Abstract
Background/Aims
Complete liver regeneration may not always be possible after liver injuries and/or partial liver resection. The present study investigated the effects of dexpanthenol, platelet-rich plasma (PRP), and thymoquinone on liver regeneration in rats after partial hepatectomy (PH).
Materials and Methods
A total of 34 Wistar albino rats, each weighing 250–280 g, were randomly separated into four groups. PH was performed, and except for the control group, intraperitoneal dexpanthenol, PRP, or thymoquinone was administered to the relevant groups for 7 days. All rats were then sacrificed, and the liver tissues were examined histopathologically and biochemically.
Results
PRP reduced all oxidant-antioxidant parameters in rats that experienced liver regeneration, but did not create histopathological improvement in the liver tissue. Dexpanthenol had a histopathological improving effect on the liver tissue, but had no effect on biochemical parameters. Thymoquinone showed no histopathological or biochemical effects on liver regeneration.
Conclusion
Although dexpanthenol did not affect biochemical oxidative parameters, it was considered to have improving effects on liver regeneration histopathologically. In addition, it was thought that PRP may be used for treatment of ischemia-reperfusion injury and cholestatic damage of the liver. Nevertheless, further studies are required on these subjects.
Keywords: Dexpanthenol, platelet-rich plasma, thymoquinone, partial hepatectomy, hepatic regeneration
INTRODUCTION
The liver is the organ with the highest capability of regeneration after resection (1). However, complete regeneration might not always be possible after liver injuries (2). Therefore, the risk of inadequate liver regeneration or total loss and shifting of the recovery process into fibrosis and development of liver deficiency as a result of this still continues to be an important problem in hepatobiliary surgery. Although several pharmacological agents have been tested in the literature to protect liver function and increase the regenerative capacity of the liver following liver injuries and/or partial resection, no effective solution has been found regarding this problem (2, 3). Moreover, to the best of our knowledge, no study was found in the literature that has investigated the effects of dexpanthenol, platelet-rich plasma (PRP), and thymoquinone on liver regeneration.
Dexpanthenol, a safe, cost-effective and easily accessible agent, is a biologically active alcohol of pantothenic acid with significant therapeutic effects on skin epithelialization and granulation due to its stimulatory effects on anti-inflammatory and fibroblast proliferation when applied locally. This agent, which increases the synthesis of glutathione (GSH), coenzyme A, and adenosine triphosphate, acts as an antioxidant to prevent ischemia-induced tissue damage, thereby contributing to wound healing (4). Pantothenate, which can contribute to the wound healing properties of this agent, also upregulates the expressions of interleukin (IL)-6, IL-8, C-C motif chemokine ligand (CCL)2, and CXCL1. It has been shown that dexpanthenol could also regulate 95 different genes (5). It has been reported in recent experimental animal studies that parenteral use of dexpanthenol could protect the animals from ischemia and reperfusion injury, lung fibrosis, acute lung injury, testicular damage, and necrotizing enterocolitis through its strong antioxidant effect (6).
Platelets play a key role in hemostasis and wound healing following tissue damage and in the subsequent stages of tissue repair. These long-term effects of platelet activation are manifested by the expression of >30 growth factors (7). PRP, which is an autologous plasma product containing three to five times more platelets (enriched) than basal plasma levels, contains elevated concentrations of autologous growth factors (e.g., vascular endothelial growth factor (VEGF), insulin-like growth factor (IGF), platelet-derived growth factor, and transforming growth factor (TGF)-1), proteins and peptides (e.g., fibrinogen, fibronectin, osteonectin, osteocalcin, vitronectin, and thrombospondin), and certain chemokines and cytokines (e.g., IL-1 and platelet factor 4). Therefore, many PRP types have been used by clinicians for many years due to their enhancing effects on wound healing, cellular mitogenesis, osteogenesis, and angiogenesis (8). Moreover, advocates of PRP treatment prefer PRP because of its beneficial effects of increasing tissue regeneration, as well as lowering infection, pain, and blood loss (7).
Thymoquinone, which is a potent inhibitor of leukotriene B4 and thromboxane B2, has been reported to have strong anti-inflammatory and antioxidant properties through scavenging activity against the superoxide anion, hydroxyl radical, and singlet molecular oxygen. It can also modulate nuclear factor-κB and TNF-α to attenuate proinflammatory responses and oxidative responses. Furthermore, it inhibits the arachidonic acid metabolism in peritoneal leukocytes by cyclooxygenase and 5-lipoxygenase pathways (9). Therefore, it has been shown that in experimental encephalomyelitis, colitis, peritonitis, edema, and arthritis, it can produce potent anti-inflammatory effects through the suppression of prostaglandins and leukotrienes. Moreover, beneficial antimicrobial, antitumor, and immunomodulatory properties enhancing the T cell and natural killer cell-mediated immune responses have been demonstrated (10).
As the therapeutic effects of the agents above-mentioned have not yet been investigated on the regeneration of liver tissue after partial hepatectomy (PH), this experimental study was conducted with the purpose of investigating the effects of dexpanthenol, PRP, and thymoquinone on the subacute stage of the regeneration of liver tissue after a PH in a rat model.
MATERIALS AND METHODS
Material
The study was approved by the local ethics committee on experimental animals (no. 25.02.2015.15/21).
A total of 39 male Wistar albino rats, each weighing 250–280 g, were included in the study. Of the 39 rats, five were separated to obtain PRP, and the remaining 34 were randomly divided into the groups described below.
