Abstract
Background:
Our lab has previously found that structural integrity in tracts from the raphe nucleus (RN) to the amygdala, measured by fractional anisotropy (FA), predicts remission to selective serotonin reuptake inhibitors (SSRIs) in major depressive disorder (MDD). This could potentially serve as a biomarker for remission that can guide clinical decision-making. To enhance repeatability and reproducibility, we replicated our study in a larger, more representative multi-site sample.
Methods:
64 direction DTI was collected in 144 medication-free patients with MDD from the Establishing Moderators and Bio signatures of Antidepressant Response for Clinical Care (EMBARC) study. We performed probabilistic tractography between the RN and bilateral amygdala and hippocampus and calculated weighted FA in these tracts. Patients were treated with either sertraline or placebo, and their change in Hamilton Depression Rating Scale (HDRS) score reported. Pretreatment weighted FA was compared between remitters and non-remitters, and correlation between FA and percent change in HDRS score was assessed. Exploratory moderator and voxel analyses were also performed.
Results:
Contrary to our hypotheses, FA was greater in nonremitters than in remitters in RN-left and right amygdala tracts (p = 0.02 and 0.01, respectively). Pretreatment FA between the raphe and left amygdala correlated with greater, not reduced, HDRS (r = 0.18, p = 0.04). This finding was found to be greater in the placebo group. Moderator and voxel analyses yielded no significant findings.
Conclusions:
We found greater FA in nonremitters between the RN and amygdala than in remitters, and a correlation between FA and symptom worsening, particularly with placebo. These findings may help reveal more about the nature of MDD, as well as guide research methods involving placebo response.
Keywords: Diffusion tensor imaging, Amygdala, Raphe nucleus, Fractional anisotropy, Remission, SSRI
1. Introduction
Major depressive disorder (MDD) is a heterogeneous mental disorder with complex etiology and a variety of negative outcomes. Despite being one of the most prevalent diseases globally, extensive research and clinical treatment trials have fallen short of consistently helping patients achieve remission (Katzman et al., 2014). Selective Serotonin Reuptake Inhibitors (SSRIs), the most common MDD treatment, only produce remission in one out of three patients (Rush et al., 2006). Ineffective SSRI treatment can often cause additional impairments due to common side effects such as insomnia, sexual dysfunction, and anxiety (Lam et al., 2012). Moreover, SSRIs often take four weeks or longer to exert antidepressant effects (Montgomery, 1997). Therefore, the ability to accurately predict response prior to treatment would help determine whether an SSRI is appropriate, or whether another form of therapy should be pursued. Such accuracy may result in higher treatment adherence, less patients lost to follow-up, and higher success rates in treating MDD.
A non-invasive, in vivo option that can be used for this purpose is Diffusion Tensor Imaging (DTI), a form of Magnetic Resonance Imaging (MRI) that measures the diffusion of water molecules in the brain (Frodl and Amico, 2014). One variable extracted from DTI is Fractional Anisotropy (FA), which is derived from the calculated mean diffusivity (MD), axial diffusivity (AD), and radial diffusivity (RD) of water molecules (Alexander et al., 2007; Soares et al., 2013). FA is a measure of the directional dependence of water diffusivity, with 0 corresponding to isotropic diffusion in all directions and 1 indicating that water diffuses completely across one axis (i.e., completely anisotropic) (O’Donnell and Westin, 2011). It is thought that high FA corresponds to dense tracts of myelinated WM axons (Du et al., 2014). Low FA values may reflect decreased myelin sheath integrity (Heckel et al., 2015), although it can be influenced by factors such as noise, artifacts, and crossing fibers. DTI can also be used to estimate the location of fiber tracts from one region (seed) to another (target).
Pretreatment FA differences between those who recover with treatment (remitters) and those who do not (non-remitters) may reflect baseline differences in structural integrity in brain regions implicated in treatment efficacy. In line with this assumption, studies have found associations between treatment response (including SSRIs, ketamine, and a selective norepinephrine reuptake inhibitor (SNRI)) and pretreatment FA in regions such as the cingulum, stria terminalis (Grieve et al., 2016; Korgaonkar et al., 2014), and frontal cortex (Vasavada et al., 2016). These regions are connected to areas known to be affected in MDD, such as the subgenual anterior cingulate cortex (Vergani et al., 2016) and amygdala (Kruger et al., 2015). However, a clinically viable marker for treatment response using DTI has not yet been validated and has only been observed in small patient samples with small effect sizes.
SSRIs’ initial chief mechanism of action involves blocking the serotonin transporter (5-HTT), preventing reuptake of serotonin and prolonging its action in the synapse (Fuller and Wong, 1977). Therefore, the efficacy of SSRIs can be affected by the overall health of the serotonin system. The principal source of serotonin in the central nervous system is the raphe nucleus (RN) (Hornung, 2003), found in the midbrain, pons, and medulla. Serotonergic fibers project from this region to nearly all areas of the brain. Therefore, FA between the RN and these regions can serve as an indication of serotonergic integrity. The amygdala is a logical choice of target: it is known to play a central role in emotional processing (LeDoux, 2003), and a decrease in serotonin levels is thought to result in over activation of emotional circuitry involving this region (Phillips et al., 2015). Antidepressant treatment has been shown to normalize such hyperactivity (Sheline et al., 2001)—thus, it has been of interest in MDD pathophysiology.
We have previously used DTI to locate tracts between the RN and the amygdala, as well as the hippocampus, which has been shown to be associated with volume loss in MDD (Schmaal et al., 2016). In this previous study, we showed that, prior to treatment, average FA in the tracts between the RN and right amygdala were significantly lower in non-remitters versus remitters (tracts between RN and left amygdala showed a marginally significant difference), while average FA in the tracts between the RN and hippocampus did not differ between those who did and did not remit (Delorenzo et al., 2013). This makes average FA in the tracts between the RN and amygdala a potential bio signature of antidepressant response. However, this was a preliminary study with a sample size of 18 patients, which may not reflect diffusion patterns in a larger cohort.
These issues can be readily addressed through the Establishing Moderators and Bio signatures of Antidepressant Response for Clinical Care (EMBARC) study (Trivedi et al., 2016). This large, multi-site study included over 140 participants who underwent 64-direction DTI prior to controlled antidepressant treatment. Thus, it provides ample resolution and statistical power to validate our preliminary findings. In this placebo-controlled MDD treatment sample, one of the largest to date with DTI imaging, we aim to replicate our finding that RN- amygdala connectivity predicts SSRI remission, while RN-hippocampus connectivity does not. We hypothesize this prediction will hold for both left and right amygdala. To analyze the role FA plays in clinical remission, we also examine whether FA in these tracts is a moderator of treatment response (i.e., a variable that influences the relationship between treatment assignment and remission) (Baron and Kenny, 1986). Finally, other studies have shown differences between remitters and non-remitters in other regions such as frontal cortex, cingulum, and stria terminalis (Alexopoulos et al., 2002, 2008; Grieve et al., 2016; Korgaonkar et al., 2014; Taylor et al., 2008). As there is no consensus, and to examine these and other regions, we perform an exploratory voxel-wise analysis to detect FA differences between remitters and non-remitters outside our a priori hypotheses, as our wellpowered sample may allow us to find such differences across the whole brain. Positive results would not only provide a tool for clinical use, but also shed light on the pathophysiology of MDD.
