Abstract
Objective:
Methacrylamide-based monomers are being pursued as novel, hydrolytically stable materials for use in dental adhesives. The impact of residual solvents, due to the chemical synthesis procedures or the need for solvated adhesives systems, on the kinetics of polymerization and mechanical properties was the aim of the present investigation.
Materials:
Two base monomers (70 wt% BisGMA or HEMAM-BDI – newly synthesized secondary methacrylamide) were combined with 30 wt% N,N-dimethylacrylamide. Eethyl acetate (EtOAc), or 75 vol% ethanol/25 vol% water (EtOH/H2O) were added as solvents in concentrations of 2, 5, 15 and 20 wt%. The resins were made polymerizable by the addition of 0.2 wt% 2,2-dimethoxy-2-phenyl acetophenone (DMPA) and 0.4 wt% diphenyliodonium hexafluorophosphate (DPI-PF6). Specimens (n=3) were photoactivated with a mercury arc lamp (Acticure 4000, 320–500 nm, 250 mW/cm2) for 5 min. Degree of conversion (DC, %) was tracked in near-IR spectroscopy in real time and yield strength and modulus of elasticity were measured in three-point bending after dry and wet storage (n=6). The data was subject to one-way ANOVA/Tukey’s Test (p ≤ 0.05), or Student’s t-test (p ≤ 0.001).
Results:
In all groups for both BisGMA and HEMAM-BDI-based materials, DC and DC at Rpmax increased and maximum rate of polymerization decreased as solvent concentration increased. Despite the increased DC, BisGMA mixtures showed a decrease in FS starting at 5 wt% EtOAc or 15 wt% EtOH/H2O. Yield strength for the HEMAM-BDI groups was overall lower than that of the BisGMA groups, but the modulus of elasticity was significantly higher.
Conclusions:
The presence of residual solvent, from manufacturing or from practitioner’s handling, affects polymerization kinetics and mechanical properties of resins. Methacrylates appear to be more strongly influenced than methacrylamides.
1. INTRODUCTION
For dental resin systems, solvent remains because it is difficult to remove prior to the start of the polymerization process. The presence of solvents within dental adhesive resins during polymerization greatly affects the reaction kinetics, and is a function of the solvent concentration and chemical structure. In dental adhesives systems, most commonly composed of methacrylates, solvents such as ethanol, water and/or acetone reduce resin viscosity and allow for the penetration of the otherwise relatively viscous and hydrophobic monomers into the hydrophilic dentin substrate. Simultaneously solvents help avoid the collapse of the collagen scaffold [1, 2]. Once the adhesive material reaches its intended depth, ideally, the solvent would be completely removed by air-drying, since adhesive properties have been shown to be inversely correlated with the solvent concentration [3]. However, in the clinical situation, even if the recommended procedures are correctly followed, between 12% and 14 wt% of the original solvent remains after air drying [4, 5]. The incomplete solvent removal is influenced by the co-monomer blend, the vapor pressure and molecular weight of the solvents, as well as the presence of water from the dentin tubules [4, 6]. Moreover, after initial solvent evaporation, the monomer concentration of the mixture increases, making the removal of any remaining solvent progressively more difficult [6]. In addition, residual solvent from the monomer synthesis may on occasion be present within the materials, being difficult to remove even after using rotary evaporators, freeze dryers, centrifugal evaporators, blow down systems, and vacuum pumps. Ethyl acetate (EtOAc) is an aprotic solvent commonly used in the synthesis of dental monomers, in part due to its , relative ease of being removed from the reaction mixtures [7].
The presence of residual solvent within the bonding interface is associated with deleterious effects, such as decreased final degree of conversion of the adhesive monomers, reduced bond strength, and increased adhesive layer permeability, which ultimately leads to early degradation of the adhesive interface [2, 5]. Most of these effects are due to the entrapment of solvent microdroplets throughout the adhesive during the solvent evaporation, especially at the bottom surface of the adhesive adjacent to the hybrid layer [8]. In addition, in the presence of solvents, phase-separation of the resin during the polymerization of the adhesive is a common occurrence [8]. Some studies have demonstrated a decrease in glass transition temperature (Tg) due to the reduction in the intensity of interactions between the polymers and an increase in the macromolecular segment mobility and altered packing of polymer chains. Greater elongation, reduced modulus, and effects on thermal behavior are commonly associated with the presence of residual solvents in the polymer network [9, 10]. Among the most common protic solvents included in dental adhesive formulations (acetone, ethanol and water) [11], the most difficult to be evaporated by air-drying and more susceptible to entrapment in the polymer network is EtOH/H2O due to its low vapor pressure.
In the dental literature, the vast majority of studies have concentrated efforts on methacrylate systems, and the influence of solvents on the properties of different types of monomers is still underexplored. This becomes relevant because, recently, adhesives based on methacrylamides have been suggested as suitable alternatives for the traditional methacrylates. Methacrylamides are being investigated because they are more resistant to hydrolysis and enzymatic degradation [12, 13]. Therefore, it is of interest to investigate the behavior of this new class of materials as a function of the type and concentration of residual solvents.
The aim of this study was to evaluate the impact of gradual additions of protic and aprotic solvents on the kinetics of polymerization and mechanical properties of methacrylate and methacrylamide monomer systems. The tested hypotheses were: (1) polymerization rate and degree of conversion of the system will decrease with an increase in solvent concentration , and (2) a system with a model methacrylamide monomer will be less affected by the presence of residual solvent than the more common methacrylates.
