Abstract
Background:
Emerging evidence suggests that pain interference (PI) and certain chronic pain conditions, including osteoarthritis (OA) may be associated with risk for Alzheimer’s disease and Related Dementias (ADRD). However, research exploring the relation of OA and PI to ADRD remains sparse.
Objective:
To assess the association of OA and PI to ADRD using cross-sectional data from a representative sample of U.S. adults aged ≥65 years.
Design:
Retrospective cross-sectional.
Study sample:
Older adults (age≥65 years) drawn from the Medical Expenditure Panel Survey (MEPS, 2009–2015).
Methods:
OA was identified using both medical conditions files and participant responses to arthritis-specific queries. ADRD was ascertained using the medical conditions files. PI was defined as reported frequent PI with normal activities (PIA). OA and PIA were categorized as a composite variable: 1) OA with PIA; 2) OA without PIA; 3) No OA with PIA; and 4) No OA and no PIA (reference group). Adjusted associations of OA and PIA to ADRD were assessed using logistic regression and adjusted for biological, demographic, socio-economic, lifestyle, and health conditions.
Results:
Overall, 27.1% had OA, of whom 47.6 % reported PIA vs. 31.1% of those without OA; 2.8% had diagnosed ADRD. Adults with PIA either with or without OA had significantly higher odds of ADRD relative to those without OA or PIA (AOR’s=1.37, 95%CI-1.01, 1.86 (p=0.04) and 1.44, 95%CI-1.13, 1.82 (p=0.003), respectively).
Conclusion:
PIA in both the presence and absence of OA remained significantly and positively associated with ADRD after adjustment for multiple confounders.
INTRODUCTION
Alzheimer’s Disease and Related Dementias (ADRD) is a constellation of neurodegenerative disorders that impose substantial clinical, humanistic, and economic burden on patients and their caregivers, payors, and healthcare systems.1,2 Many factors have been associated with ADRD risk. These include biological, socio-economic, physical and mental health conditions, genetic factors, health behaviors, and polypharmacy.2–4 There is also emerging evidence from both cross-sectional5,6 and longitudinal studies7,8 that chronic pain and pain interference with usual activities (PIA) may increase risk for ADRD. For example, in a longitudinal U.S. study of 1,114 adults (114 new onset dementia cases), pain interference was associated with significantly increased risk for dementia.7 Likewise, in a longitudinal survey study of 10,065 older U.S. adults, persistent pain was associated with both accelerated cognitive decline (a strong predictor of ADRD9) and increased risk for probable dementia.8 In contrast, longitudinal studies in a representative sample of 6,515 British adults10 and a small U.S. cohort (N=441)11 indicated a link only between high pain severity and subsequent deterioration in memory, but not in other cognitive domains.
In addition, there are limited data suggesting that risk for ADRD may be increased in adults with certain chronic pain conditions, including osteoarthritis (OA), the second leading cause of chronic pain.12 To date, only two studies have explored the relationship between OA and cognitive functioning or dementia.13,14 In a large cross-sectional study of US Appalachian adults, OA and related joint pain were strongly associated with perceived memory loss, suggesting that adults with OA and pain may be at elevated risk for ADRD.13 Broadly consistent with these findings, a nested case-control study of Taiwanese nationals reported significantly higher risk of dementia in OA patients (HR= 1.25; 95% CI, 1.10–1.43) compared to their age-sex matched non-OA counterparts.14
Many patients with OA also report pain that interferes with normal activities.12,15 In the U.S., 80% of adults with OA suffer some mobility limitations, and at least 25% are restricted in major activities of daily living.16 PIA has been associated with moderate to severe levels of pain intensity,17 suggesting that pain interference may be a proxy indicator of pain severity and burden that can be used for clinical decision making.18
Although the mechanisms underlying the putative association of OA and chronic pain/PIA to the development of ADRD remain unknown, OA may contribute to ADRD pathogenesis via both direct and indirect pathways. For example, chronic musculoskeletal conditions such as OA have been linked to adverse structural and functional changes within the Central Nervous System (CNS),19 including alterations in brain structures involved in cognitive processing6 and disruption of brain networks20 essential to cognitive function. OA has also been associated with activation of central pain processing pathways21 and hypersensitivity of the CNS,22 factors in turn linked to disruption of cognitive functioning.6,23 Peripheral factors may also play a role. For example, the proinflammatory soft tissue changes associated with OA, including nociceptor alterations in the joints, have been linked to adverse nociceptive changes in the brainstem, thalamocortical system, and elsewhere24; such alterations may, in turn, impair cognitive processing and lead to cognitive dysfunction. In addition, OA may contribute to cognitive decline and the development of dementia via effects on behavioral factors. For example, OA and pain are strongly and reciprocally related to sleep and mood impairment,25–27 both significant risk factors for ADRD.28–30
While research to date suggests that chronic pain and certain chronic pain conditions, including OA, may play a role in the development of dementia, the relation of OA and PIA to ADRD remains little explored. To help address this gap, the primary objective of this study was to examine the association of OA and PIA to ADRD among elderly (age ≥ 65 years) in the U.S., using cross-sectional data from a nationally representative survey. We hypothesize that older adults with OA who reported PIA will have higher odds of ADRD compared to older adults without OA or PIA.
METHODS
Study Design
We adopted a cross-sectional study design using data from a nationally representative household survey, the Medical Expenditure Panel Survey (MEPS). Data were pooled across multiple years.
Data Source
MEPS is a survey of U.S. non-institutionalized, civilian households and their families, conducted annually since 1996. Households for the survey are selected through a complex survey design described in detail elsewhere.31 Information on demographics, lifestyle characteristics, socioeconomic status, chronic health conditions, health care utilization and cost, prescription medications, and other factors was collected using the Computer Assisted Personal Interview questionnaire.
Study Sample
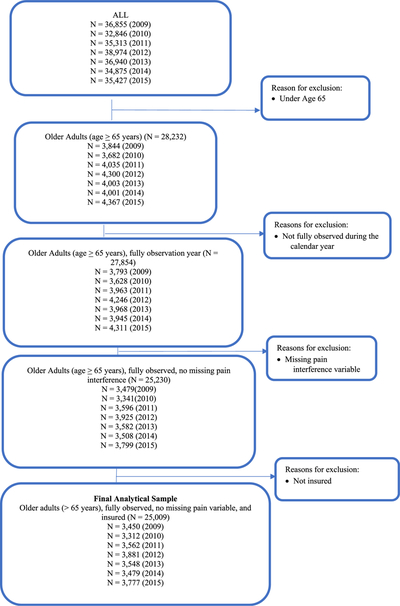
The study sample comprised adults ≥ 65 years of age who were alive during the calendar year and who did not have missing information on PIA status. We also excluded participants without health insurance (0.4%). A flow diagram of the study sample selection process is given in Appendix Figure 1. Because ADRD prevalence in non-institutionalized adults is low,32 we pooled data from multiple calendar years (2009–2015). Pooling data can reduce the relative standard error of estimates, leading to higher reliability and permitting subgroup analyses. This is a standard practice recommended by MEPS investigators and that has been commonly employed in prior studies using MEPS data.33,34 The final sample size was 25,009 (N’s by year = 3450 (2009); 3312 (2010); 3562 (2011); 3881 (2012); 3548 (2013); 3479 (2014); and 3777 (2015)).(See Appendix Figure 1).
