Abstract
Dysregulated adult hippocampal neurogenesis occurs in many temporal lobe epilepsy (TLE) models. Most dentate granule cells (DGCs) generated in response to an epileptic insult develop features that promote increased excitability, including ectopic location, persistent hilar basal dendrites (HBDs), and mossy fiber sprouting. However, some appear to integrate normally and even exhibit reduced excitability compared to other DGCs. To examine the relationship between DGC birthdate, morphology, and network integration in a model of TLE, we retrovirally birthdated either early-born [EB; postnatal day (P)7] or adult-born (AB; P60) DGCs. Male rats underwent pilocarpine-induced status epilepticus (SE) or sham treatment at P56. Three to six months after SE or sham treatment, we used whole-cell patch-clamp and fluorescence microscopy to record spontaneous excitatory and inhibitory currents from birthdated DGCs. We found that both AB and EB populations of DGCs recorded from epileptic rats received increased excitatory input compared with age-matched controls. Interestingly, when AB populations were separated into normally integrated (normotopic) and aberrant (ectopic or HBD-containing) subpopulations, only the aberrant populations exhibited a relative increase in excitatory input (amplitude, frequency, and charge transfer). The ratio of excitatory-to-inhibitory input was most dramatically upregulated for ectopically localized DGCs. These data provide definitive physiological evidence that aberrant integration of post-SE, AB DGCs contributes to increased synaptic drive and support the idea that ectopic DGCs serve as putative hub cells to promote seizures.
SIGNIFICANCE STATEMENT Adult dentate granule cell (DGC) neurogenesis is altered in rodent models of temporal lobe epilepsy (TLE). Some of the new neurons show abnormal morphology and integration, but whether adult-generated DGCs contribute to the development of epilepsy is controversial. We examined the synaptic inputs of age-defined populations of DGCs using electrophysiological recordings and fluorescent retroviral reporter birthdating. DGCs generated neonatally were compared with those generated in adulthood, and adult-born (AB) neurons with normal versus aberrant morphology or integration were examined. We found that AB, ectopically located DGCs exhibit the most pro-excitatory physiological changes, implicating this population in seizure generation or progression.
Keywords: adult neurogenesis, dentate granule cell, epileptogenesis, hippocampus, retroviral birth dating, temporal lobe epilepsy
Introduction
Reorganization of the dentate gyrus network is a prominent feature of temporal lobe epilepsy (TLE) models. Ectopic migration, persistent hilar basal dendrites (HBDs), and mossy fiber sprouting of dentate granule cells (DGCs) are potential substrates for recurrent excitation (Jessberger et al., 2007; Walter et al., 2007; Kron et al., 2010). Concomitantly, the death of interneurons reduces inhibitory synaptic input (Obenaus et al., 1993; Houser and Esclapez, 1996; Kobayashi and Buckmaster, 2003; Hofmann et al., 2016). This pro-excitatory plasticity is thought to contribute to the erosion of the “dentate gate” and the propagation of seizure activity (Pathak et al., 2007; Shao and Dudek, 2011; Krook-Magnuson et al., 2015). However, potential compensatory pro-inhibitory changes are also observed, including extensive axonal sprouting of the remaining inhibitory neurons (Zhang et al., 2009; Thind et al., 2010; Peng et al., 2013) and death of excitatory projection neurons from entorhinal cortex (Du et al., 1993, 1995).
Anatomical data implicates DGCs that develop after an epileptogenic insult as the most likely to exhibit aberrant, pro-excitatory features (Scharfman and McCloskey, 2009; Althaus and Parent, 2014). Importantly, this population is exceptionally heterogeneous; some DGCs born postinsult appear morphologically normal, while others have various dendritic, axonal, and somatic abnormalities (Jessberger et al., 2007; Walter et al., 2007; Kron et al., 2010; Murphy et al., 2011). In contrast, cells that are mature at the onset of epileptogenesis primarily maintain normal morphology (Walter et al., 2007; Kron et al., 2010).
Aberrant integration of adult-born (AB) DGCs is associated with increased spontaneous seizures (Hester and Danzer, 2013) and aberrant postnatal DGC development appears sufficient to drive spontaneous seizures in otherwise intact mice (Pun et al., 2012). Aberrantly integrated (ectopic) DGCs also receive more excitatory and less inhibitory synaptic input than their normotopic counterparts in a rat TLE model (Zhan et al., 2010). Thus, a prevailing hypothesis posits that seizure-induced neurogenesis promotes hippocampal network hyperexcitability and spontaneous seizure activity. This idea was supported by recent studies in which suppression of adult neurogenesis reduced spontaneous seizures (Cho et al., 2015; Hosford et al., 2016).
Relatively few studies have measured the physiology of AB DGCs in an epileptic network. Recordings from ectopic DGCs have found subtle changes in intrinsic excitability, such as a less polarized resting membrane potential, which may shift network dynamics in ways that are difficult to predict from studies of individual DGCs (Scharfman et al., 2000; Zhan and Nadler, 2009; Althaus et al., 2015). These ectopic DGCs also receive a greater proportion of excitatory inputs relative to normotopic DGCs in a rat TLE model (Zhan et al., 2010). Additionally, DGCs which develop after status epilepticus (SE) in a mouse model mature and integrate into the network faster than their AB counterparts in healthy mice, thereby contributing to increased network excitability (Overstreet-Wadiche et al., 2006). In contrast to the pro-excitatory hypothesis of post-SE born DGCs, a study using an electrical SE model suggested that postinsult born DGCs exhibit reduced excitatory and increased inhibitory synaptic inputs when compared to cells that were presumably mature at the time of SE (Jakubs et al., 2006). However, this study did not include ectopically integrated DGCs, which comprise up to 25% of postinjury born DGCs (Walter et al., 2007; Kron et al., 2010).
To investigate the role of DGC age and aberrant morphology on synaptic input in a rat model of TLE, we used a green fluorescent protein (GFP)-expressing retrovirus to birthdate DGCs born at either postnatal day (P)7 [early-born (EB)] or P60 (AB). Rats underwent either sham treatment (control) or pilocarpine-induced SE at P56. DGCs labeled with GFP were then identified in hippocampal brain slices obtained from rats three to six months later. Using whole-cell patch-clamp to record spontaneous EPSCs (sEPSCs) and sIPSCs, we report that aberrantly integrated, AB DGCs exhibit the greatest imbalance of excitatory-to-inhibitory synaptic input, which may promote network hyperexcitability after SE.
Materials and Methods
Animals.
Animal procedures were performed using protocols approved by the Institutional Animal Care and Use Committee of the University of Michigan.
Animals were purchased from Charles River and kept under a constant 12/12 h light/dark cycle with access to food and water ad libitum. Epileptic animals and sham controls were generated as described previously (Kron et al., 2010). Briefly, P56 male Sprague Dawley rats were pretreated with atropine methylbromide (5 mg/kg, i.p.; Sigma) 20 min before receiving pilocarpine hydrochloride (340 mg/kg, i.p.; Sigma) for epileptic animals, or an equivalent volume of 0.9% saline for sham animals. Seizures were monitored behaviorally and after 90 min of convulsive SE (several episodes of rearing and falling followed by continuous facial and forelimb clonus), animals received diazepam (10 mg/kg, i.p.; Hospira Inc) to terminate SE. Sham controls were treated with diazepam 2 h after the saline injection. After recovery and before brain tissue was obtained for slicing, at least one spontaneous behavioral seizure was observed in all rats undergoing SE. Acute slice recordings were made three to six months after SE/sham treatment.
Bilateral intrahippocampal retroviral (RV) injections.
High-titer, replication-incompetent, pseudotyped CAG or synapsin promoter-driven GFP- or YFP-expressing RV was generated and injected bilaterally into the dentate gyrus at either P7 or P60 as described previously (Kron et al., 2010). Briefly, to label EB DGCs, male P7 rat pups were anesthetized on ice and placed on an ice-cold neonatal rat stereotax adaptor (Stoelting) in a Kopf stereotaxic frame. Bilateral burr holes were drilled in the skull, and 1 μl of RV was injected using a Hamilton syringe and microinjection pump at 0.1 μl/min into each hemisphere with the following coordinates: caudal 2.0, lateral 1.5, and depth 2.7 (in mm from bregma or mm below the skull). To label AB DGCs, P60 male rats were anesthetized with a ketamine/xylazine mixture and placed in a Kopf stereotaxic frame. Bilateral burr holes were drilled in the skull and 2.5 μl of RV was injected at 0.1 μl/min into each hemisphere with the following coordinates: caudal 3.9, lateral 2.3, and depth 4.2 (in mm from bregma or mm below the skull).
Slice preparation.
