Abstract
OBJECTIVES
The mitochondrial DNA (mtDNA) point mutation m.3243A>G is known to express the following two syndromes among others: maternally inherited diabetes and deafness (MIDD) and mitochondrial encephalopathy, lactic acidosis, and stroke-like episodes (MELAS). Sensorineural hearing loss (SNHL) is the most frequent symptom in individuals harboring the m.3243A>G mutation. However, dysfunction of the vestibular organs has been scarcely examined. Therefore, the present study aimed to study the impact of the m.3243A>G mutation on the inner ear.
MATERIALS and METHODS
A total of 8 subjects harboring the blood-verified m.3243A>G mutation underwent thorough audiological and vestibular examinations, including tone and speech audiometry, video head impulse test (vHIT), ocular and cervical vestibular-evoked myogenic potential (oVEMP and cVEMP), and full otoneurological examination. The subjects also answered a Dizziness Handicap Inventory (DHI) questionnaire.
RESULTS
SNHL was identified in all the 8 subjects, with a mean pure-tone average-4 (PTA-4) of 59 dB. Speech discrimination score (n=7) ranged from 24% to 100% (mean 74%), and vHIT (n=42) detected pathology in nine lateral semicircular canals (SCCs), five posterior SCCs, and one anterior SCC, whereas three measurements were inconclusive. All oVEMPs (n=14 ears) were absent, nine cVEMPs were absent, and two were inconclusive. Based on the DHI scores, 6 subjects reported none to mild dizziness, 1 reported moderate, and 1 reported severe dizziness.
CONCLUSION
Our study population had pathological findings from every audiological and vestibular end organs. The results indicated that the pathological findings originated from within the end organs themselves and not within the superior and inferior vestibular or cochlear nerve.
Keywords: MELAS syndrome, mitochondrial diseases, sensorineural hearing loss, vestibular disease, inner ear disease
INTRODUCTION
Mitochondria play a critical role in energy production in the eukaryotic cells. Each eukaryotic cell in the human body comprises a variable number of mitochondria, usually between 100 and 1000, and each single mitochondrion comprises a variable number of mitochondrial DNA (mtDNA) molecules, ranging between 2 and 10 copies. mtDNA is a circular double-stranded molecule stretching 16.569 base pairs. It encodes for 22 transfer RNA (tRNA), 2 ribosomal RNA (rRNA), and 13 peptides, accounting for a total of 37 genes.
It is important to understand the basis of mitochondrial genetics and how it differs from traditional Mendelian genetics and nuclear DNA. The key differences are: maternal inheritance, heteroplasmy, and mitotic segregation. Maternal inheritance: only the mitochondria from the oocyte will pass on to the zygote; therefore, the mitochondrial diseases derived from mutations in mtDNA are solely inherited from the mother. Heteroplasmy: the presence of mitochondria with different genetic constitutions within a cell or organism is called heteroplasmy. Individuals inherit hundreds of mitochondria from their mother, and all carriers of mitochondrial mutations have mutations as part of heteroplasmy. The level of heteroplasmy can vary within mitochondria, cells, and tissues. Mitotic segregation: during cell mitosis, the division of mutant mtDNA and normal mtDNA does not have to be destribuated equally between daughter cells, making it possible to have a change in heteroplasmy degree within daughter cells during each generation of cells.
The m.3243A>G (A3243G) mutation is a point mutation at base pair number 3243 in mtDNA, resulting in an adenosine to guanine switch in the tRNA-leu (UUR) encoded by the MT-TL1 gene [1, 2].
Epidemiological studies in European populations have reported a prevalence of the m.3243A>G mutation between 2:100,000 [3] and 16:100,000 [4]. A more recent study conducted in an Australian population has reported a prevalence of 240:100,000 [5].
The phenotypic expression of the m.3243A>G mutation can be extremely variable, ranging from asymptomatic to highly debilitating syndromes. A large mitochondrial disease patient cohort in the United Kingdom (n=129 patients) demonstrated that 30% of the patients had maternally inherited diabetes and deafness (MIDD) syndrome, 10% had mitochondrial encephalopathy, lactic acidosis, and stroke-like episodes (MELAS) syndrome [6], 6% had MELAS/MIDD, 28% had atypical syndromes, and 13% had other syndromes or overlaps associated with the m.3243A>G mutation [7]. In 1990, two independent groups described the m.3243A>G mutation as a cause of MELAS syndrome, and in 1994, the MIDD syndrome was linked to the same mutation [8]. A clear correlation between the degree of heteroplasmy in muscles and the severity of clinical symptoms has been delineated [9].