2. Methods and materials
2.1. Participants
Data for all participants were taken from the EMBARC project, which enrolled subjects at University of Michigan, Columbia University Medical Center, Massachusetts General Hospital, and University of Texas Southwestern Medical Center. EMBARC was designed to find pretreatment bio markers of treatment response. All four sites had their protocols reviewed and approved by their Institutional Review Boards, and all participants provided written informed consent.
Eligibility for each participant was determined based on a psychiatric assessment, review of medical history, chart review, clinical interview, physical examination, routine blood tests, pregnancy test, and urine toxicology. Detailed inclusion/exclusion criteria can be found in Trivedi et al. (2016). Briefly, inclusion criteria comprised: (1) age 18–65; (2) met criteria for a major depressive episode according to the Structured Clinical Interview for the DSM IV (First et al., 1995); (3) scored at least 14 on the Quick Inventory of Depressive Symptoms (QIDS-SR) (Rush et al., 2003); and (4) had early onset (< 30 years old) and chronic (> 2 years in duration) MDD or recurrent MDD (2 or more recurrences). The study comprised 309 participants. Of these, 270 had structural MRI images that passed stringent quality control measures and 172 of these had DTI images that passed strict quality control measures, examined by trained technicians (see below). To ensure that remission was clinically significant, the sample was further restricted to patients with a Hamilton Depression Rating Scale (HDRS) 17-item (Hamilton, 1960) score of 15 or greater at baseline, as there is evidence that response correlates with depression severity and in mild depression there is a failure to differentiate placebo from drug response (Fournier et al., 2010). This final set comprised 144 patients (Table 1) Participants were classified as remitters if their final HDRS scores were less than or equal to 7 after 8 weeks of treatment on either sertraline or placebo; if these criteria were not met, they were classified as non-remitters. In addition, patients were considered responders if their HDRS score improved by at least 50% over 8 weeks and non-responders if they did not.
Table 1.
Patient demographics separated by treatment condition.
| Variable | Level | Totala | Placebo | Sertraline | P-valueb |
|---|---|---|---|---|---|
| Age | All participants (74 placebo vs 70 sertraline) | 37.27 ± 13.71 | 36.38 ± 13.36 | 38.21 ± 14.10 | 0.42 |
| Sex | Female | 90 (62.50%) | 43 (47.78%) | 47 (52.22%) | 0.26 |
| Male | 54 (37.50%) | 31 (57.41%) | 23 (42.59%) | ||
| Race | American Indian/Alaska Native | 1 (0.69%) | 1 (100.00%) | 0 (0.00%) | 0.86 |
| Asian | 11 (7.64%) | 7 (63.64%) | 4 (36.36%) | ||
| Black or African American | 24 (16.67%) | 12 (50.00%) | 12 (50.00%) | ||
| Other | 9 (6.25%) | 5 (55.56%) | 4 (44.44%) | ||
| White | 99 (68.75%) | 49 (49.49%) | 50 (50.51%) | ||
| Hispanic | No | 120 (83.33%) | 61 (50.83%) | 59 (49.17%) | 0.77 |
| Yes | 24 (16.67%) | 13 (54.17%) | 11 (45.83%) | ||
| Site | CU | 46 (31.94%) | 22 (47.83%) | 24 (52.17%) | 0.76 |
| MG | 31 (21.53%) | 18 (58.06%) | 13 (41.94%) | ||
| TX | 43 (29.86%) | 23 (53.49%) | 20 (46.51%) | ||
| UM | 24 (16.67%) | 11 (45.83%) | 13 (54.17%) | ||
| MDD severityc | High | 74 (51.39%) | 36 (48.65%) | 38 (51.35%) | 0.50 |
| Low | 70 (48.61%) | 38 (54.29%) | 32 (45.71%) | ||
| MDD chronicityd | Chronic | 73 (50.69%) | 34 (46.58%) | 39 (53.42%) | 0.24 |
| Non chronic | 71 (49.31%) | 40 (56.34%) | 31 (43.66%) | ||
| Remitter | No | 91 (63.19%) | 49 (53.85%) | 42 (46.15%) | 0.44 |
| Yes | 53 (36.81%) | 25 (47.17%) | 28 (52.83%) | ||
| Week 0 HDRS score | All participants | 19.83 ± 3.59 | 19.55 ± 3.49 | 20.11 ± 3.70 | 0.35 |
| Week 8 HDRS score | All participants | − 8.38 ± 7.36 | − 7.62 ± 7.30 | − 9.17 ± 7.40 | 0.21 |
| % Change in HDRS score | All participants | − 42 ± 37 | − 40 ± 38 | − 45 ± 36 | 0.43 |
| Weighted FA between raphe and left amygdala | All participants | 0.42 ± 0.04 | 0.42 ± 0.03 | 0.43 ± 0.04 | 0.43 |
| Weighted FA between raphe and right amygdala | All participants | 0.43 ± 0.04 | 0.42 ± 0.04 | 0.43 ± 0.04 | 0.21 |
| Weighted FA between raphe and left hippocampus | All participants | 0.44 ± 0.05 | 0.43 ± 0.05 | 0.44 ± 0.05 | 0.22 |
| Weighted FA between raphe and right hippocampus | All participants | 0.45 ± 0.05 | 0.44 ± 0.05 | 0.46 ± 0.05 | 0.03e |
| Concurrent medication use | Yes | 78 (54.17%) | 39 (50%) | 39 (50%) | 0.74 |
| No | 66 (45.83%) | 35 (53.03%) | 31 (46.97%) |
Total displayed: mean ± SD for continuous variables; N (%) for categorical variables.
For continuous variables, p-values were calculated from Welch’s t-tests; for categorical variables, exact p-values were calculated from the Monte Carlo simulation. All p-values are calculated between placebo and sertraline groups.
Defined by HDRS ≥ 20 and QIDS-SR of ≥ 16.
Chronic is defined as > 2 years.
Not significant after multiple comparisons correction.
2.2. MRI acquisition
MRI scans were performed at Columbia University Medical Center (GE Signa HDx), Massachusetts General Hospital (Siemens Trio Tim), University of Michigan (Phillips Ingenia), or University of Texas Southwestern Medical Center (Phillips Achieva). Structural MRIs were acquired using the following parameters: TR (repetition time):5.9–8.2 ms, TE (echo time): 2.4–3.7 ms, Flip Angle: 9°–12°, voxel dimensions: 1 mm × 1 mm × 1 mm, acquisition matrix: 240 × 240, 256 × 243 or 256 × 256, acceleration factor: 2, and 174–178 sagittal slices. Scan parameters can be found in Supplementary Table 1, and have been published previously (Fournier et al., 2017). Diffusion images were acquired using a single-shot EPI (echo planar imaging) sequence. Scan parameters were as follows: TR=8310–9500 ms, TE=95–96.3 ms, Flip Angle= 90°, voxel dimensions = 2.5 mm ×2.5 mm × 2.5 mm or 1.9 mm× 1.9 mm × 2.5 mm, acquisition matrix = 96 × 96, b value = 1000 s/mm2, and 64 diffusion directions with 1 or 5 non-weighted images (b = 0). DTI acquisition time was approximately 10 min. These parameters were selected prior to data acquisition to standardize acquisition between scanners as much as possible (Zhu et al., 2011). To adjust for any residual effects, site was also included as a covariate.
2.3. MRI processing
T1-weighted structural MRI images were assessed for quality and processed at Stony Brook University by trained technicians. Quality inspection included checking for common artifacts such as field inhomogeneity and poor skull stripping. Images were approved if the amygdala, hippocampus, and brainstem (all regions used in this work) were devoid of artifacts that would interfere with region identification.