At three to six months after SE/sham treatment, animals were anesthetized with isoflourane (VetOne) and transcardially perfused for 60 s with ice-cold cutting solution containing the following (in mm): 206 sucrose, 2.8 KCl, 1 MgCl2′6H2O, 1.25 NaH2PO4, 1 CaCl2, 10 D-glucose, 26 NaHCO3, and 0.4 ascorbic acid, pH 7.4. After decapitation, brains were rapidly removed and rested for 2 min in ice-cold oxygenated, cutting solution. Brains were then blocked to isolate the hippocampus and 400-μm-thick coronal slices were cut with a vibrating blade microtome (VT1000S; Leica Microsystems Inc) in ice-cold, oxygenated cutting solution. Slices were allowed to recover for 15 min in 34°C, oxygenated N-methyl D-glutamine (NMDG)-based solution containing the following (in mm): 92 NMDG (Sigma), 2.5 KCl, 1.2 NaH2PO4, 30 NaHCO3, 20 HEPES, 25 glucose, 5 sodium ascorbate (Sigma), 2 thiourea (Sigma), 3 sodium pyruvate (Gibco Life Technologies), 10 MgSO4·7H2O (Sigma), and 0.5 CaCl2·2H2O (pH adjusted to 7.35 with 10N HCl). We find that recovery in this solution provides a significant improvement in the health of slices made from aged, epileptic rats (Zhao et al., 2011). Slices were then rested for at least 1 h at room temperature in aCSF containing the following (in mm): 124 NaCl, 2.8 KCl, 2 MgSO4 1.25 NaH2PO4, 2 CaCl2, 10 D-glucose, 26 NaHCO3, and 0.4 ascorbic acid, pH 7.4, before being transferred individually to the recording chamber.
Electrophysiological recordings.
Slices were continuously perfused (∼1.5 ml/min) with heated (32°C), oxygenated aCSF containing 100 μm picrotoxin (PTX) for sEPSCs or 50 μm CNQX and 10 μm d-APV for sIPSCs (all drugs from Sigma-Aldrich). We chose to pharmacologically isolate sEPSCs and sIPSCs to avoid issues of neurotoxicity that can occur when cells are held at depolarized potentials for extended periods (e.g., holding a cell at 0 mV for 5 min to record sIPSCs). GFP-labeled DGCs were first identified under epifluorescence (525-nm emission filter), then visualized and patched using infrared differential interference contrast (IR-DIC) optics. Recordings were obtained using borosilicate glass electrodes (Sutter Instruments) with a 4- to 7-MΩ open tip resistance. For sEPSCs, pipettes contained cesium gluconate-based internal solution (in mm, all from Sigma): 100 gluconic acid, 0.2 EGTA, 5 MgCl2, 40 HEPES, 2 Mg-ATP, 0.3 Na-GTP, and 0.3% biocytin with a pH 7.2 obtained by titrating concentrated CsOH. For sIPSCs, pipettes contained cesium chloride-based internal solution (in mm, all from Sigma): 135 CsCl, 0.2 EGTA, 2 MgCl2, 10 HEPES, 2 Mg-ATP, 0.3 Na-GTP, and 0.3% biocytin with a pH 7.2 obtained by titrating concentrated CsOH. Seal resistances of >1 GΩ were achieved before breaking into whole-cell configuration. All sEPSC and sIPSC recordings (gap-free, 5 min) were obtained in voltage-clamp mode at a holding potential of −70 mV with either a Dagan Cornerstone (Dagan) or Multiclamp 700B (Molecular Devices) amplifier under control of pClamp 10 software (Molecular Devices). Data were filtered at 3 kHz and digitized at 10 kHz (Digidata 1440; Molecular Devices).
Immunohistochemistry and microscopy.
Following recording, slices were immediately placed in 4% paraformaldehyde (PFA; Sigma) in PBS, pH 7.4, and refrigerated for up to one week. For biocytin visualization, slices were rinsed with PBS, and endogenous peroxidase activity was quenched with 0.1% hydrogen peroxide in 10% methanol and PBS. After a second wash in PBS, slices were permeabilized with 2% Triton X-100 (Sigma) in PBS and then incubated at room temperature in avidin/biotin enzyme complex (Vector Laboratories). After 1–2 d, slices were rinsed with PBS and then reacted with 3,3′-diaminobenzidine (DAB; Invitrogen) until the cells could be visualized. Slices were slide-mounted before cresyl violet counterstaining and coverslipping. Images were acquired on a Leica DSM-IRB inverted microscope (Leica Microsystems Inc) connected to a SPOT Flex digital camera (SPOT Imaging Solutions).
To confirm identity of hilar ectopic cells, immunofluorescence histochemistry of fixed sections was performed eight weeks after injection of RV-GFP. Animals were anesthetized with pentobarbital and transcardially perfused with cold 0.9% saline followed by 4% PFA. Brains were removed and post-fixed overnight at 4°C in 4% PFA and cryoprotected in 30% sucrose. Frozen coronal sections of 40-μm thickness were cut using a sliding microtome. Sections were processed with standard fluorescent immunohistochemical techniques using the following primary antibodies: chicken anti-GFP (1:1000; Aves), rabbit (Rb) anti-Prospero homeobox 1 (Prox1; 1:1000, a gift from Sam Pleasure, University of California, San Francisco, San Francisco, CA) or Rb anti-GABA (1:500; Sigma). Secondary antibodies (Alexa Fluor, 1:400; Invitrogen) used were as follows: goat (Gt) anti-chicken 488 and Gt anti-rabbit 647. Nuclear counterstain was performed using bisbenzamide. Confocal microscopic images were acquired with a Leica TCS SP5X confocal microscope using a 20× objective at 1.0× optical zoom. Images were captured using a 1-μm step size with the pinhole set at 1 airy unit.
Analysis and statistics.
Detection of spontaneous events was performed off-line using MiniAnalysis 6.0 (Synaptosoft). sEPSCs were identified manually as having a fast rise time to a peak that was >3× the root mean square of the noise. sIPSCs were also identified manually by having a peak amplitude of >3× the root mean square of the noise. sEPSC and sIPSC amplitudes were measured as the maximum (peak) change in current relative to baseline, instantaneous frequency was calculated by taking the inverse of the interevent interval, and total charge transfer was calculated as the sum of the integrated area of each event from onset to when the event decayed back to baseline over the entire 5-min recording for each cell. The numbers of animals, cells, and events recorded and summary information for each variable analyzed for all groups are shown in Table 1.
Table 1.
Summary information for all groups from which recordings were made
| sEPSCs | #Events | #Cells | #Animals | Amplitude (pA), all events |
Amplitude (pA), by cells |
Frequency (Hz), all events |
Frequency (Hz), by cells |
Charge transfer (pA*ms) |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Median ± SD | Range | Average of medians ± SEM | Range | Median ± SD | Range | Average of medians ± SEM | Range | Average of sums ± SEM | Range | ||||
| EB sham | 1195 | 10 | 6 | 5.8 ± 5.1 | 2.7–92.9 | 5.8 ± 0.3 | 4.6–7.9 | 0.8 ± 14.2 | 0.03–311.5 | 0.7 ± 0.1 | 0.2–1.8 | 1621 ± 256.2 | 224.7–2626 |
| EB SE | 1386 | 5 | 4 | 7.0 ± 5.6 | 2.8–61.6 | 6.4 ± 0.5 | 5.3–8.0 | 2.1 ± 24.7 | 0.03–285.7 | 1.5 ± 0.4 | 0.5–2.8 | 5866 ± 2323 | 746.1–10,948 |
| AB sham | 924 | 9 | 5 | 5.7 ± 2.8 | 2.9–32.4 | 5.5 ± 0.3 | 4.1–7.1 | 0.8 ± 17.2 | 0.03–396.8 | 0.6 ± 0.2 | 0.9–1.3 | 1268 ± 393.1 | 47.5–3712 |
| AB norm | 1655 | 16 | 9 | 6.1 ± 3.1 | 2.1–39.5 | 6.1 ± 0.3 | 4.4–8.4 | 1.0 ± 44.7 | 0.02–1235.0 | 0.9 ± 0.2 | 0.1–2.4 | 1335 ± 318.9 | 302.5–5090 |
| AB ab | 16,088 | 11 | 7 | 9.4 ± 6.5 | 2.3–87.8 | 9.1 ± 1.2 | 4.6–19.0 | 21.9 ± 83.0 | 0.05–8333.0 | 9.0 ± 3.7 | 0.8–31.5 | 32,991 ± 13,670 | 925.1–125,810 |
| AB ect | 12,529 | 5 | 3 | 9.6 ± 6.2 | 2.3–87.8 | 11.7 ± 1.9 | 8.3–19.0 | 27.1 ± 91.5 | 0.14–8333.0 | 13.8 ± 6.8 | 1.1–31.5 | 54,574 ± 24,274 | 4333–125,810 |
| AB HBD | 3559 | 6 | 5 | 8.3 ± 7.5 | 2.3–57.2 | 7.0 ± 1.0 | 4.6–9.6 | 6.2 ± 28.6 | 0.05–357.1 | 4.2 ± 1.5 | 0.8–4.5 | 11,409 ± 4330 | 925.1–23,451 |
| sIPSCs | #Events | #Cells | #Animals | Amplitude (pA), all events |
Amplitude (pA), by cells |
Frequency (Hz), all events |
Frequency (Hz), by cells |
Charge transfer (pA*ms) |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Median ± SD | Range | Average of medians ± SEM | Range | Median ± SD | Range | Average of medians ± SEM | Range | Average of sums ± SEM | Range | ||||
| EB sham | 5843 | 11 | 4 | 39.8 ± 60.1 | 5.8–728.3 | 48.3 ± 5.9 | 15.8–55.1 | 5.3 ± 53.2 | 0.03–924.2 | 3.8 ± 0.8 | 1.0–8.7 | 142,108 ± 41,278 | 24,813–416,224 |
| EB SE | 1751 | 7 | 3 | 58.0 ± 65.5 | 12.2–679.4 | 52.4 ± 5.4 | 25.3–50.4 | 2.1 ± 33.2 | 0.04–666.7 | 1.8 ± 0.6 | 0.5–4.4 | 61,430 ± 18,118 | 21,096–155,587 |
| AB sham | 4232 | 8 | 6 | 50.0 ± 53.5 | 7.5–800.4 | 48.1 ± 8.1 | 13.9–47.8 | 4.3 ± 44.0 | 0.06–1041.0 | 3.6 ± 0.8 | 0.9–7.4 | 128,920 ± 43,152 | 26,064–363,675 |
| AB norm | 16,461 | 14 | 6 | 39.7 ± 63.9 | 3.9–940.9 | 41.2 ± 8.9 | 7.1–134.9 | 14.8 ± 49.8 | 0.03–695.4 | 7.0 ± 2.2 | 0.3–23.4 | 396,385 ± 128,190 | 1161–1,712,463 |
| AB ab | 19,277 | 13 | 7 | 48.1 ± 75.7 | 3.3–889.8 | 43.9 ± 6.1 | 10.0–55.5 | 22.3 ± 65.4 | 0.03–3610.0 | 12.7 ± 4.9 | 0.5–55.9 | 628,383 ± 299,644 | 5308–3,873,926 |
| AB ect | 6606 | 6 | 5 | 29.5 ± 33.5 | 4.7–594.6 | 27.3 ± 5.5 | 10.0–25.1 | 13.4 ± 67.9 | 0.03–680.7 | 13.3 ± 8.7 | 0.7–55.9 | 295,332 ± 191,874 | 5308–1,241,813 |
| AB HBD | 12,671 | 7 | 5 | 62.4 ± 86.0 | 3.3–889.8 | 58.1 ± 6.5 | 23.9–55.5 | 26.5 ± 64.1 | 0.05–3610.0 | 12.3 ± 5.7 | 0.5–42.0 | 913,856 ± 527,036 | 28,266–3,873,926 |
The numbers of animals, cells, and events recorded along with summary information for each variable analyzed are shown. In general, median values (instead of means) were used because they better represent non-normally distributed data. For “all events” columns, calculations were performed with all individual events from all recordings within each group considered together (n = number of events shown in the left column). For “by cell” columns, calculations were first performed only on events recorded from individual cells; then data from each cell was tabulated to generate measures for each group (n = number of cells). In this case, error was propagated mathematically from the variance of events for each individual recording. Birthdate = EB (early born) or AB (adult born); treatment = SE (status epilepticus) or sham control; location = ect (ectopic) or norm (normotopic). HBD denotes cells that maintained hilar basal dendrites.