Control group
PH was applied, but no experimental agent was administered to the rats (n=9).
DX group
PH was performed on the rats, and a single daily dose of 50 mg/kg intraperitoneal dexpanthenol (dexpanthenol 500 mg/2 mL; Bayer, Berlin, Germany) was administered for 7 days (n=9).
PR group
PH was performed on the rats, and 1 mL/kg intraperitoneal PRP was administered for 7 days (n=7).
TQ group
PH was performed on the rats, and 10 mg/kg intraperitoneal thymoquinone (thymoquinone; Santa Cruz Biotechnology, CA, USA) was administered for 7 days (n=9).
Before surgical intervention, anesthesia was achieved in all rats with the intramuscular administration of 90 mg/kg ketamine (Ketalar®; Pfizer) and 10 mg/kg xylazine (Rompun®; Bayer, Leverkusen, Germany).
Surgery
After performing standard midline abdominal laparotomy, the standard 70% PH technique described by Higgins and Anderson (11) was applied. For this purpose, first, the left lateral lobe peduncle and then the left medial lobe peduncle of the liver were extracted after the base of the lobe was tied en bloc with 4/0 silk, and only the right lobe and the caudate lobe of the liver were left. After checking for bleeding, the abdominal wall was closed up in compliance with the anatomical planes. Following the operation, a single-dose per day of 50 mg/kg intraperitoneal dexpanthenol was administered to the DX group for 7 days, a single-dose per day of 1 ml/kg intraperitoneal PRP was administered to the PR group for 7 days, and a single-dose per day of 10 mg/kg intraperitoneal thymoquinone was administered to the TQ group for 7 days. On postoperative day 7, abdominal re-laparotomy was applied to all the rats again from the old incision under anesthesia, approximately 5–6 cc of blood samples were collected from the vena cava inferior, and the samples were placed in dry tubes, tubes containing heparin, and tubes containing ethylenediaminetetraacetic acid. The liver tissue was then completely resected. Half of the tissue was kept at a temperature of −40°C for biochemical analysis, and the other half was placed in 10% buffered formaldehyde for histopathological examination. After the procedure, all the experimental animals were sacrificed by the administration of high-dose anesthesia.
PRP preparation
Five rats were used to obtain PRP. The venous blood that was collected from the vena cava inferior after applying laparotomy as described above was placed in tubes containing 3.2% sodium citrate (Merck, Darmstadt, Germany). The blood samples were centrifuged at 400g for 10 min. The supernatant that was obtained after centrifugation was centrifuged again at 800g for 10 min. After centrifugation, two-thirds of serum at the top was discarded, and the remaining part was accepted as PRP (12). These rats were then euthanized under high-dose anesthesia and excluded from the study.
Histopathological analysis
The tissue samples that were collected to assess liver regeneration were embedded in paraffin blocks, 5 μm thick sections were obtained, and these were stained with routine hematoxylin and eosin. The obtained preparates were examined under a microscope, and to reveal regeneration, “ductus proliferation,” “inflammatory cell density values in regenerated liver tissue (ICD-RT),” and “inflammatory cell density values in regeneration zone of the liver tissue (ICD-RZ)” were assessed using the scoring grade as follows (Figure 1) (13):
Figure 1.
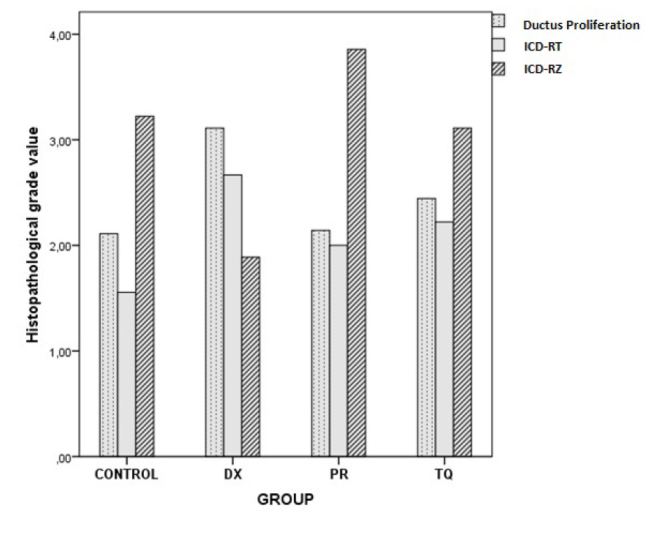
Each bar represents the histopathological grade scores of each group. ICD-RT, inflammatory cell density in regenerated liver tissue; ICD-RZ, inflammatory cell density values in regeneration zone of the liver tissue
Grade 0: none
Grade 1: minimal
Grade 2: mild
Grade 3: medium
Grade 4: severe.
Biochemical analysis
The enzyme-linked immunosorbent assay (ELISA) method was used to analyze the levels of the following in the liver tissue samples and blood serum samples obtained from the experimental animals:
hydroxyproline (HP) (Hydroxyproline ELISA kit; Eastbiopharm Co., Ltd.),
8-isoprostane (ISO-8) (8-isoprostane ELISA kit; Abbexa Ltd.),
8-hydroxy-2′-deoxyguanosine (8-OHdG) (8-OHDG ELISA kit; MyBioSource),
malondialdehyde (MDA) (MDA ELISA kit; Eastbiopharm Co., Ltd.),
GSH (GSH ELISA kit; SunRed Biotechnology Co.),
catalase (CAT) (CAT ELISA kit; SunRed Biotechnology Co.).
