Abstract
Atypical Protein Kinase C (aPKC) isozymes, PKCλ/ι and PKCζ, are now considered fundamental regulators of tumorigenesis. However, the specific separation of functions that determine their different roles in cancer is still being unraveled. Both aPKCs have pleiotropic context-dependent functions that can translate into tumor promoter or suppressive functions. Here, we review early and more recent literature to discuss how the different tumor types, and their microenvironments, might account for the selective signaling of each aPKC isotype. This is of clinical relevance because a better understanding of the roles of these kinases is essential for the design of new anti-cancer treatments.
Introduction
The extended protein kinase C (PKC) family comprises three subfamilies: the atypical (aPKCs; PKCζ and PKCλ/ι), the conventional (cPKCs; PKCα, PKCβ, and PKCγ), and the novel (nPKCs; PKCδ, PKCε, PKCη, and PKCθ) (Griner and Kazanietz, 2007; Newton and Brognard, 2017). The aPKCs differ from the other members of the extended PKC family in that they lack functional C2 and C1 domains, which accounts for their insensitivity to Ca2+ and diacylglycerol (DAG) (Ono et al., 1988; Ono et al., 1989). In contrast, the aPKCs harbor a Phox and Bem 1 (PB1) domain, which is a highly conserved protein interaction module that governs their binding to other PB1-containing proteins such as the polarity regulator PAR6 (Moscat et al., 2006; Moscat et al., 2009), or the autophagy receptor and signaling adaptor p62 (Moscat and DiazMeco, 2009; Moscat et al., 2016; Reina-Campos et al., 2018) (Figure 1; Box 1). From the very beginning, the aPKCs have been implicated in the control of critical cellular functions important in cancer (Diaz-Meco and Moscat, 2012; Moscat and Diaz-Meco, 2000). However, establishing the specific roles for each aPKC in tumor transformation has been challenging. This is mostly due to the high degree of sequence homology between PKCζ and PKCλ/ι, the lack of isoform-specific tools to modulate and measure their isotype-specific expression and activity, and the discordance that sometimes exists between the amounts of mRNA and protein (Kim et al., 2013). In fact, many studies have relied on the utilization of non-selective small molecule inhibitors, peptides based on the pseudosubstrate sequence, which is common to both aPKC isoforms, and antibodies that were not isotype-specific for each aPKC. This has resulted in attributing both tumor suppressor and tumor promoter functions to both aPKCs, which has hampered the adequate use of potential aPKC inhibitors in the clinic. Here, we focus on more conclusive experiments that stem from observations obtained from the analysis of human data, and the use of physiologically relevant genetically modified mouse models (GEMM), which selectively ablate each specific aPKC isotype in a cell type-dependent manner. In addition, we discuss here, when necessary, the potential pitfalls of earlier studies to help rationalize and clarify existing conflicting results. Therefore, this perspective aims to provide a comprehensive updated picture of the most definitive contemporary evidence on the roles and mechanisms of action of the aPKCs in cancer and discuss potential future therapeutic strategies.
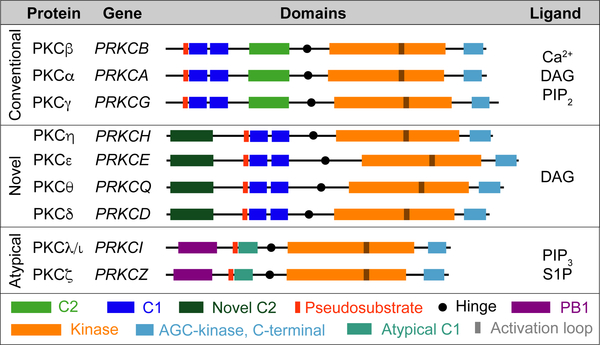
Figure 1. The PKC Family.
Classification of PKC isozymes by subfamily; classical, novel, and atypical with gene names, protein domains and their ligands.
