Abstract
A photoreductive protocol utilizing [Ru(bpy)3]2+ photocatalyst, blue light LEDs, and ascorbic acid (AscH2) has been developed to reduce nitro N-heteroaryls to the corresponding anilines. Based on experimental and computational results and previous studies, we propose that the reaction proceeds via proton-coupled electron transfer between AscH2, photocatalyst, and the nitro N-heteroaryl. The method offers a green catalytic procedure to reduce, e.g., 4-/8-nitroquinolines to the corresponding aminoquinolines, substructures present in important antimalarial drugs.
Reduction of an aromatic nitro compound is a prototypical way to prepare arylamines that are common substructures in pharmaceuticals, agrochemicals, dyes, and pigments and in a variety of other fine and specialty chemicals.1 This fundamental transformation can be performed using a plethora of synthetic and catalytic protocols: conventional stoichiometric reagents, e.g., SnCl2, TiCl3, Raney Ni, Zn, Sn, Fe, and Na2S, are appropriate for specific lab-scale conversion,2 while transition-metal-catalyzed hydrogenations (e.g., Pd, Au, and Pt on various supports: C, TiO2, etc.) dominate industrial applications.3 Lately, heterogeneous photocatalytic nitroarene reductions on various materials, e.g., semiconductors, nanoparticles, and nanocomposites, have also been under intensive development.4
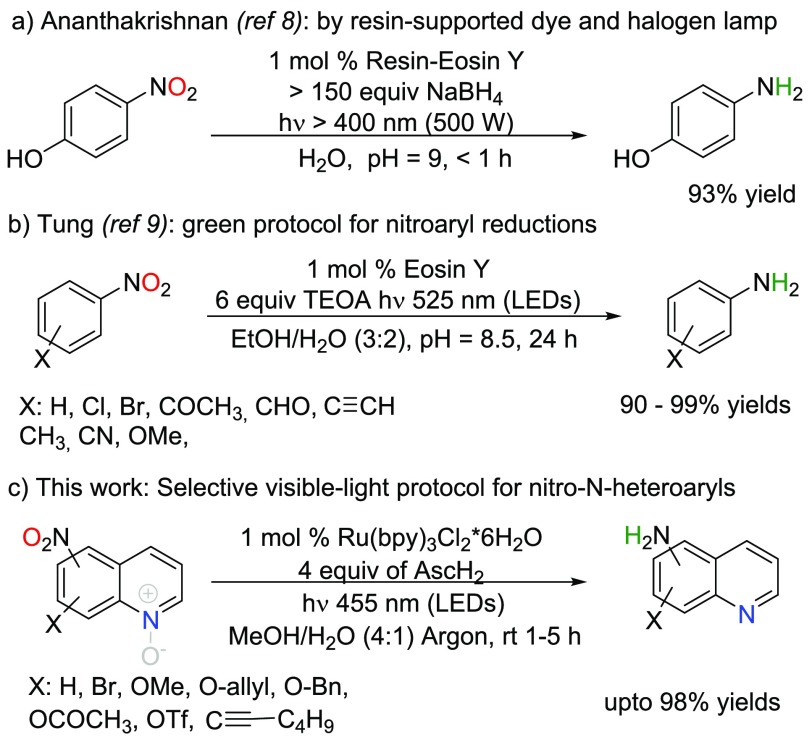
Recent advances in photoredox catalysis (e.g., metal complexes and organic dyes) have enabled new strategies for reductive organic conversions.5 The early photoreductions of nitrobenzenes to anilines were carried out under intense xenon lamp irradiation including UV wavelengths. In a pioneering example, Fukuzumi and co-workers used an excess of dihydroacridine as a reductant for triplet excited PhNO2 in the presence of HClO4/H2O additives in MeCN.6 Later on, Hirao and co-workers developed a more convenient method, exploiting a Ru(bpy)2(MeCN)2(PF6)2 photosensitizer and hydrazine as reductant, while the most intense UV light was filtered out (<300 nm).7 Ananthakrishnan and co-workers employed visible light in 4-nitrophenol photoreduction using resin-supported eosin Y with a large excess of NaBH4 (Scheme 1a).8 Tung and co-workers developed the method further to extend the substrate scope by utilizing green light-emitting diodes (LEDs) as a light source, eosin Y as a photosensitizer, and triethanolamine (TEOA) as a reductant (Scheme 1b).9
Scheme 1. Visible Light Nitroaryl Photoreduction Reduction Protocols.
