Abstract
Recent studies suggest a potential role of bioactive lipids in acute kidney injury induced by lipopolysaccharide (LPS). The current study was designed to determine the profiling activities of various polyunsaturated fatty acid (PUFA) metabolizing enzymes, including lipoxygenases (LO), cyclooxygenase, and cytochrome P450 in the plasma of LPS-injected mice using LC-MS. Heat map analysis revealed that out of 126 bioactive lipids screened, only the 12/15-LO metabolite, 12-HETE, had a significant (2.24 ± 0.4) fold increase relative to control (P= 0.0001) after Bonferroni Correction (BCF α = 0.003). We then determined the role of the 12/15-LO in LPS-induced acute kidney injury using genetic and pharmacological approaches. Treatment of LPS injected mice with the 12/15-LO inhibitor, baicalein, significantly reduced levels of renal injury and inflammation markers including urinary thiobarbituric acid reactive substance (TBARs), urinary monocyte chemoattractant protein-1 (MCP-1), renal interleukin-6 (IL-6), and tumor necrosis factor-α (TNF-α). Similarly, knocking-out of 12/15-LO reduced levels of renal inflammation and injury markers elicited by LPS injection. Next, we tested whether exogenous supplementation with docosahexaenoic acid (DHA) as a substrate would divert the role of 12/15-LO from being pro-inflammatory to anti-inflammatory via increased production of the anti-inflammatory metabolite. DHA treatment restored the decreased in plasma level of resolvin D2 (RvD2) and reduced renal injury in LPS-injected mice whereas DHA treatment failed to provide any synergistic effects in reducing renal injury in LPS injected 12/15-LO knock-out mice. The ability of RvD2 to protect kidney against LPS-induced renal injury was further confirmed by exogenous RvD2 which significantly reduced the elevation in renal injury in LPS injected mice. These data suggest a double-edged sword role of 12/15-LO in LPS-induced acute renal inflammation and injury, depending on the type of substrate available for its activity.
Keywords: 12/15-lipoxygenase, baicalein, DHA, resolvin, LPS, renal injury, inflammation
Introduction
Inflammation is a vital protective response triggered by infection or tissue injury. Inflammation serves to heal injured tissue, defend against infection and repair damaged tissue. However, aberrant activation of inflammatory pathways induces deleterious effects on different body organs as manifested by various disorders associated with inflammation such as in cardiovascular diseases [1].
Lipopolysaccharide (LPS), is a major glycolipid endotoxin within the outer membrane of Gram-negative bacteria. LPS is a potent inducer of the host immune system, causing endotoxemia [2]. LPS initially binds to toll-like receptor 4 (TLR4) [3], a specific LPS receptor that plays crucial role in innate immunity and inflammation, to trigger the activation of nuclear factor kappa B (NFκB) and activator protein-1 (AP-1) signal transduction pathways. NF-κB activation in turn increases the expression of various genes related to innate immunity and inflammatory responses [4] such as monocyte chemotactic protein-1 (MCP-1), interleukin-1β (IL-1β), IL-6, and tumor necrosis factor-α (TNF-α) [5]. The elevated levels of these inflammatory mediators could result in a systemic inflammatory status, multiple dysfunction and/or injury of various body organs including kidney [6]. Therefore, injection of LPS to experimental animals triggers acute inflammation and this model is now considered an established model for various acute inflammatory conditions including hepatitis [7], arthritis [8], acute kidney injury [9] and uveitis [10].
Acute kidney injury (AKI), is a rapid renal dysfunction that is associated with systemic inflammation. Despite various medical interventions, the combination of systemic inflammation and AKI accounts for great mortality rate [11]. Because the production of inflammatory mediators is important in the pathophysiology of renal injury, extensive studies have investigated the role of inflammatory mediators involved in the incidence and progression of AKI [12].
In addition to cyclooxygenase (COX) and cytochrome P450, lipoxygenases (LOs) are important polyunsaturated fatty acid (PUFA) metabolizing enzymes. LOs are a family of iron-containing enzymes that act on arachidonic acid to produce hydroxyeicosatetraenoic acids (HETEs). LOs are classified as 5-, 8-, 12-, and 15-LOs according to the carbon atom of arachidonic acid at which oxygen is inserted. There are three major isoforms of 12-LO; platelet-type 12-LO, macrophage- or leukocyte-type 12-LO (12/15-LO), and epidermal-type 12-LO [13]. Human and rabbit 15-LOs as well as the leukocyte-type 12-LO have high homology, and are classified as 12/15-LO since they can form both 12-HETE and 15-HETE from arachidonic acid [14, 15]. 12-HETE and 15-HETE promote pro-inflammatory effects [13], angiogenesis [16], proliferation, anti-apoptotic, and vasoconstrictive effects [17]. In rodents, the leukocyte-type 12/15-LO predominates to synthesize mainly 12-HETE which mediate growth factor effects in vascular smooth muscle cells, fibroblasts, and mesangial cells, as well as responses to vascular injury [14, 15]. In human, platelet 12/15-LO predominates to synthesize mainly 15-HETE which has similar pathophysiological function of 12-HETE [14, 15]. Our group has previously shown that 12/15-LO plays a pivotal role in the incidence and progression of diabetes-induced renal and retinal injuries as its inhibition reduced retinal and renal inflammation and injury in diabetic mice [13, 18–20].
Docosahexaenoic acid (DHA) is a biologically active polyunsaturated ω−3 fatty acid. DHA is endogenously metabolized into bioactive derivatives such as resolvins and protectins, which play crucial role in resolving inflammation [21]. DHA has antioxidant and anti-inflammatory properties in part, by reducing leukocyte-derived cytokine formation and modulation of eicosanoid synthesis [22]. DHA could also increase pro-resolving lipid mediators (SPMs) that are synthesized by transcellular processes involving enzymes of epithelial cells and leukocytes [22]. SPMs derived from DHA are known as D-series resolvins. The D-series resolvins (RvD1, RvD2, RvD3, RvD4, RvD5, and RvD6) are synthesized from DHA by acetylated COX-2 or 15-LO [22]. DHA has numerous health benefits against malignant, inflammatory, proliferative, and vascular diseases [23]. Recent epidemiological studies and clinical trials revealed the importance of the balance between the levels of DHA and arachidonic acid in modulating inflammation and fibrosis [24]. For example, DHA could be metabolized by 12/15-LO to produce the anti-inflammatory resolvin D [25]. Resolvins (RVs) are bioactive small molecules that are enzymatically derived from ω−3 fatty acids. RVs could be categorized into E-series RVs, D-series RVs, and Aspirin-triggered RV [26]. Accumulating evidence suggested that RVs are involved in attenuating inflammation and tissue injury [27]. DHA has a promising role in preventing renal inflammation; however; its mechanism remains unclear.
In the present study, we investigated whether 12/15-LO inhibition reduces acute renal inflammation and injury elicited by LPS injection and we also tested the hypothesis that DHA supplementation would divert the role of 12/15-LO from being pro-inflammatory to anti-inflammatory via the generation of the anti-inflammatory metabolite RvD2, which could protect the kidney against LPS-induced acute renal inflammation and injury.
Materials and Methods
Animal preparation and experimental design
12–15 week old male wild-type C57BL/6J (WT) mice were obtained from The Jackson Laboratory (Bar Harbor, ME). All procedures with animals were performed in accordance with the Public Health Service Guide for the Care and Use of Laboratory Animals (Department of Health, Education, and Welfare publication, NIH 80–23) and Augusta University guidelines. To evaluate the changes in bioactive lipids metabolism during acute inflammation, male WT mice were injected with single dose of LPS (4 mg/kg i.p., Sigma, St Louis, MO). After 3 days, control and LPS injected WT mice were terminated for blood collection (N=4–5). Blood samples were collected in polypropylene tubes containing EDTA (final concentration, 1 mg/ml of blood); plasma was promptly separated by centrifugation (2,500 g, 20 min, 4°C) and stored at −80°C until being analyzed by the lipidomics core facility (Wayne State University, Detroit, MI) using liquid chromatography/mass spectrometry (LC/MS) as previously described [18, 28]. Briefly, plasma samples were spiked with 10 ng of five internal standards covering all classes of oxygenated polyunsaturated fatty acid (PUFA) metabolites. These internal standards are [15(S)-HETE-d8, Leukotriene B4-d4, Prostaglandin E1-d4, RvD2-d5, and 14(15)-EpETrE-d11], and they used for recovery and quantitation. The spiked samples were mixed thoroughly and then loaded on C18 columns for extracting PUFA metabolites. LC-MS analysis was performed on a Prominence XR system (Shimadzu) using Luna C18 (3μ, 2.1×150 mm) column. The mobile phase consisted of a gradient between A: methanol-water-acetonitrile (10:85:5 v/v) and B: methanol-water-acetonitrile (90:5:5 v/v), both containing 0.1% ammonium acetate. The gradient program with respect to the composition of B was as follows: 0–1 min, 50%; 1–8 min, 50–80%; 8–15 min, 80–95%; and 15– 17 min, 95%. The flow rate was 0.2 ml/min. The eluate was directly introduced to ESI source of QTRAP5500 mass analyzer (ABSCIEX) in the negative ion mode with following conditions: Curtain gas: 35 psi, GS1: 35 psi, GS2: 65 psi, Temperature: 600 °C, Ion Spray Voltage: −1500 V, Collision gas: low, Declustering Potential: −60 V, and Entrance Potential: −7 V. The eluate was monitored by Multiple Reaction Monitoring (MRM) method to detect unique molecular ion – daughter ion combinations. Optimized Collisional Energies (18–35 eV) and Collision Cell Exit Potentials (7–10 V) were used for each MRM transition. The data was collected using Analyst 1.5.2 software and the MRM transition chromatograms were quantitated by MultiQuant software (both from ABSCIEX). The internal standard signals in each chromatogram were used for normalization, recovery and relative quantitation of each analyte. The average recovery using this procedure was > 90%. Metabolite concentrations were normalized to the total plasma protein content. Matrix2png Viewer (http://www.chibi.ubc.ca/matrix2png/) was used to analyze and represent the lipid metabolomic data as a heat map.
