Abstract
The aim of this report is to provide international recommendations for functional ear reconstruction in patients with microtia and aural atresia. All patients with microtia and external auditory atresia should be seen in the setting of a multidisciplinary team and agreed treatment outcomes should be measured, so that techniques, approaches, and results can be compared. The methods are expert opinion from the members of the International Microtia and Atresia Workgroup (IMAW). The consensus recommendations reported herein take into account the variability in practice patterns present among experts in the field; the degree of consensus was quantified by presenting the percentage of above authors who agree or partially agree with each statement. Recommendations include the definition and classification of microtia/atresia, treatment of microtia, treatment of congenital aural atresia, flowchart of functional ear reconstruction, and future research directions. Patients with microtia and aural atresia can be guided by the consensus recommendations provided herein.
Keywords: Microtia, aural atresia, ear reconstruction
Consensus Objectives
The objectives of this report are to provide recommendations for functional ear reconstruction in patients with microtia and aural atresia. All patients with microtia and external auditory atresia should be seen in the setting of a multidisciplinary team and agreed treatment outcomes should be measured, so that techniques, approaches, and results can be compared.
Target Population
Patients with microtia and aural atresia are the target population.
Intended Users
The consensus recommendations are targeted at the following:
1. General practitioners and plastic surgeons who commonly evaluate patients with ear malformations;
2. Otolaryngologists and subspecialty-trained otolaryngologists (pediatric otolaryngologists and otologists/neurotologists) who manage patients with microtia and aural atresia;
3. Audiologists, speech pathologists, and other hearing professionals who manage patients with hearing loss from microtia and aural atresia; and
4. Patients with microtia and aural atresia and their families.
Methods
Expert-opinion methods from the members of the International Microtia and Atresia Workgroup (IMAW) were used. The mission of the IMAW is to develop expertize-based consensus recommendations for the management of microtia and aural atresia with functional ear reconstruction. The consensus recommendations reported herein take into account the variability in practice patterns present among experts in the field; the degree of consensus was quantified by presenting the percentage of above authors who agree or partially agree with each statement.
Recommendations and Justification
The recommendations are outlined in the following sections.
Section 1. The Definition and Classification of Microtia/Atresia
Section 2. Treatment of Microtia
Section 3. Treatment of Congenital Aural Atresia
Section 4. Flowchart of Functional Ear Reconstruction
Section 5. Future Research Directions
Disclaimer
This report has been prepared by the members of IMAW. Consensus recommendations are based on the collective opinions of the members of the group. Any practitioner seeking to apply or consult the report is expected to use independent medical judgment in the context of individual patient and institutional circumstances. Any patient or patient family member seeking to apply or consult this report is expected to make individual decisions regarding management of microtia and aural atresia in consultation with his/her physician.
The definition and Classification of Microtia/Atresia
Classification of Microtia
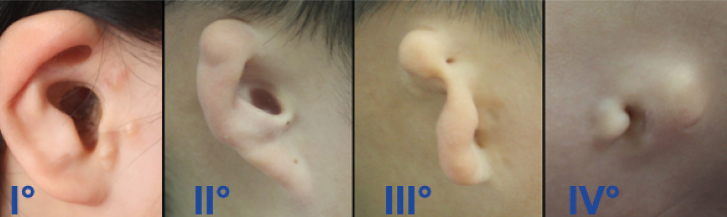
Microtia is a term used to describe a smaller and usually malformed auricle. One widely adopted classification system, originally described by Weerda and simplified by Aguilar, assigns a grade from I to III based on the severity of the deformity (Figure 1). Grade I depicts a slightly smaller than normal ear with essentially normal features. Grade II represents an auricle that is rudimentary and malformed but contains some recognizable components. Grade III includes the classic “peanut” ear, which is severely attenuated with a small lump of deformed tissue often containing some cartilage, and Grade IV includes anotia.
Figure 1.
Classification of microtia.
