Abstract
Purpose
Nonadherence to indicated therapy reduces treatment effectiveness and may increase cost of care. HUMIRA Complete, a Patient Support Program (PSP), aims to reduce nonadherence in patients prescribed adalimumab (ADA). The objective of this study was to assess the relationship between participation in the PSP and prescription abandonment rates among ADA-treated patients.
Patients and methods
This longitudinal study using patient-level data from AbbVie’s PSP linked with medical and pharmacy claims data included patients ≥18 years with an ADA-approved indication, ≥1 pharmacy claim for ADA, and available data ≥3 months before and ≥6 months after the index date (defined as the initial ADA claim [01/2015 to 02/2017]). Abandonment was defined as reversal of initial ADA prescription with no paid claim during 3-month follow-up. Abandonment rates were compared between PSP and non-PSP cohorts using multivariable logistic regression controlling for potentially confounding baseline characteristics.
Results
In 17,371 patients (9,851 PSP; 7,520 non-PSP), the overall abandonment rate was 10.8–16.8% across indications. The odds of ADA abandonment were 70% less for PSP vs non-PSP patients (5.6% vs 20.4%, odds ratio [OR]=0.30, [95% confidence interval (CI)=0.27–0.33] P<0.001), 38% less for patients using specialty vs retail pharmacy (OR=0.62, 95% CI=0.56–0.69, P<0.001), 20% less for those with income of $50–99K vs $0–49K (OR=0.80, 95% CI=0.69–0.92, P<0.01), and 78% greater for those with copayment of $26–100 vs $0–25 (OR=1.78, 95% CI=1.55–2.05, P<0.001).
Conclusion
Participation in the PSP, higher income, and using a specialty pharmacy were associated with lower odds of abandoning ADA therapy, whereas increased copayments were associated with greater abandonment. PSPs should be considered to improve initiation of ADA therapy.
Keywords: adherence, drug utilization, managed care, outcomes research/analysis, patient education, personnel management
Introduction
As health care costs continue to increase, efforts are being made to provide patient-centered care that is responsive to patients’ needs and preferences while managing costs.1 This is particularly true for patients with chronic diseases that require long-term medical management. Rheumatic diseases can be difficult to diagnose and early referral to a rheumatologist can shorten time to diagnosis and provide quicker access to appropriate treatment.2 For patients with immune-mediated inflammatory diseases, the availability of biologic therapies has had a significant positive impact including attainment of clinical remission,3–9 reduced risk of extra-articular complications,10 and improved health-related quality of life.11–17 However, to effectively treat these diseases, patients not only need to have access to therapy, but they also need to adhere to a specific treatment regimen. Non-adherence rates for biologic therapies among patients with inflammatory bowel disease and rheumatoid arthritis are estimated to be 25–34%.18–20 Among patients with inflammatory bowel disease, nonadherence to therapy is associated with a greater rate of hospitalization, higher medical costs, and increased disability.21–23
One component of nonadherence is failure to initiate therapy after a prescription has been written.24,25 Several studies have shown that 17–28% of patients do not obtain new prescriptions from the pharmacy despite a written prescription from their physician,26–29 and 39% of patients with rheumatoid arthritis do not fill new prescriptions for injectable biologic disease-modifying antirheumatic drugs.25 Patients may abandon their first prescription for several reasons including concerns about costs, decisions regarding self-administration versus outpatient injection or infusion of medication, fear of needles, extent of patient/physician communication, lack of education about the benefits and side effects of the medication, and/or concerns about administering a complex medication regimen to manage their medical condition.25–27
In an attempt to improve outcomes, patient support programs (PSPs) have been developed to assist patients with chronic diseases and provide them with the knowledge and support to actively participate in the management of their own care.30–34 In the US, AbbVie offers a PSP to all patients treated with adalimumab (ADA) designed to provide one-to-one, personalized support to increase medication adherence by behavior modification. Since the launch, more than 300,000 patients with prescriptions for ADA have opted-in to the PSP. In 2015, the Nurse Ambassador component was added to the PSP and the program, called HUMIRA Complete,35 was launched at the national level in the US to provide educational resources and support to empower patients to actively manage their treatment and understand various aspects of their chronic disease. The team of Nurse Ambassadors includes more than 400 registered nurses who provide unique, high-touch, coordinated care for ADA-treated patients. Individuals who opt-in to the Nurse Ambassador component of the PSP are assigned to a dedicated Ambassador, a trained nurse who does not provide medical advice, but provides personalized one-on-one educational support to ensure that patients can access treatment-related educational resources and understand the importance of following their ADA treatment plan as prescribed by their physician. The Nurse Ambassador quickly reaches out to these patients to motivate and educate them, so they understand how to initiate the prescribed treatment plan and navigate the insurance and financial assistance processes. The Nurse Ambassador program supplements other components of the PSP, such as injection training and medication reminders in an effort to empower patients to adhere to their prescribed treatment plan.
The 2015 nationwide availability of AbbVie’s PSP offers a unique opportunity to study the effectiveness of the patient-centered care offered by the PSP on abandonment of ADA treatment initiation across all ADA-approved indications. This is accomplished by providing a means to collect relevant patient-level data in the PSP and linking the collected data to pertinent outcomes. The objective of this study was to assess the association between participation in the PSP and the rate of ADA treatment abandonment across autoimmune diseases including inflammatory bowel disease (Crohn’s disease or ulcerative colitis), rheumatoid arthritis, ankylosing spondylitis, psoriatic arthritis, psoriasis, hidradenitis suppurativa, and uveitis. The relationship between PSP participation and continuation of ADA treatment after initiation was also examined.
Methods
Data source
A longitudinal, retrospective cohort study was conducted using patient-level data obtained by linking AbbVie’s PSP database (AbbVie Inc., North Chicago, IL, USA) with the Symphony Health Solutions (SHS, Horsham PA, USA) administrative claims database. The PSP database contains the dates that patients opted-in to each component (assistance with medication cost and insurance questions, face-to-face interactions with an Ambassador, a nurse-led injection training program [to supplement the initial injection training provided by the physician], resources to familiarize patients with the self-injection process, instructions on the disposal of used pens and syringes, reminders to take medication as prescribed, and an insulated bag to keep ADA at the required temperature when traveling short distances) of the AbbVie PSP that can be used to identify the first PSP opt-in date for each patient.
The SHS administrative claims database contains longitudinal, patient-level information on medical and pharmacy claims from a geographically diverse large set of electronic claims processors across the United States and includes International Classification of Diseases, Ninth and Tenth Revisions (ICD-9/10) codes, service dates, charge amounts, procedure codes, National Drug Codes, and pharmacy types. Pharmacy data contain a claim status code that distinguishes paid, rejected, or reversed claims. A reversed claim indicates a claim was approved by the payer, but the prescription was not picked up at the pharmacy by the patient.
Each data provider with SHS used a proprietary de-identification engine to create a series of unique patient tokens that replace personally identifiable data. These tokens or artificial identifiers were then used to link the claims data from SHS to the PSP data. The de-identification engine (SynomaTM) is a Health Insurance Portability and Accountability Act of 1996 (HIPAA) compliant industry standard encryption engine supplied by SHS and is designed to protect the anonymity of the data. An external HIPAA statistician evaluated the risk associated with linked data content and certified that the resulting files ensured patient anonymity. Because these data were completely de-identified before providing the dataset to the researchers for analysis, institutional review board approval of this study was deemed not necessary.