To determine the role of 12/15-LO in LPS-induced renal injury, three groups of age and weight matched male C57BL/6J WT mice (12–15 week old, n=6–8 per group) were treated daily for 3 days before and 3 days after single LPS injection as follows; (1) WT control injected with saline, (2) WT LPS group; mice were injected with LPS (4 mg/kg i.p on day 3: Sigma, St Louis, MO) [29], (3) WT LPS treated with the 12/15-LO inhibitor baicalein (20 mg/kg i.p for 6 days: Cayman Chemical, Ann Arbor, MI) [30]. Additionally, two groups of age and weight matched male 12/15-LO knock-out (KO) mice (B6.129S2-Alox15tm1Fun/J, Jackson laboratory) were injected with saline as control group or with LPS (4mg/kg i.p). To explore whether DHA supplementation could be utilized as an external substrate to divert 12/15-LO from being pro-inflammatory to anti-inflammatory via the generation of anti-inflammatory metabolite RvD2, male WT and 12/15-LO KO mice were injected with LPS and treated with DHA (50 mg/kg i.p daily for 6 days: Cayman Chemical, Ann Arbor, MI) 3 days before and 3 days after LPS injection [31, 32]. To explore potential protective effect of RvD2, an additional set of age and weight matched male WT C57BL/6J (n-6–8 per group) were injected with LPS (4 mg/kg i.p) and treated daily with RvD2 (100 ng, i.p for 6 days: Cayman Chemical, Ann Arbor, MI) 3 days before and 3 days after LPS injection [33]. Mice were placed in metabolic cages for 24 hour urine collection before being terminated for blood and tissue collection.
Assessment of the biochemical parameters
Urinary and plasma creatinine levels were assessed and creatinine clearance was calculated to reflect renal injury using picric acid method. Urinary protein excretions were measured as an index of renal injury using the Bradford method for protein determination (Bio-Rad, Hercules, CA). Microalbumin (Exocell, PA, USA) excretion levels were also measured as a marker of renal injury. Urinary Thiobarbituric Acid Reactive Substances (TBARs) (Cayman Chemical, Ann Arbor, MI) and urinary Monocyte Chemotactic Protein-1 (MCP-1) (BD Biosciences, San Jose, CA) excretion levels were assessed as markers of oxidative stress and inflammation, respectively.
Quantitative Reverse-Transcriptase Polymerase Chain Reaction
RNA was extracted using RNA extraction kit (Qiagen Inc., USA). Polymerase chain reaction (PCR) was carried out using TaqMan one step PCR master mix reagents kit (Applied Biosystems Inc, USA) with pre-designed TaqMan primers (Catalog#: ICAM-1: Mm00516023_m1; 12-LO: Mm00545833_m1; TNF-α: Mm00443258_m1; IL-6: Mm00446190_m1; IL-1β: Mm00434228_m1; IL-10: Mm01288386_m1; and 18S: Mm03928990_g1). PCR was performed for retention time step 48°C for 15 min then Enzyme activation step 95°C for 15 min, then 40 cycles of 95°C for 15 seconds and 60°C for 1 min. Resulting CT values were normalized to 18S and analyzed using the comparative CT method to obtain fold changes in gene expression.
Histopathological examination
Kidney tissues were excised, and then fixed in 10% neutral buffered formalin. Tissues were then processed for paraffin embedding and were subsequently sectioned at 3–4μm (Reichert Jung microtome, Germany). Deparaffinized sections were stained with hematoxylin/eosin (H&E). The samples were examined for any renal pathophysiological changes using Zeiss M2 light microscope at 200 X magnification under the supervision of Dr. Abdelsayed, an expert clinical pathologist, in a blind fashion.
Statistical analysis
All data are presented as mean ± SEM. Strain and drug treatment comparisons were made using a one-way ANOVA or two-way ANOVA followed by Tukey’s post hoc test for multiple group comparisons depending on the question. Analyses were performed using Graph Pad Prism Version 4.0 software (Graph Pad Software Inc., La Jolla, CA). For all comparisons, P<0.05 was considered statistically significant.
Results
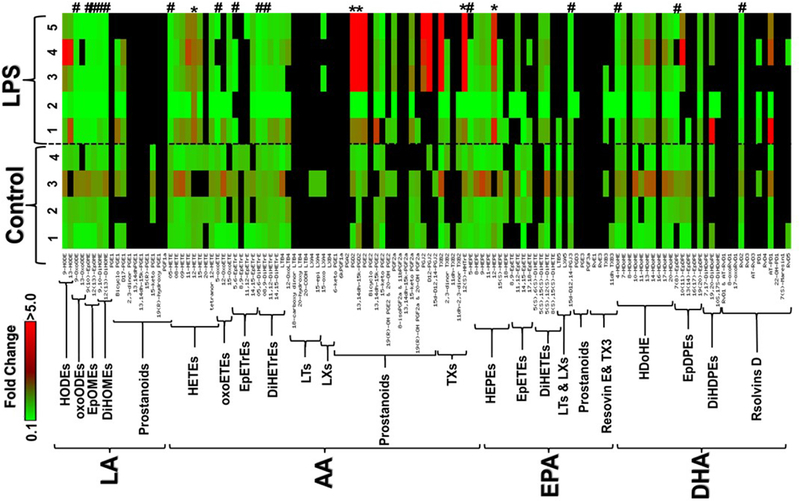
In order to evaluate the potential role of bioactive lipids in acute kidney injury induced by LPS, we first sought to determine the lipid profiling activities of PUFA metabolizing enzymes, including 12/15-LO, COX and cytochrome P450 in the plasma of LPS-treated mice using LC-MS. Figure 1 depicts a heat map for fold-change in bioactive lipid metabolites in the plasma of LPS-treated mice relative to control. These metabolites were grouped by their PUFA origin into four major groups to represent those derived from linoleic acid (LA), arachidonic acid (AA), eicosapentaenoic acid (EPA), or docosahexaenoic acid (DHA). This grouping was followed by sub-grouping the metabolites within each PUFA group according to their structure and hence enzymatic biosynthesis pathways. Interestingly, out of 124 bioactive lipids screened, only 5 metabolites were significantly increased and 13 metabolites were significantly decreased after LPS injection (Table1). However, after the Bonferroni correction for multiple comparisons, the P-values of these significantly changed metabolites failed to exceed significant threshold (BCFα = 0.05/124 = 0.0004) except for 12-HETE which remained significant with a P value of 0.0001.
Figure 1.
A clustered heat map of bioactive lipid metabolites in the plasma of LPS-treated mice relative to normal control. These metabolites were grouped into four major groups: linoleic acid (LA), arachidonic acid (AA), eicosapentaenoic acid (EPA), or docosahexaenoic acid (DHA) metabolites followed by subgrouping according to their enzymatic biosynthetic pathways. The significant (P<0.05) increased metabolites were indicated by * whereas decreased metabolites were indicated by #. Data shown for the comparison are the fold change of 4 control and 5 LPS-injected mice relative to the average of control. The highest fold change is represented by the red color, while the lowest fold change is represented by the green color. The undetected metabolite is indicated by the black color.