Structural MRIs were processed using Free surfer 5.3 (http://surfer.nmr.mgh.harvard.edu/). Amygdala and hippocampus ROIs were derived from Free surfer’s atlases. The RN ROI was derived from a previous sample from our laboratory, in which 52 healthy controls were scanned using the Positron Emission Tomography (PET) tracer [11C]-WAY100635, a serotonin 1A antagonist (Forster et al., 1995). Voxel binding maps were calculated for each subject and warped into MNI space. A threshold binding potential of 18 mL/cc was then applied to the midbrain region of these images to differentiate the RN, as this area has higher serotonin 1A binding than surrounding regions. This RN ROI was then inverse-warped to individual MRIs using Advanced Normalization Tools (ANTs) (Avants et al., 2011). We have used this method to delineate the RN previously (Delorenzo et al., 2013).
2.4. DTI processing
DTI images were also assessed for quality and processed at Stony Brook University. Diffusion weighted images were assessed for quality by trained technicians to check for artifacts such as venetian blind, gradient-wise motion, ghost, ringing, and slice-wise intensity artifacts (Liu et al., 2010). Images were approved if the ventral area encompassing the amygdala, hippocampus, and brainstem was devoid of artifacts that would interfere with probabilistic tractography. A relatively large number of studies were excluded in this process (58) for reasons stated above, due in part to the fact that DTI is sensitive to small motions. When demographics, HDRS scores, and remission status was compared between patients with approved DTI and HDRS score > 15 (144 total) and those with HDRS score > 15 who did not have approved DTI, no significant differences were found.
Images were corrected for motion and gradient coil-induced distortions using the eddy current correction routine in FSL (FMRIB Software Library, http://www.fmrib.ox.ac.uk/fsl/). Non brain tissue was removed through FSL’s Brain Extraction Tool. After this, FA was computed in Camino (http://web4.cs.ucl.ac.uk/research/medic/camino/) using the least squares fit diffusion tensor with nonlinear optimization using a Levenburg–Marquardt algorithm, constrained to positive values by fitting its Cholesky decomposition (Alexander and Barker, 2005).
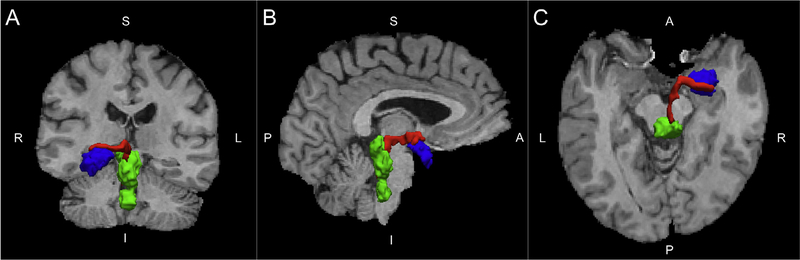
Probabilistic Tractography was performed using the FMRIB Diffusion Toolbox (FDT) (Behrens et al., 2007). FDT computes streamlines through each voxel by repeated sampling from each principal diffusion direction. This method yields the probability of connections from the seed (RN) to post-synaptic targets in the brain (amygdala and hippocampus). FDT was run with 5000 samples, a maximum of 2000 steps per sample, a step length of 0.5 mm, and a tract curvature threshold of 0.2 mm. We used this to compute the weighted average FA within the subject-specific tractography-defined tracts from the RN to the amygdala and hippocampus (Fig. 1). To include only voxels with a high probability of being in the defined tract, weighted average FA was calculated by multiplying voxel-based FA measures by the probability of connection at each voxel, summing the products, and dividing by the sum of the probabilities (Bonnelle et al., 2012; Hagler et al., 2009; Hua et al., 2008). Similar weighting procedures were used for mean, radial, and axial diffusivity.
Fig. 1.
Coronal (A), Sagittal (B), and axial (C) images of a representative tract (red) from the raphe nucleus (green) to the amygdala (blue). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
2.5. Statistics
All p-values for demographics comparisons were derived using Monte-Carlo simulations (Agresti et al., 1979). A linear mixed model was used to compare weighted FA between remitters and non-remitters after co varying for age, sex, site, and treatment (placebo vs. sertraline). The two-way interaction term among the remission measures and regions was considered in order to account for possible different region specific relationships in the left and right amygdala and hippocampus. This model yields many more samples to estimate residual errors, increasing detection power and allowing us to measure both region-specific effects and group differences between regions. Akaike Information Criteria (AIC) was used when selecting dependence structures for modeling the correlation among imaging measures from different regions but the same patient. Possible covariance structures considered were unstructured (UN), compound symmetry (CS), heterogeneous CS (CSH), Toeplitz (TOEP); CSH was selected according to AIC. Similar models were used to test mean, radial, and axial diffusivity. To test for correlations of FA with decrease in MDD symptoms, a similar linear mixed model was used, but with percent change in HDRS score as an outcome measure, defined as:
By this formulation, an x value of 0 indicates no change, a value < 0 indicates improvement, and a value > 0 indicates worsening. Note that this is the opposite as our pilot study (Delorenzo et al., 2013), where positive values indicate improvement—we performed the analysis this way to denote a change from baseline in any direction. Partial correlation coeffcients were calculated to quantify the correlation between FAs and decrease in MDD symptoms after removing possible confounding effects of age, sex and site through the linear mixed model.
Multiple linear regression models and multiple logistic regression models were also fitted to test if tracts from the raphe to the left amygdala or right amygdala are treatment response moderators by examining the interaction term between FA values and treatment groups. Possible covariates included age, sex and site, which were significantly related to treatment response in the univariate data analysis. Statistical significance was set at 0.05. Mixed model analysis was performed using SAS 9.4 (SAS Institute Inc., Cary, NC). Multiple comparisons were corrected for via the Bonferroni method for the left and right amygdala.
2.6. Voxel analysis
An exploratory voxel analysis of FA differences was carried out using SPM8 (http://www.fil.ion.ucl.ac.uk/spm/) – this test compared FA voxel values across all subjects in standard space using a full factorial model with age, sex, and site added as covariates. Cluster size was thresholded at four voxels, and family-wise error correction was used. For this analysis, only participants for whom MRI and DTI processing for all regions had been approved were included—this yielded a smaller sample than our a priori analyses, which only required approval of amygdala, hippocampus, and RN. The sample comprised 98 participants. A significance threshold of p < 0.05 family-wise error corrected threshold was used.
3. Results
3.1. Fractional anisotropy in remitters and non-remitters
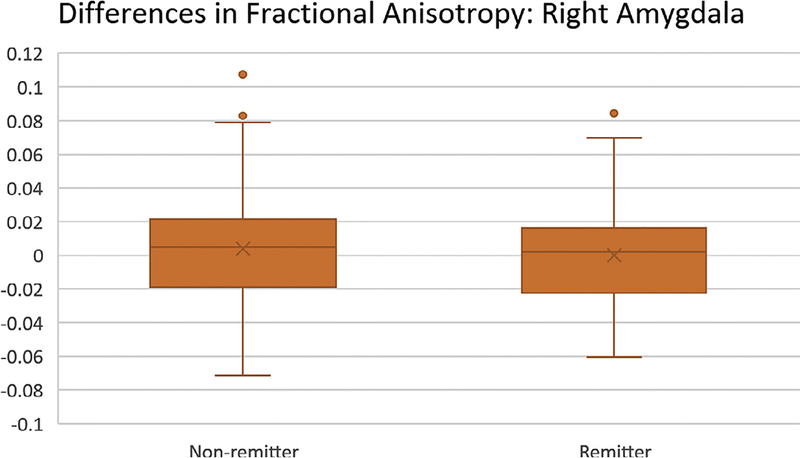
All comparisons were performed across both placebo and sertraline groups unless stated otherwise. Group wise comparisons of remitters vs. non-remitters showed significant differences in fractional anisotropy (FA) in tracts from the raphe nucleus (RN) to both left and right amygdala (p = 0.02 and 0.01, respectively, Bonferroni corrected; see Fig. 2). No significant difference was found in tracts from RN to left (p = 0.16, uncorrected) or right (p = 0.82, uncorrected) hippocampus. Average difference in FA between groups was 0.02 in the left and right amygdala, 0.01 in the left hippocampus, and less than 0.01 in the right hippocampus (Table 2)—in all cases, remitters had lower FA than non-remitters.
