Abstract
There are no Food and Drug Administration approved pharmacotherapies for methamphetamine (METH) overdose, thus identifying novel drug targets to prevent this devastating adverse event is a public-health imperative. Previous research suggests that serotonin and sigma receptors may contribute to the adverse effects of METH. The present study assessed whether pretreatment with the 5-HT2A receptor antagonist M100907 or the sigma 1 (σ1) receptor antagonist BD 1047 attenuated METH-induced lethality, hyperthermia, convulsions, and seizures. Male Swiss Webster mice received intraperitoneal injections of M100907 (1 and 10 mg/kg), BD 1047 (10 mg/kg), or a combination of M100907 (1 mg/kg) and BD 1047 (10 mg/kg) prior to treatment with METH (78 mg/kg). Convulsions and lethality were assessed by observation, core body temperature was assessed by surgically implanted telemetric probes, and seizures were assessed by electroencephalography. M100907 reduced METH-elicited lethality from 67% to 33%, BD1047 reduced METH-elicited lethality from 67% to 50%, and combined administration of both agents eliminated lethality in all mice tested. Similarly, both agents and their combination reduced METH-elicited seizures and convulsions. None of the treatments decreased METH-induced hyperthermia. This research suggests that reducing METH-induced seizures is an important factor in reducing lethality associated with METH overdose. However, future studies should examine whether M100907 and BD 1047 modulate METH-induced hypertension and other adverse effects that may also contribute to METH overdose. Our data support the continued investigation of compounds that target 5-HT2A and σ1 receptors in METH-induced overdose, including their potential to yield emergency reversal agents.
Keywords: M100907, BD 1047, methamphetamine, overdose, seizure, hyperthermia, convulsions, lethality
Introduction
Methamphetamine (METH) is a highly abused psychostimulant and its prevalence and abuse liability are a significant global problem. METH abuse can lead to hypertension, heart attack, stroke, organ failure, hyperthermia, convulsions, seizures and death. Moreover, METH-associated acute overdose is a potentially life-threatening condition requiring immediate medical attention. In 2016, the Centers for Disease Control and Prevention reported there were more than 64,000 drug overdose deaths in the United States, of which 7,663 were due to METH (Seth et al., 2018). While overshadowed by the present opioid crisis, there is a current resurgence in METH abuse due to inexpensive, high-quality product flooding the market (The Lancet, 2018). Several states are experiencing a significant surge in METH abuse and there has been a dramatic increase in METH confiscations of the illicit agent entering the United States. This is notable for several reasons, but in particular, because there is no Food and Drug Administration (FDA) approved pharmacotherapy for the treatment of METH overdose, unlike the naloxone formulations available for opioid overdose.
METH-induced overdose can be indicated by signs and symptoms such as hyperthermia and convulsions that may be addressed with the development of life-saving reversal agents. The symptoms of METH-induced overdose overlap with those of serotonin syndrome (SS), which also results in hyperthermia and convulsions. SS is a consequence of toxic levels of serotonin (5-HT) overly stimulating peripheral and central postsynaptic 5-HT1A and 5-HT2A receptors (Alusik et al., 2014, Beakley et al., 2015), suggesting that 5-HT receptors may be a target for reducing critical symptoms of METH-induced overdose. A number of previous studies have linked the 5HT2A receptor to thermoregulation (Ootsuka and Blessing, 2006, Pawlyk et al., 2006, Sipe et al., 2004), and it has been shown that 5-HT2A receptor antagonism attenuates hyperthermia and lethality associated with SS (Nisijima et al., 2001). The 5-HT2A receptor has also been tied to stimulant-induced convulsions and seizures. Co-administration of the 5-HT releaser fenfluramine potentiates cocaine-induced convulsions (Schechter and Meehan, 1995), and 5-HT2A receptor antagonism attenuates convulsions induced by cocaine (O'Dell et al., 2000). These are important findings as METH-induced convulsions are not reliably controlled by standard anticonvulsant medications. Hanson and colleagues observed that while cocaine-related seizures were controlled by phenytoin, METH-induced seizures were refractory to this therapy. Moreover, diazepam and valproate were only partially effective in attenuating METH-induced seizures (Hanson et al., 1999). In another study, diazepam, MK-801, and propranolol reduced METH-induced convulsions, but none of these agents reduced METH-induced lethality (Derlet et al., 1990). These findings warrant additional research to establish the role of 5-HT2A receptors in stimulant overdose, with particular relevance for METH.
Another treatment target for METH overdose is the σ1 receptor, which is an endoplasmic reticulum membrane protein that is expressed in the heart and lungs, and has particularly high expression in specific regions of the central nervous system, such as the cerebellum, nucleus accumbens and cerebral cortex (Bao et al., 2017, Cao et al., 2017, Weissman et al., 1988). It is known that METH binds to σ1 receptors (Nguyen et al., 2005), σ1 receptors are present in monoamine neurons, and they may play a role in modulating METH-induced hyperthermia and neurotoxicity. In addition, it has been demonstrated that σ1 receptor antagonists attenuate METH-induced neuroinflammation, apoptosis, and formation of reactive oxygen species (Kaushal et al., 2012, Robson et al., 2014) as well as METH-induced hyperthermia (Matsumoto et al., 2008, Robson et al., 2013). The σ1 receptor antagonist AC927 attenuates METH-induced locomotor stimulation, striatal dopamine depletion, and hyperthermia (Matsumoto et al., 2008). It is well established that METH causes damage and degeneration of dopamine neurons via oxidative stress, mitochondria and endoplasmic reticulum dysfunction, and inflammation (Albers and Sonsalla, 1995, Ali et al., 1996, Courtney and Ray, 2014, Fujikawa et al., 2016, Krasnova and Cadet, 2009, Matsumoto et al., 2014). σ1 receptors play a role in generating reactive oxygen species and regulation of intracellular Ca2+ stores (Kaushal and Matsumoto, 2011, Yasui and Su, 2016), both of which may contribute to METH toxicity. In addition, previous studies have shown that σ1 receptor antagonists attenuate cocaine-induced convulsions and lethality (McCracken et al., 1999). These findings provide a rationale to investigate the σ1 receptor as a potential pharmacotherapeutic target for treating METH overdose.
In the present study, we examined the role of 5-HT2A and σ1 receptors in attenuating the effects on METH-induced overdose. We evaluated the effects of the 5-HT2A receptor antagonist M100907 (Hall et al., 2000) and the σ1 receptor antagonist BD 1047 (McCracken et al., 1999), alone and in combination on METH-induced lethality, hyperthermia, convulsions, and seizures. Hyperthermia studies determined the association between elevated body temperatures and lethality. Convulsion studies assessed the latency to a convulsion (i.e., a sudden, irregular movement of a limb or of the body, caused by contraction of muscles), and the proportion of animals that exhibited a convulsion following METH administration. Seizure studies assessed the intensity of a seizure (i.e., a sudden burst of electrical activity in the brain).
Materials and Methods
Animals
The test subjects were male Swiss Webster mice (Charles River Laboratories, Inc., Wilmington, MA) that weighed between 27–33 grams and ranged in age from 2–3 months. The mice were housed 3–4 per cage in a temperature regulated room. Swiss-Webster mice were chosen for these studies because these mice are a general purpose strain that has been used extensively to study behavior, physiology, and neurochemistry (Murnane et al., 2019, Murnane et al., 2012b, Murphy and Murnane, 2019, Oppong-Damoah et al., 2019, Ray et al., 2018). For the core body temperature experiments, an additional 12 mice were prepared with Starr Life Sciences G2 E-Mitters (see telemetry probe surgery section below). These mice weighed between 25–30 grams and ranged in age from 2–3 months at the time of the surgery and were kept singly housed for the duration of the study. All mice had ad libitum access to food and water. All experiments were approved by the Mercer University Institutional Animal Care and Use Committee.
