Abstract
Petrous apex cholesterol granulomas (PACG) are rare disorders that can lead to patient morbidity and must, occasionally, be drained by either endoscopic endonasal (EN) or open procedures (OP). The objective of our study was to complete a review of the literature to compare the EN and OP approaches on multiple levels, notably on safety and effectiveness. Ovid MEDLINE and Embase were used to perform a thorough literature review of all cases of PACG treated by either EN or OP dating from January 1948 to August 2017. In total, 49 articles were selected including 23 for EN (n=76) and 26 for OP (n=210). Differences were found in the incidence of preoperative hearing loss (HL) (EN 18.4%, OP 57.3%; p<0.001), headache (EN 48.7%, OP 31.2%; p=0.007), and disequilibrium (EN: 14.5%, OP 26.1%; p=0.04). Differences in lesion proximity to the sphenoid sinus (EN 23.6%, OP: 1.0%; p<0.001), clivus (EN 11.8%, OP 4.7%; p=0.03), otic capsule (EN 0.0%, OP 5.2%; p=0.03), internal auditory canal (EN 2.6%, OP 10.9%; p=0.01), and internal carotid artery (ICA) (EN 9.2%, OP 2.8%; p=0.02) were found on preoperative imaging. The EN procedure had better hearing improvement rates (EN 85.7%, OP 23.4%; p<0.001), lower complication rates (EN 7.9%, OP 17.6%; p=0.04), shorter median follow-up (EN:13.5 months, OP:37.2 months; p<0.001), and shorter time to recurrence (EN 3 months, OP 22.6 months; p=0.002) than the known OP. No differences were found in age, preoperative size, recurrence rate, operative time, stent placement, or improvement of other symptoms. Endoscopic nasal approaches, when feasible, should be favored to open procedures for PACG drainage given their better hearing improvement and less complication rates.
Keywords: Petrous apex, cholesterol granuloma, infralabyrinthine, infracochlear, middle fossa, suboccipital, translabyrinthine, endonasal
INTRODUCTION
Petrous apex cholesterol granulomas (PACG) are rare disorders that affect 0.6 per 1 million persons [1]. The cause of these inflammatory lesions remains a controversial topic. Originally, it was thought that they were caused by hemorrhage in the petrous apex (PA) space. According to this theory, red blood cells are broken down secondary to the negative pressure of the space and cholesterol is released. This then triggers an inflammatory reaction and forms a cyst [2]. In 2003, Jackler et al. [3] proposed a new theory where the bleeding and inflammatory cascade would be caused by contact between the skull base bone marrow and the aerated PA space.
Some have advocated for the conservative management of these lesions when they are incidentally found on imaging [4]. However, when these become cumbersome and lead to morbid symptoms such as headaches and cranial neuropathies, they must be surgically drained or excised.
Several approaches have been described over the years, which can be separated into two categories: the endonasal (EN) and open (OP) approaches. Three variations of EN approaches are described: 1) the transsphenoid (TS) approach, 2) the transclival (TC) approach with or without internal carotid artery (ICA) lateralization, and 3) the transpterygoid infrapetrous (IP) approach [5]. Conversely, the open approaches include the infracochlear (IC) [6], infralabyrinthine (IL) [7], middle fossa (MF), suboccipital (SO) [8], and translabyrinthine (TL) approaches [9].
Stenting has been used to theoretically diminish the risks of recurrence. However, their benefit remains unproven and controversial. Of note, an alternative to stenting, proposed by Paluzzi et al. [10], is the nasoseptal mucoperiosteal miniflap.
In a cadaver dissection study, Scopel et al. [11] demonstrated that EN approaches were able to reach the posterior-inferior PA in 90% and the superior and anterior-inferior PA in all specimens. With this in mind, EN approaches may not be limited to lesions approximating the sphenoid sinus.
The objective of our study was to complete a review of the literature of all the aforementioned approaches to compare the EN and OP approaches on multiple levels, notably on safety and effectiveness.
MATERIALS AND METHODS
Literature Review
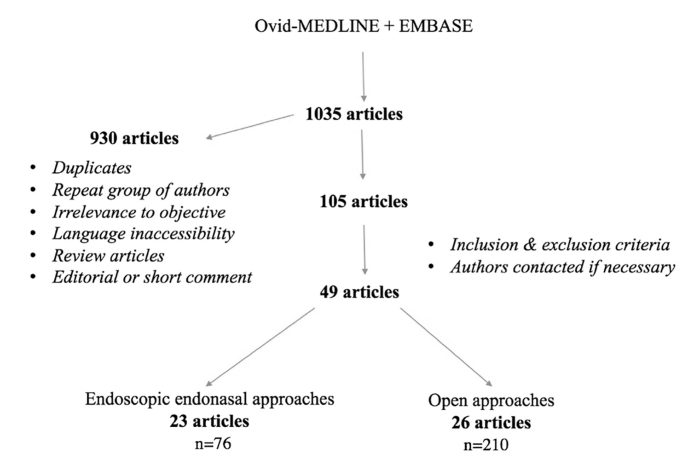
The systematic review was completed according to Preferred Reporting Items for Systematic Reviews guidelines. Pubmed, Ovid MEDLINE, and EMBASE databases were used to perform a literature review of English language publications from January 1948 to August 2017. The keyword combinations included the following: petrous apex cholesterol granuloma AND infralabyrinthine OR infracochlear OR middle fossa OR suboccipital OR translabyrinthine OR transsphenoid OR transclival OR transpterygoid. An outline of our review methodology can be seen in Figure 1.
Figure 1.
Study methodology.
