Abstract
Although highly active antiretroviral therapy has lead to improved prognosis and alleviation of some HIV-related disease complications, it has not provided complete protection against HIV-associated dementia. As the population of persons living with HIV grows older and aged persons represent a significant number of new infections, it is important to understand how HIV may affect the aged brain. In the current study, both adult and aged mice were treated with HIV gp120 and trained in a reference memory version of the water maze. Analysis of probe data revealed that aged animals treated with gp120 demonstrated profound decrements in water maze performance compared to gp120 treated young animals and saline treated aged or young animals. Additionally, we examined the neuroinflammatory responses in the aged and adult brain 4 h after treatment with gp120. Pro-inflammatory cytokines associated with neuroinflammation are known to be antagonistic to learning and memory processes and aged and adult animals treated with gp120 demonstrated similar increases in IL-1 β and IL-6 in the hippocampus and cortex. Additionally, gp120 treatment was associated with an increase in MHCII gene expression, a marker of microglial activation, in the hippocampus. Although, the aged brain demonstrated a similar inflammatory profile at the time point measured, aged animals were more sensitive to cognitive dysfunction related to gp120 treatment. This finding supports the theory that aging may be a significant risk factor in the development of HIV-associated dementia.
Keywords: HIV-1, HIV-associated dementia, gp120, aging, neuroinflammation, cognition
INTRODUCTION
Following the development of highly active antiretroviral therapy (HAART), persons living with human immunodeficiency virus type 1 (HIV) have had a greatly improved prognosis and life expectancy. However, while HAART has provided a treatment that has greatly benefitted patients, it has failed to completely afford protection against development of HIV-associated dementia (HAD)(Stauch et al., 2017). HAD is the most severe condition of the spectrum of cognitive impairment comprised in HIV-associated neurocognitive disorders (HAND) and is characterized by cognitive and motor dysfunctions that may result in profound changes in concentration, memory impairments, and discoordination (Harezlak et al., 2011; Kaul et al., 2005; Price and Brew, 1988).
While a number of factors such as nutrition, overall health, or history of drug abuse may influence the development of HAD, aging has emerged as a prominent predictive risk factor. The incidence of HAD has increased during recent years (Dore et al., 1999) and this increase may be representative of the shifting age demographics of HIV-infected persons, which are associated with improved life expectancy. Surprisingly, a quarter of the persons infected with HIV are now over age 50. Additionally, this age group accounts for 15% of new HIV infections (Simone and Appelbaum, 2008) and they may be at an increased risk for development of HAD compared to younger adults infected with HIV (Wallace et al., 2017).
A growing body of evidence suggests that the aged brain may be “primed” to react in a manner that could hasten the progression of HAD and/or magnify its symptomology (for review see Sparkman and Johnson, 2008). Therefore, these changes in reactivity with the aged brain may be a critical factor propagating the development of HAD. Early in infection, the HIV virus is able to enter the brain. As current HAART drugs do not readily cross the blood brain barrier, the brain may act as a protected reservoir in which the virus persists and replicates (Ellis et al., 2002). Although the virus has not been shown to effectively infect neurons, it can readily invade microglia and astrocytes (Flynn et al., 2003; Vallat et al., 1998). In turn, infected glia can actively shed neurotoxic viral components including the envelope glycoprotein 120 (gp120). Gp120 is capable of binding to chemokine receptors on glial cells and neurons, increasing reactive oxygen species and interleukin-1 beta (IL-1 β) eliciting neuronal cell death (Viviani et al., 2001). Furthermore, gp120 may further exacerbate related neuroexcitotoxicity by acting on NMDA receptors leading to an influx of calcium ions into affected cells, leading to neuronal hyperexcitability (Sztukowski et al., 2018). Therefore the actions of gp120 may be a contributing factor to the progression and development of HAD. For example, mice that constitutively produce gp120 show neuronal injury and a reduction in long term potentiation (LTP) in the CA1 region of the hippocampus, disrupting neurophysiological function in a brain region central to learning and memory (Krucker et al., 1998). Additional studies have demonstrated that gp120 treatment in young adult animals disrupted hippocampal LTP (Sanchez-Alavez et al., 2000) and adversely affected hippocampal-dependent cognitive tests (Glowa et al., 1992; Pugh et al., 2000; Sanchez-Alavez et al., 2000).
In response to trauma, stress, or infection, pro-inflammatory cytokines genes are upregulated in the brain and have a potent impact on behavior and cognition (Gibertini et al., 1995; Pugh et al., 2001; Yirmiya et al., 2002; Shaw et al., 2001; Sparkman et al., 2006; Sparkman et al., 2005). The hippocampus may be particularly vulnerable as it contains a relatively high number of cytokine receptors (Konsman et al., 2002). Furthermore, as the brain of aged animals may be sensitized to the induction of cytokines, stimuli that upregulate pro-inflammatory gene expression in the hippocampus are associated with increased cognitive and behavioral alterations in aged animals compared to adults (Abraham and Johnson, 2009; Chen et al., 2008; Godbout et al., 2005). Importantly, Abraham and colleagues reported that intracerebroventricular (icv) infusion of gp120 increased brain inflammatory cytokines in an age-related manner and led to greater behavioral alterations in aged animals compared to adult animals treated with gp120(Abraham et al., 2008). Thus, gp120-induced pro-inflammatory cytokines may contribute or mediate some aspects of cognitive impairment associated with HIV infection and may interact with aging to exacerbate or hasten the progression of HAD.