Some symptoms are more frequent and often present earlier than others [10]. The most frequent symptom experienced is hearing loss (HL), especially progressive, bilateral, symmetric sensorineural HL (SNHL) [11–13]. Dysfunction of the vestibular organs, including the three pairs of semicircular canals (SCCs) and the paired otolithic organs (the utricles and saccules), has been described in the literature more scarcely [14,15,16].
With this research, we aimed to study the impact of the m.3243A>G mutation on the inner ear by performing a full audiological and vestibular examination with state-of-the-art equipment.
MATERIALS AND METHODS
Candidate Recruitment
All subjects were former or current patients at Clinical Genetics Department at Aalborg University Hospital, Aalborg, Denmark. Potential subjects were identified with either the MELAS, MIDD, or m.3243A>G mutation carrier in the department’s diagnosis database.
Inclusion Criteria
Subjects must have received genetic counseling by a clinical geneticist
Subjects must have verified the m.3243A>G mutation
Subjects must be legally competent
Subjects must provide written informed consent
A total of 8 subjects fulfilled all the inclusion criteria.
Genetic Testing
An allele-specific PCR amplification with DNA isolated from venous blood was performed by a tertiary Molecular Genetics Department to evaluate the m.3243A>G mutation.
Audiological Testing
Subjects underwent standard pure-tone audiometry as well as standard speech audiometry. A trained audiologist performed all the tests. Air conduction and bone conduction pure-tone audiometry were conducted at 125, 250, 500, 1000, 2000, 4000, and 8000 Hz. The pure-tone average-4 (PTA-4) was calculated as the average threshold at four specific frequencies (0.5, 1, 2, and 4 kHz). Bone conductivity was measured to evaluate inner ear hearing thresholds, calculate a potential air–bone gap, and thereby quantify a conductive HL. Speech audiometry comprised two tests: speech discrimination score (DS or word recognition) and speech reception threshold. When measuring DS, the subjects had to recall multiple single words phonetically. At approximately 40 dB above each subject’s PTA, the words were presented and the score was calculated as a percentage of words repeated correctly. The score was calculated as the hearing threshold at which the subjects could recite 50% of the presented numbers. HL was graded according to the American Speech–Language–Hearing Association guidelines (www.asha.org 2016). Ipsilateral and contralateral acoustic reflexes were measured in both ears at 500, 1000, and 2000 Hz. Acoustic reflexes were expected to be stimulated approximately 75 dB above the hearing threshold, and in the case of recruitment, the reflex would be triggered at a relatively lower threshold. Measurements where two or more reflexes were triggered were further examined for events such as recruitment or were considered inconclusive otherwise.
Vestibular Testing
Dizziness Handicap Inventory (DHI)
Each subject was asked to fill out a DHI questionnaire [17] comprising 25 items divided into three subcategories: seven physical, nine emotional, and nine functional questions. Each question has three possible answers: No (0 points), Sometimes (2 points), or Yes (4 points). A total score within each category was calculated (0–28 in physical and 0–36 in both emotional and functional), and it ranged from 0–100 points. The total DHI scores were divided as following: mild, 0–30 points; moderate, 31–60 points; and severe, 61–100 points.
Ocular Vestibular-Evoked Myogenic Potential (oVEMP)
oVEMP was used to evaluate and stimulate the utricles and superior vestibular nerves [18] and was recorded on the contralateral inferior oblique muscle (IF). It uses air conducted (AC) sound stimuli at 100 dB to alter the muscular electric potential. Examination was performed with the Eclipse application (Interacoustics, Middelfart, Denmark). A single electrode was placed on subjects’ forehead, two additional electrodes were caudally placed to each inferior eyelid (palpebral inferior), and a fourth electrode was placed at subjects’ chin. Subjects received sound stimuli on the side being tested. Sound was presented at a frequency of 500 Hz and an intensity of 100 dB with a burst rate of 5/second. Subjects were instructed to maintain an upward gaze to ensure proper stretching of the IF and to position the muscle closer to the electrode. oVEMPs were bilaterally evaluated by the following parameters: amplitude, latency, and asymmetry.
Cervical Vestibular-Evoked Myogenic Potential (cVEMP)
The cVEMP was used to evaluate and stimulate the saccules and inferior vestibular nerves [19] and was recorded on the ipsilateral sternocleidomastoid muscle (SCM). cVEMP uses AC sound stimuli to alter muscular electric potentials in the ipsilateral SCM. Electrodes were placed on the subjects’ forehead, chin, and on both SCMs using the Eclipse application (Interacoustics, Middelfart, Denmark). During testing, subjects received audiological stimulation in both ears one at a time. Testing was performed at a frequency of 500 Hz and an intensity of 100 dB. While being tested, subjects were instructed to turn their head 90° in the horizontal plane, forcing contraction of the ipsilateral SCM. cVEMPs were evaluated based on the same parameters used to evaluate oVEMPs.
