Abstract
Background:
3D Breast Magnetic Resonance Fingerprinting (MRF) technique enables T1 and T2 mapping in breast tissues. Combined repeatability and reproducibility studies on T1 and T2 relaxometry are lacking.
Purpose:
To assess test-retest and two-visit repeatability and inter-scanner reproducibility of 3D Breast MRF technique in a single-institution setting.
Study type:
Prospective
Subjects:
Eighteen women(median age 29 years, 22-33 years)underwent visit 1 scans on scanner 1. Ten of these women underwent test-retest scan repositioning after 10-minute interval. 13 women had visit 2 scans within 7-15 days in same menstrual cycle. Remaining 5 women had visit 2 scans in same menstrual phase in next menstrual cycle. Five women were also scanner at scanner 2 at both visits for inter-scanner reproducibility.
Field Strength/Sequence:
Two 3T MR scanners with 3D Breast MRF technique
Assessment:
T1 and T2 MRF maps of both breasts.
Statistical Tests:
Mean T1 and T2 values for normal fibroglandular tissues were quantified at all scans. For variability, between and within-subjects coefficients of variation (bCV and wCV respectively) were assessed. Repeatability was assessed with Bland-Altman analysis and coefficient of repeatability (CR). Reproducibility was assessed with inter-scanner CoV and Wilcoxon signed-rank test.
Results:
The bCV at test-retest scans was 9–12% for T1, 7-17% for T2, wCV was<4% for T1, and<7% for T2. For two visits in same menstrual cycle, bCV was 10–15% for T1, 13-17% for T2, wcV was< 7% for T1 and< 5% for T2 .For two visits in same menstrual phase, bCV was 6-14% for T1, 15-18% for T2, wCV was< 7% for T1 and < 9% for T2. For test-retest scans, CR for T1 and T2 were 130 ms and 11 ms. For two visit scans, CR was <290 ms for T1 and 10-14 ms for T2.Inter-scanner CoV was 3.3-3.6% for T1 and 5.1-6.6% for T2 with no differences between inter-scanner measurements (p=1.00 for T1, p=0.344 for T2).
Conclusion:
3D Breast MRF measurements are repeatable across scan timings and scanners and may be useful in clinical applications in breast imaging.
Keywords: Magnetic Resonance Fingerprinting, Relaxometry, Repeatability, Breast, Quantitative Imaging
INTRODUCTION
Quantitative property mapping techniques can complement conventional anatomical magnetic resonance (MR) imaging by allowing more objective assessment of pathophysiology and facilitating development of imaging biomarkers(1–3). Diffusion weighted imaging is routinely employed in breast MR imaging as a quantitative measure of tissue cellularity while dynamic contrast enhanced-MRI is primarily used as a semi-quantitative technique in breast imaging perfusion. Measurement of T1 and T2 relaxation times can provide additional information about normal and diseased breast tissue(4, 5). Previous studies on breast relaxometry have observed significant differences in T1 and T2 relaxation times between normal fibroglandular breast tissue and benign and malignant breast tumors(5, 6). Changes in T2 relaxation time has also been used to characterize response to chemotherapy in breast cancers(7–9). However the T1 and T2 values reported for normal breast tissue show a wide variation due to the differences in mapping techniques and field strengths(5, 10, 11). Similarly, studies on response assessment to treatment used different T2 mapping techniques, which affects comparability of these results(7–9). For quantitative imaging techniques to be successfully used in practice, assessment of measurement variability and repeatability are crucial to interpret results. While studies have been published on repeatability and reproducibility of breast tissue apparent diffusion coefficient (ADC) values, fibroglandular tissue density and fibroglandular tissue enhancement (12–14) and a recent study assessed repeatability and reproducibility metrics for both T1 mapping and ADC in breast(15), similar repeatability and reproducibility studies on combined T1 and T2 breast relaxometry are lacking.
Recently, a 3D Magnetic Resonance fingerprinting (MRF) technique was described for breast imaging that allows simultaneous and volumetric quantification of T1 and T2 relaxation times for both breasts in less than 6 minutes of scan time (16), at a resolution that is comparable to that for clinically employed ADC mapping. The ability to obtain rapid, volumetric and simultaneous T1 and T2 maps represents a significant advantage over previously described breast relaxometry methods, that typically measured only property at a time, required long acquisition times and had limited organ coverage. The T1 and T2 values measured with 3D breast MRF have shown to be accurate and comparable to literature with phantom and in-vivo validation respectively(16). Clinically, measurements obtained from 3D breast MRF may be useful for lesion characterization or assessment of treatment response. However, prior to using this technique for clinical applications, it is important to understand the variability and repeatability of this technique. Hence, the objective of this study was to assess the repeatability and reproducibility of 3D breast MRF technique in normal breast tissues.
MATERIALS AND METHODS
Scanning Protocol
In this Institution Review Board approved prospective study, 18 asymptomatic pre-menopausal women were scanned with 3D breast MRF after obtaining written informed consent. Scans were done on two 3T MR scanners (Siemens, Verio (MR1) and Skyra (MR2) as detailed below.