Selection of patients
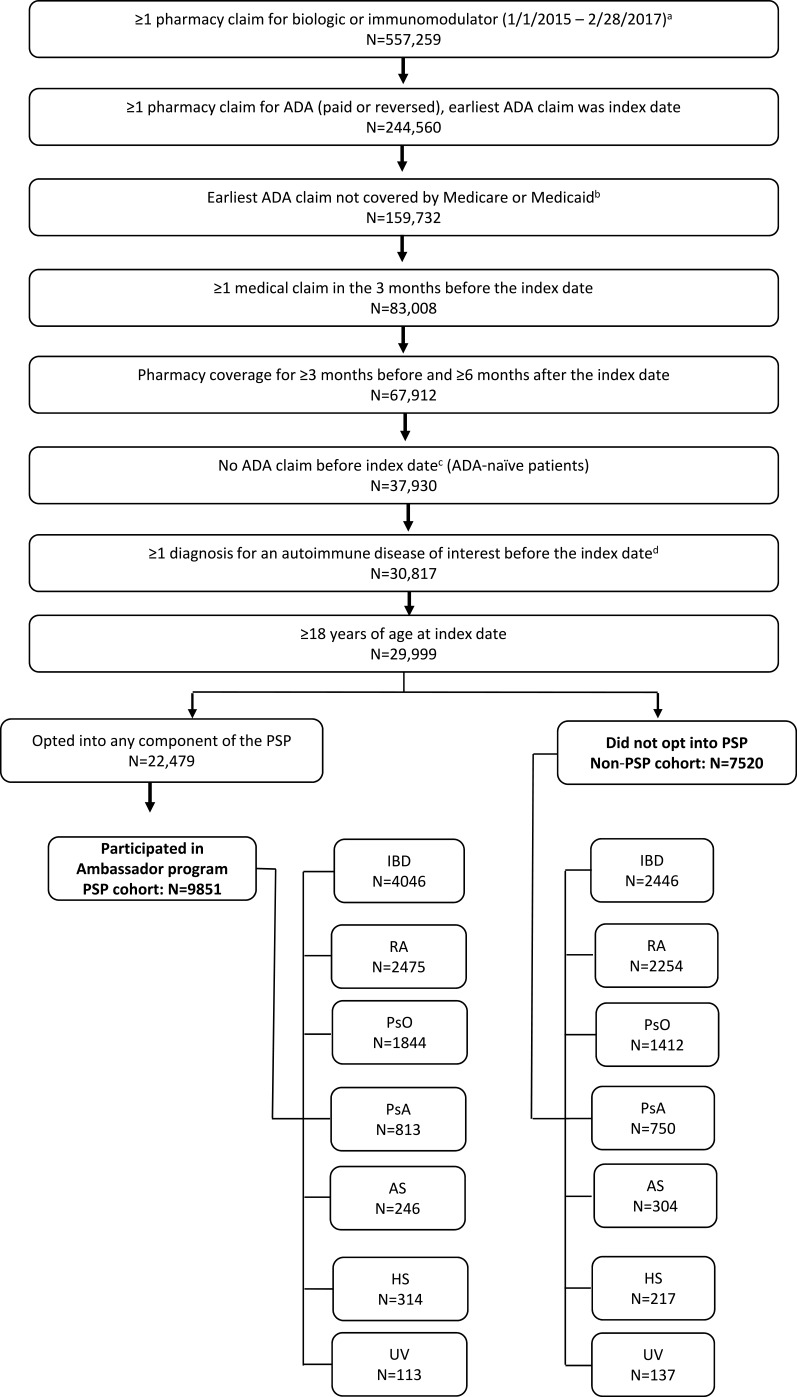
Patients ≥18 years of age with a first ADA prescription claim (paid or reversed) from January 2015 to February 2017 were selected for the analysis (Figure 1). Patients whose initial ADA claim was reimbursed by the government (Medicare or Medicaid) were also excluded because receipt of copayment cards is prohibited for Medicare/Medicaid patients; therefore, these patients were not able to utilize all components of the PSP. The index date was defined as the initial ADA claim. Patients with an ADA claim prior to 2015 were excluded from the study because the Nurse Ambassador component was not added to the PSP until 2015. Patients were required to have a diagnosis for an autoimmune disease of interest (inflammatory bowel disease [Crohn’s disease or ulcerative colitis], rheumatoid arthritis, psoriasis, psoriatic arthritis, ankylosing spondylitis, hidradenitis suppurativa, or uveitis) prior to the index date based on the ICD-9/10 codes listed in Table S1. Assignment to an indication cohort was based on a diagnosis for that indication and no subsequent diagnosis for another autoimmune disease before the index ADA claim (eg, patients with a diagnosis for rheumatoid arthritis had no subsequent diagnosis for any other autoimmune disease before the initial ADA claim). Patients had to have at least 1 medical claim in the 3 months prior to the index date to assess baseline characteristics and have pharmacy data coverage for ≥3 months before and ≥6 months after the index date to observe treatment and abandonment history. The SHS data do not contain an enrollment or eligibility file, so data coverage was determined as an observed claim before and after the study period.36
Figure 1.
Study sample selection.
Notes: aDirect feed claims consisted of filled claims only and were excluded from abandonment analysis. The following treatments were included: etanercept, infliximab, certolizumab, golimumab, anakinra, tocilizumab, secukinumab, ixekizumab, ustekinumab, abatacept, rituximab, natalizumab, vedolizumab, tofacitinib, and apremilast. bClaims with government-provided insurance were excluded as these patients were prohibited from using all components of the PSP. cDetermined using all available claims from 1/1/2006 to the index date. dAutoimmune diseases included rheumatoid arthritis, Crohn’s disease, ulcerative colitis, psoriasis, psoriatic arthritis, ankylosing spondylitis, hidradenitis suppurativa, and uveitis.
Abbreviations: ADA, adalimumab; AS, ankylosing spondylitis; HS, hidradenitis suppurativa; IBD, inflammatory bowel disease; PsA, psoriatic arthritis; PsO, psoriasis; PSP, patient support program; RA, rheumatoid arthritis; UV, uveitis.
The PSP cohort included patients who opted-in to the PSP within 30 days pre- and post-index date and participated in the Ambassador program with an initial call and at least one follow-up with the nurse. Opting-in to the PSP was assessed after the initial claim to allow for delays in registration and include patients who opted-in to the PSP after realizing the expected copayment amount. The non-PSP cohort included patients who did not opt-in to any PSP component during the 30 days before or after the index date. Patients who opted-in to the PSP within 30 days of their index date but did not participate in the Ambassador program were excluded.
Outcomes
The primary outcome measure was prescription abandonment assessed as reversal of the initial ADA claim (ie, patient did not take possession of the medication) with no paid ADA claim (pharmacy or medical) in the following 3-month period after the index date.37,38 The secondary outcome measure was occurrence of a second ADA prescription fill after ADA initiation in the 6 months following the initial ADA claim.
