Abstract
Background/Aims
Anorectal diseases, including fecal incontinence, are prevalent and have an enormous impact on the quality of life. Therefore, investigating their etiological factors may help to reduce the incidence and/or the severity of the underlying diseases.
Materials and Methods
Referral complaints (constipation, strained defecation, and incontinence) and medical and anorectal manometry records of 883 (562 female/321 male, ages 45.17±1.00 and 48.41±0.63 years, respectively) patients were evaluated retrospectively. Maximal resting pressure (MRP) and maximal squeeze pressure (MSP) measured by stationary pull-through technique, volume of rectoanal inhibitory reflex, and sensory threshold to rectal balloon distention (ST) were obtained by water perfusion system. Data were compared according to referral complaints, age, gender, parity, and underlying diseases.
Results
Incontinence was the most frequent referral complaint in 61.2% of females and 67.6% of males. MRP and MSP were significantly lower in incontinent females than in the other groups. In incontinent males, MSP was lower than the strained defecation group, and ST was higher than the constipation group. Age was negatively correlated with MRP for both of the genders and in all groups. Obstetric trauma (85%) and number of parity (3.40±2.59) were significantly higher in incontinent females. Moreover, the most prevalent underlying disease was diabetes in incontinent females (13.7%) and neurological diseases, including traumas, in incontinent males (41.5%).
Conclusion
Increasing awareness of labor safety, controlling diabetes mellitus, and preventing obstetric traumas may reduce the prevalence of fecal incontinence.
Keywords: Anorectal manometry, fecal incontinence, constipation, gender
INTRODUCTION
Fecal incontinence affects up to 7%–15% of individuals worldwide and is a medico-social problem that deteriorates the quality of life enormously. Its prevalence and etiology vary according to the studied population (1). In these patients, tests of anorectal function are known to be useful and can influence the management of defecation disorders.
Gender and age alter the physiology and pathology of the gastrointestinal system. Anorectal manometric parameters are also influenced by gender and age in a healthy population and in patients with defecation disorders (2–4). Sphincter pressures tend to be lower in females than in males (3–8), and age leads to a consistent reduction in anal function (3–8).
The effect of parity on anorectal manometric parameters has not been well described. No relationship was found between parity and anal pressures in healthy females (2,6). In one study, parous healthy females have lower maximal resting pressure (MRP) than nulliparous healthy females (7). Mahony claimed that increasing age and parity, instrumental delivery, an anal canal resting pressure, and internal anal sphincter injury are significantly related to the presence of fecal incontinence (9). Eogan found that premenopausal females who had undergone cesarean delivery have significantly higher manometry pressures than those who delivered vaginally (10).
There are not enough published data about anal manometric parameters in common anorectal diseases in our country. Therefore, to determine the etiology of common anorectal diseases and impact of age, gender, and parity on those diseases in our population, we retrospectively evaluated data of our patients referred for motility study.
MATERIAL AND METHODS
Records of 883 (562 female and 321 male) patients who were subjected to anorectal manometry due to any anorectal complaint between 2001 and 2011 in Marmara University Department of Gastroenterology Motility Unit were evaluated retrospectively. Referral complaints, full medical history, digital rectal examination, and anal manometry findings were assessed. Informed consent was obtained from all participants before the manometric examination.
Participants were grouped according to their referral complaints (constipation, strained defecation, and incontinence) and their age at the time of the manometric investigation (younger/older than 50 years). Patients complaining of defecation <3 times/week were categorized as the constipation group, and patients reporting uncontrolled passage of fecal material (solid, liquid, and mucus) were defined as the incontinence group (urge or passive). Urge incontinence was defined as the inability to control impending bowel movement, and passive incontinence was defined as an unconscious loss of stool. Patients complaining of either incomplete evacuation or obstructed defecation most of the time for at least 6 months were categorized as the strained defecation group. Criteria for functional gastrointestinal diseases (11) were not used, because we did not exclude patients with organic diseases.
Females according to parity were classified into three groups: nullipara, those with ≤3 children, and those with ≥4 children. Females reporting to have a tear and instrumentation during vaginal delivery were classified as the group with obstetric trauma. For each group, anal manometric data were evaluated.
