Abstract
Background:
In acute respiratory distress syndrome, pulmonary vascular permeability increases, causing intravascular fluid and protein to move into the lung’s interstitium. The classic model describing the formation of pulmonary edema suggests that fluid crossing the capillary endothelium is drawn by negative interstitial pressure into the potential space surrounding extra-alveolar vessels and, as interstitial pressure builds, is forced into the alveolar air space. However, the validity of this model is challenged by animal models of acute lung injury in which extra-alveolar vessels are more permeable than capillaries under a variety of conditions. In the current study we sought to determine whether extra-vascular fluid accumulation can be produced due to increased permeability of either the capillary or extra-alveolar endothelium and whether different pathophysiology results from such site-specific increases in permeability.
Materials and methods:
We perfused isolated lungs with either the plant alkaloid thapsigargin which increases extra-alveolar endothelial permeability, or with 4α-phorbol 12, 13-didecanoate (4αPDD) which increases capillary endothelial permeability.
Results:
Both treatments produced equal increases in whole-lung vascular permeability, but caused fluid accumulations in separate anatomical compartments. Light microscopy of isolated lungs showed that thapsigargin caused fluid cuffing of large vessels, while 4αPDD caused alveolar flooding. Dynamic compliance was reduced in lungs with cuffing of large vessels, but not in lungs with alveolar flooding.
Conclusions:
Phenotypic differences between vascular segments resulted in site specific increases in permeability which have different patho-physiologic outcomes. Our findings suggest that insults leading to ARDS may increase permeability in extra-alveolar or capillary vascular segments, resulting in different patho-physiologic sequela.
Keywords: Pulmonary Mechanics, Vascular Permeability, Endothelial Heterogeneity, Acute Respiratory Distress Syndrome, Pulmonary Edema
Introduction
The classic model describing development of pulmonary edema suggests that fluid transverses the capillary endothelium and flows, according to a gradient of interstitial pressure, along the peri-vascular interstitium to accumulate around large pulmonary vessels. Such fluid accumulation is seen, pathologically, as peri-vascular cuffs, and represents one of two compartments in which fluid can collect. The second compartment is the alveolar air space which, according to this model, fills after peri-vascular cuffs are formed, as a result of rising interstitial pressure that forces fluid across the alveolar epithelium into the air spaces (1). Fluid filling of the alveolar air spaces is thought to result in alveolar collapse, in turn causing decreased compliance and decreased blood oxygenation, even though fluid accumulation is present both around large blood vessels and in the alveoli in ARDS. Several lines of evidence suggest that this model incompletely describes the edema that forms in the acute respiratory distress syndrome (ARDS).
Studies of pulmonary edema in animals suggest that not all extra-vascular fluid moves across the capillary endothelium. In a number of animal models of acute lung injury, extra-alveolar vessels are more permeable than capillaries, causing fluid to accumulate in peri – vascular cuffs as the result of extra-alveolar, rather than capillary, permeability (2-5). These studies emphasize that in focusing on the accumulation of fluid in the septal compartment during in ARDS, the role that increased extra-alveolar vascular permeability plays in the pathophysiology of ARDS may be ignored. Similarly, because focus has been on events occurring at the alveolar level, alveolar flooding and inactivation of surfactant is believed to exclusively determine mechanical properties of the edematous lung. However, surfactant replacement in acute respiratory distress syndrome (ARDS) patients does not decrease peak pressure or increase tidal volume (6), suggesting that factors other than surfactant inactivation can decrease compliance in ARDS. An alternative hypothesis is that extra – alveolar fluid accumulation in peri – vascular cuffs decreases compliance. This alternative hypothesis is supported by the observation that during experimental hydrostatic edema, compliance decreases prior to alveolar flooding (7). Studies describing increased airway resistance in animal models of pulmonary edema (8-10) and expiratory flow limitation in ARDS patients (11) suggest that the pathophysiology of ARDS causes decreased flow in extra-alveolar airways. Thus, alveolar flooding alone is not a sufficient explanation for decreased compliance due to increased extra-vascular pulmonary fluid in ARDS.
While the classic model of pulmonary edema formation describes capillaries as the source of extra-vascular fluid, extra-capillary vessels may be more permeable than capillaries in a variety of conditions, and extra-alveolar forces may contribute to the changes in pulmonary mechanics associated with ARDS. Our current study sought to determine whether extra-vascular fluid accumulates as a result of either increased extra-alveolar vessel or capillary permeability and, if so, whether these two sites of fluid accumulation differentially effect pulmonary compliance.