Drugs
Methamphetamine hydrochloride was purchased from Sigma-Aldrich (St. Louis, MO). M100907 was synthesized by Kenner Rice (Ullrich and Rice, 2000). BD 1047 dihydrobromide was purchased from Sigma-Aldrich. M100907 was dissolved in sterile water with dropwise addition of 0.1 N hydrochloric acid and sonication until it dissolved. All other drugs were dissolved in physiological saline. All drugs were administered by intraperitoneal (IP) injection at a volume of 0.01 ml per g body weight of each mouse. All doses are reported in the salt form 30 minutes prior to treatment with METH at 78 mg/kg (LD67), mice were administered M100907 (1 and10 mg/kg), BD 1047 (10 mg/kg), or a combination of M100907 (1 mg/kg) and BD 1047 (10 mg/kg), which we subsequently refer to as M100907/BD 1047 (1/10 mg/kg).
Lethality
In preliminary experiments, a full dose-effect determination was conducted to assess the lethal effects of METH. To reduce the number of animals used, in some cases, animals in the lethality study also underwent observations for convulsions (as described below). Lethality was recorded at 2 hours following the METH challenge (N = 6 per group). The dependent measure examined was the percent lethality post-treatment. All animals were euthanized at 2 hours whether or not drug-induced lethality occurred to promote animal welfare.
Hyperthermia
As some literature suggests that hyperthermia may contribute to METH-induced lethality, and we have consistently observed hyperthermia in METH-treated animals (Murnane et al., 2012b), we examined whether 5-HT2A or σ1 receptors played a role in METH-induced hyperthermia and whether there is an association between hyperthermia and lethality. Mice were prepared with Starr Life Sciences G2 E-Mitters (N = 6 per group). Given the practical necessity of surgically implanting telemetry probes (see section below) to study core body temperature, we choose to initially study modulation of METH-induced hyperthermia at a dose that did not induce lethality (18 mg/kg). In a limited set of additional experiments, we examined modulation of hyperthermia induced by 78 mg/kg of METH, as this was the same dose used in the lethality experiments. These mice were housed individually over receivers designed to collect signals from the probes. Core body temperature was collected over 5 minute intervals and then averaged into 15 minute bins. Pretreatments were administered 30 minutes prior to METH, M100907 and BD 1047 challenges, and core temperature was recorded for 3 hours following all administrations, as this captures the entire time course of each treatment following IP injection.
Telemetry Probe Surgery
Subjects underwent implantation of a small, wireless G2 E-Mitter telemetry probe (Starr Life Sciences, Oakmont, PA) into their peritoneal space to collect continuous core body temperature data. The surgery was accomplished by anesthetizing each mouse with inhaled isoflurane (1–3% induction, maintenance to effect). After achievement of an appropriate level of anesthesia, the abdominal hair was removed with a grooming kit and depilatory. The surgical area was cleansed by wiping with gauze pads, soap, and water. Each subject was then placed in a supine position with limbs gently tied off. For each animal, the area of surgical incision was disinfected with Hibiclens (chlorhexidine gluconate 4% w/v) and isopropyl alcohol (70%), and 2–5 minutes later, a midline abdominal incision of < 2 cm was made, approximately 1 cm below the diaphragm. The underlying muscle was then separated via blunt dissection. A pre-sterilized probe (Benz-all®, benzalkonium chloride 12.9%, diluted 1:50; Moore Medical LLC, Farmington, CT according to the manufacturer’s instruction) was implanted ventral to the digestive organs. Following placement of the probe, the incision was closed with simple interrupted absorbable sutures (5–0 Vicryl), and the area cleansed with Hibiclens or isopropyl alcohol. Subjects were returned to their home cages when they recovered from sternal recumbency. Cephazolin (10 mg/kg) and ketoprofen (2 mg/kg) were administered subcutaneously at the outset of surgery for antibiotic and non-steroidal anti-inflammatory (NSAID) prophylaxis, respectively. These agents were administered once daily for 2 days post-surgery. All mice were allowed to recover for at least 7 days following surgery before drug treatments commenced.
Convulsions
All mice in the convulsion and lethality experiments were injected with a specified pre-treatment followed by METH, and then individually placed in acrylic chambers. A video camera placed above the chamber recorded behavior for offline scoring. The behavior of each mouse was recorded for 45 minutes following the METH challenge. The dependent measures were the proportion of mice exhibiting a convulsion and the latency to the first tonic-clonic convulsion, based on a scale that we have developed for measuring stimulant-induced convulsions (Table 1).
Table 1:
Overview of the custom seizure scale used in this study
| Category | Value | Behavior |
|---|---|---|
| Normal | 1 | Grooming, walking, tearing up bedding, scratching |
| Impaired | 2 | Not moving and obviously impaired, minor tremors, minor twitches, head turning from side to side, stereotyped behavior |
| Tonic Still | 3 | Extension of the tail over the back, limbs tonically extended |
| Tonic-Clonic | 4 | Rhythmic limb movements, stiff muscles, and jerking movements |
| Clonic Run Jump | 5 | Rhythmic limb movements, rapid locomotion, repeated jumping |
| Loss of Righting | 6 | Laying on the side or back for longer than two seconds |
Electroencephalography (EEG) Head mount Surgery
Each mouse received EEG and electromyography (EMG) electrodes and an 8-pin socket connector head mount with stainless steel leads (8201-SS; Pinnacle Technology Inc, Lawrence, KS). Under isoflurane anesthesia (1–3%, to effect), 3 sterile epidural screw electrodes (0.10” length, with stainless steel wire leads) were surgically implanted into the skull for EEG/EMG recordings. The two recording electrodes were placed over the right frontal cortex (2 mm anterior to bregma and 1.2 mm lateral to the midline) and the right the parietal cortex (1.5 mm posterior to bregma and 1.2 mm lateral to the midline). The recording electrodes were referenced to a common electrode placed over the contralateral parietal cortex. Electrodes were connected by stainless steel wire and soldered to the head mount, and the entire assembly was secured with dental acrylic. For EMG analysis, two fine-wire electrodes were placed in the nuchal muscle. After surgery, mice were allowed to recover for 7 days before the initiation of experiments, and all mice received ketoprofen as described above.
EEG Recordings
Prior to EEG recordings, the head mount was connected—via a lightweight tether and commutator—to an amplifier and PC running the Sirenia acquisition software (Pinnacle Technology Inc, Lawrence, KS). This enabled complete freedom of movement throughout a circular acrylic cage (240 mm diameter). Baseline EEG recordings were collected for 120 minutes. Mice were connected to the tether and placed in the cage at least 60 minutes before any drug administration. Video monitoring of behavior, including convulsions, was conducted continuously during EEG/EMG data collection.
EEG Analysis
For drug-induced seizures, all EEG/EMG data and video recordings were saved and analyzed off-line using the Sirenia Seizure Pro 1.8 software package (Pinnacle Technology Inc, Lawrence, KS). Seizures were initially identified by line length, heat map, and power analysis, which was used for subsequent automated seizure detection. Spectral power analysis was performed using custom written functions in IGOR Pro 7 (WaveMetrics Inc., Lake Oswego, OR). Power spectral analysis was accomplished by applying a fast Fourier transform (FFT, 0.1 Hz frequency resolution) to 90 minutes of EEG data. To quantify the seizure data, average spectral power in the 0.5 – 4 Hz range was calculated by applying a fast Fourier transformation to raw EEG waveforms. All epochs in the 90 min recordings were included in this analysis. Power is expressed as a percentage of the total spectral power in the EEG signal (0.5–40 Hz) during that time period.