2. MATERIALS AND METHODS
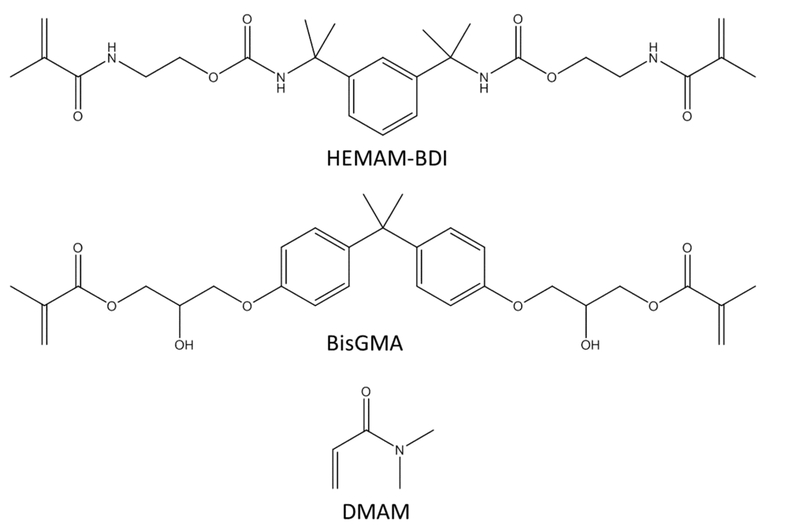
A traditional, commercially available difunctional methacrylate, bisphenol A glycidyl methacrylate (BisGMA, from Sigma Aldrich), and a newly synthesized secondary dimethacrylamide, HEMAM-BDI (Figure 1) were used as base monomers. HEMAM-BDI was chosen as a model methacrylamide because of its similar molecular weight, similar viscosity, and same difunctionality compared to BisGMA, and also because it was possible to achieve high yield in the synthesis procedure without the use of solvent.
Figure 1.
Chemical structure of the newly synthesized secondary dimethacrylamide - HEMAM-BDI (MW = 502.61g/mol). BisGMA and DMAM were also used in this study.
For HEMAM-BDI synthesis 2.1 mols of N-(2-Hydroxyethyl)methacrylamide, (HEMAM, Dajac) were mixed with 1 mol of 1,3-bis(1-isocyanato-1-methylethyl)benzene, (BDI, Esstech) in a round-bottom flask under magnetic stirring at room temperature. The condensation reaction was carried out in a solvent-free environment (neat), catalyzed by dibutyltin dilaurate. The compound was characterized by mid-IR spectroscopy with the disappearance of the isocyanate peak at 2270 cm−1 indicating completion of the reaction, and subsequently by 1H and 13C NMR.
BisGMA or HEMAM-BDI were combined at 70 wt% with a tertiary acrylamide diluent N,N-dimethylacrylamide (DMAM, from Sigma Aldrich) to form resin mixtures. The mixtures were made polymerizable by the addition of 0.2 wt% 2,2-dimethoxy-2-phenyl acetophenone (DMPA) and 0.4 wt% diphenyliodonium hexafluorophoshate (DPI-PF6). 0.1 wt% butylated hydroxytoluene (BHT) was added as inhibitor.
Ethyl acetate (EtOAc) or a 75 vol% aqueous solution of 200 proof ethanol (EtOH/H2O) were added to the monomer mixtures at 0, 0.5, 1, 2, 5, 15, and 20 wt% to assess the effect of different levels of residual solvent on polymerization and properties. The resins were subjected to the different assays immediately after mixing.
The partition coefficient (log P) and solubility (log S) of each monomer and solvent used in this study were calculated using ChemDraw (ChemBioDraw Ultra, v14, Perkin Elmer, Waltham, MA, USA).
2.1. Kinetics of Polymerization
Disc samples (0.8 mm thickness by 6 mm diameter, n=3) were prepared by placing the monomer mixtures in silicon molds between glass slides and photoactivating with a mercury arc lamp positioned 2 cm away from the specimen (Acticure 4000, 320–500 nm, irradiance reaching the specimen: 250 mW/cm2) for 300s. Degree of conversion (DC, %) was monitored in real time for the entire 300s by the disappearance of the vinyl double-bond in near-IR (6165 cm−1), and Rpmax was calculated as the first derivative of the DC vs. time curve, then normalized to initial vinyl concentration to account for differences in starting monomer concentrations among groups.
2.2. Mechanical Properties
Bar specimens (2×2×25 mm, n=5) were obtained in polyvinyl siloxane molds sandwiched between glass slides, photoactivated for two min on both sides (top and bottom surfaces) at 250 mW/cm2 irradiance reaching the specimen, with the tip of the light source 7 cm away to guarantee sufficient spot size to cover the entire sample in one shot, followed by 10 min post-curing on both sides (630 mW/cm2). All photocuring procedures were carried out by the mercury arc lamp, as described previously. Half of the specimens were removed from molds and stored for 48 hrs in dark and dry conditions before testing, and the other half were placed in 20 mL of millipore grade water for 7 days before testing. 3-Point bend testing was conducted with a 20 mm span at 1 mm/min crosshead speed (MTS Criterion, Model 42). Elastic modulus (E, GPa) and flexural strength (FS, MPa) were evaluated for all groups, before and after water storage according to ISO 4049. The yield strength (YS) for materials demonstrating gradual plastic deformation (non-brittle failure) was determined as the point where the stress-strain curve no longer obeyed a linear relation. This was calculated by applying a 2% strain offset line with slope equal to the modulus of the stress-strain curve. The stress value where the experimental data and the offset line intersect was used as the yield strength.
2.3. Statistical Analysis
After normality and homocedasticiy tests, data were analyzed with ANOVA and Tukey’s test. First, a three-way ANOVA was conducted to determine possible interaction between the different factors (monomer type, solvent type and solvent concentration). Then, to facilitate data interpretation, each of the monomers was analyzed separately using two-way ANOVA and Tukey’s test. The variables analyzed were: degree of conversion, maximum rate of polymerization (Rpmax), degree of conversion at Rpmax, yield strength and elastic modulus (dry and wet storage, analyzed separately). Student’s t-Test was performed to assess the effect of storage conditions on the mechanical properties. Linear regression plots were built to correlate the kinetic parameters to the type and concentration of solvent for the different monomers tested. Regression slopes were compared using t-test analysis based on the standard error of the regression models. An overall level of significance of α=5% was set for all tests.
3. RESULTS
The partition coefficient and solubility values calculated for the monomers and solvents used in this study are shown in Table 1. No statistical analysis applies to this set of data.