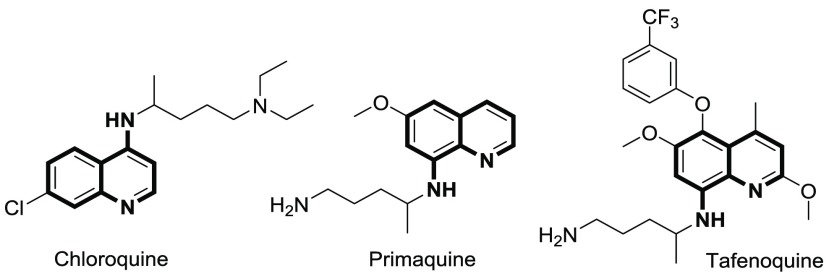
Our interest has been in the development of catalytic methods for quinoline modifications. Previously, we developed a protocol for photoreductive removal of O-benzyl groups from oxyarene N-heterocycles.10 At the beginning of the current study, we observed that even the eosin Y/TEOA photoreduction protocol (Scheme 1b) was widely tolerant for nitrophenyl functional groups, the method was incompatible with nitroquinolines (Table S7). Therefore, we developed here a photocatalytic protocol for reducing nitro groups in N-(oxo)heterocyclic nitroaryls (Scheme 1c) leading to aminoquinoline structures present in many important antimalarial pharmaceuticals, e.g., in traditional chloroquine11 and primaquine12 as well as recently FDA-approved tafenoquine13 (Figure 1).
Figure 1.
Examples of 4-/8-aminoquinoline-containing antimalarial drugs.
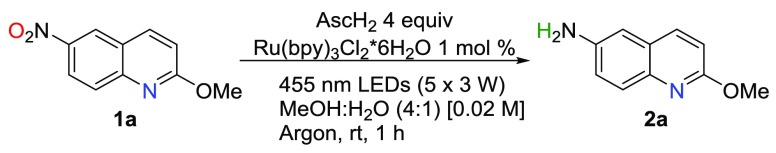
First, we studied the photoreduction of 2-methoxy-6-nitroquinoline 1a in the presence of ascorbic acid (AscH2) as the reductant and Ru(bpy)3Cl2 as the photosensitizer according to earlier successful protocols for reductions by us and others.14 Optimization of the reaction conditions, i.e., photocatalyst, reductant amount, solvent, concentration, and reaction time, provided aniline 2a with 83% yield after 1 h irradiation with blue LEDs light (455 nm) in the presence of 4 equiv of AscH2 and 1 mol % of the [Ru] catalyst in MeOH/H2O (0.02 M) at rt (Tables S1–S7). Varying the reaction conditions from the optimal shows that reaction does not take place without light, AscH2, or photocatalyst and the reaction is sensitive to air atmosphere (entries 2, 8, 4, and 3 Table 1). The reaction was also sensitive to the amount of reactants (entries 5, 9, and 10), and interestingly, the optimal solvent compositions proved to be 4:1 MeOH/H2O and 1:1 EtOH/H2O. The reaction proceeds poorly or not at all in polar aprotic solvents, e.g., DMF and MeCN, even though MeCN was employed as solvent in the previous AscH2 and [Ru]-photocatalyst studies.14
Table 1. Effect of Deviation from Standard Reaction Conditionsa.
| entry | variation from standard conditions | yieldb (%) |
|---|---|---|
| 1 | none | 83 (82)c |
| 2 | no light | 0 |
| 3 | air atmosphere | 28 |
| 4 | no photocatalyst | 0 |
| 5 | 0.5% [Ru] loading | 52 |
| 6 | (Ir[dF(CF3)ppy]2(dtbbpy))PF6 (1 mol %) | 28 |
| 7 | [Ir(dtbbpy)(ppy)2]PF6 (1 mol %) | 16 |
| 8 | no AscH2 | 0 |
| 9 | AscH2 (2 equiv) | 18 |
| 10 | AscH2 (3 equiv) | 79 |
| 11 | Na-ascorbate instead of AscH2 | 0 |
| 12 | 30 min reaction time | 36 |
| 13 | MeOH | 29 |
| 14 | MeOH/H2O (10:1) | 41 |
| 15 | MeOH/H2O (6:1) | 62 |
| 16 | MeOH/H2O (2:1) | 71 |
| 17 | EtOH/H2O (1:1) | 81 |
| 18 | AcOH additive (10 vol %) | 58 |
| 19 | DMF | 11 |
| 20 | MeCN | 0 |
Full reaction optimization in Tables S1–S7.
