Abstract
Background
Occupational and environmental exposures to toxic metals are established risk factors for the development of hypertension and kidney disease in adults. There is some evidence of developmental metal nephrotoxicity in children and from animal studies; however, to our knowledge no previous studies have examined associations between co-exposure to nephrotoxic environmental metals and children’s kidney health.
Objective
The objective of this study was to assess the association between co-exposure to lead (Pb), cadmium (Cd), mercury (Hg), and arsenic (As), measured in urine and blood, and kidney parameters in US adolescents.
Methods
We performed a cross-sectional analysis of a subsample of 2,709 children aged 12–19 participating in the National Health and Nutrition Examination Survey (NHANES) between 2009 and 2014. We analyzed urine levels of 4 nephrotoxic metals selected a priori (As, Cd, Pb and Hg), Umix, and 3 nephrotoxic metals in blood (Cd, Pb, and Hg), Bmix, using a weighted quantile sum (WQS) approach. We applied WQS regression to analyze the association of Bmix and Umix with estimated glomerular filtration rate (eGFR), serum uric acid (SUA), urine albumin, blood urea nitrogen (BUN), and systolic blood pressure (SBP), adjusting for sex, race/ethnicity, age, head of household’s education level, height, BMI, serum cotinine, and NHANES cohort year. Umix and urine albumin models were also adjusted for urine creatinine, and Bmix models were also adjusted for fish consumption. Subanalyses included stratification by sex and an arsenic-only model including six speciated forms of As measured in urine.
Results
In WQS regression models, each decile increase of Umix was associated with 1.6% (95%CI: 0.5, 2.8) higher BUN, 1.4% (95%CI: 0.7, 2.0) higher eGFR, and 7.6% (95%CI: 2.4, 13.1) higher urine albumin. The association between Umix and BUN was primarily driven by As (72%), while the association with eGFR was driven by Hg (61%), and Cd (17%), and the association with urine albumin was driven by Cd (37%), Hg (33%), and Pb (25%).There was no significant relationship between Umix and SUA or SBP. In WQS models using the combined blood metals, Bmix, each decile increase of Bmix was associated with 0.6% (95%CI: 0.0, 1.3) higher SUA; this association was driven by Pb (43%), Hg (33%), and Cd (24%) and was marginally significant (p=0.05). No associations were observed between Bmix and urine albumin, eGFR, BUN, or SBP.
Conclusions
The findings suggest metals including As, Pb, Hg, Cd and their combinations may affect renal parameters, although potential reverse causation cannot be ruled out due to the cross-sectional study design. Implications of early life low-level exposure to multiple metals on kidney function may have far-reaching consequences later in life in the development of hypertension, kidney disease, and renal dysfunction. Longitudinal studies should further evaluate these relationships.
Keywords: arsenic, lead, cadmium, mercury, mixture, childhood, kidney, systolic blood pressure, blood urea nitrogen, glomerular filtration
1. INTRODUCTION
Hypertension and renal diseases are prevalent in the US population and worldwide, and have generally increased in children and adolescents since the 1980s [1]. While there is some evidence of overall stabilization in recent years, there remains a high prevalence among minority children [2]. Moreover, poorer kidney function is a major risk factor for cardiovascular disease, a leading cause of mortality in the US [3]. A growing body of evidence supports that adult cardiorenal health and disease status may be predicted by childhood and adolescence, including hypertensive blood pressure trajectories that emerge as early as 7 years of age and persist into poorer cardiometabolic risk indicators in adulthood [4, 5]. A recent study of 2,658,238 adolescents in Israel showed that well-established hypertension during adolescence was associated with a doubling of the risk for future end-stage renal disease in an otherwise healthy population [6]. Additionally, the American Heart Association (AHA) estimates that in 2015, 85.7 million American adults ages 20 and older had high blood pressure with associated direct and indirect costs (annual average) estimated at $53.2 billion [7]. Thus, the potential burden of adolescent kidney dysfunction presents both immediate and later-life health concerns, and prevention of early life environmentally-associated kidney disease and dysfunction wields immense public health and economic advantages. Yet, the contributions of low-level environmental toxicant exposures on adolescent cardiorenal health and function are relatively understudied [8–10].
Toxic metals are paradigmatic nephrotoxicants that are understudied in human populations, and no prior studies have examined the mixed or additive effects of combined exposures with early life kidney parameters. It is estimated that in adult populations arsenic (As) and lead (Pb) exposures account for 7% and 5% of the population attributable risk for high blood pressure [11]. This is important since essential hypertension among children (in the absence of renal abnormality) is multi-factorial in origin attributable to complex cardiovascular and renal factors [12]. Further, single toxicant exposures (i.e. Pb and As) provide some evidence for developmental nephrotoxicity demonstrated by altered estimated glomerular filtration rate (eGFR) and other kidney biomarkers such as serum creatinine and urea in children [13, 14]. The absence of studies considering metal co-exposure in the literature is important because the effects of putative toxicants may be additive in combination, or otherwise interdependent on co-exposures.
In this cross-sectional study we examined urine and blood metal levels in US adolescents age 12–19 participating in the National Health and Nutrition Examination Survey (NHANES). We selected 4 primary toxic metals (As, Cd, Pb, and Hg) based on 1) demonstrated developmental nephrotoxicity in animal models [15–17], and 2) evidence from existing literature that identified associations with kidney parameters in children [9, 10]. We applied a two-tiered approach in our modeling strategy, first developing a mixtures strategy to consider the combined effects of multiple exposures as measured in blood and urine in this sample, followed by a survey-weighted single chemical analysis. The former approach enables evaluation of exposure-related effects in the mixed context in which they occur, while the survey-weighted latter approach may yield effect estimates more generalizable to a larger population. We applied weighted quantile sum (WQS) regression in our mixtures modeling approach, which accounts for correlation among the components of a mixture via supervised dimensionality-reduction [18, 19]. This ensemble approach assesses the combined effects of multiple exposures via an empirically-weighted index which both standardizes exposure concentrations and weights the contribution of individual mixture components according to their relation to the kidney parameters of interest, which included eGFR, systolic blood pressure (SBP), blood urea nitrogen (BUN), serum uric acid (SUA), and urine albumin.
2. METHODS
2.1. Study population
We analyzed NHANES data from 2009–2014, which were the study years with available blood and urine metals exposure and renal outcome data. Our analyses included eGFR, BUN, SUA, urine albumin and SBP measures. Participant selection is shown in Figure S1. Only subjects with complete data for all covariates (head of household education, race/ethnicity, BMI, height, sex, age, fish consumption, and serum cotinine), either complete data for the 4 metals in urine (As, Cd, Pb and Hg) or 3 metals in blood (Cd, Pb, and Hg), and at least one of the kidney outcomes were included in our analyses. We analyzed renal outcomes as continuous variables rather than categorically by clinical cutoffs due to the low prevalence of clinical kidney dysfunction in this cohort, and because our primary interest is in subclinical population-level shifts in adolescents. For example, we did not exclude individuals based on low eGFR since a single eGFR measurement is not sufficient to diagnose kidney disease (only 5 participants had eGFR levels < 75 mL/min/1.73m2). Our study included 2,709 adolescents aged 12–19 years who participated during the 2009–2010, 2011–2012, and 2013–2014 cycles. Our study used publicly available data and was exempted from review by the Icahn School of Medicine at Mount Sinai’s Institutional Review Board (#1702145). Statistical analyses were conducted in SAS 9.4.