Measures
Dependent variable: ADRD status - Yes/No
The presence or absence of ADRD was ascertained using the medical conditions file. Medical conditions were reported for each household member seeking care either from inpatient settings, outpatient clinics, emergency rooms, or other provider settings. These medical conditions were recorded “verbatim and converted by professional coders into fully-specified International Classification of Diseases, 9th Edition, clinical modification (ICD-9-CM) codes”.31 To protect confidentiality, MEPS releases only 3-digit ICD-9-CM codes. We used ICD-9-CM codes 290.xx, 294.xx, and 331.xx to identify ADRD based on published literature.35–37
Key Independent Variable: OA and PIA Categories
Identification of OA:
MEPS investigators consider arthritis a priority condition. Therefore, all household members over 18 years of age were queried whether or not they have been ever diagnosed with arthritis and, if so, which type of arthritis (Rheumatoid arthritis versus OA). Participants reporting diagnosed OA in response to these queries or who had a record of OA diagnosis in the medical conditions file were considered to have OA. As MEPS groups ICD-9-CM codes into a manageable number of conditions using clinical classification codes software (CCS),38 we used the CCS code (203) to identify OA in the medical conditions file.
PIA:
A mail survey was administered to collect information on PIA and other domains of health-related quality of life. PIA was measured using the one-item from the MOS Short Form (SF)-12. This item asked how much pain “interfered with normal work (including both work outside the home and housework)” during the past 4 weeks prior to the interview. Responses were recorded using a 5-item Likert scale: 1) ‘Not at all’; 2) ‘A little bit’; 3) ‘Moderately’; 4) ‘Quite a bit’; and 5) ‘Extremely’. We recoded participant responses into a binary variable indicating presence or absence of pain interference activity as follows: 1) No PIA (not at all or a little bit); and 2) PIA (moderately, quite a bit, and extremely). Combining PIA using one-item from the MOS Short Form (SF)-12 is a common practice in published literature.39–41
We then combined the binary OA and PIA variables to create a composite variable with 4 categories: 1) OA with PIA; 2) OA without PIA; 3) No OA with PIA and 4) No OA and No PIA.
Other Explanatory Variables:
In our adjusted analyses, we also included other factors previously linked to OA and/or ADRD. These included: biological characteristics, i.e.: sex (female/male); age category (65–69, 70–74, 75–79, and 80 years and older) and race (Non-Hispanic White, African American, Hispanic, and other); demographics and socio-economic status, i.e.: marital status (married, divorced/separated, widowed, single); education (Less than 12 years, 12 years/GED, and >12 years) and income as measured by percentage of federal poverty line (FPL) (poor (< 100% FPL), near poor/low income (≥100–<200% FPL), middle income (>200–<400% FPL), and high income (≥400% FPL)); lifestyle/behavioral factors, i.e.: current smoking status (smoker/non-smoker), exercise (yes/no); body mass index (BMI, calculated as weight in kg/height in m2), categorized as underweight/normal (<25), overweight (25–29.9), and obese (≥30)); access to healthcare services, measured by health insurance (private versus public health insurance); chronic conditions, including diabetes, heart disease, high cholesterol, depression, and anxiety (Y/N); and polypharmacy (defined as prescriptions of ≥6 medications (yes/no)). We also included as covariates region of residence (Northeast, Midwest, South, and West) and survey calendar year (2009–2015). In addition, we also added any use of prescribed analgesics during the year (NSAIDS and opioids) as covariates in separate ancillary multivariable analyses.
Statistical analysis
Statistically significant subgroup differences in categorical variables by OA and PIA groups with ADRD status were determined by Rao-Scott chi-square tests. Multivariable logistic regression models were used to assess the cross-sectional association of OA and PIA to ADRD. These regression analyses controlled for biological factors (sex, age, race), demographics and socio-economic status (marital status, education, income, and insurance type); chronic physical and mental health conditions (e.g. diabetes, heart disease, depression, anxiety); polypharmacy; and behavioral/lifestyle factors (obesity, smoking, and exercise), as well as region of residence and MEPS year.
As MEPS adopted a complex survey design, all analyses were performed using SAS survey procedures (SAS v 9.4 (SAS Institute, Inc)). To obtain annualized weighted numbers, we divided the annual weights by the number of years pooled, again a standard practice in the published literature.42 As data for this study included information from self-administered questionnaires, we used weights that were appropriate for responses derived from self-administered questionnaires.43
RESULTS
The number of older participants in our study represented an estimated average of 42.7 million older adults (age ≥ 65 years). A majority of the sample were female (55.8%), white (78.4%), and married (56.0%). Only 39.1% had high family income (income ≥ 400% of the FPL) (Data not presented in tabular form). Overall, 27.1% of this study sample of older adults reported having OA and 35.6% reported moderate, severe, and extreme PIA. When examined by the four categories of OA and PIA, 12.9% reported OA with PIA; 14.2% reported OA without PIA; 22.7% reported No OA with PIA; and 50.2% reported No OA and No PIA (Data not presented in tabular form).
In this sample, the four OA and PIA groups (OA with PIA, OA without PIA, No OA with PIA, No OA and No PIA) differed significantly in all measured biological, demographic, and socio-economic characteristics, lifestyle/behavioral factors, and chronic physical and mental health conditions (see Appendix Table 1). Notably, those with OA but without PIA had significantly better profiles with respect to certain socioeconomic, behavioral/lifestyle, and health-related factors than those without OA or PIA (and dramatically better profiles with respect to virtually all factors than those with OA and PIA) (Appendix Table 1).
Table 1 summarizes the characteristics of older adults by ADRD status. Overall, 2.8% of older adults had ADRD. Relative to elders with No PIA and No OA, ADRD was significantly more common in those reporting OA with PIA (4.3 vs. 2.0%) and those with PIA and No OA (4.6 vs. 2.0%) (p’s < 0.001). Treated prevalence of ADRD increased significantly with age and was significantly higher in older adults with vs. without polypharmacy (4.5 vs. 1.8%), diabetes (3.4 vs. 2.6%), heart diseases (3.4 vs. 2.4%), depression (5.4 vs. 2.4%), and anxiety (4.7% vs. 2.5%) (p’s <0.05). ADRD was also significantly more common in sedentary than in physically active seniors (4.1 vs. 1.1%).
Table 1.