Statistical analysis
Statistical Package for Social Sciences 21 software (IBM Corp.; Armonk, NY, USA) was used for statistical analyses. To analyze the differences among all the groups, one-way analysis of variance was used for parametric data, and Kruskal-Wallis test was used for non-parametric data. Tukey multiple comparisons test and Mann-Whitney U test were used for binary comparisons of the groups. A p value of <0.05 was accepted as statistically significant for all analysis results.
RESULTS
Histopathological analysis
The ductus proliferation (X2=8.494, p=0.037), ICD-RT (X2=9.641, p=0.022), and ICD-RZ (X2=15.312, p=0.002) parameters were significantly different among the groups (Table 1, 2; Figure 1).
Table 1.
Descriptive table for results of histopathological scoring
| Group | Variable | Min | Max | Median | SD |
|---|---|---|---|---|---|
| Control | Ductus proliferation | 1 | 3 | 2 | 0.60 |
| ICD-RT | 1 | 2 | 2 | 0.52 | |
| ICD-RZ | 1 | 4 | 4 | 1.09 | |
| DX | Ductus proliferation | 2 | 4 | 3 | 0.78 |
| ICD-RT | 2 | 4 | 3 | 0.70 | |
| ICD-RZ | 1 | 3 | 2 | 0.78 | |
| PR | Ductus proliferation | 1 | 3 | 2 | 0.69 |
| ICD-RT | 1 | 3 | 2 | 0.57 | |
| ICD-RZ | 3 | 4 | 4 | 0.37 | |
| TQ | Ductus proliferation | 1 | 3 | 3 | 0.72 |
| ICD-RT | 1 | 3 | 2 | 0.83 | |
| ICD-RZ | 2 | 4 | 3 | 0.78 |
Min: minimum; Max: maximum; SD: standard deviation; ICD-RT: inflammatory cell density in regenerated liver tissue; ICD-RZ: inflammatory cell density values in regeneration zone of the liver tissue
Table 2.
Histopathological scores deviated differently among all the groups
| Variable | X2 | p |
|---|---|---|
| Ductus proliferation | 8.494 | 0.037 |
| ICD-RT | 9.641 | 0.022 |
| ICD-RZ | 15.312 | 0.002 |
Kruskal-Wallis test, p<0.05
X2: chi-square; ICD-RT: inflammatory cell density in regenerated liver tissue; ICD-RZ: inflammatory cell density values in regeneration zone of the liver tissue
After the binary comparisons, significant differences were found between the control and DX groups (Z=−2.504, p=0.012) and between the PR and DX groups (Z=−2.186, p=0.029) with regard to ductus proliferation values (Figure 2).
Figure 2. a, b.
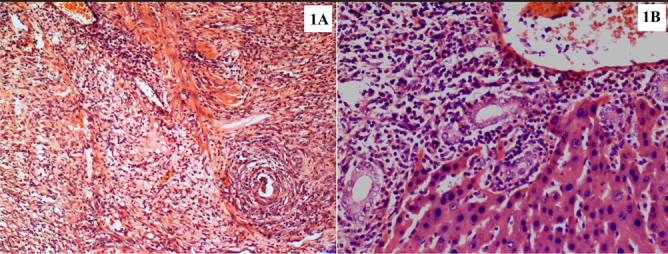
Histopathological images demonstrate the grade 1 (a) and grade 4 (b) ductus proliferation in the hepatectomized tissue samples. Panel A refers to the control group specimen, and B refers to the DX group specimen (H&E, ×20)
There was a significant difference between the control and DX groups (Z=−2.912, p=0.004) with regard to ICD-RT (Figure 3).
Figure 3. a, b.
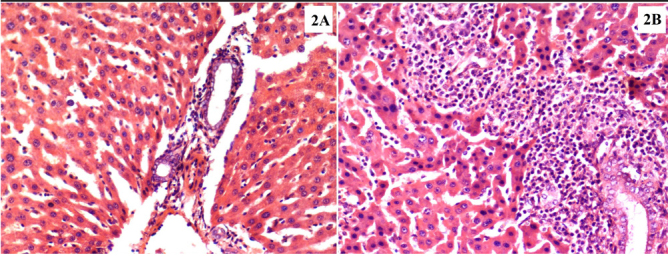
Histopathological images demonstrate the grade 1 (a) and grade 4 (b) liver parenchyma inflammatory cell density in the hepatectomized tissue samples. Panel A refers to the control group specimen, and B refers to the DX group specimen (H&E, ×20)
With regard to ICD-RT, there were significant differences between the control and DX groups (Z=−2.462, p=0.014), the DX and PR groups (Z=−3.362, p=0.001), and the DX and TQ groups (Z=−2.624, p=0.009). There was also a significant difference between the PR and TQ groups (Z=−2.081, p=0.037) (Table 3, Figure 4).
Table 3.
The histopathological recovery in the DX group where dexpanthenol was administered was better than that in the other groups
| Variable | Group (I/J) | Z | p |
|---|---|---|---|
| Ductus proliferation | DX/control | −2.504 | 0.012 |
| DX/PR | −2.186 | 0.029 | |
| ICD-RT | DX/control | −2.912 | 0.004 |
| ICD-RZ | PR/TQ | −2.081 | 0.037 |
| DX/control | −2.462 | 0.014 | |
| DX/PR | −3.362 | 0.001 | |
| DX/TQ | −2.624 | 0.009 |
Mann-Whitney U test, p<0.05
Z: Z score; ICD-RT: inflammatory cell density in regenerated liver tissue; ICD-RZ: inflammatory cell density values in regeneration zone of the liver tissue
Figure 4. a, b.