Fig. 2.
Boxplot of residual fractional anisotropy (FA) after taking age, sex, and site into account. Group means are demarcated by the “X”. Right amygdala is plotted as this is the region with the greatest difference between remitters and non-remitters.
Table 2.
Comparison of DTI measures between remitters and non-remittersa.
| Seed to target pair | Average FA (Remitters) | Average FA (Non-remitters | Average FA Differenceb | 95% CI lower | 95% CI upper | Cohen’s D | P-value |
|---|---|---|---|---|---|---|---|
| Raphe to left amygdala | 0.41 | 0.43 | −0.02 | − 0.03 | > −0.01 | 0.39 | 0.02* |
| Raphe to right amygdala | 0.41 | 0.43 | −0.02 | − 0.03 | − 0.01 | 0.54 | 0.01* |
| Raphe to left hippocampus | 0.43 | 0.44 | −0.01 | − 0.03 | <0.01 | 0.29 | 0.16 |
| Raphe to right hippocampus | 0.45 | 0.45 | < −0.01 | − 0.02 | 0.01 | 0.10 | 0.82 |
DTI: diffusion tensor imaging, FA: fractional anisotropy, CI: confidence interval. P-values are from linear mixed model and are Bonferroni corrected for left and right amygdala. Differences and p-values were calculated from linear mixed model after adjusting for age, gender and study site.
Difference is calculated as mean FA(remitters) – mean FA(non-remitters).
p < 0.05.
Similar analysis performed between responders and non-responders revealed no significant differences in tracts from the RN to the left or right amygdala (p = 0.29 and 0.18, respectively, uncorrected), or to the left or right hippocampus (p = 0.69 and >0.99, respectively, uncorrected, Supplementary Table 2).
3.2. Mean, radial, and axial diffusivity between remitters and non-remitters
Neither mean diffusivity, nor radial diffusivity, nor axial diffusivity differed significantly between remitters and non-remitters, or between responders and non-responders in any of the tracts examined. Moreover, all differences in means for these measures were less than 0.00001 (Supplemental Tables 3–5).
3.3. Moderator analysis of left amygdala and right amygdala
No interaction term between FA values and treatment group (sertraline vs. placebo) was significant, i.e., there is no evidence that FA influences the relationship between treatment group and remission (p = 0.59 and 0.95 for tracts terminating in the left and right amygdala, respectively). Similarly, there was no significant treatment interaction in the relative change in HDRS score for left or right amygdala (p = 0.27 and 0.91, respectively).
3.4. Correlation of fractional anisotropy with symptom improvement
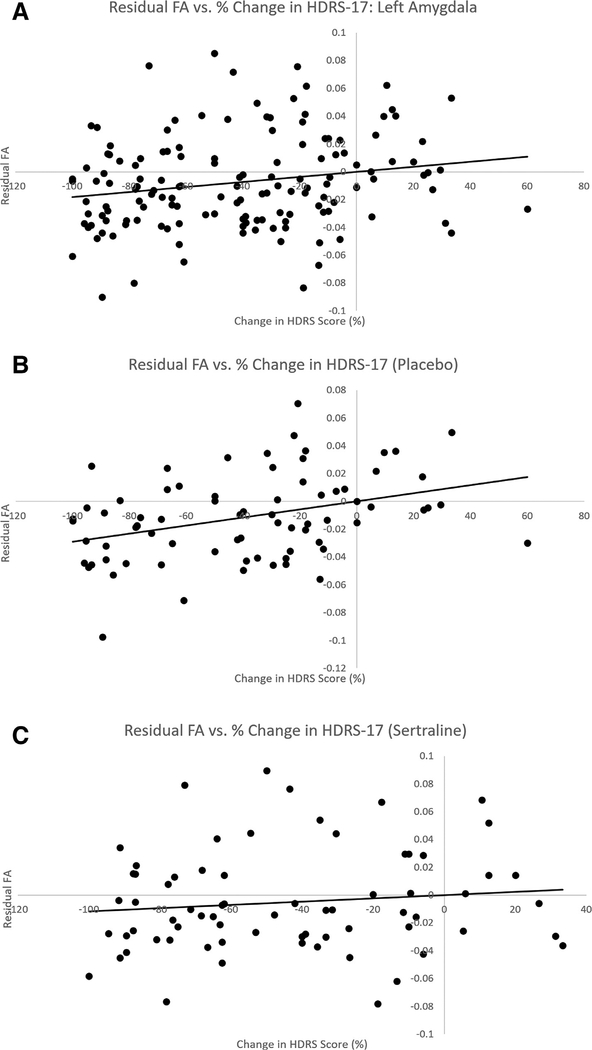
When the correlation between average FA in the defined tracts and percent change in HDRS was examined, a significant positive correlation was found in average FA of tracts connecting the RN to the left amygdala (r = 0.18, p = 0.04, Bonferroni corrected)—that is, a higher FA was associated with increased symptom severity, while low FA was associated with symptom improvement. There was no significant correlation between HRDS and average FA in the tracts between the RN and right amygdala (r = 0.09, p = 0.42), left hippocampus (r = 0.05, p = 0.44), or right hippocampus (r = −0.05, p = 0.69; see Table 3). Subgroup analysis showed that this correlation between average FA in tracts from the RN to left amygdala and depression severity was only significant in the placebo group (r = 0.32, p = 0.02), and not the sertraline group (r = 0.06, p = 0.39, see Fig. 3). However, these independent correlations were not significantly different (Z = 1.59, ns).
Table 3.
Correlation of fractional anisotropy with percent change in HDRS.
| Seed to target pair | Partial correlation coefficient | 95% CI lower | 95% CI upper | P-value |
|---|---|---|---|---|
| Raphe to left amygdala | 0.18 | 0.01 | 0.33 | 0.04* |
| Raphe to right amygdala | 0.09 | − 0.08 | 0.25 | 0.42 |
| Raphe to left hippocampus | 0.05 | − 0.12 | 0.21 | 0.44 |
| Raphe to right hippocampus | − 0.05 | − 0.22 | 0.11 | 0.69 |
HDRS: Hamilton Depression Rating Scale, CI: confidence interval. P-values are from linear mixed model and are Bonferroni corrected for left and right amygdala. Partial correlation coeffcients were calculated from linear mixed model after adjusting for age, sex and study site.
p < 0.05.
(p=0.59 and 0.95 for tracts terminating in the left and right amygdala, respectively). Similarly, there was no significant treatment interaction in the relative change in HDRS score for left or right amygdala (p=0.27 and 0.91, respectively).
Fig. 3.
Output of linear mixed models relating change in symptom severity to fractional anisotropy (FA) between the raphe nucleus and left amygdala for all participants (A), placebo group only (B), and sertraline group only (C). The placebo group drives the overall correlation. None of other correlations examined were significantly different. HDRS-17: Hamilton Depression Rating Scale, 17 Item.
