Abstract
Allogeneic hematopoietic stem cell transplant (HSCT) remains the mainstay in treating many hematologic malignancies. T-cell–depleted grafts designed to reduce graft-vs.-host disease (GVHD) may be complicated by severe viral infections that increase morbidity and mortality. Despite the use of antiviral pharmacologic therapy, challenges in controlling viral infections include drug resistance and/or side-effect intolerability. Virus-specific T-cell (VST) therapy is a promising targeted therapy for treating severe or drug-refractory viral infections after HSCT. An integrative review was conducted to inform advanced practitioners of the adverse effects associated with VST. A total of 836 articles were identified using PubMed, Scopus, and CINAHL databases, with 7 included in this review. Studies reviewed indicate that the adverse effects associated with VST therapy are limited and generally treatable. These studies reported low rates of adverse events of mild to moderate severity, including acute, recurrent, chronic, and de novo GVHD; cytokine release syndrome; infusion toxicity; and other adverse events. No deaths were attributed to VSTs in these studies.
Allogeneic hematopoietic stem cell transplant (HSCT) has become the standard of care in treating many hematologic malignancies and other immunodeficiency disorders, with over 1 million HSCTs performed in the past 50 years (Gratwohl et al., 2015; Singh & McGuirk, 2016). According to the Center for International Blood and Marrow Transplant Research (CIBMTR), the use of HSCT has been steadily increasing, with over 8,000 allogeneic transplants performed annually in the United States (D’Souza & Fretham, 2017). Hematopoietic stem cell transplants are divided into two categories: autologous, in which the patient is treated with their own stem cells, and allogeneic, in which the patient receives stem cells from another person, such as a sibling, parent or child, or unrelated donor. The most common indications for autologous HSCT in the United States are multiple myeloma and lymphomas, while acute leukemia and myelodysplastic syndrome account for 72% of allogenic HSCTs performed in the United States. Allogeneic transplant recipients experience unique complications such as graft-vs.-host disease (GVHD) or severe infection prompting intensive monitoring and treatment. There is a growing need for methods to treat these complications; as such, this review will focus on allogeneic HSCT recipients.
Despite the advances in HSCT since its inception, the procedure is not without risk. Of those who die in the first 100 days after allogeneic HSCT, 27% will die from relapsed disease, 20% from infection, and 16% from organ failure, followed by 8% from GVHD, 2% from hemorrhage, and 27% from other causes (D’Souza & Fretham, 2017). In patients who die after the first 100 days, 58% will die from relapsed disease, while only 9% and 7% will die from infection or organ failure, respectively, highlighting the importance of primary disease management followed by the prevention and management of infection and GVHD (D’Souza & Fretham, 2017). While bacterial infections occur more frequently, early and late viral infections also complicate HSCT recovery. The five most common viruses that present after HSCT include cytomegalovirus (CMV), Epstein-Barr virus (EBV), BK virus (BKV), adenovirus (AdV), and human herpesvirus 6 (HHV-6; Gratwohl et al., 2015). The standard of care for managing viruses after HSCT includes balancing antiviral pharmacotherapy with a reduction in immunosuppressive therapy (Ljungman et al., 2008; O’Reilly, Koehne, Hasan, Doubrovina, & Prockop, 2015; Styczynski et al., 2016; Tomblyn et al., 2009). Many antiviral pharmacotherapy options are renal toxic, may be ineffective, generate resistance, or are unable to confer long-term protection (Houghtelin & Bollard, 2017; Tomblyn et al., 2009).
A lack of VST immunity after HSCT allows for reactivation of viral infections such as CMV or EBV (Sellar & Peggs, 2012). Current antiviral treatments available to treat opportunistic infections are often inadequate to cure or control viral infections due to toxicity or the development of drug resistance, predisposing the HSCT recipient to a recurrence of viral infections (Sutrave, Blyth, & Gottlieb, 2017). Antiviral pharmacotherapy also does not confer long-term immunologic memory to a specific virus, so the recipient is at risk for recurrence of viral infection after the cessation of drug therapy (Sutrave et al., 2017).
Virus-specific T-cell (VST) therapy, which has been under exploration for the past 2 decades, is a targeted therapy used to treat severe or drug-refractory viral infections after HSCT. T cells are obtained from viral-experienced allogeneic donors for common viruses, such as CMV, and infused into the HSCT recipient to reconstitute antiviral immunity, allowing the patient to clear a viral infection (Houghtelin & Bollard, 2017). Investigators have improved the isolation and expansion of antigen-specific T cells, which has greatly improved the efficacy of VST therapy, with complete overall response rates ranging from 60% to 100% across multiple viral targets (Sutrave et al., 2017). This development paves the way for the use of multivalent VSTs that target multiple pathogens with a single infusion, as well as off-the-shelf human leukocyte antigen (HLA)–matched VSTs, which are available for immediate use after recognition of a viral infection. Currently, VST therapy is administered as an intravenous infusion in the inpatient setting within the context of a clinical trial. These VSTs may be single-valent, designed to target one specific virus, or multivalent, such as the broad-spectrum T cell explored by Papadopoulou and colleagues (2014) targeting AdV, EBV, CMV, BKV, and HHV-6 in one single infusion. The purpose of this review is to inform the advanced practitioner of the scope and severity of the side effects associated with VST therapy in allogeneic HSCT recipients in anticipation of the expanded use of this therapy in the future.
