Abstract
Brivaracetam (BRV), an analog of levetiracetam (LEV), was discovered during a target-based rational drug discovery program that aimed to identify potent synaptic vesicle protein 2A (SV2A) ligands. Among the 12,000 compounds screened in vitro, BRV was found to have 15–30 times greater affinity for SV2A and faster brain permeability than LEV. Although preclinical and post-marketing studies suggest broad spectrum of efficacy, BRV is currently only approved as monotherapy and adjunctive therapy of focal-onset seizures in patients age 4 years and older. This review examines the use of BRV as add‐on (5–200 mg/day) therapy for epilepsy with a particular emphasis on the six regulatory randomized clinical trialsinvolving 2399 participants. Participants receiving BRV add‐on at doses of 50–200 mg/day were more likely to experience a 50% or greater reduction in seizure frequency (pooled risk ratio [RR]) 1.79 with 95% CI of 1.51–2.12) than those receiving placebo. Participants receiving BRV were also more likely to attain seizure freedom (57 [3.3%] vs 4 [0.5%]; RR 4.74, 95% CI 2.00–11.25) than those receiving placebo. In addition, BRV demonstrated a favorable safety profile similar to placebo across all BRV doses. Treatment emergent adverse events significantly associated with BRV were irritability, fatigue, somnolence, and dizziness. Post-hoc analysis of regulatory trials, post-marketing studies, and indirect comparison meta-analyses demonstrated equivalent efficacy and better tolerability of BRV when compared to other antiseizure drugs. Further, these studies appear to suggest that behavioral adverse events are likely to be less frequent and less severe with BRV than LEV. Therefore, switching to BRV may be considered for patients who have seizure control with LEV, but who cannot tolerate its behavioral adverse effects. In this setting, immediate switch from LEV to BRV at a 10:1–15:1 ratio without titration is feasible. Further research is needed to examine the long-term tolerability and efficacy of BRV as well as its role in the treatment of other types of epilepsies, particularly dementia-related epilepsy and brain tumor-related epilepsy.
Keywords: antiepileptic drugs; brivaracetam, drug-resistant epilepsy, focal epilepsy, levetiracetam, psychiatric adverse events
Video abstract
Point your SmartPhone at the code above. If you have a QR code reader the video abstract will appear. Or use:
Introduction
Epilepsy is one of the most common, serious neurologic diseases, affecting over 65 million people worldwide.1 Despite the expansion of the anti-seizure drug (ASD) repertoire, with over 16 new ASDs having become available during the past three decades,2 one-third of patients with epilepsy do not respond to ASD treatment.3 Further, many patients suffer from adverse effects of ASDs, which is responsible for poor adherence and discontinuation of ASD therapy, contributing to increased risk of death and increased utilization of unscheduled care.4 Therefore, the need to discover new ASDs with improved efficacy and better tolerability profiles continues. Brivaracetam (BRV) (Briviact, UCB, Inc.), a propyl analog of levetiracetam (LEV), is a rationally discovered anticonvulsant with high-affinity binding to synaptic vesicle protein 2A (SV2A). It was initially approved in 2016 by the Food and Drug Administration (FDA) for adjunctive treatment of focal seizures in patients aged 16 years and older. In 2017, the FDA approved a supplemental new drug application for BRV as monotherapy for focal seizures in patients 16 years and older with epilepsy. Since 2018 BRV has been approved as monotherapy and adjunctive therapy in the treatment of partial onset (focal) seizures in patients age 4 years and older. In the European Union, BRV is only approved as an adjunctive therapy in the treatment of focal-onset seizures with or without secondary generalization in patients 16 years of age and older with epilepsy. This review discusses pharmacological properties, details of efficacy, tolerability, and safety profiles of BRV. In contrast to the previous reviews,5–11 the current review focuses on the clinical trial data and most recent post-market studies of BRV.
Discovery
BRV, a member of racetam family of anticonvulsants, was discovered during a target-based rational drug discovery program that was initiated with the purpose of identifying selective, high‐affinity SV2A ligands possessing antiepileptic properties superior to LEV.12 Approximately 12,000 compounds were screened in vitro for SV2A binding affinity. BRV and seletracetam were the two anticonvulsant candidates chosen for clinical testing,12 however, only BRV entered clinical trials, due to its wider spectrum of activity in animal models than seletracetam, and its pronounced ability to inhibit neuronal hyperexcitability.13
Pharmacokinetics
Dosage and administration
BRV is available for oral (film-coated tablets of 10, 25, 50, 75, and 100 mg or oral solution 10 mg/mL) and intravenous (injection or infusion, 50 mg/5 mL) use. A recent crossover study demonstrated these formulations to be bioequivalent.14 BRV 100 mg intravenous bolus also had the same bioavailability to that of 50 and 100 mg tablets. BRV has a favorable pharmacokinetic profile over a wide dose range (10–800 mg/day) due to its linear and dose-dependent kinetics.15 BRV is rapidly absorbed after oral administration with close to 100% bioavailability.16 High-fat meals may delay absorption, and prolong peak time from 1 to 3 hrs.15 For tablets the median time to peak (tmax) after oral intake is approximately 2 hrs, whereas oral solution shows faster absorption with a tmax of 37.8 mins.16
Metabolism
BRV is highly lipophilic and can rapidly enter the brain (much faster than LEV).17 It crosses the blood–brain barrier (BBB) by passive diffusion without involvement of transporters, and engages with SV2A within minutes.17 BRV is weakly bound to plasma proteins (<20%) with volume of distribution of 0.6 L/kg.18 The mean elimination half-life of BRV is around 7–8 hrs, which does not vary with dose.16 The steady-state concentration is typically achieved after 2 days of repeated dosing.18 BRV is extensively metabolized in the liver to three inactive metabolites.18,19
Elimination
Elimination of unchanged BRV and its metabolites occurs via kidneys within 72 hrs, with 8.6% of administered dose eliminated unchanged.15,16 The renal drug clearance approximates 1.68 L/h in healthy subjects.16 Studies have demonstrated that the pharmacokinetic profile of BRV in elderly and renally impaired subjects is similar to that in healthy subjects.20 Conversely, severe impairment of liver functions dictates reduction of BRV dose by one third, with a maximal daily dose of 150 mg.21,22 The pharmacokinetic properties of BRV (vs LEV) are summarized in Table 1.
Table 1.
The pharmacokinetic properties of brivaracetam in comparison to levetiracetam
| Brivaracetam | Levetiracteam | |
|---|---|---|
| Dosage formulations Oral Intravenous |
25 mg, 50 mg, 75 mg, 100 mg 50 mg/5 mL |
250 mg, 500 mg, 750 mg, 1000 mg 500 mg/5 mL; 500 mg/100 mL; 1500 mg/5 mL |
| Bioavailability | 100%* (may be delayed with high-fat meal) | >95% |
| Time to peak, median (range) | 2 hr (1–4 hrs) | 1 hr (1–2 hrs) |
| Protein binding | 15–20% | <10% |
| Metabolism | Hydrolysis-primary metabolism Hydroxylation (CYP2C19)-16% Unchanged-9% |
34% metabolized (hydrolysis) 66%-unchanged |
| Involvement of CYP450 enzymes | Yes (CYP2C19) | No |
| Elimination half-life (t1/2) | 7–8 hrs | 6–8 hrs |
| Time for steady state | 2 days of repeated dosing | 24–48 hrs of repeated dosing |
| Clearance | 95% via kidney (8–10% unchanged) | 100% via kidney (66% unchanged) |
| Dose adjustment in renal failure/dialysis | Not required | Required (50% supplemental dose following HD) |
| Dosing adjustment in liver failure | Reduce dose by 1/3 may be needed | Not required |
| Relevant drug–drug interaction | Reduced by co-administration of rifampin Reduce combined OCPs by 20–30% at 400 mg/day |
None |
Abbreviations: CYP450, cytochrome P450; HD, hemodialysis; OCP, oral contraceptive pills.