Table 1:
P values for statistical analysis of detectable plasma bioactive lipid metabolites’ heat map for LPS injected vs. normal WT mice (n-4–5 per group, P<0.05)
| Lipid | P value |
|---|---|
| 9-HODE | 0.438602 |
| 13-HODE | 0.311852 |
| 9-OxoODE | 0.045173 |
| 13-OxoODE | 0.100687 |
| 9(10)-EpOME | 0.04937 |
| 12(13)-EpOME | 0.009935 |
| 9,10-DiHOME | 0.025747 |
| 12(13)-DiHOME | 0.053636 |
| Bicyclo PGE1 | 0.799488 |
| D17-PGE1 | 0.892394 |
| 05-HETE | 0.000815 |
| 08-HETE | 0.6641 |
| 09-HETE | 0.804799 |
| 11-HETE | 0.281181 |
| 12-HETE | 0.000115 |
| 15-HETE | 0.623869 |
| tetranor 12-HETE | 0.348176 |
| 5-oxoETE | 0.019733 |
| 12-OxoETE | 0.924546 |
| 15-OxoETE | 0.329043 |
| 5,6-EpETrE | 0.055558 |
| 14,15-EpETrE | 0.833161 |
| 05,6-DiHETrE | 0.002514 |
| 08,9-DiHETrE | 0.010184 |
| 11,12-DiHETrE | 0.377131 |
| 14,15-DiHETrE | 0.350737 |
| LTB4 | 0.543987 |
| 12-OxoLTB4 | 0.874644 |
| PGD2 | 0.049756 |
| 13,14dh-15k-PGD2 | 0.015391 |
| PGE2 | 0.070936 |
| 13,14dh-15k-PGE2 | 0.754061 |
| 15-keto PGE2 | 0.06607 |
| 15-keto PGF2a | 0.476513 |
| TXB2 | 0.183213 |
| 2,3-dinor TXB2 | 0.261345 |
| 11dh-2,3-dinor TXB2 | 0.849725 |
| 12(S)-HHTrE | 0.023156 |
| 5-HEPE | 0.008551 |
| 8-HEPE | 0.494382 |
| 9-HEPE | 0.889219 |
| 11-HEPE | 0.456891 |
| 12-HEPE | 0.047089 |
| 15(S)-HEPE | 0.187591 |
| 11,12-EpETE | 0.621576 |
| 14,15-EpETE | 0.320877 |
| 17,18-EpETE | 0.564459 |
| 5(S),12(S)-DiHETE | 0.811389 |
| 15d-D12,14-PGJ3 | 0.042806 |
| 4-HDoHE | 0.038112 |
| 7-HDoHE | 0.368239 |
| 10-HDoHE | 0.778248 |
| 11-HDoHE | 0.540704 |
| 13-HDoHE | 0.673073 |
| 14-HDoHE | 0.809889 |
| 17-HDoHE | 0.958034 |
| 20-HDoHE | 0.379586 |
| 7(8)-EpDPE | 0.04977 |
| 10(11)-EpDPE | 0.156664 |
| 19(20)-EpDPE | 0.335547 |
| 19,20-DiHDoPE | 0.538739 |
| RvD2 | 0.029191 |
| AT-PD1 | 0.22964 |
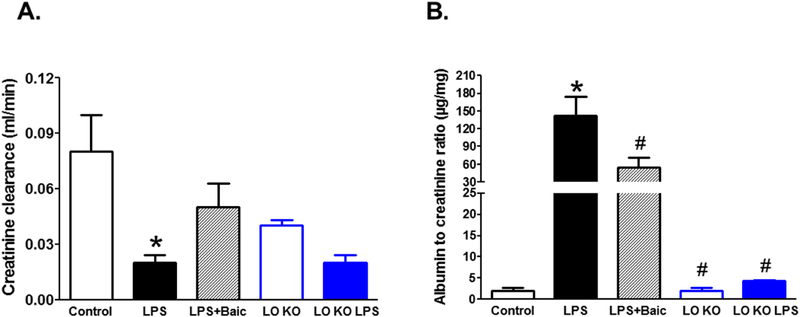
These data provide a rationale to explore the role of the 12/15-LO pathway in LPS-induced acute kidney injury. Toward this goal, we used two complementary approaches; pharmacological and genetic. Pharmacological inhibition of 12/15-LO with baicalein improved creatinine clearance and significantly reduced albumin excretion in LPS injected WT mice (Figure 2). Similarly genetic manipulation of 12/15-LO prevented the significant decrease in creatinine clearance and the elevation in albuminuria in LPS injected 12/15- LO KO when compared to 12/15-LO KO control (Figure 2).
Figure 2.
Effects of pharmacological inhibition of 12/15-LO with baicalein or genetic manipulation of 12/15-LO using 12/15-LO KO mice on LPS-induced changes in renal injury markers; creatinine clearance (A) and microalbuminuria (B). LPS injection significantly decreased creatinine clearance and increased albuminuria. baicalein treatment increased creatinine clearance and reduced albumin excretion levels in LPS injected WT mice. 12/15 LO inhibition prevented the significant decrease in creatinine clearance and the increase in albuminuria in LPS injected 12/15-LO KO mice (n=6–8, *P<0.05 vs. control group and #P<0.05 vs. LPS group).
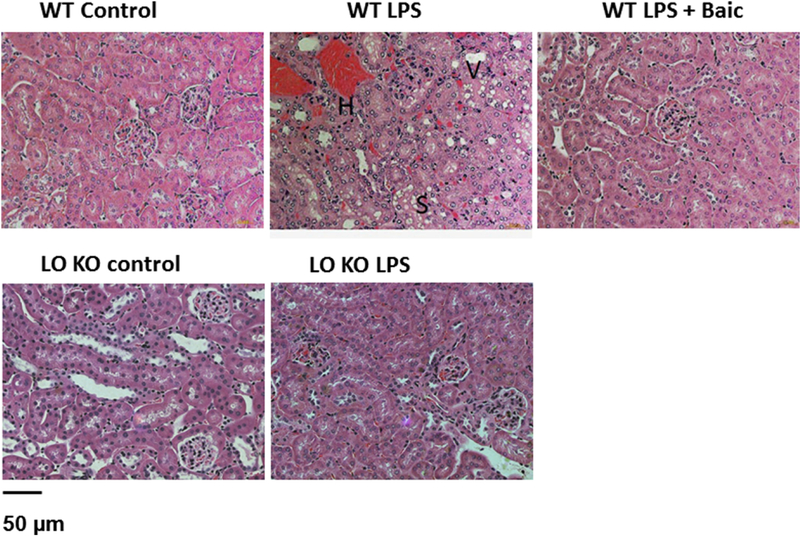
Histopathological examination of kidney sections from LPS-injected WT mice revealed that LPS induced tubular degeneration, surface blebs, hemorrhage in interstitial tissue, vaculated cells, and sloughing of tubular epithelial cells. Treatment of LPS-injected WT mice with baicalein showed less tubular degeneration and vaculated cells vs. untreated LPS-injected WT mice (Figure 3). Similar to pharmacological inhibition of 12/15-LO, 12/15 KO mice showed a marked decrease in renal tubular degeneration, vaculated cells, and interstitial hemorrhage after LPS injection (Figure 3).
Figure 3.
A. Representative images (200X) for H & E staining of the kidney sections in control and LPS injected WT mice with or without baicalein treatment or LPS injected 12/15-LO KO mice. LPS injected WT mice showed renal tubular degeneration and surface blebs, hemorrhage in interstitial tissue (H), vaculated cells (V) and sloughing of tubular epithelial cells (S). Baicalein treatment or knocking-out of 12/15-LO reduced tubular degeneration in LPS injected mice (n=4).
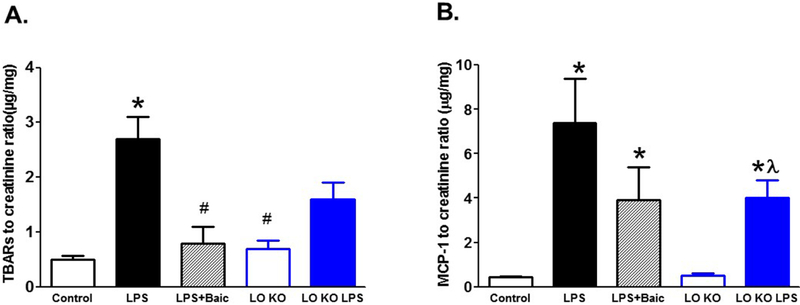
To unravel the cellular and molecular mechanisms whereby 12/15-LO inhibition abates LPS-induced renal injury, we assessed urinary TBARs and MCP-1 excretion levels as indicative of renal oxidative stress and inflammation, respectively. Injection of LPS increased renal TBARs and MCP-1 excretion levels (Figure 4). Pharmacological inhibition of 12/15-LO with baicalein treatment decreased TBARs excretion (P< 0.05) without a significant decrease in MCP-1 excretion in LPS injected WT mice (Figure 4). Knocking-out of 12/15-LO prevented the significant increase in TBARs excretion after LPS injection compared to LPS injected WT mice (Figure 4A). Although MCP-1 excretion was significantly elevated in 12/15-LO KO mice after LPS injection compared to 12/15-LO control, this elevation remained significantly less than LPS injected WT mice (Figure 4B).
Figure 4.
Effects of baicalein or knocking-out of 12/15-LO on renal oxidative stress marker TBARs and inflammatory marker MCP-1 excretion levels in LPS injected mice. Baicalein treatment significantly reduced TBARs excretion (A) in LPS injected WT mice. Similarly, knocking-out of 12/15-LO prevented the increase in TBARs excretion in LPS injected 12/15-LO KO mice. The renal inflammatory marker MCP-1 excretion (B) remained significantly higher in LPS injected WT and 12/15-LO mice when compared to control (n=6–8; *P<0.05 vs. control WT group, #P<0.05 vs. WT LPS group and λP<0.05 vs. control LO group).