Scans Performed at Visit 1:
Baseline scans: All 18 women underwent scans on scanner 1 (MR1) for baseline data
Test-retest repeatability: Ten of these women also underwent test-retest scans on MR1, in which 3D breast MRF was performed before and after repositioning with a 10 minute inter-scan interval to assess test-retest repeatability.
Inter-scanner reproducibility: Five of these eighteen women also underwent same-day scans on second scanner (MR2).
Scans Performed at Visit 2:
Two-visit repeatability in different menstrual phases: Thirteen women were scanned again on MR1 within 7–15 days in different menstrual phases within the same menstrual cycle.
Two-visit repeatability in same menstrual phase: Remaining five women were scanned again on MR1 in the next menstrual cycle in the same menstrual phase as the baseline scans.
The same five women scanned for inter-scanner reproducibility in the first visit also underwent same-day scans on MR2 for second-visit assessment of inter-scanner reproducibility.
All scans were performed using a breast coil with eight receive channels. The 3D MRF sequence used in this study was based on a 2D Fast Imaging with Steady State Free Precession (FISP) based MRF acquisition(17), modified for 3D breast imaging (16). The data were acquired sequentially in 48 partitions, with each partition containing 12 segments; each segment comprising of a) magnetization-preparation module (three inversion recovery modules with inversion recovery times of 20, 100 or 250 ms and six T2 preparation modules with effective echo times of 50 or 90 ms), b) spectral-selective fat-saturation module to suppress the fat signal in breast adipose tissue and c) data acquisition window with variable flip angles ranging from 5° to 12°. Other settings included: FOV: 400×400 mm2, matrix size 256 × 256 mm2, TR/TE: 6.1/0.9ms, in-plane resolution: 1.6×1.6 mm2, partition thickness: 3 mm, craniocaudal coverage: 14 cm, number of partitions: 48, partial Fourier in the partition direction: 6/8(16). The overall acquisition time for 48 partitions was approximately 5 minutes 30 seconds. No subject-specific shimming sequence was performed for the MRF acquisition, as the FISP readout is less sensitive to B0 field inhomogeneity as compared to the balanced steady state free precession (SSFP) readout(18). As described previously (16), the effects of B1 heterogeneity are also compensated in the MRF acquisition scheme.
In addition to the 3D breast MRF sequence, a 3D gradient echo T1w fat saturated (FS) image was obtained for anatomic localization (scan parameters: FOV 300 × 300 mm2, TR/TE: 4.76/1.62 ms, flip angle: 10 degrees, fat saturation, resolution 0.8 × 0.9 × 1.0 mm3, acquisition time: 1 minute 29 seconds). The total table time per subject including the sagittal localizer, 3D T1w FS sequence and 3D breast MRF in each sitting was approximately 9 minutes.
Data Processing and Image Analysis
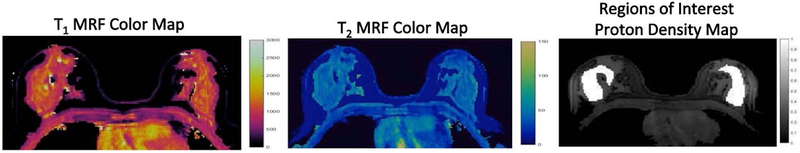
A dictionary including the signal evolutions from a wide range of T1 and T2 values (T1, 60 to 5000 ms; T2, 10 to 500 ms) was first calculated using Bloch equation simulations. The raw MR fingerprinting data were processed offline using Matlab (Matlab 2014a; MathWorks, Natick, Mass) and co-registered T1, T2 and proton density maps were generated using a pattern-matching process(16). The 3D breast MRF partition containing the largest homogenous area of normal fibroglandular breast tissue was selected. Region of Interests (ROIs) were manually drawn by one radiologist (8 years radiology experience) on proton density maps. Separate ROIs were drawn for right and left breast (Figure 1) and both T1 and T2 values were simultaneously obtained. For retest and repeatability analysis, ROIs were drawn on the partition showing similar distribution of breast fibroglandular tissue as compared to the reference scan. The cross-sectional anatomic landmarks (cardiac/breast) were additionally used as references for confirming the accuracy of partitions selected for repeat ROI annotations. The T1 and T2 values from all voxels within each ROI were extracted from quantitative T1 and T2 maps and averaged to obtain the mean T1 and T2 for each ROI.
Figure 1:
Representative T1 and T2 MR Fingerprinting color maps of normal breast tissue in a healthy volunteer. Regions of interest (ROIs) were drawn over both breasts on the proton density maps and both T1 and T2 relaxation times were simultaneously obtained.
Statistical Analysis
The mean T1 and T2 values were used for statistical analysis. The means of T1 and T2 were compared between right and left breast using Wilcoxon tests for related samples. The variability and repeatability statistics in this study are similar to those used previously in studies in normal breasts(12, 15). The variability of T1 and T2 measurements was assessed by coefficient of variation (CV). The between-subject CV (bCV) at each visit and for each scan session was expressed as a percentage ratio of standard deviation (SD) to the mean of measurements for each breast and for all breasts, the equation is as follows:
The within-subject CV (wCV) was defined as the percentage ratio of the within-subject SD (wSD) to the overall mean of measurements at each visit and each scan session. For measurements 1 and 2 (m1 and m2 respectively) at both visit 1/visit 2 and for test/retest scans for a total of n measurements in each analysis, the equations are as follows
The bCV and wCV values were computed separately for each side and both sides together.