Drug–drug interactions
BRV has a low potential for clinically relevant drug–drug interactions. In regulatory clinical trials, BRV was not demonstrated to be effective in reducing seizure frequency by 50% or more when added to patients who were simultaneously taking LEV.23 Further, recent observational studies caution against the concomitant use of BRV and LEV due to concerns of severe behavioral disturbance.24 Concomitant use of BRV and LEV could also unmask masked depression,24 an atypical depression in patients with unexplained somatic symptoms, even if the symptoms of depression were either absent or present at lesser intensity and were not of primary concern.25 A dose-dependent and reversible inhibition of carbamazepine epoxide hydrolase (CBZ-E) by BRV could occur when it is co-administrated with CBZ,26 however a post-hoc analysis of three regulatory randomized clinical trials (RCTs) (N01252, N01253, and N01358) did not support this association.27 Further, analysis of the five regulatory RCTs (N01114, N01193, N01252, N01253, and N01358) has demonstrated that BRV does not affect steady-state plasma concentrations of LEV, CBZ, lacosamide (LCM), lamotrigine (LTG), 10-hydroxyoxcarbazepine, phenobarbital (PB), pregabalin (PGB), phenytoin (PHT), topiramate (TPX), valproate (VPA), or zonisamide (ZNS).28 Despite the possibility for plasma concentrations to increase when BRV is given with CYP2C19 inhibitors of CYP2C19 (eg, fluconazole and fluvoxamine), adverse clinical consequence is not likely.5,29–31
Mechanism of action in preclinical profile
SV2A is an integral transmembrane glycoprotein expressed throughout the central nervous system and plays a significant role in regulating neurotransmitter release, although the exact mechanism remains unknown.32 It has been proposed that SV2A could act like a transporter or modulate exocytosis of transmitter-containing SVs and modify synaptic function.32 Early studies have demonstrated that SV2A-deficient mice experience severe seizures.33 Reduced SV2A expression has also been found in brain tissue obtained from animal models of epileptogenesis and patients with pharmaco-resistant temporal lobe epilepsy.34
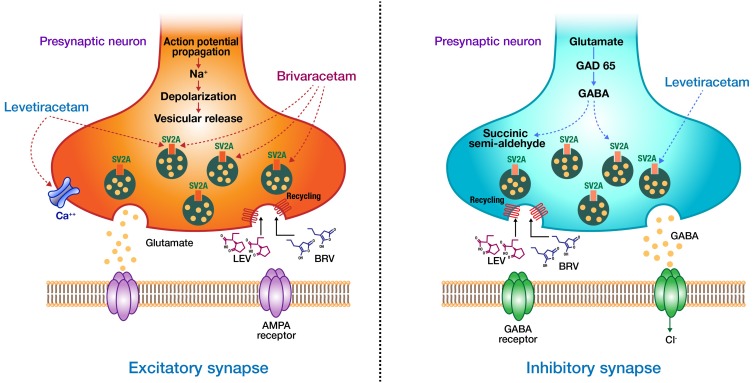
BRV and LEV bind to the human SV2A protein at closely related sites, however they interact at different binding sites, or interact with different conformational states of the protein.35 BRV has much more selective binding with the SV2A protein than LEV; a 15–30-fold higher binding affinity.36 Several animal studies demonstrated that BRV also has a much faster rate of brain penetration, SV2A occupancy (SO), and onset of action than LEV.17 Recently, using a positron emission tomography study, BRV was shown to achieve high SO more rapidly than LEV (when intravenously administered at therapeutic doses in humans).37 Similar to LEV, BRV is postulated to exert its anticonvulsant action by binding the SV2A and modulating its effect on neurotransmitter release.38 Although the details of how the binding to SV2A result in its anticonvulsant effect are not known, it is hypothesized that BRV binding may stabilize the conformation SV2A, enabling the protein to fulfill a protective role during seizures.39 BRV was suggested to inhibit voltage-gated sodium channels (VGSC), however, the reported inhibition of BRV on VGSC currents is not believed to contribute to its anticonvulsant properties.40 Unlike LEV, BRV does not modulate inhibitory or excitatory postsynaptic ligand-gated receptors at therapeutic brain concentrations, supporting the notion that BRV is a more selective and specific SV2A ligand.41 The proposed mechanism of action for BRV and LEV is shown in Figure 1.
Figure 1.
Proposed mechanism of action of brivaracetam (BRV) and levetiracetam (LEV). BRV and LEV bind to the human SV2A protein at closely related sites. BRV has a 15–30-fold higher binding affinity than LEV. Unlike LEV, BRV does not Inhibit high-voltage-gated calcium currents or modulate inhibitory or excitatory postsynaptic ligand-gated receptors at therapeutic brain concentrations.
Abbreviations: AMPA, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid; GABA, gamma-aminobutyric acid; GAD65, glutamate decarboxylase 65; SV2A, synaptic vesicle protein 2A.
The increased affinity for SV2A of BRV over LEV seen in in vitro studies correlated well with its higher potency and efficacy in various animal models of focal, generalized, and drug-resistant seizures.12 BRV has been shown to provide more potent protection than LEV against secondary generalized seizures in models of focal epilepsy, including cornea-kindled mice, hippocampus-kindled rats, amygdala-kindled rats, audiogenic seizure-susceptible mice, and the 6 Hz cornea-kindling model mice.42–44 BRV has also demonstrated seizure protection in the classical maximal electroshock stimulation and subcutaneous pentylenetetrazole seizure models, albeit at relatively high doses.45 In addition, BRV demonstrated a complete suppression of seizures in the genetic model of audiogenic seizure-susceptible mice, and in the genetic absence epilepsy rat from Strasbourg.46 Further, BRV displayed more potent anti-seizure and anti-myoclonic activity in an established rat model of cardiac arrest-induced post-hypoxic myoclonus than LEV.47 Lastly, BRV was found to have a potent anticonvulsant effect in animal models of self-sustaining status epilepticus (SE).48 These findings suggest a broad-spectrum efficacy of BRV as compared with LEV.12
Clinical trials
Clinical efficacy
Several double-blind RCTs have reported the safety and efficacy of various doses of oral BRV as adjunctive therapy for uncontrolled focal-onset seizures with or without secondary generalization.
Phase I studies
The findings of Phase I study (N01297) in 16 healthy volunteers suggested that the profile of cognitive, subjective, and electrophysiologic effects of BRV is similar to LEV and placebo.49 In a double-blind, placebo-controlled, parallel-group sequential cohort study of three successive panels of 12 healthy male subjects, BRV was well tolerated at doses of 200–800 mg daily for 2 weeks. Additionally, BRV demonstrated a favorable pharmacokinetic profile, characterized by rapid absorption, volume of distribution limited to total body water, apparent single-compartment elimination, and dose proportionality.19 In a Phase I randomized open-label, 5-year crossover study involving 25 patients the bioequivalence of oral and intravenous formulations of BRV was established.14 In a double-blind, randomized, three-way crossover study that explored the potential pharmacokinetic and pharmacodynamic interactions between ethanol and BRV in 18 healthy males, BRV approximately doubled ethanol effects on psychomotor function, attention, and memory.50 Therefore, the authors advised against intake of alcohol with BRV.
Phase II studies
An exploratory, Phase IIb, double-blind, randomized, parallel-group, placebo-controlled, dose-ranging study in patients 16–65 years old (N01193) found that adjunctive BRV at a daily dose of 50 mg (but not at 5 or 20 mg doses) was associated with significant reductions in focal seizure frequency per week.51 In another Phase IIb, double-blind, randomized, placebo-controlled, parallel-group, dose-ranging study (N01114), the primary efficacy analysis did not reach statistical significance, however statistically significant differences were observed as compared with placebo on several secondary efficacy outcomes with BRV at a 50 mg daily dose.52 Details of BRV efficacy results from these clinical trials are summarized in Table 2.
Table 2.
Summary of efficacy outcomes from the six regulatory RCTs of brivaracetam as adjunctive therapy in adult patient with drug-resistant seizures
| Study | Study design | Population | N (ITT) | Treatment arms | Age mean (years) | Primary outcome focal- onset seizure frequency/week vs placebo) | Median % reduction from baseline | Secondary endpoints >50% responder rate | Complete seizure freedom |
|---|---|---|---|---|---|---|---|---|---|
| French et al, 2010 (study N01193)51 | Phase IIb, double-blind RCT, 4-week prospective baseline, 7-week treatment period | Patients with uncontrolled focal seizures despite 1–2 ASDs | 208 | Placebo BRV 5 mg BRV 20 mg BRV 50 mg |
33.6 32.7 35,3 30.9 |
NA 9.8% (p=0.240) 14.9% (p=0.062) 22.1% (p=0.004) |
21.7% 29.9 (p=0.086) 42.6% (p=0.014) 53.1% (p<0.001) |
16.7% 32.0% (p=0.047) 44.2% (p=0.002) 55.8% (p=0.001) |
1 (1.9%) 4 (8.0%) 4 (7.7%) 4 (7.7%) |
| Van Paesschen et al, 2013 (study N01114)52 | Phase IIb, double-blind RCT, 4-week prospective baseline, 10-week treatment period (3-week up-titration plus 7-week maintenance) | Patients with uncontrolled focal seizures despite 1–2 ASDs | 157 | Placebo BRV 50 mg BRV 150 mg |
40 38.2 34.4 |
NA 14.7% (p=0.093) 13.6% (p=0.124) |
18.9% 38.2 (p=0.017) 34.9 (p=0.111) |
17.3% 35.8% (p=0.038) 30.8% (p=0.114) |
1 (1.9%) 5 (9.4%) 3 (5.8%) |
| Ryvlin et al, 2014 (study N01252)53 | Phase III, double-blind RCT, 8-week prospective baseline, 12-week treatment period | Patients with uncontrolled focal seizures despite 1–2 ASDs | 398 | Placebo BRV 20 mg BRV 50 mg BRV 100 mg |
36.4 35.7 38.9 38.0 |
NA 6.8 (p=0.239) 6.5% (p=0.261) 11.7% (p=0.037) |
17% 30.0% (p=0.019) 26.8% (p=092) 23.5% (p=0.004) |
20% 27.3% (p=0.339) 27.3 (p=0.372) 36.0% (p=0.023) |
None 2 (2%) None 4 (4%) |
| Biton et al, 2014 (study N01253)54 | Phase III, double-blind RCT, 8-week prospective baseline, 12-week treatment period | Patients with uncontrolled focal seizures despite 1–2 ASDs | 396 | Placebo BRV 5 mg BRV 20 mg BRV 50 mg |
37.5 38.9 37.3 38.9 |
NA -0.9% (p=0.885) 4.1 (p=0.492) 12.8% (p=0.025) |
17.8% 20.0% (p=NS) 22.5% (p=NS) 30.5 (p=0.003) |
16.7% 21.9% (p=NS) 23.2% (p=NS) 32.3% (p=NS) |
None 1 (1.1%) 1 91%) 4 (4%) |
| Kwan et al, 2014 (study N01254)55 | Phase III, double-blind RCT, 4-week prospective baseline, 16-week treatment period (8-week dose-finding plus 8-week stable dose maintenance) | Patients with uncontrolled focal (n=431) or generalized (n=49) seizures despite 1–3 ASDs | 480 | Placebo BRV titration up to 150 mg |
36.5 35.6 |
NA 7.3% (p=0.125) |
18.9% 26.9% (p=0.070) |
16.7% 30.3 (p=0.006) |
None 2 (1.5%) |
| Klein et al, 2015 (study N01358)56 | Phase III, double-blind RCT, 8-week prospective baseline, 12-week treatment period | Patients with uncontrolled focal seizures despite 1–2 ASDs | 760 | Placebo BRV 100 mg BRV 200 mg |
39.8 39.1 39.8 |
NA 22.8% (p<0.001) 23.2 (p<0.001) |
17.6% 37.2% (p<0.001) 35.6% (p<0.001) |
21.6% 38.9% (p<0.001) 37.8% (p<0.001) |
0.8% 5.3% 4.0% |
Note: The p-values are bold where they are significant.
