Abstract
Background/Aims
Bacteria species, which are used as probiotics, are lactic acid bacteria. The majority of them are under the genera Bifidobacterium and Lactobacillus. The aim of the present study was to isolate and identify Bifidobacterium and to evaluate the effects of their 24 h and 120 h cell-free supernatants (CFS) from both cultures on colon cancer cell line.
Materials and Methods
In the present study, 84 samples of dairy products, infant feces, and probiotic capsule were collected, and Bifidobacterium was isolated. Gram stain, biochemical tests, and molecular identification were done for the isolation and identification of Bifidobacterium. Cytotoxicity effects of CFS derived from both cultures of isolated Bifidobacterium were assessed on colon cancer cell lines.
Results
In the present study, 17 isolates of Bifidobacterium were identified. The results show that Bifidobacterium was most frequently associated with infant feces and dairy products, whereas the lowest rate was associated with local milk. After the effects of CFS on colon cancer cell line, two isolates were identified from infant feces and probiotic capsule; they had the highest ability in inhibiting the growth of cancer cells. Bifidobacterium bifidum was effective in combating cancer cells and was associated with a substantial improvement in gastrointestinal cancer.
Conclusion
The study has shown that the regular ingested probiotics could prevent the development of colorectal cancer. During the present study, the produced CFS could inhibit the growth of colon cancer cells. In conclusion, probiotics have good potential to be introduced as a new approach to colon cancer treatment.
Keywords: Bifidobacterium, dairy, infant feces, colon cancer
INTRODUCTION
Colon cancer is a significant health problem and the leading cause of cancer mortality worldwide. Colon cancer prevalence has rapidly increased as dietary patterns have modified to blending low carbohydrate, high protein, low fiber, and high fat (1). Colorectal cancer (CRC) can affect the entire length of the large intestine and rectum. The intestinal microbiota composition has been observed to be a risk factor for the development of CRC. The intestinal microbiota can influence many aspects of the intestinal health, including its cellular features, physiology, metabolism, development, and immune homeostasis. Therefore, modifying the intestinal microbiota composition by probiotics when ingested in adequate amounts may prevent the development of CRC because these microorganisms both have influence on the microbiota and potentially afford health benefits to the host (2). Bifidobacterium genus is Gram-positive, non-motile, non-spore, Y- or V-shaped, anaerobic bacteria that manufacture lactic and acetic acids from carbohydrates lacking the CO2 generation. Bifidobacterium isolated from humans is known to grow at optimal temperature values between 36°C and 38°C and at optimal pH values ranging from 6.5 to 7; however, they are inhibited by temperature values <25°C and >45°C. Bifidobacterium is able to synthesize amino acids, thiamin, and riboflavin (3). Probiotic is a living microorganism that affects the host favorably by microbial balance and improving nutrition in the digestive system. Selective lactic acid bacteria that have been extensively used in probiotic fermented food production and are considered as preparations include antibiotic tolerance, as well as the generally recognized as safe organisms and are safe for use (4). Most probiotics commercially available today belong to the genera Lactobacillus and Bifidobacterium (5). The main products of the substrate fermentation in the intestine are short-chain fatty acids (SCFAs) that interact with the host cell and the intestinal microbiota (6). These probiotic impacts decrease serum cholesterol, reduce colon cancer, increase the immune response, and manufacture antimicrobial substances that control disease-causing pathogens and undesirable diarrhea in the human intestine. The dietary consumption of Bifidobacterium lactis HN019 enhances natural immunity in healthy elderly people. In addition, viable or heat-killed Bifidobacterium and Lactobacillus species, as well as certain of their cell components, are able to stimulate the production of nitric oxide, hydrogen peroxide, and cytokines, such as tumor necrosis factor-α and interleukin-6 in macrophage cell lines (1). Bifidobacterium species are able to manufacture biotin, vitamin B2, and vitamin B6. Antitumor activity detects peptidoglycans isolated from the Bifidobacterium infantis strain, ATCC 15697 (1). The objective of the present study was to assess the effects of Bifidobacterium isolated on human colon cancer cell lines in in vitro condition.
MATERIALS AND METHODS
The present study was conducted on 50 infants from south-central Iran in 2016. The study protocol was approved by the Ethics Committee of Islamic Azad University. Written informed consent was obtained from the mothers of infants who participated in the present study.
Sample collection
This was a cross-sectional study conducted in winter 2016. Samples were isolated from different origins. Overall, 33 samples of local and pasteurized dairy products, 50 samples of infant feces (who were breast-fed), and a Familact capsule sample were collected in Falcon sterile containers, transported to the laboratory in packed ice, and kept at refrigerator temperature for the experiment. Standard strain of Bifidobacterium bifidum [1644] was provided from the Persian Type Culture Collection (PTCC, Iran) that was tested at the same time.