In order to test the hypothesis that aging may be a key factor in the development of HAD associated cognitive dysfunction, aged and adult mice were injected icv with gp120 or saline and then trained in a modified water maze task. The important findings indicate that while gp120 produced a similar neuroinflammatory profile in both the aged and adult mouse brain at the time point measured, aged animals appear to be more sensitive to cognitive deficits associated with gp120 administration.
Materials and methods
Animals
Adult (3–6 months) and aged (22–24 months) male BALB/c mice from either our in-house colony or purchased from Taconic and aged were used. Mice were housed in polypropylene cages and maintained at 23°C under a reverse phase 12-h light: 12-h dark cycle (lights off 7:00 am/ lights on 7:00 pm) with ad libitum access to water and rodent chow. All animals were handled everyday throughout the course of the experiment and habituated to light restrain techniques for icv injections. All behavior and injections were executed during the “dark” (from 8:00 am and 12:00 pm) phase under red light. At the end of the study, mice were examined postmortem for gross signs of disease (e.g., splenomeglia and tumors). Data from mice determined to be unhealthy were excluded from analysis. A total of 33 mice were tested: aged gp120-treated n=8; aged saline-treated animals n=8; young gp120-treated animals n=9; young saline-treated animals n=8). All procedures were in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals and were approved by the University of Texas at Dallas or University of Illinois Institutional Animal Care and Use Committee.
Intracerebroventricular cannulation
The intracerebroventricular cannulation was performed as previously described (Godbout et ai., 2005a). In brief, mice were deeply anesthetized using ketamine and xylazine (1 mg and 0.1 mg/10 g by body weight, intraperitoneal, respectively) and the surgical cutaneous site was shaved and sterilized. Mice were positioned in a stereotaxic instrument so that the plane formed by the frontal and parietal bones was parallel to the table top. An incision, 1 cm in length, was made on the cranium to reveal the bregma and a 26-gauge stainless-steel guide cannula was placed in the lateral cerebral ventricle using the following stereotaxic coordinates: Lateral 1.6 mm, anteroposterior 1mm to the bregma, and vertical −2mm from the dura mater. Two anchoring cranial screws were inserted adjacent to the cannula and the cannula was secured with cranioplastic cement. A dummy cannula was inserted in the guide cannula to prevent occlusion and infection.
Mice were injected subcutaneous with buprenorphine (1 mg/10 g BW) following surgery and 12 h afterwards. Mice were provided a minimum of 7 days to recover before any gp120 treatment was administered. For a portion of the animals, accurate placement of the cannulas was confirmed after euthanasia by icv injection of trypan blue dye and gross physical examination of dye diffusion throughout the ventricles. Using trypan blue in a subset of animals, we were able to determine that i.c.v. delivery into the cerebrospinal fluid allowed the drug to be dispersed throughout the lateral ventricle and associated brain regions (e.g. hippocampus, thalamus, cerebral cortex). For animals that brain tissues were collected from, cannula placement was verified by inserting an injection cannula into the guide cannula and verifying gravity flow of sterile saline 48 h prior to the start of the experiment.
Animal experimentation
HIV-1 SF162 (M-tropic) gp120 (Cat. No. 7363; NIH Aids Research & Reference Reagent Program) was dissolved in sterile saline immediately prior to an experiment. Under light restraint, mice were infused icv with 2μl of vehicle or gp120 (100 ng) over a 30s period using a 28-gauge injection cannula, Hamilton syringe and syringe pump (World Precision Instruments). Four hours after the third daily icv infusion of gp120, the first session of water maze training was conducted. This time point corresponded with the onset of sickness behavior in a previous study (Abraham et al., 2008). Acquisition training and injections continued for another 4 days. Animals received a final injection 4 hours prior to the probe trials. Immediately following the second probe trial, mice were killed by C02 asphyxiation and tissues were collected.