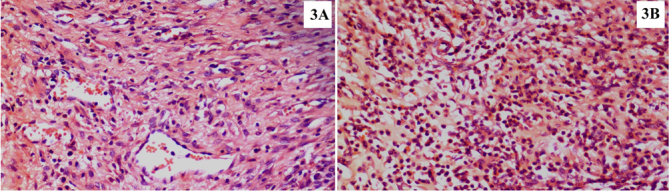
Histopathological images demonstrate the grade 1 (a) and grade 4 (b) regeneration zone inflammatory cell density in the hepatectomized tissue samples. Panel A refers to the DX group specimen, and B refers to the control group specimen (H&E, ×20)
Results of biochemical analysis
There were statistically significant differences among all the groups with regard to the biochemical variables that were analyzed in the tissues. No significant difference was determined among the groups with respect to the biochemical parameters measured in the sera (p>0.05) (Table 4).
Table 4.
Biochemical variables analyzed in the tissues were significantly different among all the groups
| Variable | F | p | |
|---|---|---|---|
| Hydroxyproline | Tissue | 3.900 | 0.018 |
| Serum | 2.539 | 0.075 | |
| 8-Isoprostane | Tissue | 3.899 | 0.018 |
| Serum | 2.539 | 0.075 | |
| 8-Hydroxy-2′-deoxyguanosine | Tissue | 3.900 | 0.018 |
| Serum | 2.539 | 0.075 | |
| Malondialdehyde | Tissue | 3.900 | 0.018 |
| Serum | 2.539 | 0.075 | |
| Glutathione | Tissue | 3.899 | 0.018 |
| Serum | 2.539 | 0.075 | |
| Catalase | Tissue | 3.899 | 0.018 |
| Serum | 2.539 | 0.075 |
One-way analysis of variance, p<0.05 F: F score
As a result of the binary comparisons, it was observed that all the biochemical parameters that were analyzed for liver tissues were significantly higher in the control group than in the PR group (p<0.05) (Table 5; Figure 5–10).
Table 5.
There was a significant decrease in all oxidant-an-tioxidant biochemical parameters in the PR group where PRP was administered in comparison with the other groups
| Variable | Group (I/J) | Mean difference | p |
|---|---|---|---|
| Hydroxyproline | Control/PR | 497.17 | 0.010 |
| 8-Isoprostane | Control/PR | 4.97 | 0.010 |
| 8-Hydroxy-2′-deoxyguanosine | Control/PR | 55.24 | 0.010 |
| Malondialdehyde | Control/PR | 39.77 | 0.010 |
| Glutathione | Control/PR | 13.81 | 0.010 |
| Catalase | Control/PR | 5.68 | 0.010 |
Tukey multiple comparisons test, p<0.05
Figure 5.
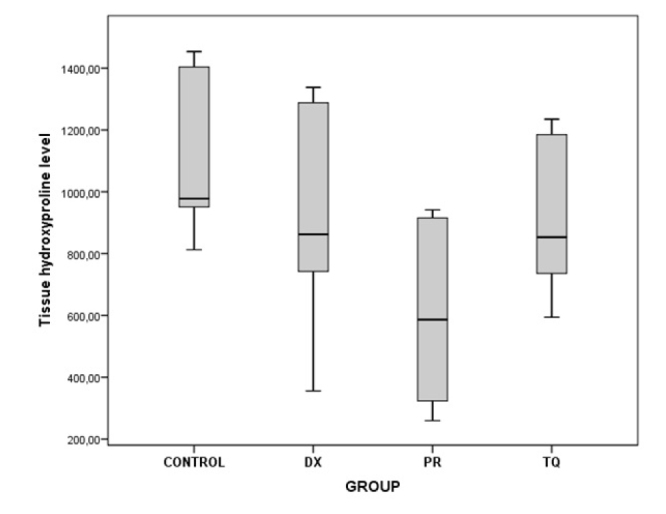
The chart presents the tissue hydroxyproline level values of each group
Figure 6.
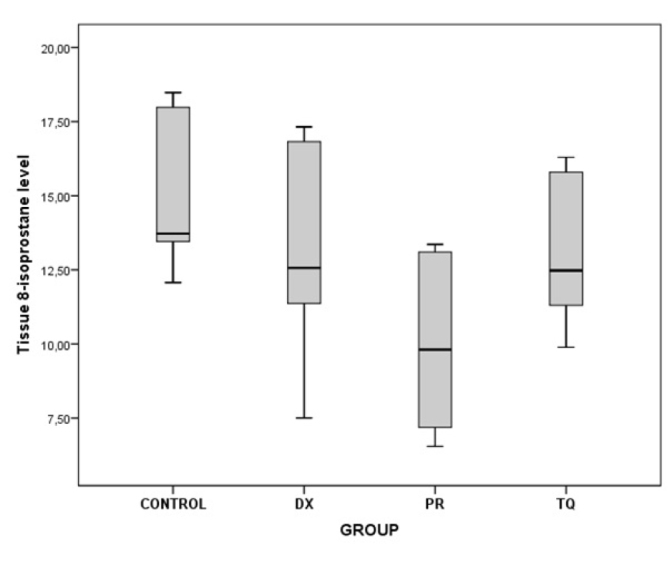
The chart presents the tissue 8-isoprostane level values of each group
Figure 7.
