Abstract
OBJECTIVES
This study aims to compare the electrical auditory brainstem response (EABR) following cochlear implant (CI) surgery in pediatric subjects with cochlear malformation and a normal cochlea, in order to assess the sensitivity of EABR and to evaluate the surgery outcome.
MATERIALS and METHODS
A total of 26 pediatric subjects who were deaf and scheduled for CI surgery were enrolled into this case control study. Group A (n=20) included subjects with a normo-conformed cochlea. Group B (n=6) included subjects with cochlear malformation. Subjects were evaluated with EABR immediately (T0) and 6 months (T1) post-CI surgery. The EABR Waves III and V average amplitude and latency were compared across time, separately for each group, and across groups, separately for each time.
RESULTS
Auditory brainstem response (ABR) could only be recorded in Group A. We were able to record EABR from all subjects at T0 and T1, and waves III and V were present in all the recorded signals. There were no statistically significant differences between T0 and T1 in EABR Waves III and V in terms of average amplitude and latency in neither group. When comparing Groups A and B, the only statistically significant difference was the average amplitude of wave V, both at T0 and T1.
CONCLUSION
EABR is a valid tool to measure the auditory nerve integrity after CI surgery in patients with a normal and malformed cochlea, as shown by its ability to measure waves III and V when ABR is absent. The EABR testing should be performed before and after CI surgery, and EABR should be used as a measure of outcome, especially in patients with a malformed cochlea.
Keywords: Cochlear malformation, cochlear implant, EABR, ABR, cochlear nerve conduction
INTRODUCTION
The cochlea can be affected by a number of malformations, [1–4] ranging from absence of a single turn (Mondini syndrome) to complete absence of all turns (common cavity) [2, 4]. In patients with cochlear malformation, cochlear implantation is technically possible, [1–3] but different malformations require specific CI surgical techniques [2] as standard techniques (e.g., posterior tympanotomy, cochleostomy, and electrode insertion by round window) [2] are not viable. Nonetheless, in these patients, clinical outcome of CI surgery is unpredictable and variable, [5] especially in terms of hearing performance. For example, Buchman et al. [6] showed that patients with the constellation of an incompletely partitioned (IP) cochlea, enlarged vestibular aqueduct, and a dilated vestibule (i.e., Mondini’s malformation) achieved relatively good levels of speech perception, while patients with total semicircular canal aplasia, isolated IP, cochlear hypoplasia, or common cavity showed impaired levels. Thus, whether patients with a malformed cochlea should be considered candidates for CI surgery remains controversial [1–3].
An outcome of CI surgery is typically assessed using a battery of tests. The speech perception test assesses the patient’s ability to both perceive and discriminate speech information. While widely used, scores are influenced by several factors, including the intelligence quotient, ability to focus, and age [7]. These limitations highlight the need for more objective outcome measures. The auditory brainstem response (ABR) test is a tool to determine the patient’s ability to hear and measures the way the hearing nerve responds to different acoustic stimuli [8]. The electrical auditory brainstem response (EABR) testing is similar to ABR testing, but it uses an electrical stimulus delivered directly to the cochlea, which makes EABR more effective than ABR for quantifying the auditory pathways nerve conduction (from spiral ganglions to the auditory cortex) [9]. EABR can objectively measure the CI function and responsiveness of the peripheral auditory neurons/nerve up to the level of the brainstem; additionally, these signals can be recorded even when excessive stimulus artifacts preclude successful acquisition of the electrically evoked compound action potentials (ECAPs) [10]. In patients with a malformed cochlea, EABR can measure proper transmission of signals from the ear to the brain with high sensitivity and accuracy, as shown by Kim et al. [11].
This study aims to quantitatively compare EABR recorded from CI subjects with a normal cochlea and with cochlear malformation, with the goal to evaluate the EABR sensitivity and the outcome of cochlear implantation. While previous studies have shown that EABR can be used to record the signal from the ear to the brain in CI patients with cochlear malformations, how EABR recorded from these patients compare to EABR recorded from CI patients with a normal cochlea has not been investigated. Such a comparison might help understand to which extent outcome of CI surgery in patients with a malformed and normal cochlea is comparable.