Materials and Methods
Lung isolation and perfusion.
All animal experiments were approved by the Animal Care and Use Committee of the University of South Alabama. Adult male Sprague-Dawley rats weighing 250 – 350 g were anesthetized with intraperitoneal pentobarbitol (40 mg/kg). The trachea was cannulated with p60 tubing connected to a ventilator delivering 10cc/kg containing 5% CO2 enriched room air at 60 breaths per minute and 2 cm H2O positive end expiratory pressure. The heart and lungs were exposed, and the pulmonary artery and left ventricle were cannulated. Lungs were perfused via these cannulae with Earl’s balanced salt solution containing NaHCO3 and 4% BSA. After the cannulae were secured, lungs were suspended from a force transducer to measure weight gain. A baseline filtration coefficient (Kf) was measured after an isogravemetric state was achieved (12). Lung volume was derived from integration of flow monitored by a spirometer (AD Instruments) connected just proximal to the tracheal cannula. During the experiment lung weight, pulmonary artery pressure, left ventricular pressure, tidal volume, tracheal pressure, dynamic compliance, and pressure / volume curves were constantly recorded (Power Lab, AD Instruments). Preparations with evidence of hemorrhage or edema at this point in the experiment were not used. Either 4αPDD (3 μM) (13) or thapsigargin (50 nM) (4) was added to the perfusate reservoir and allowed to circulate for 20 minutes before a second Kf was measured. In control experiments, the same procedure was followed using DMSO as a vehicle control. The area under the dynamic compliance curve was calculated during 5 breaths at the end of each Kf measurement using Chart 5 software (AD Instruments, Colorado Springs).
Saline filled lungs.
Heart and lungs were removed in-block and, via the tracheal cannula, lungs were filled with normal saline to the level of the transected trachea. The lungs were then attached to the ventilator and allowed to float in a saline filled beaker. Compliance data was recorded after the fifth ventilation.
Tween rinsed lungs.
Heart and lungs were removed in-block and three cc of 0.5% Tween 20 were injected into the trachea. The lungs were inflated and deflated three times and then as much Tween solution as possible was aspirated. The lungs were hung by the tracheal cannula and ventilated. Compliance data was recorded after the fifth ventilation.
Light Microscopy.
Lungs were perfusion fixed with 3% glutaraldehyde in 0.1 M cacodylate buffer under 14-15 cm H2O venous pressure and 10 ml / kg end - inspiratory volume. Lungs were then cut into smaller pieces, and immersed in fixative. One millimeter portions of the lung were rinsed in cacodylate buffer, post-fixed for 1 hour with 1% osmium tetroxide, dehydrated through a graded alcohol series, and embedded in PolyBed 812 resin (Polysciences Inc., Warrington, PA). Thick sections (1 micron) were cut with glass knives and stained with 1% toluidine blue. Thick sections were examined and photographed using a Nikon E600 light microscope (Nikon Instruments Inc., Melville, NY).
Transmission Electron Microscopy
Thin sections (80 nm) were cut with a diamond knife and then stained with uranyl acetate and Reynold’s lead citrate. Cells were examined and photographed using a Philips CM 100 transmission electron microscope (FEI Company, Hillsboro, OR).
Results
In the current study, we sought to determine whether equal increases in permeability may be achieved in discrete vascular compartments using thapsigargin and 4αPDD (fig 1) and, if so, whether different physiologic sequelae result. We first identified the thapsigargin and 4αPDD concentrations that produce equal increases in permeability by measuring the filtration coefficient (Kf). Kf can be determined by monitoring vascular pressures and weight gain in isolated and perfused lungs (23). In our experiments, untreated lungs (vehicle control DMSO < 0.5%) maintained a Kf of 0.13 mlmin−1 cm H2O−1100g−1, and exhibited no increase in extra-vascular fluid accumulation. Lungs perfused with a 50 nM thapsigargin solution displayed cuffing of large vessels and no accumulation of fluid in the capillary compartment, and an approximately 2.5 – fold increase in Kf versus control lungs (fig 2). In contrast, 4αPDD also increased Kf 2.5 folḍ, but did not produce cuffing of extra-alveolar vessels. Rather, in lungs treated with 4αPDD, fluid accumulated in the alveolar air space, but not around large vessels (fig 3). Transmission electron microscopy confirms the presence of fluid within the alveoli in 4αPDD treated lungs. Alveolar flooding and air space collapse were seen in alveoli (fig 4a) with a type II pneumocyte at its periphery (fig 4b), and floating surfactant (fig 4c and 4d). At higher magnification the endothelium and an endothelial junction appear intact (fig 4d). Though intact endothelium may seem unlikely in the presence of obvious alveolar flooding, previous authors have described an intact endothelial barrier in edematous lungs from ARDS patients (24, 25). This paradox may be explained by considering that relatively minor ultra-structural changes in endothelial architecture increase permeability (26), and that lung microvasculature possesses and extraordinary repair capacity (25).