METHODS
Eligibility Criteria
This review included international publications of phase I and II clinical research trials in patients who developed viral infections after allogeneic HSCT, refractory to standard antiviral therapy and/or a reduction in immunosuppressive therapy who were subsequently treated with VSTs. To be included, studies had to include adult patients who had undergone allogeneic HSCT for hematologic malignancies and developed a viral infection refractory to standard of care. Study outcomes included rates of acute, chronic, recurrent, and de novo GVHD; cytokine release syndrome (CRS); infusion toxicity; and other adverse events. Articles published prior to 2012 were excluded, and results were limited to the English language. Publications other than clinical trials were excluded. Studies that evaluated the use of VST therapy in only pediatric patients or nonmalignant conditions were excluded. Research studies related to the engineering of VSTs and their efficacy are beyond the scope of this review and were not included.
Search Strategy
The authors conducted an extensive search of PubMed, Scopus, and CINAHL using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Moher, Liberati, Tetzlaff, & Altman, 2009). Studies were eligible for inclusion if they were published from January 2012 to December 2017. The databases were searched on December 14, 2017, using the Medical Subject Heading (MeSH) terms "BK virus," "adenovirus," "adenoviridae infections," "herpesvirus," "herpesviridae," "cytomegalovirus," "Epstein-Barr," "viral infection*," "T-lymphocytes," "immunotherapy," "T cell*," "hematopoietic stem cell transplantation," and "hematopoietic stem cell." A research librarian assisted in the literature search.
In total, 844 records were identified (Figure 1). After duplicates were removed, 836 publications were available for review. Articles were initially screened by title and then by abstract, with 56 articles remaining for full-text review. A secondary review of references for relevancy was included, and no additional studies were identified. Of the remaining 56 articles, a total of 49 articles were excluded for the following reasons: studies were not related to hematopoietic stem cell transplant, did not report on adverse effects, focused on antiviral medication management, emphasized T-cell engineering, were implemented in solid organ transplant or primary immunodeficiency disorders, were limited to the pediatric population, or were unrelated to cancer diagnoses. Seven articles met both the inclusion and exclusion criteria for analysis in this review (Table 1).
Figure 1.
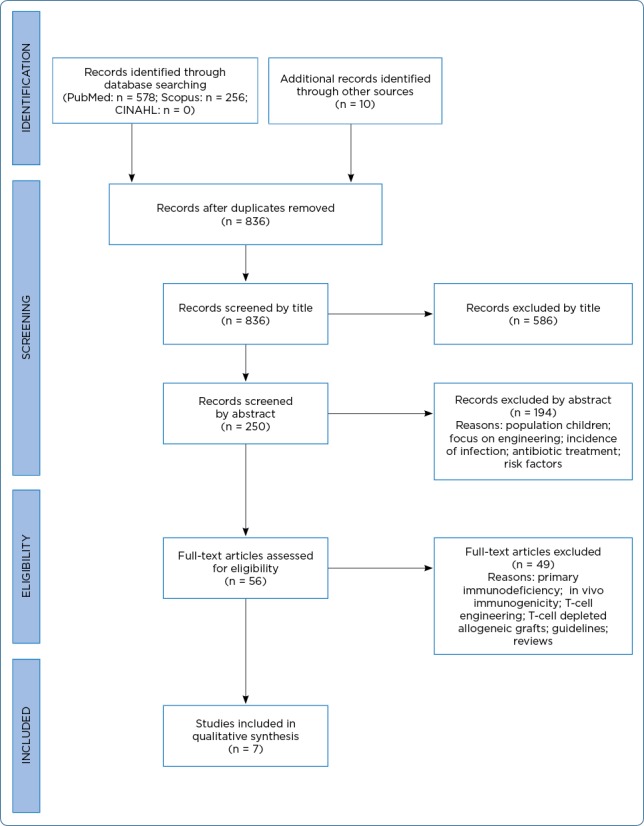
PRISMA flow diagram. Adapted from Moher, Liberati, Tetzlaff, and Altman (2009)
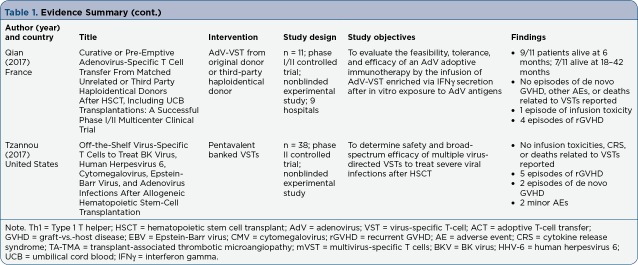
Table 1.
Evidence Summary
Table 1(cont.).
Evidence Summary (cont.)
RESULTS
The articles examined in this review addressed several outcomes, from which four themes were identified for the purpose of this review. A synthesis of the outcomes is organized by side effects: GVHD, CRS, infusion toxicity, and other adverse events (Table 2).