3.5. Exploratory voxel analysis
An exploratory voxel analysis found no significant differences in FA between remitters and non-remitters, including in previously delineated regions of frontal cortex, cingulum, and striaterminalis (Alexopoulos et al., 2002, 2008; Grieve et al., 2016; Korgaonkar et al., 2014; Taylor et al., 2008).
4. Discussion
Successful replication in neurophysiological research has often proven elusive, and there is a broad effort in the scientific community to address this (Kappenman and Keil, 2017). As part of this effort, we sought to replicate our pilot study in a larger, more representative sample. Similar to our previous study, we found a significant difference in FA between remitters and non-remitters, but we found the opposite pattern; rather than positively correlating with symptom improvement, fractional anisotropy (FA) in the tracts between the raphe nucleus (RN) and amygdala positively correlated with symptom worsening, and nonremitters had higher FA between the raphe and amygdala than remitters. A reversal such as this may have been due to the effect size—with a correlation coeffcient of 0.18, our previous study of 18 participants was likely underpowered and may have found a positive correlation by chance. As our present study has nearly ten times the number of participants, our statistical power is greatly improved. There are some differences in study design from our preliminary analysis: unlike our previous study, this was a placebo-controlled trial (although examination of the SSRI arm alone revealed no significant association with remission or symptom improvement). This also represents a difference from most clinical scenarios, where a patient is aware of what medicine they are receiving. In addition, while our previous study used a combined midbrain and RN ROI, we used the raphe by itself for our current analysis as our improved DTI resolution allowed us to explore this region. When we repeated the analysis with the combined ROI, however, we replicated our current results (data not shown). It is important to note that DTI cannot provide contrast for brainstem nuclei in of itself—therefore the integrity of the region depends on its quality of delineation from PET studies. We have used this method previously (Delorenzo et al., 2013). While an MRI-based method would be valuable, this has not yet been developed. One advantage of our PET based method is that it allows the raphe delineation to be subject-specific.
While this finding is in contrast to studies showing higher pretreatment FA in frontal cortex in remitters (Alexopoulos et al., 2002, 2008) our results resemble those of a similar analysis performed in frontal cortex in MDD patients—in that study, higher FA in the superior frontal gyrus and anterior cingulate was associated with a poorer outcome to sertraline (Taylor et al., 2008). Another study found that higher FA in the stria terminalis predicted non-remission, while higher FA in the cingulum predicted remission (Korgaonkar et al., 2014). This was replicated when the authors used the ratio of cingulum to stria terminalis binding to predict remission vs. non-remission (Grieve et al., 2016). While we were not able to replicate these findings in our own voxel analysis, the stria terminalis is particularly relevant in that it contains efferent fibers from the amygdala (Wakana et al., 2004). Given findings of increased amygdala activity in MDD (Sheline et al., 2001), it may be that connectivity to this region is detrimental to treatment. However, no significant differences in FA were observed between remitter and non-remitter in our exploratory whole-brain voxel analysis, suggesting that the effect size may not be large enough to withstand multiple corrections in an exploratory analysis. Indeed, the difference between groups in FA between the RN and amygdala was only 0.02.
While these findings cannot be applied on an individual basis at this time, our study reveals group-level findings that may give some insight into pathophysiology. Our previous hypothesis was that patients with higher FA would show greater clinical improvement, possibly by capitalizing on more robust serotonergic pathways. Our current results suggest that the opposite may be true. One possibility for this may be that SSRIs are more beneficial for patients who do not have robust serotonergic fibers, and that these drugs act to enhance these pathways. Longitudinal analysis of FA in these pathways would be needed to support this hypothesis.
A previous meta-analysis examined factors that predicted remission with placebo—these included patients with less severe MDD (lower Hamilton Depression Rating Scale scores), younger age, less anxiety, and shorter MDD episode duration (Nelson et al., 2012). Our placebo group happened to be slightly younger, have slightly lower HDRS scores, and have a smaller proportion with chronic MDD (Table 1). Despite the fact that none of these reached significance, they may have individually contributed to the lack of differences in outcomes among treatment groups.
Despite the moderate effect sizes, the absolute difference between groups was small and the predictive value of FA in these regions was only slightly better than chance (area under the curve using ten rounds of five-fold cross-validation was 0.57, while random chance would be 0.5—data not shown). In addition, there appears to be no moderating effect of FA either on remission or on changes in depression severity. Therefore, these findings can be taken as negative on a clinical level, and do not replicate previous results. It should be noted that these findings were negative with and without covariates (data not shown). This parallels previous studies reporting negative findings in FA between patients with MDD and healthy controls (Choi et al., 2014; Olvet et al., 2015). As this study had a large sample size, it raises important limitations about the ability of FA to influence clinical decisions.
Given that well-powered DTI studies have failed to find clinically significant differences between healthy controls and patients with MDD (Choi et al., 2014) or, in our sample, between remitters and non-remitters, research into biomarkers of diagnosis and prognosis may require further design considerations. For example, MDD is a heterogeneous disorder. Such heterogeneity may have confounded the search for a single marker across all subjects. Therefore, a more symptomatic approach, such as studying clinical subtypes or research domain criteria (RDoC, a classification system based on translational neurobiology and observable behavior as opposed to self-reported categories of symptoms) (Cuthbert and Insel, 2013) may reduce these confounding effects. Moreover, many patients were taking concurrent medications. While our study prohibited psychoactive medication in our study, it is possible that other classes of medications had effects on DTI measures that have not yet been studied. In addition, as mentioned, DTI cannot selectively identify neurons by their emitted neurotransmitter or by the direction of transmission. This limits our interpretation of FA findings. While there is currently no in vivo method to select for tracts based on their primary neurotransmitter, a combined PET/DTI study examining binding potential in a seed and target region in conjunction with tract integrity between them may yield valuable information. However, it is important to note that across the EMBARC study a statistically significant difference in outcome between the placebo and sertraline groups in clinical outcome was not observed (Webb et al., 2019), which may have contributed to the lack of predictive power of our study.
In conclusion, in this large multi-site study, we found greater FA in non-remitters between the RN and amygdala than in remitters. Moreover, we found a correlation between FA in the tracts connecting the RN to the left amygdala and symptom worsening. While this measure may not be useful on an individual basis with current technology, these results may help reveal more about the nature of MDD. The use of symptom-specific, RDoC, and multimodal approaches may bring us closer to finding a clinically-useful prognostic marker.
Supplementary Material
Acknowledgments
We acknowledge the bio statistical computation and support provided by the Bio statistical Consulting Core at School of Medicine, Stony Brook University. We would also like to thank the image analysts at Stony Brook University’s Center for Understanding Biology using Imaging Technology (CUBIT) for processing all MRI and DTI data. All Principal Investigators including Madhukar Trivedi, M.D., Patrick McGrath, M.D., Myrna Weissman, M.D., Ramin Parsey, M.D., Ph.D., Maurizio Fava, M.D., were involved with protocol design, data collection, and funding acquisition. The EMBARC study was supported by the National Institute of Mental Health of the National Institutes of Health under award numbers U01MH092221 (Trivedi, M.H.) and U01MH092250 (McGrath, P.J., Parsey, R.V., Weissman, M.M.). Rajapillai Pillai was supported by award number F30MH109412. The funding source had no involvement in research conduct or preparation of this article.
