Reading co-opts existing cortical visual feature representation without destruction.
Abstract
Learning to read is associated with the appearance of an orthographically sensitive brain region known as the visual word form area. It has been claimed that development of this area proceeds by impinging upon territory otherwise available for the processing of culturally relevant stimuli such as faces and houses. In a large-scale functional magnetic resonance imaging study of a group of individuals of varying degrees of literacy (from completely illiterate to highly literate), we examined cortical responses to orthographic and nonorthographic visual stimuli. We found that literacy enhances responses to other visual input in early visual areas and enhances representational similarity between text and faces, without reducing the extent of response to nonorthographic input. Thus, acquisition of literacy in childhood recycles existing object representation mechanisms but without destructive competition.
INTRODUCTION
A defining characteristic of Homo sapiens as a species is its ability to invent and culturally transmit technologies that have transformed life on Earth. One such cultural invention, reading and writing, is evolutionarily too recent for dedicated cortical networks to have evolved specifically in its service. How, then, do we accomplish this remarkable feat? Learning to read requires the fine-tuning of numerous perceptual and cognitive abilities, including low-level perceptual processes; covert and overt attentional mechanisms; oculomotor and executive control; long, short, and working memory processes; phonological processing; and so forth (1). These abilities are not specific to reading, but proficient reading requires successful coordination of these skills. Reading is thought to “recycle” (2) neural networks that already have in place most of the processing capacity necessary to support this phylogenetically new culturally transmitted function. There is considerable experimental support for this notion (3–8). What remains unclear is whether and how existing, evolutionarily old, similarly culturally relevant visual abilities such as face processing are affected by the neuronal recycling process. One possibility is that recycling of preexisting cortical mechanisms induces spatial cortical competition and thus, for instance, interferes with the area of the cortex that previously had been primarily sensitive to distinct visual categories such as faces, houses, and tools (2). A landmark study (7) proposed and presented some evidence that orthographic representations expand in the ventral visual system and interfere with the expansion of faces and other visual categories (e.g., houses and tools) into the surrounding cortex. Another possibility, however, is the converse case, namely, that training with orthographic stimuli might not intrusively co-opt existing territory but might enhance local sensitivity to visual input in a more general manner. There is behavioral evidence that illiterates perform much worse than literates on immediate naming of common everyday objects both in terms of accuracy and reaction times (9). In a large-scale functional magnetic resonance imaging (fMRI) imaging study with adult participants of varying degrees of literacy, from completely illiterate to highly literate, we investigated the effects of literacy on responses to other non–language-related visual objects cross-sectionally before reading training (n = 91), as well as longitudinally (n = 22). The participants were recruited from two rural villages in an area near Lucknow, Northern India. They were of varying degrees of literacy but matched for a set of demographic and socioeconomic factors. Detailed participant information is found in the Supplementary Materials. All orthographic stimuli presented in the course of this study were printed in Devanagari script, in which the predominant local language (Hindi) is written. Devanagari is an alphasyllabic orthographic system, in which characters encode a consonant and a vowel, thus representing information at both the syllable and phoneme level.
RESULTS
We begin by describing the results of a series of cross-sectional analyses executed on the data acquired at the first time point in the experiment.
Responses to orthographic stimuli enhanced by literacy
We tested whether brain response to orthographic stimuli (sentences and nonwords, presented in independent fMRI runs) were modulated by literacy. Literacy was defined by score on a test of speeded word reading. In response to sentences, we observed a pattern of modulation in a network incorporating the occipital lobe, ventral visual areas, inferior parietal, premotor, and supplementary motor regions, with a significant local peak in the fusiform gyrus in the vicinity of classical coordinates of visual word form area (VWFA) at Montreal Neurological Institute (MNI) coordinates −42, −55, −10 (Z = 5.54; Fig. 1 and table S2), only a few millimeters from the area reported to be modulated by literacy in (7), located at −44, −50, −14. Hereafter, we refer to this as the group VWFA. We also observed modulation by literacy of a similar network for nonwords (Fig. 1), including a local maximum close to VWFA at −42, −43, −13 (Z = 4.88; see Fig. 1 and table S2). These two overlapping networks also appear to overlap with the premotor cortex in the vicinity of the Exner’s area, a region that has been found to be implicated in the motor control of handwriting (10, 11) and typing (12), as well as an adjacent area of the premotor cortex consistent with the frontal eye fields (13). In addition, reading sentences also showed modulated activity in the supplementary eye fields, a midline motor structure associated with the saccadic control during reading (13), consistent with the oculomotor demands of sentence processing. Reading letter strings further recruited a region of left inferior frontal gyrus (LIFG), the pars triangularis, associated with articulation and previously implicated in nonword reading (14). Whole-brain analyses did not reveal further significant positive or negative correlations between brain responses and literacy in any other visual category except false fonts (Fig. 1).
Fig. 1. Brain areas whose response to orthographic stimuli is modulated by literacy.
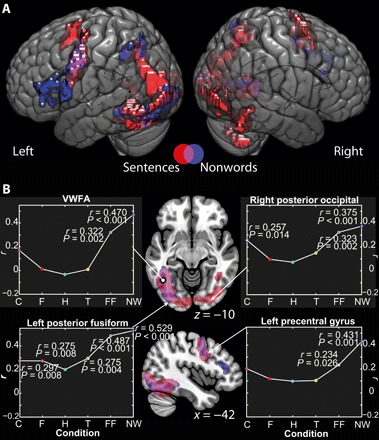
(A) Network of brain areas showing responses modulated by literacy, projected on the single-subject MNI152 template brain. Red indicates areas whose response to viewing sentences was significantly positively correlated with literacy. Blue indicates brain areas whose response to viewing nonwords was significantly positively correlated with literacy. Magenta indicates overlap between the two. (B) Images show correlations between brain response to sentences and nonwords projected into axial and sagittal planes through the VWFA (at MNI coordinates −42, −55, −10), marked with a circle. Graphs show correlation coefficients for the relationship between literacy and brain response for visual stimuli, at peaks determined from the correlations between sentence reading and literacy; arrows indicate approximate location of peaks. Significant correlations are highlighted with white dots. r and P values are provided adjacent. Correlations that reach significance at P < 0.05 when applying a Bonferroni correction corrected for multiple comparisons within region of interest (ROI) (i.e., six comparisons) are indicated with a black dot on the white highlight. C, checkerboards; F, faces; H, houses; T, tools; FF, false fonts; NW, nonwords. Activation maps thresholded at P(unc.) < 0.001 and cluster-wise threshold of P(FWE) < 0.05.
Note that the right homolog of the VWFA also showed modulation of response to sentences as a function of literacy. Although reading is typified as recruiting a left-lateralized network, some evidence exists that when reading orthographies that are more complex than the Roman alphabet, right occipitotemporal regions are engaged. Studies have shown, for example, that when reading Chinese, a right hemisphere homolog of the VWFA exhibits similar selectivity for orthographic stimuli as the left (15), and that in reading Japanese, reading the kana characters elicits greater right fusiform gyrus activation than does reading the visually simpler kanji (16). We speculate that the result we see here in these data may be the product of the relative complexity of Devanagari script.