Water maze
A reference memory version of the Morris water maze was utilized to evaluate hippocampus-dependent learning and memory (Morris, 1984). A circular tank 100 cm in diameter and 30 cm deep filled with water (24 °C) to a depth of 25 cm was used. A transparent round platform 10 cm in diameter was constructed from clear plastic and positioned approximately 0.5 cm below the surface of the water in the center of one quadrant. Numerous visual stimuli were located outside of the tank and served as spatial cues for animals to judge their relative position in the maze. A video camera mounted to the ceiling directly above the center of the maze was used in conjunction with the EthoVision tracking system (Noldus Information Technology, Wageningen, Netherlands). Prior to the start of the first trial of each session mice were placed on the platform for 30 s. To begin each trial, a mouse was pseudorandomly placed in the water in one of three preset locations 2 cm from the edge of the tank facing the wall. Mice were allowed to swim freely for a maximum of 60 s or until the platform was located. After the mouse reached the platform it was required to remain there for 30 s. If the platform was not located during the 60 s, mice were guided to the platform and allowed to remain there for 30 s. Performance parameters that were determined included swim speed, latency to the platform, and distance swam. After completion of three consecutive trials, mice were returned to their home cage and kept under a heat lamp for 10 min. Mice were trained utilizing this paradigm for 5 consecutive days. Twenty four hours after the completion of the final trial on day 5, animals were again placed in the maze with the platform absent for 30s. After 30 s had elapsed, the platform was reinserted into the maze at its original position and the mouse was allowed to climb on top of it and remain for 60 s. Mice then received a second 30 s probe and animals were killed immediately after the trial had elapsed for tissue collection.
Tissue collection
Immediately after the final probe trial (4 h post icv injection), a subset of mice from each treatment group was killed by C02 inhalation and decapitation. The brain was quickly dissected and placed in RNA later and stored at −80 °C until measuring of proinflammatory cytokines (IL-1 β, IL-6 and TNFα), a marker of microglial activation (MHCII), neurotrophin (BDNF), and protein kinase (CAMKII, MAPK) mRNA by quantitative reverse transcriptase- polymerase chain reaction (qRT-PCR).
Cytokine mRNA measurement by quantitative real-time PCR
RNA isolation
Because of tissue size and RNA amount, total hypothalamic RNA was isolated using the Arcturus PicoPure™ RNA isolation kit as described by the manufacturer. DNAse treatment was performed on a PicoPure column with a Qiagen RNAse-free DNAse set (Qiagen, Valnecia, CA). RNA from the hippocampus, cortex, striatum, and cerebellum was isolated using Tri Reagent protocol (Sigma, St. Louis, MO.) A Quanti Tect Reverse Transcription Kit (Qiagen, Valencia, CA) was used for cDNA synthesis from both RNA isolation methods with integrated removal of genomic DNA contamination according to the manufacturer’s protocol and previously described (Krzyszton et al., 2008).
Quantitative PCR
Quantitative real-time PCR was performed using the Applied Biosystems (Foster, CA) Assay-on Demand Gene Expression protocol as previously described (Krzyszton et al., 2008). In brief, cDNA was amplified by PCR where a target cDNA (IL-1 β, IL-6, TNF-α,; MHC class II (antigen E), CamK2a, BDNF, NR2B, MAPK) and a reference cDNA (glucose-3 phosphate dehydrogenase) were amplified simultaneously using an oligonucleotide probe with a 5’ fluorescent reporter dye (6-FAM) and a 3’ quencher dye (NFQ). PCR reactions were performed in triplicate under the following conditions: 50° C for 2 min, 95° C for 10 min, followed by 40 cycles of 95° C for 15 sec and 60° C for 1 min. Fluorescence was determined on an ABI PRISM 7900HT-sequence detection system (Perkin Elmer, Forest City, CA). Data were analyzed using the comparative threshold cycle (Ct) method and results are expressed as fold difference compared with saline controls.
Statistical Analysis
Session means for acquisition data from the water maze (i.e., latency, distance and speed) were subjected to a three-way (Age × Treatment × Session) repeated measures ANOVA in which post-injection test session was the within-subjects factor (i.e., repeated measure) and Age (adult or aged) and Treatment (saline or gp120) were between-subjects factors. All other data were subjected to two-way ANOVAs to determine the main effects of Age and Treatment, and the Age × Treatment interaction. Post hoc analysis consisted of Fisher’s protected least square difference to determine significant differences between groups. Additionally, percent time in the target quadrant for the probe trials were analyzed utilizing one-group T tests in order to determine if groups’ performance significantly differed from chance (25%).