Data analysis
Baseline characteristics were assessed during the 3 months before the index date. Cohorts were compared using Wilcoxon rank-sum tests and chi-square tests for continuous and categorical variables, respectively. Abandonment rate was calculated as the number of patients who abandoned ADA initiation divided by the total number of patients with a paid or reversed initial claim; comparisons between cohorts were based on two-sample z-tests of proportions.
The odds of abandoning ADA treatment were assessed using a multivariable logistic regression with abandonment of the initial ADA claim as the dependent variable, controlling for age, sex, household income, year, copayment amount, plan type, pharmacy type, indication, and comorbidity burden. Results were further stratified by primary indication so that each comparison was conducted on a unique sample. Separate models were run for each indication and odds ratios (ORs) and 95% confidence intervals (CIs) were reported overall and for each indication. In all analyses, a two-sided alpha error level of 0.05 was used to indicate statistical significance. A sensitivity analysis measuring PSP enrollment strictly prior to the initial ADA claim was performed. All analyses were conducted using SAS 9.4 software (SAS Institute Inc., Cary, NC, USA).
Results
A total of 17,371 patients (9851 in the PSP cohort and 7520 in the non-PSP cohort) were included in the analysis (Figure 1). Of the 17,371 patients in the study population, 6492 (37.4%) had inflammatory bowel disease, 4729 (27.2%) had rheumatoid arthritis, 3256 (18.7%) had psoriasis, 1563 (9.0%) had psoriatic arthritis, 550 (3.2%) had ankylosing spondylitis, 531 (3.1%) had hidradenitis suppurativa, and 250 (1.4%) had uveitis. Analysis of the baseline characteristics of the study population revealed a few significant differences between the two cohorts. Compared with the non-PSP cohort, the PSP cohort was younger (mean age 45.6 vs 46.4 years; P<0.001), and had a lower comorbidity score39 (mean Charlson Comorbidity Index: 0.38 vs 0.47; P<0.001, Table 1). Also, the expected per-patient out-of-pocket contribution for ADA was 45.1% lower ($344 vs $627; P<0.001) and the frequency of specialty pharmacy use for the first ADA prescription fill was 44.3% greater (75.6% vs 52.4%; P<0.001) in the PSP cohort compared with the non-PSP cohort.
Table 1.
Baseline characteristics of the study population
| Characteristic | PSP N=9851 | Non-PSP N=7520 | P-valuea |
|---|---|---|---|
| Age (years), mean ± SD | 45.6±13.3 | 46.4±13.9 | <0.001 |
| Male, n (%) | 3817 (38.7) | 2935 (39.0) | 0.71 |
| Household income, n (%) | |||
| $0–$49,999 | 1694 (17.2) | 1486 (19.8) | <0.001 |
| $50,000–$99,999 | 2778 (28.2) | 2106 (28.0) | 0.78 |
| >$100,000 | 2402 (24.4) | 1480 (19.7) | <0.001 |
| Unknown | 2977 (30.2) | 2448 (32.6) | <0.001 |
| Expected patient copay, mean ± SD | $344±$1601 | $627±$2042 | <0.001 |
| $0–$25, n (%) | 7685 (78.0) | 4183 (55.6) | <0.001 |
| $26–$100, n (%) | 902 (9.2) | 1581 (21.0) | <0.001 |
| $101–$500, n (%) | 437 (4.4) | 586 (7.8) | <0.001 |
| $501–$1000, n (%) | 150 (1.5) | 225 (3.0) | <0.001 |
| $1001–$2000, n (%) | 160 (1.6) | 195 (2.6) | <0.001 |
| >$2000, n (%) | 517 (5.2) | 750 (10.0) | <0.001 |
| Calendar year, n (%) | |||
| 2015 | 6152 (62.5) | 5146 (68.4) | <0.001 |
| 2016 | 3699 (37.5) | 2374 (31.6) | <0.001 |
| Primary plan, n (%) | |||
| Commercial | 3794 (38.5) | 475 (6.3) | <0.001 |
| Employer group | 584 (5.9) | 703 (9.3) | <0.001 |
| Third party administrator | 27 (0.3) | 47 (0.6) | <0.001 |
| Pharmacy benefit manager | 1887 (19.2) | 2432 (32.3) | <0.001 |
| Cash | 180 (1.8) | 361 (4.8) | <0.001 |
| Processors | 41 (0.4) | 71 (0.9) | <0.001 |
| Unspecified or missing | 3338 (33.9) | 3431 (45.6) | <0.001 |
| Initial claim at specialty pharmacy, n (%)b | 7443 (75.6) | 3938 (52.4) | <0.001 |
| Charlson Comorbidity Index, mean ± SD | 0.38±0.70 | 0.47±0.84 | <0.001 |
| Indications, n (%) | |||
| Inflammatory bowel disease | 4046 (41.1) | 2446 (32.5) | <0.001 |
| Rheumatoid arthritis | 2475 (25.1) | 2254 (30.0) | <0.001 |
| Psoriasis | 1844 (18.7) | 1412 (18.8) | 0.92 |
| Psoriatic arthritis | 813 (8.3) | 750 (10.0) | <0.001 |
| Ankylosing spondylitis | 246 (2.5) | 304 (4.0) | <0.001 |
| Hidradenitis suppurativa | 314 (3.2) | 217 (2.9) | 0.25 |
| Uveitis | 113 (1.1) | 137 (1.8) | <0.001 |
Notes: aWilcoxon rank-sum tests and chi-square tests were used to compare continuous and categorical variables, respectively. bDefined as a pharmacy type of mail order/specialty.
Abbreviations: PSP, patient support program; SD, standard deviation.
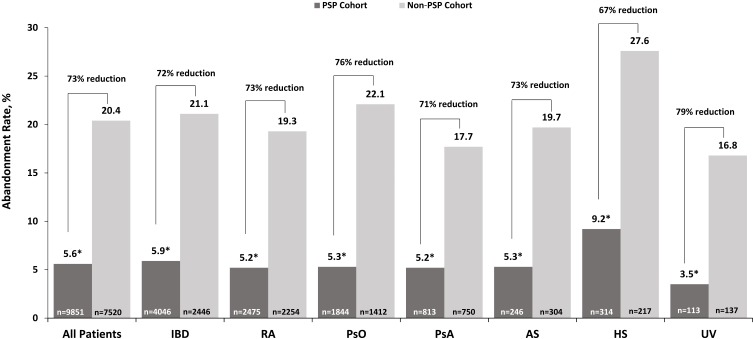
Overall, abandonment of ADA initiation was 12.0% and was consistent across calendar years (11.7% in 2015 and 12.7% in 2016) and indications (range: 10.8% for uveitis to 16.8% for hidradenitis suppurativa). As shown in Figure 2, the overall abandonment rate across all indications was reduced by 73% for the PSP cohort compared with the non-PSP cohort (5.6% vs 20.4%, respectively, P<0.001). Separate models were run for each indication and showed significant reductions in abandonment rates for each indication: 72% (5.9% vs 21.1%) for inflammatory bowel disease, 73% (5.2% vs 19.3%) for rheumatoid arthritis, 76% (5.3% vs 22.1%) for psoriasis, 71% (5.2% vs 17.7%) for psoriatic arthritis, 73% (5.3% vs 19.7%) for ankylosing spondylitis, 67% (9.2% vs 27.6%) for hidradenitis suppurativa, and 79% (3.5% vs 16.8%) for uveitis; all P<0.001.