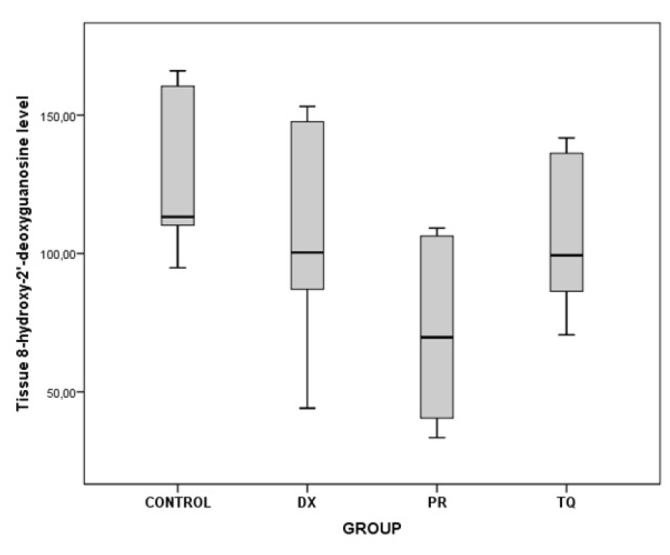
The chart presents the tissue 8-hydroxy-2′-deoxyguanosine level values of each group
Figure 8.
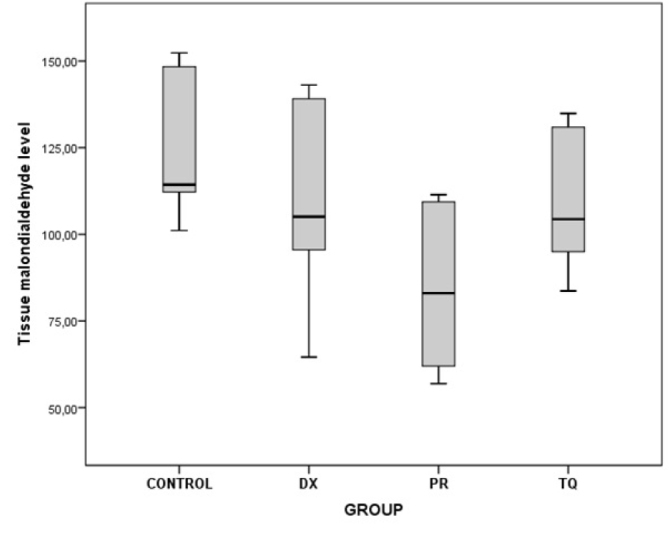
The chart presents the tissue malondialdehyde level values of each group
Figure 9.
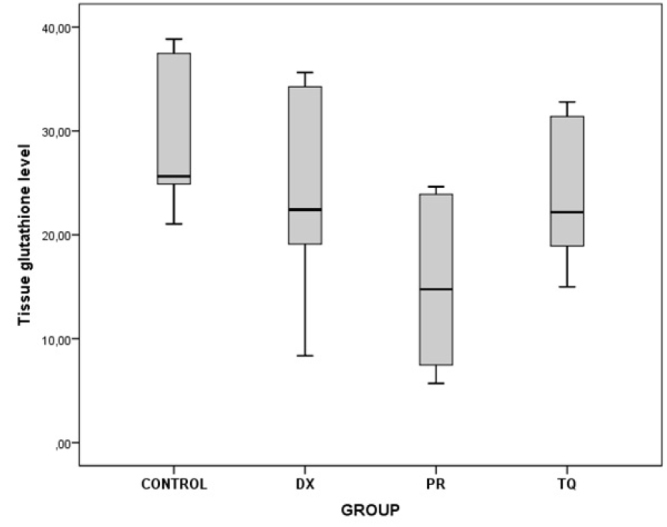
The chart presents the tissue glutathione level values of each group
Figure 10.