MATERIALS AND METHODS
This study was conducted from January 2017 to August 2018 at the Otology and Cochlear Implant Unit of a tertiary referral center. All the study procedures followed the international ethical guidelines for biomedical research involving human subjects and were approved by the institutional regulatory board. Parents/legal guardians were informed about the study procedures, and they authorized them on their children by signing a written consent.
All pediatric subjects affected by deafness who needed to undergo CI surgery were enrolled into the study. Prior to surgery, all subjects underwent a high-resolution computed tomography examination of the temporal bone and magnetic resonance imaging (MRI) of the cerebellopontine angle (CPA-MRI) with TSE T2 DRIVE sequences or 3D Fast Field Echo (FFE) T2-weighted sequences to assess cochlear abnormalities. Then, subjects were assigned to either Group A (subjects with a normal cochlea) or Group B (subjects with cochlear malformation). The otoacoustic emissions (OTOAE) and ABR tests were performed to assess the retro-cochlear function from spiral ganglions to the superior auditory area. The ABR signals were recorded with Eclipse (Interacustics; www.interacoustics.com) in clinical, automatic modality using a decreasing single click stimulus (from 100 dB to 10 dB, decreasing in steps of 10 dB). The signal was sent separately to each ear, and contralateral masking was applied in cases of asymmetric response. The ABR threshold was set to the average of the two ears’ hearing level at the frequency 2000/4000 Hz. ABR was recorded using a Socrates system (www.hederabiomedics.com), software custom sound EP 5.1, and POD interface switched to trigger. An electrode “1-22-12-11-6” was used for stimulation, and “12-1-11-6-22” for recording. The stimulation rate was 35 hearing level (Hl), the intensity was 100 dB, and the pulse width 25.
CI surgery was performed using a posterior tympanotomy and cochleostomy approach in all subjects by the same team to minimize the inter-operator variability. The surgical approach to the cochlea was the same in all patients, except for the electrode insertion, which was standard (cochleostomy) in subjects with a normal cochlea, and it used as the “releasing method for electrode positioning in subjects with a malformed cochlea.”
EABR was recorded for all subjects immediately (T0) and 6 months (T1) post-CI surgery by the same operator and under the same experimental conditions. EABR was recorded from three CI electrodes stimulating respectively at low, middle, and high frequencies, according to the configuration available for each CI model and manufacturer. The EABR signals were processed with analogical filters (high-pass filter, cut-off frequency 100 Hz, low-pass filter, cut-off frequency 2000 Hz), which define the frequency range visualized in the graph window of Eclipse, and with digital filters, designed by Interacoustics, to smooth the curve removing myogenic, electromagnetic, and stimulus artifacts. A time window of 10 ms was used for the data acquisition synchronized with the stimulus generated by the CI, using a trigger cable connected to the CI programing interface (dub box). Once the EABR graph was obtained, the marker for Waves III and V was manually inserted, and then the software calculated the waves latency and amplitude.
Statistical Analysis
The analysis of EABR focused on Waves III and V. The EABR Wave III amplitudes and latencies at T0 and T1 were compared using a two-tailed t-test separately for each group; a similar analysis was performed on Wave V. Also, EABR Wave III amplitudes and latencies of Groups A and B were compared using a two-tailed t-test, separately for T0 and T1; a similar analysis was performed on Wave V. The level of significance p for all tests was set to 0.05.
RESULTS
Subjects
Group A included 20 subjects (13 females, 7 males; average age 25 months) (standard deviation [SD], 10.9; confidence interval [CI] 95%, 12–48). Group B included 6 subjects (4 females, 2 males; average age 31.6 months old) (SD, 13.7; CI 95%, 20–40).