Data were graphed and analyzed using Prism 8.0 software (GraphPad). Group means were obtained by averaging the median value (which better represents non-normally distributed data) from each individual recording for amplitude and instantaneous frequency or by averaging the sum of the charge transfer from all events over the total 5-min period of each individual recording; statistical significance between groups was determined using the Mann–Whitney (MW) test (for two groups) or the Kruskal–Wallis (KW) test with Dunn's post hoc correction (for three groups). Cumulative histograms of amplitude and instantaneous frequency were generated using all events detected in each 5-min recording from all cells in each group and analyzed using the Kolmogorov–Smirnov (KS) test (for comparisons of two groups) or KW test with Dunn's post hoc test (for comparisons with three groups). However, because cumulative histograms consist of thousands of individual observations, the statistical power is very high and thus can overdetect significant but inconsequential differences (for example, by the KW test, all of the groups in all of the cumulative histograms we present are significantly different from each other, even when they are almost completely overlapping). Therefore, we have also calculated effect size according to Cohen's d (Cohen, 1988), which can be used to determine what differences should be considered “practically significant” (i.e., of physiological importance). In accordance with the original description and its subsequent use, we set the following cutoff values for the effect size to determine physiological significance: d < 0.2, very small effect size and negligible physiological significance; d = 0.2–0.49, small effect size and minimal physiological significance; d = 0.5–0.79, medium effect size and moderate physiological significance; d > 0.8, large effect size and major physiological significance. Finally, to evaluate the overall effect of changes in sEPSCs and sIPSCs on the total balance of synaptic drive experienced by cells in each group, we calculated an excitatory-to-inhibitory ratio for amplitude, instantaneous frequency, and charge transfer by dividing the average of the median values from individual cells for the sEPSCs by the average of the median values from individual cells for the sIPSCs; error for this ratio was propagated mathematically from the variance of events recorded in each individual cell. Ratios were compared across groups using Student's t tests (for two groups) or one-factor ANOVAs with Tukey's post hoc test (for three groups).
Results
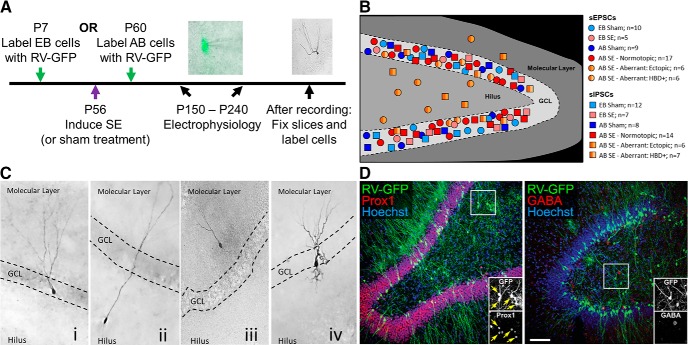
EB and AB DGCs were birthdated with a RV carrying a cytoplasmic GFP reporter as previously described (Kron et al., 2010). This allowed for identification of GFP-expressing, age-defined DGCs under epifluorescence, which could then be visualized using IR-DIC (Fig. 1A). Spontaneous synaptic currents (sEPSCs and sIPSCs) were isolated pharmacologically and thus measured from individual DGCs in separate experiments (Fig. 1B). Cell morphology and localization were observed during the recording period and confirmed with biocytin staining in a subset of cells (Fig. 1C). All cells with somas in the GCL that lacked HBDs were classified as normotopic. Cells which did not exhibit normotopic location or morphology were considered aberrant and classified into one of two subtypes: cells that had their somas in the GCL and possessed HBDs were classified as HBD+, and those with somas in the hilus or molecular layer were classified as ectopic. AB hilar ectopic, RV-GFP-labeled cells have been shown previously to be DGCs (Kron et al., 2010). To confirm this finding, we used immunofluorescence double labeling for Prox1, which labels DGCs, and GABA, which labels hilar interneurons (Fig. 1D). As expected, GFP+ hilar cells co-expressed Prox 1 (arrows in Fig. 1D inset) but not GABA, demonstrating that these cells are indeed DGCs and not interneurons. Note that aberrant cells were only found in the AB, SE-treated condition.
Figure 1.
Examples and schematic of experimental DGC recordings. A, Timeline of experimental protocol. EB cells were labeled at P7; AB cells were labeled at P60. All rats received SE or sham treatment at P56 and recordings were made between three and six months later (at P150–P240) to allow development of spontaneous recurrent seizures in pilocarpine-treated rats. An IR-DIC image of a patch-clamped DGC with GFP epifluorescence overlay and an example of biocytin staining are shown. B, Schematic diagram of the location for all DGCs that were recorded in each experimental category. GCL, granule cell layer. C, Representative images of biocytin-filled DGCs for each morphological category (i: normotopic morphology; ii, iii: ectopic location; iv: HBD+ morphology). Note that all images are from SE tissue. D, Confocal images of immunofluorescence double labeling of the dentate gyrus two months after pilocarpine treatment. In the left panel, GFP-labeled (green) hilar ectopic DGCs co-express Prox1 (red; the yellow arrows in inset denote double-labeled cells). In the right panel, GFP-labeled hilar ectopic DGCs do not co-express GABA (red). Cell nuclei are labeled with Hoechst (blue). Scale bar in D = 30 μm (C) 100 μm (D) 50 μm (D, insets).