Bifidobacterium bacteria isolation
First, samples were serially diluted 10-fold from 10−1 to 10−4, and 100 μL was spread onto selective medium agar. Bifidobacterium medium agar (QUELAB, Canada) was inoculated to enrich the specific medium of Bifidobacterium, and samples were incubated under anaerobic conditions using a jar and Gas Pack A (Merck, Germany) at 37 °C for 72 h. After 72 h, colonies of bacteria and morphology by Gram staining were examined (7).
Identification of bacteria by biochemical tests
Catalase and oxidase tests, gelatinase activities, production of indole, and production of gas from glucose were used to identify the colonies that were suspected of Bifidobacterium, and fermentable carbohydrates were determined on phenol red base broth (QUELAB) and supplemented with 1% of the following carbohydrates: glucose, sucrose, maltose, lactose, and galactose (Merck). Each tube was supplemented with two drops of sterile liquid paraffin after inoculation to ensure an anaerobic condition.
Molecular identification
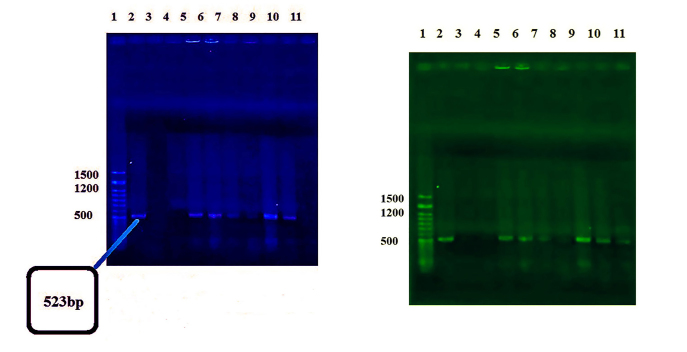
The aim of the polymerase chain reaction (PCR) is the synthesis of new DNA strands according to the string pattern that is a repeated chain. PCR is widely used in molecular biology (8). DNA extraction was performed by using a high pure DNA isolation kit (Yekta Tajhiz Azma, Tehran, Iran) according to the manufacturer’s instructions. 16S rRNA of specific primers forward (Bif164) and reverse (Bif662) Bifidobacterium that was synthesized by Malaysia 1st BASE was used for smarter identification and amplification of fragment 523 bp gene region. PCR was accomplished by a thermocycler (BioRad, USA). All components were purchased from Yekta Tajhiz Azma (Tehran, Iran). The reaction mixture contained 25 μL Master Mix (including PCR buffer at a concentration of 10 times, magnesium chloride, dNTP (10 Mm), Taq DNA polymerase enzyme (5 U/μL), 3 μl DNA (100 ng/L), 2 μL forward primer (10 pmol) (5′-GGGTGGTAATGCCGGATG-3′), 2 μL reverse primer (10 pmol) (5′-CCACCGTTACACCGGGAA-3′), and 18 μL water). In this technique, to start the polymerization process, thermal cycler machine was set at 94°C for 1 min, followed by 35 cycles of PCR performed at 94°C for 1 min, 58.8°C for 30 s, and 72°C for 1 min. Eventually, 4 min of elongation was done at 72°C. Finally, to ensure the amplification gene 16S rDNA, electrophoresis on 1% agarose gel containing TBE 1× buffer was performed for 60 min at 90 V (9). The gel results were observed by using ultraviolet (UV transilluminator machine, USA).
Metabolite preparation
Suspension bacterial cell cultures were centrifuged at 10,000 rpm for 10 min. The pH was adjusted to 7.4, and the supernatant was then sterilized using a 0.22 μm filter (Millipore, USA). Cell-free supernatants (CFS) were applied to cytotoxicity assessments (10).
Cell culture
At this level, the cells have to be subcultured by transferring them to a new plate with fresh growth medium to supply more room for continued growth. The human colon cancer cell line (SW742) was obtained from the Iran Cell Line Bank. SW742 cell was cultured in Roswell Park Memorial Institute 1640 medium (RPMI-1640), including fetal bovine serum, 1% penicillin (10,000 U/mL) [v/v], and streptomycin (10,000 U/mL) [P/S]. The culture was incubated at 37°C in a humidified atmosphere with 5% CO2. In each well of 96-well plate, 90 μL culture medium RPMI with 1×105 cell and 10 μL 24 h and 120 CFS with different concentrations of 1–1−7 mg/ml were added and incubated for 72 h. After they were grown to confluence in 75 cm2 tissue culture flasks (NunC, Denmark), the cells were detached and transferred to new cell culture dishes in a trypsin mixture (Cambrex Bio Science, USA). Cell number and viability were evaluated by the trypan blue dye-exclusion assay. The MTT method was used to evaluate the effects of CFS on colon cancer cell lines, and the results were read by an ELISA reader (Awareness, USA) at 570 nm. Cytopathic effects were photographed by an inverted microscope (Olympus, USA). For verification of the accuracy of the results of PCR samples for sequencing, they were sent to 1st BASE in Malaysia, and the results of sequencing were analyzed by the Chromas software (DNA sequencing analysis, USA) and BLAST (NCBI, USA) (11, 12).