Data Analysis
All graphical data presentations were created using GraphPad Prism (GraphPad Software Inc., La Jolla, CA, USA). All proportion data (lethality and convulsions) were analyzed by Fisher’s exact test. Telemetry data (core body temperature) were converted to area under the curve, and then analyzed by paired t-test or repeated-measures one-way analysis of variance (ANOVA), followed by Dunnett’s post-hoc test (Dunnett, 1955). The latency to the first convulsion was analyzed by one-way ANOVA, with post-hoc comparisons by Dunnett’s test. In the instances where no convulsion was observed, the maximum latency of 2700 seconds was assigned.
Results
Lethality
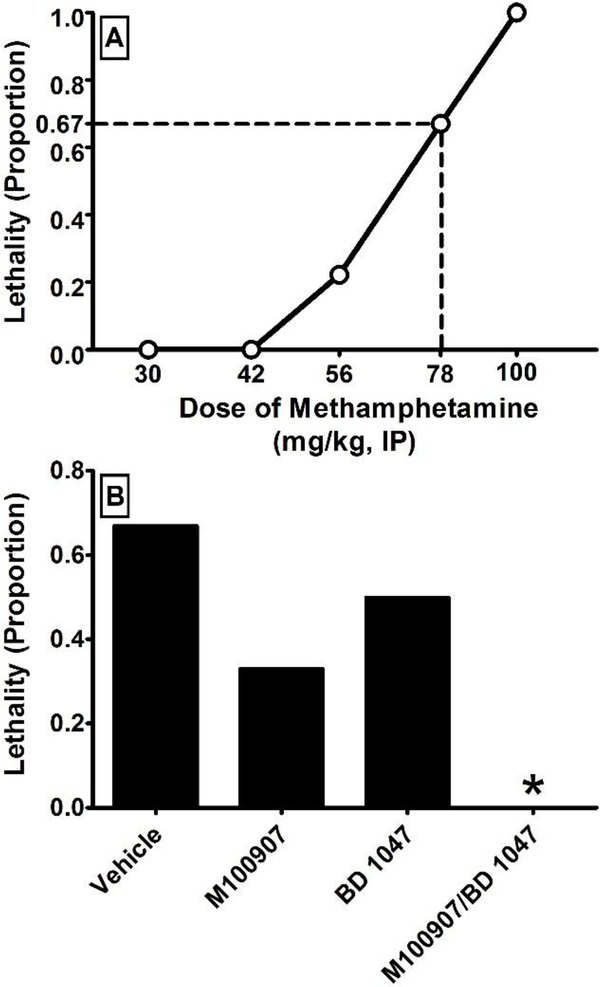
The effects of pretreatment with vehicle, M100907, BD 1047, and M100907/BD 1047 on METH-induced lethality were assessed (Figure 1). Fisher’s exact test revealed a significant reduction in lethality in animals treated with M100907/BD 1047 (odds ratio = 23.4; 95% confidence interval = 0. 89, 613.5; p = 0.03) in comparison to animals administered vehicle. In contrast, there was no significant reduction in lethality in animals administered only M100907 (odds ratio = 4.0; 95% confidence interval = 0.36, 44.14; p = 0.28) or BD 1047 (odds ratio = 2.0; 95% confidence interval = 0.19, 20.63; p = 0.50). Although not significant, it is important to note that M100907 reduced METH-elicited lethality from 67% to 33% and BD1047 reduced METH-elicited lethality from 67% to 50%.
Figure 1:
The effects of pretreatment with vehicle, M100907 (1 mg/kg), BD 1047 (10 mg/kg), and the combination of M100907/BD 1047 (1/10 mg/kg) on lethality (N = 6 per group) induced by methamphetamine (78 mg/kg). The initial dose-effect function of lethality induced by methamphetamine is presented in panel A. The LD67 dose of 78 mg/kg was chosen for all subsequent lethality experiments. The effects of pretreatment with vehicle, each agent, or their combination on lethality induced by methamphetamine is presented in panel B. All pretreatments were administered 30 minutes prior to methamphetamine. * = p < 0.05 as assessed by Fisher’s exact test.
Hyperthermia
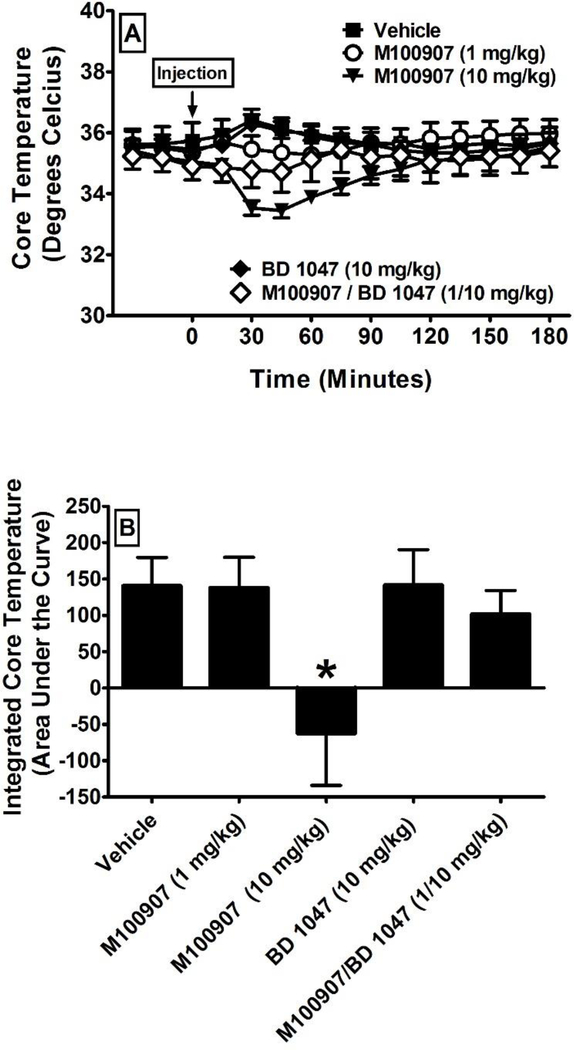
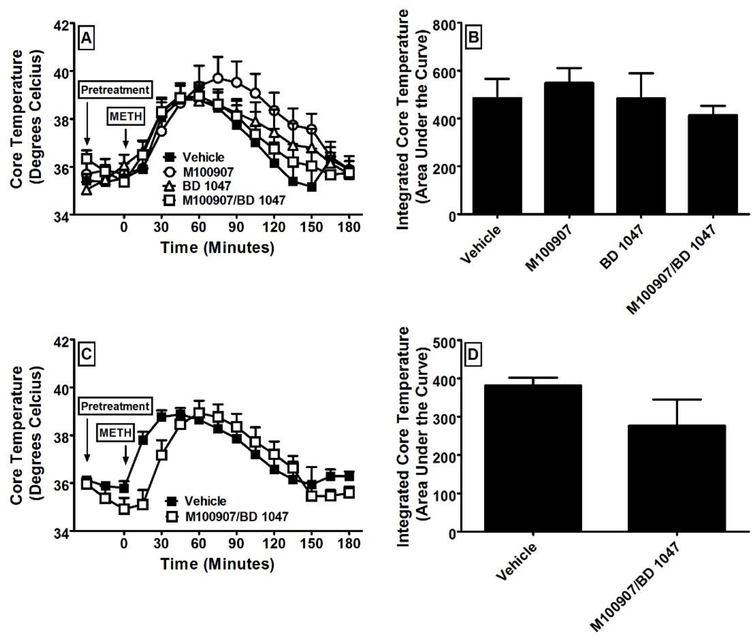
Initial experiments examined the effects of treatment with vehicle, M100907 (1 and 10 mg/kg), BD 1047 (10 mg/kg), and the combination of M100907/BD 1047 (1/10 mg/kg) on core temperature (N = 6 per group) as measured by implanted telemetry probes. The time courses of the changes in core temperature and the integrated areas under the curve of these time courses are presented in Figure 2. A one-way repeated-measures ANOVA revealed a significant main effect of treatment. Dunnett’s post-hoc analysis revealed a significant decrease in core body temperature after treatment with M100907 at 10 mg/kg (F4, 29 = 3.92; p = 0.02) in comparison to after treatment with vehicle. There was no significant difference between vehicle and any of the other pretreatments. We then examined the effects of pretreatment with vehicle, M100907 (1 mg/kg), BD 1047 (10 mg/kg), and the combination of M100907/BD 1047 (1/10 mg/kg) on hyperthermia elicited by 18 mg/kg of METH (N = 6 per group). This dose was chosen because it was found to produce robust hyperthermia in preliminary experiments in the absence of convulsions or lethality. The time courses of the changes in core temperature and the integrated areas under the curve of these time courses are presented in Figure 3. A one-way repeated-measures ANOVA revealed no significant main effect of pretreatment on METH-induced hyperthermia. We then examined the effects of pretreatment with the combination of M100907/BD 1047 (1/10 mg/kg) on hyperthermia elicited by 78 mg/kg of METH (N = 6 per group). This dose was chosen because the combination of M100907/BD 1047 did not reduce METH-induced hyperthermia at 18 mg/kg even though it significantly reduced lethality, and we therefore chose to test the same dose of METH used in the lethality experiments. The time courses of the changes in core temperature and the integrated areas under the curve of these time courses are presented in Figure 3. Unpaired t-test revealed no significant main effect of pretreatment on METH-induced hyperthermia at this dose either.