EB DGCs
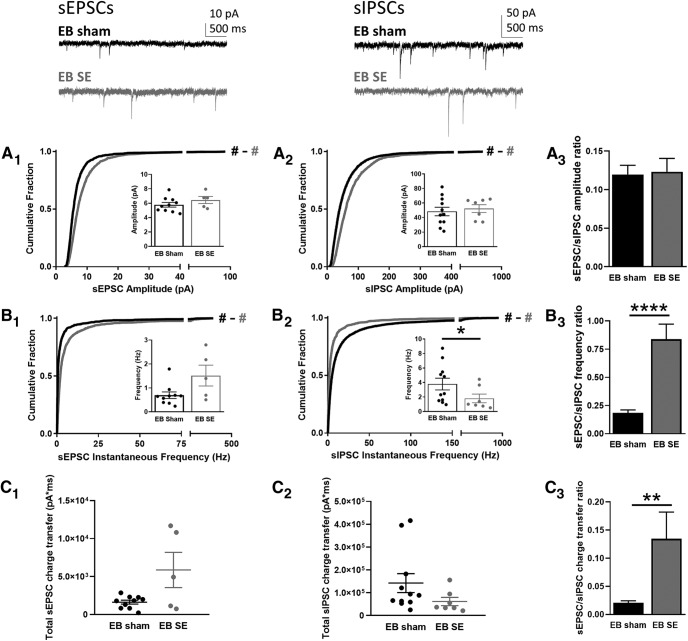
All EB DGCs from the sham and SE groups (which were mature at the time of the SE insult) exhibited normal localization and morphology. Cumulative histograms showed that the amplitudes of both sEPSCs (Fig. 2A1; KS test: D = 0.24, p < 0.0001) and sIPSCs (Fig. 2A2; KS test: D = 0.24, p < 0.0001) were significantly larger for DGCs in SE animals than DGCs in sham animals; however, the Cohen's d statistic (see Materials and Methods) revealed that the size of the effect between the sham and SE groups was small for both sEPSC amplitude [Cohen's d = 0.30 (minimal)] and sIPSC amplitude [Cohen's d = 0.35 (minimal)]. Additionally, the averages of the median amplitude values from each cell recorded in sham and SE groups were not statistically different for either sEPSCs (Fig. 2A1, inset; MW test: U = 14, p = 0.21) or sIPSCs (Fig. 2A2, inset; MW test: U = 33, p = 0.66). As a result of the relatively small and similar changes in the sEPSC and sIPSC amplitudes, the sEPSC-to-sIPSC ratio was not different between sham and SE groups (Fig. 2A3; t test: t = 0.17, p = 0.86), suggesting that the overall balance of synaptic drive in the EB SE DGCs was maintained at a level similar to that observed in the sham group.
Figure 2.
sEPSCs and sIPSCs recorded from EB DGCs. Top insets, Representative traces are shown for sEPSCs (top left) and sIPSCs (top right) from EB sham (black) and SE-treated (gray) rats. A, Cumulative histograms show the amplitude of sEPSCs (A1) and sIPSCs (A2) recorded from DGCs in EB sham (black) and SE (gray) groups. Insets show the median amplitude from each cell recorded in that group. A3, The ratio of the sEPSC-to-sIPSC amplitude was calculated using the average of the median amplitude from each cell recorded in each group; error was propagated mathematically from the variance of individual recordings. B, Cumulative histogram shows the instantaneous frequency of sEPSCs (B1) and sIPSCs (B2) recorded from DGCs in the EB sham (black) and SE (gray) groups. Insets show the median instantaneous frequency from each cell recorded in that group. B3, The ratio of the sEPSC-to-sIPSC instantaneous frequency was calculated using the average of the median amplitude from each cell recorded in each group; error was propagated mathematically from the variance of individual recordings. C, Total charge transfer, calculated as the sum of the charge transfer for each individual event over the entire 5-min recording period, is shown for each cell from each group for sEPSCs (C1) and sIPSCs (C2). C3, The ratio of the sEPSC-to-sIPSC total charge transfer was calculated using the average of the cells recorded in each group; error is SEM. Hash signs in A1, A2 and B1, B2 denote Cohen's d effect size by group comparison (black vs gray) and magnitude (# = negligible/minimal; ## = moderate; ### = major). Asterisks denote statistical significance: *p < 0.05, ****p < 0.0001.
In contrast to the similar effect on amplitudes, SE differentially affected instantaneous frequencies in EB DGCs from sham and SE groups. sEPSCs in SE animals exhibited an increase in instantaneous frequency compared to the sham group (Fig. 2B1; KS test: D = 0.37, p < 0.0001), although the effect size was small [Cohen's d = 0.22 (minimal)]. Likewise, the averages of the median frequency values for sEPSCs were not different between groups but did show a trend toward an increased frequency in the SE-treated group compared to sham (Fig. 2B1, inset; MW test: U = 10, p = 0.08). On the other hand, sIPSCs from DGCs in SE rats exhibited a decrease in instantaneous frequency (Fig. 2B2; KS test: D = 0.26, p < 0.0001), and although this effect size was also small [Cohen's d = 0.30 (minimal)], the averages of the median frequency values for sIPSCs showed a significant decrease in the SE-treated group compared to sham (Fig. 2B2, inset; MW test: U = 15, p < 0.05). Together, these concomitant changes resulted in a substantial increase in the frequency of sEPSC-to-sIPSC synaptic drive onto EB DGCs after SE (Fig. 2B3; t test: t = 16.03, p < 0.0001).
To evaluate the combined effect of changes in amplitude and frequency on overall excitatory and inhibitory drive, we calculated the total charge transfer by summing the integrated area of each event over the entire five-min recording from each cell. While there were no statistically significant differences between sham and SE-treated groups for either sEPSCs (Fig. 2C1; MW test: U = 16, p = 0.31) or sIPSCs (Fig. 2C2; MW test: U = 20, p = 0.10) alone, the ratio of the sEPSC-to-sIPSC charge transfer showed a significant increase in the SE-treated DGGs (Fig. 2C3; t test: t = 3.21, p < 0.01), resulting from the somewhat larger sEPSC charge transfer combined with the somewhat smaller sIPSC charge transfer.
AB DGCs
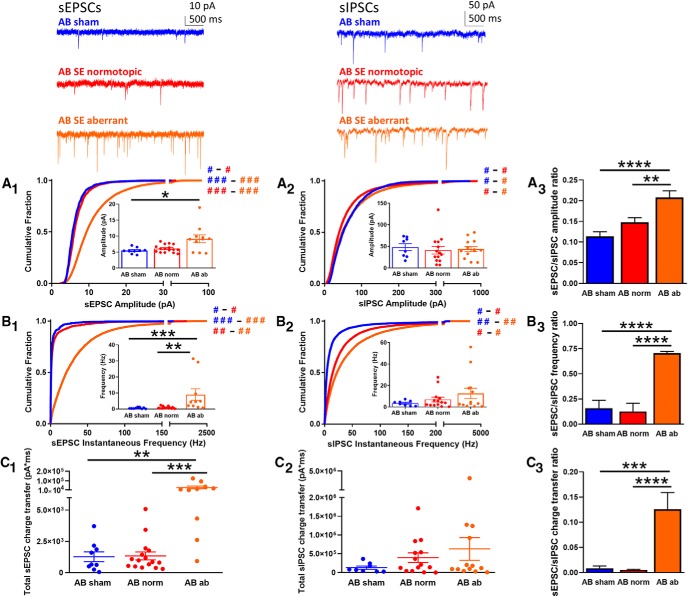
While all the AB DGCs in sham-treated rats exhibited normotopic location and morphology, AB DGCs in SE-treated animals (which were generated after the SE insult) consisted of distinct populations, including normotopic (with normal localization and morphology) and aberrant, which either had ectopic localization (soma in the dentate hilus or molecular layer) or abnormal morphology (i.e., HBDs). We therefore investigated whether DGCs in these different groups also exhibited functional differences in excitatory or inhibitory synaptic drive. The most prevalent differences were observed for sEPSCs: cumulative histograms revealed significant differences in both the amplitude (Fig. 3A1; KW test: H = 2064, p < 0.0001) and instantaneous frequency (Fig. 3B1; KW test: H = 4029, p < 0.0001), with particularly large effect sizes for aberrant DGCs compared to both sham and normotopic DGCs, suggesting that these constitute meaningful physiological differences [Cohen's d for amplitude: sham vs normotopic, 0.15 (negligible); sham vs aberrant, 1.04 (major); normotopic vs aberrant, 0.94 (major); Cohen's d for instantaneous frequency: sham vs normotopic, 0.12 (negligible); sham vs aberrant, 0.89 (major); normotopic vs aberrant, 0.56 (moderate)]. Likewise, when the averages of the median values were considered, sEPSCs from aberrant DGCs had significantly larger amplitudes than DGCs in sham animals (Fig. 3A1, inset; KW test: H = 7.15, p < 0.05; Dunn's post hoc: sham vs aberrant, p < 0.05) and significantly higher frequencies compared to both sham and normotopic DGCs (Fig. 3B1, inset; KW test: H = 16.34, p < 0.001; Dunn's post hoc: sham vs aberrant, p < 0.001; normotopic vs aberrant, p < 0.01). Finally, the combined effect of these increases in amplitude and frequency in the aberrant group resulted in a significantly larger total charge transfer compared to both the sham and normotopic groups (Fig. 3C1; KW test: H = 15.88, p < 0.001; Dunn's post hoc: sham vs aberrant, p < 0.01; normotopic vs aberrant, p < 0.001).