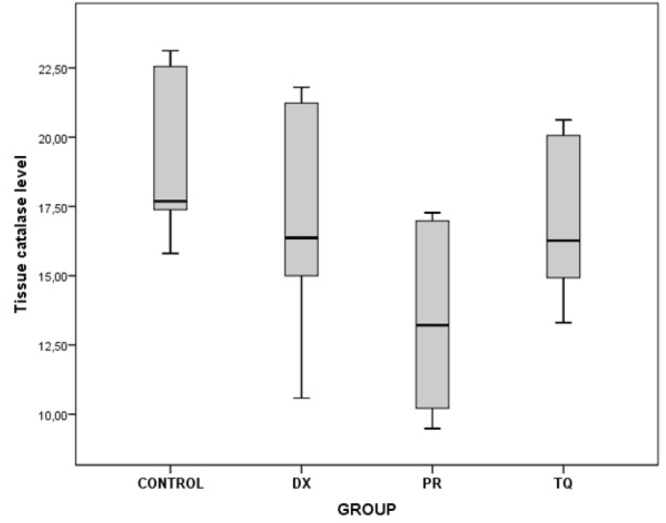
The chart presents the tissue catalase level values of each group
DISCUSSION
It has been shown that various mechanisms play a role in the recovery and regeneration process following PH, which is a complex situation, and several cytokines have activation or inhibition effects on regeneration (14). It has been reported that during regeneration, first, the number of hepatocytes increases, followed by an increase in the number of cells outside the parenchyma that are very important in providing a normal lobular architecture (15). Moreover, the triggering of apoptotic pathways after oxidative stress has been shown to be associated with reduced oxygen use, energy-dependent metabolic needs, and increases in energy-consuming inflammatory cytokines, leading to disruption of hepatocellular functions and affecting the regeneration process negatively (16). It is known that liver damage and loss of tissue after PH occur as a result of increases in reactive oxygen species (ROS). These oxygen species can not only lead to a reduction in cell growth but also reduce mitotic activity by activating proteins that inhibit the cell cycle and damage DNA synthesis (17–19). Nitric oxide (NO) secretion in particular has been shown to initiate liver regeneration (20,21), and the increase in ROS that emerge after changes in the portal blood flow may suppress liver regeneration by reducing this synthesis of NO (20,21). It has been demonstrated that isoprostanes are formed by the peroxidation of arachidonic acid, which is a polyunsaturated fatty acid found as an ester with the membrane phospholipids of animal cells. It is accepted that F2-isoprostanes are one of the most accurate indicators of in vivo oxidative stress in humans (22). Moreover, increased concentrations of MDA in the tissue and plasma are a well-known hepatocyte damage indicator that reflects lipid peroxidation levels (23). Among antioxidants, GSH, which is the cofactor of GSH peroxidase, plays a very important role in cell protection mechanisms against the effects of ROS (23). Another antioxidant, CAT, takes part in directly converting hydrogen peroxide that is formed with the activity of oxidases into water (24). In fact, the therapeutic effects of many agents (e.g., cyclosporine, mesenchymal stem cell, erythropoietin, nebivolol, tranexamic acid, and sorafenib) on the regeneration of the liver tissue after PH have been investigated in the literature (25). Currently, experimental and clinical studies are ongoing in efforts to solve this problem.
In the present study, an investigation was made of the effects of both PH and pharmacological agents that were administered after PH on oxidative stress parameters and liver regeneration. According to the results of the present study, thymoquinone did not have any histopathological effect on liver regeneration. While this active substance reduced the results of biochemical parameters numerically, the effect was not statistically significant. Based on this result, it was thought that thymoquinone did not have any positive or recovery effect on liver regeneration after PH. On the other hand, there was a significant decrease in all oxidant-antioxidant biochemical parameters (e.g., HP, ISO-8, 8-OHdG, MDA, GSH, and CAT) in the PR group where PRP was administered. However, the histopathological analysis results of this group revealed that PRP did not provide any histopathological improvement in liver regeneration. With these results, it can be stated that PRP had no significant effect on liver regeneration after PH, but it had a noticeable reduction effect on oxidative stress parameters (i.e., MDA). This is why it was considered that this pharmacological agent may be used in the treatment of ischemia-reperfusion damage of the liver or cholestatic liver damage, but it would be appropriate to conduct more advanced studies on this topic. On the other hand, the ductus proliferation and ICD of the DX group were significantly higher than those of the control group, and the ICD-RZ of this group was the lowest than all the other groups. The ICD-RZ was similar in the control group, the PR group, and the TQ group, and these levels were higher than those in the DX group. Considering the biochemical analysis results of this group, it was observed that while dexpanthenol reduced the results of biochemical parameters numerically, this effect was not statistically significant. With these findings, it was thought that dexpanthenol did not significantly affect the biochemical oxidative parameters in the PH model in rats, but it had histopathologically positive and recovering effects on liver regeneration. With these findings, a hope emerged that dexpanthenol may show positive effects on liver survival and regeneration after PH. Nevertheless, more reliable data should be obtained on this subject with histopathologically and biochemically more advanced studies that involve the use of dexpanthenol at different doses. In the literature, hepatocyte growth factor (HGF), epidermal growth factor, Fas, and TNF are known to regulate liver regeneration (26). In fact, studies demonstrated that direct contact between platelets and hepatocytes may cause the release of some growth factors that could promote liver regeneration, such as IGF-1, VEGF, and HGF from platelets. In particular, IGF-1 released from human platelets has been shown to have a proliferative effect for liver regeneration (27). On the other hand, it has been concluded that the increase of MDA levels in the liver is indicative of an enhanced lipid peroxidation that causes tissue damage and breakdown of the antioxidant defense mechanisms. During the regeneration period, hepatocytes can sense the changes in their cellular environment via chemoreceptors and mechanoreceptors present on their surfaces. Although the presence of NO in the microenvironment is a major determinant for regeneration, the regulation of shear stress caused by actinomyosin cytoskeleton, as well as the regulation of α-catenin, which is responsible for cell-cell contact tension, can also activate downstream signals that promote regeneration. It is known that liver-specific knockout of α-catenin causes hyperproliferation of hepatocytes in mice (21). In summary, these alternative mechanisms could help explain why dexpanthenol was significantly effective on microscopic regeneration indices without creating a significant reduction on oxidative stress parameters in the present study. Although PRP significantly reduced oxidative stress parameters, it had no significant effect on regeneration, which also implies that the modulation of oxidative stress is not the sole mechanism for promoting regeneration.