2.2. Laboratory analyses
Our analysis included metal exposures and renal parameters measured over three NHANES cycle years: 2009–2010, 2011–2012, and 2013–2014. Briefly, serum creatinine, BUN and SUA were analyzed on a Beckman UniCel DxC800 Synchron by Collaborative Laboratory Services, LLC. Serum creatinine was analyzed using the Jaffe rate method. SUA was assessed using a timed endpoint method. Urea nitrogen was assessed using an enzymatic conductivity rate method. Urine albumin was analyzed using a fluorescent immunoassay method on a Sequoia-Turner digital fluorometer at the University of Minnesota. Urine creatinine was analyzed via an enzymatic method on a Roche Cobas 600 Analyzer at the University of Minnesota. Clinical reference ranges for all kidney parameters, as well as the percentage of the overall cohort above or below these ranges are presented in Table A3.
Whole blood Pb, Cd, and Hg as well as spot urine As, Pb, Cd, and Hg were analyzed via inductively coupled plasma-dynamic reaction mass spectrometry (ICP-DRC-MS) at the CDC’s National Center for Environmental Health. In addition to total urinary As, speciated As including arsenous acid (As(III)), arsenic acid (As(V)), arsenobetaine (AsB), arsenocholine (AsC), dimethylarsinic acid (DMA), and monomethylarsonic acid (MMA) were analyzed in urine using high performance liquid chromatography coupled with ICP-DRC-MS. Limits of detection (LOD) for blood and urinary metals, as well as the number and percent of samples below the LOD are presented in Table A4. Values below the LOD were imputed as the lowest LOD value for each metal.
2.3. Model selection and rationale
Our primary analysis included two WQS indices, stratified by tissue matrices: 1) Umix, comprised of As, Cd, Pb and Hg levels measured in urine, and 2) Bmix, comprised of Cd, Pb, and Hg levels measured in blood. We prioritized these metals of interest based on developmental nephrotoxicity in animal models [15–17], as well as some epidemiological evidence in children [9, 10]. We additionally included As (measured in urine only) because of mounting recent evidence that As is nephrotoxic, supported by epidemiological studies [20]. Arsenic is rapidly cleared from blood, with approximately 70% of As excreted in urine, and therefore urine is the preferred matrix for capturing relevant As exposure [21]. Ultimately, our selection of total As in the main urine index (Umix) rather than one or more As species in our multi-metal index was based on several factors. The data represent a generally healthy population and inclusion of select or a single species of As may underestimate a chronic exposure. By selecting total As (comprised of all species) in human urine we consider this proxy measure assessment of combined exposure and metabolic processes [22]. We also pursued a subanalysis that examined an arsenic-only index comprised of a mixture of 6 arsenical species measured in urine (see Methods 2.6).
2.4. Single chemical analyses
We applied survey-weighted linear regression analyses for each of 4 metals in urine (As, Cd, Pb and Hg) and 3 in blood (Cd, Pb, and Hg), with separate models constructed for each metal. We examined associations between individual metals and eGFR, SUA, BUN, SBP, and urinary albumin. All models were adjusted for sex, race/ethnicity, age, serum cotinine (as a biomarker of nicotine exposure), head of household’s education level, height, BMI, and NHANES cohort year. Additionally, models with urinary metals were adjusted for urinary creatinine concentration (included as a covariate), and the model with Hg in blood was adjusted for fish consumption within the last 30 days, and blood metals models with urine albumin as an outcome were adjusted for urinary creatinine. Metal concentrations were deciled for these analyses. For all outcomes, natural log-transformed kidney parameters were normally distributed. Since height was used in the calculation of eGFR, it was not included as a covariate in these models. We calculated eGFR with serum creatinine and height measures using the Schwartz formula eGFR= [k × height in cm/ serum creatinine in mg/dL], where k is equal to 0.7 for adolescent boys age 13 years and older, and 0.55 for boys under 13 years old and adolescent girls [23]. For SBP we used the average of the first three measures (out of four available, since the 4th measure was inconsistently obtained); subjects were excluded from SBP analyses if any of the first three measures were missing. Only subjects with complete data were included in a given model. Models were implemented in SAS 9.4 (Cary, NC) via the SURVEYREG procedure. The estimation of survey-weighted means describing metal levels and kidney parameters was similarly implemented in SAS 9.4 via the SURVEYMEANS procedure.
2.5. WQS statistical analyses
To estimate the combined effects of co-occurring metals exposure, while also assessing the contributing effects of individual metals, we employed a “mixtures” approach based on WQS regression analysis, which was developed to assess combined and discrete effects of multiple predictors in the context of correlated high-dimensional mixtures. WQS regression empirically estimates an index that identifies the influential exposure variables with non-negligible weights, and tests for associations between the exposure index and an outcome in a traditional linear framework, as: g(μ) = β0 + β1 WQS + z′φ. To assess potentially nonlinear trends, this model was extended to include an additional quadratic term for the WQS index.
Here, g(μ) reflects a nonlinear link function allowing generalization to continuous, binary, and other distributions, though in this study only continuous outcomes were considered. As in typical regression approaches, β0 reflects the model intercept, while β1 reflects the parameter estimate for the co-exposure index, represented here as WQS; the significance of this parameter thus reflects a straightforward test of associations between the co-exposure index and the outcome. The WQS index is constructed such that WQS = ∑ wiqij where wi indicates a vector of empirically-estimated weights for each mixture component, and qij indicates the quantiled rank assigned to each subject per variable. In these analyses, as with the single chemical analyses, concentrations were deciled, such that qi = 0 − 9. Deciles were selected based on examination of the distribution of residuals for normality in models using varying quantile ranks of the exposures. Weight estimations were calculated across 100 bootstrapped samples, with weights constrained to sum to 1 and to vary between 0 and 1 to facilitate comparison.
We created two WQS indices stratified by tissue matrices: Umix, comprised of As, Cd, Pb and Hg levels measured in urine, and Bmix, comprised of Cd, Pb, and Hg levels measured in blood (As was not measured in blood). WQS analyses were split into training and validation sets (40:60), where the training dataset was used to estimate variable weights, and the validation dataset was used to test mixture significance. As with single chemical analyses, all outcome measures (eGFR, SUA, BUN, SBP, and urinary albumin) were natural log transformed. Models were adjusted for sex, race/ethnicity, age, head of household’s education level, height, BMI, NHANES cohort year, and serum cotinine levels (to account for tobacco smoke exposure [24]) except in cases where outcomes were calculated with covariate terms. Additionally, Umix models were adjusted for urinary creatinine concentration included as a covariate, and Bmix models adjusted for fish consumption within the last 30 days. As with the single chemical analyses, height was not used as a covariate for eGFR since this variable was a component of the outcome calculation. The percent change in a given outcome per unit change in the WQS index was calculated as (eBetaWQS-1) × 100. Supplemental analyses examined the relationships stratified by sex.
2.6. Urinary As speciated mixture and BUN
To further examine the association of BUN and Umix, we examined speciated As in urine, including arsenous acid (As(III)), arsenic acid (As(V)), arsenobetaine (AsB), arsenocholine (AsC), dimethylarsinic acid (DMA), and monomethylarsonic acid (MMA). Trimethylarsine oxide was excluded from analysis since this measure was not available for subjects in the NHANES 2013–14 cohort. The six speciated As measures were deciled and analyzed as a mixture for associations with BUN using WQS regression, as previously described. As in prior analyses, the model was adjusted for urinary creatinine, sex, race, age, head of household’s education level, height, BMI, NHANES cohort year, and cotinine in serum.