Selection Criteria
Articles explicitly reporting patients with histologically proven PACG were reviewed. The lesion had to have been either drained or excised via either EN or OP approaches. Each study was analyzed to extract all available data and assure eligibility for inclusion of all patients. Patients were separated into two groups according to the operative procedure they underwent (EN or OP). Age, preoperative symptoms, size and location of the lesion, operative times, follow-up times, postoperative symptom improvement, surgical complications, and recurrence rates with or without the use of stent placement were compared. The pediatric population was excluded. Articles describing only revision surgery were also excluded.
Titles and abstracts were reviewed by two authors (P.T. and N.S.) to discard irrelevant studies. All of these were then analyzed to include any additional articles of interest. Relevant studies were independently determined by P.T. and N.S, based on the inclusion criteria.
The study was approved by our institutional research ethics board and follows the standards of our institutional ethics committee.
Statistical Analysis
Statistical analysis was done using the Microsoft Excel software (Redmond, Washington, USA). Chi-square test was used to analyze categorical data. Fisher’s exact test was used to analyze categorical data when one of the tested groups was composed of ≤5 patients. Student’s t-test was used to analyze all continuous data. Homogeneity among studies in each group was also undertaken.
RESULTS
A total of 49 articles were included in our review: 23 described endonasal approaches [5, 10, 12–34] and 26 described open approaches [4, 6, 34–57]. Reports of 286 patients were included, 76 of whom underwent EN approaches and 210 OP approaches. The most commonly used surgical approaches are listed in Table 1.
Table 1.
Different approaches used on patients presenting with petrous apex cholesterol granuloma
| Approach | # of patients | Relative frequency (%) | |
|---|---|---|---|
| EN | Total | 76 | 26.6 |
| TS | 39 | 13.6 | |
| TC | 18 | 6.6 | |
| IP | 11 | 3.8 | |
| TC+IP | 8 | 2.8 | |
| OP | Total | 210 | 73.4 |
| IC | 62 | 21.7 | |
| IL | 78 | 27.3 | |
| MF | 33 | 11.5 | |
| TL | 16 | 5.6 | |
| SO | 6 | 2.1 | |
| Other | 15 | 5.2 |
EN: Endoscopic endonasal; OP: open; TS: Transsphenoid approach; TC: Transphenoid transclival; IP: Infrapetrous; IC: Infracochlear; IL: Infralabyrinthine; MF: Middle Fossa; TL: Translabyrinthine; SO: Suboccipital
The mean age was similar between both EN and OP groups (EN: 38.6±15 years; OP: 39.7±11.6 years; p=0.6). The most common presenting symptom was hearing loss, followed by headaches and vestibular symptoms (Table 2). Differences in preoperative symptoms between each group are shown in Table 3. No difference was found in the preoperative lesion size between both EN and OP groups (EN: 3.15±0.83 cm; OP: 2.75±0.57 cm; p=0.19). Differences in lesion proximity to important anatomic structures are shown in Table 4.
Table 2.
Presenting symptoms in patients with petrous apex cholesterol granuloma
| Symptom | Number of patients (n) | Relative frequency (%) |
|---|---|---|
| Hearing loss | 135 | 47.2 |
| Headache | 103 | 36.0 |
| Vertigo/disequilibrium | 66 | 23.1 |
| Diplopia | 39 | 13.6 |
| Facial paresis/paralysis | 24 | 8.4 |
| Facial paresthesia/pain | 21 | 7.3 |
Table 3.
Differences in preoperative symptoms between the EN and OP groups
| Symptom | EN (%) n=76 |
OP (%) n=210 |
p |
|---|---|---|---|
| Hearing loss | 18.4 | 57.3 | <0.001 |
| Headache | 48.7 | 31.2 | 0.007 |
| Vertigo/disequilibrium | 14.5 | 26.1 | 0.04 |
| Diplopia | 19.7 | 11.3 | 0.07 |
| Facial paresthesia/pain | 4.0 | 8.5 | 0.09 |
| Facial paresis/paralysis | 4.0 | 10.0 | 0.19 |
EN: Endoscopic endonasal; OP: open
Table 4.
Differences in lesion proximity to important anatomical structures between the EN and OP groups
| Location proximity | EN (n=76) (%) | OP (n=210) (%) | p |
|---|---|---|---|
| Sphenoid sinus | 23.6 | 1.0 | <0.001 |
| Clivus | 11.8 | 4.7 | 0.03 |
| Otic capsule | 0.0 | 5.2 | 0.03 |
| IAC | 2.6 | 10.9 | 0.01 |
| ICA | 9.2 | 2.8 | 0.02 |
EN: Endoscopic endonasal; OP: open; IAC: internal auditory canal; ICA: internal carotid artery
As shown in Table 5, symptom improvement was similar between EN and OP groups, except for hearing loss which responded better to EN than hearing-sparing OP (EN 85.7%, OP 23.4%; p<0.001). Table 6 demonstrates symptom improvement among the most frequently used approaches. All patients who underwent the MF approach showed complete resolution of their symptoms. The IL approach showed better hearing improvement than the IC approach (41% vs 17%; p=0.03).
Table 5.
Postoperative symptoms improvement in patients with cholesterol granuloma of the petrous apex
| Symptom | Surgical Approach | Reported symptom outcome | Symptom improvement (%) | p |
|---|---|---|---|---|
| HL | EN | 13/14 (93%) | 92.3 | <0.001 |
| OP* | 88/121 (72%) | 23.4 | ||
| Headache | EN | 33/37 (89%) | 93.9 | 0.46 |
| OP | 15/66 (23%) | 93.8 | ||
| Vertigo / | EN | 9/11 (82%) | 88.9 | 0.53 |
| disequilibrium | OP | 9/55 (16%) | 90 | |
| Diplopia | EN | 10/15 (67%) | 90 | 0.43 |
| OP | 22/24 (92%) | 86.4 | ||
| Paresis / paralysis | EN | 0/3 (0%) | N/A | N/A |
| OP | 5/21 (24%) | 50 | ||
| Paresthesia / pain | EN | 3/3 (100%) | 66.7 | 0.3 |
EN: Endoscopic endonasal; OP: open; N/A: not applicable.