There were some limitations in the present study. First, the study was completed in 7 days because it was reported that the regeneration process that occurs in the liver resection zone is completed in 7–10 days (the subacute period of liver regeneration) (13,28). The present study could have included a group to investigate the acute stage of the regeneration of the liver tissue after PH, but the aim of the study was to evaluate the longer-term beneficial effects of these agents on the regeneration of the hepatectomized tissue. Therefore, no group was formed to investigate the acute stage of the regeneration of the liver tissue after PH. Moreover, some financial restrictions did not permit the inclusion of such a group. For all these reasons, the hepatectomized livers were evaluated on day 7 in the present study (23,25). Thus, within the scope of the present study, the effects that may be caused by the studied experimental agents in the acute and/or chronic periods could not be investigated. Second, it has been reported that the number of mitotic cells may increase (2%–6%) after hepatectomy in 24–48 h in rats that have been subjected to 70% hepatic resection (29,30). However, as a mitotic increase could not be observed in the liver regeneration zone of hepatocytes on day 7, a count or a histopathological analysis method for showing these data could not be used. Third, the present study could not apply advanced histopathological methods (e.g., electron microscopy, immunohistochemistry, and fluorescence microscopy) and biochemical analysis methods (e.g., Western blot and polymerase chain reaction) that would show the regeneration in the liver. Fourth, it was observed that PRP could not be histopathologically effective on liver regeneration in the subacute period but significantly reduced biochemical parameters, whereas dexpanthenol was not effective on biochemical parameters but fixed histopathological parameters noticeably. Thus, it was thought that a group where both of these agents are used together should be included among experiment groups. Finally, it can be strongly recommended with these unexpected findings that various dosages with different administration methods (intraperitoneal or parenteral route) and time protocols of these agents should be detailed and tested in further studies.
In conclusion, it was considered that dexpanthenol did not affect biochemical oxidative parameters after PH in this rat model, but had positive and recovery effects in the histopathological sense. It was also thought that PRP may be used in the treatment of ischemia-reperfusion damage of the liver or cholestatic liver damage, but it would be appropriate to conduct more advanced studies on this subject.
Footnotes
The preliminary results of this study was presented at the 9th Congress of Surgical Research, 2017, Kocaeli, Turkey.
Ethics Committee Approval: Ethics Committee Approval has received for this study from the Kırıkkale University Ethics Committee on experimental animals (Decision Date&Number: 25.02.2015.15/21).
Informed Consent: N/A.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - O.A.; Design - O.A., F.P., G.A.; Supervision - O.A., K.A.; Data Collection and/or Processing - O.A., F.P., S.K., C.A., H.B.; Analysis and/or Interpretation - O.A., F.P., C.A., H.B., G.A., G.K.; Literature Search - O.A., G.K., G.A., K.A.; Writing Manuscript - O.A., F.P., G.A.; Critical Review - O.A., K.A.
Conflict of Interest: The authors have no conflict of interest to declare.
Financial Disclosure: The authors declared that this study was financially supported by Kirikkale University Scientific Research Project Coordination Unit (Project Number: 2015.056).
REFERENCES
- 1.Böhm F, Köhler UA, Speicher T, Werner S. Regulation of liver regeneration by growth factors and cytokines. EMBO Mol Med. 2010;2:294–305. doi: 10.1002/emmm.201000085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Palmes D, Spiegel H-U. Animal models of liver regeneration. Biomaterials. 2004;25:1601–11. doi: 10.1016/S0142-9612(03)00508-8. [DOI] [PubMed] [Google Scholar]
- 3.Karaman A, Kirimlioglu H, Tas E, et al. Effect of leflunomide on liver regeneration after partial hepatectomy in rats. Pediatr Surg Int. 2010;26:219–26. doi: 10.1007/s00383-009-2529-1. [DOI] [PubMed] [Google Scholar]
- 4.Cagin YF, Atayan Y, Sahin N, et al. Beneficial effects of dexpanthenol on mesenteric ischemia and reperfusion injury in experimental rat model. Free Radic Res. 2016;50:354–65. doi: 10.3109/10715762.2015.1126834. [DOI] [PubMed] [Google Scholar]
- 5.Heise R, Skazik C, Marquardt Y, et al. Dexpanthenol modulates gene expression in skin wound healing in vivo. Skin Pharmacol Physiol. 2012;25:241–8. doi: 10.1159/000341144. [DOI] [PubMed] [Google Scholar]
- 6.Li-Mei W, Jie T, Shan-He W, Dong-Mei M, Peng-Jiu Y. Anti-inflammatory and anti-oxidative effects of dexpanthenol on lipopolysaccharide induced acute lung injury in mice. Inflammation. 2016;39:1757–63. doi: 10.1007/s10753-016-0410-7. [DOI] [PubMed] [Google Scholar]