Financial disclosures
Dr. Madhukar H. Trivedi, is or has been an advisor/consultant and received fee from (lifetime disclosure): Abbott Laboratories, Inc., Abdi Ibrahim, Akzo (Organon Pharmaceuticals Inc.), Alkermes, AstraZeneca, Axon Advisors, Bristol-Myers Squibb Company, Cephalon, Inc., Cerecor, CME Institute of Physicians, Concert Pharmaceuticals, Inc., Eli Lilly & Company, Evotec, Fabre Kramer Pharmaceuticals, Inc., Forest Pharmaceuticals, GlaxoSmithKline, Janssen Global Services, LLC, Janssen Pharmaceutica Products, LP, Johnson & Johnson PRD, Libby, Lundbeck, Meade Johnson, MedAvante, Medtronic, Merck, Mitsubishi Tanabe Pharma Development America, Inc., Naurex, Neuronetics, Otsuka Pharmaceuticals, Pamlab, Parke-Davis Pharmaceuticals, Inc., Pfizer Inc., PgxHealth, Phoenix Marketing Solutions, Rexahn Pharmaceuticals, Ridge Diagnostics, Roche Products Ltd., Sepracor, SHIRE Development, Sierra, SK Life and Science, Sunovion, Takeda, Tal Medical/Puretech Venture, Targacept, Transcept, VantagePoint, Vivus, and Wyeth-Ayerst Laboratories. In addition, he has received grants/ research support from: Agency for Healthcare Research and Quality (AHRQ), Cyberonics, Inc., National Alliance for Research in Schizophrenia and Depression, National Institute of Mental Health and National Institute on Drug Abuse.
In the past two years, Dr. Myrna Weissman received funding from the National Institute of Mental Health (NIMH), the Sackler Foundation, the Templeton Foundation; and receives royalties from the Oxford University Press, Perseus Press, the American Psychiatric Association Press, and Multi Health Systems.
Dr. Patrick McGrath has received funding from the National Institute of Mental Health, New York State Department of Mental Hygiene, Research Foundation for Mental Hygiene (New York State), Forest Research Laboratories, Sunovion Pharmaceuticals, and Naurex Pharmaceuticals (now Allergan).
Dr. Maurizio Fava has received research support from Abbot Laboratories; Alkermes, Inc.; American Cyanamid; Aspect Medical Systems; AstraZeneca; Avanir Pharmaceuticals; BioResearch; BrainCells Inc.; Bristol-Myers Squibb; CeNeRx BioPharma; Cephalon; Clintara, LLC; Cerecor; Covance; Covidien; Eli Lilly and Company; EnVivo Pharmaceuticals, Inc.; Euthymics Bioscience, Inc.; Forest Pharmaceuticals, Inc.; Ganeden Biotech, Inc.; GlaxoSmithKline; Harvard Clinical Research Institute; Hoffman-LaRoche; Icon Clinical Research; i3 Innovus/Ingenix; Janssen R&D, LLC; Jed Foundation; Johnson & Johnson Pharmaceutical Research & Development; Lichtwer Pharma GmbH; Lorex Pharmaceuticals; Lundbeck Inc.; MedAvante; Methylation Sciences Inc.; National Alliance for Research on Schizophrenia & Depression (NARSAD); National Center for Complementary and Alternative Medicine (NCCAM); National Institute of Drug Abuse (NIDA); National Institute of Mental Health (NIMH); Neuralstem, Inc.; Novartis AG; Organon Pharmaceuticals; PamLab, LLC.; Pfizer Inc.; Pharmacia-Upjohn; Pharmaceutical Research Associates., Inc.; Pharmavite® LLC; PharmoRx Therapeutics; Photothera; Reckitt Benckiser; Roche Pharmaceuticals; RCT Logic, LLC (formerly Clinical Trials Solutions, LLC); Sanofi-Aventis US LLC; Shire; Solvay Pharmaceuticals, Inc.; Stanley Medical Research Institute (SMRI); Synthelabo; Tal Medical; Wyeth-Ayerst Laboratories; he has served as advisor or consultant to Abbott Laboratories; Acadia; Affectis Pharmaceuticals AG; Alkermes, Inc.; Amarin Pharma Inc.; Aspect Medical Systems; AstraZeneca; Auspex Pharmaceuticals; Avanir Pharmaceuticals; AXSOME Therapeutics; Bayer AG; Best Practice Project Management, Inc.; Biogen; BioMarin Pharmaceuticals, Inc.; Biovail Corporation; Brain Cells Inc; Bristol-Myers Squibb; CeNeRx BioPharma; Cephalon, Inc.; Cerecor; CNS Response, Inc.; Compellis Pharmaceuticals; Cypress Pharmaceutical, Inc.; DiagnoSearch Life Sciences (P) Ltd.; Dinippon Sumitomo Pharma Co. Inc.; Dov Pharmaceuticals, Inc.; Edgemont Pharmaceuticals, Inc.; Eisai Inc.; Eli Lilly and Company; EnVivo Pharmaceuticals, Inc.; ePharmaSolutions; EPIX Pharmaceuticals, Inc.; Euthymics Bioscience, Inc.; Fabre-Kramer Pharmaceuticals, Inc.; Forest Pharmaceuticals, Inc.; Forum Pharmaceuticals; GenOmind, LLC; GlaxoSmithKline; Grunenthal GmbH; i3 Innovus/Ingenis; Intracellular; Janssen Pharmaceutica; Jazz Pharmaceuticals, Inc.; Johnson & Johnson Pharmaceutical Research & Development, LLC; Knoll Pharmaceuticals Corp.; Labopharm Inc.; Lorex Pharmaceuticals; Lundbeck Inc.; MedAvante, Inc.; Merck & Co., Inc.; MSI Methylation Sciences, Inc.; Naurex, Inc.; Nestle Health Sciences; Neuralstem, Inc.; Neuronetics, Inc.; NextWave Pharmaceuticals; Novartis AG; Nutrition 21; Orexigen Therapeutics, Inc.; Organon Pharmaceuticals; Osmotica; Otsuka Pharmaceuticals; Pamlab, LLC.; Pfizer Inc.; PharmaStar; Pharmavite® LLC.; PharmoRx Therapeutics; Precision Human Biolaboratory; Prexa Pharmaceuticals, Inc.; Puretech Ventures; PsychoGenics; Psylin Neurosciences, Inc.; RCT Logic, LLC Formerly Clinical Trials Solutions, LLC; Rexahn Pharmaceuticals, Inc.; Ridge Diagnostics, Inc.; Roche; Sanofi-Aventis US LLC.; Sepracor Inc.; Servier Laboratories; Schering-Plough Corporation; Solvay Pharmaceuticals, Inc.; Somaxon Pharmaceuticals, Inc.; Somerset Pharmaceuticals, Inc.; Sunovion Pharmaceuticals; Supernus Pharmaceuticals, Inc.; Synthelabo; Taisho Pharmaceutical; Takeda Pharmaceutical Company Limited; Tal Medical, Inc.; Tetragenex Pharmaceuticals, Inc.; TransForm Pharmaceuticals, Inc.; Transcept Pharmaceuticals, Inc.; Vanda Pharmaceuticals, Inc.; VistaGen; he has received speaking or publishing fees from Adamed, Co; Advanced Meeting Partners; American Psychiatric Association; American Society of Clinical Psychopharmacology; AstraZeneca; Belvoir Media Group; Boehringer Ingelheim GmbH; Bristol-Myers Squibb; Cephalon, Inc.; CME Institute/Physicians Postgraduate Press, Inc.; Eli Lilly and Company; Forest Pharmaceuticals, Inc.; GlaxoSmithKline; Imedex, LLC; MGH Psychiatry Academy/Primedia; MGH Psychiatry Academy/Reed Elsevier; Novartis AG; Organon Pharmaceuticals; Pfizer Inc.; PharmaStar; United BioSource, Corp.; Wyeth-Ayerst Laboratories; he has equity holdings in Compellis and PsyBrain, Inc.; he has a patent for Sequential Parallel Comparison Design (SPCD), which are licensed by MGH to Pharmaceutical Product Development, LLC (PPD); and patent application for a combination of Ketamine plus Scopolamine in Major Depressive Disorder (MDD), licensed by MGH to Biohaven; and he receives copyright royalties for the MGH Cognitive & Physical Functioning Questionnaire (CPFQ), Sexual Functioning Inventory (SFI), Antidepressant Treatment Response Questionnaire (ATRQ), Discontinuation-Emergent Signs & Symptoms (DESS), Symptoms of Depression Questionnaire (SDQ), and SAFER; Lippincott, Williams & Wilkins; Wolkers Kluwer; World Scientific Publishing Co. Pte.Ltd.