Abbreviations: ASDs, antiseizure drugs; BRV, brivaracetam; N (ITT), number of patients in intention to treat population; NA, not applicable; NS, non-significant; RCT, randomized controlled trial.
Phase III studies
Adjunct therapy
A prospective, multicenter, randomized, double-blind, placebo-controlled, parallel-group, fixed-dose trial (N01253) reported that adjunctive BRV at a daily dose of 50 mg is associated with statistically significant reductions in seizure frequency compared with placebo.54 In another double-blind, randomized, placebo-controlled trial (N01252), the primary efficacy analysis based on the 50 mg/day dose was not statistically significant.53 In comparison, BRV at 100 mg/day reduced baseline-adjusted focal seizure frequency/week by 11.7% over placebo, achieving statistical significance (p=0.037). Secondary efficacy analyses, median percent reduction, and >50% responder rate also provided supportive evidence for the efficacy of BRV at 100 mg/day. The third pivotal Phase III randomized, double-blind, placebo-controlled, multicenter study (N01358) reported that adjunctive BRV at 100 and 200 mg/day was efficacious in reducing focal-onset seizures in adults without concomitant LEV use.56 Secondary efficacy analyses, median percent reduction, and >50% responder rate also provided supportive evidence for the efficacy of both BRV 100 mg/day and BRV 200 mg/day. Lastly, the fourth pivotal Phase III randomized, double-blind, placebo-controlled flexible dose trial (N01254) in adults 16–70 years old with uncontrolled epilepsy (up to 20% were patients with generalized epilepsy), demonstrated that adjunctive BRV given at individualized tailored doses (20–150 mg/day) was well tolerated in adults with uncontrolled epilepsy.55 Further, adjunctive BRV titrated up to 150 mg/day dose improved the 50% responder rate (secondary endpoint) by 13.6% over placebo. An efficacy result of the six regulatory RCTs is summarized in Table 2.
BRV was also evaluated in two Phase III randomized, double-blind, placebo-controlled trials (NO1187; NO1236) in 47 patients with Unverricht–Lundborg disease (EPM1).57 There was no significant improvement in action myoclonus scores (primary efficacy end-point) at 5 mg, 50 mg or 150 mg/day doses. However, the drug was well tolerated with high study completion rates (95.3% overall) and a high percentage of patients entering long-term follow-up (88.7% overall). The authors speculated that perhaps action myoclonus showed wide intra-patient variability and may not have been the optimal tool to measure severity of myoclonus in EPM1.
Monotherapy
Two Phase III, randomized, double-blind, multicenter, historical-controlled, conversion-to-monotherapy studies (N01276; N01306) were conducted to evaluate the efficacy, safety, and tolerability of conversion to BRV 50 mg/day monotherapy in adults with uncontrolled focal seizures.58 In this study, patients aged 16–75 years, with 2–40 focal seizures per 4 weeks during an 8-week baseline, and on stable doses of 1–2 ASDs were enrolled. Patients were randomized to BRV 50 or 100 mg/day (3:1) in two equal doses without titration. The treatment period comprised 1-week BRV add-on, 8-week baseline ASD tapering, and 8-week BRV monotherapy periods. Primary efficacy endpoint was Kaplan–Meier estimate of the cumulative exit rate due to pre-defined exit criteria at Day 112 (50 mg/day, efficacy population). After randomization of 150 patients, both studies were terminated as interim analysis revealed the studies were unlikely to attain a positive outcome for the efficacy analysis, however BRV 50 mg/day monotherapy demonstrated an exit rate lower than historical control.
Meta-analysis
Pooled analysis from the six regulatory RCTs studies involving 2399 participants according to intent to treat (1715 for BRV and 684 for placebo groups) demonstrated that the pooled risk ratio (RR) for the 50% responders and seizure freedom were 1.79 (1.51–2.12) and 4.74 (2.00–11.25), respectively.23 Notably, the sub-analysis by LEV status did not show a statistically significant difference in the 50% responder rate when comparing BRV with placebo in patients with concomitant administration of LEV. In safety/tolerability and efficacy data for 35 patients aged ≥65 years pooled from three randomized, double-blind, placebo-controlled, fixed-dose Phase III studies (N01252, N01253, N01358), the median percent reduction from baseline in focal seizure frequency/28 days was 14.0% for placebo vs 25.5%, 49.6%, and 74.9% for BRV 50, 100, and 200 mg/day, respectively.59 The ≥50% responder rate was 14.3% for placebo vs 25.0%, 50.0%, and 66.7% for BRV 50, 100, and 200 mg/day, respectively. These findings suggest that BRV may be a suitable adjunctive treatment for older patients with uncontrolled focal seizures.
In a post-hoc analysis pooled from the three pivotal Phase III RCTs (N01252, N01253, and N01358), BRV administration with concomitant LTG or TPM reduced seizure frequency and was generally well tolerated for BRV doses of 50–200 mg/day.60 Patients treated with BRV 100 or 200 mg/day, and either LTG or TPM, appeared more likely to achieve a ≥50% reduction in seizure frequency compared with the corresponding placebo groups. Another post-hoc study of the three pivotal Phase III RCTs (N01252, N01253, and N01358) evaluated patients receiving BRV 50–200 mg/day (n=1160) by prior exposure to LEV and three other commonly used AEDs – CBZ, TPM, and LTG.61 Study completion rates were similar in the ASD-exposed subgroups and ASD-naïve subgroups. The study found that patients previously treated by any of these four ASDs had reduced response to BRV, irrespective of the mechanism of action. The authors speculated that this observation could be due to the greater disease severity in the exposed patients. Importantly, this study also revealed that previous treatment failure with LEV should not preclude the use of BRV.61 In another study, an indirect comparison meta-analysis of 17 RCTs involving 4971 patients, the efficacy and tolerability of adjunctive BRV was compared to that of LCM, eslicarbazepine acetate (ESC), and perampanel (PER) in patients with focal-onset seizures.62 The analysis did not demonstrate a significant difference in efficacy between add-on BRV (50 mg/day) and LCM (200 mg/day), ESC (800 mg/day), or PER (12 mg/day). Further, lower adverse events were observed with high-dose BRV compared to high-dose ESC or PER. Lastly, Charokopou et al recently assessed the relative efficacy, safety, and tolerability of adjunctive BRV and other ASDs using a Bayesian network meta-analysis approach of 65 published RCTs. Their analysis demonstrated relative equivalence in efficacy, safety, and tolerability outcomes of the included ASDs (vs BRV): oxcarbazepine, PER, PGB, retigabine/ezogabine, TPM, and ZNS.63
Safety and tolerability
Overall, both oral and intravenous forms of BRV are generally well tolerated with only mild-to-moderate side effects.10 Pooled data from the six regulatory RCTs found that long-term treatment with BRV is well tolerated.23 The overall relative risk for treatment withdrawal due to treatment emergent adverse events (TEAEs) or any reason were 1.58 (1.04–2.40) and 1.27 (0.93–1.73), respectively. The TEAEs significantly associated with BRV were dizziness, somnolence, fatigue, and irritability. The most common psychiatric adverse events (PAEs) were irritability (3.2% of BRV-treated patients vs 1.1% of placebo-treated patients), insomnia (2.9% BRV vs 1.5% placebo), anxiety (2.0% BRV vs 1.3% placebo), and depression (2.0% BRV vs 1.1% placebo). The number of serious TEAEs was 73/1787 (4.1%) for subjects randomized to BRV treatment groups and 38/718 (5.3%) for placebo group. The results of the meta-analysis of TEAEs reported from the six regulatory RCTs are provided in Table 3. In another meta-analysis of eight RCTs with a total of 2505 patients, 1178 of which were randomized with respect to BRV, serious TEAEs, overall withdrawal, TEAE-related withdrawal, and PAEs were not significantly associated with BRV treatment.64 Further, there was no significant difference in the overall withdrawal rate between the BRV and placebo groups [RR 95% CI, 1.18 (0.84, 1.65); p=0.34]. TEAE-related withdrawal was also not associated with BRV treatment [RR 95% CI, 1.36 (0.91, 2.04); p=0.14]. Dizziness [RR (99% CI)=1.57 (1.13, 2.18), p=0.008], fatigue [RR (95% CI)=1.98 (1.32, 2.97), p=0.001], and back pain [RR (95% CI) =0.44 (0.20, 0.93), p=0.03] were significantly associated with BRV treatment. The comparison also showed that PAEs with BRV were not higher than with placebo [RR (95% CI)=0.88 (0.60, 1.31), p=0.54]. Lastly, a recent study consisting of 43 patients that underwent neuropsychological screening before adjunctive treatment with BRV and follow-up evaluation after 5 days or 25 weeks revealed a significant improvement with regards to attention and executive function.65
Table 3.