Selected Characteristics of older adults (≥ 65 years) by Alzheimer’s Diseases and Related Dementia Status Medical Expenditure Panel Survey, 2009–2015
| ADRD | No ADRD | |||
|---|---|---|---|---|
| N | Wt. row % | N | Wt. row % | |
| ALL | 727 | 2.8 | 24282 | 97.2 |
| OA and PIA† | ||||
| OA with PIA | 134 | 4.3 | 2,917 | 95.7 |
| OA without PIA | 44 | 1.3 | 3,031 | 98.7 |
| No OA with PIA | 287 | 4.6 | 6,092 | 95.4 |
| No OA and No PIA | 262 | 2.0 | 12,242 | 98.0 |
| Biological Factors | ||||
| Sex | ||||
| Female | 447 | 3.0 | 13,872 | 97.0 |
| Male | 280 | 2.5 | 10,410 | 97.5 |
| Race/Ethnicity† | ||||
| Non-Hispanic White | 415 | 2.7 | 14,397 | 97.3 |
| African American | 164 | 3.8 | 4,308 | 96.2 |
| Hispanic | 89 | 2.1 | 3,390 | 97.9 |
| Other-race | 59 | 3.1 | 2,187 | 96.9 |
| Age† | ||||
| 65–69 Years | 55 | 0.5 | 8,584 | 99.5 |
| 70–74 Years | 93 | 1.6 | 5,776 | 98.4 |
| 75–79 Years | 120 | 2.4 | 4,263 | 97.6 |
| 80 years or older | 459 | 7.1 | 5,659 | 92.9 |
| Socio-economic Status | ||||
| Poverty Status†* | ||||
| Poor | 128 | 3.2 | 3720 | 96.8 |
| Near Poor | 228 | 3.7 | 5968 | 96.3 |
| Middle Income | 223 | 3.0 | 7138 | 97.0 |
| High Income | 148 | 2.0 | 7456 | 98.0 |
| Education (in years)† | ||||
| <12 years | 276 | 4.5 | 6139 | 95.5 |
| 12 years/GED | 246 | 3.2 | 7539 | 96.8 |
| >12 years | 183 | 1.8 | 10320 | 98.2 |
| Life-style/Behavioral Factors | ||||
| Current Smoking† | ||||
| Current smoker | 50 | 2.1 | 2292 | 97.9 |
| Other | 661 | 2.8 | 21390 | 97.2 |
| Obesity | ||||
| Underweight/Normal | 326 | 3.6 | 7830 | 96.4 |
| Overweight | 244 | 2.7 | 8889 | 97.3 |
| Obese | 145 | 1.8 | 7164 | 98.2 |
| Exercise†** | ||||
| Yes | 117 | 1.1 | 10506 | 98.9 |
| No | 604 | 4.1 | 13607 | 95.9 |
| Treatment Factors | ||||
| Polypharmacy† | ||||
| Polypharmacy (>=6 medications)) | 401 | 4.5 | 8531 | 95.5 |
| No polypharmacy (<6 medications) | 326 | 1.8 | 15751 | 98.2 |
| Access to Healthcare | ||||
| Insurance Coverage† | ||||
| Public | 260 | 2.3 | 11451 | 97.7 |
| Private | 467 | 3.3 | 12831 | 96.7 |
| Chronic Conditions | ||||
| Diabetes† | ||||
| Yes | 229 | 3.4 | 6724 | 96.6 |
| No | 498 | 2.6 | 17556 | 97.4 |
| Heart Disease† | ||||
| Yes | 348 | 3.4 | 9307 | 96.6 |
| No | 379 | 2.4 | 14973 | 97.6 |
| High Cholesterol | ||||
| Yes | 483 | 2.8 | 15774 | 97.2 |
| No | 244 | 2.7 | 8498 | 97.3 |
| Depression† | ||||
| Yes | 159 | 5.4 | 2651 | 94.6 |
| No | 568 | 2.4 | 21631 | 97.6 |
| Anxiety† | ||||
| Yes | 121 | 4.7 | 2351 | 95.3 |
| No | 606 | 2.5 | 21931 | 97.5 |
Note: Based on pooled data from multiple years older adults (age > 65 years), who were alive during the calendar year, had health insurance and did not have missing values for the PIA variable (N in 2009 = 3450; N in 2010 = 3312; N in 2011 = 3562; N in 2012 = 3881; N in 2013 = 3548; N in 2014 = 3479; and N in 2015 = 3777).
Exercise was measured as 3 or more times/week in 2009 and 2010 and 5 or more times/week in 2011 through 2015.
Indicates statistical significance (p < .05) based on Rao-Scott Chi square tests.
Abbreviations: OA: Osteoarthritis, PIA: Pain interference with usual activity; wt.%: weighted percentage.
The results of the unadjusted logistic regression indicated a significant association between PIA and ADRD, both in the presence and absence of OA (Table 2). Compared to older adults without OA and without PIA, those with both OA and PIA were 2.3 times as likely (OR= 2.28, 95% CI= 1.68, 3.10; p< 0.0001) and those with No OA and PIA were 2.4 times as likely (OR=2.42, 95% CI= 1.92, 3.04; p < 0.0001) to report ADRD. Adjustment for biological factors (age, sex, age, and race/ethnicity) only modestly attenuated these associations (AOR’s for ADRD, for OA with PIA and No OA and PIA, respectively, = 1.88, (95% CI = 1.39, 2.54) and 1.98 (95% CI = 1.58, 2.49), p’s=0.0001), but strengthened the apparent inverse association OA without PIA to ADRD (AOR = 0. 66, 95% CI = 0.44, 0.99; p < 0.04).
Table 2.
Unadjusted Odds Ratios (UOR) and Adjusted Odds Ratios (AOR) and 95% Confidence Intervals (CI) from Logistic Regressions on Alzheimer’s Disease and related dementiasamong Older adults (≥ 65 years) Medical Expenditures Panel Survey, 2009–2015
| Model 1: Unadjusted Model | |||
| UOR | 95%CI | P-Value | |
| OA with PIA | 2.28 | [ 1.68, 3.10] | 0.0001 |
| OA without PIA | 0.70 | [0.47, 1.05] | 0.0856 |
| PIA and No OA | 2.42 | [1.92, 3.04] | 0.0001 |
| No PIA and No OA (ref) | 1.00 | - | |
| Model 2: Adjusted for Biological Factors (Sex, Age, and Race/ethnicity) | |||
|---|---|---|---|
| AOR | 95%CI | ||
| OA with PIA | 1.88 | [1.39, 2.54] | 0.0001 |
| OA without PIA | 0.66 | [0.44, 0.99] | 0.0423 |
| No OA with PIA | 1.98 | [1.58, 2.49] | 0.0001 |
| No PIA and No OA (ref) | 1.00 | - | |
| Model 3: Fully adjusted model‡ | |||
| AOR | 95%CI | ||
| OA with PIA | 1.37 | [1.01, 1.86] | 0.0408 |
| OA without PIA | 0.66 | [0.43, 0.99] | 0.0460 |
| PIA and No OA | 1.44 | [1.13, 1.82] | 0.0029 |
| No PIA and No OA (ref) | 1.00 | - | |
Note: Based on pooled data from multiple years older adults (age ≥ 65 years), who were alive during the calendar year, had health insurance and did not have missing values for PIA
Fully adjusted model controlled for age, sex, race, marital status, poverty status, education, obesity, current smoking, physical activity, polypharmacy, insurance, diabetes, heart disease, high cholesterol, depression, anxiety, region, and calendar year. The fully adjusted model had a c-statistic of 0.81.