Statistical analysis
All of the numerical data were examined through one-way analysis of variance (statistical model, USA) method using Statistical Package for Social Sciences version 18 (IBM Corp.; Armonk, NY, USA) to evaluate the experimental data. All of the mean multiple comparisons were conducted using Duncan tests. The significant differences were accepted at p≤0.05. Graphs were arranged using Microsoft Office (Excel, USA).
RESULTS
In the present study, from 84 samples collected based on the morphology of the colony, 30 isolates were collected, and according to biochemical tests, 20 isolates were identified as Bifidobacterium. After molecular identification, 17 isolates of Bifidobacterium were isolated, whereas 2 isolates had cytotoxicity effect. B. bifidum was effective in combating cancer cells and was associated with a substantial improvement in gastrointestinal cancer.
Bacterial identification
Circular, convex, milky, and small colonies were observed and collected. Results of Gram staining under an optical microscope (Nikon, Japan) were seen as Y-shaped or V-shaped Gram-positive bacteria, clubs, or curved shape in purple and are shown in Figures 1 and 2.
Figure 1.
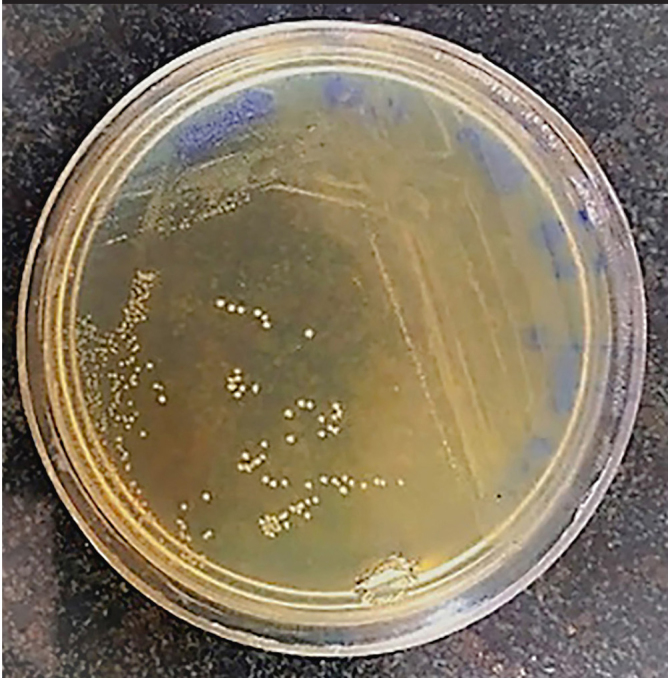
Bifidobacterium Colony on BFM.
Figure 2.
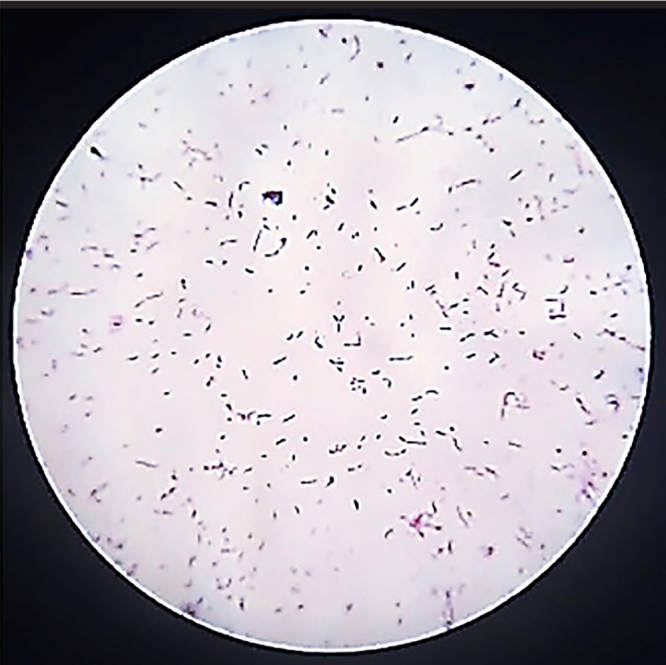
Bifidobacterium Gram staining.
Bacterial identification by biochemical and molecular tests
Catalase and oxidase tests were negative according to the standard sample. The bacteria had no movement and did not produce hydrogen sulfide. Gelatinase test was negative, and carbohydrate fermentation in strains was different. They did not have negative indole and were not able to produce gas from glucose. Biochemical test results are shown in Table 1.
Table 1.