Rate of TEAEs (%) reported by ≥5% of patients in any treatment group in the six regulatory RCTs of brivaracetam
| Side effect | Brivaracetam 20 mg | Brivaracetam 50 mg | Brivaracetam 100 mg | Brivaracetam 150 mg | Brivaracetam 200 mg | Overall incidence rate | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| RR | p | RR | p | RR | p | RR | p | RR | p | ||
| At least one TEAE | 1.06 (0.86–1.30) | 0.59 | 1.07 (0.91–1.25) | 0.42 | 1.16 (1.04–1.30) | 0.818 | 0.95 (0.73–1.22) | 0.67 | 1.13 (0.99–1.29) | 0.08 | NA |
| Headache | 0.82 (0.48–1.40) | 0.46 | 1.28 (0.82–1.99) | 0.27 | 0.86 (0.52–1.41) | 0.545 | 1.00 (0.26–3.79) | 1.00 | 0.95 (0.53–1.70) | 0.86 | 10.4% |
| Dizziness | 1.11 (0.60–2.06) | 0.74 | 1.44 (0.83–2.48) | 0.19 | 1.77 (1.01–3.09) | 0.047 | 1.67 (0.42–6.62) | 0.46 | 2.89 (1.57–5.32) | 0.001 | 9.6% |
| Somnolence | 1.47 (0.81–2.66) | 0.20 | 1.54 (0.90–2.64) | 0.12 | 2.25 (1.45–3.49) | <0.001 | 1.00 (0.21–4.73) | 1.00 | 2.19 (1.33–3.63) | 0.002 | 12.4% |
| Fatigue | 3.00 (1.21–7.47) | 0.02 | 2.38 (1.16–4.89) | 0.02 | 2.31 (1.19–4.48) | 0.014 | 0.75 (0.18–3.19) | 0.69 | 3.03 (1.51–6.08) | 0.002 | 7.7% |
| Nasopharyngitis | 5.99 (1.36–26.35) | 0.02 | 2.16 (0.77–6.08) | 0.15 | 2.00 (0.18–21.7) | 0.569 | 1.33 (0.31–5.67) | 0.69 | NA | NA | 4.2% |
| Nausea | 0.99 (0.36–2.69) | 0.98 | 0.83 (0.38–1.82) | 0.64 | 1.50 (0.44–5.16) | 0.520 | 0.67 (0.20–2.23) | 0.51 | NA | NA | 4.9% |
| Insomnia | 1.47 (0.25–8.61) | 0.67 | 2.59 (0.70–9.60) | 0.15 | NA | NA | 3.00 (0.32–27.91 | 0.33 | NA | NA | 2.5% |
| Irritability | 1.96 (0.37–10.46) | 0.43 | 2.95 (1.03–8.44) | 0.04 | 6.48 (1.17–35.9) | 0.033 | 0.50 (0.05–5.35) | 0.56 | 7.31 (0.91–58.97) | 0.062 | 2.8% |
Notes: Data from Lattanzi et al.23 The p-values are bold where they are significant.
Abbreviations: RR, Mantel-Haenszel risk ratios; TEAEs, treatment-emergent adverse events; NA, not applicable.
Although to date no head‐to‐head studies have been performed, PAEs appear less common with BRV than with LEV, and most patients who switched to BRV after experiencing behavioral adverse effects on LEV reported improvement.23 A post-hoc analysis of data pooled from the three regulatory RCTs (N01252, N01252, and N01358) suggested a lower incidence of PAEs in BRV than LEV-treated participants.61 In another study, it was shown that 93.1% of patients who switched from LEV to BRV experienced reduction in the maximum intensity of primary PAEs, while 69.0% of patients showed marked or moderate improvement.66 Therefore, the authors concluded that patients experiencing PAEs associated with LEV may benefit from switching to BRV. Significantly, in a recent prospective open-label controlled study of 37 patients, no PAEs were reported.67 BRV was also associated with better anger levels, mood scores, and quality of life. Moreover, prior use of LEV or the presence of a psychiatric background did not influence the results. In a recent case-series study involving 25 patients with drug-resistant epilepsy and psychiatric co-morbidities, 77% patients who developed PAEs with LEV did not do so on BRV, suggesting that BRV is better tolerated in patients with psychiatric co-morbidities.68 Lastly, in a recent animal study using a Kainic Acid Model, BRV-treated rats displayed significantly less aggressive behaviors (ie, they behaved like the control group) than LEV-treated rats.69 Therefore, the authors concluded that BRV could represent an effective alternative to LEV to limit problems of aggressiveness related to its use.
Post-marketing studies (Phase IV)
A multicenter study aimed to give insights into retention, efficacy, and tolerability in a large cohort of patients with different epilepsy syndromes during the first year of treatment with BRV reported that BRV in broad clinical post-marketing use is well tolerated.70 Efficacy at 3 months was 41.2% (50% responder rate) with 14.9% seizure‐free for 3 months and, at 6 months, 40.5% with 15.3% seizure‐free. The study also found that an immediate switch from LEV to BRV at a ratio of 10:1–15:1 is feasible. In addition, a recent multicenter retrospective post-marketing study involving 575 patients revealed that mean reduction in seizure frequency was 36.0%, 39.7% of patients were ≥50% responders and 17.5% were seizure‐free at 12 months.71 Lastly, post-marketing data in 34 children with focal epilepsies and BRV treatment found a 50% responder rate of 47% (29% seizure-free) at 3 months.72
BRV in the treatment of other epilepsies
Status epilepticus
BRV’s intravenous formulation and fast penetration into the brain will result in its increasing use in the management of SE.6 In a multicenter retrospective review of 205 patients with SE, 11 patients were treated with BRV for refractory or super-refractory SE; there was a cessation of SE in three patients (27%) within the first 24 hrs of BRV treatment.73 Initial BRV doses ranged between 50 and 400 mg (median 100 mg), titrated to a daily dose of 100–400 mg (median 200 mg). While taking BRV, no serious side effects were seen. Another multicenter retrospective cohort study reported that intravenous BRV with a bolus injection of 200–300 mg in two females with absence SE was well tolerated, but did not result in cessation of SE.74 A single-center retrospective study involving seven patients median-aged 68 years (range 29–79) that were treated with intravenous BRV reported immediate clinical and electrophysiological improvement in two patients (29%).75 Median loading dose was 100 mg intravenously over 15 mins (range=50–200 mg), titrated up to a median dose of 100 mg/d (range=100–300 mg). In another single-center retrospective study consisting of 14 patients, seven patients (50%) responded to intravenous BRV.76 Notably, the responders received significantly greater median loading dosage per body weight (3.3 mg/kg) compared to non-responders (1.5 mg/kg) and there were no responders with loading doses below 1.9 mg/kg. In a recent retrospective multi-center study consisting of 43 patients with SE, BRV was effective for 23 patients (54%) even when patients were already being treated with LEV.77 Lastly, a systematic review study consisting of seven studies consisting of 37 patients with SE found a 27–50% of SE cessation following the administration of BRV.78
Generalized epilepsies
Currently, BRV is only approved as adjunctive or monotherapy therapy for patients with focal-onset seizures with or without secondary generalization. However, it has been suggested that it could provide broad-spectrum efficacy given its similarity to LEV, and based on the results from preclinical and clinical studies.12 A Phase III, randomized, double-blind, placebo-controlled flexible dose trial consisting of 480 patients, conducted by Kwan et al had 49 patients with generalized seizures, mostly tonic-clonic (30 patients), absences (14 patients), and myoclonic (14 patients).55 The median percent reduction from baseline in generalized seizure days/week was 42.6% versus 20.7%, and the ≥50% responder rate was 44.4% versus 15.4% in BRV-treated and placebo-treated patients, respectively. In a recent multicenter retrospective study of 61 patients with genetic generalized epilepsies and BRV treatment, 50% responder rates of 36% (25% seizure‐free) for 3 months were reported.74 In a Phase IIA, single-blind, placebo-controlled study evaluating 18 patients with idiopathic generalized epilepsies and photosensitivity, BRV was found to have the ability to suppress generalized photoparoxysmal responses (PPR) on electroencephalogram.79 Among the evaluated dosages (10, 20, 40, or 80 mg/day), 80 mg was the most effective, resulting in long-lasting abolishment of the PPR.