Figure 2:
The effects of treatment with vehicle, M100907 (1 mg/kg), BD 1047 (10 mg/kg), and the combination of M100907/BD 1047 (1/10 mg/kg) on core temperature (N = 6 per group). The time courses of the changes in core temperature are presented in panel A. The integrated areas under the curve of these time courses are presented in panel B. All values represent the means + SEM. * = p < 0.05 as assessed by one-way analysis of variance and Dunnett’s post-hoc test in comparison to vehicle.
Figure 3:
The effects of pretreatment with vehicle, M100907 (1 mg/kg), BD 1047 (10 mg/kg), and the combination of M100907/BD 1047 (1/10 mg/kg) on hyperthermia elicited by methamphetamine as measured from core temperature (N = 6 per group). The time courses of the changes in core temperature are presented in panel A for 18 mg/kg of methamphetamine and panel C for 78 mg/kg of methamphetamine. The integrated areas under the curve of these time courses are presented in panel B for 18 mg/kg of methamphetamine and panel D for 78 mg/kg of methamphetamine. All values represent the means + SEM.
Convulsions
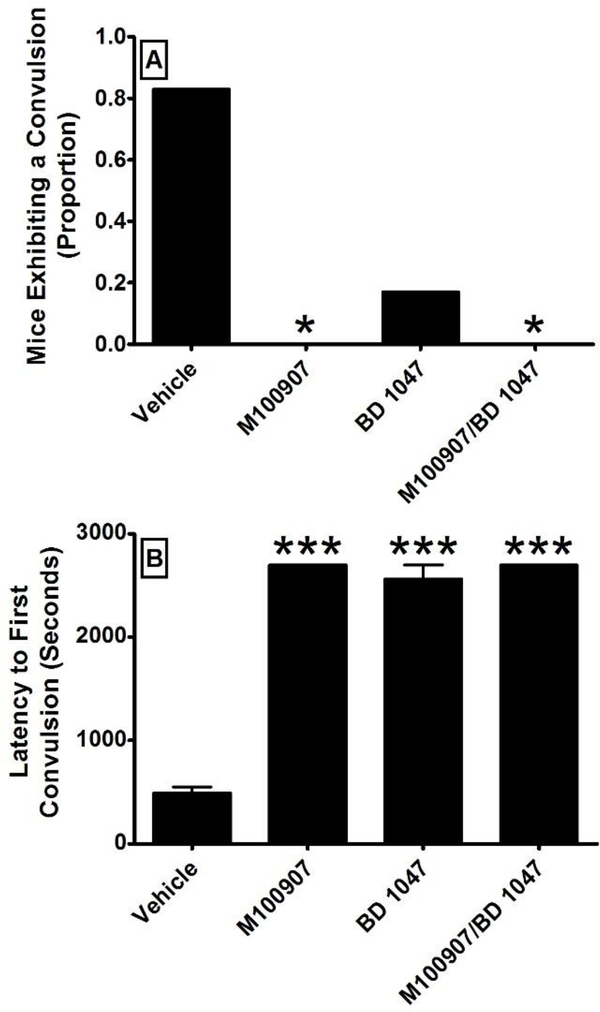
The effects of pretreatment with vehicle, M100907, BD 1047, and M100907/BD 1047 on the proportion of animals exhibiting a convulsion were assessed over a period of 45 minutes following METH administration (Figure 4A). A Fisher’s exact test revealed a significant decrease in the proportion of animals exhibiting a tonic-clonic convulsion in the animals administered M100907 (odds ratio = 47.67; 95% confidence interval = 1.59, 1424; p = 0.02) or the combination of M100907/BD 1047 (odds ratio = 47.67; 95% confidence interval = 1.59, 1424; p = 0.02) in comparison to animals administered vehicle. In contrast, there was no significant reduction in the proportion of animals exhibiting a tonic-clonic convulsion in animals administered BD 1047 alone. The effects of pretreatment with vehicle, M100907, BD 1047, and M100907/BD 1047 on the latency to the first convulsion were assessed by timing the onset of the first convulsion following the injection of METH (Figure 4B). A one-way ANOVA revealed a significant main effect of pretreatment (F3, 19 = 200.1; p < 0.001) on the latency to a tonic-clonic convulsion. Dunnett’s post-hoc analysis revealed a significant difference between pretreatment with vehicle and pretreatment with M100907 (q = 20.84; p < 0.001), BD 1047 (q = 19.57; p < 0.001), or the combination of M100907/BD 1047 (q = 20.84; p < 0.001).
Figure 4:
The effects of pretreatment with vehicle, M100907 (1 mg/kg), BD 1047 (10 mg/kg), and the combination of M100907/BD 1047 (1/10 mg/kg) on convulsions (N = 6 per group) induced by methamphetamine (78 mg/kg). The proportion of mice exhibiting a tonic-clonic convulsion is presented in panel A. The latency to the first convulsions is presented in panel B. All pretreatments were administered 30 minutes prior to methamphetamine. *= p < 0.05; *** = p < 0.001. The proportional data were analyzed by Fisher’s exact test. The latency data were analyzed by one-way analysis of variance followed by Dunnett’s test.