In a complementary analysis to examine the specificity of this response to orthographic input, we examined the relationships between literacy and the activation differences for the contrasts between nonwords and other visual categories (fig. S1). This showed clear convergence, such that the magnitude of the contrast increased with literacy not only in the left fusiform gyrus but also in the right occipital cortex and left premotor and inferior frontal cortices. This is consistent with literacy modulating response to orthographic stimuli but not to others.
Analyses of peak response location as a function of literacy
To establish whether any apparent effect of literacy on magnitude of the brain response might be due to a systematic shift in the location of individual peak responses as a function of literacy (which could manifest as a relationship between response magnitude and literacy), we determined individual peak locations (within a radius of 10 mm from the VWFA determined at the group level) for the sentence reading versus horizontal checkerboard contrast. We tested their spatial distribution for any correlation with word reading proficiency. The analysis was repeated at search radii of 20, 30, and 40 mm from the VWFA to reduce the possibility of missing an effect that was due to a more extreme literacy-related shift in peak response locus. Despite previous reports that illiterate individuals show a more lateral response to orthographic stimuli than literate individuals (7), no such effect was found here. At all search radii, the voxels corresponding to the peak sentence-checkerboard difference showed a consistent positive relationship between response to nonwords and literacy (10 mm: slope, 0.030; t89 = 4.828; P < 0.001; 20 mm: slope, 0.033; t89 = 4.932; P < 0.001; 30 mm: slope, 0.035; t89 = 4.919; P < 0.001; 40 mm: slope, 0.031; t89 = 4.536; P < 0.001). Thus, the relationship observed between word reading score and response at the group VWFA is the result of differential sensitivity to text, not the relocation of a cortical region that is equally sensitive to sentences in literate and illiterate individuals. This is compatible with the proposal that an orthographically sensitive patch of cortex emerges in left ventral temporal cortex with the development of literacy.
The impact of literacy on selectivity of visual responses in ventral temporal lobe
At the VWFA peak determined from the sentence-reading blocks, we also observed substantial responses to other visual categories compared to the rest (figs. S2 and S3). Since the VWFA is substantially more letter sensitive in literate individuals, we further investigated whether literacy displaced or impinged upon the response of ventral temporal cortex to other visual objects. We examined the correlation between literacy and the magnitude of responses to other categories at the group VWFA to determine whether literacy had a negative impact on the response of this area to nonorthographic stimuli (Fig. 1).
We also explored the hypothesis that literacy reduces the available territory for responses to nonorthographic categories. To this end, we calculated the proportion of voxels that responded significantly more to nonwords compared to all other static stimuli (faces, houses, tools, and false fonts) in each participant’s ventral temporal lobes, bilaterally. This showed a significant positive relationship with literacy in the left hemisphere region of interest (ROI) (slope, 0.0036; t89 = 3.023; P = 0.003) and no such relationship in the right hemisphere (slope, 0.0007; t89 = 1.427; P = 0.157). The same analysis was executed for the contrasts between each single visual category and the remaining static stimuli, i.e., contrasts revealing relatively category-selective voxels. This revealed no significant positive or negative relationships between the numbers of significantly differently activated voxels and literacy, for any category, in either the left or the right hemisphere (all P > 0.17). This underscores the absence of any curtailment of the extent of nonorthographic representations in the ventral temporal lobe by the acquisition of literacy. We also performed an analysis of the local selectivity of responses to faces, houses, and tools to determine whether the extent of regions sensitive to these categories, in the vicinity of VWFA, was reduced as a function of literacy. We found no such effects (see Supplementary Text).
In addition, we analyzed the relationship between literacy and lateralization of brain responses to faces and houses compared to baseline. We found a correlation between the lateralization index for faces and houses (fig. S4), such that higher reading ability predicts a more left-lateralized response (faces: slope, 0.002; t89 = 2.626; P = 0.010; houses: slope, 0.002; t89 = 2.906; P = 0.005). This result provides broad support for the notion that learning to read produces general enhancements in sensitivity to visual input in and around VWFA, rather than reducing available territory.
The mosaic in Fig. 2 shows the distribution of the significant responses to stimuli (compared to baseline) across the ventral temporal lobe, as well as plots of the relative responses to each of the visual categories, and relationships between literacy and brain response. There is no obvious decrease in the magnitude of the peak response to any category of stimulus as a function of literacy, nor is there a systematic shift in the locus of the peak response to faces when comparing literate and illiterate participants, divided according to a median split of the participants’ word recognition scores. The absence of such an effect is further supported in a more nuanced, and quantitative, fashion by the correlations between literacy and brain response.
Fig. 2. Group-level activation of ventral visual areas in response to visual categories (A to G).
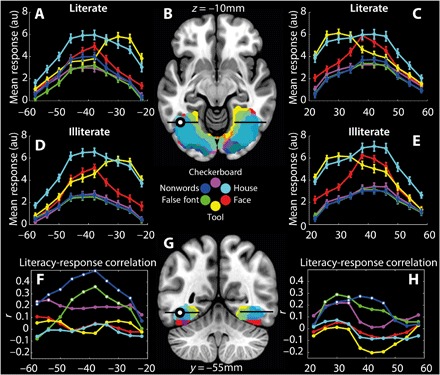
(B) and (G) show the significant responses at the group level to each category of stimulus presented during the visual run; planes of section are indicated in labels. Colors represent the indicated stimulus category in both brain projections and the graphs. The black line demarcates the range of x coordinates −58 to −22 mm (left) and 22 to 58 mm (right). The locus of the group VWFA is shown as a black dot filled in white. For clarity of display, only the top 5% of most significant voxels reaching the voxel-level threshold of P(unc.) < 0.001 and cluster-wise threshold of P(FWE) < 0.05 for the contrasts of each condition versus baseline are shown, projected on the MNI152 template brain. Note the substantial overlap between the activation maps of the different conditions (also evident in figs. S2 and S3). (A) and (D) respectively show the group mean responses of literate and illiterate individuals (±SEM) to each of the stimulus categories presented, at 4-mm intervals along the x axis of the plane through the group VWFA in the left hemisphere. (C) and (E) respectively illustrate the same data for the right hemisphere. (F) shows the correlation coefficient, r, for the relationship between literacy and brain response at the specified coordinates in the left hemisphere, and (H) shows the equivalent information for the right hemisphere; data points highlighted in white are those where there is a significant (P < 0.05) relationship between literacy and brain response. The correlation coefficients and their respective P values are tabulated in table S3. au, arbitrary units.