Table 1:
Partition coefficient (log P) and solubility (log S) calculated with ChemDraw software for all monomers and solvents used in this study.
| Monomer or solvent | logP | logS |
|---|---|---|
| Water | - | 0.158 |
| Ethanol | 0.07 | 0.3046 |
| Ethyl acetate | 0.29 | −0.5017 |
| BisGMA | 5.09 | −5.571 |
| HEMAM-BDI | 2.37 | −4.312 |
| DMAM | 0.2 | −0.3436 |
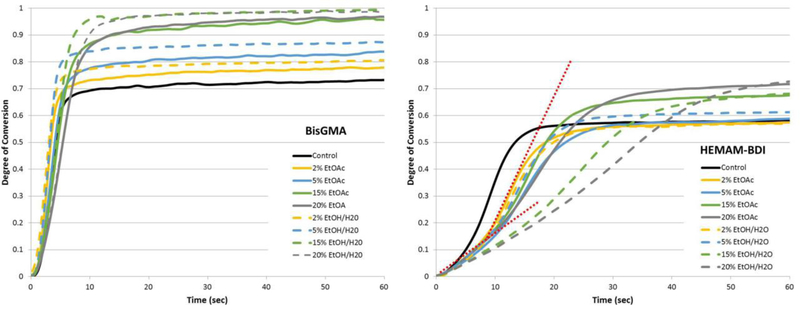
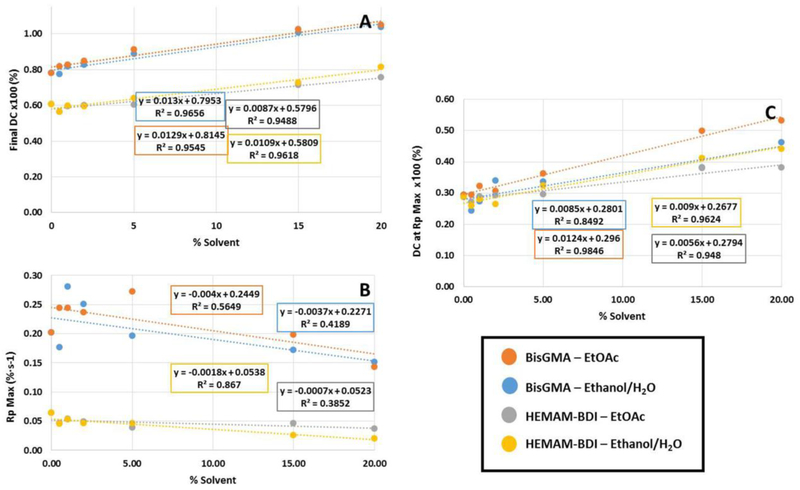
In general, the degree of conversion increased with the concentration of solvent (Figures 2 and 3). The three-way ANOVA showed significance for all factors (p<0.001) and all interactions, except for monomer vs. type of solvent (p=0.529). For BisGMA-containing mixtures, the two-way ANOVA showed significance for both factors (solvent type and concentration) and the interaction (p<0.001). For HEMAM-BDI mixtures, both factors were significant (p<0.001), but the interaction was not (p=0.065). DC was consistently higher for BisGMA compared to HEMAM-BDI. Figure 4 shows correlation plots between kinetic parameters and solvent concentration. The slopes of the DC trendlines were statistically greater for BisGMA mixtures (0.0151 and 0.0149 with EtOH/H2O and EtOAc, respectively) compared to HEMAM-BDI (0.0087 and 0.0109), according to a t-test analysis comparing the slopes for the two monomers for the same solvent system (p=0.002 and p=0.036 for EtOH/H2O and EtOAc, respectively). This indicates greater sensitivity to solvent content for the BisGMA system. 20 wt% solvent groups for BisGMA-base monomer were excluded from these curves as this mix achieved 100% conversion by 15 wt%. Additionally, in general the incorporation of EtOH/H2O resulted in equal or slightly higher degrees of conversion than EtOAc, regardless of the resin system, although this trend was not statistically significant.
Figure 2.
Degree of conversion (%) as a function of time (up to 60 s) for BisGMA-and HEMAM-BDI-based materials, containing different concentrations of ethyl acetate (EtOAc, solid lines) or Ethanol/H2O (EtOH/H2O, dashed lines). Dashed red lines on the HEMAM-BDI graph are highlighting the two-stage kinetic profile for the groups with highest solvent concentrations. The lines represent the average of three runs. Vinyl conversion was followed in real time as the materials were photocured with 250 mW/cm2 for 300 seconds. Note: due to the very similar kinetic profiles for 0.5, 1 and 2 % solvent concentrations, the 0.5 and 1% concentrations are omitted from the graph for clarity.
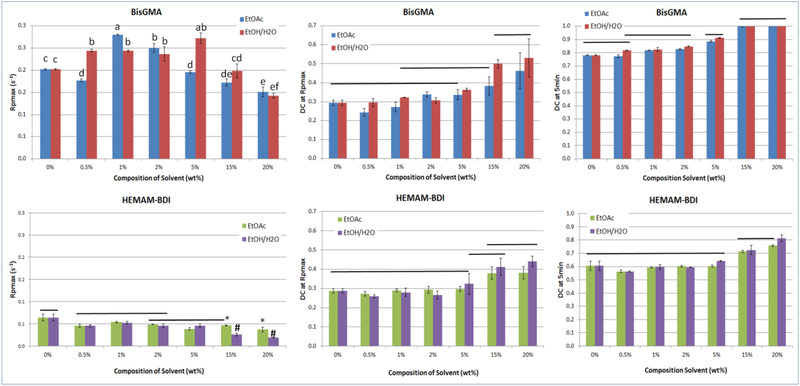
Figure 3.
Averages of maximum rates of polymerization (Rpmax, %.s−1), degree of conversion at Rpmax (DC at RP, %) and degree of conversion at 5 min (DC at 50 min, %), for all groups tested. BisGMA-based materials were statistically different from HEMAM-BDI-based materials for every variable, so comparisons were made only within each monomer system, using two-way ANOVA/Tukey’s test (solvent type and concentration as factors, α=5%). Values followed by the same superscript or connected by a horizontal bar are statistically similar. Vinyl conversion was followed in real time as the materials were photocured with 250 mW/cm2 for 300 seconds.
Figure 4.
Linear regression curves for: (A) the final degree of conversion (DC) (%), (B) maximum rate of polymerization (Rpmax) (%.s−1), and (C) degree of conversion at maximum rate of polymerization (DC at Rpmax) (%) as a function of solvent percentage incorporated in the mixtures for BisGMA and HEMAM-BDI resins.