Brain tumor-related epilepsy (BTRE)
There is no evidence that specific ASDs are more effective than others in BTRE, however LEV is the most commonly used given its clinical efficacy, low rate of medication interactions, availability of parenteral dosing, and safety profile.80 The use of LEV has also drawn attention because of its potential beneficial antitumor activity leading to increased survival.80 Unfortunately LEV-related PAEs, including agitation, anxiety, depression, emotional lability, hostility, nervousness, and psychotic symptoms, especially in patients with frontal lobe tumors receiving LEV are being increasingly recognized.81 Given the lower incidence of non‐psychotic PAEs in BRV than LEV, it would be of interest to determine whether this trend endures in BTRE.82 Interestingly, BRV showed dose-dependent cytotoxic and anti-migratory effects in an in vitro study of human glioma cells.83 This may suggest that patients with gliomas could benefit from treatment with BRV, in addition to standard ASD options (similar to LEV).
Dementia-related epilepsy
The increased prevalence of seizures in patients with Alzheimer’s disease (AD), in relation to older populations without dementia, has been widely reported. Decisions on whether to treat seizures and the choice of ASDs for an individual with AD can be challenging.84 Among the various ASDs, the use of LEV and LTG to treat seizures associated with AD is supported by the strongest evidence.84 Although the role of BRV in this patient population has not yet been investigated, in two transgenic mouse models of AD, BRV reduced spike-wave discharges and reversed memory impairments in these mice.85 These preliminary data point to a favorable cognitive profile of BRV similar to LEV86–88 with objective gains in attention and executive functions. Future studies with larger sample sizes and better controlled conditions are needed to confirm these findings.
Epileptic encephalopathies
A multicenter, retrospective cohort study of 42 patients with epileptic encephalopathy that were treated with BRV reported a 50% seizure reduction with BRV, similar to those seen in regulatory trials for focal epilepsies.89 A 50% long-term responder rate was apparent in 19 patients (43%), with two (5%) free from seizures for more than 6 months, and nine (20%, with one [2%] free from seizures) for more than 12 months. TEAEs were predominantly of psycho-behavioral nature and were observed in 16%.
Switching from LEV to BRV
Although no clinical trial has directly compared BRV with LEV in patients experiencing tolerability problems, substitution of LEV treatment by BRV is reasonable. Several studies have highlighted the safety and tolerability of switching from LEV to BRV. In a multicenter retrospective study of 575 patients, among those who switched because of PAEs from LEV therapy (n=53), only 9 (17%) reported PAEs on BRV, and only 3 (5.7%) discontinued because of PAEs.71 In another study, when patients were switched from LEV to BRV due to LEV-induced adverse reactions (mainly PAEs), 57–77% had improved tolerability with BRV.70 Although the occurrence of PAEs during previous LEV exposure predicted poor psycho-behavioral tolerability of BRV treatment, a switch to BRV was shown to alleviate LEV‐induced behavioral adverse events.70,71 An immediate overnight switch from LEV to BRV without titration, at a ratio of 10:1–15:1 is feasible.70,71 Similarly, in a multicenter study of 61 patients with genetic generalized epilepsy, immediate switch from LEV to BRV at a 15:1 ratio was feasible without titration.74 Given that the co-administration of LEV and BRV therapy could theoretically lead to competitive binding of the SV2A ligand and cause severe PAEs, it is generally advised to avoid prescribing BRV in patients concurrently taking LEV.24 A summary of pharmacological properties of BRV and LEV is provided in Table 4.
Table 4.
Comparisons of pharmacological properties of brivaracetam and levetiracetam
| Brivaracetam | Levetiracetam | |
|---|---|---|
| Discovery | Target-based rational drug discovery program | Screening in audiogenic seizure susceptible mice |
| Available formulations | Oral and intravenous | Oral and intravenous |
| Approval status (FDA) Focal-onset seizures Generalized onset seizures |
First time approval in 2016 Yes (for age >4 years old) No |
First time approval in 1999 Yes (for age >1 month old) Yes
|
| Mechanism of action | Selective binding to SV2A |
|
| Binding affinity to SVA2 | 15–30 times higher than LEV | - |
| Drug entry to the brain | Fast speed of entry (within minutes) | Longer than BRV (1 hr) |
| Clinically relevant drug interactions | With rifampin and combined oral contraceptives | None |
| Involvement of CYP450 enzymes | Yes | No |
| Dosing adjustments in liver failure | Required in severe cases | Not required |
| Dosing adjustments in renal failure | Not required | Required |
| Behavioral and psychiatric adverse events | 3% | 10–15% |
| Effect on cognition | Suspected to be similar to LEV | Neutral86 or positive87,88 effect on cognition First-line therapy for dementia-related epilepsy |
| Switching from LEV to BRV | 10:1–15:1 without titration | – |
Abbreviations: AMPA, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor; BRV, brivaracetam; CYP450, Cytochrome P450; FDA, Food and Drug Administration; GTCs, generalized tonic-clonic seizures; LEV, levetiracetam; SV2A, synaptic vesicle glycoprotein 2A.
Conclusion
BRV is a highly lipid-soluble novel member of the racetam family of anticonvulsants that inhibit calcium-dependent exocytosis of synaptic vesicles with excitatory neurotransmitters and effectively decrease frequency of focal-onset seizures. While preclinical studies suggest broad spectrum of efficacy, BRV is currently only approved as monotherapy and adjunctive therapy of focal-onset seizures. BRV has 15–30 times greater affinity for SV2A than LEV, and rapidly penetrates the BBB and engages SV2A (within minutes). BRV exhibits a linear and predictable pharmacokinetic profile with <20% plasma protein binding and elimination half-life of around 7–8 hrs. In contrast to LEV, no BRV dose adjustment is required in renal impairment; however, dose adjustment is required in severe hepatic failure. Overall, BRV has a low potential for clinically relevant drug–drug interactions. Over the last decade, 2399 patients have been studied in the regulatory clinical trial programs that demonstrated the efficacy and tolerability of BRV (for doses of 50–200 mg/day) in patients with drug-resistant epilepsy. Several post-marketing and meta-analysis studies also suggest that BRV is a safe and effective anticonvulsant. This includes patients with psychiatric comorbidities, and patients with demonstrated efficacy of LEV but intolerability of behavioral adverse effects. The better tolerability of BRV as compared with LEV, in terms of behavioral adverse events, is of great interest and requires further research to clarify the mechanism. Future studies are also needed to clarify BRV during longer-term follow-up, and establish its efficacy and tolerability in other types of epilepsies, including BTRE.
Acknowledgment
The author is supported by the Accelerator for Clinicians Engaged in Research and Neuroscience Focused Research Team Programs, Mayo Clinic. He is also a recipient of the American Epilepsy Society Research and Training Fellowship for Clinicians Award (2018/2019). The author would like to thank Katherine Kolson, BSN, RN for her assistance in language editing.
Disclosure
The author reports no conflicts of interest in this work.