Anal manometry
Anal sphincter manometry was performed by a pre-calibrated, water perfusion system using a catheter with eight holes 0.5 cm apart (Andorfer Inc., WI, USA) and a balloon inserted on the tip of the catheter 60 mm in length. Manometric data were stored and analyzed by computer software (Smartgraph Rev.3.40; Sandhill Scientific Inc., Highlands Ranch, CO, USA). After bowel preparation with enema, patients were placed comfortably in the left lateral position. MRP and maximal squeeze pressure (MSP) measurements were done. MRP was defined as the average of maximal pressures obtained from all holes by stationary pull-through technique. MSP was defined as the mean of differences between the mean pressures obtained by voluntary squeezing for 5 s and resting pressures when all of the holes were within the anal canal.
The rectal sensation and the rectoanal reflexes were evaluated by sequentially inflating the rectal balloon with a hand-held syringe using the following volumes: 10, 20, 30, 40, and 60 mL. Each inflation was maintained for 1 min. After deflation, a rest period of 2 min was allowed before reinflating the balloon. Rectoanal inhibitory reflex (RAIR) was defined as a sharp decrease in basal pressure of at least 10 cm H2O, followed by a slow recovery to the original pressure. The lowest volume to induce the RAIR was determined. The threshold of sensation (ST) is the minimal amount of air required to elicit a transient sensation of balloon distension.
Statistical analysis
Statistical analyses were performed using Prism software (GraphPad Prism 6.0, serial no. GPM6-131891-RJJW-4325E; GraphPad Software, La Jolla, CA, USA). Descriptive data were presented as percentage and mean±standard deviation. The Mann–Whitney U test was used to compare continuous variables in two independent groups. The Kruskal–Wallis test was used to compare continuous variables in three or more independent groups. Post-hoc analysis was performed by Dunn’s multiple comparison test. Chi-square test or Fisher’s exact test was used to compare categorical variables. Spearman’s correlation coefficient was used to assess the correlation of continuous variables. A p value <0.05 was considered as statistically significant.
RESULTS
The mean ages of the female and male patients were 45.17±1.00 and 48.41±0.63 years, respectively (p=0.0041). Referral complaints of the male and female patients and underlying etiological factors (diseases) are given in Table 1. The most common referral complaint was fecal incontinence (61.2% of females and 67.6% of males), followed by constipation and strained defecation in females and strained defecation and constipation in males. The rate of passive incontinence was high in males (54.8%), and the rate of urge incontinence was high in females (61.9%) (p<0.0001). Diabetes mellitus rates (13.7%) were found to be higher in female patients with incontinence (p<0.0001). Neurological disease (cerebrovascular accidents, demyelinating diseases, spinal trauma, cranial and spinal surgery, and congenital anomaly) rates (41.5%) were found to be higher in male patients with incontinence (p=0.026). Anorectal surgery rates were not different in the referral complaint groups (p=0.229) (Table 1).
Table 1.
Referral complaints and underlying etiological factors.
| Diabetes n (%) |
Neurologic diseases n (%) |
Anorectal surgery n (%) |
Anal intercourse n (%) |
n (%) | |
|---|---|---|---|---|---|
| Strained defecation (male) | 2 (3.5) | 11 (19.3) | 13 (22.8) | 0 | 57 (17.8) |
| Constipation (male) | 5 (10.6) | 14 (29.8) | 5 (10.6) | 0 | 47 (14.6) |
| Incontinence (male) | |||||
| Urge | 6 (6.1) | 29 (29.6) | 23 (23.5) | 0 | 98 (30.5) |
| Passive | 10 (8.4) | 61 (51.5) | 21 (17.6) | 1 (0.8) | 119 (37.5) |
| Total | 23 (7.2) | 115 (35.8) | 62 (19.3) | 1 (0.3) | 321 (100) |
| Strained defecation (female) | 3 (3.0) | 18 (17.8) | 16 (15.8) | 1 (1.0) | 101 (18.0) |
| Constipation (female) | 6 (5.1) | 19 (16.2) | 15 (12.8) | 4 (3.4) | 117 (20.8) |
| Incontinence (female) | |||||
| Urge | 31 (14.6) | 41 (19.2) | 27 (12.7) | 3 (1.4) | 213 (37.9) |
| Passive | 16 (12.2) | 22 (16.8) | 27 (20.6) | 1 (0.8) | 131 (23.3) |
| Total | 56 (10.0) | 100 (17.8) | 84 (14.9) | 9 (1.6) | 562 (100) |
Anorectal manometry results according to referral complaints are presented in Table 2. The magnitude of MRP (p<0.0001) and MSP (p<0.0001) was both lower, and the magnitude of ST (p=0.005) was higher in the incontinence group. The difference between the means of MRP, MSP, and ST among the groups was found to be statistically significant. Post-hoc analysis showed statistically significant differences between the strained defecation versus incontinence and constipation versus incontinence groups for MRP and MSP and constipation versus incontinence groups for ST (Table 2).