BOX 1. Role of the aPKCs in normal physiology.
Recent reviews have described in detail the role of the atypical PKCs in non-disease settings (Hong, 2018; Moscat et al., 2009). Here we intend to highlight the most relevant observations obtained in organisms genetically modified in an isotype-specific manner. The two aPKC isoforms display a 72% identity at the amino acid level, with the most similar region of homology encompassing their catalytic domain at the carboxy terminus (Figure 1). PKCλ/ι is more widely expressed and at higher levels than PKCζ, which has a much more restricted pattern of expression, with lower levels than PKCλ/ι in almost every tissue except brain, lung, and testis, where PKCζ is highly expressed (Figure 3A). The fact that mice deficient in PKCζ were grossly normal whereas PKCλ/ι deficiency in mice resulted in early embryonic lethality was an indication of the distinct and unique functions of these kinases in vivo (Leitges et al., 2001; Soloff et al., 2004). Importantly, both aPKCs have been shown to play critical roles in several biological processes under normal physiological conditions, including in cell polarity and signaling; these were mediated mostly, although not exclusively, through their interactions with the PB1-containing scaffolds PAR6 and p62, respectively (Moscat et al., 2006).
aPKCs in polarity
The role of the aPKCs as cell polarity kinases has been extensively studied (see comprehensive reviews on this matter in (Chen and Zhang, 2013; Hong, 2018; Vorhagen and Niessen, 2014). Thus, these kinases have been shown to be central to the control of the apical/lateral polarity complex that is formed by their interaction with the PB-1 adapter PAR6 and the aPKC substrate PAR3 (Figure 4). This evolutionarily conserved PAR3-PAR6-aPKC trimeric complex is important not only for the establishment of apical-basal polarity but also during the maturation of epithelial junctions, the asymmetric cell division and for cell fate determination (Chen and Zhang, 2013; Vorhagen and Niessen, 2014). These functions are well established mostly in Drosophila and Caenorhabditis elegans, in which there is only one single aPKC gene (Hong, 2018). However, their role in the control of polarity functions in mammals is much less understood, due in large part to the existence of functional redundancies between PKCζ and PKCλ/ι in mammalian cells. Furthermore, in mammalian organisms, there seems to be a tissue-specific requirement for the aPKCs in the regulation of oriented cell division, coupled with cell fate decisions and differentiation (Vorhagen and Niessen, 2014). For example, in the hematopoietic system, both aPKCs have been implicated in the establishment of T cell polarity in the immunological synapse and in the regulation of pathogen-specific CD8+ T cell activity (Chang et al., 2007; Metz et al., 2016; Yang et al., 2009). However, these kinases have been shown to be dispensable for the polarization and function in adult hematopoietic stem cells (Sengupta et al., 2011). In contrast, PKCλ/ι regulates division orientation in the mammalian epidermis where it is critical for the maintenance of stem cells and epidermal homeostasis (Niessen et al., 2013). All these pieces of evidence highlight the cell-context dependent role of the aPKCs in polarity in mammals.
aPKCs in cell signaling
The genetic inactivation of PKCζ in mice supports its key role in the control of cell survival through the activation of NF-κB both by the regulation of IKK activity and by the direct phosphorylation of RELA at Ser311 (Duran et al., 2003; Leitges et al., 2001; Martin et al., 2002). Furthermore, PKCζ also regulates NF-κB-independent pathways such as the JAK1/STAT6 cascade (Martin and Hussaini, 2005). On the other hand, PKCλ/ι has been shown to be required for the activation of Th2 transcription factors, such as NF-κB, NFATc1 and GATA3 in stimulated T cells (Yang et al., 2009), and for glucose and insulin metabolism in different metabolic tissues (Habegger et al., 2012; Sajan et al., 2014).