As shown by the MRI and CT, the cochlear nerve was intact in all subjects. Subjects in Group A had a normal cochlea. Subjects in Group B had cochlear malformation with significant alterations of different severity in the inner ear, but a normal mastoid cavity and middle ear bones (see Figures 1 and 2 for examples of a CT and MRI scan of a malformed cochlea). Specifically, in Group B, 4 subjects presented with a Type II incomplete cochlea partition (Mondini deformity) associated with a direct communication between the internal auditory canal (IAC) and cochlea basal turn; and 2 subjects presented with a Type II incomplete cochlea partition associated with an enlargement of the IAC bulbous. Besides these cochlear malformations, 3 out of the 6 Group B subjects presented with a dysmorphic profile of the vestibule and semicircular canals, and 1 subject presented with a mild dilatation of the vestibular aqueduct. Moreover, one of the subjects with the Mondini deformity showed a mild ventricular dilatation and mild macrocephaly associated with bilaterally enlarged jugular bulb. None of the other subjects in Group B had any morphological brain malformation.
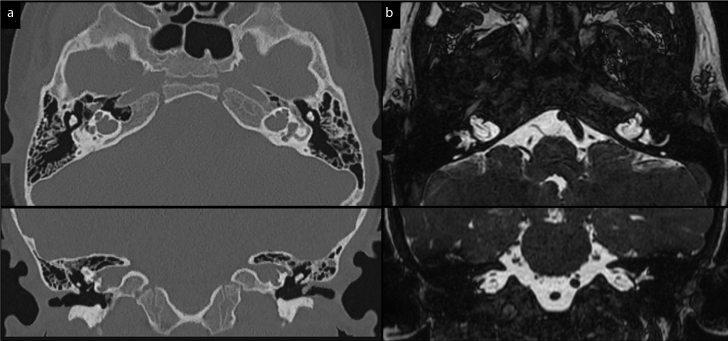
Figure 1. a, b.
Axial and coronal images of the temporal bone and cerebellopontine angle at a high-resolution CT scan (a) and MRI FFE T2-weighted sequence (b). Bilateral inner ear malformations with incomplete partition (Type III) of the cochlea and lack of separation from the dilated internal auditory canal.
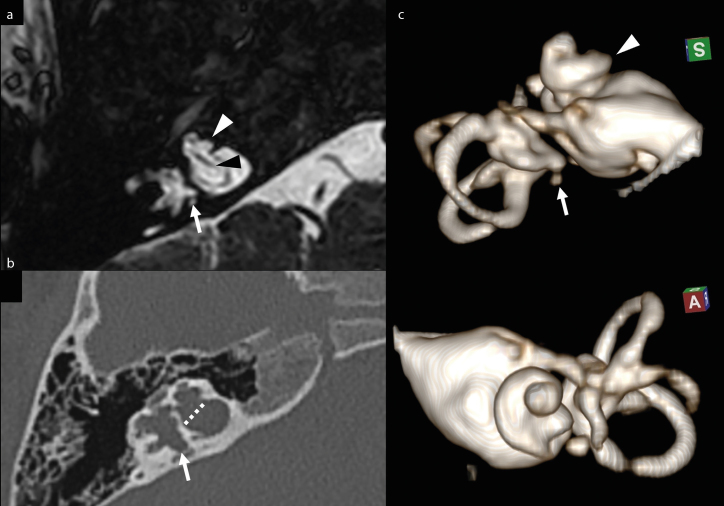
Figure 2. a–c.
Magnified axial MRI FFE T2-weighted image (a) and axial CT image (b) of right temporal bone. Volume rendering of the right internal acoustic canal and inner ear (MRI cisternography), superior and anterior view (c). Bulbous dilatation of the bottom of IAC and lack of the cribriform plate (dotted line), with direct communication between IAC and the basal turn of cochlea. The cochlea is dysmorphic (white arrowhead), with the absence of the modiolus. Note the normal cochlear nerve within IAC (black arrowhead). Also note the dysmorphic profile of the vestibule and semicircular canals, with small saccular dilatations of the wall (arrow).