Table 2.
Anorectal parameters in different referral complaint groups.
| Strained defecation | Constipation | Incontinence | p* | |
|---|---|---|---|---|
| Age (year) | 45.91±15.14 | 45.34±18.02 | 48.16±15.74 | 0.075 |
| MRP (cm H2O) | 63.50±30.06 | 60.27±31.89 | 52.50±29.56 | <0.0001 |
| MSP (cm H2O) | 103.50±66.34 | 96.92±63.78 | 79.22±57.90 | <0.0001 |
| ST (mL) | 29.25±19.72 | 27.03±18.16 | 32.55±22.80 | 0.005 |
| RAIR (mL) | 24.25±9.07 | 24.36±15.47 | 23.39±13.30 | 0.134 |
MRP: maximal resting pressure; MSP: maximal squeeze pressure; ST: sensory threshold; RAIR: rectoanal inhibitory reflex.
Kruskal-Wallis test.
Anorectal manometry results according to gender are presented in Figure 1. The magnitude of MRP (p=0.0002), MSP (p<0.0001), RAIR (p=0.03), and ST (p=0.0002) varied according to gender, and all were significantly lower in females (Figure 1).
Figure 1.
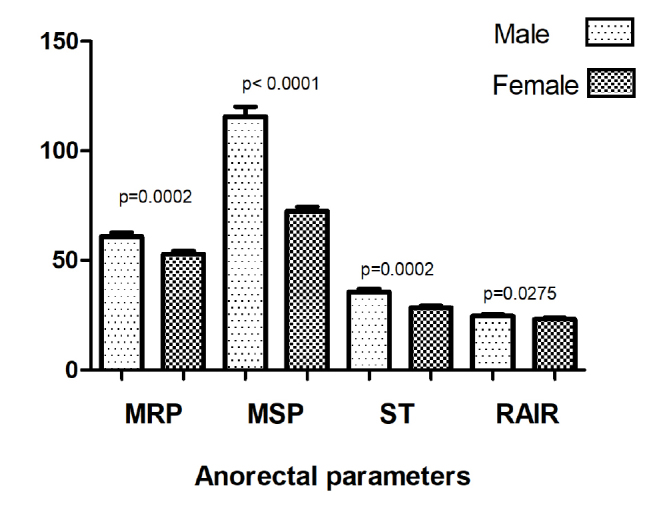
Anorectal manometry results according to gender. MRP, maximal resting pressure (cm H2O); MSP, maximal squeeze pressure (cm H2O); ST, sensory threshold (mL); RAIR, rectoanal inhibitory reflex (mL).
Anorectal manometry results of females according to their referral complaints are given in Table 3. Incontinent females were older (p<0.0001) and their sphincters were weaker (MRP (p<0.0001) and MSP (p<0.0001)) than the rest of the patients. The magnitude of RAIR was also lower in incontinent patients (p=0.01). The difference between the means of age and magnitude of MRP, MSP, and RAIR among the groups was statistically significant. Statistically significant differences were found between the strained defecation versus constipation and constipation versus incontinence groups for age, strained defecation versus incontinence and constipation versus incontinence groups for MRP and MSP, respectively, and strained defecation versus incontinence for RAIR in post-hoc analysis (Table 3).
Table 3.
Anorectal parameters of females in different referral complaint groups.
| Strained defecation | Constipation | Incontinence | p* | |
|---|---|---|---|---|
| Age (year) | 48.79±1.35 | 43.17±1.36 | 50.09±0.81 | <0.0001 |
| MRP (cm H2O) | 64.61±2.94 | 59.55±3.02 | 47.42±1.49 | <0.0001 |
| MSP (cm H2O) | 86.13±5.16 | 83.92±4.69 | 64.58±2.34 | <0.0001 |
| ST (mL) | 28.45±1.87 | 26.47±1.81 | 29.14±1.04 | 0.124 |
| RAIR (mL) | 28.85±2.27 | 24.03±1.40 | 22.79±0.78 | 0.012 |
MRP: maximal resting pressure; MSP: maximal squeeze pressure; ST: sensory threshold; RAIR: rectoanal inhibitory reflex.