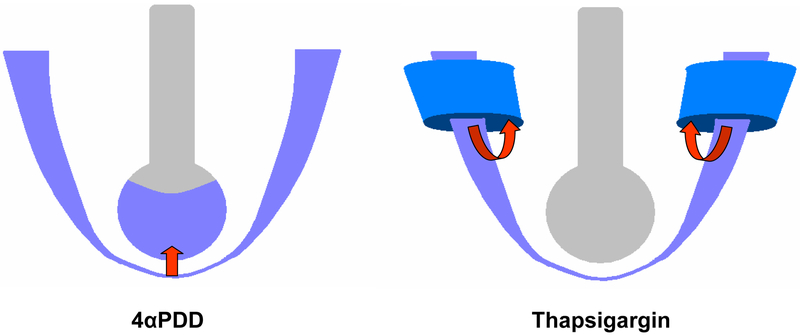
Fig 1.
Discrete sites of increased vascular permeability a) Increased capillary permeability leads to alveolar flooding in 4αPDD treated lungs. b) Increased extra-alveolar permeability leads to peri-vascular cuffs in thapsigargin treated lungs.
Fig. 2.
Vascular permeability of untreated and Tg treated lungs. a) Extra-alveolar vessel cuffing is absent in untreated lungs. b) Tg treated lungs show cuffing of arteries (A) near a bronchiole (B). The alveolar spaces (AS) are free of fluid. c) Tg increased permeability approximately two-fold versus control lungs, * p < 0.01. n = 6 in each group.
Fig. 3.
Vascular permeability of Tg and 4αPDD treated lungs. a) Tg treated lungs show extra-alveolar cuffing of an artery (A) near a bronchiole (B) Alveolar spaces (AS) are free of fluid. b) 4αPDD treated lungs have little or no extraalveolar cuffing, but do have alveolar flooding (AF) c) Tg and 4αPDD had equal increases in permeability, * p < 0.05. n = 6 in each group
Fig. 4.
SEM of 4αPDD treated lungs. a) Flooding of alveoli (AF) is seen among capillaries (C) b) A type II pneumocyte (TII) containing surfactant filled (S) vesicles is seen in a flooded alveolus. c) Surfactant granules float in a fluid filled alveolus. d) Details are seen in an enlarged view of (c): En = endothelium, Ep = epithelium, EJ = endothelial junction, S = surfactant filled vesicles, BM = basement membrane.
The pressure / volume curve has long been viewed as a sensitive descriptor of pulmonary mechanics in animal models (27) and has been studied extensively as a potential aid in diagnosis and treatment of lung disease (28, 29). Classic experiments in which pressure / volume relationships were studied in saline filled lungs, in normal air-filled lungs and in lungs rinsed of surfactant, describe pressure / volume curves in situation of very low, normal, and very high surface tension, respectively (30). We generated pressure volume curves in saline filled lungs (low surface tension), which produce a curve with a near vertical slope. In contrast, air filled lungs generate curves with an intermediate slope, and lungs rinsed of surfactant (maximal surface tension) generate curves with a small slope (fig 5). These experiments demonstrate that compliance decreases as the result of increasing surface tension within the alveoli. In fact, broncho-alveolar lavage from patients with ARDS has increased surface tension (31, 32). Together, these studies suggest that dilution or inactivation of surfactant by increased alveolar fluid causes decreased compliance in ARDS afflicted lungs. We therefore examined whether lungs treated with 4αPDD would be less compliant than control lungs and those treated with thapsigargin. Compliance can be measured in the actively ventilated lung (dynamic compliance) or during interrupted ventilation (static compliance). Both increasing airway resistance and increasing tissue resistance would be expected to increase tracheal pressure, reduce time for lung inflation and decrease dynamic compliance. However, measurements of the lung under static conditions would be sensitive only to changes in tissue resistance. In order to assess both tissue and airway influences on pulmonary mechanics induced by thapsigargin or 4αPDD, we generated dynamic pressure / volume relationships in isolated lungs treated with either thapsigargin or 4αPDD. Lungs treated with thapsigargin had significantly decreased compliance, while lungs treated with 4αPDD were not significantly different than control lungs. When we compared groups according to the percent change in the area under the curve of real-time dynamic compliance values, 4αPDD treatment did not in significantly decrease dynamic compliance. In thapsigargin treated lungs, however, there was a significant decrease in dynamic compliance of 17% versus control (fig 6). Thus, isolated rat lungs which had increased extra-alveolar vessel permeability displayed perivascular cuffing of large vessels and decreased dynamic compliance. In contrast, lungs which had equally increased permeability in the capillary endothelium showed alveolar flooding, but no decrease in dynamic compliance.