Otoacoustic Emissions and Auditory Brainstem Response
The OTOAE response was absent in all subjects. Waves III and V could only be recorded from subjects in Group A, where the average amplitude and latency were 0.14 μV (SD, 0.007; CI 95%, 0.13–0.15) and 3.62 msec (SD, 0.12; CI 95%, 3.4–3.7), respectively, for Wave III and 0.28 μV (SD, 0.013; CI 95%, 0.26–0.31) and 5.61 msec (SD, 0.14; CI 95%, 5.3–5.8) respectively for Wave V.
Electrical Auditory Brainstem Response
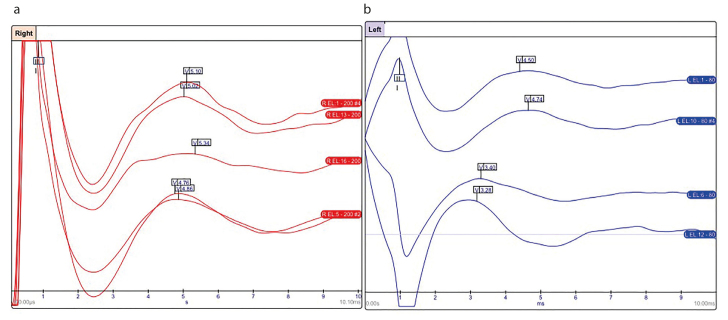
Figure 3 shows examples of typical EABRs recorded from Groups A and B at T1.
Figure 3. a, b.
(a) EABR recorded from the right ear of a patient with a normal cochlea at T1 (red). (b) EABR recorded from the left ear of a child with cochlear malformation at T1 (blue). Waves III and V are present and well recordable both in the patient with a normal cochlea and the patient with a malformed cochlea.
In Group A, the EABR recorded at T0 displayed Wave III with an average amplitude and latency of 0.81 μV (SD, 0.09; CI 95%, 0.7–1) and 1.81 msec (SD, 0.08; CI 95%, 1.7–2), respectively, and Wave V with an average amplitude and latency of 0.12 μV (SD, 0.008; CI 95%: 0.11–0.14) and 3.79 msec (SD, 0.09; CI 95%: 3.6–3.9), respectively. The EABR recorded at T1 displayed similar Wave III and Wave V average amplitude and latencies. There was no statistically significant difference between T0 and T1 in the average amplitude (p=1) nor latency (p=0.98) of Wave III. Similarly, there was no statistically significant difference between T0 and T1 in the average amplitude (p=0.97) nor latency (p=0.99) of Wave V.
In Group B, the EABR recorded at T0 displayed Wave III with an average amplitude and latency of 0.20 μV (SD, 0.01; CI 95%, 0.19–0.22) and 4.56 msec (SD, 0.1; CI 95%, 4.5–4.7), respectively, and Wave V with average amplitude and latency of 0.32 μV (SD, 0.007; CI 95%, 0.32–0.34) and 6.78 msec (SD, 0.07; CI 95%, 6.7–6.9), respectively. The EABR recorded at T1 displayed Wave III with an average amplitude and latency of 0.13 μV (SD, 0.01; CI 95%, 0.12–0.15) and 3.86 msec (SD, 0.1; CI 95%, 3.7–4), respectively, and Wave V with an average amplitude and latency of 0.25 μV (SD, 0.01; CI 95%, 0.23–0.26) and 5.8 msec (SD, 0.06; CI 95%, 5.7–5.9), respectively. There was no statistically significant difference between T0 and T1 in the average amplitude (p=0.75) nor latency (p=0.42) of Wave III. Similarly, there was no statistically significant difference between T0 and T1 in the average amplitude (p=0.51) nor latency (p=0.46) of Wave V.
There was no statistically significant difference between Groups A and B neither in average amplitude nor in latency of Wave III at T0 (p=0.35 and p=0.72, respectively), nor at T1 (p=0.08 and p=0.35, respectively). As for Wave V at T0, there was a statistically significant difference between Groups A and B in the average amplitude (p=0.002), but not in the average latency (p=0.06). Similarly, at T1 there was a statistically significant difference between Groups A and B in the average amplitude (p=0.022), but not in the average latency (p=0.18) (see Table 1). When comparing the ABR and EABR data, we found no changes in amplitude or latencies of Wave III or V in Group A; and a reduction of the amplitude and decrease of latency of Waves III and V in Group B.