3. RESULTS
Overall demographics of the 2,709 NHANES participants included in this study are shown in Table 1. The average age was 15.4 years and 48% were female. Approximately 50% of participants reported education level for head of household as at least some college education. Overall, 45% of participants indicated that they consumed fish within the last 30 days. Survey-weighted descriptive statistics for renal outcomes and metal levels by NHANES cohort year are shown in Table 2. Based on the clinical cutoffs and the single timepoint renal measurements for eGFR, SUA, BUN, and ACR, <0.5% of the cohort were outside of normal range for eGFR, 8% were outside of normal range for SUA, 8% were outside of normal range for BUN, and 18% are in the range of mild to moderately increased ACR with only 1% severely increased ACR (Table A3).
Table 1. Demographics for 2,709 adolescents aged 12–19 years participating in NHANES 2009–2014.
The table below details the mean (SD) or percentage of cohort for each variable are shown by NHANES cycle year and overall.
| NHANES cycle year |
||||
|---|---|---|---|---|
| 2009–10 n=1,102 | 2011–12 n=1,015 | 2013–14 n=592 | Overall n=2,709 | |
| Age (yrs) | 15.5 (2.2) | 15.4 (2.3) | 15.4 (2.3) | 15.4 (2.3) |
| Height (cm) | 165.8 (9.8) | 165.4 (9.7) | 164.6 (9.7) | 165.4 (9.8) |
| BMI (kg/m2) | 24.2 (5.8) | 24 (6.1) | 24.6 (6.8) | 24.2 (6.2) |
| Cotinine, serum (ng/mL) | 14.5 (51.4) | 13.4 (58.8) | 12.4 (52.4) | 13.6 (54.5) |
| Sex | ||||
| Male | 54% | 52% | 48% | 52% |
| Female | 46% | 48% | 52% | 48% |
| Race | ||||
| Hispanic | 39% | 29% | 37% | 35% |
| Black | 22% | 30% | 21% | 25% |
| White | 32% | 22% | 27% | 27% |
| Other | 7% | 19% | 16% | 13% |
| Parental Education | ||||
| HS Grad or less | 53% | 51% | 51% | 52% |
| Some College | 29% | 28% | 30% | 29% |
| College Grad | 18% | 21% | 19% | 19% |
| Fish consumption in last 30 days | 42% | 49% | 44% | 45% |
Table 2. Survey-weighted renal outcomes and metal levels for 2,709 adolescents aged 12–19 years participating in NHANES 2009–2014.
The mean (SD) of cohort for each outcome and exposure variable are shown by NHANES cycle year and overall.
| NHANES cycle years |
||||
|---|---|---|---|---|
| 2009–10 n=1,102 | 2011–12 n=1,015 | 2013–14 n=592 | Overall n=2,709 | |
| Outcomes | ||||
| Avg Sys Blood Pressure (mmHg) | 108.51 (21.79) | 109.49 (19.73) | 108.19 (13.54) | 108.84 (19.43) |
| eGFR (mL/min/1.73 m2) | 145.41 (70.72) | 144.17 (49.16) | 142.55 (35.32) | 144.32 (56.67) |
| Blood urea nitrogen (mg/dL) | 10.48 (5.15) | 10.63 (4.43) | 10.78 (7.47) | 10.6 (5.49) |
| Uric acid (mg/dL) | 5.08 (1.69) | 5.05 (1.71) | 5.12 (1.67) | 5.07 (1.71) |
| Albumin creatinine ratio (mg/g) | 30.8 (255.55) | 27.13 (109.93) | 31.55 (159.35) | 29.47 (186.00) |
| Urine albumin (ug/mL) | 37.78 (293.69) | 33.77 (147.14) | 34.3 (122.43) | 35.44 (211.44) |
| Urinary metals | ||||
| Cadmium (ug/L) | 0.10 (0.08) | 0.10 (0.15) | 0.08 (0.14) | 0.09 (0.13) |
| Lead (ug/L) | 0.41 (0.45) | 0.36 (0.54) | 0.29 (0.48) | 0.36 (0.52) |
| Mercury (ug/L) | 0.53 (0.97) | 0.42 (0.93) | 0.27 (0.8) | 0.41 (0.94) |
| Arsenic, total (ug/L) | 10.66 (19.14) | 18.28 (93.28) | 12.16 (36.71) | 13.62 (61.96) |
| Blood metals | ||||
| Cadmium (ug/L) | 0.20 (0.56) | 0.20 (0.35) | 0.19 (0.45) | 0.20 (0.46) |
| Lead (ug/dL) | 0.77 (0.86) | 0.65 (1.11) | 0.64 (1.08) | 0.70 (1.01) |
| Mercury, total (ug/L) | 0.80 (1.88) | 0.62 (1.42) | 0.55 (1.12) | 0.68 (1.56) |
3.1. Association of urine metals co-exposure with renal parameters
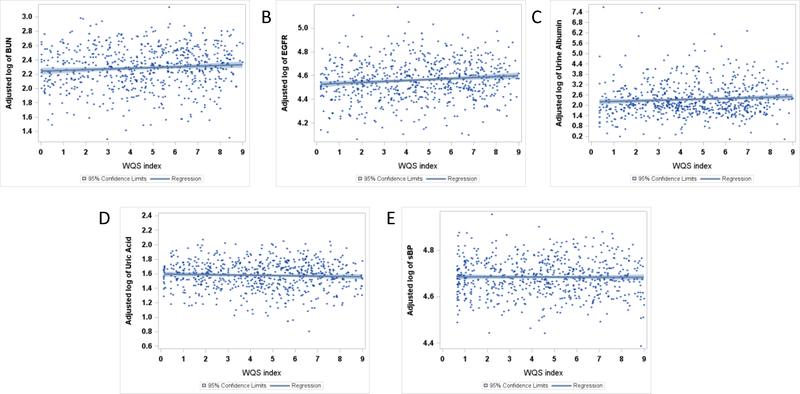
WQS regression analyses of the index Umix, the mixture of Hg, Pb, As, and Cd levels from urine, with each of the 5 renal parameters, showed evidence of association with BUN, eGFR, and urinary albumin, but not with SUA or SBP (Table 3). Specifically, per each decile increase in Umix, BUN was 1.6% higher (95%CI: 0.5, 2.8), primarily due to the contribution of As (72%) and Pb (14%) (Figure 1a). Per each decile increase in Umix, eGFR was 1.4% (95%CI: 0.7, 2.0) higher, attributable to the contribution of Hg (61%), Cd (17%), and As (13%) (Figure 1b). Per each decile increase of Umix, we found urine albumin was 7.6% (95%CI: 2.4, 13.1) higher. This relationship was driven by Cd (37%), Hg (33%), and Pb (25%) (Figure 1c).
Table 3. Association of Umixor Bmixmetals indices with renal parameters.