Excluding all nonhearing-sparing approaches.
Table 6.
Cholesterol granuloma symptom improvement by surgical approaches
| EN | OP | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||
| Symptom | TS (n=39) | TC (n=18) | IP | TC+IP (n=8) | IC (n=62) | IL (n=78) | MF (n=33) | TL (n=16) | SO (n=6) | Other (n=15) |
| Hearing loss | 6/7 (86%) | 2/2 (100%) | 3/3 (100%) | 1/1 (100%) | 5/30 (17%) | 17/41 (41%) | N/A | 0/5 (0%) | 0/4 (0%) | 3/8 (38%) |
|
| ||||||||||
| Headache | 15/16 (94%) | 6/7 (86%) | 4/4 (100%) | 6/6 (100%) | 1/1 (100%) | 6/7(86%) | 4/4 (100%) | N/A | N/A | 3/3 (100%) |
|
| ||||||||||
| Vertigo/disequilibrium | 4/4 (100%) | 3/4 (75%) | N/A | 1/1 (100%) | 2/2 (100%) | 5/5 (100%) | N/A | N/A | N/A | 0/2 (0%) |
|
| ||||||||||
| Diplopia | 6/7 (86%) | 1/1 (100%) | 2/2 (100%) | N/A | 2/4 (50%) | 5/5 (100%) | 10/10 (100%) | N/A | N/A | 2/3 (67%) |
|
| ||||||||||
| Paresis / paralysis | N/A | N/A | N/A | N/A | 2/4 (50%) | N/A | N/A | N/A | N/A | 0/1 (0%) |
|
| ||||||||||
| Paresthesia / pain | N/A | N/A | 2/3 (67%) | N/A | 1/1 (100%) | N/A | 4/4 (100%) | N/A | N/A | 2/2 (100%) |
EN: Endoscopic endonasal; OP: open; TS: Transsphenoid; TC: Transphenoid transclival; IP: Infrapetrous; IC: Infracochlear; IL: Infralabyrinthine; MF: Middle Fossa; TL: Translabyrinthine; SO: Suboccipital; N/A: not applicable.
Stenting was used in 51 patients (17.8%). Sixteen patients among the EN group (21%) underwent stent placement, including five TS, three TC, and eight the combined TC+IP approaches. Of the 35 stents placed within the OP group (16.7%), 21 used the IC approach, 12 the IL approach, and two MF approaches. Stents were specifically described as being made with Silastic in only six patients [6, 41, 42, 51]. Specific stent manufacturers were mentioned in only two articles. One article among the EN group described the stent as a 4-mm Xomed frontal sinus stent (Medtronic Inc., Jacksonville, Florida, USA) [23], and another among the OP group described it as fashioned from an angiocath (Becton, Dickinson and Co.) [48]. Furthermore, two articles mentioned T-shaped stents in the EN group [29, 30]. Graham et al. [51] described an MF approach which necessitated stent placement into the anterior tympanic cavity, and Balachandran et al. [35] described a double-barrel stenting approach which required two drilling tracts to the lesion. Finally, two articles mentioned postoperative removal of stents under general anesthesia because of granulation tissue formation [10]. No articles in our review mentioned anticoagulant therapy or its effect on patient quality of life.
EN approaches showed similar recurrence rates as OP approaches when stent placement was used (6% vs 17%; p=0.22). When comparing the recurrence rate of nonstented patient, EN approaches had a significantly higher recurrence rate (16% vs 7%; p=0.03). No difference was found in recurrence rates between stented and nonstented EN approaches (6% vs 16% p=0.20). A trend was seen toward a significantly higher recurrence rate in stented OP approaches than nonstented ones (17% vs 7%; p=0.07).
Among all collected articles, only two in the EN group [15, 24] and four in the OP group [42, 43, 53] were described as excised and not drained, all of which had positive postoperative outcomes. Given the rarity of excision, comparison between drainage and excision between groups was difficult to make.
A trend toward a significantly shorter OR time was noted between EN and OP approaches (1 h vs 2.25 h, respectively; p=0.09); however, it must be mentioned that very few operative times were reported in the literature according to our review (n=9).
Table 7 shows symptom recurrence and surgical complication rates for the most commonly used approaches. Among all patients, the presence of recurrences and complications was reported in 208/286 (72.7%) and 225/286 (78.7%), respectively. When comparing EN and OP approaches, the symptom recurrence rates were similar (12.3% vs 10.6%; p=0.57) and the complications rates were significantly higher in the OP approach group than in the EN approach group (22.5% vs 9.1%; p=0.03).
Table 7.