The Tumor Promoting Actions of PKCλ/ι
The initial evidence on the role of PKCλ/ι in tumorigenesis came from studies on its involvement in mediating the carcinogenic effects of ultraviolet irradiation and RAS transformation (Bjorkoy et al., 1997; Huang et al., 1996). Later, the tumor-promoting effects of PKCλ/ι was reported in a leukemia cell line expressing BCR-ABL (Murray and Fields, 1997). The BCR-ABL oncogene is the product of the chromosomal translocation t(9;22)(q34;q11) and is required and sufficient to drive several forms of leukemia and to promote resistance to chemotherapeutic treatments such as Paclitaxel (Bedi et al., 1995; Ren, 2005). A series of successive studies demonstrated that the upregulation of PKCλ/ι by BCR-ABL prevented drug-induced apoptosis through the NF-κB pathway in vitro (Gustafson et al., 2004; Lu et al., 2001; Murray and Fields, 1997). However, although Paclitaxel treatment promoted the upregulation of PKCλ/ι (Jamieson et al., 1999), the sole ectopic expression of this kinase was not sufficient to induce proliferation or to affect differentiation of leukemia cells (Murray and Fields, 1997). Those studies were performed in cell lines in in vitro cultures, but more recently the role of PKCλ/ι in BCR-ABL-driven leukemia has been tested in a physiologically relevant GEMM (Nayak et al., 2019). In this in vivo system, the inducible knockout (KO) of PKCλ/ι in the hematopoietic compartment completely abolished the transformation and growth of cells expressing BCR-ABL (Nayak et al., 2019). PKCλ/ι deficiency in this system was shown to impair MEK/ERK signaling concomitantly to a downregulation of the RAC GTPase activity, but independently of RAF1 or BRAF (Nayak et al., 2019). It was proposed that the reduction of MEK/ERK signaling through ETV5 abolished the repressive function of the chromatin regulator SATB2, promoting the upregulation of a B cell differentiation gene expression program that restrained B cell transformation, proliferation and survival, and halted disease progression in vivo (Nayak et al., 2019) (Figure 2). Interestingly, PKCλ/ι and PKCζ had previously been shown to be functionally dispensable for mammalian hematopoietic stem cell activity and blood formation (Sengupta et al., 2011). This is a critical observation because it suggests that inhibiting PKCλ/ι in leukemia could be a relatively non-toxic and efficient therapeutic strategy. Thus, similar to the in vitro studies with leukemia cell lines, the tumor-promoting role of PKCλ/ι appears to become more apparent when its upregulation, as a consequence of oncogenic- and/or drug-induced stress, helps block cell differentiation and promote survival and carcinogenesis (Nayak et al., 2019). This is consistent with additional studies in other tumor cell types including prostate (Win and Acevedo-Duncan, 2008), non-small cell carcinoma (Jin et al., 2005) and glioblastoma (Baldwin et al., 2006). Therefore, it seems that the tumor promoter role of PKCλ/ι is not limited to hematological malignancies. Indeed, the upregulation of PKCλ/ι has been found in most, if not all, primary solid tumors when compared to their matched normal tissues (Murray et al., 2011) (Box 2; Figure 3A). This, together with the observation that the aPKCs are important for RAS signaling in fibroblasts (Coghlan et al., 2000; Hellbert et al., 2000; Kampfer et al., 2001; Uberall et al., 1999), sparked a series of studies aimed at testing the oncogenic properties of PKCλ/ι and its link with KRAS-induced oncogenesis. In this regard, initial pioneering efforts were focused on testing whether the upregulation of PKCλ/ι in the colonic epithelium could be sufficient to induce transformation (Murray et al., 2004). However, the simple expression of a constitutively active mutant of PKCλ/ι (caPKCλ/ι), failed to induce tumorigenesis by itself although it enhanced AOM-induced carcinogenesis (Murray et al., 2004). Furthermore, inhibition of PKCλ/ι with a dominant-negative kinase-dead mutant (kdPKCλ/ι) diminished the appearance of aberrant crypt foci driven by mutant Kras expression (Murray et al., 2004). However, this effect should be considered with caution because the overexpression of a dominant-negative mutant form of PKCλ/ι has limitations since it might also impact signaling via other PKCs or disrupt interactions with adapters and substrates. In any case, these results agree with the fact that RAC1, the downstream effector of PKCλ/ι, has very weak transforming potential (Khosravi-Far et al., 1995; Lin et al., 2000).