Seizures
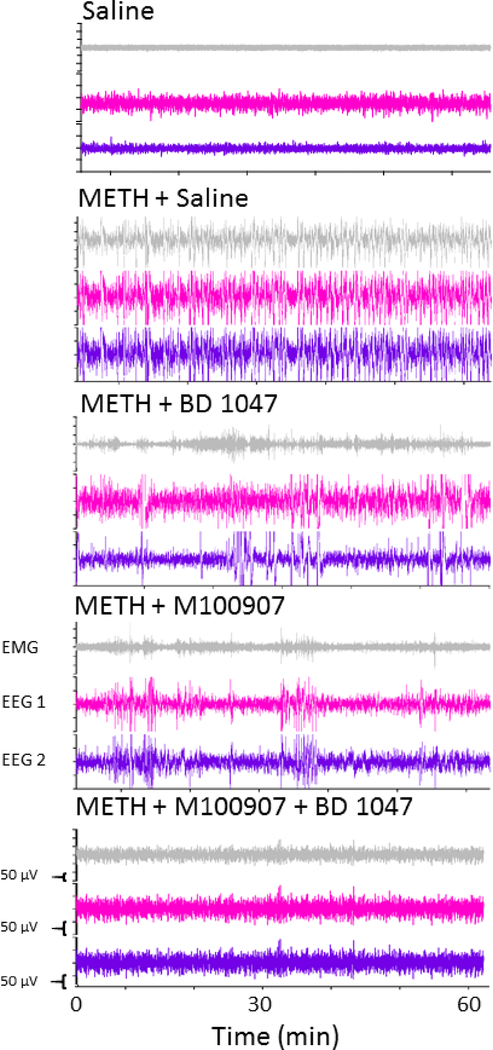
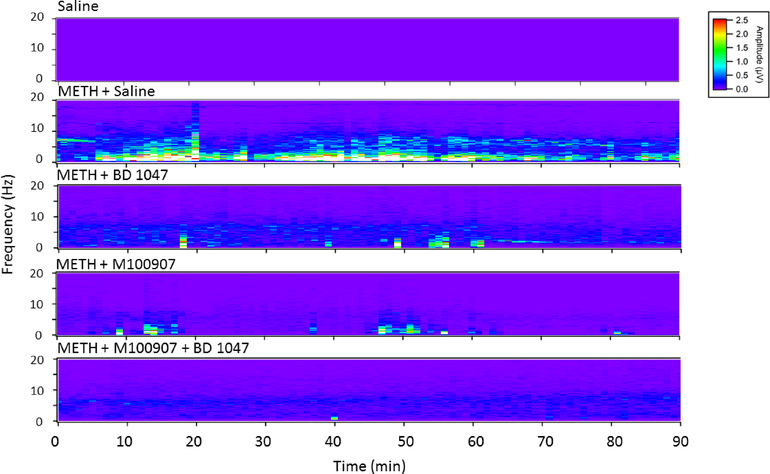
EEG waveforms were used to assess direct brain seizure activity. Representative raw tracings over a period of 60 seconds following administration of METH are shown in Figure 5. Power spectral analysis was accomplished by applying a fast Fourier transform to 90 minutes of EEG recording following METH administration. There was a substantial increase in the power observed in the higher frequency ranges following METH administration. This increase was reversed by pretreatment with either M100907 or BD 1047 given alone, and nearly eliminated by the combination of M100907 and BD 1047 (Figure 6).
Figure 5:
Representative electroencephalography waveforms over a period of 60 seconds following administration of 78 mg/kg of methamphetamine (or baseline). Data showing the effects of pretreatment with vehicle, M100907 (1 mg/kg), BD 1047 (10 mg/kg), and the combination of M100907/BD 1047 (1/10 mg/kg) on seizures are shown.
Figure 6:
Representative heat maps showing the power at each frequency in the electroencephalography data over a period of 90 minutes following administration of 78 mg/kg of methamphetamine (or baseline). Data showing the effects of pretreatment with vehicle, M100907 (1 mg/kg), BD 1047 (10 mg/kg), and the combination of M100907/BD 1047 (1/10 mg/kg) on seizures are shown.
Discussion
The major finding of the present study is that M100907 and BD 1047 reduced several important acute toxic effects of METH. A secondary finding is that there appears to be possible additive or synergistic effects of both agents in the attenuation of METH toxicity. In line with these findings, pretreatment with M100907 or BD 1047 administered alone attenuated METH-induced lethality, whereas the combination of M100907 and BD 1047 eliminated METH-induced lethality, with a change from LD67 to LD0. Surprisingly, none of these treatments had any significant effect on METH-induced hyperthermia. All three treatments reduced the latency to and the proportion of mice exhibiting a tonic-clonic convulsion, based on behavioral observations. Likewise, heat maps (power plot) and EEG (frequency-time graph) data illustrate that pretreatment with M100907 or BD 1047 alone attenuated METH-induced seizures, whereas the combination of M100907 and BD 1047 fully eliminated METH-induced seizures. Overall, these data suggest that 5-HT2A receptors and σ1 receptors may act additively or synergistically to attenuate the acute toxic effects of METH.
M100907 substantially attenuated METH-induced convulsions and lethality when given at 1 mg/kg. We therefore examined a 10-fold higher dose of M100907 and found that it completely eliminated METH-induced lethality, convulsions and hyperthermia (data not shown). However, this dose of M100907 produced a hypothermic response. This hypothermic response may be contraindicated in clinical practice, and complicates the interpretation of the elimination of METH toxicity, as it is well known that hypothermia itself can attenuate stimulant toxicity (Adori et al., 2011, Ali et al., 1994, Banks et al., 2007, Malberg and Seiden, 1998). For these reasons, and because we wanted to focus our efforts on combinations of partially effective doses, we conducted the remaining experiments using the lower dose of M100907. In addition, the presentation of this hypothermic effect is not indicative of a 5-HT2A mediated response (Ootsuka and Blessing, 2006, Pawlyk et al., 2006, Sipe et al., 2004), and suggests that M100907 loses selectivity for the 5-HT2A receptor at 10 mg/kg. M100907 has greater than 100-fold selectivity for 5-HT2A receptors over other protein targets and has low nanomolar affinity for 5-HT2A receptors (Knight et al., 2004). Nevertheless, it is important to note that doses of 1 and 10 mg/kg of M100907 are higher than the doses of this agent required to shift the dose-response curve for 5-HT2A receptor agonist induced head-twitch behavior (Canal et al., 2013) and the behavioral effects of indirect monoamine agonists (Murnane et al., 2013a, Murnane et al., 2012a, Murnane et al., 2013b). It is possible that M100907 has lost selectivity for 5-HT2A receptors across the entire dose range that we examined. As we have recently reported that a 5-HT2C receptor agonist attenuates ketamine-induced hypothermia (Murphy and Murnane, 2019), and the rank order of affinity for M100907 has the 5HT2C receptor after the 5-HT2A receptor (Knight et al., 2004), it is logical to suggest that 5-HT2C receptor antagonism may attenuate METH-induced hyperthermia, and M100907 may be acting as a dual 5-HT2A/2C receptor antagonist across the dose range we examined. The relative contribution of 5-HT2A and 5-HT2C receptors to the acute toxicity of METH should be examined in future studies.
BD 1047 has greater than 100-fold selectivity for σ1 receptors over other protein targets with some exceptions. BD 1047 has a 51-fold greater affinity for σ1 receptors compared to σ2 receptors with a Ki of below 1 nM for σ1 receptors (Matsumoto et al., 1995). However, BD 1047 does demonstrate high affinity for σ2 binding sites. In addition, BD 1047 has significant affinity for β-adrenoceptors. Nevertheless, the dose of 10 mg/kg of BD 1047 was comparable to doses that significantly attenuated the effects of METH-induced stereotypy (Kitanaka et al., 2009) and cocaine-induced convulsions (McCracken et al., 1999). Therefore, it is likely that BD 1047, at the dose administered, is producing its protective effects via σ1 receptors.