Kruskal-Wallis test.
Anorectal manometry results of males according to their referral complaints are given in Table 4. Constipated males were older (p=0.04). In incontinent males, the magnitude of MSP (p=0.03) was lower, and the magnitude of ST (p=0.01) was higher than the rest of the males. The differences between the means of age and magnitude of MSP and ST among the groups were statistically significant. Post-hoc analysis revealed statistically significant differences between the strained defecation versus constipation groups for age, strained defecation versus incontinence groups for MSP, and constipation versus incontinence groups for ST (Table 4).
Table 4.
Anorectal parameters of males in different referral complaint groups.
| Strained defecation | Constipation | Incontinence | p* | |
|---|---|---|---|---|
| Age (year) | 40.79±2.20 | 50.74±3.45 | 45.12±1.12 | 0.039 |
| MRP (cm H2O) | 61.58±4.18 | 62.00±4.62 | 60.53±2.12 | 0.966 |
| MSP (cm H2O) | 134.3±10.46 | 129.8±12.14 | 106.4±5.34 | 0.025 |
| ST (mL) | 30.74±2.93 | 28.37±2.32 | 38.78±2.04 | 0.012 |
| RAIR (mL) | 25.00±1.37 | 25.23±2.58 | 24.39±0.82 | 0.720 |
MRP: maximal resting pressure; MSP: maximal squeeze pressure; ST: sensory threshold; RAIR: rectoanal inhibitory reflex.
Kruskal-Wallis test.
The mean age of males in the strained defecation (p=0.001) and incontinence (p=0. 0005) groups was lower, but in the constipation (p=0.044) group, they were higher than that of females. The mean values of MRP, MSP, RAIR, and ST were statistically lower in females in the incontinence group (p<0.0001, p<0.0001, p=0.023, and p<0.0001, respectively). In addition, in the strained defecation and constipation groups, females had lower MSP values than males (p<0.0001).
When patients were grouped as younger and older than 50 years, mean MRP values were found to be statistically different and low only in incontinent females (55.30±28.0 and 40.94±25.31, p<0.0001) and males (66.92±30.79 and 52.07±29.27, p=0.0007), respectively. Age was well correlated with MRP in elderly females (r=−0.282, p<0.0001) and males (r=−0.155, p=0.006).
A total of 222 female patients had given birth. Of these, 26 (11.7%) patients had cesarean section, and 196 (88.3%) patients had vaginal delivery According to referral complaints, the mode of delivery was found to be statistically different between the patient groups (p=0.008). However, there was no difference between passive and urge incontinence with respect to delivery method (p=0.541). Obstetric trauma rates and mean parity values in different referral complaint groups are given in Table 5. Obstetric trauma rates (p<0.0001) and mean parity values (p=0.029) were all increased in incontinent females (Table 5).
Table 5.
Obstetric trauma rates and mean parity values in different referral complaint groups.
| Strained defecation (n=42) | Constipation (n=62) | Incontinence (n= 118) | p | |
|---|---|---|---|---|
| Obstetric trauma, n (%) | 20 (47.6) | 22 (35.5) | 100 (84.7) | <0.0001* |
| Parity (mean±SD) | 2.67±1.63 | 2.50±1.13 | 3.40±2.59 | 0.029** |
Chi-square test,
Kruskal–Wallis test
Anal manometric results of females with vaginal delivery history according to the number of parity are given in Table 6. The means of MRP (p=0.002) and ST (p=0.0004) were statistically different between the parity groups. While the number of parity increased, mean MRP (r=−0.141, p=0.0009) and mean ST (r=−0.149, p=0.0005) values decreased. However, no correlation was found between parity and other anal manometric parameters (p>0.05) (Table 6).
Table 6.
Anal manometric results according to the number of parity.
| Parity | ||||
|---|---|---|---|---|
|
|
||||
| 0 | 1–3 | >4 | p* | |
| N. of females | 340 | 162 | 60 | |
| Age (year) | 46.19±15.73 | 49.09±11.76 | 59.10±12.94 | <0.0001 |
| MRP (cm H2O) | 56.05±27.90 | 49.01±31.17 | 45.68±33.40 | 0.0018 |
| MSP (cm H2O) | 73.11±45.15 | 71.69±47.60 | 71.71±54.34 | 0.6497 |
| ST (mL) | 31.09±21.22 | 24.29±14.60 | 25.00±12.55 | 0.0004 |
| RAIR (mL) | 23.50±16.25 | 22.22±6.55 | 24.58±10.23 | 0.2038 |
MRP: maximal resting pressure; MSP: maximal squeeze pressure; ST: sensory threshold; RAIR: rectoanal inhibitory reflex.