The repeatability was assessed using Bland-Altman analysis(19). For both T1 and T2, scatterplots of the difference at two visits and for test-retest scans were plotted against the mean of measurements at two visits and for test-retest scans respectively. The SD of the difference (SDdiff) was estimated and upper and lower limits of agreement (LOA) was defined as follows: Upper LOA = mean difference + 1.96SDdiff; Lower LOA = mean difference - 1.96SDdiff. The 95% confidence intervals for mean difference; upper and lower LOAs were also reported using the appropriate statistics(19). Differences in measurements that fell within the LOA were considered clinically unimportant. The coefficient of repeatability (CR) was computed as 1.96 x √2 x SDdiff (2.77 x SDdiff). Kendall Tau Test was used to assess if there was any correlation between the difference and the mean of measurements at test/retest and two-visit scans.
Inter-scanner Reproducibility
Inter –scanner reproducibility of 3D breast MRF T1 and T2 was assessed on both scanners using Wilcoxon signed rank test to look for differences in measurements on two scanners. The coefficient of variation was estimated for all measurements on MR1 (CVMR1) and MR2 (CVMR2). The inter-scanner coefficient of variation (CVMR1-MR2) for all measurements calculated as percentage ratio of inter-scanner standard deviation [SD(MR1-MR2)] versus overall mean of measurements on both scanners. For measurements on MR1 (s1) and MR2 (s2) for a total of n measurements, the equations are as follows:
All statistical analysis was performed using statistical software package (SPSStatistics, version 20, IBM®).
RESULTS (TABLES AND PLOTS)
Subject Characteristics and Mean Statistics
The subjects’ ages ranged from 22–33 years with median age of 29 years. Table 1 details the mean T1 and T2 values for right and left breasts for all 18 subjects at visit 1. There was no statistical difference in mean T1 and T2 values between right vs. left breasts at baseline visit 1 (right vs. left; P = 0.89 for T1, P = 0.25 for T2). Figure 2 shows representative T1 and T2 MRF color maps from visit 1 test/retest and visit 2 scans from one subject. There were also no differences in the means of T1 and T2 for right and left breasts at both test/retest scans and two-visit repeatability scans (Table 2).
Table 1:
Subject characteristics, T1 and T2 values for fibroglandular tissue for right and left breasts at Visit 1 and scanner 1
| T1 at Visit 1 (ms) (Mean ± SD) |
T2 at Visit 1 (ms) (Mean ± SD) |
||||||
|---|---|---|---|---|---|---|---|
| Subject # | Age (years) |
Menstrual phase for Visit 1 |
Menstrual phase for Visit 2 |
Right Breast |
Left Breast | Right Breast |
Left Breast |
| 1 | 33 | Luteal | Proliferative | 1443 ± 524 | 1646 ± 327 | 50 ± 8 | 56 ± 13 |
| 2 | 29 | Luteal | Menstrual | 1187 ± 268 | 1110 ± 190 | 54 ± 8 | 51 ± 14 |
| 3 | 29 | Luteal | Proliferative | 1143 ± 141 | 1103 ± 201 | 41 ± 7 | 45 ± 9 |
| 4 | 29 | Proliferative | Luteal | 1282 ± 303 | 1220 ± 213 | 57 ± 8 | 56 ± 9 |
| 5 | 32 | Proliferative | Follicular | 1537 ± 240 | 1520 ± 203 | 62 ± 10 | 67 ± 12 |
| 6 | 31 | Menstrual | Proliferative | 1179 ± 212 | 1343 ± 267 | 48 ± 10 | 53 ± 9 |
| 7 | 28 | Luteal | Menstrual | 1311 ± 321 | 1104 ± 315 | 46 ± 11 | 57 ± 15 |
| 8 | 28 | Luteal | Proliferative | 1250 ± 303 | 1123 ± 341 | 47 ± 11 | 51 ± 11 |
| 9 | 30 | Menstrual | Proliferative | 1079 ± 380 | 1074 ± 384 | 40 ± 13 | 48 ± 16 |
| 10 | 22 | Proliferative | Luteal | 1127 ± 221 | 1128 ± 199 | 44 ± 8 | 41 ± 8 |
| 11 | 30 | Luteal | Menstrual | 1320 ± 235 | 1390 ± 221 | 53 ± 9 | 48 ± 9 |
| 12 | 26 | Proliferative | Luteal | 1115 ± 169 | 1151 ± 172 | 35 ± 5 | 44 ± 4 |
| 13 | 22 | Proliferative | Luteal | 1186 ± 242 | 1066 ± 117 | 55 ± 8 | 51 ± 5 |
| 14 | 23 | Luteal | Luteal | 1269 ± 189 | 1416 ± 212 | 47 ± 9 | 51 ± 8 |
| 15 | 28 | Luteal | Luteal | 1003 ± 239 | 1021 ± 185 | 49 ± 11 | 41 ± 7 |
| 16 | 30 | Proliferative | Proliferative | 1315 ± 206 | 1337 ± 262 | 49 ± 10 | 55 ± 9 |
| 17 | 30 | Menstrual | Menstrual | 1066 ± 162 | 1133 ± 150 | 36 ± 6 | 39 ± 6 |
| 18 | 24 | Luteal | Luteal | 1150 ± 262 | 1093 ± 294 | 56 ± 10 | 57 ± 10 |
| All Subjects | 1220 ± 135 | 1228 ± 174 | 48 ± 8 | 51 ± 7.0 | |||
Volunteers 1-13 underwent second visit scans within 7-15 days in the same menstrual cycle but different menstrual phases. Volunteers 14-18 were rescanned in the same menstrual phase over two successive menstrual cycles.