Results of WQS regression analysis per renal outcome for the urine and blood mixtures (Umix and Bmix). Parameter estimates (βWQS), 95% confidence intervals (CI), and p-values of the WQS mixture reported for each model. Component weights reported as percentages for mixtures with significant effects (n/a: not applicable).
| Mixture | Outcome | WQS Mixture result | Component Weights | |
|---|---|---|---|---|
| Umix | BUN | 0.0159 (0.005, 0.027) p = 0.006 | As Pb Hg Cd |
72% 14% 11% 3% |
| eGFR | 0.0137 (0.007, 0.020) p <0.0001 | Hg Cd As Pb |
61% 17% 13% 9% |
|
| SUA | −0.0073 (−0.017, 0.002) p = 0.130 | n/a | ||
| SBP | −0.0007 (−0.005, 0.003) p = 0.710 | n/a | ||
| Urine albumin | 0.0731 (0.024, 0.123) p = 0.004 | Cd Hg Pb As |
37% 33% 25% 6% |
|
| Bmix | BUN | 0.0040 (−0.003, 0.011) p = 0.265 | n/a | |
| eGFR | −0.0032 (−0.009, 0.002) p = 0.241 | n/a | ||
| SUA | 0.0063 (0.000, 0.013) p = 0.052 | Pb Hg Cd |
43% 33% 24% |
|
| SBP | 0.0010 (−0.001,0.003) p = 0.338 | n/a | ||
| Urine albumin | 0.0035 (−0.025, 0.031) p = 0.807 | n/a | ||
Figure 1.
Covariate adjusted plots with confidence limits for the mean predicted value of the metals index in urine, Umix, and the ln-transformed measures for BUN (A), eGFR (B), urine albumin (C), SUA (D), and SBP (E).
In comparison, survey-weighted single chemical analyses suggested that BUN was increased by 1.2% (95%CI: 0.0, 2.5) with each decile increase of urinary As, but not Pb. Survey weighted single chemical analyses showed eGFR was 0.6% (95%CI: 0.1, 1.0) and 1.0% (95%CI: 0.4, 1.5) higher with each decile increase in Hg and Pb, respectively, with no significant associations observed with As and Cd. Similarly, urine albumin showed a 6.9% (95%CI: 2.7, 11.3) increase per decile increase Cd and a 3.1% (95%CI: 0.3, 6.0) increase per decile increase of Pb. We found no significant association between urine Hg with albumin levels.
3.2. Association of blood metals co-exposure with renal parameters
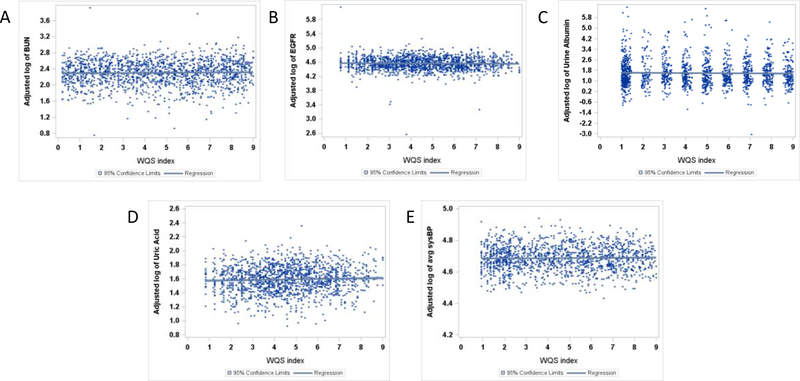
Next, we examined associations of Bmix, comprised of Hg, Pb, and Cd measured in blood, with each of the 5 renal parameters. The index Bmix was associated with SUA, but not urine albumin, BUN, eGFR, or SBP (Table 3). Specifically, per each decile increase in Bmix, SUA was 0.6% (95%CI: 0.0, 1.3) higher, with weights indicating Pb (43%) Hg (33%) and Cd (24%) all contributed to this association.
Survey-weighted single chemical analyses indicated that SUA was 0.8% (95%CI: 0.5, 1.2) higher per decile increase in blood Pb, the highest contributing metal to the Bmix mixture, but we found no significant association with blood Hg or Cd (Table A1). Additionally, a decile increase in blood Pb was associated with 0.5% (95%CI: −0.9, 0.0) lower eGFR.
3.3. Stratification by sex
Examination of potential sex-specific associations with Umix or Bmix indicated that co-exposure effects differed between males and females (Table 4). A significant association between BUN and Umix was observed among males only, where each decile increase of Umix was associated with 2.6% (95%CI: 1.2, 4.0) higher BUN. Conversely, a significant association between eGFR and Umix, was observed only in females, where eGFR was 2.0% (95%CI: 0.8, 3.2) higher for each decile increase of Umix. Similarly, the association of SUA and Bmix was present in females, with 1.7% (95%CI: 0.9, 2.5) higher SUA levels for each decile increase in Bmix, but this association was not significant among males. A positive association of urine albumin and Umix was detected in both males and females, with a respectively higher urine albumin of 12.5% (95%CI: 4.9, 20.7) and 11.8% (95% CI: 4.0, 20.2) for each decile increase of Umix.
Table 4. Association of urine or blood metals indices with renal parameters – stratified by sex.
Results of WQS regression analyses stratified by sex per renal outcome. The parameter estimates for the WQS mixture (βWQS) is reported in bold for significant WQS effects. The components with the highest weights are reported for mixtures with significant effects (n/a: not applicable).
| Mixture | Outcome | Sex | N | βwQs (95%CI) | p-value | Components with highest weight |
|---|---|---|---|---|---|---|
| Full Cohort |
1,121 | −0.0073 (−0.017, 0.002) | 0.133 | n/a | ||
| SUA | Female |
555 | −0.0025 (−0.016, 0.011) | 0.729 | n/a | |
| Male | 566 | −0.0013 (−0.018, 0.016) | 0.878 | n/a | ||
| Full Cohort |
1,130 | 0.0731 (0.024, 0.123) | 0.004 | Cd, Hg, Pb | ||
| Urine albumin | Female |
559 | 0.1116 (0.042, 0.185) | 0.002 | Cd, As, Pb | |
| Male | 571 | 0.1181 (0.048, 0.188) | 0.001 | Cd, Pb, As | ||
| Full Cohort |
1,121 | 0.0159 (0.005, 0.027) | 0.006 | As, Pb | ||
| Umix | BUN | Female |
555 | 0.0092 (−0.011,0.029) | 0.372 | n/a |
| Male | 566 | 0.0257 (0.012, 0.039) | <.001 | As | ||
| Full Cohort |
1,121 | 0.0137 (0.007, 0.020) | <.0001 | Hg, Cd, As | ||
| eGFR | Female |
555 | 0.0196 (0.008, 0.031) | 0.001 | As, Hg, Pb, Cd | |
| Male | 566 | 0.0129 (−0.001,0.027) | 0.072 | n/a | ||
| Full Cohort | 1,001 | −0.0007(−0.005, 0.003) | 0.713 | n/a | ||
| SBP | Female |
488 | −0.0016 (−0.007, 0.003) | 0.520 | n/a | |
| Male | 513 | 0.0001 (−0.005, 0.005) | 0.962 | n/a | ||
| Full Cohort |
2,634 | 0.0063 (0.000, 0.013) | 0.051 | Pb, Hg, Cd | ||
| SUA | Female |
1,266 | 0.0168 (0.009, 0.025) | <.0001 | Pb, Hg | |
| Male | 1,368 | 0.0076 (−0.001,0.016) | 0.073 | n/a | ||
| Full Cohort |
2,636 | 0.0035 (−0.025, 0.031) | 0.807 | n/a | ||
| Urine albumin | Female |
1,264 | −0.0101 (−0.056, 0.036) | 0.667 | n/a | |
| Male | 1,372 | −0.015 (−0.044, 0.014) | 0.313 | n/a | ||
| Full Cohort |
2,634 | 0.004 (−0.003, 0.011) | 0.265 | n/a | ||
| Bmix | BUN | Female |
1,266 | 0.0035 (−0.008, 0.015) | 0.540 | n/a |
| Male | 1,368 | 0.0051 (−0.007, 0.017) | 0.409 | n/a | ||
| Full Cohort |
2,634 | −0.0032 (−0.009, 0.002) | 0.241 | n/a | ||
| eGFR | Female |
1,266 | −0.0111 (−0.018, −0.004) | 0.002 | Pb, Cd | |
| Male | 1,368 | −0.0008 (−0.007, 0.005) | 0.795 | n/a | ||
| Full Cohort |
2,414 | 0.0010 (−0.001,0.003) | 0.338 | n/a | ||
| SBP | Female |
1,158 | −0.0013 (−0.004, 0.002) | 0.403 | n/a | |
| Male | 1,256 | −0.0011 (−0.005, 0.003) | 0.565 | n/a | ||
3.4. Association of speciated urinary As with BUN
To further examine the association of BUN with Umix, we examined an As-speciated WQS mixture that included speciated levels of As(III), As(V), AsB, AsC, DMA, and MMA in urine (Table A2). Each decile increase of the speciated urinary As index was associated with 2.2% (95%CI: 0.7, 3.8) higher BUN, driven by DMA (41%), AsC (33%), and AsB (24%).