Figure 2.
Prevalence of abandonment by PSP participation for the overall study population and individual indications.
Notes: Relative percent change = (PSP–Non-PSP)/Non-PSP. Asterisk (*) indicates the comparison of PSP versus non-PSP cohorts was statistically significant (P<0.001). P-value was based on 2-sample z-test of proportions.
Abbreviations: AS, ankylosing spondylitis; HS, hidradenitis suppurativa; IBD, inflammatory bowel disease; PsA, psoriatic arthritis; PsO, psoriasis; RA, rheumatoid arthritis; UV, uveitis.
After controlling for potentially confounding baseline characteristics (ie, age, sex, household income, expected patient copayment, calendar year of index date, type of insurance plan, type of pharmacy used, indication, and comorbidity burden), the odds of abandonment overall were 70% lower for the PSP cohort compared with the non-PSP cohort (OR =0.30, 95% CI=0.27–0.33, P<0.001; Table 2). Adjusted abandonment rates for each indication were significantly (all P≤0.01) lower in the PSP cohort than in the non-PSP cohort: 72% lower (OR =0.28, 95% CI=0.24–0.34) for inflammatory bowel disease, 66% (OR =0.34, 95% CI=0.27–0.42) for rheumatoid arthritis, 73% (OR =0.27, 95% CI=0.20–0.35) for psoriasis, 66% (OR =0.34, 95% CI=0.22–0.52) for psoriatic arthritis, 76% (OR =0.24, 95% CI=0.11–0.50) for ankylosing spondylitis, 75% (OR =0.25, 95% CI=0.14–0.45) for hidradenitis suppurativa, and 82% (OR =0.18, 95% CI=0.05–0.67) for uveitis (Table 3). Results of a sensitivity analysis measuring PSP enrollment strictly prior to the initial ADA claim were similar to those reported in the main analysis.
Table 2.
Multivariable analysis of the odds of abandonment of treatment with ADA
| Characteristic | All indications | |
|---|---|---|
| Odds ratio(95% CI) | P-value | |
| Opt-in to PSPa | 0.30 (0.27–0.33) | <0.001 |
| Age (years) | 1.01 (1.01–1.01) | <0.001 |
| Male | 0.92 (0.83–1.02) | 0.12 |
| Household income | ||
| $0–$49,999 | Ref | — |
| $50,000–$99,999 | 0.80 (0.69–0.92) | <0.01 |
| ≥$100,000 | 0.76 (0.65–0.90) | <0.001 |
| Unknown | 0.87 (0.75–1.00) | <0.05 |
| Expected patient copay | ||
| $0–$25 | Ref | — |
| $26–$100 | 1.78 (1.55–2.05) | <0.001 |
| $101–$500 | 2.56 (2.13–3.08) | <0.001 |
| $501–$1000 | 5.08 (3.98–6.49) | <0.001 |
| $1001–$2000 | 8.28 (6.52–10.52) | <0.001 |
| >$2000 | 9.75 (8.27–11.49) | <0.001 |
| Calendar year | ||
| 2015 | 0.89 (0.80–0.99) | 0.04 |
| 2016 | Ref | — |
| Primary plan | ||
| Commercial | Ref | — |
| Employer group | 1.47 (1.18–1.83) | <0.001 |
| Third party administrator | 1.12 (0.56–2.24) | 0.74 |
| Pharmacy benefit manager | 1.09 (0.91–1.31) | 0.35 |
| Cash | 0.87 (0.66–1.15) | 0.33 |
| Processors | 1.70 (1.07–2.70) | 0.02 |
| Unspecified or missing | 1.14 (0.96–1.36) | 0.12 |
| Initial claim at specialty pharmacy | 0.62 (0.56–0.69) | <0.001 |
| Charlson Comorbidty Index | 1.01 (0.95–1.08) | 0.74 |
| Indications | ||
| Rheumatoid arthritis | Ref | — |
| Inflammatory bowel disease | 1.37 (1.19–1.58) | <0.001 |
| Psoriasis | 1.30 (1.11–1.53) | <0.001 |
| Psoriatic arthritis | 0.99 (0.81–1.22) | 0.95 |
| Ankylosing spondylitis | 1.18 (0.88–1.58) | 0.26 |
| Hidradenitis suppurativa | 1.98 (1.50–2.61) | <0.001 |
| Uveitis | 0.92 (0.59–1.43) | 0.71 |
Note: aNon-PSP cohort was the reference.
Abbreviations: ADA, adalimumab; CI, confidence interval; PSP, patient support program; Ref, reference.
Table 3.
Multivariable analysis of the odds of abandonment of treatment with ADA by indication
| Characteristic | IBD | RA | PsO | PsA | AS | HS | UV |
|---|---|---|---|---|---|---|---|
| OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | |
| Opt-in to PSPa | 0.28 (0.24–0.34)*** | 0.34 (0.27–0.42)*** | 0.27 (0.20–0.35)*** | 0.34 (0.22–0.52)*** | 0.24 (0.11–0.50)*** | 0.25 (0.14–0.45)*** | 0.18 (0.05–0.67)* |
| Age (years) | 1.01 (1.00–1.02)** | 1.01 (1.00–1.02)* | 1.01 (1.00–1.02) | 1.02 (1.00–1.04)* | 1.02 (0.99–1.04) | 1.01 (0.99–1.04) | 1.05 (1.01–1.10)* |
| Male | 0.78 (0.65–0.92)** | 1.03 (0.82–1.31) | 1.14 (0.90–1.43) | 0.93 (0.64–1.35) | 0.57 (0.32–1.01) | 0.92 (0.51–1.67) | 2.58 (0.89–7.50) |
| Household income | |||||||
| $0–$49,999 | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| $50,000–$99,999 | 1.03 (0.80–1.32) | 0.66 (0.51–0.86)** | 0.73 (0.52–1.03) | 0.57 (0.33–0.98)* | 1.24 (0.57–2.71) | 0.72 (0.35–1.49) | 1.80 (0.32–10.17) |
| ≥$100,000 | 0.78 (0.60–1.03) | 0.65 (0.48–0.89)** | 0.92 (0.64–1.31) | 0.85 (0.49–1.47) | 0.49 (0.20–1.21) | 0.88 (0.33–2.32) | 1.30 (0.21–7.91) |
| Unknown | 0.92 (0.72–1.18) | 0.75 (0.58–0.98)* | 0.99 (0.71–1.38) | 0.94 (0.55–1.61) | 0.48 (0.20–1.16) | 0.99 (0.50–1.97) | 3.04 (0.58–15.94) |
| Expected patient copay | |||||||
| $0–$25 | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| $26–$100 | 1.82 (1.45–2.28)*** | 1.74 (1.32–2.31)*** | 2.01 (1.46–2.75)*** | 1.33 (0.77–2.30) | 1.07 (0.46–2.49) | 2.39 (1.20–4.78)* | 1.24 (0.37–4.17) |
| $101–$500 | 1.79 (1.28–2.49)*** | 2.92 (2.03–4.21)*** | 3.10 (2.11–4.55)*** | 3.16 (1.69–5.91)*** | 4.09 (1.37–12.17)* | 2.63 (1.06–6.55)* | 10.85 (2.22–53.04)** |
| $501–$1000 | 3.90 (2.41–6.30)*** | 6.15 (4.11–9.20)*** | 4.19 (2.39–7.36)*** | 7.48 (3.09–18.11)*** | 6.23 (1.75–22.14)** | 2.61 (0.29–23.37) | 55.52 (2.40–999.99)* |
| $1001–$2000 | 5.03 (3.10–8.17)*** | 11.72 (7.78–17.65)*** | 8.13 (4.89–13.50)*** | 13.34 (6.27–28.40)*** | 4.16 (0.76–22.75) | 11.78 (1.79–77.50)* | 2.18 (0.12–38.78) |
| >$2000 | 10.27 (7.95–13.27)*** | 9.60 (6.77–13.61)*** | 9.62 (6.59–14.05)*** | 13.00 (7.37–22.94)*** | 4.85 (1.59–14.79)** | 11.98 (5.31–27.00)* | 4.68 (0.39–55.75) |