Table 1.
For each subject, results of the ABR test performed at T0 and of the EABR test performed at T1 are shown. The word unchanged indicates that the EABR data at T1 were very similar (namely, within a few msecs for the latency and a few μVs for the amplitude) to the ABR data at T0
| ABR (T0) | EABR (T1) | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Cochlea | Amplitude Wave III (μV) | Latency Wave III (msec) | Amplitude Wave V (μV) | Latency Wave V (msec) | Amplitude Wave III (μV) | Latency Wave III (msec) | Amplitude Wave V (μV) | Latency Wave V (msec) |
| Normal | 0.15 | 3.6 | 0.29 | 5.6 | Unchanged | Unchanged | Unchanged | Unchanged |
|
| ||||||||
| Normal | 0.13 | 3.7 | 0.28 | 5.8 | Unchanged | Unchanged | Unchanged | Unchanged |
|
| ||||||||
| Normal | 0.15 | 3.6 | 0.27 | 5.7 | Unchanged | Unchanged | Unchanged | Unchanged |
|
| ||||||||
| Normal | 0.14 | 3.4 | 0.3 | 5.4 | Unchanged | Unchanged | Unchanged | Unchanged |
|
| ||||||||
| Normal | 0.15 | 3.6 | 0.31 | 5.5 | Unchanged | Unchanged | Unchanged | Unchanged |
|
| ||||||||
| Normal | 0.16 | 3.8 | 0.28 | 5.6 | Unchanged | Unchanged | Unchanged | Unchanged |
|
| ||||||||
| Normal | 0.15 | 3.9 | 0.27 | 5.7 | Unchanged | Unchanged | Unchanged | Unchanged |
|
| ||||||||
| Normal | 0.14 | 3.4 | 0.29 | 5.6 | Unchanged | Unchanged | Unchanged | Unchanged |
|
| ||||||||
| Normal | 0.15 | 3.6 | 0.29 | 5.6 | Unchanged | Unchanged | Unchanged | Unchanged |
|
| ||||||||
| Normal | 0,15 | 3.5 | 0.28 | 5.8 | Unchanged | Unchanged | Unchanged | Unchanged |
|
| ||||||||
| Normal | 0.14 | 3.7 | 0.3 | 5.7 | Unchanged | Unchanged | Unchanged | Unchanged |
|
| ||||||||
| Normal | 0.15 | 3.6 | 0.27 | 5.5 | Unchanged | Unchanged | Unchanged | Unchanged |
|
| ||||||||
| Normal | 0.14 | 3.8 | 0.29 | 5.4 | Unchanged | Unchanged | Unchanged | Unchanged |
|
| ||||||||
| Normal | 0,15 | 3.5 | 0.29 | 5.6 | Unchanged | Unchanged | Unchanged | Unchanged |
|
| ||||||||
| Normal | 0.15 | 3,6 | 0.29 | 5.8 | Unchanged | Unchanged | Unchanged | Unchanged |
|
| ||||||||
| Normal | 0.16 | 3.7 | 0,26 | 5,9 | Unchanged | Unchanged | Unchanged | Unchanged |
|
| ||||||||
| Normal | 0.16 | 3.6 | 0.31 | 5.3 | Unchanged | Unchanged | Unchanged | Unchanged |
|
| ||||||||
| Normal | 0.15 | 3.6 | 0.28 | 5.4 | Unchanged | Unchanged | Unchanged | Unchanged |
|
| ||||||||
| Normal | 0.15 | 3.5 | 0.29 | 5.6 | Unchanged | Unchanged | Unchanged | Unchanged |
|
| ||||||||
| Normal | 0.15 | 3.7 | 0.29 | 5.7 | Unchanged | Unchanged | Unchanged | Unchanged |
|
| ||||||||
| Malformed | 0.22 | 4.45 | 0.32 | 6.9 | 0.14 | 3.8 | 0.25 | 5.8 |
|
| ||||||||
| Malformed | 0.18 | 4.78 | 0.34 | 6.7 | 0.13 | 3.9 | 0.23 | 5.9 |
|
| ||||||||
| Malformed | 0.21 | 4.65 | 0.35 | 6.7 | 0.12 | 3.7 | 0.24 | 5.8 |
|
| ||||||||
| Malformed | 0.19 | 4.3 | 0.36 | 7 | 0.15 | 4 | 0.25 | 5.7 |
|
| ||||||||
| Malformed | 0.22 | 4.6 | 0.31 | 6.8 | 0.14 | 3.9 | 0.26 | 5.8 |
|
| ||||||||
| Malformed | 0.2 | 4.7 | 0.32 | 6.6 | 0.13 | 3.9 | 0.25 | 5.8 |
DISCUSSION