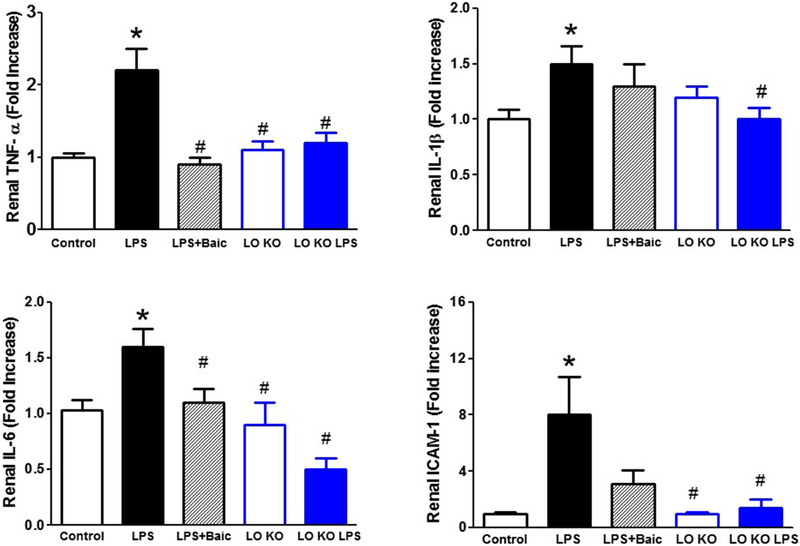
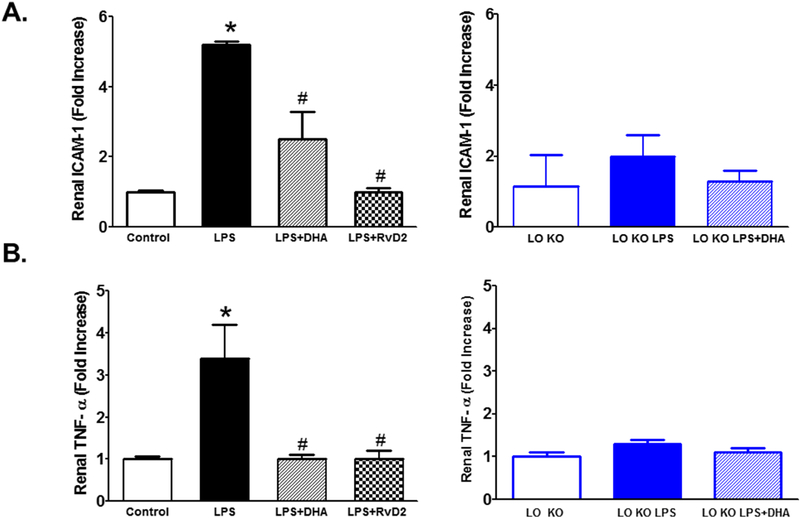
To further confirm the anti-inflammatory effects of 12/15-LO inhibition in the kidney of LPS-injected mice, we then measured renal expression levels of the inflammatory cytokines TNF-α, IL-1β, IL-6 and ICAM-1 mRNA using real time PCR. LPS increased expression levels of renal TNF-α, IL-1β, IL-6 and ICAM-1 mRNA (Figure 5). Inhibition of 12/15-LO with baicalein only significantly decreased expression levels of renal TNF-α and IL-6 mRNA in LPS injected WT mice (Figure 5). Knocking-out of 12/15-LO prevented the significant elevation in renal TNF-α, IL-1β, IL-6 and ICAM-1 mRNA in LPS injected 12/15 LO KO mice (Figure 5).
Figure 5.
Effects of baicalein and 12/15-LO knocking-out on LPS induced alteration in renal mRNA expression levels of the inflammatory cytokines TNF-α, IL-1β, IL-6 and ICAM-1. Baicalein significantly reduced the elevation in renal TNF-α and IL-6 mRNA expression in LPS injected WT mice whereas knocking-out of 12/15-LO prevented the elevation in TNF-α, IL-1β, IL-6 and ICAM-1mRNA expression in LPS injected 12/15-LO KO mice (n=6–8; *P<0.05 vs. control group and #P<0.05 vs. WT LPS group).
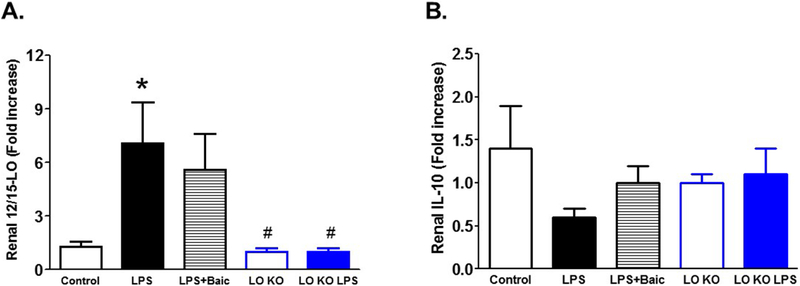
LPS injection also significantly increased expression levels of 12/15-LO mRNA and tended to decrease expression levels of the anti-inflammatory IL-10 mRNA (P < 0.08) in the kidney of LPS-injected WT mice vs. control (Figure 6). Baicalein treatment did not significantly reduce expression levels of 12/15-LO or increased IL-10 mRNA expression in the kidney of LPS-injected WT mice (Figure 6). Injection of LPS did not significantly increase 12/15-LO or decreased IL-10 mRNA expression in 12/15-LO KO mice (Figure 6).
Figure 6.
Effects of baicalein on LPS-induced increase in 12/15-LO mRNA expression (A) and decrease in the anti-inflammatory IL-10 mRNA expression (B). Baicalein improved renal IL-10 mRNA expression without a significantly decrease in 12/15-LO expression in the LPS injected mice. Knocking-out of 12/15-LO prevented the increase in 12/15-LO or the decrease in IL-10 in 12/15-LO KO mice (n=6–8; *P<0.05 vs. control group and #P<0.05 vs. WT LPS group).
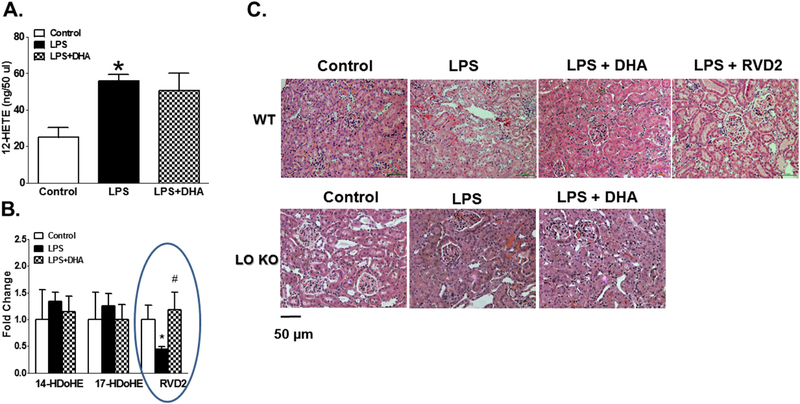
To test the hypothesis that DHA supplementation provides protective mechanisms against LPS-induced inflammation via acting as a substrate to 12/15-LO to divert its role from proinflammatory to anti-inflammatory pathway via production of the anti-inflammatory metabolites such as resolvins, we first measured plasma lipid profile of the 12/15-LO byproduct in the presence of exogenous DHA as a substrate under acute inflammatory conditions elicited by LPS injection. DHA supplementation did not significantly lower plasma 12-HETE levels in LPS injected mice (Figure 7A). Out of many detectable anti-inflammatory metabolites and out of members of Dseries resolvins, only plasma RvD2 was significantly decreased after LPS injection and DHA supplementation prevented this decrease (Figure 7B). Restoring the decrease in plasma RvD2 with DHA treatment was associated with reduction of renal inflammation and injury in LPS injected mice. On the other hand, there was no significant difference in the levels of 14-hydroxy docosahexaenoic acid (14-HDoHE) and 17-hydroxy docosahexaenoic acid (17-HDoHE), the other 12/15-LO metabolites of DHA, between control WT group, LPS-injected WT group and LPS + DHA WT group (Figure 7A).
Figure 7.
A. Plasma 12-HETE levels in as the major 12/15-LO metabolite in rodents in the presence of exogenous DHA as a substrate during LPS-induced acute inflammation. B. Plasma lipid profile of detectable anti-inflammatory 12/15-LO by-product in the presence of exogenous DHA as a substrate during LPS-induced acute inflammation. Although DHA treatment did not significantly lower plasma 12-HETE levels, it significantly restored the decrease in plasma RvD2 levels in LPS injected WT mice (n=4, *P<0.05 vs. control and #P<0.05 vs. LPS group). C. Representative images (200X) for H & E staining of the kidney sections in control WT mice, LPS injected WT mice, LPS injected WT mice plus DHA treatment, LPS injected WT mice plus RvD2 treatment, control 12/15-LO KO, LPS injected 12,15-LO KO and LPS injected 12/15-LO KO plus DHA treatment (n=4). RvD2 treatment or DHA as well as genetic inhibition of 12/15-LO reduced renal histopathological changes induced by LPS injection.