Results of biochemical test
| ROW | Samples | Oxidase | Indole | Motility | Gelatinase | Sucrose | Catalase | Gas from Glucose | Galactose | Glucose | Lactose | Maltose |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | SS*1644 | − | − | − | − | + | − | − | + | + | + | − |
| 2 | ChPSh | − | − | − | − | − | − | + | − | + | + | − |
| 3 | ChTSh | − | − | − | − | + | − | + | − | + | − | + |
| 4 | CTSh | − | − | − | − | + | − | − | − | + | + | + |
| 5 | YTSh1 | − | + | − | − | + | − | − | − | + | + | − |
| 6 | YTSh2 | − | + | − | − | + | − | − | − | − | − | V |
| 7 | ChPK | − | − | − | − | + | − | − | − | + | + | + |
| 8 | ChTKB | − | − | − | − | + | − | − | − | + | + | + |
| 9 | ChTKD1 | − | − | − | − | + | − | − | + | + | + | + |
| 10 | ChTKD2 | − | − | − | − | + | − | − | + | + | + | + |
| 11 | ChTKG | − | − | − | − | + | − | − | − | + | + | + |
| 12 | CTK1 | − | − | − | − | − | − | − | − | V | V | V |
| 13 | CTK2 | − | − | − | − | + | − | − | − | + | + | + |
| 14 | MTKB | − | − | − | − | + | − | − | − | V | + | + |
| 15 | ShTK | + | − | − | + | + | − | − | − | + | − | + |
| 16 | YPPK | − | − | − | − | + | − | − | − | + | + | + |
| 17 | YTKG | + | − | − | − | + | + | − | − | + | + | − |
| 18 | IFH12 | − | + | − | − | + | − | + | + | + | + | + |
| 19 | IFH19 | − | − | − | − | + | − | − | + | + | + | + |
| 20 | IFH31 | − | − | − | − | + | + | − | + | + | − | + |
| 21 | IFH39 | − | − | − | − | + | + | − | + | + | − | + |
| 22 | IFH41 | − | − | − | − | + | − | − | + | + | + | + |
| 23 | IFH45 | − | − | − | − | + | − | − | + | + | + | + |
| 24 | IFH46 | − | − | − | − | + | − | − | + | + | + | + |
| 25 | IFH47 | − | − | − | − | + | − | − | + | + | + | − |
| 26 | IFH48 | − | + | − | − | + | − | − | + | + | + | − |
| 27 | IFH50 (1) | − | − | − | − | + | − | − | + | + | + | + |
| 28 | IFH50 (2) | − | − | − | − | + | − | − | + | + | + | + |
| 29 | CPFD1 | − | − | − | − | − | − | − | + | + | + | + |
| 30 | CPFD2 | − | − | − | − | + | − | − | + | + | + | − |
| 31 | CPFD3 | − | − | − | − | + | − | − | + | + | + | + |
SS1644 (Standard Sample)
Seventeen isolates of Bifidobacterium were identified by PCR, and the results are given in Figure 3. Frequency percent based on biochemical and molecular tests is shown in Figure 4.
Figure 3.
PCR results on electrophoresis gel. (Left) Column 1: Yekta Tajhiz Azma Marker 50bp, 2: Negative Control and Other Bifidobacterium Isolates. (Right) Column2: Yekta Tajhiz Azma Marker 50bp and Other Bifidobacterium Isolates.
Figure 4.
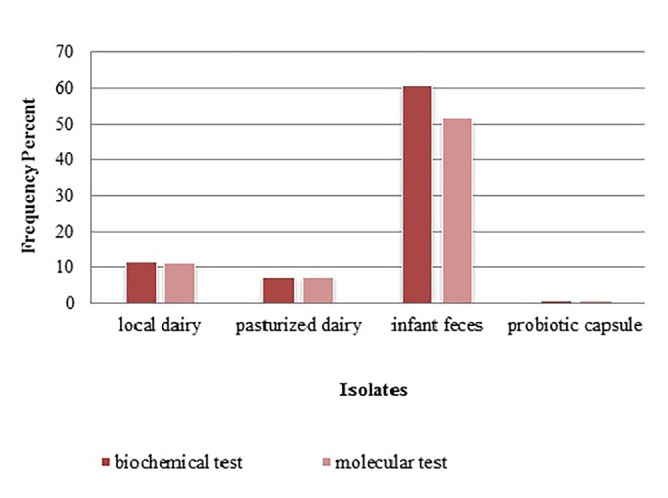
Frequency percent based on biochemical and molecular tests..