The literature is replete with studies examining the role of hyperthermia in the neurotoxic effects of stimulants (Bowyer et al., 1994, Matsumoto et al., 2014, Matsumoto et al., 2008, Robson et al., 2013). Such studies have associated METH-induced hyperthermia with damage to monoaminergic neurons in the central nervous system, contributing to the loss of tyrosine hydrolase activity, dopamine neurochemistry, and dopamine transporter expression as well as nerve terminal degeneration in the striatum (Albers and Sonsalla, 1995, Ali et al., 1996). In contrast, less attention has been paid to acute overdose, especially in the case of single large bolus dose administration. We report that M100907, BD 1047, and their combination had no effect on decreasing METH-induced hyperthermia using acute high-dose administration of METH. Although the combination of M100907 and BD 1047 did delay the onset of METH-induced hyperthermia, it did not decrease the magnitude of METH-induced hyperthermia, following area under the curve analysis. Dopamine and sertonin systems do seem to be invovled in hyperthermia following acute large doses of METH, as mice devoid of both the dopamine and the serotonin transporters showed a paradoxical hypothermic response following a single administration of METH at 45 mg/kg (Numachi et al., 2007). However, our data do not support the involvement of 5-HT2A or σ1 receptors in this response. An important caveat is that rats have been reported to only consistently exhibit lethality with repeated neurotoxic dosing regimens when their body temperature exceeds 41.3 degrees (Bowyer et al., 1994). The mice in our study did not exhibit such high increases in body temperature, perhaps leading to the discrepancies between the two outcomes. It is unclear why such large temperature differences did not occur, but may be related to the facts that we measured core body temperature whereas previous neurotoxicity previous typically used rectal temperature readings as a proxy for core temperature changes.
M100907 and BD 1047 attenuated METH-induced convulsions but not METH-induced hyperthermia – at least at doses that do not induce hypothermia. The rationale for targeting these compounds was based on the established role of their molecular targets in the effects of METH following repeated dosing regimens. It appears that the hyperthermic response to acute METH has a complex and broad pharmacological basis because mice devoid of the dopamine transporter only or the serotonin transporter only continue to exhibit a hyperthermic response following acute METH, whereas mice devoid of both the dopamine and the serotonin transporters show a paradoxical hypothermic response (Numachi et al., 2007). Likewise, it is known that factors such as route, dose, pattern of administration, and species can affect the outcome of hyperthermia experiments (Krasnova and Cadet, 2009, Numachi et al., 2007), and that temperature regulation is a complex process that involves feedback systems and a multitude of factors, such as muscle activity, cellular metabolism, and hypothalamic regulation (Murphy and Murnane, 2019). This suggests that many serotonin and dopaine receptors and systems may contribute to the acute hyperthermic response to METH, and even the combination of M100907 and BD 1047 may be insufficient to block all of these pathways that can lead to acute hyperthermia. Another possibility is that 5-HT2A or σ1 receptors are not involved in METH-induced dysregulation of body temperature, an idea supported by some previous studies (Morishima and Shibano, 1995); however, we suggest that this possibility is less likely given their modulation of METH-induced hyperthermia following repeated dosing (Matsumoto et al., 2008, Robson et al., 2013). Furthermore, we recognize that hyperthermia may still contribute to clinical METH overdose given the complex patterns of METH consumption that can lead to overdose in the clinic, and a cocktail of agents that attenuates both seizures and hyperthermia may be a particularly effective means of preventing mortality during a METH-induced overdose.
Our findings are consistent with seizures and convulsions being more strongly associated with METH lethality. This is in line with previous studies reporting that seizures are effectively elicited by administration of high doses of psychostimulants such as METH (Ceballos et al., 2009, Zagnoni and Albano, 2002). However, more research is necessary to definitively establish the relationship between METH-induced seizures and lethality. This is an important area of future study as METH-induced seizures are serious, prolonged, and not attenuated by conventional anticonvulsants (Derlet et al., 1990, Hanson et al., 1999). This study is an exciting proof of concept that M100907 and BD 1047 attenuate this aspect of the acute toxic effects of METH, and their possible additive or synergistic effects would be worth exploring in future studies.
There are several limitations of the current study. For example, the hyperthermia, seizure, and convulsion/lethality studies were conducted in different groups of animals. Given the constraints of need to surgically implant the telemetry probes, the animals in the hyperthermia experiments were slightly older, individually housed, and weighed more than the animals in the other experiments. Moreover, they were tested in a repeated measures fashion (with a 7 day wash-out period between treatments) unlike in the seizure and convulsion/lethality studies, which were conducted in younger, group housed, and drug-naïve mice, making comparisons between experiments more challenging. In addition, it is known that high METH doses can induce hypertension and stroke, and subsequent mortality, in the absence of hyperthermia. We have not elucidated the mechanisms through which the combination of M100907 and BD 1047 completely prevented lethality, and thus the examination of their modulation of other adverse effects, such as the cardiovascular adverse effects of METH, is warranted. In this regard, however, it is worth noting that σ1 receptor agonism rather than antagonism has been reported to reduce hypertension (Bhuiyan and Fukunaga, 2010, Cohen et al., 2018). In contrast, it is known that 5-HT2A receptor antagonism does prevent serotonin-induced aortic contraction and these receptors are expressed in vascular smooth muscle (Adams et al., 2008), providing a mechanism by which it may also contribute to the prevention METH-induced hypertension. In the present study, we also exclusively used pretreatments to establish the efficacy of these compounds and the role of these receptor systems in METH overdose. Future studies should examine post-treatments to determine whether these agents reverse a METH overdose once it has begun, to more closely model the clinical conditions under which such agents would be used. In addition, it would have been very informative to know whether M100907 and BD 1047 affected the pharmacokinetics and metabolism of one another. Our preferred interpretation of the reported data is that there are possible pharmacodynamic interactions between these agents, however assessing synergism and additivity requires quantitative analysis may not be possible with proportional data where an antagonist is reducing an effect. Therefore, future research is necessary to delineate potential pharmacodynamic and pharmacokinetic interactions. Likewise, it would be informative for future studies to examine whether M100907 and BD 1047 affect METH-induced hypertension or METH-induced neurotoxicity (e.g., blood brain barrier damage, dopamine terminal degeneration, and neuroinflammation). Despite these limitations, this research supports the continued investigation of the role of 5-HT2A and σ1 receptors in METH-induced overdose, including their potential to yield emergency reversal agents.
METH-induced overdose, although currently overshadowed by the present opioid epidemic, is a significant public health concern. Unlike opioids, METH-induced overdose has no approved antidote. It is therefore of public health relevance that the current study provides evidence of a role for 5-HT2A and σ1 receptors in METH-induced overdose and support their continued investigation as potential targets for pharmacotherapeutic development. The present study is an import proof of concept foray into the examination of the efficacy of M100907 and BD 1047 to reduce the acute toxic effects of METH. It is our hope that this study spurs additional research in this area.
Highlights.
The serotonin 2A receptor antagonist M100907 reduced methamphetamine-induced convulsions, seizures, and lethality
The sigma 1 receptor antagonist BD1047 reduced methamphetamine-induced convulsions, seizures, and lethality
Neither agent given alone or in combination had any effect on methamphetamine-induced hyperthermia
Combined administration of both agents seemed to be more effective than either agent alone in reducing methamphetamine-induced seizures and lethality
These data suggest that these agents may have additive or synergistic effects in the reduction of methamphetamine overdose
Acknowledgements
These studies were supported by the National Institutes of Health [DA040907] under extramural funding to KSM and CEC. The work of the Drug Design and Synthesis Section, National Institute on Drug Abuse (NIDA), and National Institute of Alcohol Abuse and Alcoholism (NIAAA) was supported by the NIH Intramural Research Programs of the National Institute on Drug Abuse (NIDA) and the National Institute of Alcohol Abuse and Alcoholism (NIAAA).