Video Head Impulse Test (vHIT)
Testing was performed to evaluate the vestibulo-ocular reflex (VOR) using the EyeSeeCam (Interacoustics, Middelfart, Denmark). The EyeSeeCam comprises an inertial measurement unit to measure movements of the head and an infrared camera to record eye movements. vHIT evaluates the VOR by testing all the three pairs of SCCs, calculates a mean gain value (ratio between head and eye velocity) for each separate SCC being tested, and also detects both overt and covert saccades. Subjects wore the EyeSeeCam goggle (Interacoustics, Middelfart, Denmark) tight to ensure that no slippage occurred during head movements. Subjects were seated approximately 1.5 meters (4 feet, 11 inches) from a wall bearing a mark at the subjects’ eye level. Before the test was initiated, the EyeSeeCam was calibrated according to the manufacturer’s guidelines. Subjects were informed that the physician would perform a series of head movements in either the transverse or in sagittal–coronal plane depending on which pair of SCCs was being tested. When testing the function of the paired lateral SCCs, small rapid head impulses in the transverse plane were performed in a random unpredictable manner for a total of minimum 10 head impulses. For testing the anterior and posterior SCCs, similar head impulses in the sagittal–coronal plane at a 45°-angle were performed. The results were categorized as either normal, inconclusive, or pathological. Normal gain values were expected to range between 0.80 and 1.20 for the lateral SCCs and between 0.70 and 1.20 for the vertical SCCs [20, 21]. The results had to include both pathological saccades and gain values lower than the cut-off values.
Statistical Analysis
Two-sided Wilcoxon signed rank test was performed using the Excel 2013 software (Microsoft Inc., Redmond, WA, USA) and Table A7 of Kirkwood et al. [22].
RESULTS
Subjects
A total of 8 subjects fulfilled the inclusion criteria and were included in the study. The subjects comprised 6 females and 2 males, with a mean age of 49.6 years (Tables 1–3). One female subject did not receive vestibular examinations because her condition resulted in poor compliance.
Table 1.
Results of audiometry and video head impulse test in 8 subjects
| Subject | Sex | Age (years) | Video head impulse test (vHIT) | Vestibular-evoked myogenic potential | Audiometry | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||||||||||||||
| Anterior semicircular canal | Lateral semicircular canal | Posterior semicircular canal | |||||||||||||||||||||||
|
|
|
|
|
|
|||||||||||||||||||||
| Gain | Saccade | Gain | Saccade | Gain | Saccade | Overt | Covert | Shape | Symmetry | Type | PTA-4 (dB) | DS (%) | |||||||||||||
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||||
| Right | Left | Right | Left | Right | Left | Right | Left | Right | Left | Right | Left | Right | Left | Right | Left | Right | Left | Right | Left | ||||||
| 1 | Male | 53 | 0.99 | 0.77 | O | O | 0.54 | 0.54 | O | O | 0.48 | 0.77 | M | M | A | A | A | A | Descending | Yes | Bilateral SNHL | 60 | 53 | 48 | 68 |
|
| |||||||||||||||||||||||||
| 2 | Female | 71 | 0.77 | 0.64 | N | N | 0.68 | 0.74 | M | N | 0.39 | 0.51 | M | M | A | A | I | I | Descending | No | Bilateral SNHL | 59 | 53 | 88 | 88 |
|
| |||||||||||||||||||||||||
| 3 | Female | 46 | 1.42 | 1.03 | N | N | 0.77 | 0.77 | O | O | 1.09 | 0.95 | O | O | A | A | A | A | Descending | No | Bilateral SNHL | 40 | 54 | 88 | 76 |
|
| |||||||||||||||||||||||||
| 4 | Male | 41 | 0.86 | 1.07 | N | N | 0.65 | 0.83 | O | M | 0.65 | 0.83 | N | N | A | A | N | A | Flat | Yes | Bilateral SNHL | 54 | 59 | 100 | 80 |
|
| |||||||||||||||||||||||||
| 5 | Female | 64 | 1.32 | 0.09 | N | N | 0.13 | 0.07 | O | O | 0.38 | 1.17 | N | N | A | A | A | A | Descending | Yes | Bilateral SNHL | 53 | 51 | 90 | 55 |
|
| |||||||||||||||||||||||||
| 6 | Female | 41 | na | na | na | na | na | na | na | na | na | na | na | na | na | na | na | na | Ascending | Yes | na | 73 | 81 | na | na |
|
| |||||||||||||||||||||||||
| 7 | Female | 35 | 1.19 | 0.88 | N | N | 1.04 | 1.01 | O | N | 1.27 | 1.24 | N | N | A | A | N | N | Flat | Yes | Bilateral SNHL | 60 | 66 | 100 | 100 |
|
| |||||||||||||||||||||||||
| 8 | Female | 56 | 1.08 | 0.79 | N | N | 0.56 | 0.81 | N | N | 0.68 | 1.33 | N | N | A | A | A | A | Descending | Yes | Bilateral SNHL | 61 | 60 | 24 | 32 |
A: Absent; I: Inconclusive; M: Mixed; na: Not assessed; N: Normal; O: Overt; Bold: Pathological measure; cursive: inconclusive measure; SNHL, Sensorineural hearing loss; PTA-4: Pure-tone average-4; DS: Speech discrimination score.