References
- 1.Moshé SL, Perucca E, Ryvlin P, Tomson T. Epilepsy: new advances. Lancet. 2015;385(9971):884–898. doi: 10.1016/S0140-6736(14)60456-6 [DOI] [PubMed] [Google Scholar]
- 2.Schmidt D, Friedman D, Dichter MA. Anti-epileptogenic clinical trial designs in epilepsy: issues and options. Neurotherapeutics. 2014;11(2):401–411. doi: 10.1007/s13311-013-0252-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brodie MJ, Barry SJ, Bamagous GA, Norrie JD, Kwan P. Patterns of treatment response in newly diagnosed epilepsy. Neurology. 2012;78(20):1548–1554. doi: 10.1212/WNL.0b013e3182563b19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Malek N, Heath CA, Greene J. A review of medication adherence in people with epilepsy. Acta Neurol Scand. 2017;135(5):507–515. doi: 10.1111/ane.12703 [DOI] [PubMed] [Google Scholar]
- 5.Klein P, Diaz A, Gasalla T, Whitesides J. A review of the pharmacology and clinical efficacy of brivaracetam. Clin Pharmacol. 2018;10:1–22. doi: 10.2147/CPAA.S114072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Willems LM, Bauer S, Rosenow F, Strzelczyk A. Recent advances in the pharmacotherapy of epilepsy: brivaracetam and perampanel as broad-spectrum antiseizure drugs for the treatment of epilepsies and status epilepticus. Expert Opin Pharmacother. 2019;2:1–11. doi: 10.1080/14656566.2019.1637420 [DOI] [PubMed] [Google Scholar]
- 7.Makke Y, Abou-Khalil B. Brivaracetam efficacy and safety in focal epilepsy. Expert Rev Neurother. 2019;24:1–10. doi: 10.1080/14737175.2019.1631160 [DOI] [PubMed] [Google Scholar]
- 8.Hellerslia V, Asistido JM, Iyamu A. Brivaracetam for epilepsy. JAAPA. 2019;32(5):21–22. doi: 10.1097/01.JAA.0000554748.77547.f8 [DOI] [PubMed] [Google Scholar]
- 9.Ferlazzo E, Russo E, Mumoli L, et al. Profile of brivaracetam and its potential in the treatment of epilepsy. Neuropsychiatr Dis Treat. 2015;11:2967–2973. doi: 10.2147/NDT.S60849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coppola G, Iapadre G, Operto FF, Verrotti A. New developments in the management of partial-onset epilepsy: role of brivaracetam. Drug Des Devel Ther. 2017;11:643–657. doi: 10.2147/DDDT.S103468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bresnahan R, Panebianco M, Marson AG. Brivaracetam add-on therapy for drug-resistant epilepsy. Cochrane Database Syst Rev. 2019;3:CD011501. doi: 10.1002/14651858.CD012521.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klitgaard H, Matagne A, Nicolas JM, et al. Brivaracetam: rationale for discovery and preclinical profile of a selective SV2A ligand for epilepsy treatment. Epilepsia. 2016;57(4):538–548. doi: 10.1111/epi.13340 [DOI] [PubMed] [Google Scholar]
- 13.Margineanu D, Klitgaard H. The novel SV2A ligands brivaracetam and seletractam manifest different effects against the epileptiform markers of field potentials in a “high K + -low CA2 + ” rat hippocampal slice model. Annual Meeting of European Congress on Epileptology; July 3; 2006; Helsinki, Finland. [Google Scholar]
- 14.Stockis A, Hartstra J, Mollet M, Hadi S. Bioavailability and bioequivalence comparison of brivaracetam 10, 50, 75, and 100 mg tablets and 100 mg intravenous bolus. Epilepsia. 2016;57(8):1288–1293. doi: 10.1111/epi.13433 [DOI] [PubMed] [Google Scholar]
- 15.Sargentini-Maier ML, Rolan P, Connell J, et al. The pharmacokinetics, CNS pharmacodynamics and adverse event profile of brivaracetam after single increasing oral doses in healthy males. Br J Clin Pharmacol. 2007;63(6):680–688. doi: 10.1111/j.1365-2125.2006.02829.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Otoul C, Watanabe S, McCabe S, Stockis A. Relative bioavailability and bioequivalence of brivaracetam 10 mg/mL oral solution and 50-mg film-coated tablet. Clin Pharmacol Drug Dev. 2017;6(3):313–317. doi: 10.1002/cpdd.275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nicolas JM, Hannestad J, Holden D, et al. Brivaracetam, a selective high-affinity synaptic vesicle protein 2A (SV2A) ligand with preclinical evidence of high brain permeability and fast onset of action. Epilepsia. 2016;57(2):201–209. doi: 10.1111/epi.13267 [DOI] [PubMed] [Google Scholar]
- 18.Sargentini-Maier ML, Espié P, Coquette A, Stockis A. Pharmacokinetics and metabolism of 14C-brivaracetam, a novel SV2A ligand, in healthy subjects. Drug Metab Dispos. 2008;36(1):36–45. doi: 10.1124/dmd.107.017129 [DOI] [PubMed] [Google Scholar]
- 19.Rolan P, Sargentini-Maier ML, Pigeolet E, Stockis A. The pharmacokinetics, CNS pharmacodynamics and adverse event profile of brivaracetam after multiple increasing oral doses in healthy men. Br J Clin Pharmacol. 2008;66(1):71–75. doi: 10.1111/j.1365-2125.2008.03158.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bialer M, Johannessen SI, Levy RH, Perucca E, Tomson T, White HS. Progress report on new antiepileptic drugs: a summary of the tenth Eilat Conference (EILATX). Epilepsy Res. 2010;92(2–3):89–124. doi: 10.1016/j.eplepsyres.2010.09.001 [DOI] [PubMed] [Google Scholar]
- 21.Stockis A, Sargentini-Maier ML, Horsmans Y. Brivaracetam disposition in mild to severe hepatic impairment. J Clin Pharmacol. 2013;53(6):633–641. doi: 10.1002/jcph.82 [DOI] [PubMed] [Google Scholar]
- 22.Strzelczyk A, Klein KM, Willems LM, Rosenow F, Bauer S. Brivaracetam in the treatment of focal and idiopathic generalized epilepsies and of status epilepticus. Expert Rev Clin Pharmacol. 2016;9(5):637–645. doi: 10.1586/17512433.2016.1156529 [DOI] [PubMed] [Google Scholar]
- 23.Lattanzi S, Cagnetti C, Foschi N, Provinciali L, Silvestrini M. Brivaracetam add-on for refractory focal epilepsy: A systematic review and meta-analysis. Neurology. 2016;86(14):1344–1352. doi: 10.1212/WNL.0000000000002545 [DOI] [PubMed] [Google Scholar]
- 24.Tiet MY, George J. Brivaracetam and levetiracetam dose adjustments leading to behavioural adverse effects. Postgrad Med J. 2017;93(1103):566. doi: 10.1136/postgradmedj-2017-134814 [DOI] [PubMed] [Google Scholar]
- 25.Swaine Z. Masked depression In: Kreutzer JS, DeLuca J, Caplan B, editors. Encyclopedia of Clinical Neuropsychology. New York: Springer; 2011:1524–1525. [Google Scholar]
- 26.Stockis A, Chanteux H, Rosa M, Rolan P. Brivaracetam and carbamazepine interaction in healthy subjects and in vitro. Epilepsy Res. 2015;113:19–27. doi: 10.1016/j.eplepsyres.2015.03.003 [DOI] [PubMed] [Google Scholar]
- 27.Brodie MJ, Fakhoury T, McDonough B, et al. Brivaracetam-induced elevation of carbamazepine epoxide levels: a post-hoc analysis from the clinical development program. Epilepsy Res. 2018;145:55–62. doi: 10.1016/j.eplepsyres.2018.06.002 [DOI] [PubMed] [Google Scholar]
- 28.Otoul C, Von Rosentiel P, Stockis A. Evaluation of the pharmacokinetic interaction of brivaracetam on other antiepileptic drugs in adults with partial-onset seizures. Annual Meeting of American Epilepsy Society; December 3; 2007; Philadelphia, PA. doi: 10.1094/PDIS-91-4-0467B [DOI] [Google Scholar]
- 29.Stephen LJ, Brodie MJ. Brivaracetam: a novel antiepileptic drug for focal-onset seizures. Ther Adv Neurol Disord. 2017; 11: 1756285617742081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stockis A, Watanabe S, Scheen AJ, et al. Effect of rifampin on the disposition of brivaracetam in human subjects: further insights into brivaracetam hydrolysis. Drug Metab Dispos. 2016;44(6):792–799. doi: 10.1124/dmd.115.069161 [DOI] [PubMed] [Google Scholar]
- 31.Stockis A, Watanabe S, Fauchoux N. Interaction between brivaracetam (100mg/day) and a combination oral contraceptive: a randomized, double-blind, placebo-controlled study. Epilepsia. 2014;55(3):e27–e31. doi: 10.1111/epi.2014.55.issue-3 [DOI] [PubMed] [Google Scholar]