GC yield: average of two runs.
Isolated yield by SiO2 column chromatography
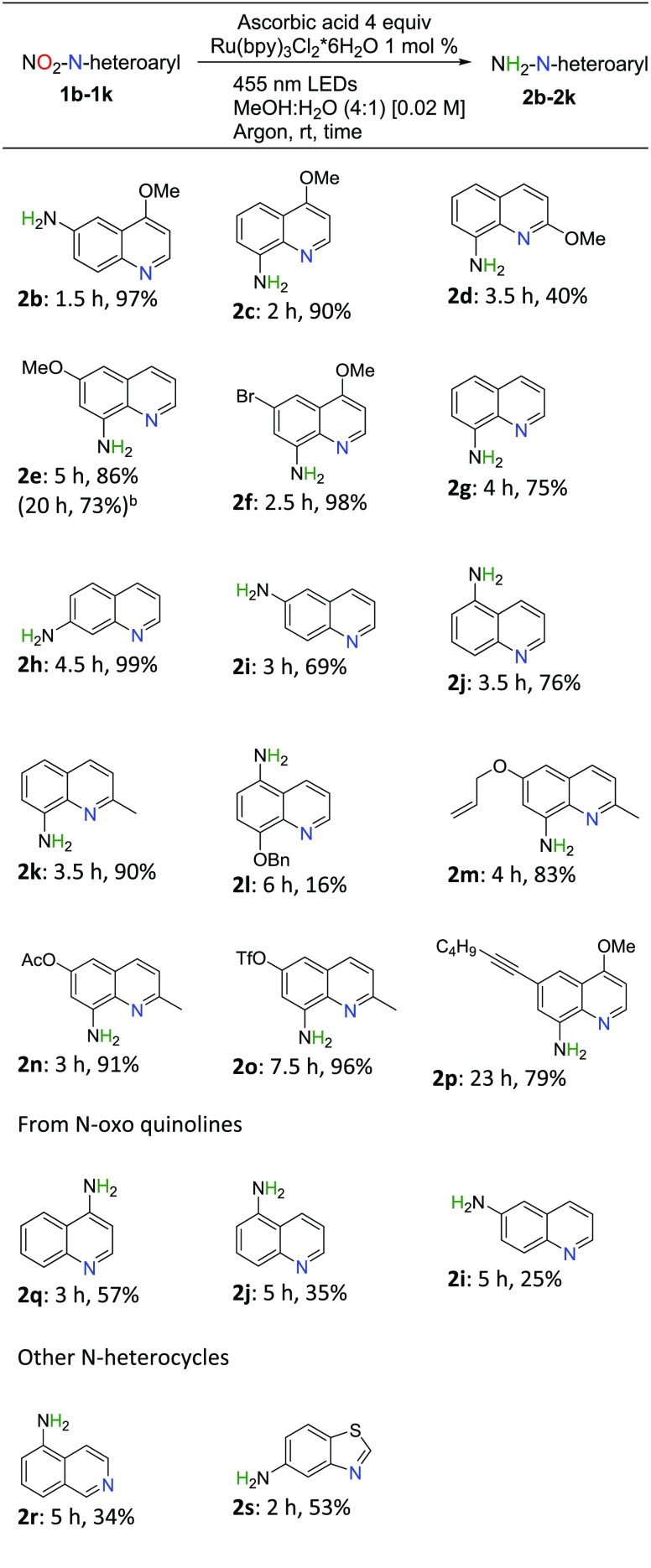
Next, we studied the scope of the reaction (Scheme 2); reduction of nitromethoxyquinolines 1b–1f to the corresponding amines 2b–2f required slightly longer reactions times, 1.5–5 h, depending on the substituent positions. The obtained yields were, however, still high (86–97%) with 2d being an exception by producing unidentified polymeric side products. The excellent yield of 2f demonstrates that halogen substituents in the nitro/amino aryl ring are also well tolerated. In addition, the monosubstituted 5-, 6-, 7-, or 8-nitroquinolines are converted into amines 2g–2j with up to quantitative yields, and the 2-methyl substituent improved the 8-aminoquinoline yield from 75 to 90% (2g vs 2k). Importantly, the protocol proved to be scalable: reduction of 1e on a larger 4 mmol (vs 0.2 mmol) scale with reduced catalyst loading ([Ru] 0.5 mol %) yielded 73% of 2e after 24 h reaction time.