The CI implantation in subjects with cochlear malformation is controversial. In fact, in these patients, the outcome of CI surgery is variable, but previous studies have shown that it can be predicted from the EABR recorded presurgery. The presence of EABR Waves III and V predicts a favorable outcome [11] and indicates that the CI properly stimulates the auditory pathways [8]. In patients with a malformed cochlea, the EABR testing prior to CI surgery not only is particularly informative, but it can also overcome the limitations of the ABR testing. In fact, in these patients, the absence of the ABR response at 100 dB might be simply due to the low number of spiral ganglions [4, 12]; to elicit an electrical response, an acoustic stimuli exciding 100 dB would need to be used, which is painful for the patient.
In this study, we could only record ABR from subjects with a normal cochlea, but we were able to record EABR from all subjects. We compared the amplitude and latency of EABRs Waves III and V recorded immediately and 6 months post-CI surgery, and we found that there was no statistically significant difference in subjects with a normal cochlea. A similar result was found for subjects with a malformed cochlea. A between-group comparison in the amplitude and latency of the EABR Wave III and V showed a statistically significant difference only for the amplitude of Wave V, which in subjects with cochlear malformation was smaller than in subjects with a normal cochlea. Such difference in the amplitude of Wave V may be due to a different brain maturity stage [13]; in fact, the subjects with a malformed cochlea were older than those with a normal cochlea for about 4 months, possibly due to the fact that surgery planning periods are typically longer and more complex in the former group (e.g., patients with a malformed cochlea typically undergo a 3D MRI rather than a traditional MRI, as well as genetic, neurological, and nonsymptomatic organ malformation consultation to prevent surgery complications) [14].
Another factor that could explain why the amplitude of the EABR Wave V was smaller in subjects with a malformed cochlea than in subjects with a normal cochlea is the possible reduction of spiral ganglion number in the modiolus [12, 15] in the former group. A malformed cochlea presents less turns and, consequently, less space for spiral ganglions, [4, 12] which can lead to a faulty transmission of signals [4, 12]. In fact, the amplitude of an EABR wave is a function of the power with which an impulse is transmitted from the periphery (cochlea) to the other structures of the auditory pathways (cochlear nuclei and brain), [16] and reduction of spiral ganglion number in the modiolus [12, 15] may lead to attenuated waves (Figure 4). Differently from Wave V, we did not see a difference in Wave III between groups. This might be due to the releasing technique we used in the CI surgery of subjects with cochlear malformation, which supposedly helped achieve a correct allocation of the electrode and, in turn, possibly yielded to a stimulation power sufficient to generate Waves III, but not high enough to produce Waves V comparable to those from normo-conformed cochlea subjects.
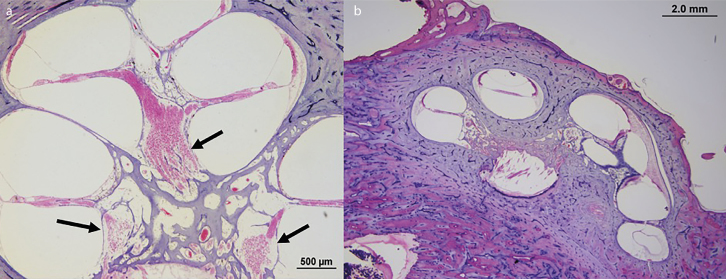
Figure 4. a, b.