Table 2.
Acoustic reflexes in 5 subjects
| Subject | Acoustic reflexes | ||
|---|---|---|---|
| Side | Ipsilateral | Contralateral | |
| 1 | Right | Absent | Recruitment |
| Left | Recruitment | Recruitment | |
| 2 | Right | Absent | Absent |
| Left | Absent | Absent | |
| 5 | Right | Recruitment | Recruitment |
| Left | Recruitment | Recruitment | |
| 7 | Right | Absent | Absent |
| Left | Absent | Absent | |
| 8 | Right | Absent | Absent |
| Left | Absent | Recruitment | |
Table 3.
Dizziness Handicap Inventory scores in 8 subjects
| Subject | Dizziness Handicap Inventory (DHI) | |||
|---|---|---|---|---|
| Physical | Emotional | Functional | Total | |
| 1 | 6 | 14 | 10 | 30 |
| 2 | 6 | 18 | 10 | 34 |
| 3 | 8 | 4 | 4 | 16 |
| 4 | 0 | 0 | 0 | 0 |
| 5 | 4 | 2 | 2 | 8 |
| 6 | 6 | 6 | 10 | 22 |
| 7 | 2 | 2 | 2 | 6 |
| 8 | 20 | 22 | 22 | 64 |
The maximum score is 28 in physical and 36 in both emotional and functional; the maximum total score is 100.
Audiological Findings
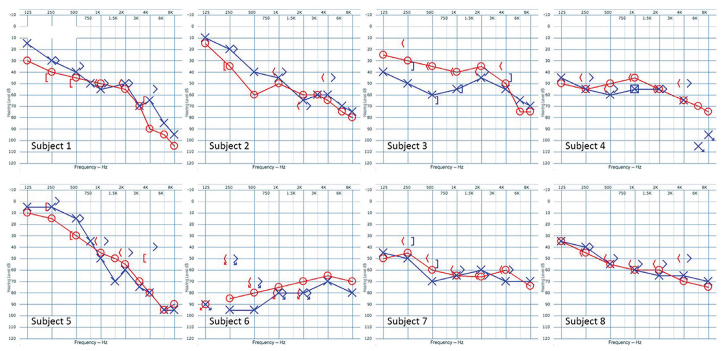
All the 8 subjects reported bilateral SNHL of variable degree. None of the subjects examined were found to have a significant air–bone gap; therefore, conductive HLs were ruled out. The PTA-4 of each individual ear was calculated as the average threshold in dB at four different frequencies (500, 1.000, 2.000, and 4.000 Hz). A total of 16 ears were tested with a mean PTA-4 of 58.6 dB. There was no significant difference between left and right sided ears (two-sided Wilcoxon signed rank test p>0.1). DS was evaluated in 7 subjects because 1 subject could not comply to the examination, giving a total of 14 ears examined with a mean DS score of 74%. Two-sided Wilcoxon signed rank test revealed no significant difference between the left and right side (p>0.1). The majority of the audiograms had a descending shape (n=5) with greater threshold at the higher frequencies than the lower frequencies, while the remaining 2 had a flat shape, and the last had a slightly ascending shape with a lower threshold at the higher frequencies than the higher frequencies. Six subjects showed symmetric HL between the ears, where two subjects showed asymmetric HL (more than 10 dB side difference on at least two neighboring frequencies). All the audiograms are presented in Figure 1. Five subjects were examined for acoustic reflexes; both their left and right sides were tested for ipsilateral and contralateral reflexes, accounting for a total of 20 reflexes. Two subjects had total absence of all reflexes, whereas another two subjects had a mixture of absent reflexes, one had three reflexes from one ear and recruitment on the other ear, the other had three and one, respectfully. One subject showed recruitment for all reflexes. All acoustic reflexes are compiled in Table 2.
Figure 1.
Audiograms of subjects affected by MELAS syndrome.