| Calendar year | |||||||
| 2015 | 0.83 (0.70–0.99)* | 0.90 (0.73–1.11) | 0.87 (0.68–1.10) | 1.15 (0.75–1.76) | 0.69 (0.37–1.28) | 1.15 (0.68–1.96) | 1.20 (0.43–3.35) |
| 2016 | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| Primary plan | |||||||
| Commercial | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| Employer group | 1.33 (0.91–1.92) | 1.33 (0.88–2.00) | 1.60 (0.95–2.68) | 2.30 (1.01–5.25)* | 1.90 (0.58–6.27) | 1.01 (0.36–2.90) | 7.14 (0.74–68.95) |
| TPA | 1.33 (0.38–4.65) | 1.26 (0.37–4.31) | 0.58 (0.11–3.16) | 3.10 (0.24–39.60) | — | 0.93 (0.06–15.01) | — |
| PBM | 1.05 (0.77–1.42) | 1.17 (0.83–1.66) | 0.95 (0.62–1.46) | 1.16 (0.57–2.36) | 1.32 (0.51–3.44) | 0.57 (0.24–1.38) | 7.40 (1.02–53.63)* |
| Cash | 0.53 (0.32–0.87)* | 0.81 (0.47–1.39) | 1.08 (0.57–2.03) | 2.98 (1.13–7.90)* | 2.52 (0.60–10.48) | 0.33 (0.07–1.66) | 4.36 (0.16–120.84) |
| Processors | 1.77 (0.92–3.40) | 1.91 (0.62–5.87) | 1.56 (0.38–6.39) | 2.14 (0.48–9.46) | 0.62 (0.03–12.08) | 0.54 (0.08–3.72) | — |
| Unspecified or missing | 1.20 (0.90–1.59) | 1.20 (0.87–1.67) | 1.10 (0.74–1.63) | 1.51 (0.79–2.92) | 0.52 (0.19–1.41) | 0.46 (0.20–1.07) | 1.67 (0.22–12.58) |
| Initial claim at SP | 0.57 (0.48–0.68)*** | 0.64 (0.53–0.79)*** | 0.73 (0.57–0.93)* | 0.57 (0.39–0.82)** | 0.35 (0.19–0.64)*** | 0.51 (0.29–0.89)* | 1.77 (0.61–5.17) |
| CCI | 0.96 (0.84–1.10) | 1.04 (0.93–1.16) | 0.92 (0.76–1.12) | 1.08 (0.88–1.32) | 1.02 (0.74–1.40) | 1.16 (0.84–1.59) | 0.73 (0.30–1.79) |
Notes: aNon-PSP cohort was the reference. *P<0.05, ** P<0.01, *** P<0.001.
Abbreviations: ADA, adalimumab; AS, ankylosing spondylitis; CCI, Charlson Comorbidity Index; CI, confidence interval; HS, hidradenitis suppurativa; IBD, inflammatory bowel disease; OR, odds ratio; PBM, pharmacy benefit manager; PsA, psoriatic arthritis; PsO, psoriasis; PSP, patient support program; RA, rheumatoid arthritis; Ref, reference; SP, specialty pharmacy; TPA, third party administrator; UV, uveitis.
Among patients who successfully initiated ADA treatment (n=15,281), the odds of a subsequent ADA fill after the first prescription fill were 36% higher for the PSP cohort compared with the non-PSP cohort (87.0% vs 82.8%; OR =1.36, 95% CI=1.23–1.51, P<0.001).
The odds of ADA prescription abandonment were 38% lower for patients obtaining their prescription from a specialty pharmacy than from a retail pharmacy (OR =0.62, 95% CI=0.56–0.69, P<0.001; Table 2), 20% lower among patients with a household income of $50–$99K than those with an income of $0–$49K (OR =0.80, 95% CI=0.69–0.92, P<0.01), and 78% greater among patients with a copayment of $26–$100 compared with a copayment of $0–$25 (OR =1.78, 95% CI=1.55–2.05; P<0.001).
Discussion
Abandonment of prescribed drug treatment has important implications for patient-centered health outcomes. Patients who abandon their initial prescription for drug therapy ultimately delay treatment initiation or forego treatment altogether. Abandoning or delaying treatment initiation could have serious consequences and result in poor clinical outcomes and increased health care costs to payers. Reasons that patients abandon prescribed treatment may include the cost burden of therapy, misconceptions about the illness, or perceived benefits of treatment.38,40–43 Because lack of motivation or knowledge regarding treatment can also influence whether patients initiate therapy,44 we assessed the effect of AbbVie’s PSP on the odds of ADA abandonment among patients with various autoimmune disorders. While all PSP patients included in the study participated in the Ambassador program, which provides face-to-face interactions to motivate and support patients to manage their treatment regimen and care as prescribed, they may have also utilized other PSP components such as insurance and financial assistance education, refresher injection training to supplement the initial training provided by the physician and resources to familiarize patients with the self-injection process, as well as ongoing treatment-related resources (including pen and sharp disposal), and medication reminders. In the overall population, the abandonment rate was 70% lower in the PSP cohort than in the non-PSP cohort. Similar reductions in abandonment rates were observed when each indication was analyzed separately. These findings suggest that medication cost assistance as well as motivating and supporting patients prior to treatment initiation may reduce the odds of prescription abandonment and are not necessarily dependent on the disease characteristics in question. The PSP provides education and copayment assistance, and both are expected to impact prescription abandonment. The PSP also provides services such as injection training and refill reminders, which are expected to impact secondary adherence. Subsequent ADA prescription refill is a measure of the latter, and results from our study indicate that among patients who successfully initiated ADA, the odds of a subsequent ADA refill after the first prescription fill was 36% higher among patients participating in the PSP program. While this could be an indication that components of the PSP, such as injection training and education, increased the likelihood of continuing treatment, the contribution of specific components was not examined in this study and continuation beyond a second fill was not evaluated, both of which could be topics of further research.