Figure 2:
Representative T1 and T2 MR Fingerprinting color maps from test-retest scans at visit 1 and visit 2 scan from one subject. The maps show similar range of numbers as seen from color-bars despite differences in breast positions and scan timings.
Table 2: Summary of fibroglandular tissue T1 and T2 values for right and left breast for all repeatability scans.
There was no difference in the means of T1 and T2 for right and left breasts at both visits using Wilcoxon tests for related samples.
| T1 relaxation time (ms) (Mean ± SD) |
T2 relaxation time (ms) (Mean ± SD) |
||||||
|---|---|---|---|---|---|---|---|
| No of subjects |
Right Breast |
Left Breast |
Right vs. Left P-value |
Right Breast |
Left Breast |
Right vs. Left P-value |
|
| Test Scan | 10 | 1154 ± 105 | 1164 ± 142 | 1.00 | 45 ± 8 | 48 ± 6 | 0.24 |
| Retest Scan | 1171 ± 112 | 1190 ± 135 | 0.89 | 44 ± 7 | 46 ± 7 | 0.41 | |
| Visit 1 Scan | 13 | 1243 ± 134 | 1259 ± 185 | 0.31 | 49 ± 7 | 52 ± 8 | 0.92 |
| Visit 2 Scan in same menstrual cycle | 1196 ± 170 | 1285 ± 184 | 0.92 | 49 ± 9 | 52 ± 8 | 0.70 | |
| Visit 1 Scan | 5 | 1161 ± 132 | 1200 ± 168 | 0.60 | 46 ± 8 | 49± 8 | 0.47 |
| Visit 2 Scan in same menstrual phase | 1183±76 | 1212 ± 168 | 0.69 | 47 ± 8 | 47 ± 7 | 0.55 | |
Between-Subject Variation and Within-Subject Variation
Visit 1Test-Retest scans (n = 10)
Figure 3 (a, b) show the scatter of T1 and T2 values at test-retest scans for both breasts. T1 values at test-retest scans ranged from 1003 ms to 1416 ms and T2 values ranged from 35 ms to 57 ms. The bCV for T1 at both scans was 9–12% and for T2 was 7–17% (Table 3). The wCV for T1 was < 4% and < 7% for T2 (Table 3).
Figure 3:
Scatter plots of repeated measurements for T1 and T2. Top row (a, b) shows results for same-day test-retest scans, middle row (c, d) shows results for two visit scans in different menstrual phases in the same menstrual cycle and bottom row (e, f) shows results for two visit scans in the same menstrual phase in the next menstrual cycle. The black line represents the identity line. Values for right and left breasts are shown separately.
Table 3:
Summary results for variability of fibroglandular tissue T1 and T2 relaxation times for test-retest scans.
| T1 relaxation time | T2 relaxation time | |||||
|---|---|---|---|---|---|---|
| bCV Test Scan (%) |
bCV Retest Scan (%) |
wCV (%) |
bCV Test Scan (%) |
bCV Retest Scan (%) |
wCV (%) |
|
| All Breasts (n = 20) | 10.77 | 10.73 | 3.16 | 7.13 | 6.76 | 4.92 |
| Right Breast (n = 10) | 9.30 | 9.72 | 3.01 | 17.16 | 14.74 | 6.48 |
| Left Breast (n = 10) | 12.22 | 11.19 | 2.87 | 12.95 | 15.41 | 5.80 |
Two-visit Scans at Different Menstrual Phases in the Same Menstrual Cycle (n = 13)
Figure 3 (c, d) show the scatter of T1 and T2 values at two visits for both breasts. T1 values at both visits ranged from 942 ms to 1646 ms and T2 values ranged from 32 ms to 67 ms. For two visits at different menstrual phases in the same menstrual cycle, the bCV for T1 was 10–15% and for T2 was 13–17% (Table 4). The wCV was < 7% for T1 and < 5% for T2 (Table 4).