Dr. Benji Kurian has received research grant support from the following organizations: Targacept, Inc., Pfizer, Inc., Johnson & Johnson, Evotec, Rexahn, Naurex, Forest Pharmaceuticals and the National Institute of Mental Health (NIMH). Mary L. Phillips has received funding from NIMH and the Emmerling-Pittsburgh Foundation.
Dr. Maria Oquendo receives royalties for use of the Columbia Suicide Severity Rating Scale and received financial compensation from Pfizer for the safety evaluation of a clinical facility, unrelated to this study. She is the recipient of a grant from Eli Lilly to support a year’s salary for the Lilly Suicide Scholar, Enrique Baca-Garcia, M.D., Ph.D.; she has received unrestricted educational grants and/or lecture fees from Astra-Zeneca, Bristol Myers Squibb, Eli Lilly, Janssen, Otsuko, Pfizer, Sanofi-Aventis, and Shire. Her family owns stock in Bristol Myers Squibb.
Over the past three years, Dr. Diego Pizzagalli has received honoraria/consulting fees from Akili Labs Interactive, Black Thorn Therapeutics, Pfizer, and Posit Science for activities unrelated to this project.
Footnotes
Clinical Trial: Establishing Moderators and Bio signatures of Antidepressant Response for Clinical Care for Depression (EMBARC), , https://clinicaltrials.gov/ct2/show/NCT01407094.
Dr. Rajapillai Pillai, Dr. Chuan Huang, Mr. Andrew LaBella, Dr. Mengru Zhang, Dr. Jie Yang, Dr. Melvin McInnis, Dr. Crystal Cooper, Dr. Christine DeLorenzo, and Dr. Ramin Parsey report no relevant or material financial interests that relate to the research described in this paper.
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jad.2019.05.055.
References
- Agresti A, Wackerly D, Boyett JM, 1979. Exact conditional tests for cross-classifications: approximation of attained significance levels. Psychometrika 44, 75–83. [Google Scholar]
- Alexander AL, Lee JE, Lazar M, Field AS, 2007. Diffusion tensor imaging of the brain. Neurotherapeutics 4, 316–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander DC, Barker GJ, 2005. Optimal imaging parameters for fiber-orientation estimation in diffusion MRI. Neuroimage 27, 357–367. [DOI] [PubMed] [Google Scholar]
- Alexopoulos GS, Kiosses DN, Choi SJ, Murphy CF, Lim KO, 2002. Frontal white matter microstructure and treatment response of late-life depression: a preliminary study. Am. J. Psychiatry 159, 1929–1932. [DOI] [PubMed] [Google Scholar]
- Alexopoulos GS, Murphy CF, Gunning-Dixon FM, Latoussakis V, Kanellopoulos D, Klimstra S, Lim KO, Hoptman MJ, 2008. Microstructural white matter abnormalities and remission of geriatric depression. Am. J. Psychiatry 165, 238–244. [DOI] [PubMed] [Google Scholar]
- Avants BB, Tustison NJ, Song G, Cook PA, Klein A, Gee JC, 2011. A reproducible evaluation of ANTs similarity metric performance in brain image registration. Neuroimage 54, 2033–2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron RM, Kenny DA, 1986. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J. Pers. Soc. Psychol. 51, 1173–1182. [DOI] [PubMed] [Google Scholar]
- Behrens TE, Berg HJ, Jbabdi S, Rushworth MF, Woolrich MW, 2007Probabilistic diffusion tractography with multiple fibre orientations: what can we gain? Neuroimage 34, 144–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnelle V, Ham TE, Leech R, Kinnunen KM, Mehta MA, Greenwood RJ, Sharp DJ, 2012. Salience network integrity predicts default mode network function after traumatic brain injury. Proc. Natl. Acad. Sci. 109, 4690–4695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi KS, Holtzheimer PE, Franco AR, Kelley ME, Dunlop BW, Hu XP, Mayberg HS, 2014. Reconciling variable findings of white matter integrity in major depressive disorder. Neuropsychopharmacology 39, 1332–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuthbert BN, Insel TR, 2013. Toward the future of psychiatric diagnosis: the seven pillars of RDoC. BMC Med. 11, 126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delorenzo C, Delaparte L, Thapa-Chhetry B, Miller JM, Mann JJ, Parsey RV, 2013. Prediction of selective serotonin reuptake inhibitor response using diffusionweighted MRI. Front. Psychiatry 4, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du H, Xie B, Lu P, Feng H, Wang J, Yuan S, 2014. Impaired white-matter integrity in photosensitive epilepsy: a DTI study using tract-based spatial statistics. J. Neuroradiol. 41, 131–135. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW, 1995. Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I/P, Version 2.0). Biometrics Research Department, New York State Psychiatric Institute, New York. [Google Scholar]
- Forster EA, Cliffe IA, Bill DJ, Dover GM, Jones D, Reilly Y, Fletcher A, 1995. A pharmacological profile of the selective silent 5-HT1A receptor antagonist, WAY- 100635. Eur. J. Pharmacol. 281, 81–88. [DOI] [PubMed] [Google Scholar]
- Fournier JC, Chase HW, Greenberg T, Etkin A, Almeida JR, Stiffer R,Deckersbach T, Weyandt S, Cooper C, Toups M, Carmody T, Kurian B, Peltier S, Adams P, McInnis MG, Oquendo MA, McGrath PJ, Fava M, Weissman M, Parsey R, Trivedi MH, Phillips ML, 2017. Neuroticism and individual differences in neural function in unmedicated major depression: findings from the EMBARC study. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2, 138–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier JC, DeRubeis RJ, Hollon SD, Dimidjian S, Amsterdam JD, Shelton RC, Fawcett J, 2010. Antidepressant drug effects and depression severity: a patient-level meta-analysis. JAMA 303, 47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frodl T, Amico F, 2014. Is there an association between peripheral immune markers and structural/functional neuroimaging findings? Prog. Neuro-psychopharmacol. Biol. Psychiatry 48, 295–303. [DOI] [PubMed] [Google Scholar]
- Fuller RW, Wong DT, 1977. Inhibition of serotonin reuptake. Fed. Proc. 36,2154–2158. [PubMed] [Google Scholar]
- Grieve SM, Korgaonkar MS, Gordon E, Williams LM, Rush AJ, 2016. Prediction of nonremission to antidepressant therapy using diffusion tensor imaging. J. Clin. Psychiatry 77, e436–e443. [DOI] [PubMed] [Google Scholar]
- Hagler DJ Jr., Ahmadi ME, Kuperman J, Holland D, McDonald CR, Halgren E, Dale AM, 2009. Automated white-matter tractography using a probabilistic diffusion tensor atlas: application to temporal lobe epilepsy. Hum. Brain Mapp. 30, 1535–1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton M, 1960. A rating scale for depression. J. Neurol. Neurosurg. Psychiatry 23,56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckel A, Weiler M, Xia A, Ruetters M, Pham M, Bendszus M, Heiland S,Baeumer P, 2015. Peripheral nerve diffusion tensor imaging: assessment of axon and myelin sheath integrity. PLoS One 10, e0130833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornung JP, 2003. The human raphe nuclei and the serotonergic system. J. Chem. Neuroanat. 26, 331–343. [DOI] [PubMed] [Google Scholar]
