Google Scholar]
- Wright KA (1991) Nematoda In: Harrison FW, Ruppert EE (eds) Microscopic anatomy of invertebrates, vol 4 John Wiley and Sons, New York, pp 111–195. [Google Scholar]
- Wurdak ES (1987) Ultrastructure and histochemistry, of the stomach of Asplanchna sieboldi. Hydrobiologia 147:361–371. doi: 10.1007/BF00025765 [DOI] [Google Scholar]
- Yonge CM (1937) Evolution and adaptation in the digestive system of the Metazoa. Biological Reviews 12:87–114 [Google Scholar]