Kruskal-Wallis test.
DISCUSSION
When we grouped our patients according to their referral complaints, internal and external sphincter pressures of incontinent subjects were lower than those with strained defecation and constipation. Rectal sensitivity was also lower in the incontinence group. Incontinent patients had significantly lower resting and squeeze pressures (8,12) and low rectal sensitivity (13). These low anorectal pressures and low rectal sensitivity may be the reason of incontinence regardless of the underlying etiology.
We found that anal sphincter pressures and RAIR were lower and rectal sensitivity was higher in females than in males. These gender differences in the anal manometric parameters result especially from the incontinence group. In the literature, squeeze pressure was lower in females in a large group of patients (3,5) and in a healthy population (4,6,7). External sphincter muscle may be more developed in males due to the testosterone effect (14), and its thickness was well correlated with squeeze pressure (8). During vaginal delivery, traction injury to the pudendal nerve that innervates the external sphincter muscle may be the other cause of decreased squeeze pressure observed in our female patients (15). The resting pressure, which is a reliable index of continence, represents the strength of the internal sphincter muscle. RAIR is the relaxation of the internal sphincter in response to rectal distention. The volume required to induce reflex anal relaxation is also lower in incontinent patients than in controls (16). Rectal sensation may be decreased (13) or increased (17) in incontinent patients. In a small healthy group, Rao (6) did not observe any gender difference in resting pressure, RAIR, and rectal sensation. Schäfer (8) showed significantly lower resting and squeeze pressures in incontinent patients. In the present study, we observed low anal pressures, decreased RAIR, and hypersensitive rectum in our female patients with incontinence.
In our study, age was negatively correlated with resting pressure in both males and females. Several studies showed that resting (2–4,8,12,18) and squeeze (2,4,18) pressures in the anal canal were significantly lower in older subjects than in younger subjects. Decreased quantity of nerves (19) and smooth muscle cells and increased collagen content of the internal sphincter muscle (20) all may cause low resting pressure and incontinence in the elderly.
In the present study, decreased resting pressure in the elderly has been observed only in the incontinence group, and this emphasizes the important role of the internal sphincter in the continence function. Mahony (9) and Fornell (21) showed that internal sphincter injury is predictive of fecal incontinence following obstetric anal sphincter injury. Schäfer (8), in patients with anorectal dysfunction (92 patients with incontinence and 32 patients with constipation), observed a negative correlation only between age and resting pressure and did not observe an age-related decrease in squeeze function. Similarly, we failed to demonstrate an age-related decrease in squeeze function. This may be due to either the low median age of our male and female patients or the predominance of patients with incontinence in our study group.
We found increased obstetric trauma rates and mean parity values in incontinent females. Data about the relationship between parity and anal manometric parameters are not consistent. Some investigators (2,6) could not find any association between parity and anal manometric parameters. Other authors (15) found reduced resting and squeeze increment pressures in parous females. Cali (7) observed that resting pressure is lower in parous females who had given birth via vaginal delivery, but the mean age of the parous group was 10 years greater than that of the nulliparous group. In our study, resting pressure was lower in the parous group, but the mean age of the parous group was again 10 years greater than that of the nulliparous group. This emphasizes the important impact of age on anal pressures. Age and internal sphincter defects were important determinants of reduced resting tone (15). Eogan observed that females with vaginal delivery history have lower anal pressures than those with cesarean delivery history, but this difference was lost after menopause (10). Mahony argued that increased age, parity, instrumental delivery, internal anal sphincter defects, and low resting pressure are related to severe fecal incontinence (9).
The prevalence of fecal incontinence in females was reported as 19% in Turkey (22). However, there is no community-based study for males. The reason of fecal incontinence was reported to be polypharmacy in the elderly (23), diabetes mellitus in males, and cerebrovascular accidents in females (24).
In the present study, diabetes mellitus rates were high in incontinent females. In one study, 13.6% of females were diabetic and 18.1% of diabetic females reported fecal incontinence and the prevalence of fecal incontinence among diabetics was higher than that among the general population (25). Acute hyperglycemia inhibited external sphincter function, decreased rectal compliance, and led to fecal incontinence (26). In type I and type 2 diabetics with incontinence, both internal and external sphincter pressures were low, and RAIR recovery time was prolonged (27).