Cell culture
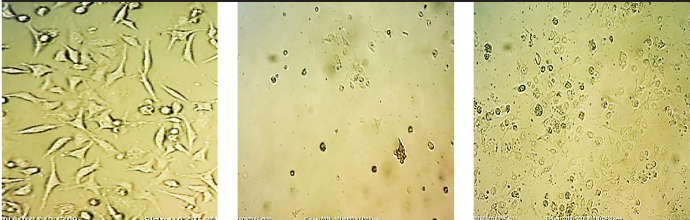
From 17 selective isolates, infant feces 47 (IFH47) and oral probiotic capsule Familact 2 (CPFD2) isolates had the greatest cytotoxicity effect on colon cancer cell line (Figure 5). These two selective isolates were treated with different concentrations of the CSF, and the highest concentration of cytotoxicity was achieved at a concentration of 1 mg/mL; the results are shown in Figure 6. The impact of CFS isolated from two selective isolates is shown in Figure 7, and the influence of CFS of two selective isolates on colon cancer cell line at 24 h and 72 h of incubation time is shown in Figure 8. After cell line treatment with Bifidobacterium CFS, the cytotoxic effects were observed and recorded by an inverted microscope. As shown in colon cancer cell line, the cells were observed in fusiform. The cytopathic effect of colon cancer cell line with 120 h CFS of two selective isolates is observed as the fragmentation of the cell, cell destruction, and reduction of cell density. The CPFD isolated cytopathic effect is greater than the IFH47, and the results are shown in Figure 9. Two isolates had the greatest cytotoxicity effect on colon cancer cell line, they were sent to 1st BASE in Malaysia, and the results of sequencing were analyzed by the Chromas software (DNA sequencing, USA) and BLAST (NCBI, USA). Analysis of data showed two isolates had 99% similarity to B. bifidum strain BXH2-3.
Figure 5.
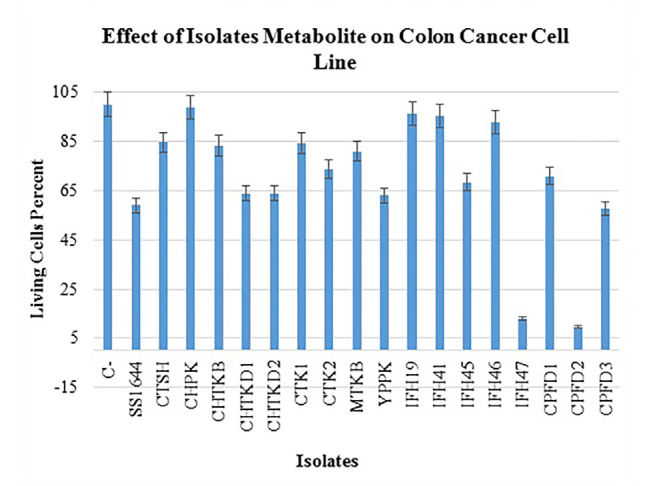
Treatment colon cancer cell line by 120 hours CFS isolated.
Figure 6.
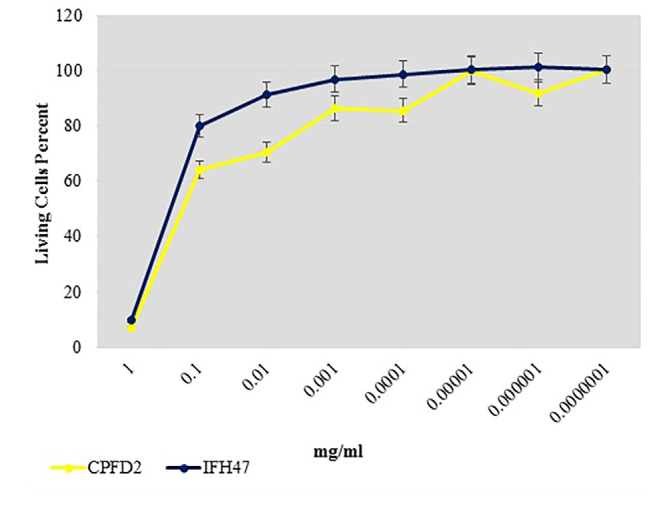
Comparison cytotoxicity effect 2 selective isolates IFH47, CPFD2 on colon cancer cell line.
Figure 7.
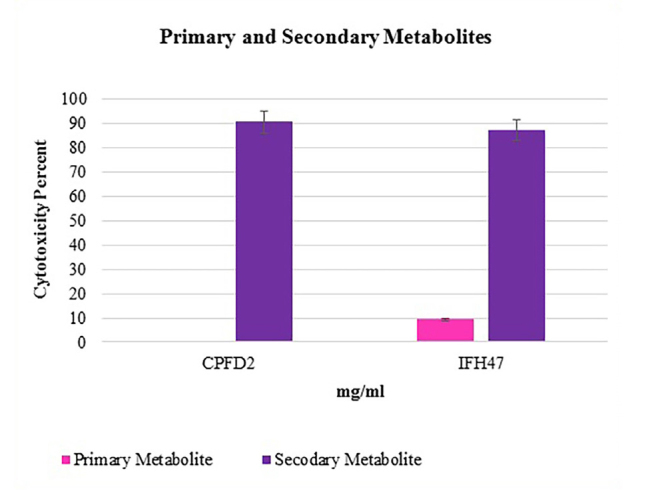
Treatment colon cancer cell line with 24 hours and 120 hours CFS of 2 selective isolates.
Figure 8.