Results
Effects of Aging and Gp120 on water maze performance
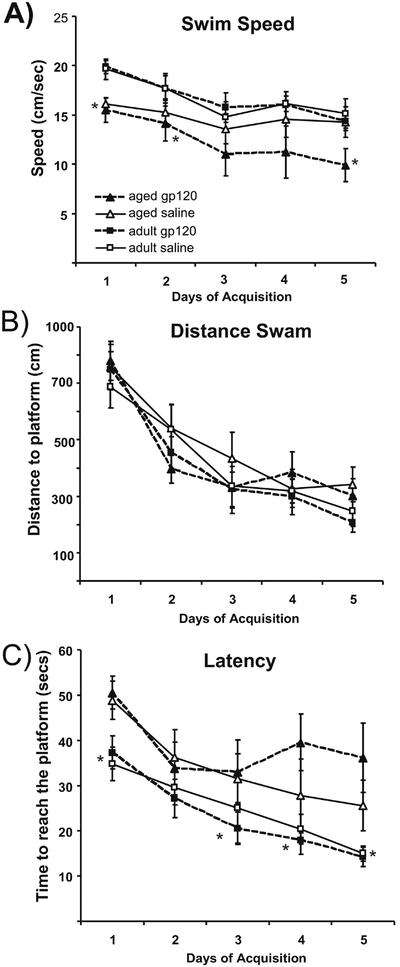
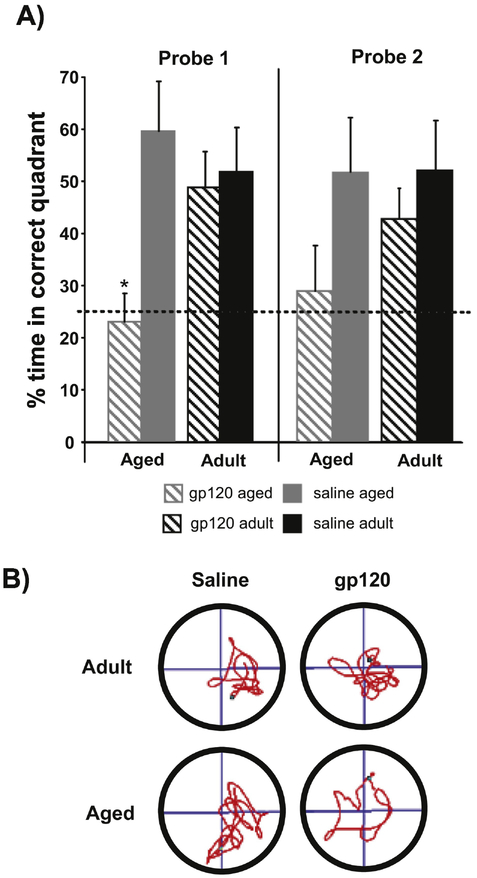
To determine if gp120 affects hippocampal-dependent learning and memory, adult and aged mice treated with saline or gp120 were trained for 5 days in a modified Morris water maze. Aged and adult animals received icv infusions of gp120 or saline 4 h prior to the start of each day of training and on the two days that immediately preceded the acquisition period. During the acquisition phase, there were no significant effects of Age or Treatment for distance swam to locate the platform, thus aged and adult animals showed no difference in their ability to efficiently locate the platform. However, there was a significant main effect of Age for swim speed (F(1,32)=7.142, p<0.05) and latency to reach the platform (F(1,32)=14.813, p<0.001) in which aged animals were slower and took longer to find the platform (Figure 1). Conservatively, these differences in swim speed may be most readily attributed to the associated changes in physical capacities that come with age rather than indicative of cognitive impairment. Twenty-four hours after the final session of acquisition, animals were subjected to two probe trials in order to test the strength of the memory for the platform location. Typically, percent time in the target quadrant is utilized to ascertain the strength of the association formed by the animal for the platform’s quadrant. During the first probe trial which measured long term retention of the platform position, there was a significant Age × Treatment interaction (F(1,32)=4.816, p<0.05) and a main effect of Treatment (F(1,32)=6.745, p<0.05) in which aged animals treated with gp120 demonstrated a significantly reduced preference for the target quadrant compared to aged or adult animals treated with saline or young animals treated with gp120 (there were no significant differences between the other groups(Figure 2). During the second probe trial, following a rest period on the platform, analysis revealed a trend for Treatment approaching significance (p=0.067) in which animals treated with gp120 showed less of a preference for the correct quadrant than saline treated animals. Interestingly, aged animals treated with gp120 did not differ from chance at either time point in their preference for the correct quadrant. All other groups’ preferences for the target quadrant were significantly greater than chance during both trials (Figure 2). These data suggests that gp120 had a greater impact upon the aged animals’ memory for the platform location compared to adult animals.
Figure 1:
Daily averages for A) Swim Speed, B) Distance swam to locate the platform and C) Latency to reach the platform during the 5 days of Acquisition of both young and aged animals (n = 8–9;*p<0.05). Bars represent group’s session means ± standard errors.
Figure 2:
A) Percent time spent in the correct quadrant during probe trials 24h following the last session of training and immediately following 60s spent on the platform. Aged animals treated with gp120 spent significantly less time in the target quadrant compared to the rest of the groups (n = 8–9; *p<0.05). The dashed line represents the level of performance predicted by chance. Bars represent group means ± standard errors. B) Representative path tracings for mice during the initial 24h probe trial. The platform had been located in the bottom right quadrant for all groups.