In our study, the overall treatment abandonment rate was 12%, which is less than the abandonment rate of 38.6% reported in the study of Harnett et al (2016).25 There are some differences between Harnett et al (2016) and our study that may explain the lower abandonment rate we observed. Harnett et al (2016) used electronic health records as the data source and the analysis assessed all written prescriptions including those that patients may not have taken to the pharmacy to fill as well as prescription claims that were denied by the insurer.25 In contrast, we used a claims database and assessed abandonment as prescriptions that were approved by the insurer and filled by the pharmacy, but not picked up by the patient.
A few studies have reported a strong association of higher cost sharing with abandonment of specialty drug prescriptions for various indications, including multiple sclerosis, rheumatoid arthritis, and cancer.37,38,43 Results of our multivariable analysis also indicate that the odds of prescription abandonment increase as the patient’s copayment increases. In the current study, the mean patient copayment was $344 and $627 for the PSP and non-PSP cohorts, respectively. These mean copays are near the costs expected to substantially increase abandonment rates.37,38,43 Other factors that may influence prescription abandonment include fear of side effects associated with starting a new medication, fear of self-injecting the medication, or a lack of understanding of the long-term benefits of therapy. Engaging patients to become partners in their own treatment and providing educational information regarding setting long-term therapy goals with their physician and addressing quality-of-life issues may reduce the effect of these other factors. For patients with chronic disease (eg, rheumatoid arthritis, multiple sclerosis, diabetes), positive benefits in terms of increased medication adherence and improved quality of life have been demonstrated through medication-focused PSPs.30–34,45 PSPs accomplish these goals by helping patients self-manage their condition by providing individualized medication counseling, training, and educational materials.34 Indeed, results from our study indicate that engaging patients through the PSP not only reduces initial ADA prescription abandonment but also increases the odds that a second ADA prescription will be filled.
Though our study did not examine long-term outcomes, the refill likelihood results are consistent with prior studies demonstrating that providing patient support positively influences patient behavior throughout the first year of treatment. A recent study showed that over a 12-month period, ADA adherence was 14% greater in patients participating in AbbVie’s PSP and the discontinuation rate for ADA was 14% lower among patients participating in AbbVie’s PSP compared with those who were not participants.36 In addition, 12-month medical costs (excluding biologic treatment costs) and disease-related medical costs were 23% and 22% lower, respectively, for PSP patients than non-PSP patients.36 As the AbbVie PSP has evolved over time, additional research has shown consistent results regarding adherence and medical costs and demonstrated the continued value of the PSP for ADA-treated patients.46 A separate study47 showed that providing educational information and encouraging patients to adhere to a newly prescribed statin resulted in greater dispensation of the statin prescription in the intervention group compared with controls (42.3% vs 26.0%). Both studies demonstrate the importance of interacting with patients to improve adherence to a treatment regimen and ultimately improve quality of care for patients.
This is the first study to assess the relationship between participation in a comprehensive PSP and ADA abandonment rates across autoimmune diseases in the US. One of the strengths of this analysis is that the claims data provide results for a large, commercial patient population that includes multiple indications treated with ADA. Another strength of this study is that we could examine the temporal relationship between opting into the PSP and ADA treatment abandonment because the database contains longitudinal, patient-level information on medical and pharmacy claims. Finally, unlike the data obtained in highly controlled clinical trials, the claims data accurately reflect real-world patterns of nonadherence.
Limitations
There are some limitations to this analysis that should be noted. First, because this was an observational study and not a randomized controlled clinical trial, any unobservable factors in data may not be balanced between the study groups. For example, sociodemographic disparities in access to prescribed treatment and enrollment in the support program could not be comprehensively evaluated because these data were not available in the source data, but they should be a topic of future research. Though we have controlled for an extensive list of observable characteristics, because of the potential for unobservable heterogeneity, results should be interpreted as the association between PSP participation and prescription abandonment, not necessarily a causal effect. As with all observational studies, there is a possibility of selection bias as patients who chose to opt-in to the PSP may have unique characteristics that affect the probability of initiating therapy. Second, opting-in to the PSP may occur after the initial claim during the period when abandonment is assessed; however, a sensitivity analysis which focused on patients who opted-in to the PSP prior to the initial claim had similar findings. Third, non-electronic prescriptions abandoned prior to submission to a pharmacy are not observed in the data, which may result in underestimating the true abandonment rate. Fourth, many patients first present a copay card at the time of fill, and thus the data may not capture copay cards for patients who abandon their script without appearing at the pharmacy. As such, the expected copay for these patients may be overestimated. Fifth, the contributions of specific components and the benefits of increased use of the PSP were not estimated and analysis of patients who were excluded (eg, those who received government-provided insurance) are areas of continued research. Sixth, the study only considered prescription fills to 6 months following the initial ADA claim, so conclusions cannot be made about long-term treatment patterns or consequences of abandonment. Finally, an enrollment or eligibility file was not available for the SHS data, so a subsequent pharmacy claim was required to ensure a continuous period of data coverage, which may have affected the observed abandonment rate.
Conclusions
Abandonment of ADA initiation is a significant problem potentially affecting patient outcomes across autoimmune indications. This study demonstrated that participation in AbbVie’s PSP was associated with substantially lower abandonment of the first prescription and increased odds of subsequent prescription fills by engaging and supporting patients before treatment initiation. Future studies are needed to assess whether reducing treatment abandonment through the PSP leads to better clinical outcomes and helps to control health care costs in patients with chronic inflammatory diseases requiring long-term care.
Acknowledgment
Medical writing support was provided by Joann Hettasch, PhD, of JK Associates, Inc. (a member of the Fishawack Group of Companies), Conshohocken, PA, and was funded by AbbVie. Financial support for this study was provided by AbbVie. AbbVie participated in the interpretation of data, review, and approval of the publication. All authors contributed to the development of the publication and maintained control over the final content.
Disclosure
Diana Brixner received consulting fees from AbbVie, AstraZeneca, Becton Dickinson, Millcreek Outcomes Group, Sanofi, and UCB Pharma. Manish Mittal is an employee and stockholder of AbbVie. Dr David T Rubin received consulting fees from AbbVie, Abgenomics, Allergan, Inc., Arena Pharmaceuticals, Biomica, Boehringer Ingelheim, Ltd., Bristol-Myers Squibb, Celgene Corp/Syneos, Check-cap, Dizal Pharmaceuticals, GalenPharma/Atlantica, Genentech/Roche, Gilead Sciences, Glenmark Pharmaceuticals, Janssen Pharmaceuticals, Lilly, Mahana Therapeutics, Medtronic, Narrow River Mgmt, Pfizer, Prometheus Laboratories, Reistone, Seres Therapeutics, Shire, Takeda, Target PharmaSolutions, Inc., and investigator-initiated grant support from Takeda. Philip Mease received grant/research support from AbbVie, Amgen, BMS, Celgene, Janssen, Lilly, Novartis, Pfizer, SUN Pharma, UCB; received consulting fees from AbbVie, Amgen, BMS, Celgene, Galapagos, Gilead, Janssen, Lilly, Merck, Novartis, Pfizer, Sun Pharma, and UCB; and was on the speakers bureau for AbbVie, Amgen, BMS, Celgene, Genentech, Janssen, Lilly, Novartis, Pfizer, and UCB. Matthew Davis is an employee of Medicus Economics, which received payment from AbbVie to participate in this research. Arijit Ganguli is an employee and stockholder of AbbVie.