Figure 3.
sEPSCs and sIPSCs recorded from AB DGCs. Top insets, Representative traces are shown for sEPSCs (top left) and sIPSCs (top right) from AB DGCs in sham (blue) and AB normotopic (red) or aberrant (orange) DGCs in SE-treated rats. A, Cumulative histogram shows the amplitude of sEPSCs (A1) and sIPSCs (A2) recorded from DGCs in the AB sham (blue), SE normotopic (norm, red), and SE aberrant (orange) groups. Insets show the median amplitude from each cell recorded in that group. A3, The ratio of the sEPSC-to-sIPSC amplitude was calculated using the average of the median amplitude from each cell recorded in each group; error was propagated mathematically from the variance of individual recordings. B, Cumulative histogram of sEPSC (B1) and sIPSC (B2) instantaneous frequency recorded from DGCs in the AB sham (blue), SE normotopic (red), and SE aberrant (orange) groups. Insets show the median instantaneous frequency from each cell recorded in that group. B3, The ratio of the sEPSC-to-sIPSC instantaneous frequency was calculated using the average of the median amplitude from each cell recorded in each group; error was propagated mathematically from the variance of individual recordings. C, Total charge transfer, calculated as the sum of the charge transfer for each individual event over the entire 5-min recording period, is shown for each cell from each group for sEPSCs (C1) and sIPSCs (C2). C3, The ratio of the sEPSC-to-sIPSC total charge transfer was calculated using the average of the cells recorded in each group; error is SEM. Hash signs in A1, A2 and B1, B2 denote Cohen's d effect size by group comparison (color) and magnitude (# = negligible/minimal; ## = moderate; ### = major). Asterisks denote statistical significance: *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
Recordings of sIPSCs, in contrast, displayed far less robust differences than those of sEPSCs. Although the cumulative histograms of the sIPSC amplitudes recorded in the three populations were statistically different from each other (Fig. 3A2; KW test: H = 478.4, p < 0.0001), the effect sizes were very small, suggesting that these differences are of little physiological relevance [Cohen's d for amplitude: sham vs normotopic, 0.12 (negligible); sham vs aberrant, 0.09 (negligible); normotopic vs aberrant, 0.18 (negligible)]. In agreement with this finding, the averages of the median sIPSC amplitudes revealed no statistically significant differences between any of the groups (Fig. 3A2, inset; KW test: H = 1.42, p = 0.49). Similarly, the cumulative histograms of the sIPSC instantaneous frequencies were statistically different from each other (Fig. 3B2; KW test: H = 3203, p < 0.0001), but only showed modest effect sizes, suggesting these changes have minor physiological relevance [Cohen's d for instantaneous frequency: sham vs normotopic, 0.33 (minimal); sham vs aberrant, 0.52 (moderate); normotopic vs aberrant, 0.23 (minimal)]. Again, there were no significant differences between any of the groups when the averages of the median sIPSC frequencies were compared (Fig. 3B2, inset; KW test: H = 0.60, p = 0.74). The lack of a substantial effect of SE treatment or aberrant features on inhibitory synaptic drive is further evidenced by a comparison of the total charge transfer (which takes both amplitude and frequency into account) between each of the AB DGC groups, which shows that these are not statistically different from each other (Fig. 3C2; KW test: H = 1.90, p = 0.39).
Together, the large changes in sEPSCs with relatively small or no changes in sIPSCs in the aberrant DGC population results in a significant increase in the excitatory-to-inhibitory ratio for all three measures. First, amplitude ratios indicate a relative increase in excitatory synaptic strength for aberrant AB DGCs (Fig. 3A3; ANOVA: F = 12.06, p < 0.0001; Tukey's post hoc: sham vs aberrant, p < 0.0001; normotopic vs aberrant, p < 0.01). Second, the higher instantaneous frequency ratio in the aberrant population suggests an increase in the prevalence of excitatory synaptic input (Fig. 3B3; ANOVA: F = 323.4, p < 0.0001; Tukey's post hoc: sham vs aberrant, p < 0.0001; normotopic vs aberrant, p < 0.0001). Lastly, the higher total charge transfer ratio suggests an increase in total excitatory drive onto aberrant DGCs (Fig. 3C3; ANOVA: F = 14.69, p < 0.0001; Tukey's post hoc: sham vs aberrant, p < 0.001; normotopic vs aberrant, p < 0.0001).
Aberrant subpopulations
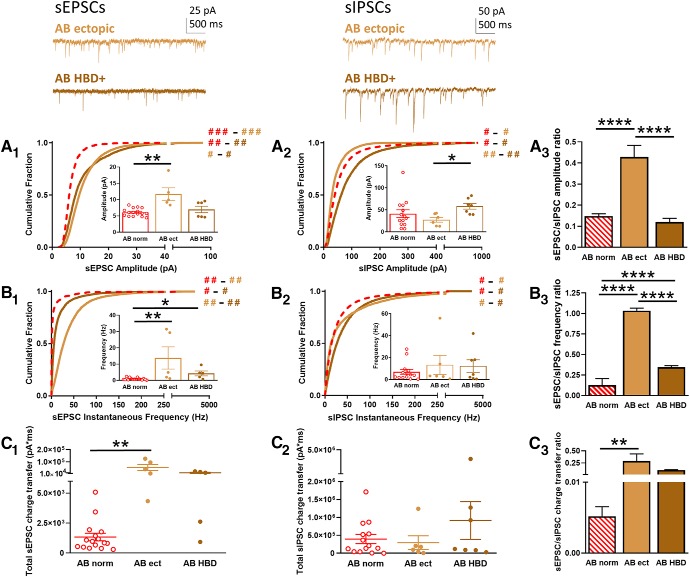
The aberrant DGCs consisted of two subpopulations: those with mislocalized cell bodies (where the soma was located ectopically in the hilus or molecular layer instead of the granule cell layer) and those with morphological abnormalities (where cells exhibited HBDs). These subsets were analyzed individually and compared to normotopic AB DGCs in the SE group to determine how aberrant location or morphology impacted sEPSC and sIPSC input.
Interestingly, ectopic and HBD+ DGCs exhibited functional differences in excitatory and inhibitory input. The cumulative histograms for sEPSC amplitude showed that ectopic and HBD+ cells were significantly different from normotopic DGCs (Fig. 4A1; KW test: H = 76.59, p < 0.0001) with large effect sizes [Cohen's d: normotopic vs ectopic, 0.96 (major); normotopic vs HBD+, 0.77 (moderate)], although the effect size between ectopic and HBD+ groups was very small [Cohen's d, 0.05 (negligible)]. Further, a comparison of the averages of the median amplitudes showed that ectopic DGCs had significantly larger sEPSCs compared to normotopic DGCs (Fig. 4A1, inset; KW test: H = 9.90, p < 0.01; Dunn's post hoc: normotopic vs ectopic, p < 0.01), and a trend toward being larger than HBD+ sEPSC amplitudes (Dunn's post hoc: ectopic vs HBD+, p = 0.06). These results suggest that both types of aberrant DGCs contribute to the overall increase in sEPSC amplitude, but that ectopic cells are the primary drivers. Conversely, the amplitudes of the sIPSCs were differentially altered in the aberrant subpopulations: ectopic DGCs had smaller sIPSCs but HBD+ cells had larger sIPSCs compared to the normotopic group [Fig. 4A2; KW test: H = 6.31, p < 0.0001; Cohen's d: normotopic vs ectopic, 0.37 (minimal); normotopic vs HBD+, 0.38 (minimal); ectopic vs HBD+, 0.78 (moderate)]. Analyzing the average of the median sIPSC amplitudes showed that, while neither subgroup was statistically different from the normotopic DGCs, ectopic and HBD+ cells were significantly different from each other (Fig. 4A2, inset; KW test: H = 6.31, p < 0.001; Dunn's post hoc: ectopic vs HBD+, p = 0.05), suggesting that the inhibitory inputs in these two subpopulations differentially modulate the observed increased in excitatory drive. Indeed, when the ratio was calculated, the increase in sEPSC amplitude combined with the decrease in sIPSC amplitude in ectopic DGCs had a synergistic effect, resulting in a marked overall increase in the relative strength of excitatory-to-inhibitory input (Fig. 4A3; ANOVA: F = 38.79, p < 0.0001; Tukey's post hoc: normotopic vs ectopic, p < 0.0001; ectopic vs HBD+, p < 0.0001). Conversely, in HBD+ cells, the increase in sIPSC amplitude offset the increase in sEPSC amplitude, resulting in little net change in the relative strength of excitatory-to-inhibitory synaptic drive compared to normotopic DGCs (Fig. 4A3; Tukey's post hoc: normotopic vs HBD+, p = 0.66).
Figure 4.
sEPSCs and sIPSCs recorded from aberrant AB DGCs. Top insets, Representative traces are shown for sEPSCs (top left) and sIPSCs (top right) from AB SE ectopic (light brown) or AB SE HBD+ (dark brown) DGCs. A, Cumulative histogram shows the amplitude of sEPSCs (A1) and sIPSCs (A2) recorded from DGCs in the AB SE ectopic (ect, light brown) and SE HBD+ (dark brown) groups, as well as the AB SE normotopic group (norm, dashed red). Insets show the median amplitude from each cell recorded in that group. A3, The ratio of the sEPSC-to-sIPSC amplitude was calculated using the average of the median amplitude from each cell recorded in each group; error was propagated mathematically from the variance of individual recordings. B, Cumulative histogram shows the instantaneous frequency of sEPSCs (B1) and sIPSCs (B2) recorded from DGCs in the AB SE ectopic (light brown) and AB SE HBD+ (dark brown) groups, as well as the AB SE normotopic group (dashed red). Insets show the median instantaneous frequency from each cell recorded in that group. B3, The ratio of the sEPSC-to-sIPSC instantaneous frequency was calculated using the average of the median amplitude from each cell recorded in each group; error was propagated mathematically from the variance of individual recordings. C, Total charge transfer, calculated as the sum of the charge transfer for each individual event over the entire 5-min recording period, is shown for each cell from each group for sEPSCs (C1) and sIPSCs (C2). C3, The ratio of the sEPSC-to-sIPSC total charge transfer was calculated using the average of the cells recorded in each group; error is SEM. Hash signs in A1, A2 and B1, B2 denote Cohen's d effect size by group comparison (color) and magnitude (# = negligible/minimal; ## = moderate; ### = major). Asterisks denote statistical significance: *p < 0.05, **p < 0.01, ****p < 0.0001.