We observed that incontinent females were older and their sphincters were weaker than other female groups. The rate of urge incontinence was high in females. Pudendal neuropathy due to either diabetes mellitus (28) or obstetric trauma may be the reason of the high frequency of urge incontinence observed in our female patients.
In incontinent males, squeeze pressure and anal sensitivity were lower than the rest of the males. The rate of passive incontinence was high in males. Increased trauma and neurological disease rates observed in our male incontinent subjects may explain external sphincter weakness, anal hyposensitivity, and high frequency of passive incontinence.
In the present study, in a middle-aged population, we found that diabetes mellitus and increased parity in females and neurological traumas in males were common etiological factors for incontinence. Our study had several limitations. The major limitation was the retrospective nature of the study. We had to rely on self-report data from subjects about their delivery history, and recall bias on the modes of delivery might exist. Finally, the participants in the present study were enrolled from a multidisciplinary tertiary clinic, which might limit the generalizability of our findings.
CONCLUSION
In the present study, squeeze pressure was lower in females than in males. Resting pressure, sensory threshold, and RAIR were all lower in females in the incontinence group. Diabetes mellitus rates in females and spinal cord trauma and neurological disease rates in males were high in the incontinence group. Increasing awareness of labor safety, controlling diabetes mellitus, and preventing obstetric traumas may reduce the prevalence of fecal incontinence.
Acknowledgements
The authors would like to thank Fatih Tarhan for statistical analysis and Ayfer Ürün for her technical assistance.
Footnotes
Ethics Committee Approval: Ethics committee approval was received from the Ethics Committee of Marmara University School of Medicine.
Informed Consent: Written informed consent was obtained from the patients who participated in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - S.T.T., N.İ.; Design - S.T.T., N.İ; Supervision - N.İ., Ö.A.; Resources - S.T.T., Ö.A., N.İ., A.G.; Data Collection and/or Processing - Ö.A., A.G., N.İ.; Analysis and/or Interpretation - Ö.A., A.G., N.İ., S.T.T.; Literature Search - S.T.T.; Writing Manuscript - S.T.T., N.İ.; Critical Review - S.T.T., N.İ.
Conflict of Interest: The authors have no conflict of interest to declare.
Financial Disclosure: The authors declared that this study has received no financial support.
REFERENCES
- 1.Bharucha AE, Dunivan G, Goode PS, et al. Epidemiology, pathophysiology, and classification of fecal incontinence: state of the science summary for the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) workshop. Am J Gastroenterol. 2015;110:127–36. doi: 10.1038/ajg.2014.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ryhammer AM, Laurberg S, Sørensen FH. Effects of age on anal function in normal women. Int J Colorect Dis. 1997;12:225–9. doi: 10.1007/s003840050094. [DOI] [PubMed] [Google Scholar]
- 3.Lee HR, Lim SB, Park JY. Anorectal manometric parameters are influenced by gender and age in subjects with normal bowel function. Int J Colorectal Dis. 2014;29:1393–9. doi: 10.1007/s00384-014-1961-4. [DOI] [PubMed] [Google Scholar]
- 4.Gundling F, Seidl H, Scalercio N, Schmidt T, Schepp W, Pehl C. Influence of Gender and Age on Anorectal Function: Normal Values from Anorectal Manometry in a Large Caucasian Population. Digestion. 2010;81:207–13. doi: 10.1159/000258662. [DOI] [PubMed] [Google Scholar]
- 5.Schuld J, Kollmar O, Schluter C, Schilling MK, Richter S. Normative values in anorectalmanometry using microtip technology: a cohort study in 172 subjects. Int J Colorectal Dis. 2012;27:1199–205. doi: 10.1007/s00384-012-1499-2. [DOI] [PubMed] [Google Scholar]