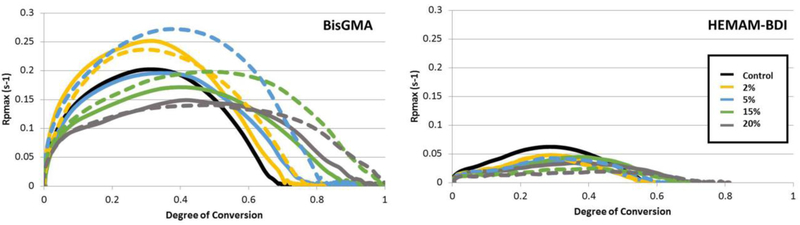
The maximum rate of polymerization (Rpmax) was consistently higher for the BisGMA matrix compared to the HEMAM-BDI matrix system, regardless of solvent content (Figures 3 and 5). The three-way ANOVA showed significance for all factors and all interactions (p<0.001). The two-way ANOVA showed significance for both factors (solvent type and concentration) and the interaction (p<0.001) for both monomers. Rpmax for BisGMA were approximately 3 times greater (and up to 7 times greater for 20 wt% solvent mixtures) than their corresponding rates with HEMAM-BDI. For BisGMA groups, Rpmax increased up to 5% solvent concentration, and decreased for solvent concentration above 5%. For HEMAM-BDI groups, solvent concentration had a much less pronounced effect on Rpmax. In general, as solvent content increases, the maximum rate achieved shifts to higher DC.
Figure 5.
Polymerization rate (%.s−1) as a function of conversion (%) for BisGMA-and HEMAM-BDI-based materials, containing different concentrations of ethyl acetate (EtOAc, solid lines) or Ethanol/H2O (EtOH/H2O, dashed lines). The lines represent the average of three runs. Vinyl conversion was followed in real time as the materials were photocured with 250 mW/cm2 for 300 seconds. Note: due to the very similar kinetic profiles for 0.5, 1 and 2 % solvent concentrations, the 0.5 and 1% concentrations are omitted from the graph for clarity.
Degree of conversion at the maximum rate of polymerization (DC at Rpmax) increased with solvent concentration for both resin systems (Figure 3 and 4). The three-way ANOVA showed significance for all factors (p<0.001) and the interaction solvent type vs. concentration (p=0.010). None of the other interactions were significant (p>0.05). For BisGMA, the two-way ANOVA showed significance for both factors (solvent type and concentration) but not for the interaction (p=0.103). For HEMAM-BDI, only the solvent concentration was significant (p<0.001). For EtOAC there is a significant positive correlation between the solvent percentage and the DC at Rpmax for both resin systems. For Ethanol/H2O there was a significant positive correlation for HEMAM-BDI, but not as strong a correlation for BisGMA. In general, the difference between the systems was slighter more marked for DC at Rpmax in comparison to final DC and Rpmax.
Results for mechanical properties are shown in Table 2. Due to very similar conversion results, the groups containing 0.5 or 1 % solvent were not tested for mechanical properties. For the dry yield strength, the three-way ANOVA showed significance for the factors “monomer type” and “solvent concentration” (p<0.0001), and for the dual interactions involving the monomer (p>0.001). The triple interaction (p=0.793) and the solvent type vs. concentration interactions were not significant (p>0.05). For the dry flexural modulus, the three-way-ANOVA showed significance for the factors “monomer type” and “solvent concentration” (p<0.0001), and for the dual interactions involving the solvent type (p>0.001). The triple interaction (p=0.793) and the monomer type vs. concentration interactions were not significant (p>0.05). The three-way ANOVA was not conducted for the wet properties because it was not possible to test the bars for the materials containing HEMAM-BDI.
Table 2.
Modulus of elasticity (GPa) and yield strength (MPa) tested dry and after 7 days storage in water (wet) for all tested experimental groups. HEMAM-BDI specimens could not tested after water storeage. Values followed by the same superscript within the same column are statistically similar (two-way ANOVA within column). The asterisk symbol * indicates statistical difference between dry and wet results for the same group (t-test). For all tests, α=5%.
| Solvent | BisGMA | HEMAM-BDI | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Type | Concentration (wt%) | Yield strength (MPa) | Flexural modulus (GPa) | Yield strength (MPa) | Flexural modulus (GPa) | ||||
| dry | wet | Reduction (%) | dry | wet | Reduction (%) | dry | dry | ||
| Control | 0 | 78.1±14.4 b | 48.2±2.1 b* | 38 | 3.55±0.10 b | 2.31±0.20 b* | 35 | 51.5±14.0 b | 5.40±0.59 a |
| EtOAc | 2 | 85.8±12.9 ab | 42.3±3.3 b* | 51 | 3.42±0.34 b | 2.13±0.23 b* | 38 | 70.1±6.3 a | 4.89±0.31 ab |
| 5 | 71.4±3.8 bc | 45.8±5.0 b* | 36 | 3.17±0.22 bc | 2.19±0.18 b* | 31 | 70.1±14.8 a | 4.94±0.44 a | |
| 15 | 76.0±3.0 b | 53.7±7.6 b* | 29 | 3.18±0.07 bc | 2.82±0.43 b | 11 | 86.5±21.0 a | 5.10±0.17 a | |
| 20 | 63.0±5.8 c | 53.5±5.7 b | 15 | 2.77±0.41 c | 2.90±0.48 b | −5 | 70.1±8.7 a | 4.57±0.81 bc | |
| EtOH/H2O | 2 | 101.3±6.6 a | 87.2±13.8 a | 14 | 4.64±0.47 a | 4.23±0.47 a | 9 | 68.1±7.0 ab | 5.01±0.65 a |
| 5 | 89.7±3.7 | 88.1±5.4 a | 2 | 4.25±0.44 | 4.10±0.27 | 4 | 53.9±17.5 | 5.16±0.36 | |
| ab | a | a | b | a | |||||
| 15 | 64.3±9.2 c | 55.2±10.3 b | 14 | 3.31±0.61 b | 2.59±0.36 b | 22 | 62.2±15.3 ab | 3.97±0.34 c | |
| 20 | 54.0±5.4 c | 52.6±3.4 b | 3 | 3.00±0.35 bc | 2.89±0.11b | 4 | 47.9±13.4 b | 3.20±0.12 c | |
For BisGMA, the two-way ANOVA on the dry yield strength data showed that the solvent type was not significant (p=0.464), but the solvent concentration and the interaction were (p<0.001). For the dry modulus and all wet properties, all the factors and interactions were significant (p<0.001). For HEMAM-BDI, the dry yield strength was significantly affected by neither the factors (p>0.05) and the interaction was not significant either (p<0.05). The two-way ANOVA performed on the dry flexural modulus data showed significance for both factors (solvent type and concentration, p<0.0001), but the interaction was not significant (p=0.129). In general, HEMAM-BDI groups under dry storage conditions did not present differences in yield strength as solvent content was changed (Table 2), while the elastic modulus was negatively affected by the addition of 15 and 20 wt% of ethanol/H2O (3.97 and 3.20 GPa, respectively). The yield strength of the BisGMA resins was negatively affected by the addition of 20 wt% EtOAc and ethanol/H2O (Table 2). The incorporation of 15 wt% ethanol/H2O and 20 wt% of both solvents was responsible for decreasing the modulus of elasticity. The addition of 2 and 5 wt% of ethanol/H2O increased the mechanical properties of the BisGMA mixtures. In general, water storage significantly decreased the mechanical properties (t-test, p≤0.001) (Table 2). This effect was especially pronounced for the methacrylamide systems, in which the wet storage resulted in bars that were extremely flexible and could not be tested in flexure.