Table 2.
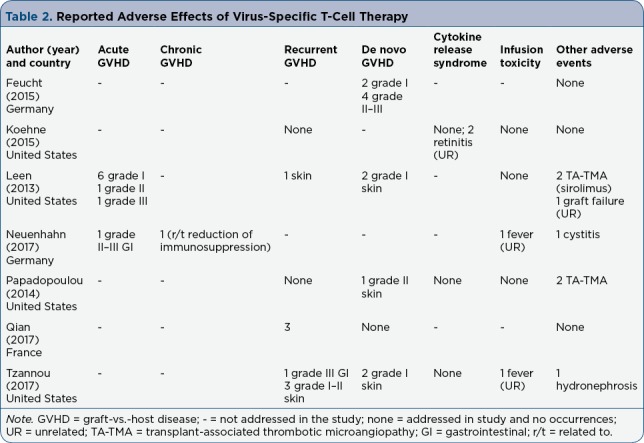
Reported Adverse Effects of Virus-Specific T-Cell Therapy
Graft-vs.-Host Disease
Graft-vs.-host disease occurs when donor cells begin to recognize host cells as foreign and attack, resulting in mild to severe symptoms. Graft-vs.-host disease is roughly divided into two categories, acute or chronic, based partially on the type and onset of symptoms. The risk of developing GVHD is based on patient and donor risk factors such as HLA matching, gender, age, and prophylactic immunosuppressive regimen (Finke et al., 2012).
All of the studies included in this review evaluated rates of GVHD after VST therapy and discussed the likelihood of attribution. The results were divided into four categories: acute, chronic, recurrent, and de novo GVHD (Table 2). Graft-vs.-host disease is a potentially fatal complication after the introduction of donor immune cells through allogeneic HSCT, but it also may occur as a result of VST therapy.
Acute GVHD
A phase I/II multicenter clinical trial examining the safety and efficacy of banked, multivalent VSTs in patients with drug-refractory CMV, AdV, or EBV infections after allogeneic HSCT was conducted by Leen and colleagues (2013). In order to receive VST therapy, patients had to have previously received standard-of-care treatment, including a reduction in immunosuppressive therapy and antiviral medication, without resolution of viremia or clinical infection. Eight occurrences of acute GVHD (aGVHD), defined as the development of symptoms within 45 days according to the Common Terminology Criteria for Adverse Events (CTCAE; National Cancer Institute, 2006) version 3.0 were reported (n = 50): six with grade 1, one with grade 2, and one with grade 3. Six of those patients had a history of GVHD, which had resolved with steroid therapy prior to receiving VST therapy. Of note, Leen and colleagues (2013) reported the standard taper of immunosuppressive therapy was likely related to six out of eight of the aGVHD flares.
Neuenhahn and colleagues (2017) prospectively assessed the efficacy and safety of donor-derived or third-party single-valent VSTs for the treatment of persistent, drug-refractory CMV infections in a phase I/II multicenter clinical trial. The authors reported one episode of gastrointestinal (GI) aGVHD grade 2 to 3 (n = 17), but clarified that the patient had received a natural killer cell transfusion and an unselected donor lymphocyte infusion—two additional confounding variables—21 and 8 days before VST therapy, respectively. The patient’s episode of GI aGVHD resolved after steroid therapy.
Chronic GVHD
Neuenhahn and colleagues (2017) reported one reactivation of chronic GVHD (cGVHD; n = 17). The patient’s cGVHD was of the skin and liver, with wasting syndrome that became worse after an attempted reduction in immunosuppressive therapy 50 days after VST administration. The cGVHD resolved after reinitiating immunosuppressive medications, with the authors noting that a causal relationship to VST therapy was unlikely (Neuenhahn, et al., 2017).
Recurrent GVHD
A phase II single-site clinical trial evaluating the feasibility, safety, and efficacy of banked, multivalent VSTs to treat severe and drug-refractory infections after HSCT was conducted by Tzannou and colleagues (2017). The multivalent VSTs were generated for off-the-shelf use and targeted AdV, CMV, and EBV, as well as two previously untargeted viruses, BKV and HHV-6. The authors reported one episode of grade 3 GI recurrent GVHD (rGVHD) per the CTCAE version 4.0 (n = 38; National Cancer Institute, 2010), which was attributed to rapid corticosteroid taper. The patient’s rGVHD resolved after reinitiating steroid therapy. Additionally, three episodes of grades 1 to 2 skin rGVHD occurred, with prompt resolution after administration of topical steroids (Tzannou et al., 2017).
In a phase I/II multicenter trial, Qian and colleagues (2017) examined the toxicity and response of donor-derived or third-party haploidentical AdV-specific VSTs after HSCT. Three episodes of early onset rGVHD (n = 11), defined as reactivation within the first month after AdV-VST, were reported (Qian et al., 2017). One recipient received VSTs derived from his previous mismatched unrelated donor (MMUD; 9/10 alleles), and two received VSTs from a third-party haploidentical donor (Qian et al., 2017). Due to the high risk of GVHD in MMUD and haploidentical donor grafts, these patients received a combination immunosuppressive regimen including either ciclosporin A plus mycophenolate mofetil or ciclosporin A plus methotrexate. The authors noted the cause was multifactorial, citing time of onset and dose of infused VSTs to confound the interpretation. Due to the number of possible causes, the authors concluded the use of VST therapy was not clearly associated with the patients’ rGVHD (Qian et al., 2017).