Central mRNA expression
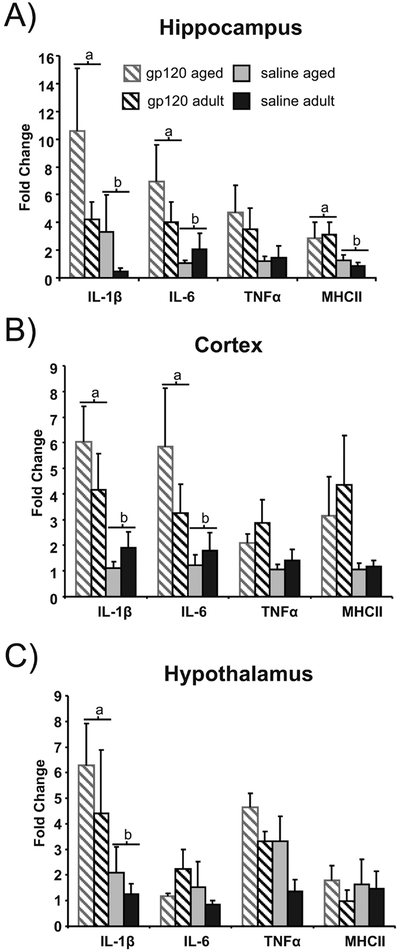
Analysis revealed that gp120 significantly increased IL-1 β mRNA levels in the cortex, hippocampus and hypothalamus of aged and adult mice (n=16) compared to saline treatment (n=14) (p<0.05). IL-6 mRNA expression was significantly greater in the cortex and hippocampus of gp120 treated animals compared to saline treated controls (p<0.05). While not significant, TNF-α mRNA levels tended to be higher in animals treated with gp120 in all three brain regions examined (cortex, p=.09; hippocampus, p=.07; hypothalamus, p=.08). Additionally, compared to saline treated animals gp120 treatment up regulated MHC-II gene expression, a marker of microglial activation, in the hippocampus (p<0.05) and tended to do so in the cortex (p=0.07). There were no significant age effects or significant age by treatment interactions. Expression of mRNA for genes associated with the cellular underpinnings of learning and memory processes (BDNF, CAMKII or MAPK) were also measured in hippocampus and cortex; however, there were no observable alterations due to gp120 treatment or aging (data not shown).
DISCUSSION
The development of HAD in aged individuals often correlates with length of infection when compared to younger individuals, but it is prudent to disentangle the contributing variables of length of infection and age in order to better understand the progression of HAD. As the population of aged individuals acquiring new HIV infections is growing and is expected to grow in the future, it is important to consider how HIV infection may differentially affect the aged brain without regards to the duration of the infection. Additionally, transgenic rodent models for studying HAD cannot currently control for developmental or cumulative effects associated with length of exposure when examining the effects of aging. The current study examines HIV- related alterations of learning and memory in aged mice and effectively controls for length of exposure within younger cohorts.
In order to evaluate how aging may influence the development of HAD, the current study utilized both aged and adult animals that received either saline or gp120 icv injections and assessed performance in the water maze. Although aged animals swam slower than adult animals during acquisition, there were no evident effects on spatial learning and memory as both aged and adult animals were equally efficient in the ability to locate the platform regardless of treatment (Figure 1). It should be noted that during the acquisition phase, animals were allowed 30s on the platform immediately prior to each swim and therefore may have been more dependent on short term memory than during the initial probe trial. A probe trial was conducted 24h after the final trial of the acquisition phase in order to determine the strength of the animals’ long term memory for the platform location. It is assumed that an increased preference for the target quadrant is indicative of animals’ ability to effectively encode spatial information during the acquisition trials and retain it (Morris, 1984). During the initial probe of long-term memory for the platform, aged animals that had been treated with gp120 during the acquisition period showed no preference for the platform location. Notably, aged gp120-treated animals preference did not differ significantly from chance. All other groups displayed a significant preference for the platform quadrant (Figure 2a & b). Therefore, gp120 may significantly interact with aging so that learning and formation of longer-term memories (i.e. consolidation) are more negatively impacted in aged animals compared to young animals that received the same exposure. Interestingly, there were no differences between groups during the training phases which may point to differences in the underlying neurophysiololgy of the required memory systems. During the initial training period, all trials were preceded by a 30s exposure to the platform, allowing the animals to reacquire the platform location and trial performance was dependent on short-term (immediate) recall. However, during the initial probe trial, animals had to rely upon memory of the platform location from 24 hours prior. Longer-term memories require both the development of LTP and require protein synthesis for effective memory consolidation. Both of these processes have been shown to be impaired by neuroinflammatory stimuli including gp120. Although, all animals did show evidence of learning during the training period, the probe trial revealed that the strength of conditioning (i.e. amount of time spent searching in the platform quadrant; as indication of memory strength or learning) was significantly reduced in gp120-treated aged animals compared to the other groups.
During aging, the brain shifts from a balance of pro-inflammatory and antiinflammatory cytokines towards a pro-inflammatory state and may predispose aged animals to increased behavioral alterations and cognitive deficits following immune activation (Abraham et al., 2008; Chen et al., 2008; Streit, 2004). However, at 4 h post gp120 treatment, there was not a significant interaction of age and gp120 treatment for mRNA expression levels of pro-inflammatory cytokines IL-1 β, IL-6 or TNF-α in any of the brain regions examined (Figure 3). This observation was unexpected due to a previous report from this lab which found increased pro-inflammatory cytokine levels 24h post icv injection of gp120 in aged animals compared to young (Abraham et al., 2008).