A Mark Fendrick reports personal fees from Merck, AstraZeneca, Trizetto, Johnson & Johnson, Sanofi, AbbVie, Amgen, Centivo, Community Oncology Association, Department of Defense, EmblemHealth, Exact Sciences, Freedman Health, Health at Scale Technologies, Health Management Associates, Lilly, MedZed, PenguinPay, Risalto, Sempre Health, State of Minnesota, Wellth, and Zansors. He also reports grants from AHRQ, Boehringer-Ingelheim, Gary and Mary West Health Policy Center, Laura & John Arnold Foundation, National Pharmaceutical Council, PCORI, PhRMA, RWJ Foundation, and the State of Michigan/CMS. The authors report no other conflicts of interest in this work.
References
- 1.Institute of Medicine. Value in Health Care: Accounting for Cost, Quality, Safety, Outcomes, and Innovation: Workshop Summary. Washington, DC: The National Academies Press; 2010. [PubMed] [Google Scholar]
- 2.Deodhar A, Mittal M, Reilly P, et al. Ankylosing spondylitis diagnosis in US patients with back pain: identifying providers involved and factors associated with rheumatology referral delay. Clin Rheumatol. 2016;35(7):1769–1776. doi: 10.1007/s10067-016-3231-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kekow J, Moots RJ, Emery P, et al. Patient-reported outcomes improve with etanercept plus methotrexate in active early rheumatoid arthritis and the improvement is strongly associated with remission: the COMET trial. Ann Rheum Dis. 2010;69(1):222–225. doi: 10.1136/ard.2008.102509 [DOI] [PubMed] [Google Scholar]
- 4.Colombel JF, Sandborn WJ, Ghosh S, et al. Four-year maintenance treatment with adalimumab in patients with moderately to severely active ulcerative colitis: data from ULTRA 1, 2, and 3. Am J Gastroenterol. 2014;109(11):1771–1780. doi: 10.1038/ajg.2014.242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rutgeerts P, Sandborn WJ, Feagan BG, et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2005;353(23):2462–2476. doi: 10.1056/NEJMoa050516 [DOI] [PubMed] [Google Scholar]
- 6.Panaccione R, Colombel JF, Sandborn WJ, et al. Adalimumab maintains remission of Crohn’s disease after up to 4 years of treatment: data from CHARM and ADHERE. Aliment Pharmacol Ther. 2013;38(10):1236–1247. doi: 10.1111/apt.12499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smolen JS, Han C, van der Heijde DM, et al. Radiographic changes in rheumatoid arthritis patients attaining different disease activity states with methotrexate monotherapy and infliximab plus methotrexate: the impacts of remission and tumour necrosis factor blockade. Ann Rheum Dis. 2009;68(6):823–827. doi: 10.1136/ard.2008.090019 [DOI] [PubMed] [Google Scholar]
- 8.Breedveld FC, Weisman MH, Kavanaugh AF, et al. The PREMIER study: a multicenter, randomized, double-blind clinical trial of combination therapy with adalimumab plus methotrexate versus methotrexate alone or adalimumab alone in patients with early, aggressive rheumatoid arthritis who had not had previous methotrexate treatment. Arthritis Rheum. 2006;54(1):26–37. doi: 10.1002/art.21519 [DOI] [PubMed] [Google Scholar]
- 9.Hanauer SB, Feagan BG, Lichtenstein GR, et al. Maintenance infliximab for Crohn’s disease: the ACCENT I randomised trial. Lancet. 2002;359(9317):1541–1549. doi: 10.1016/S0140-6736(02)08512-4 [DOI] [PubMed] [Google Scholar]
- 10.Wendling D, Joshi A, Reilly P, Jalundhwala YJ, Mittal M, Bao Y. Comparing the risk of developing uveitis in patients initiating anti-tumor necrosis factor therapy for ankylosing spondylitis: an analysis of a large US claims database. Curr Med Res Opin. 2014;30(12):2515–2521. doi: 10.1185/03007995.2014.969368 [DOI] [PubMed] [Google Scholar]
- 11.Armuzzi A, Lionetti P, Blandizzi C, et al. anti-TNF agents as therapeutic choice in immune-mediated inflammatory diseases: focus on adalimumab. Int J Immunopathol Pharmacol. 2014;27(1 Suppl):11–32. doi: 10.1177/03946320140270S102 [DOI] [PubMed] [Google Scholar]
- 12.Kuek A, Hazleman BL, Ostor AJ. Immune-mediated inflammatory diseases (IMIDs) and biologic therapy: a medical revolution. Postgrad Med J. 2007;83(978):251–260. doi: 10.1136/pgmj.2006.052688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Olivieri I, Cortesi PA, de Portu S, et al. Long-term costs and outcomes in psoriatic arthritis patients not responding to conventional therapy treated with tumour necrosis factor inhibitors: the extension of the Psoriatic Arthritis Cost Evaluation (PACE) study. Clin Exp Rheumatol. 2016;34(1):68–75. [PubMed] [Google Scholar]
- 14.Staples MP, March L, Lassere M, Reid C, Buchbinder R. Health-related quality of life and continuation rate on first-line anti-tumour necrosis factor therapy among rheumatoid arthritis patients from the Australian Rheumatology Association database. Rheumatology (Oxford). 2011;50(1):166–175. doi: 10.1093/rheumatology/keq322 [DOI] [PubMed] [Google Scholar]
- 15.Brandt J, Braun J. Anti-TNF-alpha agents in the treatment of psoriatic arthritis. Expert Opin Biol Ther. 2006;6(2):99–107. doi: 10.1517/14712598.6.2.99 [DOI] [PubMed] [Google Scholar]
- 16.Lapadula G, Marchesoni A, Armuzzi A, et al. Adalimumab in the treatment of immune-mediated diseases. Int J Immunopathol Pharmacol. 2014;27(1 Suppl):33–48. doi: 10.1177/03946320140270S103 [DOI] [PubMed] [Google Scholar]
- 17.Gisondi P, Girolomoni G. Impact of TNF-alpha antagonists on the quality of life in selected skin diseases. G Ital Dermatol Venereol. 2013;148(3):243–248. [PubMed] [Google Scholar]
- 18.Fidder HH, Singendonk MM, van der Have M, Oldenburg B, van Oijen MG. Low rates of adherence for tumor necrosis factor-alpha inhibitors in Crohn’s disease and rheumatoid arthritis: results of a systematic review. World J Gastroenterol. 2013;19(27):4344–4350. doi: 10.3748/wjg.v19.i27.4344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wentworth BJ, Buerlein RCD, Tuskey AG, Overby MA, Smolkin ME, Behm BW. Nonadherence to biologic therapies in inflammatory bowel disease. Inflamm Bowel Dis. 2018. doi: 10.1093/ibd/izy102 [DOI] [PubMed] [Google Scholar]
- 20.Lopez A, Billioud V, Peyrin-Biroulet C, Peyrin-Biroulet L. Adherence to anti-TNF therapy in inflammatory bowel diseases: a systematic review. Inflamm Bowel Dis. 2013;19(7):1528–1533. doi: 10.1097/MIB.0b013e31828132cb [DOI] [PubMed] [Google Scholar]
- 21.Kane SV, Chao J, Mulani PM. Adherence to infliximab maintenance therapy and health care utilization and costs by Crohn’s disease patients. Adv Ther. 2009;26(10):936–946. doi: 10.1007/s12325-009-0069-7 [DOI] [PubMed] [Google Scholar]
- 22.Carter CT, Waters HC, Smith DB. Impact of infliximab adherence on Crohn’s disease-related healthcare utilization and inpatient costs. Adv Ther. 2011;28(8):671–683. doi: 10.1007/s12325-011-0048-7 [DOI] [PubMed] [Google Scholar]