A normal human cochlea is shown in Figure a). The black arrows show the modiolus, that is, the cochlear area that contains spiral ganglions (pink round structures inside the modiolus). Figure b) shows a malformed cochlea with absent modiolus partition.
In both Group A and Group B, it was possible to record Waves III and V after CI. The fact that we were able to record the EABR Waves III and V from subjects with a malformed cochlea suggests that nonstandard surgical techniques are necessary to achieve good CI outcomes in these patients [2]. Although we did not find a statistically significant change between T0 and T1 in either group (possibly due to the fact that the follow-up was too short), our data suggest that EABR may be used to evaluate changes in the auditory pathways of children who have undergone CI surgery. Compared to diffusor tensor imaging proposed by other authors, [17] EABR is less expensive, and its efficacy to detect even minimal changes (e.g., due to a demyelination) in signal transmission over the auditory pathways has been widely shown [18].
Limitations of this study include lack of pre-surgery EABR recordings. Furthermore, the age difference (and thus the different stages of brain maturation) between the groups may have partially affected the features of the EABR Waves III and V. Finally, the sample of patients with a malformed cochlea was small, and it displayed different cochlear malformations.
Future studies should focus on analyzing the correlation between the EABR Waves III and V and clinical outcomes such as speech and psychological assessment scores, and on further investigating whether these variables can predict the outcome of CI surgery.
CONCLUSION
Our results show that CI is able to stimulate the hearing pathways correctly, not only in patients with a normal cochlea, but also in patients with cochlear malformation, provided that a suitable CI surgery technique is used. We recommend including EABR as part of the patient examination performed during CI surgery planning, especially in patients with a malformed cochlea. We also recommend using EABR to monitor reconnection of the auditory pathways and development of the brain function after CI.
Acknowledgements
Special thanks to the children parents that allow this study.
Footnotes
Ethics Committee Approval: Ethics committee approval was received for this study from Ethics Committee of Santobono-Posilipon Hospital.
Informed Consent: Written informed consent was obtained from the patients’ parents who participated in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept – A.D.S., A.d.V.; Design – A.D.S.; Supervision – A.d.V.; Resource – A.d.V., G.R., F.T.; Materials – A.D.S., A.d.V., L.D.; Data Collection and/or Processing – F.M., A.D.L., V.P.; Analysis and/or Interpretation – A.D.S., A.d.V., L.D.; Literature Search – A.D.S., F.M., L.D.; Writing – A.D.S.; L.D.; Critical Reviews - A.d.V., G.R.
Conflict of Interest: ADS is a consultant for Oticon Medical. The other authors have no conflict of interest to declare.
Financial Disclosure: The authors declared that this study has received no financial support.
REFERENCES
- 1.Slattery WH, 3rd, Luxford WM. Cochlear implantation in the congenital malformed cochlea. Laryngoscope. 1995;105:1184–7. doi: 10.1288/00005537-199511000-00008. [DOI] [PubMed] [Google Scholar]
- 2.Sennaroglu L, Sarac S, Ergin T. Surgical Results of Cochlear Implantation in Malformed Cochlea. Otol Neurotol. 2006;27:615–23. doi: 10.1097/01.mao.0000224090.94882.b4. [DOI] [PubMed] [Google Scholar]
- 3.Kabatova Z, Profant M, Simkova L, Groma M, Nechojdomova D. Cochlear implantation in malformed inner ear. Bratisl Lek Listy. 2009;110:609–13. [PubMed] [Google Scholar]