Scheme 2. Reaction Scope Study.
Average isolated yields of two runs after SiO2 chromatography.
Up-scaled (4 mmol) reaction under the same light source, 0.5 mol % of [Ru] catalyst.
A poor yield of 16% received for 2l indicates that the combination of 6-nitro-8-ether substitution is unfavorable for the reaction. On the other hand, 2-methyl-8-nitroquinolines with allyl ether, acyl, or triflyl at the 6-position gave high yields of 83%, 91%, and 96% for 2m, 2n, and 2o, respectively, although the TfO compound (2o) required longer reaction time (7.5 h). The chemoselectivity of the method was further highlighted with a 79% yield of 6-alkyne-substituted 8-amino-4-methoxyquinone 2p after 23 h reaction time.
Another attractive class of target molecules is 4-nitroquinolines (Scheme 2), which are commonly prepared by nitration of N-oxoquinolines and require the use of trivalent phosphorus compounds for removal of the N-oxo groups.15 Our received yields of 57%, 35%, and 25% for 2q, 2i, and 2j, respectively, using N-oxo compounds as starting materials are therefore highly encouraging.
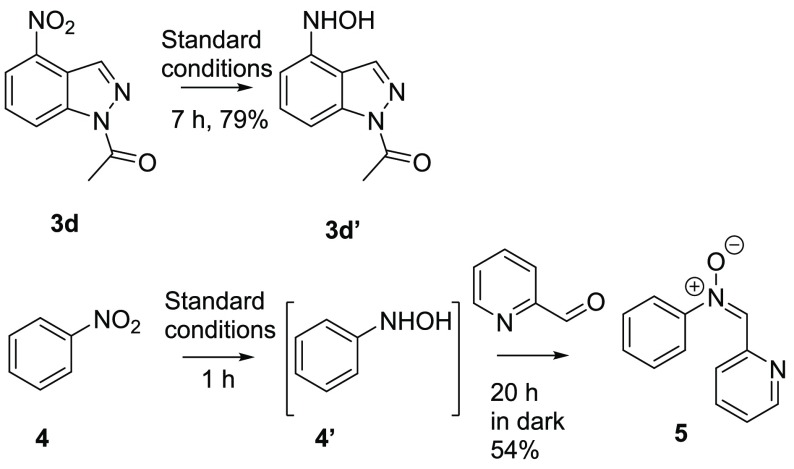
Last, we also tested the substrate scope for producing 6-aminoisoquinoline 2r and 5-aminobenzothiazole 2s, which worked with decent to good yields (Scheme 2). Other tested N-heterocycles fell into the category of unsuitable substrates (3a–3h, SI), being either unreactive, stopping at hydroxylamine intermediate stage (3d, Scheme 3), or leading to side products and oligomerization, which limit the scope of the method.
Scheme 3. Synthesis of Hydroxylamine and Nitrone.
Interestingly, for 1-acyl-4-nitroindazole 3d and nitrobenzene 4,very good yields (79%) of hydroxylamine 3d′ and (54%) nitrone 5, respectively, were obtained instead of their amine products (Scheme 3). Therefore, we decided to study the reaction mechanism to understand why the reaction is not driven to amine formation in these cases.