Multivariate representational similarity of different visual object categories as a function of literacy
It has recently been suggested that in childhood, ventral temporal cortex contains labile cortical regions that may develop sensitivity to orthographic stimuli, with appropriate exposure, and that the VWFA thus emerges (17). In the absence of this exposure, these areas will not become orthographically sensitive and will rather be invaded by other categories (e.g., faces or tools). The results presented above indicate that literacy does not appear to have an impact on the extent of cortex recruited by categories other than readable stimuli. Nonetheless, there is a patch of cortex (namely, VWFA) that reliably shows a response to orthographic stimuli in literate, but not illiterate, individuals. If this does not emerge as a result of displacing, encroaching upon, or preventing the emergence of selectivity to other, otherwise neighboring categories, then one possibility is that VWFA develops sensitivity to orthographic stimuli in addition to other categories. To probe this possibility, we investigated the multivariate representation of the different categories of static stimuli in the visual runs (faces, houses, tools, false fonts, and nonwords) and examined the between-category similarity. If literacy impinges upon the establishment of, for example, face-sensitive areas, then this should be manifested as a literacy-related reduction in the representational similarity of faces and nonwords. To test this, we established the representational similarity between the response vectors for each of the categories faces, houses, tools, false fonts, and nonwords in ROIs, defined as all the voxels in individually defined masks of the gray matter (GM) of the ventral temporal lobes (left and right separately; see the Supplementary Materials) and a left ventral temporal cluster, functionally defined as the cluster surrounding the group VWFA established in the correlation between literacy and sentence reading. We then analyzed representational similarity for a relationship with literacy, revealing several significant relationships.
In the left ventral temporal ROI, significant relationships were found for the following pairs of conditions: faces and false fonts (slope, 0.003; t89 = 2.996; P = 0.004), faces and nonwords (slope, 0.003; t89 = 2.882; P = 0.005), houses and false fonts (slope, 0.002; t89 = 2.466; P = 0.016), tools and false fonts (slope, 0.002; t89 = 2.553; P = 0.012), tools and nonwords (slope, 0.003; t89 = 3.172; P = 0.002), and false fonts and nonwords (slope, 0.005; t89 = 3.606; P = 0.001). In the right ventral temporal lobe, such an effect was observed only for the pair false fonts and nonwords (slope, 0.003; t89 = 2.631; P = 0.010). Within the VWFA ROI, the representational similarity between the following pairs all significantly increased with literacy: faces and false fonts (slope, 0.002; t89 = 2.212; P = 0.030), faces and nonwords (slope, 0.003; t89 = 2.603; P = 0.011), tools and nonwords (slope, 0.003; t89 = 2.602; P = 0.011), and false fonts and nonwords (slope, 0.005; t89 = 3.892; P < 0.001). In all cases, the pairs of categories that were significantly affected by literacy involved text or text-like stimuli, and in all cases, the relationships with literacy are positive.
This suggests that voxels, and by implication, the neural populations therein, that become tuned to orthographic stimuli as a result of the acquisition of literacy in childhood do not necessarily lose their responsiveness to nonorthographic stimuli and may even contribute additionally to the representation of nonorthographic categories after they have developed a sensitivity to text. It is further intriguing that rather than becoming more differentiated from one another, the categories nonwords and false fonts actually become more similar as a function of literacy. It is possible that the VWFA, during the acquisition of literacy, begins to encode visual features that are relevant to orthographic stimuli but that are shared with the false fonts. Given that the false fonts used in the present study were very similar to Devanagari Akshara but that their similarity was not systematically manipulated, we must limit our speculation in this direction. Nevertheless, it remains an intriguing possibility that learning to read sensitizes visual occipitotemporal cortical areas to the features of the learned orthographic code.
General increases in sensitivity
Closer examination of the response profiles at the voxels showing the greatest modulation of responses to nonwords and sentences by literacy further revealed that in some regions, literacy may have a less than specific effect in enhancing visual responses. Figure 1B illustrates the responses to various visual categories at selected significant peaks. It can be seen that, while not significant at whole brain levels of significance, certain areas whose response to readable stimuli displays a relationship with literacy also show increased responses to other stimulus categories as a function of literacy. Notably, the left posterior fusiform gyrus (a visual association area) exhibits a significant positive correlation between literacy and brain response not only to nonwords and sentences but also to checkerboards, faces, and tools (see Fig. 1B). The right posterior fusiform gyrus shows an increased response to checkerboards as a function of literacy. Consistent with literacy not seeming to provoke a contraction of the extent of tissue that is sensitive to specific categories, these response profiles suggest that literacy may be associated with nonspecific increase in cortical response to visual stimuli and, in the left hemisphere, in relatively early visual areas. In the interest of completeness, fig. S5 shows correlations between brain response to all presented stimulus categories and literacy for a number of a priori ROIs found in the left perisylvian language areas, which have previously been identified as being involved in reading (7, 18). These loci do not show a convincing pattern of response enhancement as a function of literacy, except in a limited number of cases, that is, to sentences, nonwords, and false fonts, as well as to vertical and circular checkerboards in the left anterior superior temporal sulcus.
The consequences of learning to read Devanagari script
To establish the cerebral consequences of improved literacy, we scanned participants again after training to evaluate whether there were any systematic changes in brain responses to sentences. Comparing the group’s pretraining and posttraining brain responses to sentences revealed increased responses in a subset of the reading network revealed in the analysis of the impact of literacy on responses to sentence reading at baseline (Fig. 3 and table S4). A peak in activation change is found within 8.5 mm of the group VWFA (at MNI coordinates −39, −49, −10), confirming that this region has a responsivity to orthographic stimuli that is modulated by literacy, even when acquired in adulthood. However, a similar significant change is not seen for the response to nonwords. This does not permit us to rule out the possibility that this response may be purely due to the recognition of words learned during training rather than the change in response to arbitrary nonwords. Previous reports of the response profile of VWFA have suggested that it is sensitive to nonwords, which has been taken to exclude the possibility that it is sensitive only to known strings (19). This peak locus is consistent with a recent report (20) in which a group of literate individuals learned to read a font composed of faces (“FaceFont”) whose mappings to orthography were trained, alongside a separate group who learned to read Hangul characters. After training, both groups showed enhanced cortical responses (manifest as a group-by-font interaction) to their trained character set, peaking at MNI coordinates −40, −51, −16 (coordinates originally published in Talairach space: −40, −50, −11 and converted using tal2mni MATLAB code retrieved from https://github.com/openroc/eeglab/blob/master/tags/EEGLAB7_1_5_17beta/plugins/dipfit2.2/mni2tal.m), supporting the notion that this region is susceptible to change as a function of learning visual to phonological mappings.
Fig. 3. Increased response to reading sentences in trained participants after training.
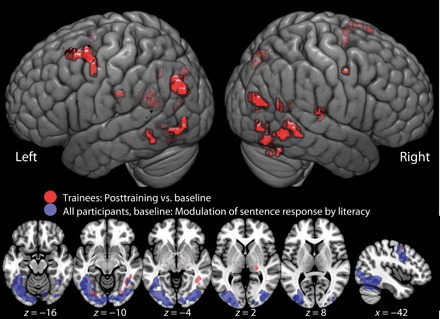
Data projected on the MNI152 brain template at a voxel-wise statistical threshold of P < 0.001 uncorrected for multiple comparisons. Bottom: The brain areas implicated for the most part constitute a subset of the regions implicated in sentence reading and were found to have a level of response modulated by literacy at baseline.