4. DISCUSSION
Free radical polymerization in the presence of solvents is a common challenge faced with dental adhesive resins. Whether they are residual from solvents that have been purposely added but cannot be completely removed or as impurities from the synthesis reaction, the presence of solvents may affect the polymerization process. Solvents increase the mobility of the molecules within the reaction medium, which can affect the kinetics of polymerization differently depending on the initial viscosity and reactivity of the monomers being polymerized [14, 15]. In terms of mechanical properties, if the final conversion is not a factor, it is generally accepted that higher solvent concentrations will negatively affect mechanical behavior [16]. However, there is likely a critical solvent concentration at which the polymer mobility (and consequent increase in conversion) is counter-balanced by the compromise in mechanical properties. This critical point has, to date, not been determined for dental methacrylate systems, and is also not known for novel methacrylamides being proposed as alternative adhesive monomers. Therefore, this study investigated the sensitivity of methacrylate and methacrylamide resins in the presence of ethyl acetate and EtOH/H2O in terms of polymerization kinetics and bulk mechanical properties. Because of the high viscosity of neat BisGMA and the fact that the model dimethacrylamide HEMAM-BDI is a solid, a low viscosity, highly reactive diluent monomer, DMAM, was selected and the mixtures were tested in co-polymerizations. Potential issues with the co-polymerization of acrylamides with both methacrylates and methacrylamides will be addressed in the pertinent sections of the discussion.
BisGMA showed consistently higher Rpmax and final DC than HEMAM-BDI. The nitrogen atom in the amide bond provides stronger resonance stabilization to the vinyl, making acrylamides inherently less reactive than acrylates [17]. One important aspect to consider is the fact that the two monomers were co-polymerized with a very low viscosity, highly reactive acrylamide. This was done to allow for the handling and polymerization of the HEMAM-BDI monomer, which presents as a solid at room temperature. Assuming BisGMA is more reactive than the methacrylamide (for the reasons already explained), the differential in reactivity compared to DMAM (the acrylamide) was much more marked for the methacrylamide groups. In that case, the chances for formation of interpenetrating polymer networs (IPNs), rather than co-polymerizations, was much greater [18]. In fact, as will be explored in detail later, the materials containing methacrylamides became visually hazy during polymerization, a strong indication of the formation of domains with different compositions [19]. In addition, the apparent viscosity of the HEMAM-BDI groups was greater than the BisGMA-containing groups, which may also have contributed to early limitations to diffusion and decreased rate and conversion. Therefore, the lower reactivity of the methacrylamide at every solvent concentration is fully justified.
A strong positive correlation was observed between degree of conversion and increased solvent concentration for both base monomers. The solvents contribute to a decrease in the baseline viscosity, which in turn plays a crucial role in determining the kinetics of polymerization [20]. High viscosity systems are generally less reactive due to the intrinsic diffusional limitations to molecular mobility. But extremely low viscosity systems tend toward a delayed onset of diffusional limitations to termination, which competes with propagation events such that autoacceleration at faster rates is generally not observed [21]. In fact, synergistic effects in co-polymerization of highly viscous monomers, such as BisGMA, with lower viscosity diluents, such as TEGDMA, have been widely demonstrated in the literature [22]. In the present study, the addition of solvents increased the initial mobility of the reacting species, which ultimately led to higher double-bond conversion [23].
However, as expected, the effect of solvent addition on the rate of polymerization was far more complex. The incorporation of 2% and 5% of either solvent increased the rate of polymerization for the methacrylate resin. However, the addition of higher amounts of solvent (15% and 20%) resulted in decreased rate of polymerization. For the methacrylate system, up to a certain threshold in solvent concentration (5%), the increased initial mobility favored propagation over termination events. Beyond that threshold, the increase in mobility allowed for prolonged competition between propagation and termination events, and as already explained, that led to an overall decrease in the rate of polymerization [20]. Ultimately, the inclusion of solvents causes a shift in the sol-gel transition to higher degrees of conversion due to the enhanced molecular diffusion [24]. For the methacrylamide, the overall rate of polymerization was lower when either solvent was added at any concentration. This was somewhat surprising, since the initial viscosity of the methacrylamide materials was actually higher than the methacrylate, so it was expected that the methacrylamide would also benefit from the presence of some amount of solvent. One of the factors that need to be considered to explain that apparent contradiction relates to the Hildebrand solubility parameters of the compounds in the mixture. Ethyl acetate’s value is 18.2 MPa1/2, ethanol’s is 26.5 MPa1/2 and water’s is 48 MPa1/2 [25, 26], which makes the Hildebrand’s parameter of a 75/25 ethanol/water solution to be about 31.875 MPa1/2. Previous studies showed that BisGMA’s value is around 20.2 MPa1/2 and it is solubilized by solvents with Hildebrand parameters between 18 and 30 MPa1/2 [26, 27], and therefore, in line with either solvent system used here. The Hildebrand parameter for the newly synthesized monomer is not known. Alternatively, the monomers can be compared in terms of partition coefficient (logP) and solubility (logS) values, easily calculated using ChemDraw software. Log P defines the tendency of a compound to partition preferentially into the aqueous or organic phases of a solvent system, and the lower its value, the more hydrophilic the compound [28]. LogS determines the solubility of a substance (in this case, in water), calculated as the ratio of the free energy of solvation (ΔGsolv) and the temperature (multiplied by a fudge factor and the gas constant – [29]). These two parameters provide a simplistic overview of the miscibility of compounds, since they only take into account the polarity, but disregard other factors such as molecular weight, degree of branching, size of side chains, etc. [30], which certainly affected the results. Therefore, based solely on the logP/logS results, it would be expected that methacrylamide-containing mixtures would be more efficiently solvated by either solvent system. That would explain why the rates of polymerization decreased in relation to the control even at the lowest solvent concentrations. For the BisGMA-containing mixtures, other factors such as the hydrogen bonding strength and rigidity of the backbone were likely compounded with the solubility (in theory, worse than for the methacrylamides) to determine the effect of solvent concentration on rate of polymerization. Furthermore, EtOAc has a vapor pressure of 73.911 mmHg at room temperature (20°C), while ethanol is 43.7005 mmHg and water 17.4733 mmHg. It is possible that during sample preparation or photoactivation procedures a significant amount of EtOAc was evaporated, which means the concentrations of that solvent might have actually be overestimated.