Next, we determined whether exogenous supplementation of DHA or RvD2 reduces renal inflammation and injury in LPS injected WT mice. DHA or RvD2 treatment decreased renal tubular degeneration, interstitial hemorrhage and vaculated cells in LPS-injected WT mice (Figure 7C) similar to the inhibition of 12/15-LO. The improvement of renal histopathological changes induced by LPS injection was also associated with significance increases in creatinine clearance and decreases in proteinuria in LPS injected WT mice treated with either DHA or RvD2 compared to untreated LPS injected WT mice (Figure 8). DHA restored the decrease in creatinine clearance and decreased proteinuria in LPS injected WT mice to levels similar to control WT mice whereas DHA treatment failed to restore the decrease in creatinine clearance or to reduce proteinuria in LPS injected 12/15-LO KO mice to levels similar to control 12/15-LO KO mice (Figure 8).
Figure 8.
Effect of DHA or RvD2 treatment and 12/15-LO knocking-out on creatinine clearance and proteinuria in LPS injected mice. Either DHA or RvD2 treatment increased creatinine clearance and decreased proteinuria in LPS injected WT mice. Although knocking-out of 12/15-LO also decreased creatinine clearance and increased proteinuria in LPS injected 12/15-LO KO mice, these changes were less than LPS injected WT mice and were not further improved with DHA treatment (n=6–8, *P<0.05 vs. control and #P<0.05 vs. LPS group).
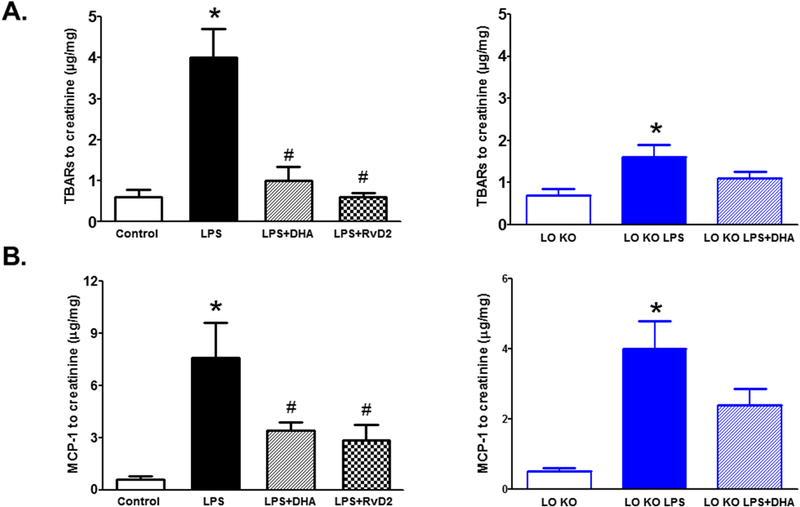
DHA or RvD2 treatment significantly reduced the elevation in urinary TBARs and MCP-1 excretion levels in LPS injected WT mice whereas DHA treatment did not significantly decrease TBARs or MCP-1 expression in LPS injected 12/15-LO KO mice compared to untreated LPS injected 12/15-LO KO mice (Figure 9). The reduction in TBARs excretion as a marker of renal oxidative stress and MCP-1 excretion as a marker of inflammation were also associated with reduction in renal ICAM-1 and TNF-α mRNA expression in both DHA and RvD2 treated LPS injected WT mice (Figure 10). Renal TNF-α and ICAM-1 mRNA expression levels did not significantly increase in LPS injected 12/15-LO KO mice and DHA treatment failed to significantly lower renal TNF-α and ICAM-1 mRNA expression than either LPS injected 12/15LO KO mice or control 12/15-LO KO mice (Figure 10). Based on the findings that DHA failed to augment the protective effect of 12/15-LO inhibition in LPS injected 12/15-LO KO mice, our data suggest that exogenous DHA supplementation could lower renal injury and inflammation possibility via its utilization as a substrate by 12/15-LO to produce RvD2.
Figure 9.
Effects of DHA or RvD2 treatment and 12/15-LO inhibition on urinary excretion levels of TBARs and MCP-1. DHA or RvD2 administration significantly attenuated TBARs and MCP-1 excretion levels in LPS injected WT mice. Knocking-out of 12/15-LO also increased TBARs and MCP-1 excretion in LPS injected 12/15-LO KO mice compared to control 12/15-LO KO mice; however, these changes were not significantly reduced with DHA treatment in LPS injected 12/15-LO KO mice (n=6–8; *P<0.05 vs. control WT or 12/15-LO KO group, #P<0.05 vs. WT LPS group).
Figure 10.
Effects of DHA or RvD2 treatment and 12/15-LO inhibition on renal TNF-α and ICAM-1 mRNA expression levels in LPS injected mice. DHA or RvD2 administration significantly reduced the elevation in renal TNF-α and ICAM-1 mRNA expression levels in LPS injected WT mice. Knocking-out of 12/15-LO prevented the elevation in renal TNF-α and ICAM-1 compared to LPS injected WT mice and these effects were not further decreased with DHA treatment in LPS injected 12/15-LO KO mice (n=6–8; *P<0.05 vs. control WT or 12/15-LO KO group, #P<0.05 vs. WT LPS group).
Discussion
We and others have previously demonstrated that 12/15-LO has an established role in the incidence and progression of diabetic nephropathy and retinopathy through increased inflammation and oxidative stress [13, 18]. The polyunsaturated ω−3 fatty acid DHA is endogenously metabolized into bioactive derivatives including resolvins, which is involved in reducing inflammation [21]. Accordingly, the current study extend our previous findings to determine role of 12/15-LO in acute renal injury elicited by LPS injection and whether DHA could prevent the incidence of renal injury after LPS injection possibility via acting as a substrate to 12/15-LO to increase anti-inflammatory resolvins. Injection of LPS increased renal injury markers in WT mice as it increased albuminuria together with decreased creatinine clearance. The increase in renal injury could be a consequence of elevated renal oxidative stress and inflammation as injection of LPS also increased TBARs and MCP-1 excretion levels as wells increased renal TNFα, IL-1β, IL-6 and ICAM-1 mRNA expression levels. Inhibition of 12/15-LO using genetic manipulation or pharmacological inhibition with baicalein reduced renal inflammation and injury elicited by LPS injection. We also found that RvD2 levels decreased in the plasma of LPS-injected mice and this decrease was restored by exogenous DHA treatment. Exogenous DHA or RvD2 supplementation also reduced renal inflammation and injury in LPS injected WT mice. However, DHA supplementation failed to exacerbate the protective effect of 12/15-LO inhibition in LPS injected 12/15-LO KO mice. Taken together these data suggest that 12/15-LO is involved in LPS-induced renal inflammation and injury and DHA provides renal protection in LPS-induced acute renal injury, at least in part, via acting as a substrate for 12/15-LO to increase the anti-inflammatory RvD2 levels.
LPS induced systemic inflammatory response in animal models is accompanied by multiple organs injury. For instance, LPS injection results in kidney, lung and liver injury [34–36]. Moreover, LPS has been reported to activate retinal microglia with subsequent neurotoxicity [37]. Several mechanisms have been proposed to explain LPS-induced acute organ injury, including the kidney such as increased production of pro-inflammatory cytokines and increased oxidative stress [36, 38]. However, the exact pathophysiological mechanisms have not been fully elucidated. We have previously shown that inhibition of 12/15-LO reduced renal inflammation and injury in diabetic mice [13]. Renal 12-LO expression increased following LPS injection and this effect was mitigated by 12/15-LO inhibition with baicalein [13]. Based on our observations that 12- HETE was elevated after LPS injection and inhibition of 12/15-LO with baicalein reduced LPS-induced renal injury, our data in the present study suggest a potential role of 12/15-LO activation and increased production of 12-HETE in the incidence of LPS-induced acute renal inflammation.
Bioactive lipids are potent signaling molecules that incredibly modulate wide range of cellular activities. Various classes of bioactive lipids are derived from different polyunsaturated fatty acids (PUFAs). Accumulating evidences indicate the ability of many classes of bioactive lipids to modulate immunity and inflammation [39]. Significant aspects of the polyunsaturated fatty acid bioactivities are mediated through their transformation to specific lipid mediators via various enzymes such as cyclooxygenase, lipoxygenase and cytochrome P450 where these enzymes could catalyze PUFA peroxidation-mediated bioactive metabolites production [15, 22]. Causal relationship between eicosanoids and inflammation is now firmly established. HETEs, leukotrienes and prostaglandins are inflammatory bioactive lipid mediators produced from arachidonic acid. Interestingly, epoxyeicosatrienoic acids (EETs) are anti-inflammatory cytochrome P450 epoxygenase-derived product [40]. Herein, analysis of plasma lipid profile in LPS injected mice revealed an elevation in the cyclooxygenase metabolites PGE2, PGD2 and TXB2 compared to control which is consistent with previous findings of Willenberg et al. and Paul et al. where they showed that plasma PGE2 was elevated following LPS injection in mice [41, 42]. LPS injection also increased the inflammatory eicosanoids 9-, 11-, 12- and 15-HETE and decrease in the anti-inflammatory eicosanoids 5,6- and 11,12-EETs. Because levels of 12- and 15-HETE, the products of arachidonic acid metabolism by 12/15-LO, showed a marked increase in plasma of LPS injected mice, we postulate a significant role for 12/15-LO pathway in LPS-induced inflammation. Our data are consistent with previous studies which reported implication of 12/15-LO in various inflammatory disorders [13, 43]. Studies have also shown that 12/15-LO up-regulation elevates 12- and 15-HETEs production which could amplify inflammatory cascades to exacerbate organ damage [44, 45].