Footnotes
Conflicts of Interest
The authors have no conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Azizi Ray, Department of Pharmaceutical Sciences, Mercer University College of Pharmacy, Mercer University Health Sciences Center, Atlanta, GA USA.
Clinton E. Canal, Department of Pharmaceutical Sciences, Mercer University College of Pharmacy, Mercer University Health Sciences Center, Atlanta, GA USA
J. Christopher Ehlen, Department of Neurobiology, Morehouse School of Medicine, Atlanta, GA USA.
Kenner C. Rice, Drug Design and Synthesis Section, National Institute on Drug Abuse and National Institute on Alcohol Abuse and Alcoholism, Rockville, MD USA
Kevin Sean Murnane, Department of Pharmaceutical Sciences, Mercer University College of Pharmacy, Mercer University Health Sciences Center, Atlanta, GA USA.
References
- Adams JW, Ramirez J, Ortuno D, Shi Y, Thomsen W, Richman JG, et al. Anti-thrombotic and vascular effects of AR246686, a novel 5-HT2A receptor antagonist. Eur J Pharmacol. 2008;586:234–43. [DOI] [PubMed] [Google Scholar]
- Adori C, Ando RD, Balazsa T, Soti C, Vas S, Palkovits M, et al. Low ambient temperature reveals distinct mechanisms for MDMA-induced serotonergic toxicity and astroglial Hsp27 heat shock response in rat brain. Neurochemistry international. 2011;59:695–705. [DOI] [PubMed] [Google Scholar]
- Albers DS, Sonsalla PK. Methamphetamine-induced hyperthermia and dopaminergic neurotoxicity in mice: pharmacological profile of protective and nonprotective agents. J Pharmacol Exp Ther. 1995;275:1104–14. [PubMed] [Google Scholar]
- Ali SF, Newport GD, Holson RR, Slikker W Jr., Bowyer JF. Low environmental temperatures or pharmacologic agents that produce hypothermia decrease methamphetamine neurotoxicity in mice. Brain Res. 1994;658:33–8. [DOI] [PubMed] [Google Scholar]
- Ali SF, Newport GD, Slikker W Jr. Methamphetamine-induced dopaminergic toxicity in mice. Role of environmental temperature and pharmacological agents. Ann N Y Acad Sci. 1996;801:187–98. [DOI] [PubMed] [Google Scholar]
- Alusik S, Kalatova D, Paluch Z. Serotonin syndrome. Neuro endocrinology letters. 2014;35:265–73. [PubMed] [Google Scholar]
- Banks ML, Sprague JE, Kisor DF, Czoty PW, Nichols DE, Nader MA. Ambient temperature effects on 3,4-methylenedioxymethamphetamine-induced thermodysregulation and pharmacokinetics in male monkeys. Drug Metab Dispos. 2007;35:1840–5. [DOI] [PubMed] [Google Scholar]
- Bao Q, Zhao M, Chen L, Wang Y, Wu S, Wu W, et al. MicroRNA-297 promotes cardiomyocyte hypertrophy via targeting sigma-1 receptor. Life sciences. 2017;175:1–10. [DOI] [PubMed] [Google Scholar]
- Beakley BD, Kaye AM, Kaye AD. Tramadol, Pharmacology, Side Effects, and Serotonin Syndrome: A Review. Pain physician. 2015;18:395–400. [PubMed] [Google Scholar]
- Bhuiyan S, Fukunaga K. Stimulation of Sigma-1 receptor by dehydroepiandrosterone ameliorates hypertension-induced kidney hypertrophy in ovariectomized rats. Experimental biology and medicine (Maywood, NJ). 2010;235:356–64. [DOI] [PubMed] [Google Scholar]
- Bowyer JF, Davies DL, Schmued L, Broening HW, Newport GD, Slikker W, Jr., et al. Further studies of the role of hyperthermia in methamphetamine neurotoxicity. J Pharmacol Exp Ther. 1994;268:1571–80. [PubMed] [Google Scholar]
- Canal CE, Booth RG, Morgan D. Support for 5-HT2C receptor functional selectivity in vivo utilizing structurally diverse, selective 5-HT2C receptor ligands and the 2,5-dimethoxy-4iodoamphetamine elicited head-twitch response model. Neuropharmacology. 2013;70:112–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Z, Xiao Q, Dai X, Zhou Z, Jiang R, Cheng Y, et al. circHIPK2-mediated sigma-1R promotes endoplasmic reticulum stress in human pulmonary fibroblasts exposed to silica. Cell death & disease. 2017;8:3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceballos NA, Bauer LO, Houston RJ. Recent EEG and ERP findings in substance abusers. Clinical EEG and neuroscience. 2009;40:122–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JB, Perlis ML, Townsend RR. Systolic blood pressure as a potential target of sigma-1 receptor agonist therapy. Journal of clinical hypertension (Greenwich, Conn). 2018;20:416–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtney KE, Ray LA. Methamphetamine: an update on epidemiology, pharmacology, clinical phenomenology, and treatment literature. Drug Alcohol Depend. 2014;143:11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derlet RW, Albertson TE, Rice P. Antagonism of cocaine, amphetamine, and methamphetamine toxicity. Pharmacol Biochem Behav. 1990;36:745–9. [DOI] [PubMed] [Google Scholar]
- Dunnett CW. A Multiple Comparison Procedure for Comparing Several Treatments with a Control. Journal of the American Statistical Association. 1955;50:1096–121. [Google Scholar]
- Fujikawa DG, Pais ES, Aviles ER Jr., Hsieh KC, Bashir MT. Methamphetamine-induced neuronal necrosis: the role of electrographic seizure discharges. Neurotoxicology. 2016;52:84–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall H, Farde L, Halldin C, Lundkvist C, Sedvall G. Autoradiographic localization of 5-HT(2A) receptors in the human brain using [(3)H]M100907 and [(11)C]M100907. Synapse (New York, NY. 2000;38:421–31. [DOI] [PubMed] [Google Scholar]
- Hanson GR, Jensen M, Johnson M, White HS. Distinct features of seizures induced by cocaine and amphetamine analogs. Eur J Pharmacol. 1999;377:167–73. [DOI] [PubMed] [Google Scholar]
- Kaushal N, Elliott M, Robson MJ, Iyer AK, Rojanasakul Y, Coop A, et al. AC927, a sigma receptor ligand, blocks methamphetamine-induced release of dopamine and generation of reactive oxygen species in NG108–15 cells. Molecular pharmacology. 2012;81:299–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaushal N, Matsumoto RR. Role of sigma receptors in methamphetamine-induced neurotoxicity. Current neuropharmacology. 2011;9:54–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitanaka J, Kitanaka N, Tatsuta T, Hall FS, Uhl GR, Tanaka K, et al. Sigma1 receptor antagonists determine the behavioral pattern of the methamphetamine-induced stereotypy in mice. Psychopharmacology. 2009;203:781–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight AR, Misra A, Quirk K, Benwell K, Revell D, Kennett G, et al. Pharmacological characterisation of the agonist radioligand binding site of 5-HT(2A), 5-HT(2B) and 5-HT(2C) receptors. Naunyn-Schmiedeberg's archives of pharmacology. 2004;370:114–23. [DOI] [PubMed] [Google Scholar]
- Krasnova IN, Cadet JL. Methamphetamine toxicity and messengers of death. Brain research reviews. 2009;60:379–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malberg JE, Seiden LS. Small changes in ambient temperature cause large changes in 3,4methylenedioxymethamphetamine (MDMA)-induced serotonin neurotoxicity and core body temperature in the rat. J Neurosci. 1998;18:5086–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto RR, Bowen WD, Tom MA, Vo VN, Truong DD, De Costa BR. Characterization of two novel sigma receptor ligands: antidystonic effects in rats suggest sigma receptor antagonism. European journal of pharmacology. 1995;280:301–10. [DOI] [PubMed] [Google Scholar]