Abbreviations: AOR: Adjusted odds ratios; CI: 95% Confidence Intervals; OA: Osteoarthritis; PIA: Pain interference with usual activity;
Additional adjustment for demographics, socio-economic status, behavioral/lifestyle factors, insurance type, chronic physical and mental health conditions, polypharmacy, region, and calendar year further reduced but did not eliminate the significant, positive associations of OA with PIA (AOR = 1.37, 95% CI = 1.01, 1.86; p=0.041) and PIA without OA to ADRD (AOR = 1.44, 95% CI =1.13, 1.82, p<0.003); however, additional adjustment for these factors did not appreciably alter the inverse association of OA without PIA to ADRD (AOR = 0.66, 95% CI= 0.43, 0.99; p =0.046). (Table 2). Inclusion of analgesics in the ancillary adjusted model did not alter any of the observed associations of OA and PIA to ADRD (data not shown).
Other variables significantly and positively associated with ADRD (Appendix Table 2) in the fully adjusted analyses included: age (AOR for 80+ vs. 65–69 years= 10.90, 95% CI= 6.92, 17.17; p < 0.01), African American race (AOR=1.56, 95% CI= 1.15, 2.11; p=0.0045), lack of physical activity (AOR=2.36, 95% CI = 1.80, 3.10; p < 0.001), and lower education (AOR for less than 12 years =1.64, 95% CI = 1.17, 2.31; p= 0.004). ADRD also showed a significant positive relationship to depression (AOR =2.06, 95% CI= 1.57, 2.70; p=0.0001), anxiety (AOR= 1.46, 95% CI= 1.04, 2.05; p= 0.0309), and polypharmacy (AOR=1.90, 95% CI= 1.45, 2.47; p=0.0001). In ancillary adjusted analysis (data not presented), any use of prescription analgesic medications (opioids and NSAIDs) was not significantly associated with the odds of ADRD (NSAIDs p = 0.8516; opioids p = 0.6883).
DISCUSSION
In this first population-based cross-sectional US study on OA, PIA and ADRD, nearly one in four (27.1%) community-dwelling older adults reported osteoarthritis and 2.8% reported ADRD. We found that PIA in OA and no OA was strongly and positively associated with ADRD after adjustment for multiple correlates. Our findings are broadly consistent with those of a nested-case control study of Taiwanese Nationals indicating an increased risk for dementia in adults with OA14 and a cross-sectional study of Appalachian adults, which documented a strong association of OA and related joint pain to perceived memory loss.13 Our findings are also in general agreement with cross-sectional,5,6,23 case-control,44,45 and longitudinal studies7,8,11 documenting significant associations of non-specific chronic pain5–8,11 and other (non-OA) chronic pain conditions5,6,44,45 to impairment in cognitive functioning. Notably, in one recent study of a small US cohort followed for an average of 4.4 years, pain interference was associated with significantly increased risk for incident dementia.7 In contrast, in their 4-year longitudinal study of British adults, Veronese et al. found no association between non-specific chronic pain and overall decline in cognitive function, although, severe pain was associated with significantly greater memory decline.10
While mechanisms underlying the observed association of OA and PIA to ADRD remain unknown, OA and PIA may contribute to cognitive decline and subsequent development of ADRD via both direct and indirect pathways. For example, as discussed above, pain, both in the presence and absence of OA, may lead to adverse changes in brain structure and function and in CNS activation and sensitivity that increase risk for cognitive dysfunction and ultimately, ADRD.6,19,21–23 PIA in OA may also impair sleep, contribute to depression, and lead to lifestyle changes such as reduced physical activity that further increase the risk for cognitive decline and dementia.
In our study, older adults with OA without PIA appeared to have lower odds of ADRD compared to those without OA and without PIA. The reasons for this finding are not clear. However, it may reflect either effective treatment of OA or asymptomatic OA status. As we could not assess effectiveness of OA treatment, we were unfortunately unable to control for this factor in our analyses. However, we adjusted for prescription analgesics (NSAIDs and opioids) and inclusion of these variables did not appreciably alter odds ratios. It has to be noted that all types of analgesics use are not captured in MEPS (e.g., non-prescription NSAIDS were not recorded). Notably, NSAIDS have been associated with reduced risk for incident cognitive impairment and dementia in several observational studies46,47 and with lower risk for cognitive decline in a recent meta-analysis of 11 prospective cohort studies,48 although studies regarding the relation of opioid use to subsequent cognitive function have produced inconsistent findings.13 As most OA patients rely on NSAIDs and other pain medications, failure to control adequately for this factor may have biased observed AOR estimates, and may help explain the apparent inverse association of OA to ADRD. In addition, those with OA and without PIA had significantly better profiles with respect to certain socioeconomic, behavioral/lifestyle, and health conditions than those without OA or PIA. Those with OA and without PIA also had dramatically better profiles with respect to virtually all factors than those with OA and PIA (Appendix Table 1), which may also have contributed to the observed inverse AOR of ADRD in this group.
Associations of other factors with ADRD observed in this study were consistent with those reported in prior published studies. For example, age was strongly and positively associated with the likelihood of ADRD in this study, consistent with findings of previous epidemiological studies.2,49 Likewise, the elevated risk of ADRD in African Americans observed in our sample (OR=1.56) is in agreement with findings of a recent meta-analysis of population-based studies, which indicated increased risk for both prevalent and incident Alzheimer’s disease risk in African American vs. Caucasian Americans (AOR =1.56 and 1.64, respectively).50 We also observed higher odds of ADRD in older adults who reported less education (AOR = 1.64), physical inactivity (AOR= 2.36), depression (AOR=2.06), anxiety (AOR = 1.46) and polypharmacy (AOR = 1.90). These findings are consistent with the previous cross-sectional and longitudinal studies.2–4,51
This study has several strengths, including the population-based design and the large, nationally representative sample of older, ethnically diverse, community-dwelling U.S. adults. In our analyses, we were able to adjust for many covariates associated with OA and/or ADRD, reducing the likelihood of confounding. However, our study also has several important limitations. The cross-sectional nature of the data does not allow the determination of causal relationships, and reverse causality, while unlikely, cannot be ruled out. Much of the data gathered were reliant on self-report, introducing the possibility of recall and other forms of information bias. Given that both OA and chronic pain have been linked to increased mortality risk.52–54 survival bias may also have influenced our findings, potentially attenuating observed risk estimates. Moreover, the differential under-reporting of pain common in patients with dementia55 may likewise have biased risk estimates toward the null. Furthermore, the pain interference question captured pain experienced within the past 4 weeks. Our study sample was restricted to adults 65 years and older, limiting generalizability to other age groups. Although we were able to adjust for many potential confounders, we cannot rule out the possibility of unmeasured confounding.
Conclusion:
In this large, cross-sectional study in a representative sample of U.S. adults, PIA both in the presence and absence of OA was associated with significantly increased likelihood of ADRD after adjustment for multiple demographic, socioeconomic, lifestyle, health related, and other factors. Future population-based studies that track individuals over time are needed to confirm and extend these findings and to explore potential underlying mechanisms.
Acknowledgements:
Research reported in this publication was supported in part by the National Institute of General Medical Sciences of the National Institutes of Health under Award Number 2U54GM104942–02, WVCTSI and in part by the Alzheimer’s Research and Prevention Foundation (ARPF). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the ARPF.