Leen and colleagues (2013) reported a flare of skin rGVHD in one patient (n = 50) with no discussion of treatment, resolution, or impact on VST outcome. Koehne and colleagues (2015) and Papadopoulou and colleagues (2014), whose studies will be further discussed, included rGVHD as an outcome; however, there were no reported episodes. The initiation of immunosuppressive, specifically steroid, therapy resulted in the resolution of rGVHD according to Neuenhahn and colleagues (2017), Tzannou and colleagues (2017), and Qian and colleagues (2017). Of note, Tzannou and colleagues (2017) and Neuenhahn and colleagues (2017) also reported rGVHD was unlikely to be related to the use of VSTs in their studies.
De Novo GVHD
Feucht and colleagues (2015) reported on a phase II multicenter clinical trial analyzing the safety and efficacy of donor-derived AdV-specific VSTs utilized after HSCT. Participants were eligible for the trial if they had failed treatment with standard antiviral drugs over 14 days without GVHD greater than grade 3 (Feucht et al., 2015). If an onset or aggravation of GVHD occurred within 8 weeks of receiving VSTs, it was arbitrarily attributed to the VSTs. The authors reported two patients (n = 30) who developed de novo grade 1 GVHD within 2 weeks, and four patients who developed de novo grades 2 to 3 GVHD at least 7 weeks after receipt of VST therapy. All episodes of GVHD responded to steroid therapy and the authors stated they could find no difference in the incidence of GVHD between treatment responders and nonresponders (Feucht et al., 2015).
Two episodes of de novo grade 1 skin GVHD (n = 50) were reported by Leen and colleagues (2013), which were mild and well controlled with topical steroids. The authors noted that the low incidence of de novo GVHD could be attributed to the specific lineage of T cells used, which showed no evidence of destructive alloreactivity (Leen et al., 2013).
In a phase I, single-site clinical trial, Papadopoulou and colleagues (2014) explored the safety and efficacy of banked, broad-spectrum, multivalent VSTs that recognized 12 immunogenic antigens from five viruses: EBV, AdV, CMV, BKV, and HHV-6. The VSTs were derived from a curated bank and used as prophylaxis or treatment. Prior to use in patients, the banked VSTs were tested for evidence of alloreactivity by measuring cytotoxicity against recipient or haploidentical blood samples, and none were found. The authors reported one episode of de novo grade 2 skin GVHD (n = 11), which improved with topical steroids.
Tzannou and colleagues (2017) reported two episodes of de novo grade 1 skin GVHD (n = 38), which resolved with topical steroids. Feucht and colleagues (2015), Leen and colleagues (2013), Papadopoulou and colleagues (2014), and Tzannou and colleagues (2017) reported no cases of severe (grade 3 or higher) de novo GVHD and that all cases resolved with steroid therapy. Of note, Qian and colleagues (2017) included de novo GVHD as an outcome but reported no occurrences.
Cytokine Release Syndrome
Three studies addressed CRS, a complex hyperimmune response syndrome that may occur after receipt of donor T-cell therapy, such as that found in CAR T-cell therapy, and ranges from mild constitutional symptoms to severe, life-threatening multiorgan dysfunction (Neelapu et al., 2018). Koehne and colleagues (2015) published a phase I, single-center clinical trial exploring the efficacy and toxicity of escalating doses of CMV-specific donor-derived VSTs after HSCT in patients with drug-refractory viremia or clinical infection. The authors speculated that the use of VST therapy in invasive viral disease, such as interstitial CMV pneumonia, could trigger an inflammatory response within the infected tissue, resulting in infiltrative tissue damage, which may result in chronic lung disease. This assumption was challenged when Koehne and colleagues (2015) reported two cases of CMV retinitis (n = 17) that cleared without any residual postinflammatory retinal damage. Tzannou and colleagues (2017) included CRS as a potential outcome; however, no occurrences were noted. Papadopoulou and colleagues (2014) reported no elevations in plasma cytokines after VST therapy, implying no development of CRS.
Infusion Toxicity
Five studies evaluated infusion-related reactions, or an exaggerated immune response resulting from the introduction of a foreign substance as seen with monoclonal antibodies. Infusion toxicity may result in mild constitutional symptoms such as fever, rigors, and cough, or can progress to life-threatening multiorgan damage or death (Vogel, 2010). Neuenhahn and colleagues (2017) reported one fever (n = 17), for which the patient was admitted to the hospital and found to have bacterial cystitis; therefore, the fever was not attributed to VST therapy. Tzannou and colleagues (2017) reported one patient with an isolated fever within 24 hours of infusion, but no other immediate toxicities were observed in this patient or any other patient (n = 38). Koehne and colleagues (2015), Leen and colleagues (2013), and Papadopoulou and colleagues (2014) included infusion toxicity as a potential outcome but reported no episodes.