Vestibular Findings
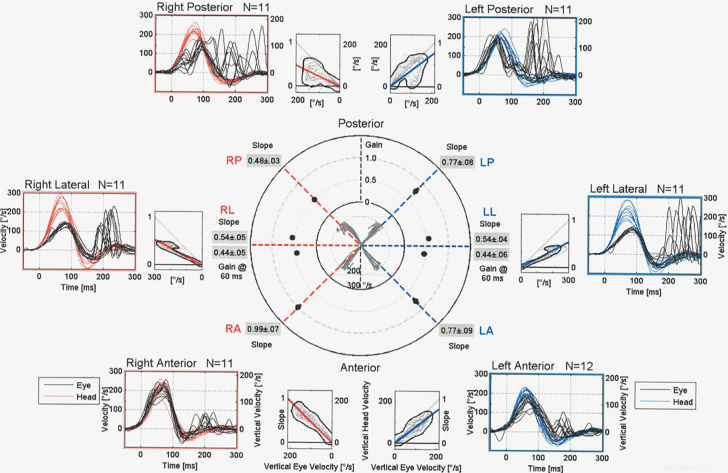
A total of 7 subjects underwent vestibular examinations, which comprised vHIT of all six SCCs, oVEMP, and cVEMP. A total of 42 SCCs were examined, divided equally into 14 lateral, 14 posterior, and 14 anterior canals. Among the 42 canals examined, 15 were evaluated as having a pathological mean gain value, and among these 15 canals, 13 also had either overt, covert, or mixed saccades. Three gain values were inconclusive. Of the 14 lateral SCCs examined (seven pairs), 9 were found as having a pathological gain value and all having saccades. Four subjects (8 SCCs) had bilateral pathological gain values, whereas one had a unilateral pathological gain value. Among the posterior canals (n=14), 5 were found to have a pathological gain value, 2 subjects had a bilateral pathology, whereas 1 had unilateral pathology. None of the gain values for the posterior SCCs were evaluated as inconclusive. The anterior SCCs (n=14) were the least affected with only 1 pathological gain value. One subject had bilateral inconclusive gain values without saccades.
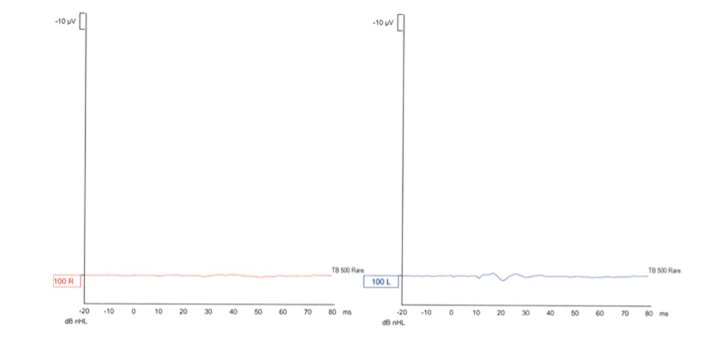
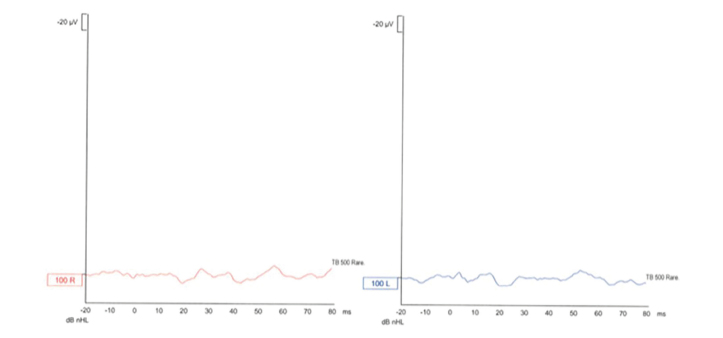
The oVEMP was used to evaluate the function of the utricles and superior vestibular nerves. All the 7 subjects showed an absence of measured potentials bilaterally. The cVEMP was used to evaluate the function of the saccule and inferior vestibular nerve. Four subjects showed bilateral absence of evoked potentials, one had unilateral absence of potential, and one had bilaterally measured potentials. The last subject’s measurements were evaluated as inconclusive because the measurements seemed similar to expected potentials values but were no greater than the background noise in amplitude.
All subjects (n=8) completed the DHI questionnaire. Among the 8 subjects, 6 reported none to mild self-perceived dizziness, 1 reported moderate, and 1 reported severe self-perceived dizziness (Table 3).
The vHIT, oVEMP, and cVEMP values of subject 1 are shown in Figures 2–4, respectively.
Figure 2.
Video head impulse test (vHIT) of subject 1.
Figure 3.
Ocular vestibular-evoked myogenic potential (oVEMP) of subject 1.
Figure 4.
Cervical vestibular-evoked myogenic potential (cVEMP) of subject 1.