Appendices
Appendix Fig. 1.
Study Sample Selection: Medical Expenditure Panel Survey (MEPS), 2009–2015.
Appendix Table I.
ICharacteristics of Older Adults (≥65 years) By Osteoarthritis and PIA Status (Column Percentages) Medical Expenditure Panel Survey (MEPS), 2009–2015
| ALL | OA with PIA | OA without PIA | No OA with PIA | No OA and No PIA | ||||
|---|---|---|---|---|---|---|---|---|
| N | Wt. % | N | Wt. % | N | Wt. % | N | Wt.% | |
| 3,051 | 100 | 3,075 | 100 | 6,379 | 100 | 12,504 | 100 | |
| Biological Factors | ||||||||
| Sex* | ||||||||
| Female | 2,169 | 68.2 | 2,082 | 66.3 | 3,654 | 55.0 | 6,414 | 50.1 |
| Male | 882 | 31.8 | 993 | 33.7 | 2,725 | 45.0 | 6,090 | 49.9 |
| Race* | ||||||||
| White | 2,081 | 83.9 | 2,279 | 87.1 | 3,171 | 71.6 | 7,281 | 77.6 |
| African American | 455 | 6.8 | 375 | 5.7 | 1,478 | 11.5 | 2,164 | 8.4 |
| Latino | 320 | 5.1 | 225 | 3.3 | 1,134 | 10.4 | 1,800 | 7.7 |
| Other-race | 195 | 4.3 | 196 | 3.8 | 596 | 6.4 | 1,259 | 6.2 |
| Age* | ||||||||
| 65–69 Years | 900 | 27.0 | 1,024 | 33.4 | 1,977 | 29.7 | 4,738 | 36.8 |
| 70–74 Years | 666 | 23.1 | 757 | 24.9 | 1,394 | 21.1 | 3,052 | 24.8 |
| 75–79 Years | 576 | 19.0 | 560 | 17.9 | 1,158 | 18.6 | 2,089 | 16.7 |
| 80 years or older | 909 | 30.9 | 734 | 23.8 | 1,850 | 30.5 | 2,625 | 21.7 |
| Demographic Characteristics | ||||||||
| Marital Status* | ||||||||
| Married | 1,394 | 51.3 | 1,680 | 58.8 | 2,953 | 50.1 | 6,816 | 59.0 |
| Widowed | 979 | 29.9 | 850 | 25.3 | 2,023 | 30.1 | 3,173 | 23.6 |
| Separated/Divorced | 531 | 15.0 | 415 | 12.2 | 1,056 | 15.4 | 1,848 | 13.1 |
| Never married | 147 | 3.8 | 130 | 3.7 | 347 | 4.4 | 667 | 4.3 |
| Socio-Economic Status | ||||||||
| Poverty Status*,† | ||||||||
| Poor | 507 | 10.0 | 306 | 6.1 | 1,357 | 12.1 | 1,678 | 7.5 |
| Near Poor | 838 | 26.3 | 632 | 18.6 | 1,959 | 29.8 | 2,767 | 19.9 |
| Middle Income | 865 | 28.4 | 897 | 27.9 | 1,790 | 30.4 | 3,809 | 29.6 |
| High Income | 841 | 35.2 | 1,240 | 47.4 | 1,273 | 27.6 | 4,250 | 42.9 |
| Education*(in years) | ||||||||
| <12 years | 723 | 17.1 | 450 | 10.7 | 2,357 | 27.7 | 2,885 | 15.8 |
| 12 years/GED | 1,052 | 36.8 | 925 | 28.9 | 2,005 | 33.9 | 3,803 | 30.6 |
| >12 years | 1,259 | 45.6 | 1,686 | 60.1 | 1,892 | 37.0 | 5,666 | 52.9 |
| Life-style/Behavioral Factors | ||||||||
| Body Mass Index* | ||||||||
| Underweight/Normal | 822 | 28.1 | 998 | 33.8 | 1,875 | 30.6 | 4,461 | 36.5 |
| Over weight | 913 | 29.4 | 1,155 | 38.3 | 2,140 | 34.9 | 4,925 | 40.9 |
| Obese | 1,275 | 42.5 | 874 | 28.0 | 2,226 | 34.5 | 2,934 | 22.7 |
| Current Smoking* | ||||||||
| Current smoker | 270 | 8.2 | 168 | 5.6 | 715 | 11.2 | 1,189 | 9.2 |
| Other | 2,705 | 89.8 | 2,852 | 93.0 | 5,483 | 86.4 | 11,011 | 88.8 |
| Exercise*,† | ||||||||
| Yes | 880 | 29.2 | 1,519 | 50.8 | 1,937 | 31.8 | 6,287 | 52.2 |
| No | 2,156 | 70.2 | 1,545 | 48.9 | 4,388 | 67.4 | 6,122 | 47.2 |
| Treatment Factor | ||||||||
| Polypharmacy* | ||||||||
| Polypharmacy ≥ 6 | 1,697 | 56.5 | 1,078 | 35.4 | 3,051 | 49.3 | 3,106 | 25.0 |
| No polypharmacy <6 | 1,354 | 43.5 | 1,997 | 64.6 | 3,328 | 50.7 | 9,398 | 75.0 |
| Access to Healthcare | ||||||||
| Insurance Coverage* | ||||||||
| Public | 1,383 | 52.3 | 1,725 | 61.7 | 2,346 | 44.9 | 6,257 | 57.2 |
| Private | 1,668 | 47.7 | 1,350 | 38.3 | 4,033 | 55.1 | 6,247 | 42.8 |
| Chronic Conditions | ||||||||
| Diabetes* | ||||||||
| Yes | 1,015 | 30.6 | 630 | 17.5 | 2,352 | 33.7 | 2,956 | 20.9 |
| No | 2,036 | 69.4 | 2,445 | 82.5 | 4,026 | 66.3 | 9,547 | 79.1 |
| Heart Disease* | ||||||||
| Yes | 1,557 | 52.9 | 1,201 | 40.8 | 2,964 | 48.8 | 3,933 | 33.4 |
| No | 1,493 | 47.1 | 1,874 | 59.2 | 3,415 | 51.2 | 8,570 | 66.6 |
| High Cholesterol* | ||||||||
| Yes | 2,205 | 72.4 | 2,038 | 66.7 | 4,475 | 70.2 | 7,539 | 61.6 |
| No | 844 | 27.6 | 1,036 | 33.3 | 1,901 | 29.8 | 4,961 | 38.4 |
| Depression* | ||||||||
| Yes | 621 | 20.9 | 350 | 12.2 | 931 | 15.8 | 908 | 8.2 |
| No | 2,430 | 79.1 | 2,725 | 87.8 | 5,448 | 84.2 | 11,596 | 91.8 |
| Anxiety* | ||||||||
| Yes | 480 | 15.9 | 339 | 11.7 | 817 | 14.0 | 836 | 7.4 |
| No | 2,571 | 84.1 | 2,736 | 88.3 | 5,562 | 86.0 | 11,668 | 92.6 |
| Region and Year of Interview | ||||||||
| Region* | ||||||||
| Northeast | 499 | 19.5 | 560 | 21.1 | 922 | 16.6 | 2,098 | 19.1 |
| Midwest | 687 | 23.0 | 749 | 24.3 | 1,111 | 20.1 | 2,526 | 22.3 |
| South | 1,226 | 38.5 | 1,117 | 36.5 | 2,771 | 40.7 | 4,631 | 35.3 |
| West | 639 | 19.1 | 649 | 18.1 | 1,575 | 22.6 | 3,249 | 23.3 |
| Calendar Year | ||||||||
| 2009 | 433 | 13.5 | 385 | 11.7 | 926 | 12.9 | 1,706 | 12.9 |
| 2010 | 406 | 13.7 | 423 | 13.1 | 833 | 12.7 | 1,650 | 12.9 |
| 2011 | 443 | 13.3 | 451 | 14.7 | 904 | 13.6 | 1,764 | 13.5 |
| 2012 | 471 | 13.8 | 454 | 13.9 | 1,034 | 15.1 | 1,922 | 14.4 |
| 2013 | 422 | 15.0 | 445 | 15.1 | 873 | 14.8 | 1,808 | 14.6 |
| 2014 | 454 | 16.3 | 428 | 14.9 | 846 | 14.8 | 1,751 | 15.7 |
| 2015 | 422 | 14.3 | 489 | 16.6 | 963 | 16.2 | 1,903 | 16.1 |
Note: Based on pooled data from multiple years older adults (age > 65 years), who were alive during the calendar year, had health insurance and did not have missing values for the PIA variable (N in 2009 = 3450; N in 2010 = 3312; N in 2011 = 3562; N in 2012 = 3881; N in 2013 = 3548; N in 2014 = 3479; and N in 2015 = 3777).