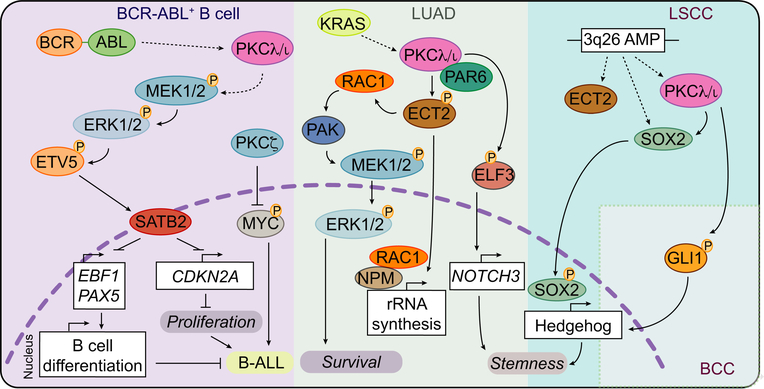
Figure 2. The Tumor Promoting Actions of PKCλ/ι.
PKCλ/ι is activated downstream of the BCR-ABL oncogene and triggers a MEK-ERK-ETV5 signaling cascade that promotes SATB2-dependent inhibition of proliferation and B cell differentiation that contributes to the development of B-ALL. Additionally, PKCλ/ι is activated downstream of KRAS in LUAD and directs three signal transduction programs; (1) ECT2-RAC1-PAK-MEK1/2-ERK1/2 to promote cell survival; (2) rRNA biosynthesis through ECT2; (3) NOTCH3 transcriptional activation that contributes to stemness. Also, PRKCI is commonly amplified (3q26 AMP) in LSCC together with SOX2 and ECT2 to drive a Hedgehog-dependent transcriptional program that promotes stemness. Finally, PKCλ/ι maintains Hedgehog activation through GLI1 phosphorylation in BCC.
BOX 2. Transcriptomics and genetics of the aPKCs in cancer.
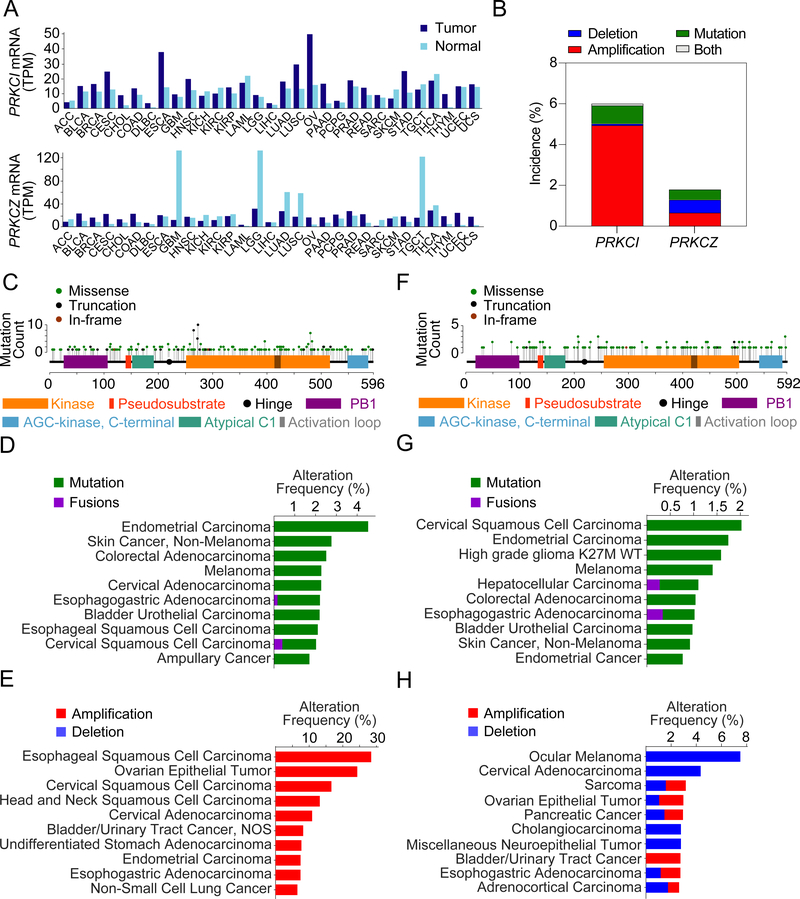
The aPKCs are highly dysregulated at the mRNA level in cancer. More than half of the tumor types compiled by the TCGA show upregulation of PRKCI expression, except for a two-fold downregulation in skin cutaneous melanoma (SKCM) (Figure 3A). PRKCZ expression is similarly upregulated in approximately half of the cancer types but is strikingly downregulated in brain (GBM, LGG), lung (LUAD, LUSC) and testicular germ cell tumors (TGCT) (Figure 3A). The PRKCI gene is altered in ~6% of samples, with common gene amplifications (~5%), and very rare deletions (<0.7%) (Figure 3B). The mutation incidence of PRKCI, while low on average (~1%), can be 4 times as high in specific tumor types, such as endometrial carcinoma (~4%), non-melanoma skin cancer (2.7%) and CRC (~2.5%) (Figures 3B–3D). PRKCI harbors recurrent hot spot mutations in the upstream region of the catalytic domain, including a truncating mutation that is expected to render the enzyme inactive (Figure 3C). Additionally, it