We next examined instantaneous frequency of spontaneous postsynaptic currents. These data revealed changes in a similar directional for the two aberrant subpopulations. For sEPSCs, the cumulative histograms showed that both ectopic and HBD+ cells had an increased frequency compared to normotopic DGCs, although the effect size was larger for ectopic DGCs than for HBD+ DGCs [Fig. 4B1; KW test: H = 297.2, p < 0.0001; Cohen's d: normotopic vs ectopic, 0.66 (moderate); normotopic vs HBD+, 0.21 (minimal); ectopic vs HBD+, 0.52 (moderate)]. Likewise, when the averages of the median frequencies were considered, sEPSCs from both aberrant populations had significantly higher instantaneous frequencies than normotopic DGCs (Fig. 4B1, inset; KW test: H = 12.58, p < 0.01; Dunn's post hoc: normotopic vs ectopic, p < 0.01; normotopic vs HBD+, p < 0.05). Conversely, neither ectopic nor HBD+ cells exhibited a major change in the sIPSC instantaneous frequency. While there were significant differences between cumulative histograms, none of the effect sizes were substantial [Fig. 4B2; KW test: H = 313.1, p < 0.0001; Cohen's d: normotopic vs ectopic, 0.20 (minimal); normotopic vs HBD+, 0.24 (minimal); ectopic vs HBD+, 0.03 (negligible)]. Moreover, none of the averages of the medians were significantly different from each other (Fig. 4B2, inset; KW test: H = 0.15, p = 0.93). As a result of the increase in the prevalence of excitatory input and the relatively unchanged inhibitory input, the ratio of the excitatory-to-inhibitory frequency of events was significantly increased in both subpopulations, with the strongest effect observed in the ectopic DGCs due to the large increase in sEPSC frequency in this group (Fig. 4B3; ANOVA, F = 449.7, p < 0.0001; Tukey's post hoc: normotopic vs ectopic, p < 0.0001; normotopic vs HBD+, p < 0.0001, ectopic vs HBD+, p < 0.0001).
Finally, for sEPSCs, the combined effect of the increased amplitude and frequency in the ectopic group resulted in a significantly larger total charge transfer, while the increase in the total charge transfer was somewhat mitigated in the HBD+ group because of the more modest increase in the instantaneous frequency (Fig. 4C1; KW test: H = 14.05, p < 0.001; Dunn's post hoc: normotopic vs ectopic, p < 0.01; normotopic vs HBD+, p = 0.06). The lack of substantial changes in the sIPSC amplitudes and frequencies resulted in a relatively unchanged total sIPSC charge transfer for both the ectopic and HBD+ groups (Fig. 4C2; KW test: H = 0.51, p = 0.78). Because of the increase in the charge transfer for sEPSCs with no change in the sIPSC charge transfer, the ratio of the excitatory-to-inhibitory charge transfer was significantly increased in the ectopic DGCs (Fig. 4C3; ANOVA: F = 7.355, p < 0.01; Tukey's post hoc: normotopic vs ectopic, p < 0.01), suggesting that this population is the primary driver of SE-induced functional changes in the DG circuit.
Several additional comparisons between the different groups examined here are warranted. First, the AB normotopic group, despite being generated and maturing in an epileptic brain, was not significantly different from the EB sham group with respect to the overall excitatory-to-inhibitory synaptic drive (compare Figs. 2, 3; for amplitude ratio, ANOVA: F = 8.65, p < 0.0001; Tukey's post hoc: EB sham vs AB normotopic, p = 0.49; for instantaneous frequency ratio, ANOVA: F = 229.0, p < 0.0001; Tukey's post hoc: EB sham vs AB normotopic, p = 0.27; for total charge transfer ratio, ANOVA: F = 8.15, p < 0.0001; Tukey's post hoc: EB sham vs AB normotopic, p = 0.98). Conversely, the AB aberrant population exhibited a dramatic increase in the excitatory-to-inhibitory synaptic drive compared to the EB sham condition (compare Figs. 2, 3; for amplitude ratio, ANOVA: F = 8.65, p < 0.0001; Tukey's post hoc: EB sham vs AB aberrant, p < 0.0001; for instantaneous frequency ratio, ANOVA: F = 229.0, p < 0.0001; Tukey's post hoc: EB sham vs AB aberrant, p < 0.0001; for total charge transfer ratio, ANOVA: F = 8.15, p < 0.0001; Tukey's post hoc: EB sham vs AB aberrant, p < 0.01). When the aberrant population was further examined and separated into ectopic and HBD+ subgroups, it was clear that the overall increase in excitatory-to-inhibitory drive was due almost exclusively to increased excitatory input with little to no change in inhibitory input onto ectopic DGCs, while HBD+ cells had much less of an impact. Both ectopic and HBD+ DGCs exhibited significantly larger sEPSC amplitudes (see Fig. 4A1), but HBD+ DGCs also exhibited larger sIPSC amplitudes (Fig. 4A2) which compensated for the increase in excitation resulting in a ratio that was not different from the EB sham condition (compare Figs. 2A3, Fig. 4A3; ANOVA: F = 28.62, EB sham vs AB HBD+, p > 0.99). On the other hand, the ectopic DGCs exhibited a decrease in sIPSC amplitude, which further exacerbated the overall increase in excitatory input, resulting in a significantly different amplitude ratio (compare Figs. 2A3, 4A3; ANOVA: F = 28.62, EB sham vs AB ectopic, p < 0.0001). Similarly, both ectopic and HBD+ DGCs displayed an increase in the instantaneous frequency of sEPSCs (see Fig. 4B1), with little to no change in the frequency of sIPSCs (see Fig. 4B2), and thus both contributed to the overall increase in the excitatory-to-inhibitory frequency ratio (compare Figs. 2B3, 4B3; ANOVA: F = 220.8, p < 0.0001; Tukey's post hoc: EB sham vs AB ectopic, p < 0.0001; EB sham vs AB HBD+, p < 0.001). However, due to the significantly higher sEPSC frequency in the ectopic DGCs compared to HBD+ DGCs (Fig. 4B3; Tukey's post hoc: AB ectopic vs AB HBD+, p < 0.0001), it is clear that ectopic cells represent the primary drivers of the overall increase in the frequency of excitatory events.
Discussion
Mounting evidence suggests that aberrant integration of neonatally or adult-generated DGCs can promote abnormal network function leading to seizures. Studies using rodent TLE models have reported apparent discrepancies regarding the net impact of seizure-induced DGC neurogenesis, with some reports suggesting that neurogenesis may be protective (Jakubs et al., 2006) and others suggesting it is likely pathological (Jessberger et al., 2007; Kron et al., 2010; Cho et al., 2015; Hosford et al., 2016). A parsimonious explanation of these discrepant findings is that seizure-induced neurogenesis leads to heterogeneous populations of DGCs that exert differential influences on the epileptic dentate gyrus network. To further investigate this heterogeneity, we birthdated both AB and EB DGCs and recorded sEPSCs and sIPSCs in GFP-labeled DGCs after pilocarpine-induced SE. We found that seizure-induced neurogenesis produces distinct populations of DGCs that may be protective or neutral (normotopic) and DGCs that may be pathological (aberrant).
Recording spontaneous synaptic inputs onto DGCs provides insight into the cellular mechanisms underlying the changes in excitatory and inhibitory drive in DG networks. Differences in current amplitudes are typically thought to reflect intrinsic characteristics of the postsynaptic cell, such as receptor number or turnover at the synapse (Katz, 1962; O'Brien et al., 1998; Turrigiano et al., 1998), receptor subunit composition (Swanson et al., 1997; Liu and Cull-Candy, 2002), or channel properties (Benke et al., 1998). While glutamatergic and GABAergic receptor expression levels in DGCs have been shown to be altered by SE in an age- and maturity-dependent matter (Brooks-Kayal et al., 1998; Porter et al., 2006), little is known about the impact of morphology or ectopic migration on postsynaptic neurotransmitter receptors. Our results suggest that receptor expression is differentially regulated in distinct cell populations, with little change in either type of receptor in EB DGCs, but with significant pro-excitatory adaptations in the aberrant AB DGCs, and especially in the ectopic population. Differences in current frequencies are thought to result from changes in the presynaptic cell or network structure, such as the probability of vesicle release or number of synapses (Katz, 1962; Han and Stevens, 2009). Significant cell death, which has been observed after SE, can greatly reduce the number of synaptic contacts (Shao and Dudek, 2005; Thind et al., 2010), while axonal sprouting and synaptogenesis can increase the number. Sprouting of axons from hilar mossy cells and CA3 pyramidal cells, which is a well-characterized form of post-SE reorganization, results in aberrant excitatory synaptic input onto DGCs (Scharfman et al., 2001; Wuarin and Dudek, 2001; Pierce et al., 2007; Zhang et al., 2012; Du et al., 2017). More recently, GABAergic cells have been shown to exhibit axonal sprouting after SE, with new inhibitory synapses being formed on both EB and AB DGCs (Thind et al., 2010; Matthews et al., 2013; Du et al., 2017). Our results suggest that these distinct mechanisms are engaged in a maturity- and cell-specific manner: the reduced sIPSC frequency in EB DGCs is consistent with loss of GABAergic interneurons and inhibitory contacts, but the increased sIPSC frequency in AB DGCs is consistent with axon sprouting or increased firing of surviving inhibitory neurons, perhaps to compensate for the initial neuronal loss. On the other hand, sEPSC frequency is significantly increased only in the AB aberrant population, in both the HBD+ DGCs and even more strongly in the ectopic DGCs, consistent with sprouting of excitatory afferents.