- Matsumoto RR, Seminerio MJ, Turner RC, Robson MJ, Nguyen L, Miller DB, et al. Methamphetamine-induced toxicity: an updated review on issues related to hyperthermia. Pharmacol Ther. 2014;144:28–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto RR, Shaikh J, Wilson LL, Vedam S, Coop A. Attenuation of methamphetamine-induced effects through the antagonism of sigma (sigma) receptors: Evidence from in vivo and in vitro studies. Eur Neuropsychopharmacol. 2008;18:871–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCracken KA, Bowen WD, de Costa BR, Matsumoto RR. Two novel sigma receptor ligands, BD1047 and LR172, attenuate cocaine-induced toxicity and locomotor activity. Eur J Pharmacol. 1999;370:225–32. [DOI] [PubMed] [Google Scholar]
- Morishima Y, Shibano T. Evidence that 5-HT2A receptors are not involved in 5-HT-mediated thermoregulation in mice. Pharmacology, biochemistry, and behavior. 1995;52:755–8. [DOI] [PubMed] [Google Scholar]
- Murnane KS, Andersen ML, Rice KC, Howell LL. Selective serotonin 2A receptor antagonism attenuates the effects of amphetamine on arousal and dopamine overflow in non-human primates. J Sleep Res. 2013a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murnane KS, Guner OF, Bowen JP, Rambacher KM, Moniri NH, Murphy TJ, et al. The adrenergic receptor antagonist carvedilol interacts with serotonin 2A receptors both in vitro and in vivo. Pharmacol Biochem Behav. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murnane KS, Kimmel HL, Rice KC, Howell LL. The neuropharmacology of prolactin secretion elicited by 3,4-methylenedioxymethamphetamine ("ecstasy"): a concurrent microdialysis and plasma analysis study. Horm Behav. 2012a;61:181–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murnane KS, Perrine SA, Finton BJ, Galloway MP, Howell LL, Fantegrossi WE. Effects of exposure to amphetamine derivatives on passive avoidance performance and the central levels of monoamines and their metabolites in mice: correlations between behavior and neurochemistry. Psychopharmacology (Berl). 2012b;220:495–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murnane KS, Winschel J, Schmidt KT, Stewart LM, Rose SJ, Cheng K, et al. Serotonin 2A Receptors Differentially Contribute to Abuse-Related Effects of Cocaine and Cocaine-Induced Nigrostriatal and Mesolimbic Dopamine Overflow in Nonhuman Primates. J Neurosci. 2013b;33:13367–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy TJ, Murnane KS. The serotonin 2C receptor agonist WAY-163909 attenuates ketamineinduced hypothermia in mice. Eur J Pharmacol. 2019;842:255–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen EC, McCracken KA, Liu Y, Pouw B, Matsumoto RR. Involvement of sigma (sigma) receptors in the acute actions of methamphetamine: receptor binding and behavioral studies. Neuropharmacology. 2005;49:638–45. [DOI] [PubMed] [Google Scholar]
- Nisijima K, Yoshino T, Yui K, Katoh S. Potent serotonin (5-HT)(2A) receptor antagonists completely prevent the development of hyperthermia in an animal model of the 5-HT syndrome. Brain Res. 2001;890:23–31. [DOI] [PubMed] [Google Scholar]
- Numachi Y, Ohara A, Yamashita M, Fukushima S, Kobayashi H, Hata H, et al. Methamphetamine-induced hyperthermia and lethal toxicity: role of the dopamine and serotonin transporters. Eur J Pharmacol. 2007;572:120–8. [DOI] [PubMed] [Google Scholar]
- O'Dell LE, Kreifeldt MJ, George FR, Ritz MC. The role of serotonin(2) receptors in mediating cocaine-induced convulsions. Pharmacol Biochem Behav. 2000;65:677–81. [DOI] [PubMed] [Google Scholar]
- Ootsuka Y, Blessing WW. Thermogenesis in brown adipose tissue: increase by 5-HT2A receptor activation and decrease by 5-HT1A receptor activation in conscious rats. Neurosci Lett. 2006;395:170–4. [DOI] [PubMed] [Google Scholar]
- Oppong-Damoah A, Zaman RU, D'Souza MJ, Murnane KS. Nanoparticle encapsulation increases the brain penetrance and duration of action of intranasal oxytocin. Horm Behav. 2019;108:20–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawlyk AC, Cosmi S, Alfinito PD, Maswood N, Deecher DC. Effects of the 5-HT2A antagonist mirtazapine in rat models of thermoregulation. Brain Res. 2006;1123:135–44. [DOI] [PubMed] [Google Scholar]
- Ray A, Chitre NM, Daphney CM, Blough BE, Canal CE, Murnane KS. Effects of the second-generation "bath salt" cathinone alpha-pyrrolidinopropiophenone (alpha-PPP) on behavior and monoamine neurochemistry in male mice. Psychopharmacology (Berl). 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robson MJ, Seminerio MJ, McCurdy CR, Coop A, Matsumoto RR. sigma Receptor antagonist attenuation of methamphetamine-induced neurotoxicity is correlated to body temperature modulation. Pharmacol Rep. 2013;65:343–9. [DOI] [PubMed] [Google Scholar]
- Robson MJ, Turner RC, Naser ZJ, McCurdy CR, O'Callaghan JP, Huber JD, et al. SN79, a sigma receptor antagonist, attenuates methamphetamine-induced astrogliosis through a blockade of OSMR/gp130 signaling and STAT3 phosphorylation. Experimental neurology. 2014;254:180–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schechter MD, Meehan SM. Serotonergic mediation of cocaine seizures in mice. Pharmacol Biochem Behav. 1995;51:313–6. [DOI] [PubMed] [Google Scholar]
- Seth P, Scholl L, Rudd RA, Bacon S. Overdose Deaths Involving Opioids, Cocaine, and Psychostimulants - United States, 2015–2016. MMWR Morbidity and mortality weekly report. 2018;67:349–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sipe K, Leventhal L, Burroughs K, Cosmi S, Johnston GH, Deecher DC. Serotonin 2A receptors modulate tail-skin temperature in two rodent models of estrogen deficiency-related thermoregulatory dysfunction. Brain Res. 2004;1028:191–202. [DOI] [PubMed] [Google Scholar]
- Ullrich T, Rice KC. A practical synthesis of the serotonin 5-HT2A receptor antagonist MDL 100907, its enantiomer and their 3-phenolic derivatives as precursors for [11C]labeled PET ligands. Bioorganic & medicinal chemistry. 2000;8:2427–32. [DOI] [PubMed] [Google Scholar]
- Weissman AD, Su TP, Hedreen JC, London ED. Sigma receptors in post-mortem human brains. J Pharmacol Exp Ther. 1988;247:29–33. [PubMed] [Google Scholar]
- Yasui Y, Su TP. Potential Molecular Mechanisms on the Role of the Sigma-1 Receptor in the Action of Cocaine and Methamphetamine . Journal of drug and alcohol research. 2016;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagnoni PG, Albano C. Psychostimulants and epilepsy. Epilepsia. 2002;43 Suppl 2:28–31. [DOI] [PubMed] [Google Scholar]