We also tested whether the change in response to literacy found over the group was significantly modulated by the amount of improvement (as indexed by individual change in word reading performance). This correlation revealed that a set of left inferior frontal and bilateral occipital areas showed an increase in response to sentence reading that was correlated with the improvement in reading performance (Fig. 4 and table S5). These regions are not consistent with the VWFA but include primary visual cortex and extrastriate visual cortices (visual association areas), suggesting a role for enhanced early visual processing of sentences as a function of the acquisition of literacy. This result suggests that at early stages of adult literacy acquisition, successful readers make use of a broader visual network, incorporating areas not typically associated with orthographic processing. This is consistent with other studies of skill learning, in which learners at early stages of skill acquisition might recruit broader networks than do experts (21, 22). In addition, the LIFG shows changes in activation modulated by improvement in literacy. The LIFG has been shown to be implicated in semantic and syntactic processes, consistent with increased improvements in reading leading to increased linguistic processing of sentences.
Fig. 4. Significant correlation between word reading improvement and change in brain response to sentence reading in trained participants after training.
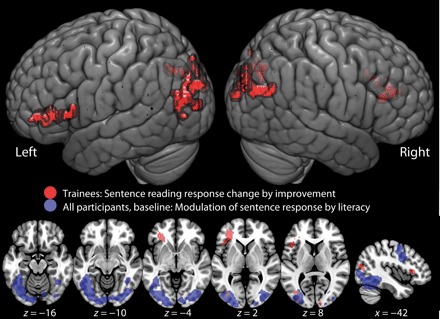
Data projected on the MNI152 brain template at a voxel-wise statistical threshold of P < 0.001 uncorrected for multiple comparisons and significant at a cluster-level FWE-corrected threshold of P < 0.05 for multiple comparisons.
To test whether this emergence of orthographic sensitivity had an impact on the representation of other visual input, we also compared pretraining brain responses to posttraining brain responses for faces, houses, tools, false fonts, and checkerboards. This revealed no significant differences. This suggests that over the time course of the experiment and with the instruction received, the acquisition of increasing literacy had no substantial impact on the representation of visual objects.
DISCUSSION
Reading, a recent cultural invention on the evolutionary time scale, exists under strong evolutionary anatomical and connectional constraints and thus must make use of neural networks that are close to the required function but also sufficiently plastic to reorient a considerable part of their neural functioning to the new skill (2). This study provides further evidence that a portion of the left ventral temporal cortex becomes more sensitive to orthographic stimuli as a function of literacy (3–7). However, does this reading-related functional reorganization of neural networks induce cortical competition with other visual categories?
The present study thoroughly tested this question. We found no evidence of displacement of peak cortical responses to faces, houses, or tools, nor to the extent of tissue significantly responsive to these categories as a function of literacy. We found no evidence that literacy and the development of VWFA encroached upon the territory sensitive to other categories. Notably, we found that the representational similarity between nonwords and faces is enhanced by literacy in the VWFA, and the representational similarity between nonwords and faces and nonwords and tools is enhanced in the left ventral temporal lobe. These results indicate that far from cannibalizing the territory of its neighbors, the VWFA is rather overlaid upon these and not only remains responsive to other categories of visual stimuli but also recruits an assembly of neural populations in much the same way, regardless of whether the input is orthographic, albeit with a lesser overall response magnitude.
Our findings also suggest that literacy may have a general effect in enhancing early visual responses rather than provoking a contraction of the extent of tissue that is sensitive to specific visual categories. Several areas such as the left posterior fusiform gyrus and right occipital cortex that showed literacy-related responses to orthographic stimuli also show literacy-related increased responses to other stimulus categories. Our finding that the lateralization of responses to faces and houses is leftward as a function of literacy further suggests that learning to read has a general positive impact on the responsiveness of left ventral occipitotemporal cortex to nonorthographic and orthographic stimuli.
According to the recently proposed revised cortical recycling model for reading (17), literacy acquisition during development blocks the expansion of face responses in the left hemisphere and restricts growth of patches responsive to faces to the right hemisphere. This is because it is assumed that category-specific MRI responses arise from patches of neurons that are responsive to each category (e.g., faces, houses, and tools) and clustered in reproducible areas of the cortex (23–25). Category selectivity is thought to develop early and expand during development (26–29), resulting in a cortical organization in children’s ventral visual cortex that contains sites that are specialized and sites that are not or less specialized (17). Crucially, it is argued that, without schooling, weakly specialized patches are progressively invaded by categories localized nearby such as faces, houses, and tools (17). The current data show no evidence in support of the notion that illiteracy in adulthood positively affects the extent of the cortical representation of the visual categories faces, houses, and tools. It is suggested that during development, the same MRI voxel appears to contain patches for not only visual word forms but also other visual categories, i.e., some considerable “superposition” of categories (17). In later stages of development, this superposition is thought to give way to cortical competition (2, 7, 17). The data presented here suggest superposition without subsequent competition.
Last, our longitudinal analyses suggest that literacy-related enhanced general responses to visual categories may take some time to develop. Our comparison of pretraining brain responses to posttraining brain responses for faces, houses, tools, false fonts, and checkerboards revealed that, over the time course of the 6-month training study, the acquisition of increasing literacy had no extensive immediate impact on the representation of visual objects. Our findings fundamentally challenge the notion that literacy impinges upon the area of the cortex receptive to nonorthographic categories, as well as the notion of literacy-induced cortical competition more generally. In contrast, data are most compatible with the notion that literacy has a general effect in enhancing early visual responses rather than provoking a contraction of the extent of tissue that is sensitive to specific visual categories. Large-scale longitudinal intervention studies with developing and mature participants will have to be the gold standard for attempts to find the exact mechanisms of cortical specialization during reading acquisition.
MATERIALS AND METHODS
Participants
Participants were recruited from two villages near the city of Lucknow in the Northern Indian state of Uttar Pradesh as part of a study that was approved by the ethics committee of the Centre of Biomedical Research, Lucknow. After giving informed consent, 91 healthy right-handed human volunteers without a known history of psychiatric disease or neurological condition took part in the study (see “Demographic and behavioral data” section for more details). Twenty-nine completely illiterate participants (mean age, 31; range from 23 to 39; two males; average monthly income, 1776 rupies) from the same societal community in two villages in a rural area near Lucknow, India were recruited to take part in a 6-month literacy training program in which they learned some basic reading and writing of Devanagari script. None of them had ever undergone any formal schooling. Some of the participants had some literate family members, but others had no literate family members (average number of literate family members, 2.3). An illiterate no-training control group (n = 24; mean age, 29; range from 19 to 40; 8 males; average number of literate family members, 2.64; average monthly income, 2250 rupies) and literate no-training control group (n = 38; mean age, 26; range from 18 to 40; 25 males; average number of literate family members, 2; average monthly income, 2276 rupies) were matched to the training group in terms of socioeconomic background and were recruited from the same societal community in the two villages. There were no significant differences between the groups in terms of age, numbers of literate family members, or income. Although participants were recruited and assigned to groups, note that most of the analyses presented in this paper do not rely upon this grouping, focusing instead upon regressions with literacy as a continuous factor.