represent significant group differences by osteoarthritis PIA groups based on Rao-Scott Chi square tests.
Exercise was measured as 3 or more times/week in 2009 and 2010 and 5 or more times/week in 2011 through 2015.
PIA = Pain Interference with usual activity, wt. = weighted column percentage.
Appendix Table II.
Adjusted Odds Ratios (AOR) and 95% Confidence Intervals (CI) of Biological, Demographic, Socio-economic, Lifestyle/Behavioral, and Health Conditions from Logistic Regression on Alzheimer’s Diseases and Related Dementias in adults ≥65 years, Medical Expenditure Panel Survey, 2009e2015
| AOR | 95%CI | Significance | |
|---|---|---|---|
| Biological Factors | |||
| Sex | |||
| Male (ref) | |||
| Female | 0.89 | [0.68, 1.16] | 0.3903 |
| Race | |||
| White Non-Hispanic (ref) | |||
| African American | 1.56 | [1.15, 2.11] | 0.0045 |
| Hispanic | 0.66 | [0.43, 1.00] | 0.0498 |
| Other-race | 1.21 | [0.75, 1.96] | 0.4280 |
| Age | |||
| 65–69 Years (ref) | |||
| 70–74 Years | 2.97 | [1.87, 4.70] | 0.0001 |
| 75–79 Years | 4.12 | [2.58, 6.58] | 0.0001 |
| GE 80 years | 10.9 | [6.92, 17.17] | 0.0001 |
| Demographic and Socio-Economic status | |||
| Marital Status | |||
| Married (ref) | |||
| Widowed | 1.00 | [0.76, 1.31] | 0.9728 |
| Separated/Divorced | 1.19 | [0.82, 1.72] | 0.3707 |
| Never married | 0.77 | [0.42, 1.43] | 0.4102 |
| Poverty Status | |||
| High Income (ref) | |||
| Poor | 0.68 | [0.50, 0.93] | 0.0167 |
| Low Income | 0.83 | [0.63, 1.09] | 0.1882 |
| Middle Income | 0.91 | [0.69, 1.21] | 0.5137 |
| Education (in years) | |||
| >12 years (ref) | |||
| <12 years | 1.64 | [1.17, 2.31] | 0.0043 |
| 12 years/GED | 1.34 | [1.00, 1.78] | 0.0481 |
| Behavioral/Lifestyle Factors | |||
| Obesity | |||
| Underweight/Normal (ref) | |||
| Over weight | 0.86 | [0.69, 1.07] | 0.1704 |
| Obese | 0.52 | [0.37, 0.74] | 0.0003 |
| Current Smoking | |||
| Other (ref) | |||
| Current smoker [Continued] | 0.96 | [0.65, 1.43] | 0.8402 |
| Exercise* | |||
| Yes (ref) | |||
| No | 2.36 | [1.80, 3.10] | 0.0001 |
| Treatment Factor | |||
| Polypharmacy | |||
| <6 prescription meds (ref) | |||
| ≥6 prescription meds | 1.9 | [1.45, 2.47] | 0.0001 |
| Access to healthcare | |||
| Insurance Coverage | |||
| Private (ref) | |||
| Public Insurance | 0.84 | [0.66, 1.08] | 0.1734 |
| Chronic Conditions | |||
| Diabetes | |||
| No (ref) | |||
| Yes | 1.16 | [0.88, 1.53] | 0.2968 |
| Heart Disease | |||
| No (ref) | |||
| Yes | 0.83 | [0.65, 1.07] | 0.1542 |
| High Cholesterol | |||
| No (ref) | |||
| Yes | 0.88 | [0.71, 1.09] | 0.2492 |
| Depression | |||
| No (ref) | |||
| Yes | 2.06 | [1.57, 2.70] | 0.0001 |
| Anxiety | |||
| No (ref) | |||
| Yes | 1.46 | [1.04, 2.05] | 0.0309 |
Note: Based on pooled data from multiple years (N in 2009 = 3450; N in 2010 = 3312; N in 2011 = 3562; N in 2012 = 3881; N in 2013 = 3548; N in 2014 = 3479; and N in 2015 = 3777), who were alive during the calendar year, had health insurance and did not have missing values for the PIA variable.
Abbreviations: ADRD: ref = Reference group, P-values based on survey logistic regression.
Exercise was measured as 3 or more times/week in 2009 and 2010 and 5 or more times/week in 2011 through 2015.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: The authors report no conflicts of interest.
Contributor Information
Mohammad Ikram, Department of Pharmaceutical Systems and Policy, West Virginia University School of Pharmacy, Robert C. Byrd Health Sciences Center [North], P.O. Box 9510 Morgantown, WV 26506–9510.
Kim Innes, Department of Epidemiology, West Virginia University School of Public Health, Morgantown, WV 26506.
Usha Sambamoorthi, Department of Pharmaceutical Systems and Policy, West Virginia University School of Pharmacy, P.O. Box 9510 Morgantown, WV 26506–9510.