Importantly, during the polymerization of HEMAM-BDI-containing materials, evidence for phase-separation was observed, with the samples becoming visually hazy, a strong indication of the formation of domains with different compositions [19] mentioned. In addition, the reaction kinetics presented a characteristic two-stage profile (as highlighted in Figure 2), corroborating the presence of two phases polymerizing separately [31]. The presence of this secondary maximum rate of polymerization (shown as a “shoulder” in the curve) may be associated with differences in reactivity between HEMAM-BDI – a crystalline-solid secondary methacrylamide, and DMAM – a highly reactive, low molecular weight tertiary acrylamide. While the monomers are miscible at room temperature, upon light exposure, the faster reacting acrylamide undergoes homopolymerization, surrounded by unreacted (or slower polymerizing) HEMAM-BDI. The increased molecular weight of the acrylamide network likely increased the free energy of mixing in the system, making it thermodynamically unfavorable for the two phases (DMAM-rich and HEMAM-rich) to form a solution. This likely drove phase separation even in the unsolvated system [31], though this was less evident. The HEMAM-BDI-rich phase eventually “catches up” and polymerization proceeds in its own domain. The fact that these two compositionally-distinct phases are somewhat independently polymerizing leads to the double-staged profile of the kinetics curve, where two distinct autoacceleration slopes are observed. In the presence of solvents with dissimilar Hildenbrand parameters compared to the main monomer, the polymerization-induced phase separations (PIPS) are compounded with solvent-induced phase separation (SIPS), potentiating the increase in free energy of mixing. Therefore, in spite of the increased mobility of the reactive species due to the reduction of the system viscosity, the secondary maximum rate of polymerization decreased progressively with the increase in the solvent content. In summary, in SIPS the increased mobility of monomers and reactive species promoted by the solvent addition is responsible for enhancing microgel precipitation, which is translated into mechanical phase separation and a direct correlation between the increase of the solvent amount and the decrease in the maximum rate of polymerization [32, 33].
In terms of mechanical properties, in general BisGMA systems had higher yield strength but lower modulus of elasticity than HEMAM-BDI. As discussed above, HEMAM-BDI is able to undergo more hydrogen bonding than BisGMA (δh ≅ 20.0 MPa1/2 and δh ≅ 10.0 MPa1/2, respectively) and these interactions reinforce the mechanical properties of crosslinked polymer systems [34]. On the other hand, the flexural strength for BisGMA compounds was significantly higher due to its rigid aromatic backbone and high crosslink density [34]. For BisGMA, the inclusion of EtOAc did not affect the mechanical properties, except that the addition of 20% solvent resulted in lower yield strength and modulus of elasticity. However, the incorporation of small amounts of ethanol/water (2% and 5%) increased the mechanical properties, which is in agreement with the higher degrees of conversion observed for these two groups. While water and ethanol can participate in hydrogen bonds and serve as a source of protons, EtOAc is an aprotic solvent, i.e., it cannot act as a hydrogen bond donor. In other words, EtOAc does not participate in reactions and serves only as the medium, but water and ethanol tend to show high dielectric constants and dipoles, and may participate actively in hydrogen bonding. Therefore, the inclusion of EtOH/H2O seems to have helped the network development by decreasing the initial viscosity just enough to assist the polymeric chains to form crosslinks more homogeneously. Additionally, the absence of reduction in mechanical properties after 7 days water storage for groups containing EtOH/H2O indicates that the ethanol may get adsorbed to the polymer, helping to increase intermolecular cross-linking interactions via hydrogen bonding. The addition of higher amounts greater than the observed threshold (15% and 20%), however, resulted in reduced mechanical properties, in spite of the significantly increased degree of conversion. This was an expected effect derived from the plasticization by water [35]. For the methacrylamide resin, in general the addition of solvents did not influence the yield strength, and the inclusion of higher amounts of EtOH/H2O (15% and 20%) resulted in significant lower elastic modulus. There are two possible reasons for that, the first one is based on the water plasticization effect on the polymeric network, and the second is that the high hydrogen bonding potential responsible for intermolecular interactions and ultimately translated in higher modulus was compromised by the greater spacing between the molecules due to the solvent addition.