As a consequence of cultural factors at the study site, there was an imbalance in the sex of the participants as a function of literacy status, with illiterate participants being more likely to be female. The potential consequences of this are not readily predictable. There is little evidence for sex differences in the neurophysiology of normal reading, although sex differences in the cerebral organization of language have been reported [e.g., in phonological tasks during reading (30)], and a recent review (31) concluded that the neural basis of developmental dyslexia may be different in male and female children. Nevertheless, existing findings do not suggest that the VWFA is systematically differently localized in female samples compared to male samples. We therefore do not believe that this limitation would account for the effects that we observed.
All of the participants were right-handed and examined by a medical doctor. None of the participants had any known neurological impairments. Participants were interviewed about their educational background. A word reading test and a letter identification task were administered.
Literacy program
Participants in the illiterate reading group were paid to attend three times per week (for approximately 2 hours per day), a 6-month literacy training program in which they learned basic reading and writing of Devanagari script. The Indic scripts such as Devanagari derive from Brahmic script, which emerged around 300 CE from a Semitic script source. Devanagari, which is written from left to right, is used for over 100 languages other than Hindi—including Bengali, Nepali, Tibetan, and Burmese—and used by hundreds of millions of people. It is an alphasyllabic writing system that represents spoken units at both the syllable and phoneme level. The basic graphic unit is the syllable (e.g., V, CV, CCV, etc.) and is termed Akshara. Unmarked consonants are followed by an inherent vowel (or “schwa”). Vowelless consonants (to indicate consonant clusters or consonants in word-final position) are marked by a number of consonant markers. Vowels in Devanagari other than the inherent vowel are marked by means of diacritic signs attached directly to the consonants. Vowels are attached on the left, right, top, or bottom of the consonant. Syllable and phoneme level information is represented thus in parallel, i.e., vowels and consonants are not ordered sequentially as independent letter units in words. Alphasyllabaries are similar to logographic writing systems in that they comprise large sets of visually complex symbols that are larger than phonological units and are indivisible in that some of the component parts (e.g., the diacritic signs) cannot stand alone. Alphasyllabaries are similar to alphabetic writing systems in that the symbols predominantly convey a word’s phonology. It has distinct graphic elements that correspond to vowels and consonants and graphic units that correspond to individual phonemes rather than syllables or words.
The teaching instructor was a professional teacher following a local method of reading instruction. During the first month of instruction, the Hindi alphabet was taught (vowels followed by consonants). Reading and writing of Devanagari script were taught simultaneously. The teaching of the alphabet was followed by two-letter words. Approximately 200 words were taught in the first month. During the second month, participants were taught to read and write simple sentences containing mostly of two-letter words. Teaching in the third month of reading instruction primarily involved combinations of three-letter words and the reading and writing of simple sentences. For the remaining 3 months of the program, more complex words and some basic grammar rules (e.g., the difference between nouns, pronouns, verbs, proverbs, and adjectives and basic rules involving tense and gender) were taught. The teacher monitored learning progress continuously throughout the program.
Demographic and behavioral data
Participants were matched for age, gender, handedness, income, number of literate family members, and nonsymbolic intelligence (table S1). Information about age, income, and number of literate family members was obtained in an interview. Right-handedness was also verified in an interview by asking the participants which hand they used for common activities (e.g., drawing). Raven progressive matrices were administered to test for nonverbal abilities. Two measures of literacy were taken, namely, letter identification (knowledge of the 46 primary Devanagari characters) and word reading ability (knowledge of 86 words of varying syllabic complexity). In the letter identification task, participants received a recorded spoken instruction. In the spoken instruction, they were told that they would be presented with the letters of the Hindi alphabet one by one. They were told that each letter would be shown for 5 s, followed by a question mark. The instruction specified that when the question mark was on the screen, they were supposed to say the name of the letter out loud. The response was recorded. The recording terminated automatically after 10 s. The 46 Akshara of Devanagari were presented in font Mangal (size 96 point). After the letter identification task, participants received the word reading test. Eighty-six words were presented in font Mangal (size 96 point) one by one. The words were of differing syllabic complexity (26 monosyllabic, 30 disyllabic, and 30 trisyllabic words) and were presented in a pseudorandomized order. Each word was displayed on the screen for 10 s, followed by a question mark. A spoken recorded instruction specified that when the question mark was on the screen, participants should say aloud the word that they had been presented with. Participants’ responses were recorded. The recording terminated automatically after 30 s. Word reading scores were subsequently used as the reference score for literacy. This score was chosen, as it is the most representative of the ability to read in the sense of functionally composing sequences of phonemes from orthographic input.
Procedure
Tasks were presented in blocks. In the localizer run, there were 10 blocks for each task, which were arranged in a different pseudorandom order for each participant. Stimulus presentation was controlled using E-Prime (Psychology Software Tools). Most of the tasks were modeled on those featured in a previous, highly influential publication (7) on the impact of literacy on the visual system.
Localizer
Four stimulus categories were presented in the localizer block: visual sentences, auditory sentences, horizontal checkerboards, and vertical checkerboards. Ten blocks of each stimulus were presented in a randomized order (randomized per participant). In each visual sentence block, each trial consisted of a simple sentence, which was shown on four successive screens with one to three words on each screen. Each screen was shown for 400 ms with an interval of 100 ms between each screen. Between each sentence, there was a 500-ms pause. All words were displayed in font Mangal (size 86 point). The words were shown at the center of the screen. Participants received a recorded auditory instruction to read the sentences. Blocks lasted 33 s.
For auditory sentences, each block consisted of 10 sentences. Sentences were presented auditorily with four audio sequences for each sentence and one to three words in each sequence. Participants were asked to listen to the sentences carefully. Blocks lasted approximately 60 s (mean, 59.55 s; range, 57.75 to 66.19 s).
Vertical and horizontal checkerboard blocks each consisted of 30 flashing vertical checkerboards. The checkerboards changed their contrast after 400 ms. Each block lasted 12 s (30 × 400 ms).