Another commonly cited classification system, proposed by Nagata, categorizes microtia according to the vestigial structures present. Lobule-type microtic ears have a remnant ear and lobule but lack a concha, acoustic meatus, and tragus; these usually correspond to Grade III. Concha-type microtic ears possess some degree of a lobule, concha, acoustic meatus, tragus, and incisura tragica; these usually correspond to Grade II, according to the previously described system. Small concha-type microtic ears contain the remnant ear and lobule with a small indentation for a concha.
Classification of Congenital Aural Atresia
Congenital aural atresia is a birth defect that is characterized by hypoplasia or aplasia of the external auditory canal (EAC), often in association with dysmorphic features of the auricle, middle ear, and, occasionally, inner ear structures. The classification of congenital aural atresia recommended here is based on both clinical and surgical observations [1, 2].
Type A. Stenosis
Stenosis is narrowing of the fibrocartilaginous or bony parts of the EAC, in which the tympanic membrane is present, but it is smaller than normal, with slight deformity. The ossicular chain generally develops normally but is often fixed; most cases have a mild to moderate conductive hearing loss.
Type B. Partial Atresia
Some part of the fibrocartilaginous or bony EAC is present. There is a bony atretic plate, and the tympanic membrane is missing or may be rudimentary. The tympanic membrane is often not attached to the ossicular chain, which may also be underdeveloped. The patient generally has a moderate or moderate–severe conductive hearing loss.
Type C. Total Atresia
There is complete aplasia of the external auditory canal with a bony atretic plate. Both fibrocartilaginous and bony parts of the EAC are absent. The tympanic membrane is also absent. There is some degree of underdevelopment of the middle ear and its associated structures. The patient has a moderate or moderate–severe conductive hearing loss.
Treatment of Microtia
The reconstructive options for microtia are broadly divided into: 1, no treatment; 2, a subcutaneously placed autologous costal cartilage framework; 3, implanted artificial material including a porous polyethylene implant placed either subcutaneously or under a vascularized fascial flap and skin graft; and 4, a prosthetic ear affixed to the skin with a medical grade adhesive or by osseointegrated implants. Patients should be observed by a multidisciplinary team where options for both hearing and pinna reconstruction must be considered and coordinated. This surgery should be undertaken by experienced teams as there is a steep learning curve.
Surgeons performing the autologous costal cartilage method must consider the development of costal cartilage, which occurs at least from 6 years old; ideally performing the method in 9 years old or higher would meet the requirements of framework carving. Different surgical techniques have different age requirements [3]. Puberty, usually occurring at 12–15 years, is not the suitable age for inexperienced surgeons, because the costal cartilage is prone to hollow during the rapid pubertal growth and care must be taken to maintain consistency of the cartilage framework.
In adulthood, costal cartilage becomes calcified with gradually worsening elasticity, which is not optimal for surgery. Costal cartilage B-ultrasound can be used to determine whether the costal cartilage is hollow or calcified. If the patient is a candidate for aural atresia repair and wishes to pursue a canal reconstruction surgery, the canal surgery must be combined with or follow the autologous rib cartilage microtia repair in order to prevent compromise to the often tenuous vascularity of the skin flap covering the cartilage graft reconstruction [4].
For porous polyethylene microtia repair, surgical reconstruction can be performed in as young as 3 years old, but IMAW recommends this surgery after 5 years of age. If the patient is a candidate for aural atresia repair and wishes to pursue the canal reconstruction surgery, the canal surgery must be performed before the porous polyethylene microtia repair. Canal surgery following previous polyethylene repair risks infection and/or extrusion of the polyethylene graft [5].
The quality of the tissues and post-auricular skin must be assessed as this will affect how best to approach the surgical procedure and the results. Loose skin with good laxity is optimal for autologous cartilage microtia reconstruction. The laxity of the skin and presence of previous incisions or trauma will determine if further measures are required such as temporoparietal fascial flaps with skin grafts or skin expansion may be considered to help cover the framework.
Agreed clinical and patient-reported outcomes should be routinely collected to assess the benefit to the patient and to compare techniques.
Treatment of Congenital Aural Atresia
Stenosis
Congenital aural stenosis can be divided into three subgroups: (1) stenosis combined with cholesteatoma, (2) stenosis combined with hearing loss, and (3) stenosis without cholesteatoma with normal hearing. The degree of stenosis (diameter) and curvature of the osseous EAC are risk factors for EAC cholesteatoma formation [6, 7].