4. DISCUSSION
Several studies have examined whether individual metal exposures are associated with adverse effects on kidney function, biomarkers of kidney damage, or blood pressure changes in children or adolescents (reviewed in [9, 10]); however, to our knowledge, this is the first study to examine the association between combined metals exposures (measured in blood or urine) and renal parameters in adolescents. Our modeling strategy was inherently two-tiered and stratified, in that we pursued both mixtures and single-chemical analyses, and discrete analyses of urine and blood exposure biomarkers. In these contexts, we identified relationships between adolescents’ co-exposures to toxic metals and renal parameters and found that: i) metals assessed in urine (e.g. As, Pb, Hg, and Cd) were associated with increased eGFR, BUN, and urine albumin; ii) blood levels of Pb, Hg, and Cd were associated with increased SUA; iii) there were sex-specific associations of urine metal levels (e.g. Cd and Pb) with increased eGFR, BUN, as well as with blood metals and increased SUA.
Our findings suggest an association between combined exposures to Hg, As, Cd, and Pb, as measured in urine, and higher eGFR. We observed positive associations with eGFR when urine metals were combined as an index of co-exposure, and when assessed separately. One explanation of the findings is that joint metals exposure may lead to glomerular hyperfiltration. Although prior studies of metals and glomerular filtration report mixed findings [25–29], there is some evidence of associations between trace metal exposure (to As, Cd, and Pb for example) and hyperfiltration [30–33]. Glomerular hyperfiltration is an absolute increase in GFR well described for diabetes, obesity, and other conditions associated with damage to the glomerular filtration barrier, as well as physiological processes such as high protein loading. Hyperfiltration may precede albuminuria clinically, and is often a precursor of chronic kidney disease (CKD) [34]. As evidenced in studies of adults [35], hyperfiltration may precede later life reduction in GFR and renal decline by several years. Thus, early life hyperfiltration may be an important indicator of lifelong renal health. Another possible interpretation of our findings is that the observed positive associations are indicative of reverse causality, previously observed for single urinary metal analyses in NHANES [33, 36]. In this case, reduced renal function may affect metal excretion. Our findings are similar to those reported by Buser et al. (2016) who showed that increased urinary Cd and Pb levels were associated with increased eGFR, whereas an inverse relationship was observed with Cd levels measured in blood [36]. Similarly, these authors concluded that this relationship may either reflect hyperfiltration due to renal function decline or an adverse effect of low-level Cd exposure on kidney function. Still, there is a need for future studies to examine the contributions of combined metals exposure to eGFR in prospective cohorts and populations, and to examine the longitudinal impact of early life hyperfiltration on adult renal function.
We also observed an association between co-exposure to As and Pb measured in urine with higher BUN. To our knowledge, no prior studies have analyzed the combined effect of these metals on serum urea in children or adolescents. In single-metal analyses, we observed a marginally significant association with urine As and BUN, but no association with Pb. These findings underscore the potential for unique health effects resulting from exposures to combinations of metals (i.e. metal co-exposures) that would otherwise be missed in single-metal analyses, and highlight the utility of WQS regression to detect complex associations. Additionally, our findings align with a study of rats treated with As via drinking water which showed increased BUN and serum creatinine, as well as elevated markers of liver toxicity [37]. In our analysis of the urinary “arsenic index”, urinary DMA, AsC, and AsB contributed the highest weights to the arsenic index associated with BUN. Interestingly, among these top-weighted components DMA and AsB were also described in a previous study of the NHANES population [22]. This supports the hypothesis that both organic and inorganic species may play a role in kidney dysfunction although the mechanisms remain unclear. Our findings further highlight the utility of the WQS co-exposure approach to detect complex associations.
In single-metal analyses, urine Pb and Cd concentrations were also associated with increased urine albumin. Two previous studies including adults ( ≥ 20 years) participating in NHANES 1999–2006 and 2007–2012 showed that chronic exposure to low levels of Cd was associated with changes in albumin and eGFR, and with coexposure to Pb, an increased risk for albuminuria and CKD [38, 39]. It is well-established that one of the earliest signs of Cd-induced renal damage is characterized by the presence of tubular proteinuria such as β2-microglobulin as well as glomerular proteinuria such as albuminuria [40–42]. Intriguingly, we observed a significant association between the urinary metals mixture and urine albumin in males mainly driven by Cd concentrations. However, prospective longitudinal studies are needed to clarify the link between Cd exposure and renal biomarkers in adolescents, including sex-specific associations.
We observed no association between metals exposure and SBP. This is consistent with a number of studies on childhood environmental exposure and BP reviewed elsewhere [9]. However, we and others have previously demonstrated associations between in utero Pb exposure and subsequent childhood BP [43, 44]. Thus, it may be that prenatal and early childhood metal exposures contribute to changes in SBP that are no longer influenced by exposures in adolescence; however, prospective longitudinal studies are needed to explore this possibility.
Our study had many strengths. We assessed the relationship between multiple metal co-exposures and adolescent kidney function in a large, nationally representative population with carefully collected exposure, covariate and kidney parameter data. We included a breadth of kidney parameters and adjusted for factors that can influence metals exposure or kidney parameters among adolescents. For example, we adjusted for cotinine, a biomarker of nicotine exposure [24] which can co-occur with exposures to Pb, Cd, and As and other nephrotoxic contaminants found in tobacco products. Further we stratified by sex to examine sex-specific effects of metals with kidney parameters. Our statistical approach included both blood and urine metal biomarkers, separated into indices based on the respective matrix, as well as a separate arsenic-only index of subspecies measured in urine. We acknowledge that these distinct exposure biomarker matrices (blood and urine) and the specific mixture subcomponents (metals or speciated arsenic compounds) represent varying toxicological profiles for metabolism and excretion. For example, the biological half-lives of Hg, Cd, and Pb are respectively longer (days to years [45, 46] [47] [48]) than that of As (hours) which is rapidly excreted in urine [21]. We note that given these considerations, a direct comparison of the two matrices is inappropriate. A strength of WQS regression is that the weights of individual metals shift with each model, allowing for different resultant indices reflecting effect sizes or potential underlying mechanism(s). Our findings support prior observations that associations of single metal exposures with renal outcomes is matrix-dependent [33]; we also observed this for mixed metal exposures. There are many ways we could have performed this analysis and we present the Umix and Bmix approach as one. We are further encouraged by the consistency of results in the individual metal analyses and mixtures based approaches. Our selection of a mixtures-based strategy allowed us to simultaneously assess the combined effects of multiple co-exposures in this sample, which we complement with survey-weighted single-chemical analyses which emphasize mostly consistent effects that may be expected to generalize to a larger population. The WQS statistical approach ensures that our results are not confounded by issues of exposure collinearity and enables identification of major contributors (i.e. higher weighted metals) to the association with kidney parameters.