To evaluate the pathophysiological role of 12/15-LO in LPS-induced acute renal inflammation, we first assessed the effect of LPS injection on renal expression of 12/15-LO. We found that LPS injection induced a significant increase in renal 12/15-LO expression levels. Plasma 12 HETE levels were also significantly elevated in LPS injected mice. Our knowledge of the biological role of various LO isoforms in particular 15-LO and 12-LO is still somewhat limited. AlOX15 gene encodes for the 12/15-LO which is highly expressed in eosinophils and bronchoalveolar epithelium whereas ALOX12 gene encodes for the platelets type 12-LO which is expressed at high levels in blood platelets and in the skin [14, 15]. In rodents ALOX15 gene encodes for leukocyte 12-LO whereas ALOX12 genes encode for platelets 12-LO [14, 15]. The predominant major metabolic product of LO in rodent is 12-HETE whereas the predominant major metabolic product of LO in human is 15-HETE [14, 15]. It is worthy to mention that analysis of completely sequenced genome of mammalian species did not provide any evidence for the simultaneous existence of separate ALOX15 and leukocyte type ALOX12 genes in a single mammalian species [14, 15]. We then targeted 12/15-LO pathway using baicalein. Baicalein, a 12/15-LO inhibitor, is a flavonoid isolated from the roots of a Chinese herbal medicine. Many reports confirmed the efficacy of baicalein against various pathological inflammatory conditions [46, 47]. Baicalein has antioxidant and anti-inflammatory properties independent on 12/15-LO inhibition which could mediate, at least in part, the reno-protective mechanisms against LPS induced acute renal injury [13]. However, we and other have previously shown that baicalein could also inhibit 12/15-LO and reduce 12- and 15-HETE production which could explain why baicalein reduces oxidative stress and inflammation particularly HETEs products of 12/15-LO, which have been reported to elicit pro-inflammatory and -oxidative effect [19, 48]. Indeed, we previously reported beneficial protective effect of baicalein in diabetic complications [13, 18]. LPS induced renal injury and inflammation was significantly reduced by 5-lipoxygenase inhibition with zileuton or target disruption of the 5-lipoxygenase gene utilizing 5-lipoxygenase knock-out mice suggesting a role of lipoxygenase in LPS induced acute renal injury which is consistent with our data in the current study [49].
To further confirm the role of 12/15-LO pathway in LPS-induced inflammation, we also induced acute inflammation by injection of LPS in 12/15-LO KO mice. Consistent with pharmacological inhibition of 12,15-LO with baicalein, knocking-out 12/15-LO prevented LPS-induced renal injury and prevented the elevation in renal oxidative stress marker TBARs excretion as well as renal inflammatory cytokines (TNF-α, IL-6, IL-1β and ICAM-1) expression, establishing a fundamental role for 12/15-LO in acute inflammatory response associated with LPS injection.
The metabolic transformation of fatty acids to form oxylipids using 12/15-LO can promote either resolving or non-resolving inflammation. However, the mechanism of how 12/15-LO interacts with polyunsaturated fatty acids (PUFA) remains unclear. Halade et al. recently showed that deletion of 12/15-LO and acute PUFA intake amplified the reparative lipid mediator epoxyeicosatrienoic acids (EETs) levels, thereby promoting neutrophil clearance and improved wound healing post-myocardial infarction [50]. Kain et al. also demonstrated that genetic deletion of 12/15-LO reduced 12(S)-HETE and activated CYP2J epoxygenase-derived EETs to promote effective resolution of inflammation post- myocardial infarction leading to reduced cardiac rupture, improved LV function, and better survival. These data suggest that importance of epoxygenase pathway in resolving inflammation post-MI when 12/15-LO pathway is compromised.
Previous findings suggest that DHA could have a promising role in reducing inflammation via acting as substrate for 12/15-LO to produce anti-inflammatory resolvin D [51, 52]. In fact, a recent study suggest that DHA reduced renal injury in mice model of hyperhomocysteinemia via inhibition of NLRP3 inflammasome activation [53]. Halade el al. previously demonstrated that DHA, but not EPA, is the most potent n-3 fatty acid that suppresses glomerulonephritis and extends life span of systemic lupus erythematosus-prone short-lived B x W mice, possibly via inhibition of IL-18 induction and IL-18-dependent signaling [54]. In our study, acute LPS injection was associated with a significant degree of renal injury as manifested by increased albuminuria and decreased creatinine clearance as well as structural cellular damage and tubular degeneration and these changes were reduced by DHA supplementation. Moreover, LPS injected mice showed a marked increase in the renal oxidative stress marker TBARs and the inflammatory marker MCP1 beside increased renal inflammatory cytokines and adhesion molecules expression. DHA supplementation also reduced markers of renal oxidative stress and inflammation in LPS injected mice. In our study, the dose of DHA was selected based on the previous findings of Kielar el al. where they demonstrated that Intraperitoneal DHA injection at this dose range ameliorated murine ischemic acute renal failure [32]. Because the inflammatory response generated by the oxygenated metabolites of arachidonic acid is known to be mediated via pro-inflammatory cytokines and adhesion molecules [55], We postulate that LPS injection leads to renal activation of 12/15-LO pathway which in turn results in elevation of oxidative stress, pro inflammatory cytokines and adhesion molecules.
The long chain ω−3 fatty acids DHA has antioxidant and anti-inflammatory properties in part, by reducing leukocyte-derived cytokine formation and modulation of eicosanoid synthesis [22, 55, 56]. Studies have also shown that enzymatic oxidation of DHA also produced the anti-inflammatory resolvins and protectins which could plays a role in DHA protective effects [57]. DHA could also increase pro-resolving lipid mediators (SPMs) that are synthesized by transcellular processes involving enzymes of epithelial cells and leukocytes [22]. SPMs derived from DHA are known as D-series resolvins. The D-series resolvins (RvD1, RvD2, RvD3, RvD4, RvD5, and RvD6) are synthesized from DHA by acetylated COX-2 or 15-LO [22]. D-Series resolvin synthesis results via formation of 17-hydroperoxydocosahexaenoic acid (17-HpDHA) and 17-hydroxydocosahexaenoic acid (17-HDHA) [22]. The actions of SPMs in disease models have been extensively reviewed and included controlling inflammatory pain (RvD1, 17R-RvD1 and RvD2), accelerating wound healing in diabetes (RvD1), inhibiting secondary thrombosis and necrosis in burn injury (RvD2) and preventing colitis (RvD1, and RvD2) [22]. In our experimental design, out of detectable anti-inflammatory metabolites and out of member of D-series resolvins, only RvD2 was significantly decreased after LPS injection and DHA supplementation prevented this decrease. The increased in RvD2 production with DHA treatment was associated with reduction of renal inflammation and injury in LPS injected mice. RvD2 is a potent lipid mediator which is synthesized from DHA during resolution phase of inflammation [58]. Thus, the current study was designed to test if DHA supplementation would divert the role of 12/15-LO from being pro-inflammatory to anti-inflammatory via the generation of the anti-inflammatory metabolite RvD2.
To further confirm protective effect of RvD2 in LPS-induced acute renal injury, we found that treatment of LPS injected mice with RvD2 improved creatinine clearance, reduced renal injury and prevented the elevation in renal TNF-α and ICAM-1 mRNA expression levels. These protective effects in acute LPS-induced renal injury are similar to those produced by DHA treatment. Our data are in consistent with the previous findings of Croasdell et al. where they showed that RvD2 reduced LPS-induced cytokines production and TLR4 expression in human monocyte [59]. In rat model of major burn and endotoxin insult, Inoue et al. previously demonstrated that the pathologic changes in kidney and liver tissues were ameliorated by RvD2 [60]. RvD1 also demonstrated to provide renal protection against LPS-induced acute kidney injury via down regulation of the inflammatory NF-κB signaling and the inhibition of apoptosis [26]. In our study, RvD2 provided similar protective effect to DHA even though DHA produces other anti-inflammatory metabolites. This could be related to bioavailability and the used dose of DHA versus RvD2 in the current study.