In a modified meatoplasty procedure with an endaural-conchal incision, in which two local rotation flaps and a transposition split-thickness scalp flap can be used to widen the stenotic EAC and reconstruct the tympanic membrane to prevent recurrent disease, canal skin is preserved, and a split thickness skin graft can be employed to cover exposed bone. When necessary, tympanoplasty or ossicle mobilization/reconstruction is performed [8].
Partial Atresia
In patients with partial atresia, some part of the fibrocartilaginous or bony EAC is present; a bony atretic plate is also present, and the tympanic membrane is hypoplastic or absent. There may also be some degree of mild ossicular hypoplasia or even a normal ossicular chain that is fixed.
In these patients, bony canalplasty must be performed with removal of the atretic plate to liberate the ossicular chain. Once liberated, a tympanic membrane graft can be used over the freed ossicular chain or an underlay graft can be used if the patient retains a small rudimentary tympanic membrane. The tympanic membrane also can be reconstructed using cartilage to avoid lateralization, but the hearing outcomes will be affected. Split thickness skin grafting is generally required in addition to the patient’s own EAC skin to cover exposed bone. A modified meatoplasty procedure with an endaural-conchal incision to reconstruct the meatus is then performed. Patients with partial atresia still have certain EAC skin containing ceruminous glands; this skin should be preserved to prevent post-operative stenosis.
Total Atresia
Not all patients with complete aural atresia are candidates for atresia repair. The patient must have normal inner ear function (mild bone conduction hearing loss may be due to the absence of inertia of ossicular chain) [9] and well-developed middle ear anatomy to support reconstruction. The IMAW supports the use of the Jahrsdoerfer grading scale for assessment of middle ear anatomy and recommends surgery for children scoring 7 or higher [10].
Technological options for hearing habilitation include bone conduction hearing devices on a soft or hard band or adhesive gel pad in the youngest patients. After the age of five, bone conduction devices or middle ear implants can be proposed.
The IMAW very strongly recommends bone conduction technology for children with bilateral aural atresia to support speech and language development. The decision to place a bone conduction device on a child with unilateral aural atresia is less clear and should be made by the family in conjunction with the otolaryngologist/otologist, hearing specialist/audiologist, speech therapist, and school specialists. Even though the benefits have not been definitively proven, bone conduction devices have been shown to have some benefit in unilateral hearing loss patients in noisy environments, and families should be given an option to trial these devices.
The IMAW recommends a careful computed tomography (CT) scan evaluation of the temporal bones and precise evaluation of the type of hearing loss, and of preoperative motivation with the softband. A surgical team should be able to propose all devices to satisfy a personalized technological option. Currently, Vibrant Soundbridge (MED-EL, North Carolina, USA), [11, 12] Bone Bridge (MED-EL, North Carolina, USA), transcutaneous bone conduction device like Sophono Alpha 1–2 (Medtronic, Jacksonville, USA) and BAHA Attract (Cochlear, NSW, Australia), and percutaneous osseointegrated bone conduction device like BAHA Connect (Cochlear, NSW, Australia) and Ponto implant (Oticon, Copenhagen, Denmark) are viable options.
The IMAW strongly recommends that the otolaryngologist/otologist/pediatric otolaryngologist discuss placement of the osseointegrated bone conduction hearing device with the microtia surgeon to ensure that the processor does not interfere with the microtia reconstruction.
For children with hearing and anatomy that supports surgical reconstruction (normal inner ear function and Jahrsdoerfer score 7 or higher), the IMAW recommends that atresia surgery be performed in children older than 6 years with the understanding that revision surgery may need to be performed if new bone growth during puberty compromises the canal and hearing. To be safe, atresia surgery after puberty lowers the risk of new bone growth and revision surgery.
As noted above, the strategy of atresia surgery is to enhance hearing as well as improve esthetics. The IMAW strongly recommends atresia repair in combination with or AFTER autologous rib graft microtia repair and BEFORE porous polyethylene microtia repair. The IMAW also recommends families to seek atresia surgeons with experience to minimize complications including ear canal stenosis, moisture/infection, lateralization of the tympanic membrane, facial nerve injury, poor hearing outcomes, and reduce the need for revision surgery [13].