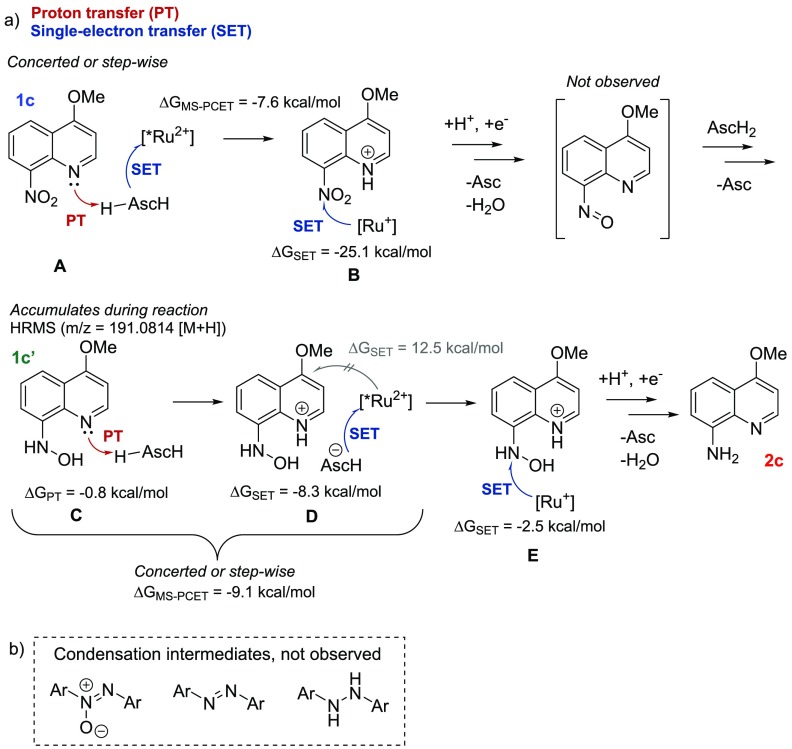
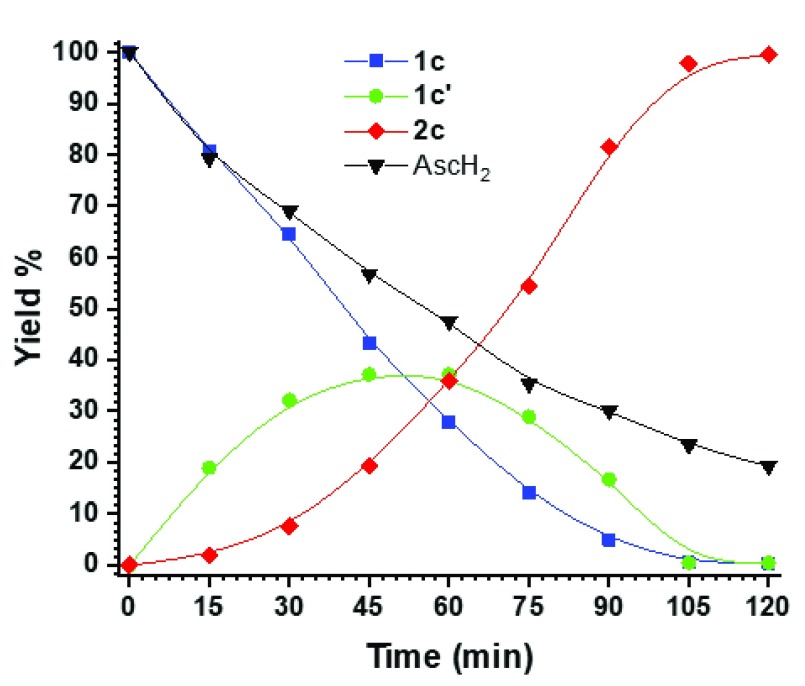
First, the mechanism of the photoreduction was studied by monitoring the conversion of 1c to 2c with NMR. Hydroxylamine intermediate (1c′) was increasingly formed and consumed in the course of the reaction (Figures 2a and 3 and SI) in accordance with the previously proposed mechanism for the nitrobenzene reduction.16,17 The acquired NMR data showed no traces of other species, indicating that the reduction proceeds via the direct route (Figure 2a and SI) rather than through the condensation of hydroxylamine and nitroso intermediates (Figure 2b).
Figure 2.
(a) Schematic representation of the nitro reduction to amine. (b) Condensation intermediates.
Figure 3.
Conversion of 1c to 2c via observed hydroxylamine intermediate 1c′ followed by 1H NMR monitoring (SI). Structures are presented in Figure 2a.