- 23.Perry J, Chen A, Kariyawasam V, et al. Medication non-adherence in inflammatory bowel diseases is associated with disability. Intest Res. 2018;16(4):571–578. doi: 10.5217/ir.2018.00033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fallis BA, Dhalla IA, Klemensberg J, Bell CM. Primary medication non-adherence after discharge from a general internal medicine service. PLoS One. 2013;8(5):e61735. doi: 10.1371/journal.pone.0061735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harnett J, Wiederkehr D, Gerber R, Gruben D, Bourret J, Koenig A. Primary nonadherence, associated clinical outcomes, and health care resource use among patients with rheumatoid arthritis prescribed treatment with injectable biologic disease-modifying antirheumatic drugs. J Manag Care Spec Pharm. 2016;22(3):209–218. doi: 10.18553/jmcp.2016.22.3.209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fischer MA, Stedman MR, Lii J, et al. Primary medication non-adherence: analysis of 195,930 electronic prescriptions. J Gen Intern Med. 2010;25(4):284–290. doi: 10.1007/s11606-010-1253-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shah NR, Hirsch AG, Zacker C, et al. Predictors of first-fill adherence for patients with hypertension. Am J Hypertens. 2009;22(4):392–396. doi: 10.1038/ajh.2008.367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Karter AJ, Parker MM, Moffet HH, Ahmed AT, Schmittdiel JA, Selby JV. New prescription medication gaps: a comprehensive measure of adherence to new prescriptions. Health Serv Res. 2009;44(5 Pt 1):1640–1661. doi: 10.1111/j.1475-6773.2009.00989.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hohl CM, Abu-Laban RB, Brubacher JR, et al. Adherence to emergency department discharge prescriptions. CJEM. 2009;11(2):131–138. [DOI] [PubMed] [Google Scholar]
- 30.Stockl KM, Shin JS, Gong S, Harada AS, Solow BK, Lew HC. Improving patient self-management of multiple sclerosis through a disease therapy management program. Am J Manag Care. 2010;16(2):139–144. [PubMed] [Google Scholar]
- 31.Stockl KM, Shin JS, Lew HC, et al. Outcomes of a rheumatoid arthritis disease therapy management program focusing on medication adherence. J Manag Care Pharm. 2010;16(8):593–604. doi: 10.18553/jmcp.2010.16.8.593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ali M, Schifano F, Robinson P, et al. Impact of community pharmacy diabetes monitoring and education programme on diabetes management: a randomized controlled study. Diabet Med. 2012;29(9):e326–e333. doi: 10.1111/j.1464-5491.2012.03725.x [DOI] [PubMed] [Google Scholar]
- 33.Al Hayek AA, Robert AA, Al Dawish MA, Zamzami MM, Sam AE, Alzaid AA. Impact of an education program on patient anxiety, depression, glycemic control, and adherence to self-care and medication in type 2 diabetes. J Family Community Med. 2013;20(2):77–82. doi: 10.4103/2230-8229.114766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ganguli A, Clewell J, Shillington AC. The impact of patient support programs on adherence, clinical, humanistic, and economic patient outcomes: a targeted systematic review. Patient Prefer Adherence. 2016;10:711–725. doi: 10.2147/PPA.S101175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.HUMIRA Patient Support Program. Available from https://www.humira.com/humira-complete. Updated August 18, 2019. Accessed August 21, 2019.
- 36.Rubin DT, Mittal M, Davis M, Johnson S, Chao J, Skup M. Impact of a patient support program on patient adherence to adalimumab and direct medical costs in Crohn’s disease, ulcerative colitis, rheumatoid arthritis, psoriasis, psoriatic arthritis, and ankylosing spondylitis. J Manag Care Spec Pharm. 2017;23(8):859–867. doi: 10.18553/jmcp.2017.16272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gleason PP, Starner CI, Gunderson BW, Schafer JA, Sarran HS. Association of prescription abandonment with cost share for high-cost specialty pharmacy medications. J Manag Care Pharm. 2009;15(8):648–658. doi: 10.18553/jmcp.2009.15.8.648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Starner CI, Alexander GC, Bowen K, Qiu Y, Wickersham PJ, Gleason PP. Specialty drug coupons lower out-of-pocket costs and may improve adherence at the risk of increasing premiums. Health Aff (Millwood). 2014;33(10):1761–1769. doi: 10.1377/hlthaff.2014.0497 [DOI] [PubMed] [Google Scholar]
- 39.Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130–1139. [DOI] [PubMed] [Google Scholar]
- 40.Hopson S, Saverno K, Liu LZ, et al. Impact of out-of-pocket costs on prescription fills among new initiators of biologic therapies for rheumatoid arthritis. J Manag Care Spec Pharm. 2016;22(2):122–130. doi: 10.18553/jmcp.2016.14261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shrank WH, Choudhry NK, Fischer MA, et al. The epidemiology of prescriptions abandoned at the pharmacy. Ann Intern Med. 2010;153(10):633–640. doi: 10.7326/0003-4819-153-10-201011160-00005 [DOI] [PubMed] [Google Scholar]
- 42.Silverman E. Increased abandonment of prescriptions means less control of chronic conditions. Manag Care. 2010;19(6):33–36. [PubMed] [Google Scholar]
- 43.Streeter SB, Schwartzberg L, Husain N, Johnsrud M. Patient and plan characteristics affecting abandonment of oral oncolytic prescriptions. J Oncol Pract. 2011;7(3 Suppl):46s–51s. doi: 10.1200/JOP.2011.000316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Horne R, Chapman SC, Parham R, Freemantle N, Forbes A, Cooper V. Understanding patients’ adherence-related beliefs about medicines prescribed for long-term conditions: a meta-analytic review of the necessity-concerns framework. PLoS One. 2013;8(12):e80633. doi: 10.1371/journal.pone.0080633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marshall JK, Bessette L, Thorne C, et al. Impact of the adalimumab patient support program’s care coach calls on persistence and adherence in Canada: an observational retrospective cohort study. Clin Ther. 2018;40(3):415–429.e416. doi: 10.1016/j.clinthera.2018.02.001 [DOI] [PubMed] [Google Scholar]
- 46.Brixner D, Rubin DT, Mease P, et al. Patient support program increased medication adherence with lower total health care costs despite increased drug spending. J Manag Care Spec Pharm. 2019;25(7):770–779. doi: 10.18553/jmcp.2019.18443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Derose SF, Green K, Marrett E, et al. Automated outreach to increase primary adherence to cholesterol-lowering medications. JAMA Intern Med. 2013;173(1):38–43. doi: 10.1001/2013.jamainternmed.717 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- HUMIRA Patient Support Program. Available from https://www.humira.com/humira-complete. Updated August 18, 2019. Accessed August 21, 2019.