- Hua K, Zhang J, Wakana S, Jiang H, Li X, Reich DS, Calabresi PA, Pekar JJ, van Zijl PC, Mori S, 2008. Tract probability maps in stereotaxic spaces: analyses of white matter anatomy and tract-specific quantification. Neuroimage 39, 336–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappenman ES, Keil A, 2017. Introduction to the special issue on recentering science: replication, robustness, and reproducibility in psychophysiology. Psychophysiology 54, 3–5. [DOI] [PubMed] [Google Scholar]
- Katzman MA, Anand L, Furtado M, Chokka P, 2014. Food for thought: understanding the value, variety and usage of management algorithms for major depressive disorder. Psychiatry Res. 220 Suppl (1), S3–14. [DOI] [PubMed] [Google Scholar]
- Korgaonkar MS, Williams LM, Song YJ, Usher wood T, Grieve SM, 2014Diffusion tensor imaging predictors of treatment outcomes in major depressive disorder. Br. J. Psychiatry 205, 321–328. [DOI] [PubMed] [Google Scholar]
- Kruger O, Shiozawa T, Kreifelts B, Scheffer K, Ethofer T, 2015. Three distinct fiber pathways of the bed nucleus of the stria terminalis to the amygdala and prefrontal cortex. Cortex 66, 60–68. [DOI] [PubMed] [Google Scholar]
- Lam RW, Michalak EE, Bond DJ, Tam EM, Axler A, Yatham LN, 2012. Which depressive symptoms and medication side effects are perceived by patients as interfering most with occupational functioning? Depress. Res. Treat. 2012, 630206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux J, 2003. The emotional brain, fear, and the amygdala. Cell. Mol. Neurobiol. 23,727–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Wang Y, Gerig G, Gouttard S, Tao R, Fletcher T, Styner M, 2010. Quality control of diffusion weighted images. In: Proceedings of SPIE. International Society for Optical Engineering, pp. 7628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery SA, 1997. Fast-onset antidepressants. Int. Clin. Psychopharmacol. 12 Suppl (3), S1–S5. [DOI] [PubMed] [Google Scholar]
- Nelson JC, Zhang Q, Deberdt W, Marangell LB, Karamustafalioglu O, Lipkovich IA, 2012. Predictors of remission with placebo using an integrated study database from patients with major depressive disorder. Curr. Med. Res. Opin. 28, 325–334. [DOI] [PubMed] [Google Scholar]
- O’Donnell LJ, Westin CF, 2011. An introduction to diffusion tensor image analysis. Neurosurg. Clin. N. Am. 22, 185–196 viii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olvet DM, Delaparte L, Yeh FC, DeLorenzo C, McGrath PJ, Weissman MM, Adams P, Fava M, Deckersbach T, McInnis MG, Carmody TJ, Cooper CM, Kurian BT, Lu H, Toups MS, Trivedi MH, Parsey RV, 2015. ′’. A comprehensive examination of white matter tracts and connectometry in major depressive disorder. Depress. Anxiety. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips ML, Chase HW, Sheline YI, Etkin A, Almeida JR, Deckersbach T,Trivedi MH, 2015. Identifying predictors, moderators, and mediators of antidepressant response in major depressive disorder: neuroimaging approaches. Am. J. Psychiatry 172, 124–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rush AJ, Trivedi MH, Ibrahim HM, Carmody TJ, Arnow B, Klein DN,Markowitz JC, Ninan PT, Kornstein S, Manber R, Thase ME, Kocsis JH, Keller MB, 2003. The 16-item Quick Inventory of Depressive Symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biol. Psychiatry 54, 573–583. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, Niederehe G, Thase ME, Lavori PW, Lebowitz BD, McGrath PJ, Rosenbaum JF, Sackeim HA, Kupfer DJ, Luther J, Fava M, 2006. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am. J. Psychiatry 163, 1905–1917. [DOI] [PubMed] [Google Scholar]
- Schmaal L, Veltman DJ, van Erp TG, Sämann P, Frodl T, Jahanshad N, Loehrer E, Tiemeier H, Hofman A, Niessen W, 2016. Subcortical brain alterations in major depressive disorder: findings from the ENIGMA major depressive disorder working group. Mol. Psychiatry 21, 806–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI, Barch DM, Donnelly JM, Ollinger JM, Snyder AZ, Mintun MA, 2001. Increased amygdala response to masked emotional faces in depressed subjects resolves with antidepressant treatment: an fMRI study. Biol. Psychiatry 50, 651–658. [DOI] [PubMed] [Google Scholar]
- Soares JM, Marques P, Alves V, Sousa N, 2013. A hitchhiker’s guide to diffusion tensor imaging. Front. Neurosci. 7, 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor WD, Kuchibhatla M, Payne ME, Macfall JR, Sheline YI, Krishnan KR, Doraiswamy PM, 2008. Frontal white matter anisotropy and antidepressant remission in late-life depression. PLoS One 3, e3267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivedi MH, McGrath PJ, Fava M, Parsey RV, Kurian BT, Phillips ML, Oquendo MA, Bruder G, Pizzagalli D, Toups M, 2016. Establishing moderators and biosignatures of antidepressant response in clinical care (EMBARC): rationale and design. J. Psychiatr. Res. 78, 11–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasavada MM, Leaver AM, Espinoza RT, Joshi SH, Njau SN, Woods RP, Narr KL, 2016. Structural connectivity and response to ketamine therapy in major depression: a preliminary study. J. Affect. Disord. 190, 836–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergani F, Martino J, Morris C, Attems J, Ashkan K, Dell’Acqua F, 2016. Anatomic connections of the subgenual cingulate region. Neurosurgery 79, 465–472. [DOI] [PubMed] [Google Scholar]
- Wakana S, Jiang H, Nagae-Poetscher LM, van Zijl PC, Mori S, 2004. Fiber tractbased atlas of human white matter anatomy. Radiology 230, 77–87. [DOI] [PubMed] [Google Scholar]
- Webb CA, Trivedi MH, Cohen ZD, Dillon DG, Fournier JC, Goer F, Fava M, McGrath PJ, Weissman M, Parsey R, Adams P, Trombello JM, Cooper C, Deldin P, Oquendo MA, McInnis MG, Huys Q, Bruder G, Kurian BT, Jha M, DeRubeis RJ, Pizzagalli DA, 2019. Personalized prediction of antidepressant v. placebo response: evidence from the EMBARC study. Psychol. Med. 49 (7), 1118–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu T, Hu R, Qiu X, Taylor M, Tso Y, Yiannoutsos C, Navia B, Mori S, Ekholm S, Schifitto G, Zhong J, 2011. Quantification of accuracy and precision of multicenter DTI measurements: a diffusion phantom and human brain study. Neuroimage 56, 1398–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.