Other Adverse Events
Transplant-Associated Thrombotic Microangiopathy. Transplant-associated thrombotic micro-angiopathy (TA-TMA) is a complex disorder resulting from systemic vascular endothelial injury affecting multiple organ systems and occurring in up to 30% of patients undergoing HSCT (Rosenthal, 2016). Transplant-associated thrombotic microangiopathy is marked by a set of diagnostic criteria that includes complement activation, proteinuria, and hypertension, and is associated with high mortality rates (Jodele et al., 2016; Rosenthal, 2016). This complication is primarily treated with supportive care, which includes the elimination of toxic agents, such as calcineurin inhibitors and sirolimus (two commonly used immunosuppressive medications), as well as preservation of renal function and adequate antimicrobial treatment (Ho et al., 2005; Rosenthal, 2016).
Two studies reported on TA-TMA as a potential adverse effect of VST therapy. Papadopoulou and colleagues (2014) reported two patients (n = 11) who developed TA-TMA at weeks 11 and 19, respectively. The authors specified TA-TMA is a known complication of allogeneic HSCT, and the delayed toxicity was unrelated to the VST infusions (Papadopoulou et al., 2014). Leen and colleagues (2013) also reported that TA-TMA is a complication that occurs in allogeneic HSCT recipients, particularly in those receiving sirolimus. The authors reported two patients who developed TA-TMA (n = 50), both of whom were receiving sirolimus (Leen et al., 2013). The authors did not discuss whether this outcome was directly related to VST administration.
Genitourinary Complications. Tzannou and colleagues (2017) reported one patient treated for BKV-associated hemorrhagic cystitis developed transient hydronephrosis with a decrease in renal function after VST treatment. The patient was found to have a concomitant bacterial urinary tract infection that resolved within 2 weeks, and the complication was deemed unrelated to the VST infusion (Tzannou et al., 2017). Neuenhahn and colleagues (2017) reported one patient diagnosed with cystitis and that it was unlikely to be caused by the VST therapy.
Graft Failure. Graft failure is a rare complication of allogeneic HSCT, most commonly attributed to disease recurrence, which may lead to failure of treatment and death (Rondón et al., 2008). Leen and colleagues (2013) reported one patient who experienced secondary graft failure concomitant with leukemic relapse. The banked, multivalent VSTs were not attributed to graft failure in this patient. Additionally, Qian and colleagues (2017) observed no toxicity against the hematopoietic stem cell grafts after receipt of VST therapy.
DISCUSSION AND IMPLICATIONS FOR PRACTICE
The clinical trials included in this review found few mild-to-moderate adverse events (AEs) associated with VST therapy. Adverse events reported in these studies included acute, chronic, recurrent, and de novo GVHD; CRS; infusion toxicity; and other outcomes, including TA-TMA, genitourinary complications, and graft failure. All of the described adverse events are incidents that can occur among HSCT recipients due to their donor graft. Generally, the AEs resolved with standard treatment or were deemed unrelated to VST therapy, and no deaths were attributed to the use of VSTs.
Graft-vs.-host disease was a commonly identified outcome discussed in the research included in this review (Table 2). Despite multiple studies reporting cases of grades 1 to 3 GVHD, Feucht and colleagues (2015), Koehne and colleagues (2015), Leen and colleagues (2013), Qian and colleagues (2017), and Tzannou and colleagues (2017) stated that no clinically significant cases of GVHD resulted from the use of VSTs in their studies. Additionally, Feucht and colleagues (2015) commented on the difficulty of distinguishing if the development of GVHD was in direct response to the use of VSTs, as opposed to the receipt of HSCT alone. Advanced practitioners should exercise caution when considering patients for VST therapy due to the indeterminate risk of GVHD. Despite GVHD being a known adverse event that occurs in allogeneic HSCT recipients, there is limited knowledge about the risk factors associated with the development of GVHD after VST therapy.
No evidence of CRS was identified in this review (Table 2). Cytokine release syndrome is a known toxicity of some T-cell therapies that typically first manifests with constitutional symptoms, including fever, malaise, anorexia, and myalgia (Neelapu et al., 2018). Cytokine release syndrome can affect any body system, including nervous, respiratory, cardiovascular, gastrointestinal, hepatic, renal, hematologic, and integumentary systems (Brudno & Kochenderfer, 2016; Lee et al., 2014; Neelapu et al., 2018). Cytokine release syndrome presentation ranges from vague constitutional symptoms to a high-grade syndrome associated with life-threatening multiorgan dysfunction (Neelapu et al., 2018). Quick intervention and management with best supportive care and anticytokine, specifically anti-interleukin 6, pharmacotherapy is indicated to reduce morbidity (Neelapu et al., 2018).