Recurrence and complications rate comparison between EN and open approaches
| Approach | Reported complications | Complication rate (%) | Complications | Reported recurrences | Recurrence rate (%) |
|---|---|---|---|---|---|
| Total | 65/76 (85.5%) | 5/55 (9.1%) | 57/76 (75.0%) | 7/57 (12.3%) | |
| TS | 30/39 (76.9%) | 2/30 (6.6%) | Epistaxis; pneumocephalus | 28/39 (71.8%) | 6/28 (21.4%) |
| TC | 17/18 (94.4%) | 1/17 (5.8%) | Transient abducens nerve palsy | 13/18 (72.2%) | 0 (0%) |
| IP | 10/11 (90.9%) | 0 (0%) | 9/11 (81.8%) | 1/9 (11.1%) | |
| TC+IP | 8/8 (100%) | 2/8 (25%) | Chronic otitis media; eye dryness | 7/8 (87.5%) | 0 |
| Total | 160/210 (76.1%) | 36/160 (22.5%) | 151/210 (71.9%) | 16/151 (10.6%) | |
| IC | 48/62 (77.4%) | 7/48 (14.5%) | Tympanic perforation; facial paralysis; CSF leak/meningitis; SNHL | 47/62 (75.8%) | 9/47 (19.1%) |
| IL | 58/78 (74.4%) | 18/58 (31%) | Facial paralysis (x4), CSF leak (x2), superficial wound infection (x2), stitch abscess; labyrinth injury, cochlea aqueduct injury, carotid artery injury | 58/78 (74.4%) | 4/58 (6.9%) |
| MF | 26/33 (78.8%) | 5/26 (19.2%) | CSF leak, meningitis, seizures | 24/33 (72.7%) | 2/24 (8.3%) |
| TL | 10/16 (62.5%) | 0* | 8/16 (50.0%) | 0/8 (0%) | |
| SO | 5/6 (83.3%) | 3/5 (60%) | Facial nerve palsy, abdominal hematoma, swallowing dysfunction | 5/6 (83.3%) | 0/5 (0%) |
| Other | 13/15 (86.6%) | 3/13 (23.0%) | Facial nerve paralysis; carotid artery injury | 9/15 (60%) | 1/9 (11.1%) |
EN: Endoscopic endonasal; OP: open; TS: Transsphenoid; TC: Transphenoid transclival; IP: Infrapetrous; IC: Infracochlear; IL: Infralabyrinthine; MF: Middle fossa; TL: Translabyrinthine; SO: Suboccipital; CSF: Cerebrospinal fluid; SNHL: Sensorineural hearing loss.
Postoperative hearing loss in TL was not considered a complication
The EN approach was also found to have shorter median follow-up (EN: 13.5 months, OP: 37.2 months; p<0.001) and shorter time to recurrence (EN: 3 months, OP: 22.6 months; p=0.002).
A study of homogeneity was attempted but was determined to be unreliable given the low sample size within each case series.
DISCUSSION
The indications for a given approach were rarely explicitly expressed within each article; however, the differences in preoperative symptoms and lesion location may explain why a certain procedure was favored over another. Of note, hearing loss and proximity to the internal auditory canal (IAC) and otic capsule seemed to favor the OP approach. Extension to the internal auditory canal has previously been stated as a contraindication to EN procedures [47]; nonetheless, two patients in our review underwent EN resection of PACG extending to IAC [23, 29]. Interestingly, they did not present any postoperative complications and were symptom-free at 3-and 10-month follow-ups. Of the 11 patients with PACG approximating to the otic capsule, one had no reported symptom improvement, and of the remaining 10 patients, 7 (70%) had improved hearing after an OP approach. This entails that the approximation to the otic capsule does not entail the lack of hearing improvement postoperatively and that these patients should be included in the hearing outcome comparison.
Furthermore, many authors have limited the use of EN approaches to medial lesions either protruding or abutting the sphenoid sinus; however, the use of ICA lateralization or a transpterygoid infrapetrous approach has made a more deep and lateral dissection possible [11]. Paluzzi et al. [10] reported five patients necessitating ICA lateralization and eight necessitating an infrapetrous approach. None of these patients had either ICA injury or permanent vision disturbances. These findings are corroborated by Zanation et al. [5] who also reported three patients with ICA lateralization and no associated complications. All these patients were recurrence-free at long-term follow-up.
Even though some have advised against the use of EN procedures in cases of dural involvement because of the risk of secondary meningitis [47], Georgalas et al. reported a case of PACG excised from the sella turcica, the dura of the posterior cranial fossa, ICA, and optic nerves which showed no evidence of meningitis or ICA injury [15]. In fact, no patients who underwent the EN approach in our review showed complications with meningitis.
As for subjective clinical outcomes, EN approaches have shown promising results in symptom improvement. First, successful treatment seems to be similar to the more invasive OP approaches as shown by comparable symptom improvement scores and recurrence rates (Tables 5 and 7). Even when including only hearing-sparing OP procedures, EN showed better hearing loss improvement. Unfortunately, no articles reported postoperative facial paralysis outcomes.
Finally, the EN procedure complication rate was significantly lower than the OP approach. Complications in EN included epistaxis [10, 31], pneumocephalus [21], transient abducens nerve palsy, chronic serous otitis media, and eye dryness.
The infracochlear (IC) approach carries a complication rate of 14.5%, which included mostly minor complications such as tympanic perforation and temporary facial paralysis [6]. One patient had a severe complication of CSF leak and subsequent meningitis [52]. Furthermore, the IC approach has a theoretic risk of carotid artery injury during drilling [39]. This complication was, however, never reported during draining of a PACG. The recurrence rate of this approach was higher than overall EN, IL, and MF approaches. These results lead us to prefer EN procedures over IC ones when the former is deemed possible.
The IL approach, which necessitates complete mastoidectomy, presents with similar recurrence rates to the other OP approaches but has a much higher complication rate than the EN and IC approaches. This may be due to the dangerous boundaries of the infralabyrinthine cells whose limits are the sigmoid sinus, the third portion of the facial nerve, posterior semicircular canal, and jugular bulb [6]. Of all complications encountered after the IL approach, four were of facial paralysis [41, 45, 47]. Even though hearing improvement was significantly better with the IL approach than the IC approach, the EN approach should be favored, when feasible, in patients presenting with this symptom.