Our study has several limitations. Due to the cross-sectional study design, we cannot draw conclusions regarding temporality of the relationship between metals exposure and renal outcomes. Reverse causality remains a concern given that as renal function declines, changes in the excretion rate of metals in urine could contribute to elevated urinary metal levels [49]. This is a particular concern for Cd and As since these metals are eliminated from the body primarily in urine [31, 36]. Thus, as discussed above, observed associations between higher levels of urinary metals and renal parameters could be interpreted in two ways: 1) exposure to these metals resulted in altered renal function, or 2) urine metal levels are elevated due to increased filtration among individuals who already have poorer renal function. Another limitation is that NHANES collected a single spot urine sample for metals and urine albumin analysis. We acknowledge that blood and urine sample collection times were not standardized in NHANES and that metal levels as well as renal biomarkers can fluctuate over time [50]. Similarly, due to the cross-sectional study design, measures were collected at only a single time point, and longitudinal assessment of eGFR or blood pressure are required to define clinical kidney disease or pediatric hypertension, respectively. Challenges remain in defining the best approaches in studies of metals and kidney function [51], and interpretation of findings is especially in limited cross-sectional studies where directionality cannot be determined.
5. CONCLUSION
To our knowledge, this is the first study to examine combined metal co-exposures measured in blood or urine with renal parameters in adolescents. The findings suggest metals including As, Pb, Hg, Cd and their combinations may affect renal parameters, although potential reverse causation cannot be ruled out due to the cross-sectional study design. Our results support previous observations that urinary metal assessment presents challenging interpretations in studies of kidney function. Implications of early life low-level exposure to multiple metals on kidney function may have far-reaching consequences later in life in the development of hypertension, kidney disease, and renal dysfunction. Longitudinal studies should further evaluate whether the combined effects of metals and other nephrotoxicants may be risk factors of early life subclinical dysfunction.
Supplementary Material
Figure 2.
Covariate adjusted plots with confidence limits for the mean predicted value of the metals index in blood, Bmix, and the ln-transformed measures for BUN (A), eGFR (B), urine albumin (C), SUA (D), and SBP (E).
Highlights.
Co-exposure to metals is associated with altered renal parameters in adolescents.
Urine As, Pb, Hg, and Cd were associated with increased eGFR, BUN, and albumin.
Combined blood levels of Pb, Hg, and Cd were associated with increased SUA.
Sex-specific effects were observed with increased eGFR, BUN, and SUA.
6. ACKNOWLEDGEMENTS
We thank the participants and staff of NHANES.
7 FUNDING: This work was supported in part by funding from the Mount Sinai Children’s Center Foundation and NIH/NIEHS: K99ES027508, R00ES027508, P30ES023515, R01ES013744, R01ES020268, and R01ES021357.
Abbreviations
- As(III)
arsenous acid (arsenite)
- As(V)
arsenic acid (arsenate)
- AsB
arsenobetaine
- AsC
arsenocholine
- As
arsenic
- BUN
blood urea nitrogen
- Cd
cadmium
- DMA
dimethylarsinic acid
- eGFR
estimated glomerular filtration rate
- Hg
mercury
- MMA
monomethylarsonic acid
- NHANES
National Health and Nutrition Examination survey
- Pb
lead
- SBP
systolic blood pressure
- SUA
serum uric acid
9. APPENDIX A
Table A1. Comparison of results from the survey-weighted single chemical analyses and WQS regression of the matrix specific metal mixtures per renal outcome for the urine and blood mixtures (Umix and Bmix).
The parameter estimate (β) is reported in bold for significant single chemical or WQS mixture effects. The components with the highest weights are reported for mixtures with significant effects (ns: not significant).
| Single chemical regression survey-weighted | WQS regression | ||||||
|---|---|---|---|---|---|---|---|
| Outcome | Matrix | Metal | βmetal (95%CI) | p-value | βWQS (95%CI) | p-value | Components with highest weight |
| SUA | Urine | Cd | −0.0006 (−0.007, 0.006) | 0.844 | −0.0073 (−0.017, 0.002) | 0.133 | n/a |
| Pb | −0.0054 (−0.012, 0.001) | 0.115 | |||||
| Hg | −0.0051 (−0.011,0.001) | 0.103 | |||||
| As | 0.0058 (−0.001,0.012) | 0.085 | |||||
| Blood | Cd | −0.0019 (−0.006, 0.003) | 0.409 | 0.0063 (0.000, 0.013) | 0.051 | Pb, Hg, Cd | |
| Pb | 0.0084 (0.005, 0.012) | <.0001 | |||||
| Hg | 0.0031 (−0.001,0.007) | 0.112 | |||||
| BUN | Urine | Cd | −0.0073 (−0.018, 0.003) | 0.171 | 0.0159 (0.005, 0.027 ) | 0.006 | As, Pb |
| Pb | 0.0040 (−0.005, 0.013) | 0.389 | |||||
| Hg | 0.0068 (−0.001,0.015) | 0.095 | |||||
| As | 0.0120 (0.000, 0.024) | 0.059 | |||||
| Blood | Cd | 0.0021 (−0.004, 0.008) | 0.491 | 0.004 (−0.003, 0.011) | 0.265 | n/a | |
| Pb | 0.0014 (−0.005, 0.008) | 0.657 | |||||
| Hg | 0.0059 (0.000, 0.012) | 0.069 | |||||
| eGFR | Urine | Cd | 0.0057 (0.000, 0.012) | 0.068 | 0.0137 (0.007, 0.020) | < 0001 | Hg Cd As |
| Pb | 0.0098 (0.004, 0.015) | 0.001 | |||||
| Hg | 0.0056 (0.001, 0.010) | 0.015 | |||||
| As | 0.0017 (−0.005, 0.008) | 0.595 | |||||
| Blood | Cd | −0.0025 (−0.007, 0.002) | 0.248 | −0.0032 (−0.009, 0.002) | 0.241 | n/a | |
| Pb | −0.0046 (−0.009, 0.000) | 0.049 | |||||
| Hg | −0.0008 (−0.005, 0.004) | 0.725 | |||||
| SBP | Urine | Cd | 0.0001 (−0.002, 0.003) | 0.914 | −0.0007 (−0.005, 0.003) | 0.713 | n/a |
| Pb | −0.0009 (−0.004, 0.002) | 0.507 | |||||
| Hg | 0.0001 (−0.002, 0.002) | 0.925 | |||||
| As | 0.0010 (−0.002, 0.004) | 0.495 | |||||
| Blood | Cd | 0.0010 (−0.001,0.003) | 0.216 | 0.001(−0.001,0.003) | 0.338 | n/a | |
| Pb | −0.0003 (−0.002, 0.001) | 0.712 | |||||
| Hg | 0.0002 (−0.002, 0.002) | 0.883 | |||||
| Urine Albumin | Urine | Cd | 0.0668 (0.026, 0.107) | 0.002 | 0.0731 (0.024, 0.123) | 0 004 | Cd Ha Pb |
| Pb | 0.0305 (0.003, 0.058) | 0.030 | |||||
| Hg | 0.0195 (−0.007, 0.053) | 0.219 | |||||
| As | 0.0149 (−0.022, 0.052) | 0.427 | |||||
| Blood | Cd | −0.0004 (−0.026, 0.025) | 0.974 | 0.0035 (−0.025, 0.031) | 0.807 | n/a | |
| Pb | −0.0072 (−0.028, 0.014) | 0.502 | |||||
| Hg | 0.0018 (−0.022, 0.026) | 0.881 | |||||
Table A2.