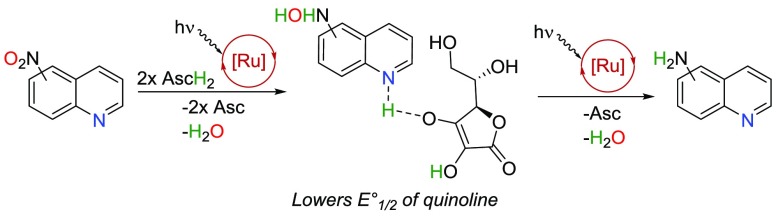
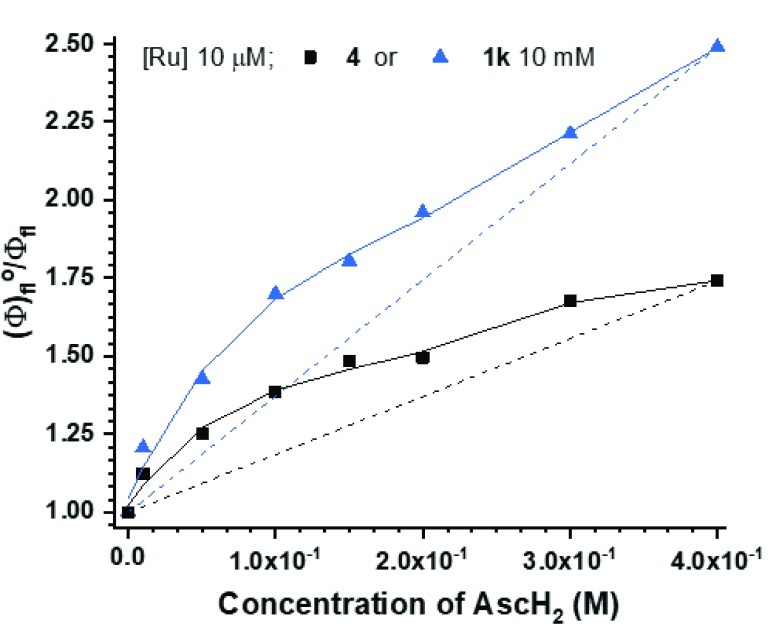
Initially, we considered the first step of the mechanism to be reduction of substrate, or protonation of the substrate followed by its reduction. However, Stern–Volmer measurements pointed out that a 1:1 mixture of 1k/AscH2 was a superior quencher of [*Ru2+] compared to either of the components alone, indicating their cooperative role (SI). Furthermore, the computed oxidation potentials indicate oxidation of ascorbic acid with [*Ru2+] to be an endergonic process (17.5 kcal/mol, SI), and neither do the experimentally observed yields correlate with the computed reduction potentials of the protonated or nonprotonated substrates (see SI for E°red and E°red,H+ values). Therefore, we propose the mechanism depicted in Figure 2a: (A) the reaction is initiated by a multisite proton-coupled electron transfer (MS-PCET)18 between the nitro quinoline, AscH2, and [*Ru2+], i.e., proton and electron are transferred from one donor to two separate acceptors. This is supported by Stern–Volmer titrations for 1k and nitrobenzene (4), in which concentrations of these substrates were fixed and the amount of AscH2 was varied (Figure 4). The nonlinear behavior in both cases indicates an MS-PCET-type reaction as suggested by Qiu and Knowles.18b Next, (B) the protonated nitro substrate is reduced by [Ru+]: reduction of protonated nitro compound with [Ru+] is a more exergonic process (ΔG ≤ −15.9 kcal/mol) than with [*Ru2+] (−12.7 [1g] ≤ ΔG ≤ −0.9 [3d] kcal/mol), and reduction of [*Ru2+] to [Ru+] by AscH– is an exergonic process (−8.3 kcal/mol). The protonation energies of reactive nitro substrates by AscH2 were calculated to vary between thermoneutral (0.7 kcal/mol for 1c) and endergonic (15.4 kcal/mol for 3d), where the endergonicity of protonation does not exclude hydrogen-bonding interactions between acid and base, which in turn can facilitate MS-PCET reactions.18
Figure 4.
Modified Stern–Volmer titration, where [Ru] and quencher 1k or 4 were kept constant and AscH2 quencher was varied.