Visual run
Stimuli of five different categories (faces, houses, tools, nonwords, and false fonts) and vertical and horizontal checkerboards were used. Twenty-four different items were used for each category. The stimuli were presented in mini blocks, which contained seven trials randomly selected from the same category. Each block lasted 10.5 s and was repeated six times. For each trial, a picture was displayed for 200 ms and then followed by a fixation cross for 200 ms. This was followed by a second picture from the same category for 500 ms and a fixation cross for 600 ms. A trial lasted 1.5 s in total. The order of the individual blocks was randomized. There was a 10-s rest period between each block. Participants’ task was to monitor for a black star and to press a key whenever the black star appeared on the screen. For color photographs of faces, we selected pictures from the Indian Institute of Technology Kanpur database of Indian faces. We selected 24 pictures (12 males and 12 females) from the database. The photos were shown in 400 pixels by 400 pixels. Color photographs of 24 Indian houses and huts were shown in 400 pixels by 400 pixels. Color photographs of 24 household tools were presented in 400 pixels by 400 pixels. Twenty-four nonwords were constructed and presented in white font on a black background in 1024 pixels by 768 pixels. All words are presented on the center of the screen with font size 72. Twenty-four false font strings were constructed and presented in white font on a black background in 1024 pixels by 768 pixels. All false fonts were presented on the center of the screen with font size 72. A flashing checkerboard (increasing in size from 184 pixels by 184 pixels to 218 pixels by 218 pixels) was displayed for 200 ms, followed by a fixation cross for 200 ms. This was followed by another flashing checkerboard presented for 500 ms (increasing in size from 184 pixels by 184 pixels to 293 pixels by 293 pixels). After that, a fixation cross was presented for 600 ms. False fonts were manually generated such that they reflected the typical composition of Devanagari characters in terms of line junctions, curvature, and visual complexity.
MRI data
Anatomical and functional data were collected before and after the literacy program using a 3.0-T Siemens MAGNETOM Skyra (Siemens AG, Germany) whole-body magnetic resonance scanner using a 64-channel radiofrequency head coil. T1-weighted three-dimensional (3D) magnetization–prepared rapid-acquisition gradient echo images were obtained using a pulse sequence with repetition time (TR) of 1.690 ms, echo time (TE) of 2.60 ms, inversion time (TI) of 1.100 ms, field of view (FOV) of 256 by 256, matrix size of 256 by 256 by 192, and voxel size of 1.0 mm by 1.0 mm by 1.0 mm. Functional images for the visual and localizer runs were acquired as continuous echo-planar imaging (EPI) (TR, 2400 ms; TE, 30 ms; 38 slices; voxel size, 3.5 mm by 3.5 mm by 3 mm; no interslice gap and interleaved slice order). Images in each run were realigned using a two-pass procedure (a mean EPI image was produced after an initial rigid body realignment, and all volumes were then realigned to the mean image). Realigned EPI volumes were coregistered with the T1 images. T1 images were normalized to the single-subject MNI T1 template provided with Statistical Parametric Mapping (SPM). Realignment parameters were applied to the coregistered realigned EPI images, and normalized EPI volumes were output-resliced to 3-mm isotropic voxels. Images were initially smoothed using an 8-mm full width at half maximum 3D Gaussian kernel. However, different analyses required different smoothing parameters, and where relevant, these are specified below.
Each functional imaging session (one for each of the localizer and visual categories) was modeled at the single-subject level using a general linear model in SPM12. The design consisted of one regressor per condition (localizer: sentence reading, sentence listening, horizontal checkerboards, and vertical checkerboards; visual: nonwords, false font strings, faces, houses, tools, and checkerboards).
Six regressors of no interest coding for scan-to-scan movement (x, y, and z translations and rotations) and a constant term were added. Stimulus blocks were modeled as epochs convolved with the canonical hemodynamic response function in SPM12.
For each subject, parameter estimates for conditions were contrasted with the baseline, and contrast images were used for second-level, random-effects analyses. Further analyses examining the relationships between literacy and brain responses to various visual inputs were carried out as described below. Statistical parametric maps of the contrasts for each of the categories of stimuli presented in the visual and localizer runs were generated at the group level and are presented in figs. S1 and S2. All visually presented stimuli evoked substantial bilateral visual cortical activation and significant activation in the ventral visual stream. Auditory stimuli evoked substantial bilateral auditory activation.
Analysis
Whole brain correlation between literacy and blood-oxygenation-level-dependent (BOLD) response
We created second-level models in which individual word reading scores (at baseline) were tested for their relationship with the magnitude of the BOLD response to each stimulus type.
Analysis of the relationship between peak location and literacy
Individual peaks were identified for target conditions within a specified radius of the locus of VWFA established as the left ventral temporal peak where activation during sentence reading was most strongly modulated by literacy (at MNI coordinates −45, −55, −10). Note that this procedure did not exclude “peaks” where response was of negative polarity. Coordinates and peak response value were stored for analysis.
To test for any systematic displacement of the individual’s peak voxels as a function of literacy, robust regressions were executed to test for a relationship between x, y, and z coordinates and word reading performance. Robust regressions here and throughout were implemented in MATLAB using the default parameters (bisquare weighting function with a tuning constant of 4.685). The use of robust regression helps to limit the impact of outliers by reducing their contribution to the regression model. Significance of the relationship was evaluated as the significance of the slope of the relationship. Slope values and their attendant t statistic and P value are reported.
To test for more subtle shifts, coordinates were converted from Cartesian x, y, z to spherical coordinates relative to the group VWFA and tested for any correlation between word reading scores and their angular components (azimuth and elevation). This was carried out using circular statistics appropriate for correlating a linear with a circular variable (32). The relationship between peak activation level and literacy was tested using robust regression to evaluate whether any significant increases or decreases in response magnitude were apparent as a function of word reading performance.
Determination of the lateralization index
The lateralization index for the contrasts of face and house against baseline was determined using the LI-tool (33), applied with the default parameters, and an exclusion mask of midline ±5 mm, with inclusion mask created in WFU_PickAtlas (34), composed of both fusiform gyri. The overall lateralization index values from the LI-tool analysis were then tested for relationship with literacy using robust regression (fig. S4).
Analysis of spatial extent of responses in ROIs
Three ROIs for this analysis were determined as follows: Masks were created on the basis of a cuboid containing the ventral temporal lobe (x, −70 to −20; y, −82 to −33; z, 28 to 4; the polarity of x coordinates was reversed for the right hemisphere); these were then refined for application to individual subjects in standard MNI space. Using grey matter (GM) probability maps derived from segmentation of T1 images (extracted using the CAT12 toolbox in SPM12), the cuboidal masks were binarized such that only voxels with a greater probability of being GM than either white matter or cerebrospinal fluid were included. The images were resliced to 3-mm isotropic voxels, and to exclude artifactual non-GM voxels that can arise from interpolation, the resliced images were binarized again, using a threshold of >0.2, thereby ensuring a conservative GM mask. The cerebellum was excluded on the basis of the cerebellar atlas SUIT3.0. (35, 36). The third ROI was created from the contiguous cluster of voxels, including the VWFA that showed significant modulation by literacy in response to sentence reading (at a voxel-wise P < 0.001 and cluster-level family-wise error–corrected P < 0.05). To maximize the spatial precision of this analysis, the fMRI data were analyzed as before, but this time, without smoothing.
Within each of the left and right ventral temporal masks, the number of voxels active in response to a given condition contrasted with all the other static visual categories (i.e., not including the flickering checkerboards) at a voxel-wise threshold of P < 0.001 was counted and then scaled as a proportion of the total number of voxels included in the mask. These proportions of activated tissue for each condition were tested for a relationship with literacy using robust regressions.