DISCUSSION
Mitochondrial point mutations, such as m.3243A>G, are known for their heterogeneous phenotypical expression. It is well known that individuals harboring this mutation often have impairments of the inner ear, especially HL. With this study, we intended to examine the function of the entire inner ear. All our subjects had reported bilateral HL. No difference between bone conduction and air conduction hearing thresholds were observed, indicating that all subjects suffer from SNHL ranging from mild to severe according to the ASHA (PTA; 40–81 dB) with different shaped audiograms. These findings correspond with those reported in the literature [11–23]. In the present study, DS was found to have a mean of 74%, ranging from 24% to 100%. Two subjects had a bilaterally low DS, whereas one subject had a unilaterally low DS, and the remaining subjects had a relatively high DS (ranging between 76% and 100%). A high DS was suggestive of cochlear HL (HL originated from the cochlea), whereas a low DS was suggestive of retro-cochlear HL. A previous study has showed that 5 of the 8 subjects with MELAS syndrome due to the m.3243A>G mutation suffered from HL [13]. Four subjects showed findings indicative of cochlear HL, whereas one subject was suspected of having retro-cochlear HL. These findings are similar to those of our study, given that SNHL combined with a high DS indicated dysfunction of the cochlea, rather than sound conduction or neural pathways, as the reason of the HL, whereas SNHL combined with a low DS indicated a retro-cochlear HL. Our DS findings with the acoustic reflex findings can further help to determine the precise site of the hearing dysfunction. In our study, 3 subjects had one or less measured reflexes and were therefore regarded as inconclusive. Two subjects were found to have three and four measured reflexes, respectively. These reflexes were found to be of the recruitment phenomenon. Taken together, these findings indicate that their HL was of a cochlear origin and not of a retro-cochlear origin.
All 14 oVEMP recordings showed bilateral absence of potentials. These findings indicate that there is a dysfunction of either the utricles and/or superior vestibular nerve [18, 24]. Further investigations of the function of the utricles are warranted, especially considering that research has found that individuals exhibit a decrease in oVEMP amplitude when they pass the age of 50 years [25].
Seven subjects underwent AC-cVEMP testing. Four of the seven subjects showed bilateral absence of potentials, one showed unilateral absence of potential with a normal measurement on the other side, one showed inconclusive cVEMP, and the last subject had a bilaterally normal cVEMP. Absence of cVEMP indicates dysfunction of the saccule and/or inferior vestibular nerve [19]. Our findings are similar to those reported in an earlier study on subjects harboring the m.3243A>G mutation [14] that examined 13 subjects using AC-cVEMP testing. To evaluate if the site of dysfunction is to be found inside the labyrinth or retro-labyrinth, our subjects would have to undergo an additional test, namely galvanic-cVEMP, which uses electric/galvanic stimuli and a similar method as AC-cVEMP [26, 27].
Our vHIT findings showed a big mixture of normal, pathological, and inconclusive values. The lateral SCCs seemed to be the most affected, followed by the posterior SCCs, with the anterior SCCs only having one affected SCC. Five subjects (two in one study and three in another study) with the m.3243A>G mutation have previously been examined [15, 16]. These subjects underwent vHIT examinations, and four had bilateral pathological values of the horizontal and posterior canals, whereas the anterior canals had values within the normal range. Only one of our subjects had bilateral pathology of both the horizontal and posterior SCCs. The horizontal and posterior SCCs of our subjects expressed higher pathological values than those expressed by the anterior SCCs.
The anterior and lateral SCCs and utricles transmit through the superior vestibular nerve, whereas the posterior semicircular canals and saccules transmit through the inferior vestibular nerve. Moreover, as the cochlea transmits through the cochlear nerve, nerve dysfunction must be considered and identified to help determine the site of pathology in individuals with the m.3243A>G mutation. In our study population, the number of pathological values was greater for the lateral SCCs than for the anterior SCC (60% and 7%, respectively; Table 1), indicating different degrees of pathology in the end organs innervating the same nerve branch. This discrepancy between the anterior and lateral SCCs with our other findings further indicates that the dysfunction itself lies within the end organs (the SCCs, otolith organs, and cochlea) and not within the vestibulocochlear nerve that they innervate.
CONCLUSION
We detected pathological findings in all the examinations performed and demonstrated that subjects harboring the m.3243A>G mutation suffer from dysfunction of both the vestibular and audiological systems despite the limited number of participants in this study. We have shown that vestibular pathologies are common among individuals with this mutation.
Acknowledgements
The authors thank the patients and their families for participating in this study.
Footnotes
This study was presented at the XIV Annual Meeting in the Danish Society of Otorhinolaryngology, Head & Neck Surgery, April 20–21, 2017, Nyborg, Denmark.
Ethics Committee Approval: Ethics committee approval was received for this study from the Ethics Committee on Health Research Ethics of North Jutland, Denmark (project N-20160068).