Fig. 5.
a) Dynamic pressure volume surves from saline, untreated and detergent rinsed lungs. Saline filled lungs were most compliant, and tween rinsed lungs were least compliant. b) Dynamic pressure / volume curves of representative thapsigargin and 4αPDD treated lungs are superimposed on those of Figure 4a. For clarity, only the inspiratory portion of the curves are shown.
Fig 6.
Dynamic compliance in Tg and 4αPDD treated lungs. Dynamic compliance was significantly lower in Tg treated lungs than in 4αPDD treated lungs. * p < 0.05 vs. 4αPDD
Discussion
Since the original histological descriptions of pulmonary edema, fluid and protein accumulations have been seen both in the interstitium surrounding large vessels and in the alveolar air spaces (14). Fluid accumulation around extraalveolar vessels may result either as fluid moves across the capillary endothelium and then distributes into interstitium (1), or may enter the extraalveolar interstitium directly from extra-alveolar vessels (2, 4, 5, 15). This latter situation, in which pulmonary edema is caused by fluid movement directly across extra-alveolar vessels, can be induced in isolated lungs with the plant alkaloid thapsigargin which activates store operated calcium entry (4). Activation of store operated calcium entry through stimulation of TRPC1 and TRPC4 (16-19) channels increases permeability of extra-alveolar vessels and of endothelial cell mono-layers cultured from those vessels, but does not increase the permeability of capillaries or mono-layers of capillary endothelial cells (4, 20). Interestingly, attenuation of this permeability response to thapsigargin is associated with decreased expression of TRPC1 and TRPC4 channels in extra-alveolar endothelium in rats with heart failure (21), suggesting that down – regulation of these channels in pulmonary endothelium decreases the permeability of vessels under conditions of increased hydrostatic pressure. Conversely, 4α - Phorbol 12, 13 - didecanoate (4αPDD) stimulates TRPV4 channels, which are osmo -, mechano - and temperature - sensitive, and are activated by arachidonic acid metabolites (22). These channels are expressed in greater numbers in capillary endothelium than in extra-alveolar endothelium, and when stimulated, increase permeability of the capillary compartment without substantially increasing the permeability of extra-alveolar vessels (13). These channels may, therefore, represent a mechanism by which circulating inflammatory mediators increase the permeability of capillaries during acute lung injury.
According to the generally accepted model that describes extra-vascular fluid accumulation within the lung, fluid transverses the capillary endothelium and flows by negative interstitial pressure into the potential space surrounding extra-alveolar vessels. This fluid is seen by microscopy as extra-alveolar peri-vascular cuffs (33). In this model pressure builds within these cuffs until fluid is forced across the alveolar epithelium into the alveolar air space (1). Our study provides evidence which is not consistent with this classic model in two respects. First, store operated calcium entry stimulated by thapsigargin causes peri-vascular cuffs to form due to increased permeability in extra-alveolar vessels, demonstrating that extra-vascular fluid accumulates without an increase in capillary permeability. Second, stimulation of TRPV4 channels with 4αPDD causes fluid to enter the alveolar compartment directly from the capillaries without first accumulating in the extra-alveolar interstitium. We also explored the physiologic effects of this site-specific fluid accumulation, and show that perivascular cuffing decreases dynamic compliance while an intermediate degree of alveolar flooding does not. This finding suggests that the ‘stiff lung’ associated with ARDS does not exclusively result from inactivation of surfactant within the alveoli.