The protonation of hydroxylamine (C) and the subsequent reduction of [*Ru2+] to [Ru+] by AscH– (D) are required for the reduction of the substrate by [Ru+] (E, Figure 2a), as the reduction of electron-rich hydroxylamine quinolines would be endergonic with *Ru(bpy)32+ (ΔG ≥ 27.9 kcal/mol, SI). The pKaH values for unreactive hydroxylamine intermediates of 3d and 4 indicates that the protonation is endergonic: 10.1 kcal/mol for 3d′ and 8.9 kcal/mol for 4′. In turn, the reactive hydroxylamine intermediates are easily protonated (C, Figure 2a) with free energies of −0.8, 2.4, and 2.0 kcal/mol for 1c, 1d, and 1g, respectively. The reduction of protonated hydroxylamine quinolines would be endergonic with [*Ru2+] (4.8 ≤ ΔG ≤ 12.5 kcal/mol, SI), while the process is exergonic with the obtained ground state [Ru+] (ΔG ≤ −2.5 kcal/mol, SI).
In summary, we have developed a chemoselective and green photoreductive protocol for reduction of nitro N-heteroaryls that exploits AscH2 as hydrogen source. The method complements the existing photoredox catalysis protocols, extending their applicability for the N-heteroaryls.
Acknowledgments
Financial support from the Academy of Finland (Project No. 129062) is acknowledged. The Finnish National Centre for Scientific Computing (CSC) is recognized for computational resources.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.orglett.9b01205.
Experimental procedures, full reaction optimization tables, 1H NMR reaction monitoring, computational procedures and details, and 1H and 13C NMR spectra for all compounds (PDF)
Author Present Address
† (M.M.) BASF SE, Carl-Bosch-Str. 38, 67056 Ludwigshafen, Germany.
The authors declare no competing financial interest.
Supplementary Material
References
- Selected reviews:; a Handbook of reagents for organic synthesis, oxidising and reducing agents; Burk S. D., Danheiser R. L., Eds.; Wiley-VCH: New York, 1999. [Google Scholar]; b Nitro compounds, aromatic. In Ullmann’s Encyclopedia of Industrial Chemistry; Booth G., Ed.; Wiley-VCH: Weinheim, 2012. [Google Scholar]
- Selected reviews:; a Hydrogenation methods; Rylander P. N., Ed.; Academic Press: New York, 1985. [Google Scholar]; b Comprehensive organic synthesis. Selectivity, strategy and efficiency in modern organic chemistry; Trost B. M., Fleming I., Eds.; Pergamon: Oxford, 1991. [Google Scholar]
- Orlandi M.; Brenna D.; Harms R.; Jost S.; Benaglia M. Recent developments in the reduction of aromatic and aliphatic nitro compounds to amines. Org. Process Res. Dev. 2018, 22, 430–445. 10.1021/acs.oprd.6b00205. [DOI] [Google Scholar]
- Selected reviews:; a Valenzuela M. A.; Albiter E.; Ríos-Bernÿ O.; Córdova I.; Flores S. O. Photocatalytic Reduction of Organic Compounds. J. Adv. Oxid. Technol. 2010, 13, 321–340. 10.1515/jaots-2010-0310. [DOI] [Google Scholar]; b Kadam H. K.; Tilve S. G. Advancement in methodologies for reduction of nitroarenes. RSC Adv. 2015, 5, 83391–83407. 10.1039/C5RA10076C. [DOI] [Google Scholar]
- Organic photoredox catalysis reviews:; a Prier C. K.; Rankic D. A.; MacMillan D. W. C. Visible light photoredox catalysis with transition metal complexes: applications in organic synthesis. Chem. Rev. 2013, 113, 5322–5363. 10.1021/cr300503r. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Shaw M. H.; Twilton J.; MacMillan D. W. C. Photoredox catalysis in organic chemistry. J. Org. Chem. 2016, 81, 6898–6926. 10.1021/acs.joc.6b01449. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Romero N. A.; Nicewicz D. A. Organic photoredox catalysis. Chem. Rev. 2016, 116, 10075–10166. 10.1021/acs.chemrev.6b00057. [DOI] [PubMed] [Google Scholar]
- Fukuzumi S.; Tokuda Y. Efficient six-electron photoreduction of nitrobenzene derivatives by 10-Methyl-9,10-dihydroacridine in the presence of perchloric acid. Bull. Chem. Soc. Jpn. 1992, 65, 831–836. 10.1246/bcsj.65.831. [DOI] [Google Scholar]