To confirm that the protective effect of DHA against LPS-induced acute renal injury, at least in part, involves 12/15-LO pathway, we treated LPS injected 12/15-LO KO mice with DHA. DHA did not exacerbate the reno-protective effect of 12/15-LO inhibition in LPS injected 12/15-LO KO mice confirming our hypothesis that DHA utilized 12/15-LO as a substrate to produce the anti-inflammatory RvD2 which could provide renal protection against LPS-induced acute renal inflammation and injury.
Collectively, our data suggest that 12/15-LO activation is involved in LPS-induced acute inflammatory responses and injury in the kidney and exogenous DHA supplementation could provide renal protection, at least in part, via utilizing 12/15-LO as an external substrate to produce the anti-inflammatory RvD2 metabolite.
Acknowledgements
This work was supported by intramural grant from Augusta University to A. Elmarakby and extramural NIH R01EY023315-award to M. Al-Shabrawey.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Medzhitov R, Origin and physiological roles of inflammation, Nature, 454 (2008) 428–435. [DOI] [PubMed] [Google Scholar]
- [2].Yu C, Qi D, Sun JF, Li P, Fan HY, Rhein prevents endotoxin-induced acute kidney injury by inhibiting NF-kappaB activities, Sci Rep, 5 (2015) 11822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Chang JH, McCluskey PJ, Wakefield D, Toll-like receptors in ocular immunity and the immunopathogenesis of inflammatory eye disease, Br J Ophthalmol, 90 (2006) 103–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Ngkelo A, Meja K, Yeadon M, Adcock I, Kirkham PA, LPS induced inflammatory responses in human peripheral blood mononuclear cells is mediated through NOX4 and Gialpha dependent PI-3kinase signalling, J Inflamm (Lond), 9 (2012) 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Guha M, Mackman N, LPS induction of gene expression in human monocytes, Cell Signal, 13 (2001) 85–94. [DOI] [PubMed] [Google Scholar]
- [6].van der Poll T, Meijers JC, Systemic inflammatory response syndrome and compensatory anti-inflammatory response syndrome in sepsis, J Innate Immun, 2 (2010) 379–380. [DOI] [PubMed] [Google Scholar]
- [7].Chen Z, Liu H, Lei S, Zhao B, Xia Z, LY294002 prevents lipopolysaccharideinduced hepatitis in a murine model by suppressing IkappaB phosphorylation, Mol Med Rep, 13 (2016) 811–816. [DOI] [PubMed] [Google Scholar]
- [8].Kalaiselvan S, Rasool MK, Triphala herbal extract suppresses inflammatory responses in LPS-stimulated RAW 264.7 macrophages and adjuvant-induced arthritic rats via inhibition of NFkappaB pathway, J Immunotoxicol, 13 (2016) 509–525. [DOI] [PubMed] [Google Scholar]
- [9].Qi M, Yin L, Xu L, Tao X, Qi Y, Han X, Wang C, Xu Y, Sun H, Liu K, Peng J, Dioscin alleviates lipopolysaccharide-induced inflammatory kidney injury via the microRNA let7i/TLR4/MyD88 signaling pathway, Pharmacol Res, 111 (2016) 509–522. [DOI] [PubMed] [Google Scholar]
- [10].Zheng C, Lei C, Chen Z, Zheng S, Yang H, Qiu Y, Lei B, Topical administration of diminazene aceturate decreases inflammation in endotoxin-induced uveitis, Mol Vis, 21 (2015) 403–411. [PMC free article] [PubMed] [Google Scholar]
- [11].Schrier RW, Wang W, Acute renal failure and sepsis, N Engl J Med, 351 (2004) 159–169. [DOI] [PubMed] [Google Scholar]
- [12].Moro K, Nagahashi M, Ramanathan R, Takabe K, Wakai T, Resolvins and omega three polyunsaturated fatty acids: Clinical implications in inflammatory diseases and cancer, World J Clin Cases, 4 (2016) 155–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Faulkner J, Pye C, Al-Shabrawey M, Elmarakby AA, Inhibition of 12/15-Lipoxygenase Reduces Renal Inflammation and Injury in Streptozotocin-Induced Diabetic Mice, Journal of diabetes & metabolism, 6 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Adel S, Karst F, Gonzalez-Lafont A, Pekarova M, Saura P, Masgrau L, Lluch JM, Stehling S, Horn T, Kuhn H, Heydeck D, Evolutionary alteration of ALOX15 specificity optimizes the biosynthesis of antiinflammatory and proresolving lipoxins, Proc Natl Acad Sci U S A, 113 (2016) E4266–4275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Kuhn H, Banthiya S, van Leyen K, Mammalian lipoxygenases and their biological relevance, Biochim Biophys Acta, 1851 (2015) 308–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Wang S, Cao W, Xing H, Chen YL, Li Q, Shen T, Jiang C, Zhu D, Activation of ERK pathway is required for 15-HETE-induced angiogenesis in human umbilical vascular endothelial cells, J Recept Signal Transduct Res, 36 (2016) 225–232. [DOI] [PubMed] [Google Scholar]
- [17].Zhu D, Ran Y, Role of 15-lipoxygenase/15-hydroxyeicosatetraenoic acid in hypoxiainduced pulmonary hypertension, J Physiol Sci, 62 (2012) 163–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Othman A, Ahmad S, Megyerdi S, Mussell R, Choksi K, Maddipati KR, Elmarakby A, Rizk N, Al-Shabrawey M, 12/15-Lipoxygenase-derived lipid metabolites induce retinal endothelial cell barrier dysfunction: contribution of NADPH oxidase, PLoS One, 8 (2013) e57254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Elmasry K, Ibrahim AS, Saleh H, Elsherbiny N, Elshafey S, Hussein KA, AlShabrawey M, Role of endoplasmic reticulum stress in 12/15-lipoxygenase-induced retinal microvascular dysfunction in a mouse model of diabetic retinopathy, Diabetologia, 61 (2018) 1220–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Ibrahim AS, Elshafey S, Sellak H, Hussein KA, El-Sherbiny M, Abdelsaid M, Rizk N, Beasley S, Tawfik AM, Smith SB, Al-Shabrawey M, A lipidomic screen of hyperglycemia-treated HRECs links 12/15-Lipoxygenase to microvascular dysfunction during diabetic retinopathy via NADPH oxidase, J Lipid Res, 56 (2015) 599–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Katakura M, Hashimoto M, Inoue T, Al Mamun A, Tanabe Y, Iwamoto R, Arita M, Tsuchikura S, Shido O, Omega-3 fatty acids protect renal functions by increasing docosahexaenoic acid-derived metabolite levels in SHR.Cg-Lepr(cp)/NDmcr rats, a metabolic syndrome model, Molecules, 19 (2014) 3247–3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Calder PC, Omega-3 fatty acids and inflammatory processes, Nutrients, 2 (2010) 355–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Yang ZH, Emma-Okon B, Remaley AT, Dietary marine-derived long-chain monounsaturated fatty acids and cardiovascular disease risk: a mini review, Lipids Health Dis, 15 (2016) 201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Morin C, Blier PU, Fortin S, Eicosapentaenoic acid and docosapentaenoic acid monoglycerides are more potent than docosahexaenoic acid monoglyceride to resolve inflammation in a rheumatoid arthritis model, Arthritis Res Ther, 17 (2015) 142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Orr SK, Palumbo S, Bosetti F, Mount HT, Kang JX, Greenwood CE, Ma DW, Serhan CN, Bazinet RP, Unesterified docosahexaenoic acid is protective in neuroinflammation, J Neurochem, 127 (2013) 378–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Zhao YL, Zhang L, Yang YY, Tang Y, Zhou JJ, Feng YY, Cui TL, Liu F, Fu P, Resolvin D1 Protects Lipopolysaccharide-induced Acute Kidney Injury by Down-regulating Nuclear Factor-kappa B Signal and Inhibiting Apoptosis, Chin Med J (Engl), 129 (2016) 11001107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Titos E, Rius B, Gonzalez-Periz A, Lopez-Vicario C, Moran-Salvador E, Martinez-Clemente M, Arroyo V, Claria J, Resolvin D1 and its precursor docosahexaenoic acid promote resolution of adipose tissue inflammation by eliciting macrophage polarization toward an M2-like phenotype, J Immunol, 187 (2011) 5408–5418. [DOI] [PubMed] [Google Scholar]
- [28].Ibrahim AS, Saleh H, El-Shafey M, Hussein KA, El-Masry K, Baban B, Sheibani N, Wang MH, Tawfik A, Al-Shabrawey M, Targeting of 12/15-Lipoxygenase in retinal endothelial cells, but not in monocytes/macrophages, attenuates high glucose-induced retinal leukostasis, Biochim Biophys Acta, 1862 (2017) 636–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Zhang W, Baban B, Rojas M, Tofigh S, Virmani SK, Patel C, Behzadian MA, Romero MJ, Caldwell RW, Caldwell RB, Arginase activity mediates retinal inflammation in endotoxin-induced uveitis, Am J Pathol, 175 (2009) 891–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Lixuan Z, Jingcheng D, Wenqin Y, Jianhua H, Baojun L, Xiaotao F, Baicalin attenuates inflammation by inhibiting NF-kappaB activation in cigarette smoke induced inflammatory models, Pulm Pharmacol Ther, 23 (2010) 411–419. [DOI] [PubMed] [Google Scholar]