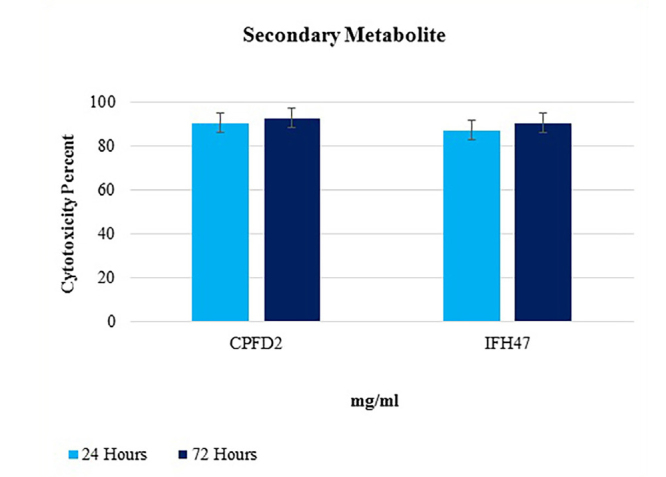
Treatment colon cancer cell line with 120 hours CFS of 2 selective isolates in 24, 72 hours.
Figure 9.
Cytopathic effects of 2 isolates CPFD2 and IFH47 on colon cancer cell line by invert microscope. (Left) Isolate CPFD2, (Right) Isolate IFH47, (Down) Negative Control.
Death of cell by apoptosis or necrosis
To evaluate apoptosis or necrosis, 1 mg/mL selective CFS was added to the culture after the cell culture. The culture was incubated at 37 °C for 24 h. After 24 h, the cells died by toxin cytopathic activities, and they were floated on broth culture. The contents of the culture were collected and centrifuged (Hettich, Germany) at 3000 rpm for 10 min. DNA of cell line extraction was performed by using a high pure DNA isolation kit (Yekta Tajhiz Azma, Tehran, Iran) according to the manufacturer’s instructions. After centrifugation, the cell genome pellet was visible at the bottom of the microtube. Cell death detection was performed by using a cell death detection kit (Sigma Aldrich, Germany) according to the manufacturer’s instructions, and extracted DNA was set with electrophoresis on 1.5% agarose gel for 60 min at 90 V. The gel results were observed by using ultraviolet (UV transilluminator machine, USA). Necrosis samples showed an overall smaller DNA signal and no trace of laddering, whereas apoptosis samples showed bigger DNA signal and trace of laddering. The result of the present study showed that samples had a smaller DNA signal and no trace of laddering on electrophoresis gel that potentially indicated necrosis of the tumor cells.
DISCUSSION
Bifidobacterium and Lactobacillus are probiotic organisms in humans and stimulate antitumor effects and immune function (1). Bifidobacterium inhibits the growth and attachment of pathogens to epithelial cells, and these organisms are constituents produced as bacteriocin and hydrogen peroxide that kill the pathogenic microorganisms in humans (13). The principal products of the metabolism of Bifidobacterium are lactic and acetic acids. These two acids lower the pH within the gut, especially in the cecum and the ascending colon. It is likely that the ability of Bifidobacterium to increase the acidity of the gut is a factor in their probiotic effects; thus, many harmful microbes are inhibited in a low pH environment (14). In this report, the effect of B. bifidum on cancer cell line SW742 was studied, and the results showed that the CFS of B. bifidum has the ability to inhibit the growth of colon cancer cells. Bifidobacterium is strictly anaerobic, but a study by Martin in 2003 found that Bifidobacterium thermophilus and B. bifidum could grow in oxygen pressure. During this study, no Bifidobacterium grew in aerobic conditions (9). Rita-Narayanan et al. (15) in 2013 for the isolation and identification of Bifidobacterium from infant feces used phenotype identification methods including morphology of colony and carbohydrate fermentation. The results of this study showed that Bifidobacterium, a Gram-positive bacterium with Y- or V-shaped colony and different biochemical tests, was reported in other species. In this study, the results were similar with previous study. In 2007, Gronlund et al. (16) reported a significant amount of Bifidobacterium in the feces of breast-fed infants. In 2014, Jost et al. (17) examined the number of anaerobic intestinal bacteria, such as Bifidobacterium, Clostridia, and Bacteroides, and found a connection between breast milk and infant feces. Bifidobacterium may keep the host from carcinogenic activity by decreasing the activity of these potential carcinogens. In 2008, Kim reviewed that the CFS of Bifidobacterium Spmo212 inhibits the expansion of human colon cancer cell lines SW480, CacoII, and HT-29 (18). In this study, from 84 selective isolates, 2 isolates had the greatest cytotoxicity effect on colon cancer cell line, and both of them were B. bifidum. The reason behind its effectivity in one of the infant feces samples is due to the differences in genetics of bacteria in different persons and different places. In addition, changing the genetics of bacteria changes the type and character of CFS that causes different effects. The role of gut flora probiotic bacteria in the prevention of colon cancer is to help defend against cancer by increasing the beneficial bacteria, reducing the level of pathogens, changes in metabolism, enzyme activity, reducing inflammation, and increasing the immune system (19). Interventional treatment of probiotics in patients with CRC was studied by Gao et al. (20), and the results demonstrated microflora improvement in mucosal intestine and a significant reduction in frequency-dependent mucosal pathogens. In 2016, Liang et al. (21) studied the effect of probiotics on bacterial overgrowth in patients with CRC. The results showed that Bifidobacterium triple viable capsule had significant improvement in cancer symptoms. In 2017, they investigated the mechanism of probiotics action in the prevention of CRC and found that the intestinal flora is the most critical risk factor in the amplification of CRC, and that probiotics are able to balance the intestinal microflora (2). In 2017, Hibberd et al. (22) reported that the intestinal microbiota is altered in patients with colon cancer and modified by probiotic intervention. The results showed promise for potential therapeutic benefits in CRC by manipulation of the microbiota).