The isolated, perfused lung model has been used extensively to study pulmonary vascular permeability. Kf is a measure which describes changes in permeability of the vasculature of isolated lungs based on rate of weight gain and increases in the static pressure difference across the vascular tree during a period of increased outflow pressure (34). Traditionally, because of the much larger surface area of the capillary endothelium, Kf was thought to describe the permeability state of the exchange vessels (23). However, later studies in isolated lungs subject to ischemia / reperfusion (3), hypoxia (5), increased hydrostatic pressure (35) and store operated calcium (4) entry establish that Kf also increases due to permeability of extra-alveolar vessels. We confirm that increased extra-alveolar vessel permeability induced by thapsigargin leads to an increase in Kf, and document the presence of peri-vascular cuffs in these lungs while, in the same lungs, documenting the absence of fluid in the alveolar compartment. Importantly, we were able to equal this increase in Kf, which is due to increased extra-alveolar vessel permeability, by increasing capillary permeability with 4αPDD. These findings are more remarkable if we consider that equal increases in permeability in these two vascular beds means that the permeability increases per surface area was necessarily much greater in extra-alveolar vessels. This finding is consistent with previous studies in uninjured lungs showing that when standardized to surface area, the arterial endothelium has a 26 - fold, and the venous endothelium a 58 - fold greater permeability than capillaries (35). Indeed, according to recent estimates, this tight capillary endothelial cell barrier function represents the most important safety factor against formation of pulmonary edema (36).
The finding that 4αPDD induces fluid accumulation in the alveolar air space without evidence of significant peri-vascular cuffs indicates that a route of fluid movement exists directly from vessels to air spaces, suggesting decreased barrier function of both the capillary endothelium and alveolar epithelium. Indeed, previous studies using 4αPDD and another TRPV4 agonist, 14,15 EET, show that both the endothelium and epithelium are damaged (13). A potential pathway for fluid movement directly from capillaries to alveolar airspace is suggested by descriptions of modeled and clinical ARDS which document substantial injury to the epithelial barrier early in the disease process (25, 37). Taken together, these findings suggest that the classic model in which fluid moves across capillary endothelium to extra-alveolar interstitium and then into air spaces after rising interstitial pressure forces a breach of the alveolar epithelium, likely does not describe the only mechanism by which alveolar flooding may occur in ARDS.
Though peri-vascular cuffs have often been noted in pathological descriptions of edematous lungs (33, 38), the patho-physiologic effects of these cuffs are largely unknown. Specifically, the effect of extra-alveolar fluid accumulation on pulmonary mechanics is largely unknown. A decrease in dynamic compliance was associated with peri-vascular cuffing which occurred prior to alveolar flooding in isolated dog lungs under conditions of increased hydrostatic pressure (7). Investigators have also suggested that post - myocardial infarction and congestive heart failure patients have increased airway closing pressure due to the presence of extra-alveolar interstitial fluid (39, 40). In the current study, we provide further support for the idea that extra-alveolar fluid accumulation may negatively affect pulmonary mechanics by documenting that extra-alveolar peri-vascular cuffs are sufficient to cause decreased dynamic compliance. The mechanism by which extra-alveolar fluid accumulation decreases dynamic compliance remains to be shown. Because dynamic compliance depends on airway resistance, it is possible that peri-vascular cuffs compress anatomically related bronchi, induce airway narrowing and increase airway resistance. Narrowed airways may also cause gas trapping (41) which would shift tidal volume to higher total lung volumes resulting in a measured decrease in dynamic compliance (42). An alternate explanation is that perivascular cuffs may interrupt the transfer of radial tension from the parynchyma to the vascular and bronchial walls and thereby increase tissue resistance. Also, the finding that dynamic compliance was not decreased in lungs with alveolar flooding warrants further discussion. Studies of the alveolar mechanics suggest that alveoli expand unequally in edematous lungs (43). Thus, volume delivered by the ventilator would likely be directed away from the relatively few collapsed alveoli and toward non-flooded alveoli which, at the low tidal volumes delivered in our studies (< 8 ml / Kg), were likely able to accommodate the increased volume along the linear portion of the pressure volume curve. In this case, there would be no decrease in compliance. Questions concerning the mechanisms which produced our results will be answered by further investigations of pulmonary mechanics in the setting of site specific increases in permeability.