- 32.Mendoza-Torreblanca JG, Vanoye-Carlo A, Phillips-Farfán BV, Carmona-Aparicio L, Gómez-Lira G. Synaptic vesicle protein 2A: basic facts and role in synaptic function. Eur J Neurosci. 2013;38(11):3529–3539. doi: 10.1111/ejn.12360 [DOI] [PubMed] [Google Scholar]
- 33.Crowder KM, Gunther JM, Jones TA, et al. Abnormal neurotransmission in mice lacking synaptic vesicle protein 2A (SV2A). Proc Natl Acad Sci U S A. 1999;96(26):15268–15273. doi: 10.1073/pnas.96.26.15268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van Vliet EA, Aronica E, Redeker S, Boer K, Gorter JA. Decreased expression of synaptic vesicle protein 2A, the binding site for levetiracetam, during epileptogenesis and chronic epilepsy. Epilepsia. 2009;50(3):422–433. doi: 10.1111/j.1528-1167.2008.01727.x [DOI] [PubMed] [Google Scholar]
- 35.Wood MD, Sands ZA, Vandenplas C, Gillard M. Further evidence for a differential interaction of brivaracetam and levetiracetam with the synaptic vesicle 2A protein. Epilepsia. 2018;59(9):e147–e151. doi: 10.1111/epi.14532 [DOI] [PubMed] [Google Scholar]
- 36.Gillard M, Fuks B, Leclercq K, Matagne A. Binding characteristics of brivaracetam, a selective, high affinity SV2A ligand in rat, mouse and human brain: relationship to anti-convulsant properties. Eur J Pharmacol. 2011;664(1–3):36–44. doi: 10.1016/j.ejphar.2011.04.064 [DOI] [PubMed] [Google Scholar]
- 37.Finnema SJ, Rossano S, Naganawa M, et al. A single-center, open-label positron emission tomography study to evaluate brivaracetam and levetiracetam synaptic vesicle glycoprotein 2A binding in healthy volunteers. Epilepsia. 2019;60(5):958–967. doi: 10.1111/epi.14701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Niespodziany I, Lukyanetz EA, Matagne A, Klitgaard H, Wolff C. Brivaracetam does not modulate the major ionic conductances in neurons. Epilepsia. 2015;56(Suppl 1):192–193. [Google Scholar]
- 39.Daniels V, Wood M, Leclercq K, Kaminski RM, Gillard M. Modulation of the conformational state of the SV2A protein by an allosteric mechanism as evidenced by ligand binding assays. Br J Pharmacol. 2013;169(5):1091–1101. doi: 10.1111/bph.12192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Niespodziany I, André VM, Leclère N, Hanon E, Ghisdal P, Wolff C. Brivaracetam differentially affects voltage-gated sodium currents without impairing sustained repetitive firing in neurons. CNS Neurosci Ther. 2015;21(3):241–251. doi: 10.1111/cns.12347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Niespodziany I, Rigo JM, Moonen G, Matagne A, Klitgaard H, Wolff C. Brivaracetam does not modulate ionotropic channels activated by glutamate, γ-aminobutyric acid, and glycine in hippocampal neurons. Epilepsia. 2017;58(11):e157–e161. doi: 10.1111/epi.13890 [DOI] [PubMed] [Google Scholar]
- 42.Detrait ER, Leclercq K, Matagne A, Klitgaard H. Protective activity of brivaracetam in the 6 Hz model of partial epilepsy: comparison with levetiracetam older antiepileptic drugs. Annual Meeting of American Epilepsy Society; December 6; 2008; Seattle, Washington. [Google Scholar]
- 43.Matagne A, Margineanu DG, Kenda B, Michel P, Klitgaard H. Anti-convulsive and antiepileptic properties of brivaracetam (ucb 34714), a high-affinity ligand for the synaptic vesicle protein, SV2A. Br J Pharmacol. 2008;154:1662–1671. doi: 10.1038/bjp.2008.198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Leclercq K, Kaminski R. Anticonvulsant effects of brivaracetam in the 6 Hz fully-kindled mice. International Epilepsy Congress; September 6; 2015; Istanbul, Turkey. [Google Scholar]
- 45.Klitgaard H, Matagne A. Mechanisms of action of levetiracetam and newer SV2A ligands In: Shorvon S, Pedley TA, editors. The Epilepsies 3. Philadelphia: Butterworth Heineman Elsevier; 2008:28–38. [Google Scholar]
- 46.Klitgaard H, Matagne A, Schachter S, et al. Animal and translational models of the epilepsies In: McArthur RA, Borsini F, editors. Animal and Translational Models for CNS Drug Discovery. New York: Academic Press; 2008:311–335. [Google Scholar]
- 47.Tai KK, Truong DD. Brivaracetam is superior to levetiracetam in a rat model of post-hypoxic myoclonus. J. Neural Transm (Vienna). 2007;114(12):1547–1551. doi: 10.1007/s00702-007-0788-3 [DOI] [PubMed] [Google Scholar]
- 48.Wasterlain C, Suchomelova L, Matagne A, et al. Brivaracetam is a potent anticonvulsant in experimental status epilepticus. Epilepsia. 2005;46:219–220. [Google Scholar]
- 49.Meador KJ, Gevins A, Leese PT, Otoul C, Loring DW. Neurocognitive effects of brivaracetam, levetiracetam, and lorazepam. Epilepsia. 2011;52(2):264–272. doi: 10.1111/j.1528-1167.2010.02746.x [DOI] [PubMed] [Google Scholar]
- 50.Kruithof AC, Watanabe S, Peeters PA, et al. Pharmacological interactions between brivaracetam and ethanol in healthy males. J Psychopharmacol. 2017;31(7):915–926. doi: 10.1177/0269881116665326 [DOI] [PubMed] [Google Scholar]
- 51.French JA, Costantini C, Brodsky A, von Rosenstiel P; N01193 Study Group. Adjunctive brivaracetam for refractory partial-onset seizures: a randomized, controlled trial. Neurology. 2010;75(6):519–525. doi: 10.1212/WNL.0b013e3181ec7f7f [DOI] [PubMed] [Google Scholar]
- 52.Van Paesschen W, Hirsch E, Johnson M, Falter U, von Rosenstiel P. Efficacy and tolerability of adjunctive brivaracetam in adults with uncontrolled partial-onset seizures: a phase IIb, randomized, controlled trial. Epilepsia. 2013;54(1):89–97. doi: 10.1111/j.1528-1167.2012.03598.x [DOI] [PubMed] [Google Scholar]
- 53.Ryvlin P, Werhahn KJ, Blaszczyk B, Johnson ME, Lu S. Adjunctive brivaracetam in adults with uncontrolled focal epilepsy: results from a double-blind, randomized, placebo-controlled trial. Epilepsia. 2014;55(1):47–56. doi: 10.1111/epi.12432 [DOI] [PubMed] [Google Scholar]
- 54.Biton V, Berkovic SF, Abou-Khalil B, Sperling MR, Johnson ME, Lu S. Brivaracetam as adjunctive treatment for uncontrolled partial epilepsy in adults: a phase III randomized, double-blind, placebo-controlled trial. Epilepsia. 2014;55(1):57–66. doi: 10.1111/epi.12433 [DOI] [PubMed] [Google Scholar]
- 55.Kwan P, Trinka E, Van Paesschen W, Rektor I, Johnson ME, Lu S. Adjunctive brivaracetam for uncontrolled focal and generalized epilepsies: results of a phase III, double-blind, randomized, placebo-controlled, flexible-dose trial. Epilepsia. 2014;55(1):38–46. doi: 10.1111/epi.12391 [DOI] [PubMed] [Google Scholar]
- 56.Klein P, Schiemann J, Sperling MR, et al. A randomized, double-blind, placebo-controlled, multicenter, parallel-group study to evaluate the efficacy and safety of adjunctive brivaracetam in adult patients with uncontrolled partial-onset seizures. Epilepsia. 2015;56(12):1890–1898. doi: 10.1111/epi.13212 [DOI] [PubMed] [Google Scholar]
- 57.Kälviäinen R, Genton P, Andermann E, et al. Brivaracetam in unverricht-lundborg disease (EPM1): results from two randomized, double-blind, placebo-controlled studies. Epilepsia. 2016;57(2):210–221. doi: 10.1111/epi.13275 [DOI] [PubMed] [Google Scholar]
- 58.Arnold S, Badalamenti V, Diaz A, et al. Conversion to brivaracetam monotherapy for the treatment of patients with focal seizures: two double-blind, randomized, multicenter, historical control, phase III studies. Epilepsy Res. 2018;141:73–82. doi: 10.1016/j.eplepsyres.2018.02.005 [DOI] [PubMed] [Google Scholar]
- 59.Brodie MJ, Whitesides J, Schiemann J, D'Souza J, Johnson ME. Tolerability, safety, and efficacy of adjunctive brivaracetam for focal seizures in older patients: A pooled analysis from three phase III studies. Epilepsy Res. 2016;127:114––118.. [DOI] [PubMed] [Google Scholar]
- 60.Benbadis S, Klein P, Schiemann J, Diaz A, Elmoufti S, Whitesides J. Efficacy, safety, and tolerability of brivaracetam with concomitant lamotrigine or concomitant topiramate in pooled phase III randomized, double-blind trials: A post-hoc analysis. Epilepsy Behav. 2018;80:129–134. doi: 10.1016/j.yebeh.2017.12.024 [DOI] [PubMed] [Google Scholar]