Table 4:
Summary results for variability of fibroglandular tissue T1 and T2 relaxation times for different menstrual phase in the same menstrual cycle two-visits scans.
| T1 relaxation time | T2 relaxation time | |||||
|---|---|---|---|---|---|---|
| bCV Visit 1 (%) |
bCV Visit 2 (%) |
wCV (%) |
bCV Visit 1 (%) |
bCV Visit 2 (%) |
wCV (%) |
|
| All Breasts (n = 26) | 12.73 | 14.38 | 5.9 | 14.23 | 16.69 | 4.96 |
| Right Breast (n = 13) | 10.78 | 14.19 | 6.3 | 15.40 | 17.33 | 4.99 |
| Left Breast (n = 13) | 14.67 | 14.30 | 5.2 | 13.54 | 16.33 | 4.53 |
Two-visit Scans in Same Menstrual Phase (n = 5)
Figure 3 (e, f) shows the scatter of T1 and T2 values at same menstrual phases over two visits for both breasts. T1 values at both visits ranged from 1021 ms to 1416 ms and T2 values ranged from 36 ms to 57 ms. For two visits in the same menstrual phases, the bCV for T1 was 6–14% and for T2 was 15–18% (Table 5). The wCV was < 7% for T1 and < 9% for T2 (Table 5).
Table 5:
Summary results for variability of fibroglandular tissue T1 and T2 relaxation times for same menstrual phase two-visits scan.
| T1 relaxation time | T2 relaxation time | |||||
|---|---|---|---|---|---|---|
| bCV Visit 1 (%) |
bCV Visit 2 (%) |
wCV (%) |
bCV Visit 1 (%) |
bCV Visit 2 (%) |
wCV (%) |
|
| All Breasts (n = 10) | 12.75 | 5.68 | 4.49 | 15.87 | 14.55 | 4.05 |
| Right Breast (n = 5) | 11.36 | 6.41 | 6.23 | 18.2 | 18.00 | 5.84 |
| Left Breast (n = 5) | 14.03 | 5.20 | 4.39 | 16.82 | 14.82 | 8.43 |
Bland-Altman Analysis
Both T1 and T2 were repeatable between test-retest scans and two-visit scans independent of the timing of the second visit with respect to the menstrual cycle or menstrual phase (Figure 4).
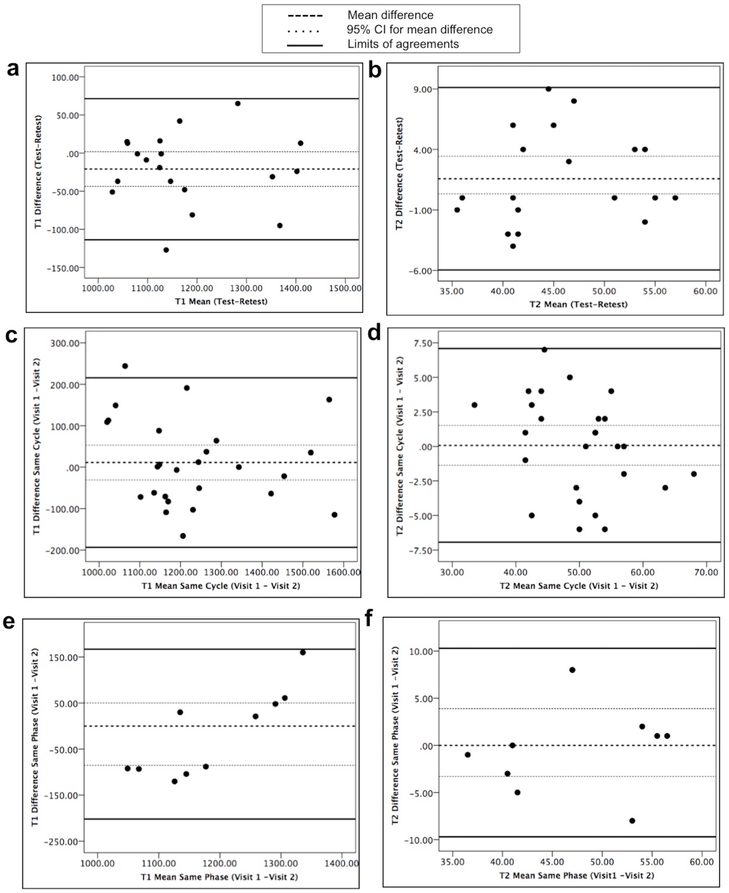
Figure 4:
Bland Altman plots for differences in measurements from two scans versus mean of measurements from two scans. Top row shows T1 (a) and T2 (b) plots for same-day test-retest scans, middle row shows T1 (c) and T2 (d) plots for two-visit scans in different menstrual phases in the same menstrual cycle and bottom row shows T1 (e) and T2 (f) plots for two-visit scans in the same menstrual phase in the next menstrual cycle. Upper and lower solid lines represent LOA, the center dashed line is the mean difference in measurement from two scans with 95% CI of mean difference in dotted lines.