REFERENCES
- 1.Deb A, Thornton JD, Sambamoorthi U, Innes K. Direct and indirect cost of managing alzheimer’s disease and related dementias in the United States. Expert Rev Pharmacoecon Outcomes Res. 2017;17(2):189–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alzheimer’s Association. 2018 Alzheimer’s disease facts and figures. Alzheimer’s Dement. 2018;14(3):367–429. [Google Scholar]
- 3.Burke SL, Maramaldi P, Cadet T, Kukull W. Associations between depression, sleep disturbance, and apolipoprotein E in the development of Alzheimer’s disease: dementia. Int psychogeriatrics. 2016;28(9):1409–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Park H-Y, Park J-W, Song HJ, Sohn HS, Kwon J-W. The Association between Polypharmacy and Dementia: A Nested Case-Control Study Based on a 12-Year Longitudinal Cohort Database in South Korea. Laks J, ed. PLoS One. 2017;12(1):e0169463. doi: 10.1371/journal.pone.0169463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Higgins DM, Martin AM, Baker DG, Vasterling JJ, Risbrough V. The Relationship Between Chronic Pain and Neurocognitive Function A Systematic Review. Clin J Pain. 2018;34(3):262–275. doi: 10.1097/Ajp.0000000000000536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moriarty O, McGuire BE, Finn DP. The effect of pain on cognitive function: a review of clinical and preclinical research. Prog Neurobiol. 2011;93(3):385–404. [DOI] [PubMed] [Google Scholar]
- 7.Ezzati Ali, Wang Cuiling, Katz Mindy J., Derby Carol A., Zammit Andrea R., Zimmerman Molly E., et al. The Temporal Relationship between Pain Intensity and Pain Interference and Incident Dementia. Curr Alzheimer Res. 2019;16(2):109–115. doi: 10.2174/1567205016666181212162424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Whitlock EL, Diaz-Ramirez LG, Glymour MM, Boscardin WJ, Covinsky KE, Smith AK. Association Between Persistent Pain and Memory Decline and Dementia in a Longitudinal Cohort of Elders. JAMA Intern Med. 2017;177(8):1146–1153. doi: 10.1001/jamainternmed.2017.1622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mendonca MD, Alves L, Bugalho P. From Subjective Cognitive Complaints to Dementia: Who is at Risk?: A Systematic Review. Am J Alzheimers Dis Other Demen. 2016;31(2):105–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Veronese Nicola, Koyanagi Ai, Solmi Marco, Thompson Trevor, Maggi Stefania, Schofield Patricia, et al. Pain is not associated with cognitive decline in older adults: A four-year longitudinal study. Maturitas. 2018;115:92–96. doi: 10.1016/j.maturitas.2018.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van der Leeuw G, Ayers E, Leveille SG, Blankenstein AH, van der Horst HE, Verghese J. The Effect of Pain on Major Cognitive Impairment in Older Adults. J Pain. July 2018. doi: 10.1016/j.jpain.2018.06.009 [DOI] [PubMed] [Google Scholar]
- 12.Arthritis Foundation. Arthritis Statistics and Facts - Book of Trusted Facts & Figures. 2018. Vol 2; 2018 https://www.arthritis.org/about-arthritis/understanding-arthritis/arthritis-statistics-facts.php. [Google Scholar]
- 13.Innes KE, Sambamoorthi U. The Association of Perceived Memory Loss with Osteoarthritis and Related Joint Pain in a Large Appalachian Population. Pain Med (Malden, Mass). 2018;9(7):1340–1356. doi:doi: 10.1093/pm/pnx107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang S-W, Wang W-T, Chou L-C, Liao C-D, Liou T-H, Lin H-W. Osteoarthritis Increases the Risk of Dementia: A Nationwide Cohort Study in Taiwan. Sci Rep. 2015;5:10145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Breivik H, Collett B, Ventafridda V, Cohen R, Gallacher D. Survey of chronic pain in Europe: prevalence, impact on daily life, and treatment. Eur J pain. 2006;10(4):287. [DOI] [PubMed] [Google Scholar]
- 16.Ma VY, Chan L, Carruthers KJ. Incidence, prevalence, costs, and impact on disability of common conditions requiring rehabilitation in the United States: stroke, spinal cord injury, traumatic brain injury, multiple sclerosis, osteoarthritis, rheumatoid arthritis, limb loss, and back pa. Arch Phys Med Rehabil. 2014;95(5):986–995.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eslami Vahid, Katz Mindy J., White Robert S., Sundermann Erin, Jiang Julie M., Ezzati Ali, et al. Pain Intensity and Pain Interference in Older Adults: Role of Gender, Obesity and High-Sensitivity C-Reactive Protein. Gerontology. 2017;63(1):3–12. doi: 10.1159/000446651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jensen MP. Measuring pain interference In: The Pain Stethoscope: A Clinician’s Guide to Measuring Pain. Springer; 2011:23–27. [Google Scholar]
- 19.Pelletier R, Higgins J, Bourbonnais D. Is neuroplasticity in the central nervous system the missing link to our understanding of chronic musculoskeletal disorders? BMC Musculoskelet Disord. 2015;16:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cauda Franco, Palermo Sara, Costa Tommaso, Torta Riccardo, Duca Sergio, Vercelli Ugo, et al. Gray matter alterations in chronic pain: A network-oriented meta-analytic approach. NeuroImageClin. 2014;4:676–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sofat N, Ejindu V, Kiely P. What makes osteoarthritis painful? The evidence for local and central pain processing. Rheumatology (Oxford). 2011;50(12):2157–2165. doi: 10.1093/rheumatology/ker283 [DOI] [PubMed] [Google Scholar]
- 22.Lluch E, Torres R, Nijs J, Van Oosterwijck J. Evidence for central sensitization in patients with osteoarthritis pain: a systematic literature review. Eur J Pain. 2014;18(10):1367–1375. doi: 10.1002/j.1532-2149.2014.499.x [DOI] [PubMed] [Google Scholar]
- 23.Baker KS, Georgiou-Karistianis N, Gibson SJ, Giummarra MJ. Optimizing Cognitive Function in Persons With Chronic Pain. Clin J Pain. 2017;33(5):462–472. doi: 10.1097/Ajp.0000000000000423 [DOI] [PubMed] [Google Scholar]
- 24.Schaible H-G. Mechanisms of chronic pain in osteoarthritis. Curr Rheumatol Rep. 2012;14(6):549–556. doi: 10.1007/s11926-012-0279-x [DOI] [PubMed] [Google Scholar]
- 25.Pickering M-E, Chapurlat R, Kocher L, Peter-Derex L. Sleep Disturbances and Osteoarthritis. Pain Pract. 2016;16(2):237–244. [DOI] [PubMed] [Google Scholar]
- 26.Harris ML. Psychological Factors in Arthritis: Cause or Consequence? In: Psychosocial Factors in Arthritis. Springer; 2016:53–77. [Google Scholar]
- 27.Alvaro PK, Roberts RM, Harris JK. A Systematic Review Assessing Bidirectionality between Sleep Disturbances, Anxiety, and Depression. Sleep. 2013;36(7):1059–1068. doi: 10.5665/sleep.2810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bellou V, Belbasis L, Tzoulaki I, Middleton LT, Ioannidis JPA, Evangelou E. Systematic evaluation of the associations between environmental risk factors and dementia: An umbrella review of systematic reviews and meta-analyses. Alzheimers Dement. 2017;13(4):406–418. [DOI] [PubMed] [Google Scholar]
- 29.Petkus AJ, Reynolds CA, Wetherell JL, Kremen WS, Pedersen NL, Gatz M. Anxiety is associated with increased risk of dementia in older Swedish twins. Alzheimer’s Dement J Alzheimer’s Assoc. 2016;12(4):399–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shi Le, Chen Si-Jing, Ma Meng-Ying, Bao Yan-Ping, Han Ying, Wang Yu-Mei, et al. Sleep disturbances increase the risk of dementia: A systematic review and meta-analysis. Sleep Med Rev. 2018;40:4–16. doi: 10.1016/j.smrv.2017.06.010 [DOI] [PubMed] [Google Scholar]
- 31.Agency for Healthcare Research and Quality (AHRQ). Medical Expenditure Panel Survey (MEPS). 2019. https://meps.ahrq.gov/mepsweb/.