also harbors recurrent mutations in R480C, previously mistakenly identified as R471C (Linch et al., 2013) (Figure 3C). PRKCI is frequently found amplified as part of the 3q26 amplicon in squamous cell lung cancers of the esophagus, cervix, head and neck, and lung, as well as in other epithelial tumors of the ovaries, endometrium, bladder and stomach, with some studies reporting up to >70% amplifications in high grade serous ovarian cancer (HGSOC). Amplifications of PRKCI have been well described in NSCLC, which occurs in 43% to 96% of LSCC (depending on patient cohort) compared to a very low incidence in the LUAD subtype (1.4%) (Figure 3E) (Balsara et al., 1997; Brass et al., 1996; Hagerstrand et al., 2013; Justilien et al., 2014; Lin et al., 2006; Racz et al., 1999; Regala et al., 2005b; Singh et al., 2002; Wang et al., 2013; Yang et al., 2008). PRKCI is not amplified in NEPC (Reina-Campos et al., 2019). PRKCZ gene is altered in ~2% of tumor samples, with similar incidences of amplifications (~0.7%) and deletions (~0.7%) (Figure 3B). PRKCZ mutations are infrequent, and none of them are hotspots (~0.5%) (Figure 3F). However, mutations of PRKCZ might be relevant in specific tumor types where its incidence is significantly increased, such as cervical squamous and endometrial carcinomas (Figure 3G). Additionally, a significant inactivating mutation in PRKCZ, S510F/C, has been found in CRC, cervical squamous carcinoma and diffuse large B cell lymphoma (cBioportal) (Wood et al., 2007) (Figure 3F). Also, PRKCZ mutations are among the top somatically altered genes influencing expression in triple-negative breast cancer (Shah et al., 2012). Deletions of PRKCZ occur in more than 7% of ocular melanomas and in ~4% of cervical adenocarcinomas, and amplifications are rare but most common in bladder/urinary tract cancers (~3%) (Figure 3H).
Figure 3. Transcriptomics and Genetics of the aPKCs in Cancer.