- 7.Pallua N, Wolter T, Markowicz M. Platelet-rich plasma in burns. Burns. 2010;36:4–8. doi: 10.1016/j.burns.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 8.Aydin O, Karaca G, Pehlivanli F, et al. Platelet-Rich Plasma May Offer a New Hope in Suppressed Wound Healing When Compared to Mesenchymal Stem Cells. J Clin Med. 2018;7 doi: 10.3390/jcm7060143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aydin O, Aydinuraz K, Agalar F, et al. The effect of thymoquinone coating on adhesive properties of polypropylene mesh. BMC Surg. 2017;17:40. doi: 10.1186/s12893-017-0239-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Salem ML. Immunomodulatory and therapeutic properties of the Nigella sativa L. seed. Int Immunopharmacol. 2005;5:1749–70. doi: 10.1016/j.intimp.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 11.Higgins G. Arch. Experimental pathology of the liver. I. Restoration of the liver of the white rat following partial surgical removal. Arch Pathol. 1931;12:186–202. [Google Scholar]
- 12.Franco D, Franco T, Schettino AM, Tavares Filho JM, Vendramin FS. Protocol for obtaining platelet-rich plasma (PRP), platelet-poor plasma (PPP), and thrombin for autologous use. Aesthetic Plast Surg. 2012;36:1254–9. doi: 10.1007/s00266-012-9957-3. [DOI] [PubMed] [Google Scholar]
- 13.Cetinkunar S, Tokgoz S, Bilgin BC, et al. The effect of silymarin on hepatic regeneration after partial hepatectomy: is silymarin effective in hepatic regeneration? Int J Clin Exp Med. 2015;8:2578. [PMC free article] [PubMed] [Google Scholar]
- 14.Sumer F, Colakoglu MK, Ozdemir Y, et al. Effect of nebivolol on liver regeneration in an experimental 70% partial hepatectomy model. Asian J Surg. 2017;40:375–9. doi: 10.1016/j.asjsur.2015.12.005. [DOI] [PubMed] [Google Scholar]
- 15.Court F, Wemyss-Holden S, Dennison A, Maddern G. The mystery of liver regeneration. Bri J Surg. 2002;89:1089–95. doi: 10.1046/j.1365-2168.2002.02166.x. [DOI] [PubMed] [Google Scholar]
- 16.Helling TS. Liver failure following partial hepatectomy. HPB. 2006;8:165–74. doi: 10.1080/13651820510035712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yao X-M, Zhao J, Li Y, Li Y. Effects of bicyclol on liver regeneration after partial hepatectomy in rats. Dig Dis Sci. 2009;54:774–81. doi: 10.1007/s10620-009-0715-6. [DOI] [PubMed] [Google Scholar]
- 18.Beyer TA, Xu W, Teupser D, et al. Impaired liver regeneration in Nrf2 knockout mice: role of ROS-mediated insulin/IGF-1 resistance. EMBO J. 2008;27:212–23. doi: 10.1038/sj.emboj.7601950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Horimoto M, Fülöp P, Derdák Z, Wands JR, Baffy G. Uncoupling protein-2 deficiency promotes oxidant stress and delays liver regeneration in mice. Hepatology. 2004;39:386–92. doi: 10.1002/hep.20047. [DOI] [PubMed] [Google Scholar]
- 20.Hsieh H-J, Liu C-A, Huang B, Tseng AH, Wang DL. Shear-induced endothelial mechanotransduction: the interplay between reactive oxygen species (ROS) and nitric oxide (NO) and the pathophysiological implications. J Biomed Sci. 2014;21:3. doi: 10.1186/1423-0127-21-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Song Z, Gupta K, Ng IC, Xing J, Yang YA, Yu H. Seminars in cell & developmental biology. Elsevier; 2017. Mechanosensing in liver regeneration; pp. 153–67. [DOI] [PubMed] [Google Scholar]
- 22.Roberts JL, Morrow JD. Measurement of F2-isoprostanes as an Index of Oxidative Stress in Vivo. Free Radic Biol Med. 2005;39:1284. doi: 10.1016/s0891-5849(99)00264-6. [DOI] [PubMed] [Google Scholar]
- 23.Kirimlioglu H, Ecevit A, Yilmaz S, Kirimlioglu V, Karabulut AB. Transplantation proceedings. Elsevier; 2008. Effect of resveratrol and melatonin on oxidative stress enzymes, regeneration, and hepatocyte ultrastructure in rats subjected to 70% partial hepatectomy; pp. 285–9. [DOI] [PubMed] [Google Scholar]
- 24.Yabe Y, Kobayashi N, Nishihashi T, et al. Prevention of neutrophil-mediated hepatic ischemia/reperfusion injury by superoxide dismutase and catalase derivatives. J Pharmacol Exp Ther. 2001;298:894–9. [PubMed] [Google Scholar]
- 25.Andersen KJ, Knudsen AR, Kannerup A-S, et al. Postoperative but not preoperative treatment with sorafenib inhibits liver regeneration in rats. J Surg Res. 2014;191:331–8. doi: 10.1016/j.jss.2014.04.019. [DOI] [PubMed] [Google Scholar]
- 26.Michalopoulos GK. Liver regeneration after partial hepatectomy: critical analysis of mechanistic dilemmas. Am J Pathol. 2010;176:2–13. doi: 10.2353/ajpath.2010.090675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Middleton KK, Barro V, Muller B, Terada S, Fu FH. Evaluation of the effects of platelet-rich plasma (PRP) therapy involved in the healing of sports-related soft tissue injuries. IOWA Orthop J. 2012;32:150. [PMC free article] [PubMed] [Google Scholar]
- 28.Karimian G, Kirschbaum M, Veldhuis ZJ, Bomfati F, Porte RJ, Lisman T. Vitamin E attenuates the progression of non-alcoholic fatty liver disease caused by partial hepatectomy in mice. PloS one. 2015;10:e0143121. doi: 10.1371/journal.pone.0143121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Laconi S, Curreli F, Diana S, et al. Liver regeneration in response to partial hepatectomy in rats treated with retrorsine: a kinetic study. J Hepatol. 1999;31:1069–74. doi: 10.1016/S0168-8278(99)80320-1. [DOI] [PubMed] [Google Scholar]
- 30.Selzner M, Clavien PA. Failure of regeneration of the steatotic rat liver: disruption at two different levels in the regeneration pathway. Hepatology. 2000;31:35–42. doi: 10.1002/hep.510310108. [DOI] [PubMed] [Google Scholar]