- 61.Asadi-Pooya AA, Sperling MR, Chung S, et al. Efficacy and tolerability of adjunctive brivaracetam in patients with prior antiepileptic drug exposure: a post-hoc study. Epilepsy Res. 2017;131:70–75. doi: 10.1016/j.eplepsyres.2017.02.007 [DOI] [PubMed] [Google Scholar]
- 62.Brigo F, Bragazzi NL, Nardone R, Trinka E. Efficacy and tolerability of brivaracetam compared to lacosamide, eslicarbazepine acetate, and perampanel as adjunctive treatments in uncontrolled focal epilepsy: results of an indirect comparison meta-analysis of RCTs. Seizure. 2016;42:29–37. doi: 10.1016/j.seizure.2016.08.007 [DOI] [PubMed] [Google Scholar]
- 63.Charokopou M, Harvey R, Srivastava K, Brandt C, Borghs S. Relative performance of brivaracetam as adjunctive treatment of focal seizures in adults: a network meta-analysis. Curr Med Res Opin. 2019;25:1–10. [DOI] [PubMed] [Google Scholar]
- 64.Zhu LN, Chen D, Chen T, Xu D, Chen SH, Liu L. The adverse event profile of brivaracetam: a meta-analysis of randomized controlled trials. Seizure. 2017;45:7–16. doi: 10.1016/j.seizure.2016.11.008 [DOI] [PubMed] [Google Scholar]
- 65.Witt JA, Elger CE, Helmstaedter C. Short-term and longer-term effects of brivaracetam on cognition and behavior in a naturalistic clinical setting-preliminary data. Seizure. 2018;62:49–54. doi: 10.1016/j.seizure.2018.09.016 [DOI] [PubMed] [Google Scholar]
- 66.Yates SL, Fakhoury T, Liang W, Eckhardt K, Borghs S, D’Souza J. An open-label, prospective, exploratory study of patients with epilepsy switching from levetiracetam to brivaracetam. Epilepsy Behav. 2015;52(Pt A):165–168. doi: 10.1016/j.yebeh.2015.09.005 [DOI] [PubMed] [Google Scholar]
- 67.Toledo M, Abraira L, Mazuela G, Quintana M, Cazorla S, Santamarina E. Effect of brivaracetam on the anger levels of epilepsy patients. A prospective open-labelled controlled study. Seizure. 2019;69:198–203. doi: 10.1016/j.seizure.2019.04.021 [DOI] [PubMed] [Google Scholar]
- 68.Theochari E, Cock H, Lozsadi D, Galtrey C, Arevalo J, Mula M. Brivaracetam in adults with drug-resistant epilepsy and psychiatric comorbidities. Epilepsy Behav. 2019;90:129–131. doi: 10.1016/j.yebeh.2018.11.032 [DOI] [PubMed] [Google Scholar]
- 69.Sanon NT, Gagné J, Wolf DC, et al. Favorable adverse effect profile of brivaracetam vs levetiracetam in a preclinical model. Epilepsy Behav. 2018;79:117–125. doi: 10.1016/j.yebeh.2017.11.019 [DOI] [PubMed] [Google Scholar]
- 70.Steinig I, von Podewils F, Möddel G, et al. Postmarketing experience with brivaracetam in the treatment of epilepsies: A multicenter cohort study from Germany. Epilepsia. 2017;58(7):1208–1216. doi: 10.1111/epi.13768 [DOI] [PubMed] [Google Scholar]
- 71.Villanueva V, López-González FJ, Mauri JA, et al. BRIVA-LIFE-A multicenter retrospective study of the long-term use of brivaracetam in clinical practice. Acta Neurol Scand. 2019;139(4):360–368. doi: 10.1111/ane.13059 [DOI] [PubMed] [Google Scholar]
- 72.Schubert-Bast S, Willems LM, Kurlemann G, et al. Postmarketing experience with brivaracetam in the treatment of focal epilepsy in children and adolescents. Epilepsy Behav. 2018;89:89–93. doi: 10.1016/j.yebeh.2018.10.018 [DOI] [PubMed] [Google Scholar]
- 73.Strzelczyk A, Steinig I, Willems LM, et al. Treatment of refractory and super-refractory status epilepticus with brivaracetam: A cohort study from two German university hospitals. Epilepsy Behav. 2017;70(Pt A):177–181. doi: 10.1016/j.yebeh.2017.03.028 [DOI] [PubMed] [Google Scholar]
- 74.Strzelczyk A, Kay L, Bauer S, et al. Use of brivaracetam in genetic generalized epilepsies and for acute, intravenous treatment of absence status epilepticus. Epilepsia. 2018;59(8):1549–1556. doi: 10.1111/epi.14476 [DOI] [PubMed] [Google Scholar]
- 75.Kalss G, Rohracher A, Leitinger M, et al. Intravenous brivaracetam in status epilepticus: a retrospective single-center study. Epilepsia. 2018;59(Suppl 2):228–233. doi: 10.1111/epi.14486 [DOI] [PubMed] [Google Scholar]
- 76.Aicua-Rapun I, André P, Rossetti AO, Decosterd LA, Buclin T, Novy J. Intravenous brivaracetam in status epilepticus: correlation between loading dose, plasma levels and clinical response. Epilepsy Res. 2019;149:88–91. doi: 10.1016/j.eplepsyres.2018.12.001 [DOI] [PubMed] [Google Scholar]
- 77.Santamarina E, Parejo Carbonell B, Sala J, et al. Use of intravenous brivaracetam in status epilepticus: A multicenter registry. Epilepsia. 2019;60(8):1593–1601. doi: 10.1111/epi.16094 [DOI] [PubMed] [Google Scholar]
- 78.Brigo F, Lattanzi S, Nardone R, Trinka E. Intravenous brivaracetam in the treatment of status epilepticus: a systematic review. CNS Drugs. 2019;33(8):771–781. doi: 10.1007/s40263-019-00652-0 [DOI] [PubMed] [Google Scholar]
- 79.Kasteleijn-Nolst Trenité DG, Genton P, Parain D, et al. Evaluation of brivaracetam, a novel SV2A ligand, in the photosensitivity model. Neurology. 2007;69(10):1027–1034. doi: 10.1212/01.wnl.0000271385.85302.55 [DOI] [PubMed] [Google Scholar]
- 80.Goldstein ED, Feyissa AM. Brain tumor related-epilepsy. Neurol Neurochir Pol. 2018;52(4):436–447. doi: 10.1016/j.pjnns.2018.06.001 [DOI] [PubMed] [Google Scholar]
- 81.Bedetti C, Romoli M, Maschio M, et al. Neuropsychiatric adverse events of antiepileptic drugs in brain tumour-related epilepsy: an Italian multicenter prospective observational study. Eur J Neurol. 2017;24(10):1283–1289. doi: 10.1111/ene.13375 [DOI] [PubMed] [Google Scholar]
- 82.Feyissa AM. Antiepileptic drug-related neuropsychiatric adverse events in brain tumor-related epilepsy: levetiracetam front and center. Eur J Neurol. 2017;24(12):1435–1436. doi: 10.1111/ene.13399 [DOI] [PubMed] [Google Scholar]
- 83.Rizzo A, Donzelli S, Girgenti V, et al. In vitro antineoplastic effects of brivaracetam and lacosamide on human glioma cells. J Exp Clin Cancer Res. 2017;36(1):76. doi: 10.1186/s13046-017-0546-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Vossel KA, Tartaglia MC, Nygaard HB, Zeman AZ, Miller BL. Epileptic activity in alzheimer’s disease: causes and clinical relevance. Lancet Neurol. 2017;16(4):311–322. doi: 10.1016/S1474-4422(17)30044-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nygaard HB, Kaufman AC, Sekine-Konno T, et al. Brivaracetam, but not ethosuximide, reverses memory impairments in an alzheimer’s disease mouse model. Alzheimers Res Ther. 2015;7(1):25. doi: 10.1186/s13195-015-0110-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lamberty Y, Margineanu DG, Klitgaard H. Absence of negative impact of levetiracetam on cognitive function and memory in normal and amygdala-kindled rats. Epilepsy Behav. 2000;1(5):333–342. doi: 10.1006/ebeh.2000.0098 [DOI] [PubMed] [Google Scholar]
- 87.Magalhães JC, Gongora M, Vicente R, et al. The influence of levetiracetam in cognitive performance in healthy individuals: neuropsychological, behavioral and electrophysiological approach. Clin Psychopharmacol Neurosci. 2015;13(1):83–93. doi: 10.9758/cpn.2015.13.1.83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhou B, Zhang Q, Tian L, Xiao J, Stefan H, Zhou D. Effects of levetiracetam as an add-on therapy on cognitive function and quality of life in patients with refractory partial seizures. Epilepsy Behav. 2008;12:305–310. doi: 10.1016/j.yebeh.2007.10.003 [DOI] [PubMed] [Google Scholar]
- 89.Willems LM, Bertsche A, Bösebeck F, et al. Efficacy, retention, and tolerability of brivaracetam in patients with epileptic encephalopathies: a multicenter cohort study from Germany. Front Neurol. 2018;9:569. doi: 10.3389/fneur.2018.00569 [DOI] [PMC free article] [PubMed] [Google Scholar]