(A) PRKCI and PRKCZ median expression across all tumor samples and paired normal tissues for 31 tumor types. Plots generated with GEPIA 2 (Tang et al., 2017). Tumor type nomenclature follows TCGA study abbreviations guidelines (https://gdc.cancer.gov).
(B) PRKCI and PRKCZ alterations in cancer using a curated set of non-redundant samples containing 159 studies with 35,693 patients and 36,917 samples for mutation data, and 158* studies with 27,410 patients and 28,457 samples for copy number variation data (cBioportal) (Cerami et al., 2012; Gao et al., 2013). *NEPC 2016 was omitted from CNV studies because it is not corrected for ploidy.
(C) Somatic mutation profile of PRKCI (cBioportal).
(D) Top 10 cancer types sorted by mutation frequency of PRKCI (cBioportal).
(E) Top 10 cancer types sorted by CNV frequency of PRKCI (cBioportal).
(F) Somatic mutation profile of PRKCZ (cBioportal).
(G) Top 10 cancer types sorted by mutation frequency of PRKCZ (cBioportal).
(H) Top 10 cancer types sorted by CNV frequency of PRKCZ (cBioportal).
Tumor Promoter Role of PKCλ/ι in KRAS-driven Cancer
In the lung, the tumor-promoting role of PKCλ/ι seems to be more relevant in non-small cell lung cancer (NSCLC), where its expression is upregulated about two-fold on average (Regala et al., 2009; Regala et al., 2005a; Regala et al., 2005b) (Box 2; Figure 3A). KRAS mutations are present in approximately 30% of NSCLC (Herbst et al., 2008), and the interrogation of a panel of KRAS mutant NSCLC cell lines confirmed the requirement of PKCλ/ι for KRAS transforming activity (Regala et al., 2005a). Interestingly, PKCλ/ι function was only required for KRAS-driven cell growth when cells were cultured in 3D (Regala et al., 2005a). It was proposed that, in this model, PKCλ/ι regulates transformation through a RAC1-PAK1-MEK-ERK signaling axis (Eblen et al., 2002; Regala et al., 2005a) (Figure 2).
An additional and widely used lung cancer model, termed LSL-KrasG12D, generates spontaneous lung tumors after the intranasal delivery of AdenoCre, which results in the expression of the KRASG12D oncogene in several lung cell types at endogenous levels (Jackson et al., 2001). Lung adenomas in the LSL-KrasG12D model showed higher protein expression levels of PKCλ/ι, recapitulating what is observed in human samples (Regala et al., 2009) (Box 2; Figure 3A). Notably, the simultaneous genetic inactivation of PKCλ/ι along with the expression of KRASG12D in LSL-KrasG12D;Prkcifl/fl mice resulted in the complete blockade of lung atypical adenomatous hyperplasia and adenoma initiation, as compared to LSL-KrasG12D mice expressing WT levels of PKCλ/ι. In fact, the few adenomas formed in the LSL-KrasG12D;Prkcifl/fl mice retained one Prkci unrecombined allele, demonstrating that the genetic inactivation of PKCλ/ι is incompatible with the expression of mutant Kras (Regala et al., 2009). From the cell mechanistic point of view, the inhibition of the tumorigenic activity of KRASG12D was proposed in that study to be a consequence of limiting the expansion of the so-called bronchioalveolar stem cells (BASC), which had been suggested by others to be the cell of origin in LSL-KrasG12D-driven adenocarcinoma (Kim et al., 2005). Whether this is through the previously proposed role of PKCλ/ι in controlling the RAC1-PAK-MEK-ERK axis in NSCLC human cell lines or by any other mechanism still needs to be clarified (Regala et al., 2009; Regala et al., 2005a) (Figure 2). In addition, it should also be kept in mind that posterior studies favor the idea that other cell types of the lung, such as club (Clara) and alveolar type II (AT2) cells, have been shown to also be cells of origin in LUAD (Desai et al., 2014; Mainardi et al., 2014; Sutherland et al., 2014; Xu et al., 2012). A more recently published study shows that the in vivo loss of PKCλ/ι in a more aggressive LUAD model (LSL-KrasG12D;Trp53fl/fl;Prkcifl/fl) decreases the emergence of BASCderived LUAD, but surprisingly does not inhibit LUAD originating from AXIN2+ AT2 cells (Yin et al., 2019). PKCλ/ι null AT2-derived LUAD mouse tumors correlate with increased β-catenin, a feature shared by a subset of human LUAD, which also displayed low expression levels of PKCλ/ι (Yin et al., 2019).