Annular analyses testing spatial extent of category-selective responses
These supplementary analyses were carried out to complement the analysis of the number of voxels responding to a given condition and to establish whether the local patches of cortex responsive to faces, house, and tools in the vicinity of the VWFA show any reduction in extent as a function of literacy. To be able to conduct spatially more specific analyses for the purposes of the annular investigation of cortical specificity, the fMRI data were analyzed using a 4-mm smoothing kernel, as opposed to 8 mm, and contrasts between condition and baseline were executed as before.
Individual’s peak voxels within specified search radii of the group VWFA were determined. Once an individual’s peak was found, voxels in successive annuli of 1 to 8 voxel city-block distance (i.e., a maximum L1 distance of 24 mm from the individual peak) were determined, and the activation of these to the specified condition was extracted and averaged, excluding any voxels that fell outside of the analyzed brain mask for that participant. The relationship with literacy was then tested by examining the significance of the slope of a robust regression between mean annular response and literacy.
Representational similarity analysis in ventral temporal cortex and VWFA
To carry out the multivariate analysis of the representation of the various visual categories in ventral temporal cortices and VWFA, unsmoothed data were analyzed. Specifically, the vector of responses of all voxels in the mask (left ventral temporal, right ventral temporal, or group VWFA; see above) was extracted for all the conditions compared to baseline. Subsequently, the correlation (Pearson’s R) between the vectors of responses over all the included voxels across conditions was calculated, giving a measure of the representational similarity of the different categories within the mask, within participant. This measure was then tested for a relationship with literacy across the group. To do this, the correlation coefficients were transformed using Fisher’s Z-transform (the arc tan hyperbolic transform) and then assessed for their relationship with literacy using robust regression. To correctly interpret these analyses, note that the similarity measure used is correlation, and therefore, does not reflect the magnitude of responses, only the distribution thereof across the vector of voxels analyzed.
Longitudinal analysis of trainees
Change in behavioral performance on reading and Akshara recognition was evaluated using a three-way analysis of variance (ANOVA), followed by post hoc pairwise comparisons (word reading: F3,63 = 4.48, P = 0.0065; Akshara recognition: F3,63 = 11.59, P < 0.001). Post hoc pairwise comparisons (one-way t tests and Bonferroni-Holm corrected for multiple comparisons) revealed that the trainees showed significant improvements in performance (word reading improvement versus illiterate controls, P = 0.031; word reading improvement versus literate controls, P = 0.004; Akshara recognition improvement versus illiterate controls, P < 0.001; Akshara recognition improvement versus illiterate controls, P < 0.001). Improvement within the group was significant for both word reading (P = 0.031) and Akshara recognition (P < 0.001); results are shown in fig. S6.
Participants who underwent literacy training were assessed for changes in brain response to orthographic stimuli in two analyses executed on the fMRI data smoothed at 8 mm. In the first analysis, a paired t test was executed in SPM to evaluate pretraining versus posttraining differences in brain response. In the second analysis, a difference image was generated by subtracting the pretraining from the posttraining contrast images for each condition against baseline. The difference images were then submitted to a regression analysis in SPM to evaluate the linear relationships between changes in literacy and brain response.
Supplementary Material
Acknowledgments
F.H. would like to thank A. Cutler. A.H.-A. would like to thank P. Hagoort. Funding: This project was funded by a Max Planck Society Strategic Innovation Grant. A.H.-A. is supported by the Swiss National Science Foundation (grant number PP00P1_163726). Competing interests: The authors declare that they have no competing interests. Author contributions: F.H. designed the research. U.K., R.K.M., V.N.T., A.G., and J.P.S. recruited participants and collected data. F.E. contributed to the data analysis. A.H.-A. carried out the data analysis and visualization. A.H.-A. and F.H. wrote the paper. Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. Additional data related to this paper may be requested from F.H.
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/5/9/eaax0262/DC1
Supplementary Text
Fig. S1. Modulation of nonword responses by literacy.
Fig. S2. Activation maps for all conditions presented in the visual run.
Fig. S3. Activation maps for all conditions presented in the localizer run.
Fig. S4. Lateralization of responses to faces and houses.
Fig. S5. Correlations between literacy and brain response in selected language-related cortical areas.
Fig. S6. Participants’ literacy scores.
Table S1. Participant demographic information and behavioral performance at both time 1 and time 2.
Table S2. Significantly modulated brain responses to orthographic stimuli.
Table S3. Correlation between literacy and brain response to visual categories in ventral temporal lobes.
Table S4. Loci of peaks showing an increased activation in response to sentences after training, for the subtraction “pretraining versus posttraining” in the participants who underwent literacy training.
Table S5. Loci of peaks showing an increase in activation in response to sentences after training, the magnitude of which is significantly correlated with the improvement in reading score in the participants who underwent literacy training.
REFERENCES AND NOTES
- 1.Huettig F., Kolinsky R., Lachmann T., The culturally co-opted brain: How literacy affects the human mind. Lang. Cogn. Neurosci. 33, 275–277 (2018). [Google Scholar]
- 2.Dehaene S., Cohen L., Cultural recycling of cortical maps. Neuron 56, 384–398 (2007). [DOI] [PubMed] [Google Scholar]
- 3.Castro-Caldas A., Miranda P. C., Carmo I., Reis A., Leote F., Ribeiro C., Ducla-Soares E., Influence of learning to read and write on the morphology of the corpus callosum. Eur. J. Neurol. 6, 23–28 (1999). [DOI] [PubMed] [Google Scholar]
- 4.Carreiras M., Seghier M. L., Baquero S., Estévez A., Lozano A., Devlin J. T., Price C. J., An anatomical signature for literacy. Nature 461, 983–986 (2009). [DOI] [PubMed] [Google Scholar]
- 5.Castro-Caldas A., Petersson K. M., Reis A., Stone-Elander S., Ingvar M., The illiterate brain. Learning to read and write during childhood influences the functional organization of the adult brain. Brain 121, 1053–1063 (1998). [DOI] [PubMed] [Google Scholar]
- 6.Petersson K. M., Reis A., Askelöf S., Castro-Caldas A., Ingvar M., Language processing modulated by literacy: A network analysis of verbal repetition in literate and illiterate subjects. J. Cogn. Neurosci. 12, 364–382 (2000). [DOI] [PubMed] [Google Scholar]
- 7.Dehaene S., Pegado F., Braga L. W., Ventura P., Filho G. N., Jobert A., Dehaene-Lambertz G., Kolinsky R., Morais J., Cohen L., How learning to read changes the cortical networks for vision and language. Science 330, 1359–1364 (2010). [DOI] [PubMed] [Google Scholar]