Figure 3:
Proinflammatory cytokine and MHCII gene expression in the A) hippocampus, B) cortex and C) hypothalamus in aged and adult mice (n=7–9) following gp120 treatment. Bars represent mean fold change ± standard error of the mean. Bars with different letters are significantly different (n= 7–9; p<0.05). Bars that share subscripts do not differ.
Interestingly, even though no measurable differences in cytokines were observed 4 h after gp120 administration, aged animals demonstrated a clear deficit in their conditioning to the target quadrant compared to aged or adult animals treated with saline or gp120 treated adult animals (Figure 2a). While gp120 led to a similar induction of cytokine mRNA in both aged groups that was similar at the time point measured, it is possible that the dynamics of cytokine upregulation and its resolution may be altered in the age animals.
For example, Abraham et. al., (2008) utilized a similar dosing regimen and observed that 24 h post gp120 treatment adult animals had pro-inflammatory cytokine levels that were comparable to controls, while gp120 treated aged animals still had increased cytokine levels compared to controls. Together with the results of the current study, it seems gp120 may elicit a similar cytokine response in the brains of aged and adult animals in the shorter term; however, the aged brain may react in a manner wherein the upregulation of pro-inflammatory genes is protracted. This may be relevant for the results of the current study in that the prolonged cytokine response may interfere with memory consolidation(and possibly memory acquisition, given that gp120 was given prior to training), a dynamic process by which shorter term memories undergo processing that transfers them to longer term memories. Memory consolidation takes place in the hours and days following the learning experience and is vulnerable to various degrees of disruption throughout the process. Pro-inflammatory cytokines, particularly IL-1 β, have been shown to be potent disrupters of memory consolidation (Barrientos et al., 2002; Pugh et al., 2000; Yirmiya et al., 2002). Therefore, it is possible that the prolonged upregulation of inflammatory genes in the aged brain may interfere with processes associated with learning and memory (e.g. long-term potentiation and memory consolidation), whereas the contracted time course of inflammatory mediators in the adult brain may not lead to the same level of interference. Moreover, several studies have shown that when pro-inflammatory cytokines, such as, IL-1 β or IL-6 are blocked after other stimuli such as LPS, mTBI, and poly l:C, in aging, that the aged population displays more robust ameliorated behavioral effects compared to adults (Abraham and Johnson, 2009; Burton et. al., 2013, Sparkman et. al., 2006; Cho et.,al. 2016).
While we measured mRNA for genes associated with the fundamental processes associated with learning and memory, we did not observe any changes in BDNF, CAMKII or MAPK (data not shown). However, as learning and memory are controlled by a number of different genes it is possible that gp120 may disrupt a different subset of genes that modulate encoding or memory formation. Alternatively, it is possible that gp120 may elicit a neurotoxic response or influence apoptotic mechanisms that may alter neuronal morphology or synaptic density that may interact with aged related declines in cognitive function. T-cell infiltration has been recently studied in the context of HAD and might play an important role on the altered aged phenotype (Hong and Banks, 2015). In addition, peripheral chronic inflammation, DNA damage and demethylation are mechanisms facilitated in aging and lead to neuronal dysfunction and cell death (Coppede and Migliori, 2010). Regardless of the mechanism, it is evident that aged animals treated with gp120 suffered more severe decrements in hippocampal-dependent learning and memory compared to adult animals that received similar treatment.
Generally, aging is associated with diminished cognitive reserve and may reflect alterations associated with decreased brain volume and decreases in neuronal number (for review see Stern, 2009). In order to ensure that aged animals could learn the task and ensure that differences were detectable among the experimental groups, we moderated the difficulty of the task by utilizing an immediate recall paradigm (massed trials) during training. The results showed that all animals could successfully navigate to the platform during shorter-term memory trials. Retention of the maze cues was evident by shorter distances across the days of training; however, when required to retain the specific platform location for 24 hours, the impact of gp 120-treatment was evident by decreased preference (i.e. conditioning) to the correct quadrant. Assuming that aged mice in the current study had diminished reserves, the gp120-related insult may have had a greater impact on the aged brain, and processes related to long-term memory formation, regardless of the lack of differential expression of proinflammatory cytokines in aged and adult mice at the 4h time point. Furthermore, when IL-1 β was administered to both aged and adult mice, aged animals suffered more serious sickness behavior and took longer to recover, indicating that the aged brain may indeed be more susceptible to the neurobehavioral properties of cytokines (Abraham and Johnson, 2009) and may represent a change in the duration of cytokine production rather than absolute magnitude. It may be that shorter-term perturbations in the CNS do not readily affect immediate short-term memory processes but may impair long-term memory formation and retention (i.e. impair LTP and protein synthesis).