There is increasing awareness that different insults may induce permeability at different sites along the arterial-capillary-venous axis. In the current investigation, we exploit phenotypic differences among endothelial segments to induce site - specific increases in permeability. Treating isolated lungs with the calcium agonists thapsigargin or 4αPDD induce fluid accumulation in the extra-alveolar interstitium or alveolar air space, respectively, by increasing the permeability of the related vascular compartment. We show that these two compartments do not necessarily communicate as suggested by the traditional model. We also demonstrate that dynamic compliance decreases in lungs with peri-vascular cuffs, but not in lungs with alveolar flooding, suggesting that mechanisms other than alveolar flooding and inactivation of surfactant are involved in producing the ‘stiff lungs’ seen in ARDS. These findings are not consistent with current paradigms concerning the pathogenesis of pulmonary edema and suggest the need for further investigations into the importance of site specific increases in permeability in the patho-physiology of ARDS.
Acknowledgements
This work is supported by NIH grants HL-66299 and HL-60024, the Center for Lung Biology and the Department of Surgery at the University of South Alabama. The authors would like to thank Freda McDonald and the histotechnologists for their technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Staub NC, Nagano H, and Pearce ML Pulmonary edema in dogs, especially the sequence of fluid accumulation in lungs. Journal of Applied Physiology. 22(2):227–40, 1967. Feb. [DOI] [PubMed] [Google Scholar]
- 2.Bohm GM Vascular permeability changes during experimentally produced pulmonary oedema in rats. Journal of Pathology & Bacteriology. 92(1):151–61, 1966. Jul. [DOI] [PubMed] [Google Scholar]
- 3.Khimenko PL, and Taylor AE Segmental microvascular permeability in ischemia-reperfusion injury in rat lung. American Journal of Physiology. 276(6 Pt 1):L958–60, 1999. June. [DOI] [PubMed] [Google Scholar]
- 4.Chetham PM, Babal P, Bridges JP, Moore TM, and Stevens T Segmental regulation of pulmonary vascular permeability by store-operated Ca2+ entry. American Journal of Physiology. 276(1 Pt 1):L41–50, 1999. January. [DOI] [PubMed] [Google Scholar]
- 5.Whayne TF Jr., and Severinghaus JW Experimental hypoxic pulmonary edema in the rat. Journal of Applied Physiology. 25(6):729–32, 1968. Dec. [DOI] [PubMed] [Google Scholar]
- 6.Spragg RG, Lewis JF, Walmrath HD, Johannigman J, Bellingan G, Laterre PF, Witte MC, Richards GA, Rippin G, Rathgeb F, Hafner D, Taut FJ, and Seeger W Effect of recombinant surfactant protein C-based surfactant on the acute respiratory distress syndrome. New England Journal of Medicine. 351(9):884–92, 2004. August 26. [DOI] [PubMed] [Google Scholar]
- 7.Noble WH, Kay JC, and Obdrzalek J Lung mechanics in hypervolemic pulmonary edema. Journal of Applied Physiology. 38(4):681–7, 1975. Apr. [DOI] [PubMed] [Google Scholar]
- 8.Hogg JC, Agarawal JB, Gardiner AJ, Palmer WH, and Macklem PT Distribution of airway resistance with developing pulmonary edema in dogs. Journal of Applied Physiology. 32(1):20–4, 1972. Jan. [DOI] [PubMed] [Google Scholar]
- 9.Ishii M, Matsumoto N, Fuyuki T, Hida W, Ichinose M, Inoue H, and Takishima T Effects of hemodynamic edema formation on peripheral vs. central airway mechanics. Journal of Applied Physiology. 59(5):1578–84, 1985. Nov. [DOI] [PubMed] [Google Scholar]
- 10.Cook CD, Mead J, Schreiner GL, Frank NR, and Craig JM Pulmonary mechanics during induced pulmonary edema in anesthetized dogs. Journal of Applied Physiology. 14(2):177–86, 1959. Mar. [DOI] [PubMed] [Google Scholar]
- 11.Koutsoukou A, Armaganidis A, Stavrakaki-Kallergi C, Vassilakopoulos T, Lymberis A, Roussos C, and Milic-Emili J Expiratory flow limitation and intrinsic positive end-expiratory pressure at zero positive end-expiratory pressure in patients with adult respiratory distress syndrome. American Journal of Respiratory & Critical Care Medicine. 161(5):1590–6, 2000. May. [DOI] [PubMed] [Google Scholar]