- 6.Rao SSC, Hatfield R, Soffer E, Rao S, Beaty J, Conklin JL. Manometric Tests of Anorectal Function in Healthy Adults. Am J Gastroenterol. 1999;94:773–83. doi: 10.1111/j.1572-0241.1999.00950.x. [DOI] [PubMed] [Google Scholar]
- 7.Cali RL, Blatchford GJ, Perry RE, Pitsch RM, Thorson AG, Christensen MA. Normal variation in anorectal manometry. Dis Colon Rectum. 1992;35:1161–4. doi: 10.1007/BF02251969. [DOI] [PubMed] [Google Scholar]
- 8.Schäfer R, Heyer T, Gantke B, et al. Anal endosonography and manometry: comparison in patients with defecation problems. Dis Colon Rectum. 1997;40:293–7. doi: 10.1007/BF02050418. [DOI] [PubMed] [Google Scholar]
- 9.Felt-Bersma RJF, Luth WJ, Janssen JJWM, Meuwissen SGM. Defecography in patients with anorectal disorders: which findings are clinically relevant? Dis Colon Rectum. 1990;33:277–84. doi: 10.1007/BF02055468. [DOI] [PubMed] [Google Scholar]
- 10.Jameson JS, Chia YW, Kamm MA, Speakman CT, Chye YH, Henry MM. Effect of age, sex and parity on anorectal function. Br J Surg. 1994;81:1689–92. doi: 10.1002/bjs.1800811143. [DOI] [PubMed] [Google Scholar]
- 11.Broens PMA, Penninckx FM. Relation between anal electrosensitivity and rectal filling. Sensation and the Influence of Age. Dis Colon Rectum. 2005;48:127–33. doi: 10.1007/s10350-004-0779-5. [DOI] [PubMed] [Google Scholar]
- 12.Akervall S, Nordgren S, Fasth S, Oresland T, Pettersson K, Hultén L. The effects of age, gender, and parity on rectoanal functions in adults. Scand J Gastroenterol. 1990;25:1247–56. doi: 10.3109/00365529008998561. [DOI] [PubMed] [Google Scholar]
- 13.Eogan M, O’Brien C, Daly L, Behan M, O’Connell PR, O’Herlihy C. The dual influences of age and obstetric history on fecal continence in parous women. Int J Gynaecol Obstet. 2011;112:93–7. doi: 10.1016/j.ijgo.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 14.Mahony R, Behan M, Daly L, Kirwan C, O’Herlihy C, O’Connell PR. Internal anal sphincter defect influences continence outcome following obstetric anal sphincter injury. Am J Obstet Gynecol. 2007;196:217.e1–217.e5. doi: 10.1016/j.ajog.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 15.Oztürk R, Rao SSC. Defecation disorders: an important subgroup of functional constipation, its pathophysiology, evaluation and treatment with biofeedback. Turk J Gastroenterol. 2007;18:139–49. [PubMed] [Google Scholar]
- 16.Matheson DM, Keighley MRB. Manometric evaluation of rectal prolapse and faecal incontinence. Gut. 1981;22:126–9. doi: 10.1136/gut.22.2.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Enck P, Kuhlbusch R, Lobke H, Frieling T, Erckenbrecht JF. Age and sex and anorectal manometry in incontinence. Dis Colon Rectum. 1989;32:1026–30. doi: 10.1007/BF02553874. [DOI] [PubMed] [Google Scholar]
- 18.Felt-Bersma RJF, Poen AC, Cuesta MA, Meuwissen SGM. Anal sensitivity test: What does it measure and do we need it? Cause or derivative of anorectal complaints? Dis Colon Rectum. 1997;40:811–6. doi: 10.1007/BF02055438. [DOI] [PubMed] [Google Scholar]
- 19.Felt-Bersma RJF, Sloots CEJ, Poen AC, Cuesta MA, Meuwissen SGM. Rectal compliance as a routine measurement: extreme volumes have direct clinical impact and normal volumes exclude rectum as a problem. Dis Colon Rectum. 2000;43:1732–8. doi: 10.1007/BF02236859. [DOI] [PubMed] [Google Scholar]
- 20.Storer TW, Woodhouse L, Magliano L, et al. Changes in muscle mass, muscle strength, and power but not physical function are related to testosterone dose in healthy older men. J Am Geriatr Soc. 2008;56:1991–9. doi: 10.1111/j.1532-5415.2008.01927.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaneko T, Nemoto T, Funahashi K, Koike J, Shibuya K, Kaneko H. Differences in innervated neurons of the internal anal sphincter based on age and sex: A histological study. Geriatr Gerontol Int. 2018;18:495–500. doi: 10.1111/ggi.13193. [DOI] [PubMed] [Google Scholar]