Linear associations between the speciated As index measured in urine and BUN. Parameter estimates, 95% CIs, p-values, and component weights of the As index are reported as percentages.
| Outcome | WQS Mixture result | Component Weights | |
|---|---|---|---|
| Blood Urea Nitrogen | βWQS= 0.0220, 95%CI (0.0068, 0.0372) p = 0.005 | DMA AsC AsB As(V) As(III) MMA |
41% 33% 24% 1% 0% 0% |
Table A3.
Number and percent of overall cohort outside of normal clinical range for eGFR, SUA, BUN, and ACR, along with the distribution of subjects within the overall cohort across clinical categories.
| N (% of overall cohort) | ||
| eGFR clinical cutoffs |
normal ≥ 75 mL/min/1.73m2 |
2684 (100%) |
|
abnormal < 75 mL/min/1.73m2 |
1 (0%) | |
|
chronic kidney disease ≤ 60 mL/min/1.73m2 |
4 (0%) | |
| SUA clinical cutoffs |
normal 12–18yo: 3.5–7.3 mg/dL 19yo: male: 3.6–8.4 mg/dL 19yo: female: 2.9–7.5 mg/dL |
2481 (92%) |
|
below normal range 12–18yo: < 3.5 mg/dL 19yo: male: < 3.6 mg/dL 19yo: female: < 2.9 mg/dL |
99 (4%) | |
|
above normal range 12–18yo: > 7.3 mg/dL 19yo: male: > 8.4 mg/dL 19yo: female: > 7.5 mg/dL |
109 (4%) | |
| BUN clinical cutoffs |
normal 12–15yo: 7–18 mg/dL 15–19yo: 6–23 mg/dL |
2484 (92%) |
|
below normal range 12–15yo: < 7 mg/dL 15–19yo: < 6 mg/dL |
190 (7%) | |
|
above normal range 12–15yo: > 18 mg/dL 15–19yo: > 23 mg/dL |
15 (1%) | |
| ACR clinical cutoffs |
normal < 20 mg/g |
2175 (81%) |
|
mild 20–30 mg/g |
165 (6%) | |
|
moderate 30–300 mg/g |
315 (12%) | |
|
severe >300 mg/g |
36 (1%) | |
Table A4.
Range of the lower limits of detection (LOD) for each of the urine and blood metals that comprise the Umix and Bmix mixtures and speciated urinary As, as reported by NHANES for cohorts between 2009 and 2014. Percent values below LOD are reported as a percentage of the combined cohort.
| Mixture | Component (units) | LOD (range) | % below LOD |
|---|---|---|---|
| Bmix | Blood cadmium (ug/L) | 0.10–0.16 | 46% |
| Blood lead (ug/dL) | 0.07–0.25 | 2% | |
| Blood mercury, total (ug/L) | 0.16–0.28 | 23% | |
| Umix | Urinary cadmium (ug/L) | 0.04–0.06 | 26% |
| Urinary lead (ug/L) | 0.03–0.10 | 4% | |
| Urinary mercury (ug/L) | 0.05–0.13 | 19% | |
| Urinary arsenic, total (ug/L) | 0.26–1.25 | 1% | |
| Speciated urinary As mixture | Urinary arsenous acid (ug/L) | 0.12–1.20 | 58% |
| Urinary arsenic acid (ug/L) | 0.79–1.00 | 98% | |
| Urinary arsenobetaine (ug/L) | 0.40–1.19 | 63% | |
| Urinary arsenocholine (ug/L) | 0.11–0.60 | 94% | |
| Urinary dimethylarsonic acid (ug/L) | 1.70–1.91 | 52% | |
| Urinary monomethylarsonic acid (ug/L) | 0.20–0.90 | 99% |
Footnotes
Competing Financial Interests:
There were no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
8. REFERENCES
- 1.Muntner P, et al. , Trends in blood pressure among children and adolescents. Jama, 2004. 291(17): p. 2107–13. [DOI] [PubMed] [Google Scholar]
- 2.Kit BK, et al. , Prevalence of and trends in dyslipidemia and blood pressure among US children and adolescents, 1999–2012. JAMA Pediatr, 2015. 169(3): p. 272–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.CDC. Heart Disease Facts & Statistics. 2017. November 28, 2017 [cited 2018 accessed 11.19.18]; Available from: https://www.cdc.gov/heartdisease/facts.htm
- 4.Theodore RF, et al. , Childhood to Early-Midlife Systolic Blood Pressure Trajectories Early-Life Predictors, Effect Modifiers, and Adult Cardiovascular Outcomes. Hypertension, 2015. 66(6): p. 1108–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Expert panel on integrated guidelines for cardiovascular health and risk reduction in children and adolescents: summary report. Pediatrics, 2011. 128 Suppl 5: p. S213–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leiba A, et al. , Association of Adolescent Hypertension With Future End-Stage Renal Disease. JAMA Intern Med, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benjamin EJ, et al. , Heart Disease and Stroke Statistics—2018 Update: A Report From the American Heart Association. Circulation, 2018. 137(12): p. e67–e492. [DOI] [PubMed] [Google Scholar]
- 8.Kataria A, Trasande L, and Trachtman H, The effects of environmental chemicals on renal function. Nature Reviews Nephrology, 2015. 11(10): p. 610–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sanders AP, et al. , Perinatal and childhood exposure to environmental chemicals and blood pressure in children: a review of literature 2007–2017. Pediatr Res, 2018. 84(2): p. 165–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zheng LY, et al. , Environmental exposures and pediatric kidney function and disease: A systematic review. Environ Res, 2017. 158: p. 625–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shiue I and Hristova K, Higher urinary heavy metal, phthalate and arsenic concentrations accounted for 3–19% of the population attributable risk for high blood pressure: US NHANES, 2009–2012. Hypertens Res, 2014. 37(12): p. 1075–81. [DOI] [PubMed] [Google Scholar]
- 12.Raj M and Krishnakumar R, Hypertension in children and adolescents: epidemiology and pathogenesis. Indian J Pediatr, 2013. 80 Suppl 1: p. S71–6. [DOI] [PubMed] [Google Scholar]
- 13.Khan DA, et al. , Lead exposure and its adverse health effects among occupational worker’s children. Toxicol Ind Health, 2010. 26(8): p. 497–504. [DOI] [PubMed] [Google Scholar]
- 14.Weidemann DK, Weaver VM, and Fadrowski JJ, Toxic environmental exposures and kidney health in children. Pediatr Nephrol, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fowler BA, et al. , Chronic low-level lead toxicity in the rat: III. An integrated assessment of long-term toxicity with special reference to the kidney. Toxicology and Applied Pharmacology, 1980. 56(1): p. 59–77. [DOI] [PubMed] [Google Scholar]
- 16.Jacobo-Estrada T, et al. , Evaluation of kidney injury biomarkers in rat amniotic fluid after gestational exposure to cadmium. J Appl Toxicol, 2016. 36(9): p. 1183–93. [DOI] [PubMed] [Google Scholar]
- 17.Daston GP, et al. , Toxicity of mercuric chloride to the developing rat kidney. III. Distribution and elimination of mercury during postnatal maturation. Toxicol Appl Pharmacol, 1986. 85(1): p. 39–48. [DOI] [PubMed] [Google Scholar]
- 18.Carrico C, et al. , Characterization of Weighted Quantile Sum Regression for Highly Correlated Data in a Risk Analysis Setting. Journal of Agricultural Biological and Environmental Statistics, 2015. 20(1): p. 100–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Czarnota J, Gennings C, and Wheeler DC, Assessment of weighted quantile sum regression for modeling chemical mixtures and cancer risk. Cancer Inform, 2015. 14(Suppl 2): p. 159–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Robles-Osorio ML, Sabath-Silva E, and Sabath E, Arsenic-mediated nephrotoxicity. Ren Fail, 2015. 37(4): p. 542–7. [DOI] [PubMed] [Google Scholar]