Ohara and Suzutani investigated the effects of intake of yogurt containing Bifidobacterium longum (BB536-y) and fructo-oligosaccharides (FOS) in preventing colorectal carcinogenesis in healthy subjects and the preventive effects of SCFAs, whose production was enhanced by the intake of BB536-y and FOS, in human colon cancer cell lines. Their findings suggested that the intake of both BB536-y and BB536-y with FOS prevents colorectal carcinogenesis (23). Borges-Canha et al. (24) studied the role of colonic microbiota in colorectal carcinogenesis. They suggested that colorectal carcinogenesis is associated with microbial dysbiosis. This information may be used to create new prophylactic, diagnostic, and therapeutic strategies for CRC. Owing to the beneficial effects of probiotics, the results of this survey and the fact that in our country there is a lesser amount of beneficial probiotic in food, development of the Cell Bank probiotics and their use in the industry can provide valuable benefits for society.
In conclusion, Bifidobacterium may become an important means of elevating gastrointestinal health and averting illness. Currently, scientific evidence suggests that Bifidobacterium exerts anti-carcinogenic activity by potential physiological mechanisms, usually co-dependent and strain-specific. The regular consumption of probiotics should not be an impediment to general health. Generally, B. bifidum can help human health and inhibit cancer cell formation through the intestinal microflora balance.
Acknowledgements
We appreciate the contributions of Samira Bahmani to this study.
Footnotes
Ethics Committee Approval: Ethics committee approval was obtained from the Ethics Committee of Islamic Azad University, Shiraz Branch.
Informed Consent: Written informed consent was obtained from parents of the infants who participated in this study.
Conflict of Interest: The authors have no conflict of interest to declare.
Financial Disclosure: The authors declared that this study has received no financial support.
REFERENCES
- 1.Lee DK, Jang S, Kim MJ, et al. Anti-proliferative effects of Bifidobacterium adolescentis SPM0212 extract on human colon cancer cell lines. BMC Cancer. 2008;8:310–7. doi: 10.1186/1471-2407-8-310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dos-Reis SA, Da-Conceicao LL, Siqueira NP, et al. Review of the mechanisms of probiotic actions in the prevention of colorectal cancer. Nutr Res. 2017;37:1–19. doi: 10.1016/j.nutres.2016.11.009. [DOI] [PubMed] [Google Scholar]
- 3.Zinedine A, Faid M. Isolation and characterization of strains of Bifidobacteria with probiotic proprieties in vitro. J Dairy Sci. 2007;2:28–34. [Google Scholar]
- 4.Issazadeh K, Aliabadi MA, Darsanaki RK, Alikhani F, Dadras H, Tajehmiri A. Isolation, Identification and Analysis of Probiotic Properties of Lactobacillus spp from Traditional Yoghurts in North of Iran. J Appl Microbiol. 2013;7:2965–71. [Google Scholar]
- 5.Tajehmiri A, Darsanaki RK, Moslem MN, Lozoumi Z, Kolavani MH, Aliabadi MA. Antimicrobial Activity of Lactobacillus spp Isolated from Commercial Yoghurts against Pathogenic Bacteria. J Appl Microbiol. 2014;8:2211–5. [Google Scholar]
- 6.Tojo R, Suarez A, Clemente MG, et al. Intestinal microbiota in health and disease: role of bifidobacteria in gut homeostasis. World J Gastroenterol. 2014;20:15163–9. doi: 10.3748/wjg.v20.i41.15163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang M, Jiang R, Liu M, et al. Study of the Probiotic Properties of Lactic Acid Bacteria Isolated from Chinese Traditional Fermented Pickles. J Food Process Preserv. 2016;41:12954–61. doi: 10.1111/jfpp.12954. [DOI] [Google Scholar]
- 8.Lorenz TC. Polymerase chain reaction: basic protocol plus troubleshooting and optimization strategies. J Vis Exp. 2012;63:3998–4007. doi: 10.3791/3998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martin R, Langa S, Reviriego C, et al. Human milk is a source of lactic acid bacteria for the infant gut. J Pediatr. 2003;143:754–8. doi: 10.1016/j.jpeds.2003.09.028. [DOI] [PubMed] [Google Scholar]