No evidence of infusion toxicity was identified in this review (Table 2). Infusion toxicity may range from rash to life-threatening organ dysfunction and can affect any body system, resulting in symptoms such as chest pain, palpitations, hypotension, tachycardia, headache, rash, rigors, diaphoresis, nausea, vomiting, arthralgias, anxiety or sense of impending doom, cough, dyspnea, nasal congestion, or rhinorrhea (Vogel, 2010). Despite the lack of CRS and infusion toxicity documented in these studies, advanced practitioners should maintain a high index of suspicion for any signs of symptoms of hypersensitivity or infusion-related reaction during the infusion of foreign cellular products.
LIMITATIONS
Limitations in the review include the use of phase I and II clinical trials and small sample sizes, both of which prevent a generalization of results to the HSCT patient population or practice change recommendations. Other limitations of the seven studies include a lack of blinding and comparison groups. Additionally, the studies implemented a wide variety of VST engineering and administration techniques. The rates of GVHD vary based on the type of donor graft and amount of HLA disparity (Jacobsohn & Vogelsang, 2007). Donor graft type and HLA matching were not controlled for in the studies included in this review and may account for the variable rates of GVHD. Importantly, the attribution of GVHD to VST therapy was either arbitrarily defined or went unmentioned in the study designs, making the comparison of outcomes across studies challenging. Trial designs varied significantly, from the number of investigating institutions, to the use of single virus-, trivalent-, and pentavalent-specific VSTs, and to the examination of outcomes at escalating cell doses of VST. Four trials examined single VSTs, and three assessed multivalent VSTs (Table 1). Although these limitations hinder the ability to make definitive generalizations, these initial trials offer insight into future research to better understand the safety of this novel therapy.
CONCLUSIONS
Viral infections remain a major complication after HSCT and are often difficult to control or eradicate with antiviral pharmacotherapy due to drug resistance and side-effect toxicity (Tomblyn et al., 2009). The use of VST therapy after HSCT demonstrates an immunotherapeutic approach to clear severe, drug-refractory viral infections, with seemingly mild-to-moderate adverse effects. This integrative review has sought to explore known adverse effects of VST therapy, with the aim of educating advanced practitioners on their significance. Evidence suggests that the adverse effects associated with VST therapy are treatable; however, limited knowledge of this novel therapy limits clinical application at this time. Further study including large, multicenter, randomized controlled trials are needed to determine the implications of the broad application of VST therapy. Future study design should consider comparing outcomes controlling for donor graft type, banked vs. direct donor VSTs, method of VST manufacturing, HLA matching, or single vs. multivalent VST therapy.
As the field of T-cell therapy continues to expand, advanced practitioners may require additional education to understand and manage these complex treatments. Despite the limitations, the outcomes reviewed, specifically GVHD, CRS, infusion toxicity, TA-TMA, graft failure, and genitourinary complications, indicate that there are little to no severe side effects associated with VST therapy, suggesting VST therapy can be safely used to treat viral infections in allogeneic HSCT recipients. These promising results should prompt additional investigation in additional patient populations.
Acknowledgments
The authors gratefully acknowledge Laurissa Gann, MSLS, AHIP, for her assistance with the literature review. Heidi Simmons completed the data collection. All authors contributed to the conceptualization and design, provided the analysis, and contributed to the manuscript preparation.
Footnotes
The authors have no conflicts of interest to disclose.
References
- 1.Brudno Jennifer N, Kochenderfer James N. Toxicities of chimeric antigen receptor T cells: recognition and management. Blood. 2016;127:3321–3330. doi: 10.1182/blood-2016-04-703751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.D’Souza A, Fretham C. Current uses and outcomes of hematopoietic stem cell transplantation: CIBMTR summary slides, 2017. 2017 Retrieved from http://www.cibmtr.org. [Google Scholar]
- 3.Feucht Judith, Opherk Kathrin, Lang Peter, Kayser Simone, Hartl Lena, Bethge Wolfgang, Matthes-Martin Susanne, Bader Peter, Albert Michael H, Maecker-Kolhoff Britta, Greil Johann, Einsele Hermann, Schlegel Paul-Gerhardt, Schuster Friedhelm R, Kremens Bernhard, Rossig Claudia, Gruhn Bernd, Handgretinger Rupert, Feuchtinger Tobias. Adoptive T-cell therapy with hexon-specific Th1 cells as a treatment of refractory adenovirus infection after HSCT. Blood. 2015;125:1986–1994. doi: 10.1182/blood-2014-06-573725. [DOI] [PubMed] [Google Scholar]