- 21.Agency for Toxic Substances and Disease Registry. Arsenic Toxicity: What is the Biologic Fate of Arsenic in the Body? Environmental Health and Medicine Education 2010 [cited 2019 4/18]; Available from: https://www.atsdr.cdc.gov/csem/csem.asp?csem=1&po=9
- 22.Caldwell KL, et al. , Levels of urinary total and speciated arsenic in the US population: National Health and Nutrition Examination Survey 2003–2004. J Expo Sci Environ Epidemiol, 2009. 19(1): p. 59–68. [DOI] [PubMed] [Google Scholar]
- 23.Schwartz GJ, et al. , New equations to estimate GFR in children with CKD. J Am Soc Nephrol, 2009. 20(3): p. 629–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jain RB, Analysis of self-reported versus biomarker based smoking prevalence: methodology to compute corrected smoking prevalence rates. Biomarkers, 2017. 22(5): p. 476–487. [DOI] [PubMed] [Google Scholar]
- 25.Loghman-Adham M, Aminoaciduria and glycosuria following severe childhood lead poisoning. Pediatric Nephrology, 1998. 12(3): p. 218–221. [DOI] [PubMed] [Google Scholar]
- 26.de Burbure C, et al. , Renal and neurologic effects of cadmium, lead, mercury, and arsenic in children: Evidence of early effects and multiple interactions at environmental exposure levels. Environmental Health Perspectives, 2006. 114(4): p. 584–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fadrowski JJ, et al. , Blood lead level and kidney function in US adolescents: The Third National Health and Nutrition Examination Survey. Arch Intern Med, 2010. 170(1): p. 75–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Filler G, et al. , High prevalence of elevated lead levels in pediatric dialysis patients. Pediatr Nephrol, 2012. 27(9): p. 1551–6. [DOI] [PubMed] [Google Scholar]
- 29.Fadrowski JJ, et al. , Blood lead level and measured glomerular filtration rate in children with chronic kidney disease. Environ Health Perspect, 2013. 121(8): p. 965–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weaver VM, et al. , Impact of urine concentration adjustment method on associations between urine metals and estimated glomerular filtration rates (eGFR) in adolescents. Environmental Research, 2014. 132: p. 226–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weidemann D, et al. , Association of arsenic with kidney function in adolescents and young adults: Results from the National Health and Nutrition Examination Survey 2009–2012. Environmental Research, 2015. 140: p. 317–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weaver VM, et al. , Associations of low-level urine cadmium with kidney function in lead workers. Occup Environ Med, 2011. 68(4): p. 250–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jin R, et al. , Associations of renal function with urinary excretion of metals: Evidence from NHANES 2003–2012. Environ Int, 2018. 121(Pt 2): p. 1355–1362. [DOI] [PubMed] [Google Scholar]
- 34.Helal I, et al. , Glomerular hyperfiltration: definitions, mechanisms and clinical implications. Nat Rev Nephrol, 2012. 8(5): p. 293–300. [DOI] [PubMed] [Google Scholar]
- 35.Bjornstad P, et al. , Rapid GFR decline is associated with renal hyperfiltration and impaired GFR in adults with Type 1 diabetes. Nephrol Dial Transplant, 2015. 30(10): p. 1706–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Buser MC, et al. , Urinary and blood cadmium and lead and kidney function: NHANES 2007–2012. Int J Hyg Environ Health, 2016. 219(3): p. 261–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mehrzadi S, et al. , Ellagic acid mitigates sodium arsenite-induced renal and hepatic toxicity in male Wistar rats. Pharmacol Rep, 2018. 70(4): p. 712–719. [DOI] [PubMed] [Google Scholar]
- 38.Buser MC, et al. , Urinary and blood cadmium and lead and kidney function: NHANES 2007–2012. International Journal of Hygiene and Environmental Health, 2016. 219(3): p. 261–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Navas-Acien A, et al. , Blood cadmium and lead and chronic kidney disease in US adults: a joint analysis. Am J Epidemiol, 2009. 170(9): p. 1156–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roels HA, et al. , Assessment of the filtration reserve capacity of the kidney in workers exposed to cadmium. Br J Ind Med, 1991. 48(6): p. 365–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.ATSDR. Toxicological Profile for Cadmium. Agency for Toxic Substances & Disease Registry - Cadmium 2012 August 14, 2018. [cited 2018 accessed 11.19.18]; CAS#: 7440–43-9]. Available from: https://www.atsdr.cdc.gov/toxprofiles/TP.asp?id=48&tid=15
- 42.Nordberg GF, et al. , Prevalence of kidney dysfunction in humans - relationship to cadmium dose, metallothionein, immunological and metabolic factors. Biochimie, 2009. 91(10): p. 1282–5. [DOI] [PubMed] [Google Scholar]
- 43.Sanders AP, et al. , Prenatal lead exposure modifies the effect of shorter gestation on increased blood pressure in children. Environ Int, 2018. 120: p. 464–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Farzan SF, et al. , Prenatal lead exposure and elevated blood pressure in children. Environ Int, 2018. 121(Pt 2): p. 1289–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Agency for Toxic Substances and Disease Registry, Evaluating Mercury Exposure: Information for Health Care Providers. 2012. [Google Scholar]
- 46.U.S. Department of Health and Human Services, P.H.S., Agency for Toxic Substances and Disease Registry,, INTERACTION PROFILE FOR: CHLORPYRIFOS, LEAD, MERCURY, AND METHYLMERCURY 2006. [PubMed] [Google Scholar]
- 47.Agency for Toxic Substances and Disease Registry. Cadmium Toxicity: What Is the Biological Fate of Cadmium in the Body? Environmental Health and Medicine Education 2013. [cited 2019 4/18]; Available from: https://www.atsdr.cdc.gov/csem/csem.asp?csem=6&po=9
- 48.Agency for Toxic Substances and Disease Registry. Lead Toxicity: What is the Biological Fate of Lead in the Body? Environmental Health and Medicine Education 2017. [cited 2019 4/18]; Available from: https://www.atsdr.cdc.gov/csem/csem.asp?csem=34&po=9.
- 49.Diamond GL, et al. , Estimates of urinary blood lead clearance and its relationship to glomerular filtration rate based on a large population survey. J Toxicol Environ Health A, 2019: p. 1–4. [DOI] [PubMed] [Google Scholar]
- 50.Yu KH, et al. , Intermittent elevation of serum urate and 24-hour urinary uric acid excretion. Rheumatology (Oxford), 2004. 43(12): p. 1541–5. [DOI] [PubMed] [Google Scholar]
- 51.Weaver VM, et al. , Challenges for environmental epidemiology research: are biomarker concentrations altered by kidney function or urine concentration adjustment? Journal of Exposure Science and Environmental Epidemiology, 2016. 26(1): p. 1–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.