- 10.Haghgshenas B, Abdullah N, Nami Y, et al. Different effects of two newly-isolated probiotic Lactobacillus plantarum 15HN and Lactobacillus lactis subsp. Lactis 44lac strain from traditional dairy products on cancer cell lines. Anaerobe. 2014;30:51–9. doi: 10.1016/j.anaerobe.2014.08.009. [DOI] [PubMed] [Google Scholar]
- 11.Michelini S, Modesto M, Oki K, et al. Isolation and identification of cultivable Bifidobacterium spp from the faeces of 5 baby common marmosets (Callithrix jacchus L) Anaerobe. 2015;33:101–4. doi: 10.1016/j.anaerobe.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 12.Moazamian E, Bahador N, Azarpira N, et al. Anti-cancer parasporin toxins of new Bacillus thuringiensis against human colon (HCT-116) and blood (CCRF-CEM) cancer cell lines. Curr Microbiol. 2018;75:1090–8. doi: 10.1007/s00284-018-1479-z. [DOI] [PubMed] [Google Scholar]
- 13.Amin M, Sheikh AF, Goodarzi H, Sormeh M. Identification of Bifidobacterium animalis, Bifidobacterium adolescentis and Bifidobacterium bifidum from Stool of Children and Detection of Their Antibacterial Properties. Adv Infect Dis. 2013;3:200–6. doi: 10.4236/aid.2013.33029. [DOI] [Google Scholar]
- 14.Picard C, Fioramonti J, Francois A, Robinson T, Neant F, Matuchansky C. Bifidobacteria as probiotic agents physiological effects and clinical benefits. Aliment Pharmacol Ther. 2005;22:495–510. doi: 10.1111/j.1365-2036.2005.02615.x. [DOI] [PubMed] [Google Scholar]
- 15.Rita-Narayanan BSS, Pugzhenthi TR. Isolation of Bifidobacteria from Infant Faeces. Indian J Med Res. 2013;3:269–71. [Google Scholar]
- 16.Gronlund MM, Gueimonde M, Laitinen K, et al. Maternal breast-milk and intestinal Bifidobacteria guide the compositional development of the Bifidobacterium microbiota in infants at risk of allergic disease. Clin Exp Allergy. 2007;37:1764–77. doi: 10.1111/j.1365-2222.2007.02849.x. [DOI] [PubMed] [Google Scholar]
- 17.Jost T, Lacroix C, Braegger CP, Rochat F, Chassard C. Vertical mother-neonate transfer of maternal gut bacteria via breastfeeding. Environ Microbiol. 2014;16:2891–902. doi: 10.1111/1462-2920.12238. [DOI] [PubMed] [Google Scholar]
- 18.Kim Y, Lee D, Kim D, et al. Inhibition of proliferation in colon cancer cell lines and harmful enzyme activity of colon bacteria by Bifidobacterium adolescentis SPM0212. Arch Pharm Res. 2008;31:468–75. doi: 10.1007/s12272-001-1180-y. [DOI] [PubMed] [Google Scholar]
- 19.Wasilewska E, Zlotkowska D, Pijagin ME. The role of intestinal microflora and probiotic bacteria in prophylactic and development of colorectal cancer. Postepy Hig Med Dosw. 2013;67:837–47. doi: 10.5604/17322693.1061847. [DOI] [PubMed] [Google Scholar]
- 20.Gao Z, Guo B, Gao R, Zhu Q, Wu W, Qin H. Probiotics modify human intestinal mucosa-associated microbiota in patients with colorectal cancer. Mol Med Rep. 2015;12:6119–27. doi: 10.3892/mmr.2015.4124. [DOI] [PubMed] [Google Scholar]
- 21.Liang S, Xu L, Zhang D, Wu Z. Effect of probiotics on small intestinal bacterial overgrowth in patients with gastric and colorectal cancer. Turk J Gastroenterol. 2016;27:227–32. doi: 10.5152/tjg.2016.15375. [DOI] [PubMed] [Google Scholar]
- 22.Hibberd AA, Lyra A, Ouwehand AC, et al. Intestinal microbiota is altered in patients with colon cancer and modified by probiotic intervention. BMJ Open Gastroenterol. 2017;4:145–57. doi: 10.1136/bmjgast-2017-000145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ohara T, Suzutani T. Intake of Bifidobacterium longum and Fructo-oligosaccharides prevents Colorectal Carcinogenesis. Euroasian J Hepatogastroenterol. 2018;8:11–7. doi: 10.5005/jp-journals-10018-1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Borges-Canha M, Portela-Cidade JP, Dinis-Ribeiro M, et al. Role of colonic microbiota in colorectal carcinogenesis: a systematic review. Rev Esp Enferm Dig. 2015;107:659–71. doi: 10.17235/reed.2015.3830/2015. [DOI] [PubMed] [Google Scholar]