Informed Consent: Written informed consent was obtained from all subjects who participated in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept – M.B.P.; Design - D.D.H., A.T.H., M.B.P.; Supervision – D.D.H., A.T.H., M.G., M.B.P.; Materials – D.D.H., M.B.P.; Data Collection and/or Processing – D.D.H., D.H.H.; Analysis and/or Interpretation – D.D.H., D.H.H., A.T.H., M.G.; Literature Search – D.H.H., M.B.P.; Writing Manuscript –D.H.H., M.B.P.; Critical Review – D.D.H., A.T.H., M.G.
Conflict of Interest: The authors have no conflict of interest to declare.
Financial Disclosure: The Research and Knowledge Center was supported by a grant from the Obelske Familiefond. D.H.H. received financial support from Aalborg University to visit the MRC Mitochondrial Biology Unit, University of Cambridge, Cambridge, United Kingdom.
REFERENCES
- 1.Goto Y, Nonaki I, Horai S. A mutation in the tRNA(Leu)(UUR) gene associated with the MELAS subgroup of mitochondrial encephalomyopathies. Nature. 1990;348:651–3. doi: 10.1038/348651a0. [DOI] [PubMed] [Google Scholar]
- 2.Kobayashi Y, Momoi MY, Tominaga K, Momoi T, Nihei K, Yanagisawa M, et al. A point mutation in the mitochondrial tRNA(Leu)(UUR) gene in MELAS (mitochondrial myopathy, encephalopathy, lactic acidosis and stroke-like episodes) Biochem Biophys Res Commun. 1990;173:816–22. doi: 10.1016/S0006-291X(05)80860-5. [DOI] [PubMed] [Google Scholar]
- 3.Chinnery PF, Johnson MA, Wardell TM, Singh-Kler R, Hayes C, Brown DT, et al. The epidemiology of pathogenic mitochondrial DNA mutations. Ann Neurol. 2000;48:188–93. [PubMed] [Google Scholar]
- 4.Majamaa K, Moilanen JS, Uimonen S, Remes AM, Salmela PI, Kärppä M, et al. Epidemiology of A3243G, the mutation for Mitochondrial Encephalomyopathy, Lactic Acidosis, and Strokelike Episodes: Prevalence of the mutation in an adult population. Am J Hum Genet. 1998;63:447–54. doi: 10.1086/301959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Manwaring N, Jones MM, Wang JJ, Rochtchina E, Howard C, Mitchell P, et al. Population prevalence of the MELAS A3243G mutation. Mitochondrion. 2007;7:230–3. doi: 10.1016/j.mito.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 6.Pavlakis SG, Phillips PC, DiMauro S, De Vivo DC, Rowland LP. Mitochondrial myopathy, encephalopathy, lactic acidosis, and strokelike episodes: a distinctive clinical syndrome. Ann Neurol. 1984;16:481–8. doi: 10.1002/ana.410160409. [DOI] [PubMed] [Google Scholar]
- 7.Nesbitt V, Pitceathly RDS, Turnbull DM, Taylor RW, Sweeney MG, Mudanohwo EE, et al. The UK MRC Mitochondrial Disease Patient Cohort Study: clinical phenotypes associated with the m.3243A>G nutation-implications for diagnosis and management. J Neurol Neurosurg Psychiatry. 2013;84:936–8. doi: 10.1136/jnnp-2012-303528. [DOI] [PubMed] [Google Scholar]
- 8.van den Ouweland JMW, Lemkes HHPJ, Trembath RC, Ross R, Velho G, Cohen D, et al. Maternally inherited diabetes and deafness is a distinct sub-type of diabetes and associates with a single point mutation in the mitochondrial tRNALeu(UUR) gene. Diabetes. 1994;43:746–51. doi: 10.2337/diab.43.6.746. [DOI] [PubMed] [Google Scholar]
- 9.Chinnery PF, Howell N, Lightowlers RN, Turnbull DM. Molecular pathology of MELAS and MERRF. The relationship between mutation load and clinical phenotypes. Brain. 1997;120:1713–21. doi: 10.1093/brain/120.10.1713. [DOI] [PubMed] [Google Scholar]
- 10.Dvorakova V, Kolarova H, Magner M, Tesarova M, Hansikova H, Zeman J, et al. The phenotypic spectrum of fifty Czech m.3243A>G carriers. Mol Genet Metab. 2016;118:288–95. doi: 10.1016/j.ymgme.2016.06.003. [DOI] [PubMed] [Google Scholar]
- 11.Yamasoba T, Oka Y, Tsukuda K, Nakamura M, Kaga K. Auditory findings in patients with maternally inherited diabetes and deafness harboring a point mutation in the mitochondrial transfer RNALeu (UUR) gene. Laryngoscope. 1996;106:49–53. doi: 10.1097/00005537-199601000-00010. [DOI] [PubMed] [Google Scholar]