Calculating the ratio of sEPSC-to-sIPSC variables distilled multifaceted changes into one measure that allowed for comparisons across different DGC populations (Zhan et al., 2010). EB cells in sham animals represent the majority of DGCs present in a healthy adult dentate gyrus (Bayer and Altman, 1974) and therefore can be considered as the “normal condition.” Interestingly, we found that normotopic DGCs generated after SE did not exhibit alterations in excitatory-to-inhibitory synaptic drive compared to the normal condition. This finding is in line with previous work in which recordings that were collected primarily from normotopic DGCs suggested that SE-induced increases in adult neurogenesis generated cells that received reduced excitatory input (both in amplitude and/or frequency), which provided a homeostatic mechanism for reducing dentate gyrus excitability (Jakubs et al., 2006; Gao et al., 2015). However, our results strongly suggest that normotopic DGCs comprise a subset of post-SE born DGCs that exhibit relatively normal physiological properties (in line with the normal condition), which are substantively different from their age-matched, aberrantly integrated counterparts. In fact, comparison of both the amplitude and frequency ratios shows that the AB aberrant DGCs have three to four times the relative excitatory-to-inhibitory synaptic drive as the normal condition, which is almost entirely driven by a large increase in excitatory drive in the ectopic DGC population, with little to no change in inhibitory input. An important point is that the ratios presented here are intended to provide a qualitative comparison of the relative excitatory-to-inhibitory balance in the DG network between experimental groups. Because sEPSCs and sIPSCs were not measured in the same cell in these experiments, one cannot know whether all cells in a group experience similar alterations in excitatory-to-inhibitory balance, or whether subsets of cells within a group are differentially affected. In addition, the pharmacologically isolated currents reflect only the influence of neurons that directly synapse onto the DGCs that we recorded and therefore do not capture putative compensatory changes that might have occurred elsewhere within the dentate gyrus network.
The data presented here indicate that ectopic DGCs receive greater pro-excitatory drive in an epileptic network than any other population of DGCs, which implicates ectopic DGCs as drivers of network hyperexcitability in TLE. An elegant study using inducible transgenic alteration of mTOR signaling to disrupt normal development of a minority of postnatal born DGCs provides a biological proof-of-principal for the idea that the aberrant development of a subset of DGCs is sufficient to induce spontaneous seizures (Pun et al., 2012). In support of this, pharmacogenetic ablation of the post-SE born DGCs significantly reduced the later development of spontaneous seizures (Cho et al., 2015; Hosford et al., 2016). One hypothesis is that these aberrantly integrated cells are acting as “hub cells” for initiating or propagating seizure activity. This hypothesis was developed using computational modeling which suggested that a network in which a small number of DGCs are hyperconnected to one another is more effective for promoting seizure-like activity than a network in which all DGCs have increased interconnectivity relative to the control condition (Morgan and Soltesz, 2008). Owing to their role in the epileptic network, these hyperconnected cells were termed hub cells (Morgan and Soltesz, 2008).
Whether hub-like cells actually exist in epileptic dentate gyrus networks is unknown, but if they do, then identifying them and their function is critical for understanding how seizures develop or propagate. Ectopic DGCs are candidates for these potential hub-like cells because of both their increased excitatory input and their relative scarcity within the network. The data we present here provides insight into synaptic inputs, but more work will be required to understand the full implications of this aberrant population on overall network excitability. For example, recent data from Hendricks et al. corroborated earlier evidence of functional recurrent synaptic connections between DGCs in a TLE model, but also suggested that AB DGCs were less likely to contribute to repetitive network activation than their EB counterparts (Hendricks et al., 2017). Critically, ectopic or HBD+ DGCs were not considered in this work. On the other hand, we have previously shown that AB, ectopic DGCs in the pilocarpine-induced TLE model exhibit a less polarized resting membrane potential and a greater firing rate than both their normotopic counterparts and AB DGCs in controls (Althaus et al., 2015). These results are consistent with increased intrinsic excitability and, combined with the enhanced pro-excitatory drive shown here, support the hypothesis that this population serves as hub-like cells to promote seizures in TLE.
Footnotes
The authors declare no competing financial interests.
This work was supported by National Institutes of Health Grants R01NS058585 (to J.M.P.) and R01AG052934 (to G.G.M.). We thank Elizabeth Messenger and J. P. Purtell for help with histology and animal surgeries. Dr. Victor Cazares' suggestion of using effect size was especially helpful in interpreting cumulative histogram data.
The authors declare no competing financial interests.
References
- Althaus AL, Parent JM (2014) Role of adult neurogenesis in seizure-induced hippocampal remodeling and epilepsy. In: Endogenous stem cell-based brain remodeling in mammals, Ed 1 (Junier M-P, Kernie SG, eds), pp 87–104. New York, NY: Springer. [Google Scholar]
- Althaus AL, Sagher O, Parent JM, Murphy GG (2015) Intrinsic neurophysiological properties of hilar ectopic and normotopic dentate granule cells in human temporal lobe epilepsy and a rat model. J Neurophysiol 113:1184–1194. 10.1152/jn.00835.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer SA, Altman J (1974) Hippocampal development in the rat: cytogenesis and morphogenesis examined with autoradiography and low-level X-irradiation. J Comp Neurol 158:55–79. 10.1002/cne.901580105 [DOI] [PubMed] [Google Scholar]
- Benke TA, Lüthi A, Isaac JT, Collingridge GL (1998) Modulation of AMPA receptor unitary conductance by synaptic activity. Nature 393:793–797. 10.1038/31709 [DOI] [PubMed] [Google Scholar]
- Brooks-Kayal AR, Jin H, Price M, Dichter MA (1998) Developmental expression of GABA(A) receptor subunit mRNAs in individual hippocampal neurons in vitro and in vivo. J Neurochem 70:1017–1028. [DOI] [PubMed] [Google Scholar]
- Cho KO, Lybrand ZR, Ito N, Brulet R, Tafacory F, Zhang L, Good L, Ure K, Kernie SG, Birnbaum SG, Scharfman HE, Eisch AJ, Hsieh J (2015) Aberrant hippocampal neurogenesis contributes to epilepsy and associated cognitive decline. Nat Commun 6:6606. 10.1038/ncomms7606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. (1988) Statistical power analysis for the behavioral sciences, Ed 2 Hillsdale, NJ: L. Erlbaum Associates. [Google Scholar]
- Du F, Whetsell WO Jr, Abou-Khalil B, Blumenkopf B, Lothman EW, Schwarcz R (1993) Preferential neuronal loss in layer III of the entorhinal cortex in patients with temporal lobe epilepsy. Epilepsy Res 16:223–233. 10.1016/0920-1211(93)90083-J [DOI] [PubMed] [Google Scholar]
- Du F, Eid T, Lothman EW, Köhler C, Schwarcz R (1995) Preferential neuronal loss in layer III of the medial entorhinal cortex in rat models of temporal lobe epilepsy. J Neurosci 15:6301–6313. 10.1523/JNEUROSCI.15-10-06301.1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du X, Zhang H, Parent JM (2017) Rabies tracing of birthdated dentate granule cells in rat temporal lobe epilepsy. Ann Neurol 81:790–803. 10.1002/ana.24946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao F, Song X, Zhu D, Wang X, Hao A, Nadler JV, Zhan RZ (2015) Dendritic morphology, synaptic transmission, and activity of mature granule cells born following pilocarpine-induced status epilepticus in the rat. Front Cell Neurosci 9:384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han EB, Stevens CF (2009) Development regulates a switch between post- and presynaptic strengthening in response to activity deprivation. Proc Natl Acad Sci USA 106:10817–10822. 10.1073/pnas.0903603106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendricks WD, Chen Y, Bensen AL, Westbrook GL, Schnell E (2017) Short-term depression of sprouted mossy fiber synapses from adult-born granule cells. J Neurosci 37:5722–5735. 10.1523/JNEUROSCI.0761-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hester MS, Danzer SC (2013) Accumulation of abnormal adult-generated hippocampal granule cells predicts seizure frequency and severity. J Neurosci 33:8926–8936. 10.1523/JNEUROSCI.5161-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann G, Balgooyen L, Mattis J, Deisseroth K, Buckmaster PS (2016) Hilar somatostatin interneuron loss reduces dentate gyrus inhibition in a mouse model of temporal lobe epilepsy. Epilepsia 57:977–983. 10.1111/epi.13376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosford BE, Liska JP, Danzer SC (2016) Ablation of newly generated hippocampal granule cells has disease-modifying effects in epilepsy. J Neurosci 36:11013–11023. 10.1523/JNEUROSCI.1371-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houser CR, Esclapez M (1996) Vulnerability and plasticity of the GABA system in the pilocarpine model of spontaneous recurrent seizures. Epilepsy Res 26:207–218. 10.1016/S0920-1211(96)00054-X [DOI] [PubMed] [Google Scholar]