- 22.Kepenekci I, Keskinkilic B, Akinsu F, et al. Prevalence of pelvic floor disorders in the female population and the impact of age, mode of delivery, and parity. Dis Colon Rectum. 2011;54:85–94. doi: 10.1007/DCR.0b013e3181fd2356. [DOI] [PubMed] [Google Scholar]
- 23.Demir N, Yuruyen M, Atay K, et al. Prevalence of fecal incontinence and associated risk factors in elderly outpatients: a cross-sectional study. Aging Clin Exp Res. 2017;29:1165–71. doi: 10.1007/s40520-017-0723-x. [DOI] [PubMed] [Google Scholar]
- 24.Aslan E, Beji NK, Erkan HA, Yalcin O, Gungor F. The prevalence of and the related factors for urinary and fecal incontinence among older residing in nursing homes. J Clin Nurs. 2009;18:3290–8. doi: 10.1111/j.1365-2702.2009.02936.x. [DOI] [PubMed] [Google Scholar]
- 25.Swash M, Gray A, Lubowski DZ, Nicholls RJ. Ultrastructural changes in internal and sphincter in neurogenic faecal incontinence. Gut. 1988;29:1692–8. doi: 10.1136/gut.29.12.1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chaliha C, Sultan AH, Emmanuel AV. Normal ranges for anorectal manometry and sensation in women of reproductive age. Colorectal Dis. 2007;9:839–44. doi: 10.1111/j.1463-1318.2007.01212.x. [DOI] [PubMed] [Google Scholar]
- 27.Fornell EU, Matthiesen L, Sjödahl R, Berg G. Obstetric anal sphincter injury ten years after: subjective and objective long-term effects. BJOG. 2005;112:312–6. doi: 10.1111/j.1471-0528.2004.00400.x. [DOI] [PubMed] [Google Scholar]
- 28.Boyle DJ, Knowles CH, Murphy J, et al. The Effects of Age and Childbirth on Anal Sphincter Function and Morphology in 999 Symptomatic Female Patients with Colorectal Dysfunction. Dis Colon Rectum. 2012;55:286–93. doi: 10.1097/DCR.0b013e31823fe7f1. [DOI] [PubMed] [Google Scholar]
- 29.Carrington EV, Brokjaer A, Craven H, et al. Traditional measures of normal anal sphincter function using high-resolution anorectal manometry (HRAM) in 115 healthy volunteers. Neurogastroenterol Motil. 2014;26:625–35. doi: 10.1111/nmo.12307. [DOI] [PubMed] [Google Scholar]
- 30.Russo A, Botten R, Kong MF, et al. Effects of acute hyperglycaemia on anorectal motor and sensory function in diabetes mellitus. Diabet Med. 2004;21:176–82. doi: 10.1111/j.1464-5491.2004.01106.x. [DOI] [PubMed] [Google Scholar]
- 31.Thiruppathy K, Bajwa A, Kuan KG, Murray C, Cohen R, Emmanuel A. Gut symptoms in diabetics correlate with components of the rectoanal inhibitory reflex, but not with pudendal nerve motor latencies or systemic autonomic neuropathy. J Dig Dis. 2015;16:342–9. doi: 10.1111/1751-2980.12244. [DOI] [PubMed] [Google Scholar]
- 32.Nieto ML, Wu JM, Matthews C, Whitehead WE, Markland AD. Factors associated with fecal incontinence in a nationally representative sample of diabetic women. Int Urogynecol J. 2015;26:1483–8. doi: 10.1007/s00192-015-2730-9. [DOI] [PubMed] [Google Scholar]
- 33.Noelting J, Ratuapli SK, Bharucha AE, Harvey DM, Ravi K, Zinsmeister AR. Normal Values for High-Resolution Anorectal Manometry in Healthy Women: Effects of Age and Significance of Rectoanal Gradient. Am J Gastroenterol. 2012;107:1530–6. doi: 10.1038/ajg.2012.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fox JC, Fletcher JG, Zinsmeister AR, Seide B, Riederer SJ, Bharucha AE. Effect of aging on anorectal and pelvic floor functions in females. Dis Colon Rectum. 2006;49:1726–35. doi: 10.1007/s10350-006-0657-4. [DOI] [PubMed] [Google Scholar]
- 35.Ryhammer AM, Laurberg S, Bek KM. Age and Anorectal Sensibility in Normal Women. Scand J Gastroenterol. 1997;32:278–84. doi: 10.3109/00365529709000207. [DOI] [PubMed] [Google Scholar]
- 36.Burnett SJD, Bartram CI. Endosonographic variations in the normal internal anal sphincter. Int J Colorect Dis. 1991;6:2–4. doi: 10.1007/BF00703951. [DOI] [PubMed] [Google Scholar]