The MF approach was the most successful intervention with regards to symptoms improvement, in fact, all patient in our review who underwent MF approach were symptom-free after their surgery. This approach unfortunately has the downfall of having a high complication rate. Complications were relatively severe and included CSF leak, meningitis [38], and seizures [53].
All patients operated via the TL approach had nonserviceable hearing and no recurrences of their lesions secondary to complete resection of the disease. In fact, given the wide exposure of the PA, a complete resection of the granuloma is possible, and therefore, a low recurrence rate is to be expected.
Of the six patients who underwent a suboccipital approach (SO) compared to the PA approach, postoperative symptom outcomes were reported only in four patients who initially presented with hearing loss and none showed postsurgical improvement. Two patients had postoperative complications, one developed a facial nerve palsy and an abdominal wound hematoma [34] and the second reported swallowing dysfunction [49]. The proximity to the brainstem origin of cranial nerves VII, IX, and X could explain these findings [58].
Interestingly, when comparing nonstented EN and OP approaches, a significantly higher recurrence rate was observed in EN which may indicate a higher necessity for stenting in these cases. In fact, the larger dissected space in the OP techniques may explain the limited usefulness of stents in these procedures. Another interesting finding is the trend toward a higher recurrence rate in stented OP patients than non-stented ones. This may be secondary to stent blockage and subsequent narrowing of the open dissected space. Therefore, we conclude that stenting is an advantageous intervention only in EN cases and may increase the risk of recurrence in OP cases.
Finally, the shorter median follow-up and time to recurrence after EN approaches may represent another advantage of such procedures. Since disease recurrence presents earlier with EN, cure from PACG may be established more quickly in these patients than in those having undergone OP procedures. The timing of complications and hearing improvement were not reported in the reviewed articles. However, most described complications can be considered acute complications normally occurring within a year of surgery. Similarly, postoperative hearing assessment is usually done within a year of surgery as well. Therefore, the shorter follow-up time in EN approaches (13.5 month) should not invalidate the differences found between the EN and OP groups.
It is important to note, however, that regardless of these different outcomes between the EN and OP approaches, we do emphasize the value of observing patients with tolerable symptoms given the possibility of spontaneous resolution [59] and the risks involved in surgery.
Limitations
First, our study is limited by the reported data within the above cited articles. As previously noted, postoperative outcomes were not systematically mentioned in every article. Furthermore, no distinction was made between symptom improvement and resolution. Also, if any authors brought subtle variations of known surgical approaches when excising or draining the cholesterol granuloma, these variations were not taken into account given their high number in variability. For example, the Brackmann’s method [6] for the infracochlear approach is not identical to the one described by Goldofsky et al. [41] No articles in the EN group and 18 articles in the OP group mentioned pure-tone audiometry (PTA) scores. Ten of the 18 articles in the OP group mentioned pre- and post-operative PTA scores. Given the limitations of quantitative data in literature, we opted to use only the mention of the presence, improvement, stability, or worsening of hearing loss. A specific threshold was not used given the lack of PTA scores in the EN group. Finally, homogeneity testing was not possible given the low sample size of each study, multiple case reports and series had to be included given the lack of literature on the topic. This permits an early interpretation of data in literature and more studies, including randomized studies, must be undertaken to further understand this topic. However, to do so, endoscopic endonasal approaches should be attempted in greater numbers and this study in an introduction to its safety and efficacy.
CONCLUSION
While the recurrence rates are similar between EN and OP procedures, the EN approach has lower complication rates as well as better hearing improvement outcomes. Also, stent placement seems to be more useful in the EN approach than in the OP approach. Given these results, we encourage to always consider EN approaches, when feasible, in patients with PACG. If an OP approach in chosen however, one should be aware of the lower recurrence rates and better hearing outcomes of the IL approach than the IC approach which has, on the other hand, less complications.
Footnotes
This study was presented at the “Annual Congress of the ORL Association of Quebec”, “October 6–28th of October 2018”, “Quebec (Qc) Canada”.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept – I.S., P.T.; Design - I.S., P.T., N.S.; Supervision – I.S.; Resource - I.S., P.T., N.S; Materials - I.S., P.T., N.S.; Data Collection and/or Processing - I.S., P.T., N.S.; Analysis and/or Interpretation - I.S., P.T.; Literature Search - I.S., P.T., N.S; Writing - I.S., P.T., N.S; Critical Reviews - I.S., P.T., N.S..
Conflict of Interest: The authors have no conflict of interest to declare.
Financial Disclosure: The authors declared that this study has received no financial support.