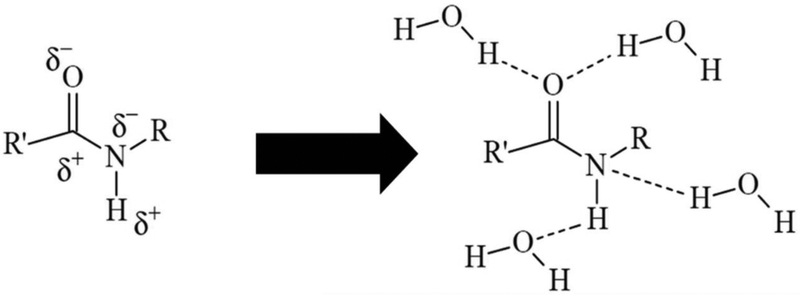
After water storage, as expected, the mechanical properties significantly decreased for all materials. However, this effect was particularly devastating for the methacrylamide/acrylamide system, to the point of rendering the bars untestable. This is explained by the susceptibility of the amides for establishing strong hydrogen bonds with water molecules, and essentially, becoming hydrated, as illustrated in Figure 6. In the water molecule, each hydrogen nucleus is covalently bound to the central oxygen atom by a pair of electrons and the other four outer-shell electrons are arranged surrounding the oxygen in two pairs. Despite being overall a neutral molecule, the partial positive charge on the hydrogen and negative charge on the oxygen are not uniformly distributed, producing a strong dipole. HEMAM-BDI, as a secondary methacrylamide, has a dipolar nature (C=O and N-H dipoles), capable of multiple hydrogen bonds with water molecules via hydrogen-bonding acceptor and also hydrogen-bond donor sites (Figure 6). Since the nitrogen atom in the amide is less electronegative than the oxygen and presents two non-bonded electrons, there is a delocalization by resonance of these electrons to the adjacent carbonyl, which makes the oxygen partially negatively charged (δ−) and susceptible to function as a hydrogen-bond acceptor, facilitating interaction with the molecule of water. Additionally, the hydrogen of the N-H dipole is partially positively charged (δ+), which allows it to function as a hydrogen-bond donor and also interact with a molecule of water. As a result of these interactions, the amides are extremely prone to absorb and retain water [36–39].
Figure 6.
Schematic representation of a secondary methacrylamide with the two dipoles: carbonyl (C=0) and amine (N-H) and the potential hydrogen bonds. (Adapted from De Ruiter2005).
Finally, in terms of the mechanical properties of the BisGMA mixtures, generally the addition of greater amounts of solvents resulted in less pronounced reduction of properties compared to HEMAM-BDI groups. Even with the incorporation of large amounts of solvents, which would be expected to decrease the glass transition temperature (Tg) and compromise mechanical properties, the lower % reduction in highly solvated mixtures may be due to an increased mobility of the polymerizable species, which resulted in higher degrees of conversion and improved packing of polymer chains in the network.
5. CONCLUSION
The presence of residual solvent affected kinetics of polymerization and mechanical properties of the two monomer systems. For either monomer system, a threshold in solvent concentration was identified up to which the rate of polymerization and conversion increased, in tandem with increases in dry mechanical properties. At the highest solvent concentrations, the materials based on either monomer had lower dry mechanical properties, as expected, but at least for the methacrylates, the reduction in properties after water storage was actually lower than for the unsolvated materials. No conclusion can be drawn in regards to the effect of the presence of solvents on wet mechanical properties for methacrylamide systems because the specimens were not testable, indicating more developments are needed before these monomers can be clinically useful in replacement of dimethacrylates for adhesive formulations. The effect of use of methacrylamides in adhesive-relevant properties (such as bond strengths) is unknown. In summary, these results demonstrate that, up to a certain threshold, which is monomer- and solvent-dependent, the presence of solvents in the polymerizing network may actually be beneficial.
Acknowledgements
The authors thank NIH-NIDCR for funding (U01-DE023756, K02-DE025280 and R01-DE026113).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Van Meerbeek B, Yoshida Y, Lambrechts P, Vanherle G, Duke ES, Eick JD, et al. A TEM study of two water-based adhesive systems bonded to dry and wet dentin. Journal of Dental Research. 1998;77:50–9. [DOI] [PubMed] [Google Scholar]
- [2].Ikeda T, De Munck J, Shirai K, Hikita K, Inoue S, Sano H, et al. Effect of evaporation of primer components on ultimate tensile strengths of primer-adhesive mixture. Dent Mater. 2005;21:1051–8. [DOI] [PubMed] [Google Scholar]
- [3].Sauro S, Pashley DH, Montanari M, Chersoni S, Carvalho RM, Toledano M, et al. Effect of simulated pulpal pressure on dentin permeability and adhesion of selfetch adhesives. Dent Mater. 2007;23:705–13. [DOI] [PubMed] [Google Scholar]
- [4].Garcia G, Fernandes KB, Garcia FC, D’Alpino PH, da Rocha Svizero N, Wang L. Solvent retention of contemporary commercial dentin bonding agents in a demineralized dentin matrix. Eur J Dent. 2010;4:293–7. [PMC free article] [PubMed] [Google Scholar]
- [5].Yiu CK, Pashley EL, Hiraishi N, King NM, Goracci C, Ferrari M, et al. Solvent and water retention in dental adhesive blends after evaporation. Biomaterials. 2005;26:6863–72. [DOI] [PubMed] [Google Scholar]
- [6].Pashley EL, Zhang Y, Lockwood PE, Rueggeberg FA, Pashley DH. Effects of HEMA on water evaporation from water-HEMA mixtures. Dent Mater. 1998; 14:6–10. [DOI] [PubMed] [Google Scholar]
- [7].Auras RA. Chapter 19 - Solubility of Gases and Vapors in Polylactide Polymers In: Letcher TM, editor. Thermodynamics, Solubility and Environmental Issues. Amsterdam: Elsevier; 2007. p. 343–68. [Google Scholar]
- [8].Van Landuyt KL, De Munck J, Snauwaert J, Coutinho E, Poitevin A, Yoshida Y, et al. Monomer-solvent phase separation in one-step self-etch adhesives. J Dent Res. 2005;84:183–8. [DOI] [PubMed] [Google Scholar]
- [9].Yoshioka A, Tashiro K. Solvent Effect on the Glass Transition Temperature of Syndiotactic Polystyrene Viewed from Time-Resolved Measurements of Infrared Spectra at the Various Temperatures and Its Simulation by Molecular Dynamics Calculation. Macromolecules. 2004;37:467–72. [Google Scholar]