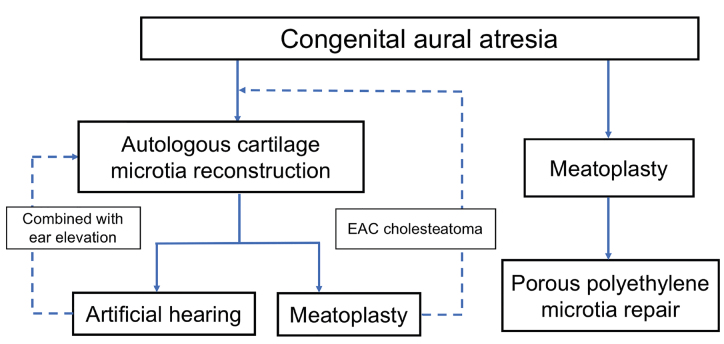
Flowchart of Functional Ear Reconstruction (Figure 2)
Figure 2.
Flowchart of functional ear reconstruction.
The IMAW strongly recommends atresia repair in combination with or AFTER autologous rib graft microtia repair and BEFORE porous polyethylene microtia repair.
In patients with microtia who also have EAC cholesteatoma and cannot be treated by non-surgical treatment, meatoplasty can be performed before autologous rib graft microtia repair.
The placement of the osseointegrated bone conduction hearing device should not interfere with the microtia reconstruction. It can be performed after autologous rib graft microtia repair or combined with ear elevation.
Future Research Directions
Future research will focus on the etiology of microtia/atresia, whether genetic or acquired, and finding possible genetic associations and even causative genetic mutations, in both syndromic and non-syndromic patients.
Tissue engineering holds great promise for auricular reconstruction; results still need long-term follow-up to verify safety and stability. This group believes tissue engineering to be the core of future research to change the approach of ear reconstruction [14].
Another area for future research is external auditory canal skin physiology and skin graft breakdown leading to mucosalization and drainage, in order to prevent stenosis and maintain the normal function of EAC in reconstructed atresia patients by developing tissue-engineered skin containing ceruminous gland.
Finally, optimizing functional hearing outcomes—atresia repair surgery, bone-conducting technology, both, or other technologies—in these patients with moderate or moderate–severe conductive hearing loss will enable children with microtia/atresia to reach their full potential.
Acknowledgements
Tian-yu Zhang (first author) was the lead author. All remaining authors are listed in alphabetical order. The authorship list follows the agreement of the members of the IMAW. All authors have contributed to the conception and design of the work, drafting and revising the consensus recommendations for important intellectual content, final approval of the version to be published, and agreement to be accountable for all aspects of the work. We would like to express our gratitude to Dr. Chenlong Li as the scientific secretary of the consensus recommendations.
Footnotes
This study was presented at the “32nd Politzer Society Meeting, May 29, 2019, Warsaw, Poland”.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept – T.Z.; Design – T.Z., K.W.C., D.J.; Supervision – T.Z.; Resource – T.Z., N.B., K.W.C., Y.S.C., H.F., D.J., B.W.K., R.S., J.M.T.; Materials – T.Z., N.B., K.W.C., Y.S.C., H.F., D.J., B.W.K., R.S., J.M.T.; Data Collection and/or Processing – T.Z.; Analysis and/or Interpretation – T.Z.; Literature Search – T.Z., B.W.K., K.W.C., D.J.; Writing - T.Z.; Critical Reviews - T.Z., N.B., K.W.C., Y.S.C., H.F., D.J., B.W.K., R.S., J.M.T.
Conflict of Interest: The authors have no conflict of interest to declare.
Financial Disclosure: The authors declared that this study has received no financial support.