- Jakubs K, Nanobashvili A, Bonde S, Ekdahl CT, Kokaia Z, Kokaia M, Lindvall O (2006) Environment matters: synaptic properties of neurons born in the epileptic adult brain develop to reduce excitability. Neuron 52:1047–1059. 10.1016/j.neuron.2006.11.004 [DOI] [PubMed] [Google Scholar]
- Jessberger S, Zhao C, Toni N, Clemenson GD Jr, Li Y, Gage FH (2007) Seizure-associated, aberrant neurogenesis in adult rats characterized with retrovirus-mediated cell labeling. J Neurosci 27:9400–9407. 10.1523/JNEUROSCI.2002-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B. (1962) The Croonian Lecture - the transmission of impulses from nerve to muscle, and subcellular unit of synaptic action. Proc R Soc Ser B Bio 155:455–477. 10.1098/rspb.1962.0012 [DOI] [Google Scholar]
- Kobayashi M, Buckmaster PS (2003) Reduced inhibition of dentate granule cells in a model of temporal lobe epilepsy. J Neurosci 23:2440–2452. 10.1523/JNEUROSCI.23-06-02440.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kron MM, Zhang H, Parent JM (2010) The developmental stage of dentate granule cells dictates their contribution to seizure-induced plasticity. J Neurosci 30:2051–2059. 10.1523/JNEUROSCI.5655-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krook-Magnuson E, Armstrong C, Bui A, Lew S, Oijala M, Soltesz I (2015) In vivo evaluation of the dentate gate theory in epilepsy. J Physiol 593:2379–2388. 10.1113/JP270056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu SJ, Cull-Candy SG (2002) Activity-dependent change in AMPA receptor properties in cerebellar stellate cells. J Neurosci 22:3881–3889. 10.1523/JNEUROSCI.22-10-03881.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews EA, Schoch S, Dietrich D (2013) Tuning local calcium availability: cell-type-specific immobile calcium buffer capacity in hippocampal neurons. J Neurosci 33:14431–14445. 10.1523/JNEUROSCI.4118-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan RJ, Soltesz I (2008) Nonrandom connectivity of the epileptic dentate gyrus predicts a major role for neuronal hubs in seizures. Proc Natl Acad Sci USA 105:6179–6184. 10.1073/pnas.0801372105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy BL, Pun RY, Yin H, Faulkner CR, Loepke AW, Danzer SC (2011) Heterogeneous integration of adult-generated granule cells into the epileptic brain. J Neurosci 31:105–117. 10.1523/JNEUROSCI.2728-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obenaus A, Esclapez M, Houser CR (1993) Loss of glutamate decarboxylase mRNA-containing neurons in the rat dentate gyrus following pilocarpine-induced seizures. J Neurosci 13:4470–4485. 10.1523/JNEUROSCI.13-10-04470.1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien RJ, Kamboj S, Ehlers MD, Rosen KR, Fischbach GD, Huganir RL (1998) Activity-dependent modulation of synaptic AMPA receptor accumulation. Neuron 21:1067–1078. 10.1016/S0896-6273(00)80624-8 [DOI] [PubMed] [Google Scholar]
- Overstreet-Wadiche LS, Bromberg DA, Bensen AL, Westbrook GL (2006) Seizures accelerate functional integration of adult-generated granule cells. J Neurosci 26:4095–4103. 10.1523/JNEUROSCI.5508-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathak HR, Weissinger F, Terunuma M, Carlson GC, Hsu FC, Moss SJ, Coulter DA (2007) Disrupted dentate granule cell chloride regulation enhances synaptic excitability during development of temporal lobe epilepsy. J Neurosci 27:14012–14022. 10.1523/JNEUROSCI.4390-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Z, Zhang N, Wei W, Huang CS, Cetina Y, Otis TS, Houser CR (2013) A reorganized GABAergic circuit in a model of epilepsy: evidence from optogenetic labeling and stimulation of somatostatin interneurons. J Neurosci 33:14392–14405. 10.1523/JNEUROSCI.2045-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce JP, Punsoni M, McCloskey DP, Scharfman HE (2007) Mossy cell axon synaptic contacts on ectopic granule cells that are born following pilocarpine-induced seizures. Neurosci Lett 422:136–140. 10.1016/j.neulet.2007.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter BE, Cui XN, Brooks-Kayal AR (2006) Status epilepticus differentially alters AMPA and kainate receptor subunit expression in mature and immature dentate granule neurons. Eur J Neurosci 23:2857–2863. 10.1111/j.1460-9568.2006.04839.x [DOI] [PubMed] [Google Scholar]
- Pun RY, Rolle IJ, Lasarge CL, Hosford BE, Rosen JM, Uhl JD, Schmeltzer SN, Faulkner C, Bronson SL, Murphy BL, Richards DA, Holland KD, Danzer SC (2012) Excessive activation of mTOR in postnatally generated granule cells is sufficient to cause epilepsy. Neuron 75:1022–1034. 10.1016/j.neuron.2012.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman HE, McCloskey DP (2009) Postnatal neurogenesis as a therapeutic target in temporal lobe epilepsy. Epilepsy Res 85:150–161. 10.1016/j.eplepsyres.2009.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman HE, Goodman JH, Sollas AL (2000) Granule-like neurons at the hilar/CA3 border after status epilepticus and their synchrony with area CA3 pyramidal cells: functional implications of seizure-induced neurogenesis. J Neurosci 20:6144–6158. 10.1523/JNEUROSCI.20-16-06144.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman HE, Smith KL, Goodman JH, Sollas AL (2001) Survival of dentate hilar mossy cells after pilocarpine-induced seizures and their synchronized burst discharges with area CA3 pyramidal cells. Neuroscience 104:741–759. 10.1016/S0306-4522(01)00132-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao LR, Dudek FE (2005) Changes in mIPSCs and sIPSCs after kainate treatment: evidence for loss of inhibitory input to dentate granule cells and possible compensatory responses. J Neurophysiol 94:952–960. 10.1152/jn.01342.2004 [DOI] [PubMed] [Google Scholar]
- Shao LR, Dudek FE (2011) Repetitive perforant-path stimulation induces epileptiform bursts in minislices of dentate gyrus from rats with kainate-induced epilepsy. J Neurophysiol 105:522–527. 10.1152/jn.00456.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson GT, Kamboj SK, Cull-Candy SG (1997) Single-channel properties of recombinant AMPA receptors depend on RNA editing, splice variation, and subunit composition. J Neurosci 17:58–69. 10.1523/JNEUROSCI.17-01-00058.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thind KK, Yamawaki R, Phanwar I, Zhang G, Wen X, Buckmaster PS (2010) Initial loss but later excess of GABAergic synapses with dentate granule cells in a rat model of temporal lobe epilepsy. J Comp Neurol 518:647–667. 10.1002/cne.22235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrigiano GG, Leslie KR, Desai NS, Rutherford LC, Nelson SB (1998) Activity-dependent scaling of quantal amplitude in neocortical neurons. Nature 391:892–896. 10.1038/36103 [DOI] [PubMed] [Google Scholar]
- Walter C, Murphy BL, Pun RY, Spieles-Engemann AL, Danzer SC (2007) Pilocarpine-induced seizures cause selective time-dependent changes to adult-generated hippocampal dentate granule cells. J Neurosci 27:7541–7552. 10.1523/JNEUROSCI.0431-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wuarin JP, Dudek FE (2001) Excitatory synaptic input to granule cells increases with time after kainate treatment. J Neurophysiol 85:1067–1077. 10.1152/jn.2001.85.3.1067 [DOI] [PubMed] [Google Scholar]
- Zhan RZ, Nadler JV (2009) Enhanced tonic GABA current in normotopic and hilar ectopic dentate granule cells after pilocarpine-induced status epilepticus. J Neurophysiol 102:670–681. 10.1152/jn.00147.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan RZ, Timofeeva O, Nadler JV (2010) High ratio of synaptic excitation to synaptic inhibition in hilar ectopic granule cells of pilocarpine-treated rats. J Neurophysiol 104:3293–3304. 10.1152/jn.00663.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Yamawaki R, Wen X, Uhl J, Diaz J, Prince DA, Buckmaster PS (2009) Surviving hilar somatostatin interneurons enlarge, sprout axons, and form new synapses with granule cells in a mouse model of temporal lobe epilepsy. J Neurosci 29:14247–14256. 10.1523/JNEUROSCI.3842-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Huguenard JR, Buckmaster PS (2012) Increased excitatory synaptic input to granule cells from hilar and CA3 regions in a rat model of temporal lobe epilepsy. J Neurosci 32:1183–1196. 10.1523/JNEUROSCI.5342-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao S, Ting JT, Atallah HE, Qiu L, Tan J, Gloss B, Augustine GJ, Deisseroth K, Luo M, Graybiel AM, Feng G (2011) Cell type-specific channelrhodopsin-2 transgenic mice for optogenetic dissection of neural circuitry function. Nat Methods 8:745–752. 10.1038/nmeth.1668 [DOI] [PMC free article] [PubMed] [Google Scholar]