- [31].Trepanier MO, Lim J, Lai TK, Cho HJ, Domenichiello AF, Chen CT, Taha AY, Bazinet RP, Burnham WM, Intraperitoneal administration of docosahexaenoic acid for 14days increases serum unesterified DHA and seizure latency in the maximal pentylenetetrazol model, Epilepsy Behav, 33 (2014) 138–143. [DOI] [PubMed] [Google Scholar]
- [32].Kielar ML, Jeyarajah DR, Zhou XJ, Lu CY, Docosahexaenoic acid ameliorates murine ischemic acute renal failure and prevents increases in mRNA abundance for both TNF-alpha and inducible nitric oxide synthase, J Am Soc Nephrol, 14 (2003) 389–396. [DOI] [PubMed] [Google Scholar]
- [33].Spite M, Norling LV, Summers L, Yang R, Cooper D, Petasis NA, Flower RJ, Perretti M, Serhan CN, Resolvin D2 is a potent regulator of leukocytes and controls microbial sepsis, Nature, 461 (2009) 1287–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].El-Mas MM, Helmy MW, Ali RM, El-Gowelli HM, Celecoxib, but not indomethacin, ameliorates the hypertensive and perivascular fibrotic actions of cyclosporine in rats: Role of endothelin signaling, Toxicology and applied pharmacology, 284 (2015) 1–7. [DOI] [PubMed] [Google Scholar]
- [35].Zhang S, Ma J, Sheng L, Zhang D, Chen X, Yang J, Wang D, Total Coumarins from Hydrangea paniculata Show Renal Protective Effects in Lipopolysaccharide-Induced Acute Kidney Injury via Anti-inflammatory and Antioxidant Activities, Front Pharmacol, 8 (2017) 872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].An X, Shang F, RA-XII exerts anti-oxidant and anti-inflammatory activities on lipopolysaccharide-induced acute renal injury by suppressing NF-kappaB and MAPKs regulated by HO-1/Nrf2 pathway, Biochem Biophys Res Commun, 495 (2018) 2317–2323. [DOI] [PubMed] [Google Scholar]
- [37].Bauer PM, Zalis MC, Abdshill H, Deierborg T, Johansson F, Englund-Johansson U, Inflamed In Vitro Retina: Cytotoxic Neuroinflammation and Galectin-3 Expression, PLoS One, 11 (2016) e0161723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Takahashi K, Mizukami H, Kamata K, Inaba W, Kato N, Hibi C, Yagihashi S, Amelioration of acute kidney injury in lipopolysaccharide-induced systemic inflammatory response syndrome by an aldose reductase inhibitor, fidarestat, PLoS One, 7 (2012) e30134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Bennett M, Gilroy DW, Lipid Mediators in Inflammation, Microbiol Spectr, 4 (2016). [DOI] [PubMed] [Google Scholar]
- [40].Levick SP, Loch DC, Taylor SM, Janicki JS, Arachidonic acid metabolism as a potential mediator of cardiac fibrosis associated with inflammation, J Immunol, 178 (2007) 641–646. [DOI] [PubMed] [Google Scholar]
- [41].Willenberg I, Rund K, Rong S, Shushakova N, Gueler F, Schebb NH, Characterization of changes in plasma and tissue oxylipin levels in LPS and CLP induced murine sepsis, Inflamm Res, 65 (2016) 133–142. [DOI] [PubMed] [Google Scholar]
- [42].Paul L, Fraifeld V, Kaplanski J, Evidence supporting involvement of leukotrienes in LPSinduced hypothermia in mice, Am J Physiol, 276 (1999) R52–58. [DOI] [PubMed] [Google Scholar]
- [43].Sacharzewska E, Bielecki P, Bernatowicz P, Niklinski J, Kowal-Bielecka O, Kowal K, The role of 12/15-lipoxygenase in production of selected eicosanoids in allergic airway inflammation, Adv Med Sci, 61 (2016) 141–146. [DOI] [PubMed] [Google Scholar]
- [44].Suzuki H, Kayama Y, Sakamoto M, Iuchi H, Shimizu I, Yoshino T, Katoh D, Nagoshi T, Tojo K, Minamino T, Yoshimura M, Utsunomiya K, Arachidonate 12/15-lipoxygenase-induced inflammation and oxidative stress are involved in the development of diabetic cardiomyopathy, Diabetes, 64 (2015) 618–630. [DOI] [PubMed] [Google Scholar]
- [45].Ibrahim AS, Tawfik AM, Hussein KA, Elshafey S, Markand S, Rizk N, Duh EJ, Smith SB, Al-Shabrawey M, Pigment epithelium-derived factor inhibits retinal microvascular dysfunction induced by 12/15-lipoxygenase-derived eicosanoids, Biochim Biophys Acta, 1851 (2015) 290–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Song L, Yang H, Wang HX, Tian C, Liu Y, Zeng XJ, Gao E, Kang YM, Du J, Li HH, Inhibition of 12/15 lipoxygenase by baicalein reduces myocardial ischemia/reperfusion injury via modulation of multiple signaling pathways, Apoptosis : an international journal on programmed cell death, 19 (2014) 567–580. [DOI] [PubMed] [Google Scholar]
- [47].Ahad A, Mujeeb M, Ahsan H, Siddiqui WA, Prophylactic effect of baicalein against renal dysfunction in type 2 diabetic rats, Biochimie, 106 (2014) 101–110. [DOI] [PubMed] [Google Scholar]
- [48].Elmasry K, Ibrahim AS, Abdulmoneim S, Al-Shabrawey M, Bioactive lipids and pathological retinal angiogenesis, Br J Pharmacol, 176 (2019) 93–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Collin M, Rossi A, Cuzzocrea S, Patel NS, Di Paola R, Hadley J, Collino M, Sautebin L, Thiemermann C, Reduction of the multiple organ injury and dysfunction caused by endotoxemia in 5-lipoxygenase knockout mice and by the 5-lipoxygenase inhibitor zileuton, J Leukoc Biol, 76 (2004) 961–970. [DOI] [PubMed] [Google Scholar]
- [50].Halade GV, Kain V, Ingle KA, Prabhu SD, Interaction of 12/15-lipoxygenase with fatty acids alters the leukocyte kinetics leading to improved postmyocardial infarction healing, Am J Physiol Heart Circ Physiol, 313 (2017) H89–H102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Koltsida O, Karamnov S, Pyrillou K, Vickery T, Chairakaki AD, Tamvakopoulos C, Sideras P, Serhan CN, Andreakos E, Toll-like receptor 7 stimulates production of specialized pro-resolving lipid mediators and promotes resolution of airway inflammation, EMBO Mol Med, 5 (2013) 762–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Li PL, Gulbins E, Bioactive Lipids and Redox Signaling: Molecular Mechanism and Disease Pathogenesis, Antioxid Redox Signal, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Li G, Chen Z, Bhat OM, Zhang Q, Abais-Battad JM, Conley SM, Ritter JK, Li PL, NLRP3 inflammasome as a novel target for docosahexaenoic acid metabolites to abrogate glomerular injury, J Lipid Res, 58 (2017) 1080–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Halade GV, Rahman MM, Bhattacharya A, Barnes JL, Chandrasekar B, Fernandes G, Docosahexaenoic acid-enriched fish oil attenuates kidney disease and prolongs median and maximal life span of autoimmune lupus-prone mice, J Immunol, 184 (2010) 5280–5286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Calder PC, Polyunsaturated fatty acids and inflammation, Prostaglandins Leukot Essent Fatty Acids, 75 (2006) 197–202. [DOI] [PubMed] [Google Scholar]
- [56].Simopoulos AP, Omega-3 fatty acids in inflammation and autoimmune diseases, J Am Coll Nutr, 21 (2002) 495–505. [DOI] [PubMed] [Google Scholar]
- [57].Dobson EP, Barrow CJ, Kralovec JA, Adcock JL, Controlled formation of mono- and dihydroxy-resolvins from EPA and DHA using soybean 15-lipoxygenase, J Lipid Res, 54 (2013) 1439–1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Zhang MJ, Sansbury BE, Hellmann J, Baker JF, Guo L, Parmer CM, Prenner JC, Conklin DJ, Bhatnagar A, Creager MA, Spite M, Resolvin D2 Enhances Postischemic Revascularization While Resolving Inflammation, Circulation, 134 (2016) 666–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Croasdell A, Sime PJ, Phipps RP, Resolvin D2 decreases TLR4 expression to mediate resolution in human monocytes, FASEB J, 30 (2016) 3181–3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Inoue Y, Yu YM, Kurihara T, Vasilyev A, Ibrahim A, Oklu R, Zhao G, Nair AV, Brown D, Fischman AJ, Tompkins RG, Irimia D, Kidney and Liver Injuries After Major Burns in Rats Are Prevented by Resolvin D2, Crit Care Med, 44 (2016) e241–252. [DOI] [PMC free article] [PubMed] [Google Scholar]