REFERENCES
- 1.Lo WW, Solti-Bohman LG, Brackmann DE, Gruskin P. Cholesterol granuloma of the petrous apex: CT diagnosis. Radiology. 1984;153:705–11. doi: 10.1148/radiology.153.3.6494466. [DOI] [PubMed] [Google Scholar]
- 2.Amedee RG, Marks HW, Lyons GD. Cholesterol granuloma of the petrous apex. Am J Otol. 1987;8:48–55. [PubMed] [Google Scholar]
- 3.Jackler RK, Cho M. A new theory to explain the genesis of petrous apex cholesterol granuloma. Otol Neurotol. 2003;24:96–106. doi: 10.1097/00129492-200301000-00020. [DOI] [PubMed] [Google Scholar]
- 4.Wick CC, Hansen AR, Kutz JW, Jr, Isaacson B. Endoscopic Infracochlear Approach for Drainage of Petrous Apex Cholesterol Granulomas: A Case Series. Otol Neurotol. 2017;38:876–81. doi: 10.1097/MAO.0000000000001422. [DOI] [PubMed] [Google Scholar]
- 5.Zanation AM, Snyderman CH, Carrau RL, Gardner PA, Prevedello DM, Kassam AB. Endoscopic endonasal surgery for petrous apex lesions. Laryngoscope. 2009;119:19–25. doi: 10.1002/lary.20027. [DOI] [PubMed] [Google Scholar]
- 6.Brackmann DE, Toh EH. Surgical management of petrous apex cholesterol granulomas. Otol Neurotol. 2002;23:529–33. doi: 10.1097/00129492-200207000-00023. [DOI] [PubMed] [Google Scholar]
- 7.Wilson DF, Hodgson RS. Transmastoid infralabyrinthine approach to petrous temporal bone. Skull Base Surg. 1991;1:188–90. doi: 10.1055/s-2008-1057005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shelton C, Brackmann DE, House WF, Hitselberger WE. Middle fossa acoustic tumor surgery: results in 106 cases. Laryngoscope. 1989;99:405–8. doi: 10.1288/00005537-198904000-00009. [DOI] [PubMed] [Google Scholar]
- 9.House WF, Belal A., Jr Translabyrinthine surgery: anatomy and pathology. Am J Otol. 1980;1:189–98. [PubMed] [Google Scholar]
- 10.Paluzzi A, Gardner P, Fernandez-Miranda JC, Pinheiro-Neto CD, Scopel TF, Koutourousiou M, et al. Endoscopic endonasal approach to cholesterol granulomas of the petrous apex: a series of 17 patients: clinical article. J Neurosurg. 2012;116:792–8. doi: 10.3171/2011.11.JNS111077. [DOI] [PubMed] [Google Scholar]
- 11.Scopel TF, Fernandez-Miranda JC, Pinheiro-Neto CD, Peris-Celda M, Paluzzi A, Gardner PA, et al. Petrous apex cholesterol granulomas: endonasal versus infracochlear approach. Laryngoscope. 2012;122:751–61. doi: 10.1002/lary.22448. [DOI] [PubMed] [Google Scholar]
- 12.Bootz F, Keiner S, Schulz T, Scheffler B, Seifert V. Magnetic resonance imaging--guided biopsies of the petrous apex and petroclival region. Otol Neurotol. 2001;22:383–8. doi: 10.1097/00129492-200105000-00019. [DOI] [PubMed] [Google Scholar]
- 13.DiNardo LJ, Pippin GW, Sismanis A. Image-guided endoscopic transsphenoidal drainage of select petrous apex cholesterol granulomas. Otol Neurotol. 2003;24:939–41. doi: 10.1097/00129492-200311000-00020. [DOI] [PubMed] [Google Scholar]
- 14.Emanuelli E, Ciorba A, Bianchini C, Bossolesi P, Stomeo F, Pelucchi S. Transnasal endoscopic management of petrous apex and clivus selected lesions. Eur Arch Otorhinolaryngol. 2013;270:1747–50. doi: 10.1007/s00405-012-2229-7. [DOI] [PubMed] [Google Scholar]
- 15.Georgalas C, Kania R, Guichard JP, Sauvaget E, Tran Ba Huy P, Herman P. Endoscopic transsphenoidal surgery for cholesterol granulomas involving the petrous apex. Clin Otolaryngol. 2008;33:38–42. doi: 10.1111/j.1749-4486.2007.01589.x. [DOI] [PubMed] [Google Scholar]
- 16.Ishi Y, Kobayashi H, Motegi H, Endo S, Yamaguchi S, Terasaka S, et al. Endoscopic transsphenoidal surgery using pedicle vascularized nasoseptal flap for cholesterol granuloma in petrous apex: A technical note. Neurol Neurochir Pol. 2016;50:504–10. doi: 10.1016/j.pjnns.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 17.Jaberoo MC, Hassan A, Pulido MA, Saleh HA. Endoscopic endonasal approaches to management of cholesterol granuloma of the petrous apex. Skull Base. 2010;20:375–9. doi: 10.1055/s-0030-1253574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karligkiotis A, Bignami M, Terranova P, Ciniglio-Appiani M, Shawkat A, Verrilaud B, et al. Use of the pedicled nasoseptal flap in the endoscopic management of cholesterol granulomas of the petrous apex. Int Forum Allergy Rhinol. 2015;5:747–53. doi: 10.1002/alr.21521. [DOI] [PubMed] [Google Scholar]
- 19.McLaughlin N, Kelly DF, Prevedello DM, Shahlaie K, Carrau RL, Kassam AB. Endoscopic endonasal management of recurrent petrous apex cholesterol granuloma. Skull Base Rep. 2011;1:27–32. doi: 10.1055/s-0031-1275253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oyama K, Ikezono T, Tahara S, Shindo S, Kitamura T, Teramoto A. Petrous apex cholesterol granuloma treated via the endoscopic transsphenoidal approach. Acta Neurochir (Wien) 2007;149:299–302. doi: 10.1007/s00701-006-1105-x. [DOI] [PubMed] [Google Scholar]