- 12.Vivero RJ, Ouyang X, Kim YG, Liu W, Du L, Yan D, et al. Audiologic and genetic features of the A3243G mtDNA mutation. Gen Test Mol Biomar. 2013;17:383–9. doi: 10.1089/gtmb.2012.0403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vandana VP, Bindu PS, Sonam K, Govindaraj P, Taly AB, Gayathri N, et al. Audiological manifestations in mitochondrial encephalomyopathy lactic acidosis and stroke like episodes (MELAS) syndrome. Clin Neurol Neurosurg. 2016;148:17–21. doi: 10.1016/j.clineuro.2016.04.024. [DOI] [PubMed] [Google Scholar]
- 14.Iwasaki S, Egami N, Fujimoto C, Chihara Y, Ushio M, Kashio A, et al. The mitochondrial A3243G mutation involves the peripheral vestibule as well as the cochlea. The Laryngoscope. 2011;121:1821–4. doi: 10.1002/lary.21879. [DOI] [PubMed] [Google Scholar]
- 15.Cardenas-Robledo S, Tehrani AS, Blume G, Kattah JC. Visual, ocular motor, and cochleo-vestibular loss in patients with heteroplasmic, Maternally-Inherited Diabetes Mellitus and Deafness (MIDD), 3243 transfer RNA mutation. J Neuro-Ophthalmol. 2016;36:134–40. doi: 10.1097/WNO.0000000000000340. [DOI] [PubMed] [Google Scholar]
- 16.Kim SH, Akbarkhodjaeva ZA, Jung I, Kim JS. Eye movement and vestibular dysfunction in mitochondrial A3243G mutation. Neurol Sci. 2016;37:1159–62. doi: 10.1007/s10072-016-2577-y. [DOI] [PubMed] [Google Scholar]
- 17.Jacobson GP, Newman CW. The development of the Dizziness Handicap Inventory. Arch Otolaryngol Head Neck Surg. 1990;116:424–7. doi: 10.1001/archotol.1990.01870040046011. [DOI] [PubMed] [Google Scholar]
- 18.Kantner C, Gürkov R. Characteristics and clinical applications of ocular vestibular evoked myogenic potentials. Hear Res. 2012;294:55–63. doi: 10.1016/j.heares.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 19.Welgampola MS, Colebatch JG. Characteristics and clinical applications of vestibular evoked myogenic potentials. Neurology. 2005;64:1682–8. doi: 10.1212/01.WNL.0000161876.20552.AA. [DOI] [PubMed] [Google Scholar]
- 20.Blödow A, Pannasch S, Walther LE. Detection of isolated covert saccades with the video head impulse test in peripheral vestibular disorders. Auris Nasus Larynx. 2013;40:348–51. doi: 10.1016/j.anl.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 21.Mossman B, Mossman S, Purdie G, Schneider E. Age dependent normal horizontal VOR gain of head impulse test as measured with video-oculography. Journal of Otolaryngology - Head and Neck Surgery. 2015;44:29. doi: 10.1186/s40463-015-0081-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kirkwood BR, Sterne JAC. Essential Medical Statistics. 2nd ed. Malden: Blackwell Science; 2003. [Google Scholar]
- 23.Hendrickx JJ, Mudde AH, ‘t Hart LM, Huygen PLM, Cremers CWRJ. Progressive sensorineural hearing impairment in maternally inherited diabetes mellitus and deafness (MIDD) Otol Neurotol. 2006;27:802–8. doi: 10.1097/01.mao.0000224091.02506.a0. [DOI] [PubMed] [Google Scholar]
- 24.Shin BS, Oh SY, Kim JS, Kim TW, Seo MW, Lee H, et al. Cervical and ocular vestibular-evoked myogenic potentials in acute vestibular neuritis. Clin Neurophysiol. 2012;123:369–75. doi: 10.1016/j.clinph.2011.05.029. [DOI] [PubMed] [Google Scholar]
- 25.Nguyen KD, Welgampola MS, Carey JP. Test-Retest reliability and age-related characteristics of the ocular and cervical vestibular evoked myogenic potential tests. Otol Neurotol. 2010;31:793–802. doi: 10.1097/MAO.0b013e3181e3d60e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Watson SR, Fagan P, Colebatch JG. Galvanic stimulation evokes short-latency EMG responses in sternocleidomastoid which are abolished by selective vestibular nerve section. Electroencephalogr Clin Neurophysiol. 1998;109:471–4. doi: 10.1016/S0924-980X(98)00033-2. [DOI] [PubMed] [Google Scholar]
- 27.Murofushi T, Takegoshi H, Ohki M, Ozeki H. Galvanic-evoked myogenic responses in patients with an absence of click-evoked vestibule-collic reflexes. Clin Neurophysiol. 2002;113:305–9. doi: 10.1016/S1388-2457(01)00738-6. [DOI] [PubMed] [Google Scholar]