REFERENCES
- 1.Li CL, Chen Y, Chen YZ, Fu YY, Zhang TY. Congenital Aural Stenosis: Clinical Features and Long-term Outcomes. Sci Rep. 2016;6:27063. doi: 10.1038/srep27063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weerda H. Chirurgie der Ohrmuschel-Verletzungen, Defekte und Anomalien. Stuttgart: Thieme; 2004. pp. 105–226.pp. 253–6. [DOI] [Google Scholar]
- 3.Wilkes GH, Wong J, Guilfoyle R. Microtia reconstruction. Plast Reconstr Surg. 2014;134:464e–479e. doi: 10.1097/PRS.0000000000000526. [DOI] [PubMed] [Google Scholar]
- 4.Siegert R. Combined reconstruction of congenital auricular atresia and severe microtia. Adv Otorhinolaryngol. 2010;68:95–107. doi: 10.1159/000314565. [DOI] [PubMed] [Google Scholar]
- 5.Tahiri Y, Reinisch J. Porous Polyethylene Ear Reconstruction. Clin Plast Surg. 2019;46:223–30. doi: 10.1016/j.cps.2018.11.006. [DOI] [PubMed] [Google Scholar]
- 6.Casale G, Nicholas BD, Kesser BW. Acquired ear canal cholesteatoma in congenital aural atresia/stenosis. Otol Neurotol. 2014;35:1474–9. doi: 10.1097/MAO.0000000000000495. [DOI] [PubMed] [Google Scholar]
- 7.Yin D, Li C, Juan H, Li J, Yang L, Zhang T, et al. Morphological Characteristics of Osseous External Auditory Canal and Its Relationship With External Auditory Canal Cholesteatoma in Patients With Congenital Aural Stenosis. Otol Neurotol. 2017;38:1528–34. doi: 10.1097/MAO.0000000000001600. [DOI] [PubMed] [Google Scholar]
- 8.Chen Y, Zhang T. Modified meatoplasty for external auditory canal stenosis with endoaural-conchal incision. Otol Neurotol. 2015;36:1–3. doi: 10.1097/MAO.0000000000000667. [DOI] [PubMed] [Google Scholar]
- 9.Zhang L, Gao N, Yin Y, Yang L, Xie Y, Chen Y, et al. Bone conduction hearing in congenital aural atresia. Eur Arch Otorhinolaryngol. 2016;273:1697–703. doi: 10.1007/s00405-015-3727-1. [DOI] [PubMed] [Google Scholar]
- 10.Shonka DC, Jr, Livingston WJ, 3rd, Kesser BW. The Jahrsdoerfer grading scale in surgery to repair congenital aural atresia. Arch Otolaryngol Head Neck Surg. 2008;134:873–7. doi: 10.1001/archotol.134.8.873. [DOI] [PubMed] [Google Scholar]
- 11.Roman S, Denoyelle F, Garabedian EN, Triglia JM. Middle ear implant in conductive and mixed congenital hearing loss in children. Int J Pediatr Otorhinolaryngol. 2012;76:1775–8. doi: 10.1016/j.ijporl.2012.08.022. [DOI] [PubMed] [Google Scholar]
- 12.Frenzel H, Sprinzl G, Widmann G, Petersen D, Wollenberg B, et al. Grading system for the selection of patients with congenital aural atresia for active middle ear implants. Neuroradiology. 2013;55:895–911. doi: 10.1007/s00234-013-1177-2. [DOI] [PubMed] [Google Scholar]
- 13.UK Care Standards for the Management of Patients with Microtia and Atresia. British Academy of Audiology, British Association of Audiovestibular Physicians, British Association of Paediatricians in Audiology, British Association of Plastic, Reconstructive and Aesthetic Surgeons, Changing Faces, ENT-UK, Microtia UK, Microtia Mingle, National Deaf Children’s Society, Paediatric Psychology Network UK; Mar, 2015. Accessible at http://microtiauk.org/docs/UK_Care_Standards_for_the_Management_of_Patients_with_Microtia_and_Atresia__March_2015.pdf. [Google Scholar]
- 14.Zhou G, Jiang H, Yin Z, Liu Y, Zhang Q, Zhang C, et al. In Vitro Regeneration of Patient-specific Ear-shaped Cartilage and Its First Clinical Application for Auricular Reconstruction. EBioMedicine. 2018;28:287–302. doi: 10.1016/j.ebiom.2018.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]