Visit 1Test-Retest scans (n = 10)
As shown in the Bland-Altman plots in Figure 4 (a, b), the mean difference for T1 at test-retest was −21 ms (95% CI −44, 1.8 ms) with upper LOA (95% CI) of 71 ms (109, 33 ms) and lower LOA (95% CI) of −113 ms (−75, −151 ms). The CR for T1 was 130 ms. The mean difference for T2 at test-retest was 1.6 ms (95% CI −0.3, 3.4 ms) with upper LOA (95% CI) of 9 ms (12.3, 6.0 ms) and lower LOA (95% CI) of −6 ms (−2.9, −9.1 ms). The CR for T2 was 11 ms.
Two-visit scans at Different Menstrual Phases in the Same Menstrual Cycle (n = 13)
As shown in the Bland-Altman plots in Figure 4 (c, d), the mean difference for T1 at two visits was 11.03 ms (95% CI −31, 53 ms) with upper LOA (95% CI) of 216 ms (289,143 ms) and lower LOA (95% CI) of −194 ms (−121, −267 ms). The CR for T1 was 289 ms. The mean difference for T2 at two visits was 0.08 ms (95% CI −1.4, 1.5 ms) with upper LOA (95% CI) of 7 ms (9.6, 4.6 ms) and lower LOA (95% CI) of −7 ms (−4.4, −9.4 ms). The CR for T2 was 10 ms.
Two-visit Scans in Same Menstrual Phase (n = 5)
As shown in the Bland-Altman plots in Figure 4 (e, f), the mean difference for T1 at two visits was −18 ms (95% CI −85, 50 ms) with upper LOA (95% CI) of 167 ms (284,50 ms) and lower LOA (95% CI) of −202 ms (−86, −319 ms). The CR for T1 was 261 ms. The mean difference for T2 at two visits was 0.3 ms (95% CI −3.3, 3.9 ms) with upper LOA (95% CI) of 10 ms (16.6, 3.9 ms) and lower LOA (95% CI) of −10 ms (−3.4, −15.9 ms). The CR for T2 was 14 ms.
Kendall Tau analysis did not reveal statistically significant coefficient values for any of the comparisons suggesting that the differences between repeated measurements were independent of the means of repeated measurements for T1 and T2.
Inter-scanner Reproducibility Results
Figure 5 shows scatter of T1 and T2 measurements on MR2 versus MR1 at both visits. There were no statistical differences between inter-scanner T1 and T2 at both visits (Visit 1: (MR1-MR2) T1, p = 1.000, Visit 1: (MR1-MR2) T2, p = 0.344, Visit 2: (MR1-MR2) T1, p = 1.000, Visit 2: (MR1-MR2) T2, p = 0.109).
Figure 5:
Scatter plots of repeated measurements for T1 (a) and T2 (b) on scanner 2 (MR2) versus scanner 1 (MR1). Top row (a, b) shows the relationships at visit 1 and bottom row (c, d) shows the relationships at visit 2. The inter-scanner CoV [CV(MR1-MR2)] for T1 and T2 was 3.6% and 6.6% at visit 1 and 3.3% and 5.1% at visit 2 respectively without significant differences in inter-scanner measurements. The black line represents the identity line.
At visit 1, for T1, the CVMR1 , CVMR2 and (CVMR1-MR2) were 12.2%, 11.78% and 3.6% respectively. For T2, the CVMR1 , CVMR2 and (CVMR1-MR2) were 16.7%, 15.8% and 6.6% respectively. At visit 2, for T1, the CVMR1 , CVMR2 and (CVMR1-MR2) were 5.6%, 6.97% and 3.3% respectively. For T2, the CVMR1 , CVMR2 and (CVMR1-MR2) were 15.6%, 10.2% and 5.1% respectively.
DISCUSSION
This work shows the repeatability and inter-scanner reproducibility of 3D breast MRF measurements in a single-institution setting. The T1 and T2 measured using 3D breast MRF were repeatable across multiple volunteers at different scan timings. The ranges of T1 and T2 for normal fibroglandular tissue observed in this study are similar to the mean T1 of 1198 ± 99 ms and 1445 ± 93 and T2 of 40 ± 5 ms and 54 ± 9 values reported previously(11, 16) with comparable T1 and T2 for right and left breasts for all subjects.
There was a higher bCV as compared to wCV, indicating a greater inter-subject variability but relatively consistent intra-subject measurements. The greater inter-subject variation may be due to the physiological differences in breast density and in timings of menstrual phases between different women. While we are unaware of previous data on bCV for T1 and T2 available for comparison, the bCV for 3D breast RF T1 (9–15%) and T2 (7–18%) across all sub-groups is comparable to the previously reported bCV of 18% for ADC and much less than bCV of 47% and 61% for fibroglandular tissue enhancement and fibroglandular density respectively (12). Thus breast relaxometry may potentially be a more reliable quantitative measure of breast fibroglandular tissue characteristics as compared to fibroglandular tissue density/enhancement metrics despite differences in scan timings between subjects and subject positioning on repeated scans.