- [10].Kostina J, Bondarenko G, Gringolts M, Rodionov A, Rusakova O, Alentiev A, et al. Influence of residual solvent on physical and chemical properties of amorphous glassy polymer films. Polymer International. 2013;62:1566–74. [Google Scholar]
- [11].Ekambaram M, Yiu CKY, Matinlinna JP. An overview of solvents in resin-dentin bonding. International Journal of Adhesion and Adhesives. 2015;57:22–33. [Google Scholar]
- [12].Delaviz Y, Finer Y, Santerre JP. Biodegradation of resin composites and adhesives by oral bacteria and saliva: a rationale for new material designs that consider the clinical environment and treatment challenges. Dent Mater. 2014;30:16–32. [DOI] [PubMed] [Google Scholar]
- [13].Moszner N, Zeuner F, Angermann J, Fischer UK, Rheinberger V. Monomers for adhesive polymers, 4: Synthesis and radical polymerization of hydrolytically stable crosslinking monomers. Macromolecular Materials and Engineering. 2003;288:621–8. [Google Scholar]
- [14].Anseth KS, Wang CM, Bowman CN. Reaction behaviour and kinetic constants for photopolymerizations of multi(meth)acrylate monomers. Polymer. 1994;35:3243–50. [Google Scholar]
- [15].Elliott JE, Anseth JW, Bowman CN. Kinetic modeling of the effect of solvent concentration on primary cyclization during polymerization of multifunctional monomers. Chemical Engineering Science. 2001;56:3173–84. [Google Scholar]
- [16].Salim Al-Ani AAS, Scarabello Stape TH, Mutluay M, Tjäderhane L, Tezvergil-Mutluay A. Incorporation of dimethyl sulfoxide to model adhesive resins with different hydrophilicities: Physico/mechanical properties. Journal of the Mechanical Behavior of Biomedical Materials. 2019;93:143–50. [DOI] [PubMed] [Google Scholar]
- [17].Tian M, Xu Y. Monomer reactivity ratios of acrylamide and 2-ethylhexyl acrylate determined by the elemental analysis method. Oxidation Communications. 2016;39:3357–69. [Google Scholar]
- [18].Chen F, Cook WD. Curing kinetics and morphology of IPNs from a flexible dimethacrylate and a rigid epoxy via sequential photo and thermal polymerization. European Polymer Journal. 2008;44:1796–813. [Google Scholar]
- [19].Serbutoviez C, Kloosterboer JG, Boots HMJ, Paulissen FAMA, Touwslager FJ. Polymerization-induced phase separation III. Morphologies and contrast ratios of polymer dispersed liquid crystals. Liquid Crystals. 1997;22:145–56. [Google Scholar]
- [20].Dickens SH, Stansbury JW, Choi KM, Floyd CJE. Photopolymerization Kinetics of Methacrylate Dental Resins. Macromolecules. 2003;36:6043–53. [Google Scholar]
- [21].Berchtold KA, Randolph TW, Bowman CN. Propagation and termination kinetics of cross-linking photopolymerizations studied using electron paramagnetic resonance spectroscopy in conjunction with near IR spectroscopy. Macromolecules. 2005;38:6954–64. [Google Scholar]
- [22].Asmussen E, Peutzfeldt A. Influence of UEDMA BisGMA and TEGDMA on selected mechanical properties of experimental resin composites. Dent Mater. 1998;14:51–6. [DOI] [PubMed] [Google Scholar]
- [23].Lovell LG, Stansbury JW, Syrpes DC, Bowman CN. Effects of Composition and Reactivity on the Reaction Kinetics of Dimethacrylate/Dimethacrylate Copolymerizations. Macromolecules. 1999;32:3913–21. [Google Scholar]
- [24].Bansil R, Herrmann HJ, Stauffer D. Computer simulation of kinetics of gelation by addition polymerization in a solvent. Macromolecules. 1984; 17:998–1004. [Google Scholar]
- [25].Nishitani Y, Yoshiyama M, Hosaka K, Tagami J, Donnelly A, Carrilho M, et al. Use of Hoy’s solubility parameters to predict water sorption/solubility of experimental primers and adhesives. European Journal of Oral Sciences. 2007;115:81–6. [DOI] [PubMed] [Google Scholar]
- [26].Barton AFM. CRC handbook of polymer-liquid interaction parameters and solubility parameters. Boca Raton: CRC Press; 1990. [Google Scholar]
- [27].Wu W, McKinney JE. Influence of Chemicals on Wear of Dental Composites. Journal of Dental Research. 1982;61:1180–3. [DOI] [PubMed] [Google Scholar]
- [28].Comer J, Tam K. Lipophilicity Profiles: Theory and Measurement In: Testa HvdW B, Folkers G and Guy R, editor. Pharmacokinetic Optimization in Drug Research: Wiley Online Library; 2007. [Google Scholar]
- [29].Palmer DS, McDonagh JL, Mitchell JBO, van Mourik T, Fedorov MV. First-Principles Calculation of the Intrinsic Aqueous Solubility of Crystalline Druglike Molecules. Journal of Chemical Theory and Computation. 2012;8:3322–37. [DOI] [PubMed] [Google Scholar]
- [30].Odian G Principles of polymerization. 4th ed. New York: Wiley Interscience; 2004. [Google Scholar]
- [31].Szczepanski CR, Pfeifer CS, Stansbury JW. A new approach to network heterogeneity: Polymerization Induced Phase Separation in photo-initiated, free-radical methacrylic systems. Polymer (Guildf). 2012;53:4694–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Abedin F, Ye Q, Good HJ, Parthasarathy R, Spencer P. Polymerization- and Solvent-Induced Phase Separation in Hydrophilic-rich Dentin Adhesive Mimic. Acta biomaterialia. 2014;10:3038–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Li L, Lee LJ. Photopolymerization of HEMA/DEGDMA hydrogels in solution. Polymer. 2005;46:11540–7. [Google Scholar]
- [34].Podgôrski M, Becka E, Claudino M, Flores A, Shah PK, Stansbury JW, et al. Ester-free thiol-ene dental restoratives—Part A: Resin development. Dental Materials. 2015;31:1255–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Frassetto A, Breschi L, Turco G, Marchesi G, Di Lenarda R, Tay FR, et al. Mechanisms of degradation of the hybrid layer in adhesive dentistry and therapeutic agents to improve bond durability—A literature review. Dental Materials. 2016;32:e41–e53. [DOI] [PubMed] [Google Scholar]
- [36].McMurry J Organic chemistry. Belmont, CA: Thomson-Brooks/Cole; 2004. [Google Scholar]
- [37].Morrison RT, Boyd RN. Organic Chemistry. 6th ed. Englewood Cliffs, NJ: Prentice Hall; 1992. [Google Scholar]
- [38].Solomons TWG, Fryhle CB. Organic Chemistry. 8th ed. Hoboken, NJ: John Wiley; 2004. [Google Scholar]
- [39].DeRuiter J Principles of Drug Action 1 - Amides and Related Fuctional Groups: Spring; 2005. [Google Scholar]