- Hirao T.; Shiori J.; Okahata N. Ruthenium–bipyridine complex catalyzed photo-induced reduction of nitrobenzenes with hydrazine. Bull. Chem. Soc. Jpn. 2004, 77, 1763–1764. 10.1246/bcsj.77.1763. [DOI] [Google Scholar]
- Gazi S.; Ananthakrishnan R. Metal-free-photocatalytic reduction of 4-nitrophenol by resin-supported dye under the visible irradiation. Appl. Catal., B 2011, 105, 317–325. 10.1016/j.apcatb.2011.04.025. [DOI] [Google Scholar]
- Yang X.-J.; Chen B.; Zheng L.-Q.; Wu L.-Z.; Tung C.-H. Highly efficient and selective photocatalytic hydrogenation of functionalized nitrobenzenes. Green Chem. 2014, 16, 1082–1086. 10.1039/C3GC42042F. [DOI] [Google Scholar]
- Todorov A. R.; Wirtanen T.; Helaja J. Photoreductive removal of O-benzyl groups from oxyarene N-heterocycles assisted by O-pyridine–pyridone tautomerism. J. Org. Chem. 2017, 82, 13756–13767. 10.1021/acs.joc.7b02775. [DOI] [PubMed] [Google Scholar]
- Vale N.; Moreira R.; Gomes P. Primaquine revisited six decades after its discovery. Eur. J. Med. Chem. 2009, 44, 937–953. 10.1016/j.ejmech.2008.08.011. [DOI] [PubMed] [Google Scholar]
- Loria P.; Miller S.; Foley M.; Tilley L. Inhibition of the peroxidative degradation of haem as the basis of action of chloroquine and other quinoline antimalarials. Biochem. J. 1999, 339, 363–370. 10.1042/0264-6021:3390363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman J.; Brown T.; Dow G.; Toovey S. Tafenoquine and primaquine do not exhibit clinical neurologic signs associated with central nervous system lesions in the same manner as earlier 8-aminoquinolines. Malar. J. 2018, 17, 1–12. 10.1186/s12936-018-2555-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Edson J. B.; Spencer L. P.; Boncella J. M. Photorelease of primary aliphatic and aromatic amines by visible-light-induced electron transfer. Org. Lett. 2011, 13, 6156–6159. 10.1021/ol202456d. [DOI] [PubMed] [Google Scholar]; b Binstead R. A.; McGuire M. E.; Dovletoglou A.; Seok W. K.; Roecker L. E.; Meyer T. J. Oxidation of hydroquinones by [(bpy)2(py)RuIV(0)]2+ and [(bpy)2(py)RuIII(OH)]2+. Proton-coupled electron transfer. J. Am. Chem. Soc. 1992, 114, 173–186. 10.1021/ja00027a025. [DOI] [Google Scholar]
- a Brown E. V. Preparation and Reactions of 2-Nitropyridine-l-oxides. J. Am. Chem. Soc. 1957, 79, 3565–3566. 10.1021/ja01570a070. [DOI] [Google Scholar]; b Emerson T. R.; Rees C. W. The deoxygenation of heterocyclic N-oxides. Part III. Kinetics of their reactions with phosphorus trichloride in chloroform. J. Chem. Soc. 1964, 0, 2319–2325. 10.1039/jr9640002319. [DOI] [Google Scholar]; c Ross W. C. The preparation of some 4-substituted nicotinic acids and nicotinamides. J. Chem. Soc. C 1966, 0, 1816–1821. 10.1039/j39660001816. [DOI] [PubMed] [Google Scholar]
- Gelder E. A.; Jackson S. D.; Lok C. M. The hydrogenation of nitrobenzene to aniline: a new mechanism. Chem. Commun. 2005, 522–524. 10.1039/b411603h. [DOI] [PubMed] [Google Scholar]
- Haber F. Über stufenweise reduktion des nitrobenzol mit begrenztem kathodpotential. Z. Elektrochem. Angew. Phys. Chem. 1898, 4, 506–514. 10.1002/bbpc.18980042204. [DOI] [Google Scholar]
- a Biczók L.; Gupta N.; Linschitz H. Coupled electron-proton transfer in interactions of triplet C60 with hydrogen-bonded phenols: Effects of solvation, deuteration, and redox potentials. J. Am. Chem. Soc. 1997, 119, 12601–12609. 10.1021/ja9727528. [DOI] [Google Scholar]; b Qiu G.; Knowles R. R. Rate-driving force relationship in multisite proton-coupled electron transfer activation of ketones. J. Am. Chem. Soc. 2019, 141, 2721–2730. 10.1021/jacs.8b13451. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.