The deficits in cognition are similar to those previously reported by D’Hooge et al (1999). In that study, mice that constitutively overproduce gp120 show neuronal injury, including loss of dendritic spines, as well as reductions in LTP in the CA1 region of the hippocampus (Krucker et al., 1998). These animals also demonstrate an age-related impairment in Morris water maze conditioning (D’Hooge et al., 1999). While, this study provides some evidence for gp120’s role in the etiology of HAD, the model does have some limitations in its application for HIV-related aging research. Foremost, as the animals express gp120 throughout the lifespan, it is impossible to delineate how this may have impacted developmental trajectories. Secondly, the study could not control for length of exposure between young and old transgenic animals. In contrast, length of exposure to gp120 in the current study was equivalent between adult and aged animals. The results of the current study indicate that the resultant neurophysiological changes associated with aging may be an important mediating factor for the development of HAD and associated decrements in learning and memory, regardless of the duration of the infection.
Future studies are needed to better elucidate the impact of gp120 on the aged brain, as current studies are yet to elucidate neuroimmune molecular mechanisms contributing to the development of HAD (Hong and Banks, 2015). While it was necessary that tissues be collected at a more proximal time point for the current study, it may be beneficial to examine the interaction of gp120 and aging at various time points after administration in order to observe the relative changes that may take place during the course of exposure. As it appears that cognitive reserve may play an appreciable role in the development of HAD, it would prove interesting and informative to examine variables that are thought to manipulate this reserve, such as environmental enrichment and social isolation.
Highlights.
HIV-Associated Dementia is becoming more prominent as individuals’ living with and contracting HIV increase in age.
Aged mice are more susceptible to HIV gp120-induced cognitive perturbations.
IL-1 β productions represents a link between aging and cognitive effects in HIV-gp120 treated animals.
Acknowledgments
Supported by NIH grants K22NS096030 (MDB) and The University of Texas STARS program research support grant (MDB).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Abraham J, et al. , 2008. Aging sensitizes mice to behavioral deficits induced by central HIV-1 gp120. Neurobiol Aging. 29, 614–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abraham J, Johnson RW, 2009. Central inhibition of interleukin-1 beta ameliorates sickness behavior in aged mice. Brain Behav Immun. 23, 396–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrientos RM, et al. , 2002. Memory for context is impaired by a post context exposure injection of interleukin-1 beta into dorsal hippocampus. Behav Brain Res. 134, 291–8. [DOI] [PubMed] [Google Scholar]
- Chen J, et al. , 2008. Neuroinflammation and disruption in working memory in aged mice after acute stimulation of the peripheral innate immune system. Brain Behav Immun. 22, 301–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppedè F, & Migliore L (2010). DNA repair in premature aging disorders and neurodegeneration. Current aging science, 3(1), 3–19. [DOI] [PubMed] [Google Scholar]
- Cho YE, Latour LL,Kim H, Turtzo LC, Olivera A, Livingston WS, Wang D, Martin C, Cashion A, Gill J, (2016) Older Age Results in Differential Gene Expression after Mild Traumatic Brain Injury and Is Linked to imaging Differences at Acute Follow-up. Front Aging Neurosci. 2016 July 13;8:168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Hooge R, et al. , 1999. Age-related behavioural deficits in transgenic mice expressing the HIV-1 coat protein gp120. Eur J Neurosci. 11, 4398–402. [DOI] [PubMed] [Google Scholar]
- Dore GJ, et al. , 1999. Changes to AIDS dementia complex in the era of highly active antiretroviral therapy. AIDS. 13, 1249–53. [DOI] [PubMed] [Google Scholar]
- Ellis RJ, et al. , 2002. Progression to neuropsychological impairment in human immunodeficiency virus infection predicted by elevated cerebrospinal fluid levels of human immunodeficiency virus RNA. Arch Neurol. 59, 923–8. [DOI] [PubMed] [Google Scholar]
- Flynn G, et al. , 2003. Regulation of chemokine receptor expression in human microglia and astrocytes. J Neuroimmunol. 136, 84–93. [DOI] [PubMed] [Google Scholar]
- Gibertini M, et al. , 1995. Legionella pneumophila-induced visual learning impairment reversed by anti-interleukin-1 beta. Proc Soc Exp Biol Med. 210,7–11. [DOI] [PubMed] [Google Scholar]
- Glowa JR, et al. , 1992. Learning impairment following intracerebral administration of the HIV envelope protein gp120 or a VIP antagonist. Brain Res. 570, 49–53. [DOI] [PubMed] [Google Scholar]