- 32.Hoffmann F, Kaduszkiewicz H, Glaeske G, van den Bussche H, Koller D. Prevalence of dementia in nursing home and community-dwelling older adults in Germany. Aging Clin Exp Res. 2014;26(5):555–559. doi: 10.1007/s40520-014-0210-6 [DOI] [PubMed] [Google Scholar]
- 33.Yu WW, Machlin SR. An examination of skewed health expenditure data from the Medical Expenditure Panel Survey (MEPS). J Econ Soc Meas. 2005;30(2, 3):127–134. [Google Scholar]
- 34.Sommers J An Examination of State Estimates Using Multiple Years of Data from the Medical Expenditure Panel Survey, Household Component. Agency for Healthcare Research and Quality Working Paper No. 06004. https://www.meps.ahrq.gov/data_files/publications/workingpapers/wp_06004.pdf. Published 2006. Accessed December 2, 2019.
- 35.Kachru N, Carnahan RM, Johnson ML, Aparasu RR. Potentially inappropriate anticholinergic medication use in older adults with dementia. J Am Pharm Assoc (2003). 2015;55(6):603–612. doi: 10.1331/JAPhA.2015.14288 [DOI] [PubMed] [Google Scholar]
- 36.Sura SD, Carnahan RM, Chen H, Aparasu RR. Prevalence and determinants of anticholinergic medication use in elderly dementia patients. Drugs Aging. 2013;30(10):837–844. doi: 10.1007/s40266-013-0104-x [DOI] [PubMed] [Google Scholar]
- 37.Chekani F, Bali V, Aparasu RR. Quality of life of patients with Parkinson’s disease and neurodegenerative dementia: A nationally representative study. Res Social Adm Pharm. 2016;12(4):604–613. doi: 10.1016/j.sapharm.2015.09.007 [DOI] [PubMed] [Google Scholar]
- 38.MEPS HC-181: 2015. Full Year Consolidated Data File.
- 39.Gaskin DJ, Richard P. The economic costs of pain in the United States. J Pain. 2012;13(8):715–724. [DOI] [PubMed] [Google Scholar]
- 40.Stockbridge EL, Suzuki S, Pagan JA. Chronic pain and health care spending: an analysis of longitudinal data from the Medical Expenditure Panel Survey. Health Serv Res. 2015;50(3):847–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nahin RL, Sayer B, Stussman BJ, Feinberg TM. Eighteen-Year Trends in the Prevalence of, and Health Care Use for, Noncancer Pain in the United States: Data from the Medical Expenditure Panel Survey. J Pain. 2019. [DOI] [PubMed] [Google Scholar]
- 42.Coughlan D, Yeh ST, O’Neill C, Frick KD. Evaluating Direct Medical Expenditures Estimation Methods of Adults Using the Medical Expenditure Panel Survey: An Example Focusing on Head and Neck Cancer. Value Heal. 2014;17(1):90–97 doi: 10.1016/j.jval.2013.10.004 [DOI] [PubMed] [Google Scholar]
- 43.MEPS HC-163: 2013. Full Year Consolidated Data File.
- 44.Yang FC, Lin TY, Chen HJ, Lee JT, Lin CC, Kao CH. Increased Risk of Dementia in Patients with Tension-Type Headache: A Nationwide Retrospective Population-Based Cohort Study. PLoS One. 2016;11(6). doi:ARTN e015609710.1371/journal.pone.0156097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tzeng Nian-Sheng, Chung Chi-Hsiang, Liu Feng-Cheng, Chiu Yu-Hsiang, Chang Hsin-An, et al. Fibromyalgia and Risk of Dementia-A Nationwide, Population-Based, Cohort Study. Am J Med Sci. 2018;355(2):153–161. doi: 10.1016/j.amjms.2017.09.002 [DOI] [PubMed] [Google Scholar]
- 46.Gorelick PB. Role of inflammation in cognitive impairment: results of observational epidemiological studies and clinical trials. Innate Inflamm Stroke. 2010;1207:155–162. doi:DOI 10.1111/j.1749-6632.2010.05726.x [DOI] [PubMed] [Google Scholar]
- 47.Cote S, Carmichael P-H, Verreault R, Lindsay J, Lefebvre J, Laurin D. Nonsteroidal anti-inflammatory drug use and the risk of cognitive impairment and Alzheimer’s disease. Alzheimers Dement. 2012;8(3):219–226. [DOI] [PubMed] [Google Scholar]
- 48.Wang W, Sun Y, Zhang D. Association Between Non-Steroidal Anti-Inflammatory Drug Use and Cognitive Decline: A Systematic Review and Meta-Analysis of Prospective Cohort Studies. Drugs Aging. 2016;33(7):501–509. [DOI] [PubMed] [Google Scholar]
- 49.Mayeux R, Stern Y. Epidemiology of Alzheimer Disease. Cold Spring Harb Perspect Med. 2012;2(8):10.1101/cshperspect.a006239 a006239. doi: 10.1101/cshperspect.a006239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Steenland K, Goldstein FC, Levey A, Wharton W. A Meta-Analysis of Alzheimer’s Disease Incidence and Prevalence Comparing African-Americans and Caucasians. J Alzheimers Dis. 2016;50(1):71–76. doi: 10.3233/JAD-150778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Baumgart M, Snyder HM, Carrillo MC, Fazio S, Kim H, Johns H. Summary of the evidence on modifiable risk factors for cognitive decline and dementia: A population-based perspective. Alzheimers Dement. 2015;11(6):718–726. [DOI] [PubMed] [Google Scholar]
- 52.Kluzek S, Sanchez-Santos MT, Leyland KM, Judge A, Spector TD, Hart D, et al. Painful knee but not hand osteoarthritis is an independent predictor of mortality over 23 years follow-up of a population-based cohort of middle-aged women. Ann Rheum Dis. 2015:annrheumdis-2015 208056. [DOI] [PubMed] [Google Scholar]
- 53.Macfarlane GJ, Barnish MS, Jones GT. Persons with chronic widespread pain experience excess mortality: longitudinal results from UK Biobank and meta-analysis. Ann Rheum Dis. 2017;76(11):1815–1822. [DOI] [PubMed] [Google Scholar]
- 54.Nuesch E, Dieppe P, Reichenbach S, Williams S, Iff S, Juni P. All cause and disease specific mortality in patients with knee or hip osteoarthritis: population based cohort study. BMJ (Clinical Res ed). 2011;342:d1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cipriani G Pain and Dementia. A Brief Review. EC Neurol. 2018;10:643–652. [Google Scholar]