- 4.Finke Jürgen, Schmoor Claudia, Bethge Wolfgang A, Ottinger Hellmut D, Stelljes Matthias, Zander Axel R, Volin Liisa, Heim Dominik A, Schwerdtfeger Rainer, Kolbe Karin, Mayer Jiri, Maertens Johan A, Linkesch Werner, Holler Ernst, Koza Vladimir, Bornhäuser Martin, Einsele Hermann, Bertz Hartmut, Grishina Olga, Socié Gérard. Prognostic factors affecting outcome after allogeneic transplantation for hematological malignancies from unrelated donors: results from a randomized trial. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2012;18:1716–1726. doi: 10.1016/j.bbmt.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 5.Gratwohl Alois, Pasquini Marcelo C, Aljurf Mahmoud, Atsuta Yoshiko, Baldomero Helen, Foeken Lydia, Gratwohl Michael, Bouzas Luis Fernando, Confer Dennis, Frauendorfer Karl, Gluckman Eliane, Greinix Hildegard, Horowitz Mary, Iida Minako, Lipton Jeff, Madrigal Alejandro, Mohty Mohamad, Noel Luc, Novitzky Nicolas, Nunez José, Oudshoorn Machteld, Passweg Jakob, van Rood Jon, Szer Jeff, Blume Karl, Appelbaum Frederic R, Kodera Yoshihisa, Niederwieser Dietger. One million haemopoietic stem-cell transplants: a retrospective observational study. The Lancet. Haematology. 2015;2:e91–100. doi: 10.1016/S2352-3026(15)00028-9. [DOI] [PubMed] [Google Scholar]
- 6.Ho Vincent T, Cutler Corey, Carter Shelly, Martin Paul, Adams Roberta, Horowitz Mary, Ferrara James, Soiffer Robert, Giralt Sergio. Blood and marrow transplant clinical trials network toxicity committee consensus summary: thrombotic microangiopathy after hematopoietic stem cell transplantation. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2005;11:571–575. doi: 10.1016/j.bbmt.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 7.Houghtelin A, Bollard C M. Virus-specific T cells for the immunocompromised patient. Frontiers in Immunology. 2017;8:1272. doi: 10.3389/fimmu.2017.01272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jacobsohn D A, Vogelsang G B. Acute graft versus host disease. Orphanet Journal of Rare Diseases. 2007;2:35. doi: 10.1186/1750-1172-2-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jodele Sonata, Dandoy Christopher E, Myers Kasiani C, El-Bietar Javier, Nelson Adam, Wallace Gregory, Laskin Benjamin L. New approaches in the diagnosis, pathophysiology, and treatment of pediatric hematopoietic stem cell transplantation-associated thrombotic microangiopathy. Transfusion and apheresis science : official journal of the World Apheresis Association : official journal of the European Society for Haemapheresis. 2016;54:181–190. doi: 10.1016/j.transci.2016.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koehne Guenther, Hasan Aisha, Doubrovina Ekaterina, Prockop Susan, Tyler Eleanor, Wasilewski Gloria, O’Reilly Richard J. Immunotherapy with Donor T Cells Sensitized with Overlapping Pentadecapeptides for Treatment of Persistent Cytomegalovirus Infection or Viremia. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2015;21:1663–1678. doi: 10.1016/j.bbmt.2015.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee Daniel W, Gardner Rebecca, Porter David L, Louis Chrystal U, Ahmed Nabil, Jensen Michael, Grupp Stephan A, Mackall Crystal L. Current concepts in the diagnosis and management of cytokine release syndrome. Blood. 2014;124:188–195. doi: 10.1182/blood-2014-05-552729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leen Ann M, Bollard Catherine M, Mendizabal Adam M, Shpall Elizabeth J, Szabolcs Paul, Antin Joseph H, Kapoor Neena, Pai Sung-Yun, Rowley Scott D, Kebriaei Partow, Dey Bimalangshu R, Grilley Bambi J, Gee Adrian P, Brenner Malcolm K, Rooney Cliona M, Heslop Helen E. Multicenter study of banked third-party virus-specific T cells to treat severe viral infections after hematopoietic stem cell transplantation. Blood. 2013;121:5113–5123. doi: 10.1182/blood-2013-02-486324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ljungman P, de la Camara R, Cordonnier C, Einsele H, Engelhard D, Reusser P, Styczynski J, Ward K. Management of CMV, HHV-6, HHV-7 and Kaposi-sarcoma herpesvirus (HHV-8) infections in patients with hematological malignancies and after SCT. Bone marrow transplantation. 2008;42:227–240. doi: 10.1038/bmt.2008.162. [DOI] [PubMed] [Google Scholar]
- 14.Moher D, Liberati A, Tetzlaff J, Altman D G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.National Cancer Institute. Bethesda, MD: National Cancer Institute.; 2006. Common Terminology Criteria for Adverse Events v3.0 (CTCAE). Retrieved from https://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf. [Google Scholar]
- 16.National Cancer Institute. Bethesda, MD: National Cancer Institute.; 2010. Common Terminology Criteria for Adverse Events v4.0 (CTCAE). https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm#ctc_40. [Google Scholar]