- 8.Skeide M. A., Kumar U., Mishra R. K., Tripathi V. N., Guleria A., Singh J. P., Eisner F., Huettig F., Learning to read alters cortico-subcortical cross-talk in the visual system of illiterates. Sci. Adv. 3, e1602612 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reis A., Petersson K. M., Castro-Caldas A., Ingvar M., Formal schooling influences two- but not three-dimensional naming skills. Brain Cogn. 47, 397–411 (2001). [DOI] [PubMed] [Google Scholar]
- 10.Planton S., Jucla M., Roux F.-E., Démonet J.-F., The “handwriting brain”: A meta-analysis of neuroimaging studies of motor versus orthographic processes. Cortex 49, 2772–2787 (2013). [DOI] [PubMed] [Google Scholar]
- 11.Longcamp M., Lagarrigue A., Nazarian B., Roth M., Anton J.-L., Alario F.-X., Velay J.-L., Functional specificity in the motor system: Evidence from coupled fMRI and kinematic recordings during letter and digit writing. Hum. Brain Mapp. 35, 6077–6087 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Higashiyama Y., Takeda K., Someya Y., Kuroiwa Y., Tanaka F., The neural basis of typewriting: A functional MRI study. PLOS ONE 10, e0134131 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jamadar S. D., Fielding J., Egan G. F., Quantitative meta-analysis of fMRI and PET studies reveals consistent activation in fronto-striatal-parietal regions and cerebellum during antisaccades and prosaccades. Front. Psychol. 4, 749 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heim S., Alter K., Ischebeck A. K., Amunts K., Eickhoff S. B., Mohlberg H., Zilles K., von Cramon D. Y., Friederici A. D., The role of the left Brodmann’s areas 44 and 45 in reading words and pseudowords. Brain Res. Cogn. Brain Res. 25, 982–993 (2005). [DOI] [PubMed] [Google Scholar]
- 15.Zhang B., He S., Weng X., Localization and functional characterization of an occipital visual word form sensitive area. Sci. Rep. 8, 6723 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ino T., Nakai R., Azuma T., Kimura T., Fukuyama H., Recognition and reading aloud of kana and kanji word: An fMRI study. Brain Res. Bull. 78, 232–239 (2009). [DOI] [PubMed] [Google Scholar]
- 17.Dehaene-Lambertz G., Monzalvo K., Dehaene S., The emergence of the visual word form: Longitudinal evolution of category-specific ventral visual areas during reading acquisition. PLOS Biol. 16, e2004103 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pinel P., Dehaene S., Beyond hemispheric dominance: Brain regions underlying the joint lateralization of language and arithmetic to the left hemisphere. J. Cogn. Neurosci. 22, 48–66 (2010). [DOI] [PubMed] [Google Scholar]
- 19.Vogel A. C., Petersen S. E., Schlaggar B. L., The VWFA: It’s not just for words anymore. Front. Hum. Neurosci. 8, 88 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moore M. W., Durisko C., Perfetti C. A., Fiez J. A., Learning to read an alphabet of human faces produces left-lateralized training effects in the fusiform gyrus. J. Cogn. Neurosci. 26, 896–913 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kelly A. M. C., Garavan H., Human functional neuroimaging of brain changes associated with practice. Cereb. Cortex 15, 1089–1102 (2005). [DOI] [PubMed] [Google Scholar]
- 22.Milton J., Solodkin A., Hluštík P., Small S. L., The mind of expert motor performance is cool and focused. Neuroimage 35, 804–813 (2007). [DOI] [PubMed] [Google Scholar]
- 23.Tsao D. Y., Freiwald W. A., Tootell R. B. H., Livingstone M. S., A cortical region consisting entirely of face-selective cells. Science 311, 670–674 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tanaka K., Columns for complex visual object features in the inferotemporal cortex: Clustering of cells with similar but slightly different stimulus selectivities. Cereb. Cortex 13, 90–99 (2003). [DOI] [PubMed] [Google Scholar]
- 25.Sato T., Uchida G., Lescroart M. D., Kitazono J., Okada M., Tanifuji M., Object representation in inferior temporal cortex is organized hierarchically in a mosaic-like structure. J. Neurosci. 33, 16642–16656 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scherf K. S., Behrmann M., Humphreys K., Luna B., Visual category-selectivity for faces, places and objects emerges along different developmental trajectories. Dev. Sci. 10, F15–F30 (2007). [DOI] [PubMed] [Google Scholar]
- 27.Golarai G., Ghahremani D. G., Whitfield-Gabrieli S., Reiss A., Eberhardt J. L., Gabrieli J. D. E., Grill-Spector K., Differential development of high-level visual cortex correlates with category-specific recognition memory. Nat. Neurosci. 10, 512–522 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cantlon J. F., Pinel P., Dehaene S., Pelphrey K. A., Cortical representations of symbols, objects, and faces are pruned back during early childhood. Cereb. Cortex 21, 191–199 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Deen B., Richardson H., Dilks D. D., Takahashi A., Keil B., Wald L. L., Kanwisher N., Saxe R., Organization of high-level visual cortex in human infants. Nat. Commun. 8, 13995 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shaywitz B. A., Shaywltz S. E., Pugh K. R., Constable R. T., Skudlarski P., Fulbright R. K., Bronen R. A., Fletcher J. M., Shankweiler D. P., Katz L., Gore J. C., Sex differences in the functional organization of the brain for language. Nature 373, 607–609 (1995). [DOI] [PubMed] [Google Scholar]
- 31.Krafnick A. J., Evans T. M., Neurobiological sex differences in developmental dyslexia. Front. Psychol. 9, 2669 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Berens P., CircStat: A MATLAB toolbox for circular statistics. J. Stat. Softw. 31, 1– 21 (2009). [Google Scholar]
- 33.Wilke M., Lidzba K., LI-tool: A new toolbox to assess lateralization in functional MR-data. J. Neurosci. Methods 163, 128–136 (2007). [DOI] [PubMed] [Google Scholar]
- 34.Maldjian J. A., Laurienti P. J., Kraft R. A., Burdette J. H., An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage 19, 1233–1239 (2003). [DOI] [PubMed] [Google Scholar]
- 35.Diedrichsen J., A spatially unbiased atlas template of the human cerebellum. Neuroimage 33, 127–138 (2006). [DOI] [PubMed] [Google Scholar]
- 36.Diedrichsen J., Balsters J. H., Flavell J., Cussans E., Ramnani N., A probabilistic MR atlas of the human cerebellum. Neuroimage 46, 39–46 (2009). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/5/9/eaax0262/DC1
Supplementary Text
Fig. S1. Modulation of nonword responses by literacy.
Fig. S2. Activation maps for all conditions presented in the visual run.
Fig. S3. Activation maps for all conditions presented in the localizer run.
Fig. S4. Lateralization of responses to faces and houses.
Fig. S5. Correlations between literacy and brain response in selected language-related cortical areas.
Fig. S6. Participants’ literacy scores.
Table S1. Participant demographic information and behavioral performance at both time 1 and time 2.
Table S2. Significantly modulated brain responses to orthographic stimuli.
Table S3. Correlation between literacy and brain response to visual categories in ventral temporal lobes.
Table S4. Loci of peaks showing an increased activation in response to sentences after training, for the subtraction “pretraining versus posttraining” in the participants who underwent literacy training.
Table S5. Loci of peaks showing an increase in activation in response to sentences after training, the magnitude of which is significantly correlated with the improvement in reading score in the participants who underwent literacy training.