- 21.Park KC, Wong G, Stephens JC, Saleh HA. Endoscopic transsphenoidal drainage of an aggressive petrous apex cholesterol granuloma: unusual complications and lessons learnt. J Laryngol Otol. 2013;127:1230–4. doi: 10.1017/S0022215113002983. [DOI] [PubMed] [Google Scholar]
- 22.Prabhu K, Kurien M, Chacko AG. Endoscopic transsphenoidal approach to petrous apex cholesterol granulomas. Br J Neurosurg. 2010;24:688–91. doi: 10.3109/02688697.2010.520766. [DOI] [PubMed] [Google Scholar]
- 23.Sade B, Batra PS, Scharpf J, Citardi MJ, Lee JH. Minimally invasive endoscopic endonasal management of skull base cholesterol granulomas. World Neurosurg. 2012;78:683–8. doi: 10.1016/j.wneu.2011.10.047. [DOI] [PubMed] [Google Scholar]
- 24.Samadian M, Akbari Dilmaghani N, Ahmady Roozbahany N, Farzin N, Bahadoram M. Endoscopic Transnasal Approach for Cholesterol Granuloma of the Petrous Apex. Case Rep Neurol Med. 2015 doi: 10.1155/2015/481231. doi: 10.1155/2015/481231. Epub 2015 Jul 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Samadian M, Vazirnezami M, Moqaddasi H, Rakhshan M, Khormaee F, Ashraf H. Endoscopic transrostral- transsphenoidal approach to petrous apex cholesterol granuloma: case report. Turk Neurosurg. 2009;19:106–11. [PubMed] [Google Scholar]
- 26.Shibao S, Toda M, Tomita T, Saito K, Ogawa K, Kawase T, et al. Petrous apex cholesterol granuloma: importance of pedicled nasoseptal flap in addition to silicone T-tube for prevention of occlusion of drainage route in transsphenoidal approach--a technical note. Neurol Med Chir (Tokyo) 2015;55:351–5. doi: 10.2176/nmc.tn.2014-0254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Terranova P, Karligkiotis A, Gallo S, Meloni F, Bignami M, Castelnuovo P. A novel endoscopic technique for long-term patency of cholesterol granulomas of the petrous apex. Laryngoscope. 2013;123:2639–42. doi: 10.1002/lary.24170. [DOI] [PubMed] [Google Scholar]
- 28.Turan N, Baum GR, Holland CM, Ahmad FU, Henriquez OA, Pradilla G. Upper Nasopharyngeal Corridor for Transnasal Endoscopic Drainage of Petroclival Cholesterol Granulomas: Alternative Access in Conchal Sphenoid Patients. J Neurol Surg Rep. 2016;77:e017–22. doi: 10.1055/s-0035-1567865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fucci MJ, Alford EL, Lowry LD, Keane WM, Sataloff RT. Endoscopic management of a giant cholesterol cyst of the petrous apex. Skull Base Surg. 1994;4:52–8. doi: 10.1055/s-2008-1058990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Presutti L, Villari D, Marchioni D. Petrous apex cholesterol granuloma: transsphenoid endoscopic approach. J Laryngol Otol. 2006;120:e20. doi: 10.1017/S0022215106009121. [DOI] [PubMed] [Google Scholar]
- 31.Griffith AJ, Terrell JE. Transsphenoid endoscopic management of petrous apex cholesterol granuloma. Otolaryngol Head Neck Surg. 1996;114:91–4. doi: 10.1016/S0194-5998(96)70289-9. [DOI] [PubMed] [Google Scholar]
- 32.Edkins O, Lubbe D, Taylor A. Endoscopic trans-sphenoidal drainage of petrous apex cholesterol granulomas. S Afr J Surg. 2010;48:94–6. [PubMed] [Google Scholar]
- 33.Tomazic PV, Nemetz U, Koele W, Walch C, Braun EM, Hammer GP, et al. Cholesterol granulomas of the petrous apex; endonasal endoscopic approach. B-ENT. 2013;9:263–7. [PubMed] [Google Scholar]
- 34.Gianoli GJ, Amedee RG. Hearing results in surgery for primary petrous apex lesions. Otolaryngol Head Neck Surg. 1994;111:250–7. doi: 10.1016/S0194-5998(94)70599-2. [DOI] [PubMed] [Google Scholar]
- 35.Balachandran R, Tsai BS, Ramachandra T, Noble JH, Dawant BM, Labadie RF, et al. Minimally invasive image-guided access for drainage of petrous apex lesions: a case report. Otol Neurotol. 2014;35:649–55. doi: 10.1097/MAO.0000000000000328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carlos C, Parkes W, James AL. Application of 3-dimensional Modeling to Plan Totally Endoscopic Per-Meatal Drainage of Petrous Apex Cholesterol Granuloma. Otolaryngol Head Neck Surg. 2015;153:1074–5. doi: 10.1177/0194599815607851. [DOI] [PubMed] [Google Scholar]
- 37.Caversaccio M, Panosetti E, Ziglinas P, Lukes A, Hausler R. Cholesterol granuloma of the petrous apex: benefit of computer-aided surgery. Eur Arch Otorhinolaryngol. 2009;266:47–50. doi: 10.1007/s00405-008-0719-4. [DOI] [PubMed] [Google Scholar]
- 38.Fong BP, Brackmann DE, Telischi FF. The long-term follow-up of drainage procedures for petrous apex cholesterol granulomas. Arch Otolaryngol Head Neck Surg. 1995;121:426–30. doi: 10.1001/archotol.1995.01890040050008. [DOI] [PubMed] [Google Scholar]
- 39.Ghorayeb BY, Jahrsdoerfer RA. Subcochlear approach for cholesterol granulomas of the inferior petrous apex. Otolaryngol Head Neck Surg. 1990;103:60–5. doi: 10.1177/019459989010300109. [DOI] [PubMed] [Google Scholar]