- 17.Neelapu Sattva S, Tummala Sudhakar, Kebriaei Partow, Wierda William, Gutierrez Cristina, Locke Frederick L, Komanduri Krishna V, Lin Yi, Jain Nitin, Daver Naval, Westin Jason, Gulbis Alison M, Loghin Monica E, de Groot John F, Adkins Sherry, Davis Suzanne E, Rezvani Katayoun, Hwu Patrick, Shpall Elizabeth J. Chimeric antigen receptor T-cell therapy - assessment and management of toxicities. Nature reviews. Clinical oncology. 2018;15:47–62. doi: 10.1038/nrclinonc.2017.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Neuenhahn M, Albrecht J, Odendahl M, Schlott F, Dössinger G, Schiemann M, Lakshmipathi S, Martin K, Bunjes D, Harsdorf S, Weissinger E M, Menzel H, Verbeek M, Uharek L, Kröger N, Wagner E, Kobbe G, Schroeder T, Schmitt M, Held G, Herr W, Germeroth L, Bonig H, Tonn T, Einsele H, Busch D H, Grigoleit G U. Transfer of minimally manipulated CMV-specific T cells from stem cell or third-party donors to treat CMV infection after allo-HSCT. Leukemia. 2017;31:2161–2171. doi: 10.1038/leu.2017.16. [DOI] [PubMed] [Google Scholar]
- 19.O’Reilly R J, Koehne G, Hasan A N, Doubrovina E, Prockop S. T-cell depleted allogeneic hematopoietic cell transplants as a platform for adoptive therapy with leukemia selective or virus-specific T-cells. Bone marrow transplantation. 2015;50 Suppl 2:S43–50. doi: 10.1038/bmt.2015.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Papadopoulou A, Gerdemann U, Katari U L, Tzannou I, Liu H, Martinez C, Leen A M. Activity of broad-spectrum T cells as treatment for AdV, EBV, CMV, BKV, and HHV6 infections after HSCT. Science Translational Medicine. 2014;6(242):242ra283. doi: 10.1126/scitranslmed.3008825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qian C, Campidelli A, Wang Y, Cai H, Venard V, Jeulin H, Bensoussan D. Curative or pre-emptive adenovirus-specific T cell transfer from matched unrelated or third party haploidentical donors after HSCT, including UCB transplantations: A successful phase I/II multicenter clinical trial. Journal of Hematology and Oncology. 2017;10(1):102. doi: 10.1186/s13045-017-0469-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rondón Gabriela, Saliba Rima M, Khouri Issa, Giralt Sergio, Chan Kawah, Jabbour Elias, McMannis John, Champlin Richard, Shpall Elizabeth. Long-term follow-up of patients who experienced graft failure postallogeneic progenitor cell transplantation. Results of a single institution analysis. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2008;14:859–866. doi: 10.1016/j.bbmt.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rosenthal Joseph. Hematopoietic cell transplantation-associated thrombotic microangiopathy: a review of pathophysiology, diagnosis, and treatment. Journal of blood medicine. 2016;7:181–186. doi: 10.2147/JBM.S102235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sellar Rob S, Peggs Karl S. Recent progress in managing graft-versus-host disease and viral infections following allogeneic stem cell transplantation. Future oncology (London, England) 2012;8:1549–1565. doi: 10.2217/fon.12.153. [DOI] [PubMed] [Google Scholar]
- 25.Singh Anurag K, McGuirk Joseph P. Allogeneic Stem Cell Transplantation: A Historical and Scientific Overview. Cancer research. 2016;76:6445–6451. doi: 10.1158/0008-5472.CAN-16-1311. [DOI] [PubMed] [Google Scholar]
- 26.Styczynski Jan, van der Velden Walter, Fox Christopher P, Engelhard Dan, de la Camara Rafael, Cordonnier Catherine, Ljungman Per. Management of Epstein-Barr Virus infections and post-transplant lymphoproliferative disorders in patients after allogeneic hematopoietic stem cell transplantation: Sixth European Conference on Infections in Leukemia (ECIL-6) guidelines. Haematologica. 2016;101:803–811. doi: 10.3324/haematol.2016.144428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sutrave Gaurav, Blyth Emily, Gottlieb David J. Cellular therapy for multiple pathogen infections after hematopoietic stem cell transplant. Cytotherapy. 2017;19:1284–1301. doi: 10.1016/j.jcyt.2017.07.012. [DOI] [PubMed] [Google Scholar]
- 28.Tomblyn Marcie, Chiller Tom, Einsele Hermann, Gress Ronald, Sepkowitz Kent, Storek Jan, Wingard John R, Young Jo-Anne H, Boeckh Michael J, Boeckh Michael A. Guidelines for preventing infectious complications among hematopoietic cell transplantation recipients: a global perspective. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2009;15:1143–1238. doi: 10.1016/j.bbmt.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tzannou Ifigeneia, Papadopoulou Anastasia, Naik Swati, Leung Kathryn, Martinez Caridad A, Ramos Carlos A, Carrum George, Sasa Ghadir, Lulla Premal, Watanabe Ayumi, Kuvalekar Manik, Gee Adrian P, Wu Meng-Fen, Liu Hao, Grilley Bambi J, Krance Robert A, Gottschalk Stephen, Brenner Malcolm K, Rooney Cliona M, Heslop Helen E, Leen Ann M, Omer Bilal. Off-the-Shelf Virus-Specific T Cells to Treat BK Virus, Human Herpesvirus 6, Cytomegalovirus, Epstein-Barr Virus, and Adenovirus Infections After Allogeneic Hematopoietic Stem-Cell Transplantation. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2017;35:3547–3557. doi: 10.1200/JCO.2017.73.0655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vogel Wendy H. Infusion reactions: diagnosis, assessment, and management. Clinical journal of oncology nursing. 2010;14:E10–21. doi: 10.1188/10.CJON.E10-E21. [DOI] [PubMed] [Google Scholar]