- 12.Townsley MI, Korthuis RJ, Rippe B, Parker JC, and Taylor AE Validation of double vascular occlusion method for Pc, i in lung and skeletal muscle. Journal of Applied Physiology. 61(1):127–32, 1986. Jul. [DOI] [PubMed] [Google Scholar]
- 13.Alvarez DF, King JA, Weber D, Addison E, Liedtke W, and Townsley MI Transient receptor potential vanilloid 4-mediated disruption of the alveolar septal barrier: a novel mechanism of acute lung injury. Circulation Research. 99(9):988–95, 2006. October 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Staub NC The pathophysiology of pulmonary edema. Human Pathology. 1(3):419–32, 1970. Sep. [DOI] [PubMed] [Google Scholar]
- 15.Iliff LD Extra-alveolar vessels and oedema development in excised dog lungs. Journal of Physiology. 207(2):85P–86P, 1970. Apr. [PubMed] [Google Scholar]
- 16.Brough GH, Wu S, Cioffi D, Moore TM, Li M, Dean N, and Stevens T Contribution of endogenously expressed Trp1 to a Ca2+−selective, store-operated Ca2+ entry pathway. FASEB Journal. 15(10):1727–38, 2001. Aug. [PubMed] [Google Scholar]
- 17.Groschner K, Hingel S, Lintschinger B, Balzer M, Romanin C, Zhu X, and Schreibmayer W Trp proteins form store-operated cation channels in human vascular endothelial cells. FEBS Letters 437: 101–106, 1998. [DOI] [PubMed] [Google Scholar]
- 18.Tiruppathi C, Freichel M, Vogel SM, Paria BC, Mehta D, Flockerzi V, and Malik AB Impairment of store-operated Ca2+ entry in TRPC4(−/−) mice interferes with increase in lung microvascular permeability.[see comment]. Circulation Research. 91(1):70–6, 2002. July 12. [DOI] [PubMed] [Google Scholar]
- 19.Wu S, Cioffi EA, Alvarez D, Sayner SL, Chen H, Cioffi DL, King J, Creighton JR, Townsley M, Goodman SR, and Stevens T Essential role of a Ca2+−selective, store-operated current (ISOC) in endothelial cell permeability: determinants of the vascular leak site. Circulation Research. 96(8):856–63, 2005. April 29. [DOI] [PubMed] [Google Scholar]
- 20.Kelly JJ, Moore TM, Babal P, Diwan AH, Stevens T, and Thompson WJ Pulmonary microvascular and macrovascular endothelial cells: differential regulation of Ca2+ and permeability. Am J Physiol Lung Cell Mol Physiol 274: L810–819, 1998. [DOI] [PubMed] [Google Scholar]
- 21.Alvarez DF, King JA, and Townsley MI Resistance to store depletion-induced endothelial injury in rat lung after chronic heart failure. American Journal of Respiratory & Critical Care Medicine. 172(9):1153–60, 2005. November 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nilius B, Vriens J, Prenen J, Droogmans G, and Voets T TRPV4 calcium entry channel: a paradigm for gating diversity. American Journal of Physiology - Cell Physiology. 286(2):C195–205, 2004. Feb. [DOI] [PubMed] [Google Scholar]
- 23.Drake R, Gaar KA, and Taylor AE Estimation of the filtration coefficient of pulmonary exchange vessels. American Journal of Physiology. 234(3):H266–74, 1978. Mar. [DOI] [PubMed] [Google Scholar]
- 24.Albertine KH Ultrastructural abnormalities in increased-permeability pulmonary edema. Clinics in Chest Medicine. 6(3):345–69, 1985. Sep. [PubMed] [Google Scholar]
- 25.Bachofen M, and Weibel ER Alterations of the gas exchange apparatus in adult respiratory insufficiency associated with septicemia. American Review of Respiratory Disease. 116(4):589–615, 1977. Oct. [DOI] [PubMed] [Google Scholar]
- 26.Tomashefski JF Jr. Pulmonary pathology of acute respiratory distress syndrome. Clinics in Chest Medicine. 21(3):435–66, 2000. Sep. [DOI] [PubMed] [Google Scholar]
- 27.Bachofen H, and Hildebrandt J Area analysis of pressure-volume hysteresis in mammalian lungs. Journal of Applied Physiology. 30(4):493–7, 1971. Apr. [DOI] [PubMed] [Google Scholar]