- 40.Giddings NA, Brackmann DE, Kwartler JA. Transcanal infracochlear approach to the petrous apex. Otolaryngol Head Neck Surg. 1991;104:29–36. doi: 10.1177/019459989110400107. [DOI] [PubMed] [Google Scholar]
- 41.Goldofsky E, Hoffman RA, Holliday RA, Cohen NL. Cholesterol cysts of the temporal bone: diagnosis and treatment. Ann Otol Rhinol Laryngol. 1991;100:181–7. doi: 10.1177/000348949110000303. [DOI] [PubMed] [Google Scholar]
- 42.Isaacson B, Kutz JW, Jr, Mendelsohn D, Roland PS. CT venography: use in selecting a surgical approach for the treatment of petrous apex cholesterol granulomas. Otol Neurotol. 2009;30:386–91. doi: 10.1097/MAO.0b013e31819d3355. [DOI] [PubMed] [Google Scholar]
- 43.Kim HC, An YS, Ahn JH. Petrous apex cholesterol granuloma presenting as endolymphatic hydrops: a case report. Clin Exp Otorhinolaryngol. 2009;2:151–4. doi: 10.3342/ceo.2009.2.3.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mattox DE. Endoscopy-assisted surgery of the petrous apex. Otolaryngol Head Neck Surg. 2004;130:229–41. doi: 10.1016/j.otohns.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 45.Mosnier I, Cyna-Gorse F, Grayeli AB, Fraysse B, Martin C, Robier A, et al. Management of cholesterol granulomas of the petrous apex based on clinical and radiologic evaluation. Otol Neurotol. 2002;23:522–8. doi: 10.1097/00129492-200207000-00022. [DOI] [PubMed] [Google Scholar]
- 46.Pietrantonio A, D’Andrea G, Famà I, Volpini L, Raco A, Barbara M. Usefulness of image guidance in the surgical treatment of petrous apex cholesterol granuloma. Case Rep Otolaryngol. 2013 doi: 10.1155/2013/257263. doi: 10.1155/2013/257263. Epub 2013 Oct 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sanna M, Dispenza F, Mathur N, De Stefano A, De Donato G. Otoneurological management of petrous apex cholesterol granuloma. Am J Otolaryngol. 2009;30:407–14. doi: 10.1016/j.amjoto.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 48.Carlton DA, Iloreta AM, Chandrasekhar SS. Endoscopic-assisted transmastoid decompression of petrous apex cholesterol granuloma. Laryngoscope. 2017;127:496–9. doi: 10.1002/lary.26100. [DOI] [PubMed] [Google Scholar]
- 49.Gilsbach JM, Sure U, Mann W. The supracondylar approach to the jugular tubercle and hypoglossal canal. Surg Neurol. 1998;50:563–70. doi: 10.1016/S0090-3019(97)00378-9. [DOI] [PubMed] [Google Scholar]
- 50.Gherini SG, Brackmann DE, Lo WW, Solti-Bohman LG. Cholesterol granuloma of the petrous apex. Laryngoscope. 1985;95:659–64. doi: 10.1288/00005537-198506000-00005. [DOI] [PubMed] [Google Scholar]
- 51.Graham MD, Kemink JL, Latack JT, Kartush JM. The giant cholesterol cyst of the petrous apex: a distinct clinical entity. Laryngoscope. 1985;95:1401–6. doi: 10.1288/00005537-198511000-00021. [DOI] [PubMed] [Google Scholar]
- 52.Brodkey JA, Robertson JH, Shea JJ, 3rd, Gardner G. Cholesterol granulomas of the petrous apex: combined neurosurgical and otological management. J Neurosurg. 1996;85:625–33. doi: 10.3171/jns.1996.85.4.0625. [DOI] [PubMed] [Google Scholar]
- 53.Eisenberg MB, Haddad G, Al-Mefty O. Petrous apex cholesterol granulomas: evolution and management. J Neurosurg. 1997;86:822–9. doi: 10.3171/jns.1997.86.5.0822. [DOI] [PubMed] [Google Scholar]
- 54.Cristante L, Puchner MA. A keyhole middle fossa approach to large cholesterol granulomas of the petrous apex. Surg Neurol. 2000;53:64–70. doi: 10.1016/S0090-3019(99)00163-9. [DOI] [PubMed] [Google Scholar]
- 55.Hoa M, House JW, Linthicum FH., Jr Petrous apex cholesterol granuloma: maintenance of drainage pathway, the histopathology of surgical management and histopathologic evidence for the exposed marrow theory. Otol Neurotol. 2012;33:1059–65. doi: 10.1097/MAO.0b013e31825d63ea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Thedinger BA, Nadol JB, Jr, Montgomery WW, Thedinger BS, Greenberg JJ. Radiographic diagnosis, surgical treatment, and long-term follow-up of cholesterol granulomas of the petrous apex. Laryngoscope. 1989;99:896–907. doi: 10.1288/00005537-198909000-00003. [DOI] [PubMed] [Google Scholar]
- 57.Rihani J, Kutz JW, Jr, Isaacson B. Hearing Outcomes after Surgical Drainage of Petrous Apex Cholesterol Granuloma. J Neurol Surg B Skull Base. 2015;76:171–5. doi: 10.1055/s-0034-1395489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fraenkel J, Hunt JR, Woolsey G, Elsberg CA. I. Contribution to the Surgery of Neurofibroma of the Acoustic Nerve: With Remarks on the Surgical Procedure. Ann Surg. 1904;40:293–319. doi: 10.1097/00000658-190409000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yawn RJ, Sweeney AD, Carlson ML, Wanna GB. Spontaneous resolution of a petrous apex cholesterol granuloma. Am J Otolaryngol. 2016;37:452–4. doi: 10.1016/j.amjoto.2015.10.013. [DOI] [PubMed] [Google Scholar]