In this study, wCV was low for both T1 and T2. The wCV for 3D breast MRF T1 is comparable to the wCV of T1 mapping with variable flip angle technique (15). Additionally, the low wCV for T2 obtained with 3D breast MRF can allow repeated and reliable mapping of both T1 and T2 in the same subjects on follow-up scans. The wCV for 3D breast MRF T1 and T2 are also comparable to the 5–11% wCV reported for ADC (12–15, 20) and lesser than 13–22% wCV reported for fibroglandular tissue density and fibroglandular tissue enhancement respectively (12). In this study, we scanned a few women in same menstrual phase and the variability in T1 and T2 metrics was similar to repeat scans in different menstrual phases. The small within-subject variability in measurements may make this technique useful for quantitative assessment in longitudinal studies wherein baseline and follow-up clinical scans in patients may be scheduled at different menstrual phases. Prior studies on ADC mapping have shown that the overall fluctuation in normal breast ADC is relatively small (20) and there were no differences in ADC and fibroglandular tissue density in relation to menstrual cycle(12, 13). There is less contemporary evidence on menstrual variation on T1 and T2. Older studies have shown no significant alterations in T1 values in different stages of menstrual cycles (21, 22) while for T2 values, the results have been mixed, with one study reporting no change (23) while another reporting significant change between second and 3rd week of cycles(21). However, the studies on T2 relaxometry were performed using older mapping techniques and at extremely low field scanners (0.02 T) and results may not reflect true breast physiology. Thus 3D breast MRF may also be a useful technique in simultaneously evaluating the physiologic variability in T1 and T2 over menstrual cycles on the current high field scanners used in clinical practice.
In general, for test-retest scans, there was lesser variability and lower CR as compared to two-visit scans. The 5 women scanned in same menstrual phase showed a slightly greater difference in T2 values on follow-up scans compared to 13 women scanned in the different menstrual phase and this is likely due to the smaller number of subjects and lesser number of measurements in the same-menstrual phase group compared to different-menstrual phase cohort. Overall, based on Bland-Altman plots and CR, a T1 difference of < 290 ms and T2 difference of 10–14 ms on repeat scanning is likely to be physiologic and clinically unimportant. Conversely, an effect size greater than this would represent a true measurable difference due to pathology or disease.
The 3D breast MRF technique also had a low inter-scanner variation, ranging from 3–4% for T1 and 5–7% for T2 at two separate visits. To our knowledge, there has been no prior work on inter-scanner reproducibility of both T1 and T2 together. A recent work explored the inter-site reproducibility of B1-corrected variable flip angle T1 mapping technique and showed an average difference of 8.4% for fibroglandular tissue and 7.5% for adipose tissue(15), both of which are slightly higher than the differences obtained for 3D breast MRF T1. Moreover, 3D breast MRF does not require prior subject-specific shimming or B1 correction as the low-flip angles used in the acquisition scheme mitigates the effect of B1 inhomogeneity (16). This may make the technique less prone to T1 mapping errors due to B1 inhomogeneity and easier to apply across scanners and sites in practice (24). As 3D breast MRF acquisition scheme also uses a fat suppression module; breast fat matches to lowest dictionary entry and the maps generated depict the quantitative distribution of T1 and T2 fibroglandular tissue. However, imperfect fat suppression or partial volume effects may still theoretically affect measurements and this may be better evaluated with larger sample population in the future.
This study has several limitations. First, all volunteers were healthy young premenopausal women. There may be some physiological variation in relaxation times due to loss of hormonal stimulation or decreased fibroglandular tissue volume with age. While one study showed a slight increase in T1 relaxation times with age (21), in the study by Chen et al(16), older patients had a slightly lower T1 in healthy contralateral breast compared to younger, healthy volunteers with a mean difference of < 200 ms while T2 values were similar in both groups. Second, single-partition ROIs were drawn instead of volumetric ROIs. While partitions selected for drawing repeat ROIs were anatomically similar to the partition used for baseline data, in the future volumetric ROIs may allow more precision in measurements. Third, this was a single-institution, single observer study. However, our sample size is comparable to some of the other previously published single–institution repeatability studies in breast imaging (12, 15, 20). This is an initial feasibility study on the repeatability and reproducibility of this breast relaxometry technique before expanding it to larger populations and multiple institutions, or employing it for clinical applications in breast oncologic imaging.
In conclusion, 3D breast MRF T1 and T2 measurements in normal breast tissue are repeatable across various scan timings and scanners and may potentially be useful in clinical applications in breast imaging and longitudinal follow-up of individual subjects.