- 28.Matamis D, Lemaire F, Harf A, Brun-Buisson C, Ansquer JC, and Atlan G Total respiratory pressure-volume curves in the adult respiratory distress syndrome. Chest. 86(1):58–66, 1984. Jul. [DOI] [PubMed] [Google Scholar]
- 29.Lemaire F ARDS and PV curves: the inseparable duet?. Intensive Care Medicine. 26(1):1–2, 2000. Jan. [DOI] [PubMed] [Google Scholar]
- 30.Hoppin Frederic G., S. JC, Greaves Ian A., Lai Yih-Loong, Jacob Hildebrandt Lung recoil: elastic and rheological properties In Fishman AP (Ed.), Handbook of Pysiology. Bethesda: American Physiological Society, 1986. Pp. 195–215. [Google Scholar]
- 31.Pison U, Seeger W, Buchhorn R, Joka T, Brand M, Obertacke U, Neuhof H, and Schmit-Neuerburg KP Surfactant abnormalities in patients with respiratory failure after multiple trauma. American Review of Respiratory Disease. 140(4):1033–9, 1989. Oct. [DOI] [PubMed] [Google Scholar]
- 32.Gregory TJ, Longmore WJ, Moxley MA, Whitsett JA, Reed CR, Fowler AA 3rd, Hudson LD, Maunder RJ, Crim C, and Hyers TM Surfactant chemical composition and biophysical activity in acute respiratory distress syndrome. Journal of Clinical Investigation. 88(6):1976–81, 1991. Dec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Teplitz C The core pathobiology and integrated medical science of adult acute respiratory insufficiency. Surgical Clinics of North America. 56(5):1091–1133, 1976. Oct. [DOI] [PubMed] [Google Scholar]
- 34.Parker JC, and Townsley MI Evaluation of lung injury in rats and mice. American Journal of Physiology - Lung Cellular & Molecular Physiology. 286(2):L231–46, 2004. Feb. [DOI] [PubMed] [Google Scholar]
- 35.Parker JC, and Yoshikawa S Vascular segmental permeabilities at high peak inflation pressure in isolated rat lungs.[see comment]. American Journal of Physiology - Lung Cellular & Molecular Physiology. 283(6):L1203–9, 2002. Dec. [DOI] [PubMed] [Google Scholar]
- 36.Parker JC, Stevens T, Randall J, Weber DS, and King JA Hydraulic conductance of pulmonary microvascular and macrovascular endothelial cell monolayers. American Journal of Physiology - Lung Cellular & Molecular Physiology. 291(1):L30–7, 2006. Jul. [DOI] [PubMed] [Google Scholar]
- 37.Mitsuoka H, Sakurai T, Unno N, Kaneko H, Suzuki S, Nakamura S, Baba S, and Terakawa S Intravital laser confocal microscopy of pulmonary edema resulting from intestinal ischemia-reperfusion injury in the rat.[see comment]. Critical Care Medicine. 27(9):1862–8, 1999. Sep. [DOI] [PubMed] [Google Scholar]
- 38.Teplitz C Pathogenesis of Pseudomonas Vasculitis and Septic Legions. Archives of Pathology & Laboratory Medicine. 80:297–307, 1965. Sep. [PubMed] [Google Scholar]
- 39.Interiano B, Hyde RW, Hodges M, and Yu PN Interrelation between alterations in pulmonary mechanics and hemodynamics in acute myocardial infarction. Journal of Clinical Investigation. 52(8):1994–2006, 1973. Aug. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hales CA, and Kazemi H Small-airways function in myocardial infarction. New England Journal of Medicine. 290(14):761–5, 1974. April 4. [DOI] [PubMed] [Google Scholar]
- 41.Vieillard-Baron A, Prin S, Schmitt JM, Augarde R, Page B, Beauchet A, and Jardin F Pressure-volume curves in acute respiratory distress syndrome: clinical demonstration of the influence of expiratory flow limitation on the initial slope.[erratum appears in Am J Respir Crit Care Med 2002 Dec 1;166(11):1517]. American Journal of Respiratory & Critical Care Medicine. 165(8):1107–12, 2002. April 15. [DOI] [PubMed] [Google Scholar]
- 42.Volgyesi GA, Tremblay LN, Webster P, Zamel N, and Slutsky AS A new ventilator for monitoring lung mechanics in small animals.[see comment]. Journal of Applied Physiology. 89(2):413–21, 2000. Aug. [DOI] [PubMed] [Google Scholar]
- 43.Schiller HJMD, McCann UGIIMD, Carney DEMD, Gatto LAP, Steinberg JMDO, and Nieman GFBA Altered alveolar mechanics in the acutely injured lung. [Article]. Critical Care Medicine May 2001;29(5):1049–1055. [DOI] [PubMed] [Google Scholar]