- Godbout JP, et al. , 2005. Exaggerated neuroinflammation and sickness behavior in aged mice following activation of the peripheral innate immune system. Faseb J. 19, 1329–31. [DOI] [PubMed] [Google Scholar]
- Harezlak Jarek, et al. “Persistence of hiv- associated cognitive impairment, inflammation and neuronal injury in era of highly active antiretroviral treatment.” AIDS (London, England) 25.5 (2011): 625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S, & Banks WA (2015). Role of the immune system in HIV-associated neuroinflammation and neurocognitive implications. Brain, behavior, and immunity, 45, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaul M, et al. , 2005. HIV-1 infection and AIDS: consequences for the central nervous system. Cell Death Differ. 12 Suppl 1, 878–92. [DOI] [PubMed] [Google Scholar]
- Konsman JP, et al. , 2002. Cytokine-induced sickness behaviour: mechanisms and implications. Trends Neurosci. 25, 154–9. [DOI] [PubMed] [Google Scholar]
- Krucker T, et al. , 1998. Transgenic mice with cerebral expression of human immunodeficiency virus type-1 coat protein gp120 show divergent changes in short- and long-term potentiation in CA1 hippocampus. Neuroscience. 83, 691–700. [DOI] [PubMed] [Google Scholar]
- Morris R, 1984. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods. 11, 47–60. [DOI] [PubMed] [Google Scholar]
- Price RW, Brew BJ, 1988. The AIDS dementia complex. J Infect Dis. 158, 1079–83. [DOI] [PubMed] [Google Scholar]
- Pugh CR, et al. , 2000. Human immunodeficiency virus-1 coat protein gp120 impairs contextual fear conditioning: a potential role in AIDS related learning and memory impairments. Brain Res. 861, 8–15. [DOI] [PubMed] [Google Scholar]
- Pugh RC, et al. , 2001. The immune system and memory consolidation: a role for the cytokine IL-1 beta. Neurosci Biobehav Rev. 25, 29–41. [DOI] [PubMed] [Google Scholar]
- Rottman JB, 1997. Cellular localization of the chemokine receptor CCR5. Correlation to cellular targets of HIV-1 infection. Am. J. Pathol 151, 1341–1351. [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Alavez M, et al. , 2000. HIV- and FIV-derived gp120 alter spatial memory, LTP, and sleep in rats. Neurobiol Dis. 7, 384–94. [DOI] [PubMed] [Google Scholar]
- Shaw KN, et al. , 2001. Lipopolysaccharide causes deficits in spatial learning in the watermaze but not in BDNF expression in the rat dentate gyrus. Behav Brain Res. 124, 47–54. [DOI] [PubMed] [Google Scholar]
- Simone MJ, Appelbaum J, 2008. HIV in older adults. Geriatrics. 63, 6–12. [PubMed] [Google Scholar]
- Sparkman NL, et al. , 2006. Interleukin-6 facilitates lipopolysaccharide-induced disruption in working memory and expression of other proinflammatory cytokines in hippocampal neuronal cell layers. J Neurosci. 26, 10709–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparkman NL, Johnson RW, 2008. Neuroinflammation associated with aging sensitizes the brain to the effects of infection or stress. Neuroimmunomodulation. 15, 323–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparkman NL, et al. , 2005. Bacterial endotoxin-induced behavioral alterations in two variations of the Morris water maze. Physiol Behav. 86, 244–251. [DOI] [PubMed] [Google Scholar]
- Stauch KL, Emanuel K, Lamberty BG, Morsey B, & Fox HS (2017). Central nervous system-penetrating antiretrovirals impair energetic reserve in striatal nerve terminals. Journal of neurovirology, 23(6), 795–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern Y, 2009. Cognitive reserve. Neuropsychologia. 47, 2015–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streit WJ, 2004. Microglia and Alzheimer’s disease pathogenesis. J Neurosci Res. 77,1–8. [DOI] [PubMed] [Google Scholar]
- Sztukowski K, Nip K, Ostwald PN, Sathler MF, Sun JL, Shou J, … & Hofmann F (2018). HIV induces synaptic hyperexcitation via cGMP-dependent protein kinase II activation in the FIV infection model. PLoS biology, 16(7), e2005315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallat AV, et al. , 1998. Localization of HIV-1 co-receptors CCR5 and CXCR4 in the brain of children with AIDS. Am J Pathol. 152, 167–78. [PMC free article] [PubMed] [Google Scholar]
- Vigorito M, LaShomb AL, & Chang SL (2007). Spatial learning and memory in HIV-1 transgenic rats. Journal of Neuroimmune Pharmacology, 2(4), 319–328. [DOI] [PubMed] [Google Scholar]
- Viviani B, Corsini E, Binaglia M, Galli CL, & Marinovich M (2001). Reactive oxygen species generated by glia are responsible for neuron death induced by human immunodeficiency virus-glycoprotein 120 in vitro. Neuroscience, 107(1), 51–58. [DOI] [PubMed] [Google Scholar]
- Wallace LM, Ferrara M, Brothers TD, Garlassi S, Kirkland SA, Theou O, … & Guaraldi G (2017). Lower frailty is associated with successful cognitive aging among older adults with HIV. AIDS research and human retroviruses, 33(2), 157–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yirmiya R, et al. , 2002. Brain interleukin-1 is involved in spatial memory and passive avoidance conditioning. Neurobiol Learn Mem. 78, 379–89. [DOI] [PubMed] [Google Scholar]