- 4.Sennaroğlu L, Bajin MD. Classification and Current Management of Inner Ear Malformations. Balkan Med J. 2017;34:397–411. doi: 10.4274/balkanmedj.2017.0367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Graham JM, Phelps PD, Michaels L. Congenital malformations of the ear and cochlear implantation in children: review and temporal bone report of common cavity. J Laryngol Otol Suppl. 2000;25:1–14. doi: 10.1258/0022215001904842. [DOI] [PubMed] [Google Scholar]
- 6.Buchman CA, Copeland BJ, Yu KK, Brown CJ, Carrasco VN, Pillsbury HC., 3rd Cochlear implantation in children with congenital inner ear malformations. Laryngoscope. 2004;114:309–16. doi: 10.1097/00005537-200402000-00025. [DOI] [PubMed] [Google Scholar]
- 7.Di Stadio A, Dipietro L, Toffano R, Burgio F, De Lucia T, Ippolito V, et al. Working memory function in children with single side deafness using a Bone Anchored Hearing Implant: a case-control study. Audiol Neurootol. 2018;23:238–44. doi: 10.1159/000493722. [DOI] [PubMed] [Google Scholar]
- 8.Wang Y, Pan T, Zhou N, Ma F. Electrophysiological characteristics of EABR and its value assessment of cochlear implant. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2013;27:8–12. [PubMed] [Google Scholar]
- 9.Seo YJ, Kwak C, Kim S, Park YA, Park KH, Han W. Update on Bone-Conduction Auditory Brainstem Responses: A Review. J Audiol Otol. 2018;22:53–8. doi: 10.7874/jao.2017.00346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shujiro Minami Kimitaka Kaga. Modern Otology Book Springer Link. 2016. EABR of Inner Ear Malformation and Cochlear Nerve Deficiency After Cochlear Implantation in Children; pp. 97–109. [DOI] [Google Scholar]
- 11.Kim AH, Kileny PR, Arts HA, El-Kashlan HK, Telian SA, Zwolan TA. Role of electrically evoked auditory brainstem response in cochlear implantation of children with inner ear malformations. Otol Neurotol. 2008;29:626–34. doi: 10.1097/MAO.0b013e31817781f5. [DOI] [PubMed] [Google Scholar]
- 12.Rask-Andersen H, Liu W, Erixon E, Kinnefors A, Pfaller K, Schrott-Fischer A, et al. Human cochlea: anatomical characteristics and their relevance for cochlear implantation. Anat Rec (Hoboken) 2012;295:1791–811. doi: 10.1002/ar.22599. [DOI] [PubMed] [Google Scholar]
- 13.Thai-Van H, Cozma S, Boutitie F, Disant F, Truy E, Collet L. The pattern of auditory brainstem response wave V maturation in cochlear-implanted children. Clin Neurophysiol. 2007;118:676–89. doi: 10.1016/j.clinph.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 14.Pakdaman MN, Herrmann BS, Curtin HD, Van Beek-King J, Lee DJ. Cochlear implantation in children with anomalous cochleovestibular anatomy: a systematic review. Otolaryngol Head Neck Surg. 2012;146:180–90. doi: 10.1177/0194599811429244. [DOI] [PubMed] [Google Scholar]
- 15.Di Stadio A, Ralli M, Ishai R, D’Ascanio L, Trabalzini F, Della Volpe A, et al. Nucleolus vs Nucleus Count for Identifying Spiral Ganglion in Human Temporal Bone. J Int Adv Otol. 2018;14:181–9. doi: 10.5152/iao.2018.5517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Verhulst S, Jagadeesh A, Mauermann M, Ernst F. Individual Differences in Auditory Brainstem Response Wave Characteristics Relations to Different Aspects of Peripheral Hearing Loss. Trends Hear. 2016;20 doi: 10.1177/2331216516672186. 2331216516672186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tarabichi O, Kozin ED, Kanumuri VV, Barber S, Ghosh S, Sitek KR, et al. Diffusion Tensor Imaging of Central Auditory Pathways in Patients with Sensorineural Hearing Loss: A Systematic Review. Otolaryngol Head Neck Surg. 2018;158:432–42. doi: 10.1177/0194599817739838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Di Stadio A, Dipietro L, Ralli M, Meneghello F, Minni A, Greco A, et al. Sudden hearing loss as an early detector of multiple sclerosis: a systematic review. Eur Rev Med Pharmacol Sci. 2018;22:4611–24. doi: 10.26355/eurrev_201807_15520. [DOI] [PubMed] [Google Scholar]