Acknowledgments
Grant Support and Conflicts of Interest: Siemens Healthineers and NIH grants 1R01CA208236, 1R01EB016728, 1R01DK098503, 1R01EB017219
REFERENCES
- 1.Boone JM: Radiological interpretation 2020: Toward quantitative image assessment. Med Phys 2007; 34:4173–4179. [DOI] [PubMed] [Google Scholar]
- 2.Sullivan DC: Imaging as a quantitative science. Radiology 2008; 248:328–332. [DOI] [PubMed] [Google Scholar]
- 3.Yankeelov TE, Abramson RG, Quarles CC: Quantitative multimodality imaging in cancer research and therapy. Nat Rev Clin Oncol 2014; 11:670–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McSweeney MB, Small WC, Cerny V, Sewell W, Powell RW, Goldstein JH: Magnetic resonance imaging in the diagnosis of breast disease: use of transverse relaxation times. Radiology 1984; 153:741–744. [DOI] [PubMed] [Google Scholar]
- 5.Merchant TE, Thelissen GR, de Graaf PW, Nieuwenhuizen CW, Kievit HC, Den Otter W: Application of a mixed imaging sequence for MR imaging characterization of human breast disease. Acta Radiol Stockh Swed 1987 1993; 34:356–361. [PubMed] [Google Scholar]
- 6.Liu L, Yin B, Shek K, et al. : Role of quantitative analysis of T2 relaxation time in differentiating benign from malignant breast lesions. J Int Med Res 2018:0300060517721071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tan PC, Pickles MD, Lowry M, Manton DJ, Turnbull LW: Lesion T(2) relaxation times and volumes predict the response of malignant breast lesions to neoadjuvant chemotherapy. Magn Reson Imaging 2008; 26:26–34. [DOI] [PubMed] [Google Scholar]
- 8.Manton DJ, Chaturvedi A, Hubbard A, et al. : Neoadjuvant chemotherapy in breast cancer: early response prediction with quantitative MR imaging and spectroscopy. Br J Cancer 2006; 94:427–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu L, Yin B, Geng DY, Lu YP, Peng WJ: Changes of T2 Relaxation Time From Neoadjuvant Chemotherapy in Breast Cancer Lesions. Iran J Radiol 2016; 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Edden RAE, Smith SA, Barker PB: Longitudinal and Multi-Echo Transverse Relaxation Times of Normal Breast Tissue at 3 Tesla. J Magn Reson Imaging JMRI 2010; 32:982–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rakow-Penner R, Daniel B, Yu H, Sawyer-Glover A, Glover GH: Relaxation times of breast tissue at 1.5T and 3T measured using IDEAL. J Magn Reson Imaging JMRI 2006; 23:87–91. [DOI] [PubMed] [Google Scholar]
- 12.Aliu SO, Jones EF, Azziz A, et al. : Repeatability of Quantitative MRI Measurements in Normal Breast Tissue. Transl Oncol 2014; 7:130–137. [The Quantitative Imaging Network] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O’Flynn EAM, Morgan VA, Giles SL, deSouza NM: Diffusion weighted imaging of the normal breast: reproducibility of apparent diffusion coefficient measurements and variation with menstrual cycle and menopausal status. Eur Radiol 2012; 22:1512–1518. [DOI] [PubMed] [Google Scholar]
- 14.Newitt DC, Zhang Z, Gibbs JE, et al. : Test–retest repeatability and reproducibility of ADC measures by breast DWI: Results from the ACRIN 6698 trial. J Magn Reson Imaging ; 0 DOI: 10.1002/jmri.26539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sorace AG, Wu C, Barnes SL, et al. : Repeatability, reproducibility, and accuracy of quantitative mri of the breast in the community radiology setting. J Magn Reson Imaging ; 0 DOI: 10.1002/jmri.26011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen Y, Panda A, Pahwa S, et al. : Three-dimensional MR Fingerprinting for Quantitative Breast Imaging. Radiology 2018:180836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hamilton JI, Jiang Y, Chen Y, et al. : MR fingerprinting for rapid quantification of myocardial T1 , T2 , and proton spin density. Magn Reson Med 2017; 77:1446–1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jiang Y, Ma D, Seiberlich N, Gulani V, Griswold MA: MR Fingerprinting Using Fast Imaging with Steady State Precession (FISP) with Spiral Readout. Magn Reson Med 2015; 74:1621–1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bland JM, Altman DG: Statistical methods for assessing agreement between two methods of clinical measurement. Lancet Lond Engl 1986; 1:307–310. [PubMed] [Google Scholar]
- 20.Partridge SC, McKinnon GC, Henry RG, Hylton NM: Menstrual cycle variation of apparent diffusion coefficients measured in the normal breast using MRI. J Magn Reson Imaging JMRI 2001; 14:433–438. [DOI] [PubMed] [Google Scholar]
- 21.Delille J-P, Slanetz PJ, Yeh ED, Kopans DB, Garrido L: Physiologic changes in breast magnetic resonance imaging during the menstrual cycle: perfusion imaging, signal enhancement, and influence of the T1 relaxation time of breast tissue. Breast J 2005; 11:236–241. [DOI] [PubMed] [Google Scholar]
- 22.Dean KI, Majurin M-L, Komu M: Relaxation times of normal breast tissues. Acta Radiol 1994; 35:258–261. [PubMed] [Google Scholar]
- 23.Martin B, el Yousef SJ: Transverse relaxation time values in MR imaging of normal breast during menstrual cycle. J Comput Assist Tomogr 1986; 10:924–927. [DOI] [PubMed] [Google Scholar]
- 24.Park B, Choi BS, Sung YS, et al. : Influence of B1-Inhomogeneity on Pharmacokinetic Modeling of Dynamic Contrast-Enhanced MRI: A Simulation Study. Korean J Radiol 2017; 18:585–596. [DOI] [PMC free article] [PubMed] [Google Scholar]