In keeping with the notion that PKCλ/ι is important for TIC function, additional studies found that PKCλ/ι was required for the growth of TIC isolated from human LUAD cell lines when grown in 3D cultures and through a mechanism different from the RAC1-controlled pathway but implicating a NOTCH3-dependent signaling cascade (Ali et al., 2016) (Figure 2). In agreement with an important role of PKCλ/ι in these TIC, downregulation of this aPKC not only impaired their ability to grow in vitro but also to form tumors when implanted orthotopically into the lungs of immunodeficient mice (Eramo et al., 2008; Justilien et al., 2014). An interesting proposal is that the imbalance between symmetric and asymmetric cell division favors the depletion of TICs upon PKCλ/ι inactivation in these cells (Ali et al., 2016). However, it would be important to define the precise relationship between the proposed role of PKCλ/ι in NOTCH3 signaling with its role in controlling polarity (Ali et al., 2016; Knoblich, 2008). Furthermore, an additional important question that needs to be clarified is how the regulation of the NOTCH3-dependent signaling by PKCλ/ι relates to its implication in the RAC1-PAK-MEK-ERK mechanism required for the growth of LUAD cell lines in 3D cultures (Regala et al., 2005a). Intriguingly, in contrast to the previously reported increase in mRNA and protein levels of PKCλ/ι associated to KRAS expression (Regala et al., 2005a), the PKCλ/ι phosphorylation status, but not its total protein or mRNA amount, was altered in oncospheres expressing mutant KRAS (Ali et al., 2016). It is possible that the way PKCλ/ι is regulated in TIC varies from how it is regulated in differentiated tumor cells. Overall, these observations established the requirement of PKCλ/ι for KRAS-driven tumorigenesis in lung and opened the possibility that it might also be important in other tumor types in which mutations in KRAS are highly prevalent. Indeed, PKCλ/ι is highly expressed in human pancreatic cancers, a type of neoplasia that is driven by mutant KRAS (Scotti et al., 2010). Similar to studies in LUAD, targeted deletion of PKCλ/ι had no effect on the growth of pancreatic ductal adenocarcinoma (PDAC) cells under adherent cell culture conditions but did impair their anchorage-independent growth, as well as the proliferation of orthotopically implanted human pancreatic tumors in immunocompromised mice (Scotti et al., 2010). The mechanism here seems to be mediated through ERK activation by KRAS (Scotti et al., 2010). While these data are consistent with previous studies showing the requirement of the PKCλ/ι RAC1-PAK-MEK-ERK axis in 3D growth of KRAS-driven NSCLC cell lines (Regala et al., 2005a), it is not clear whether NOTCH3 signaling also identified in KRAS-driven LUAD TICs could be playing a similar role in pancreatic cancer. Also, the contribution of PKCλ/ι in physiologically relevant immunocompetent mouse models of PDAC still needs to be addressed.
Tumor Promoter Role of PKCλ/ι in Lung Squamous Cell Carcinoma
Although these studies strongly suggest that PKCλ/ι is an essential component in the machinery of KRAS-induced lung and pancreatic tumorigenesis, research on the squamous subtype of NSCLC (LSCC), in which KRAS mutation very rarely occurs, suggests that PKCλ/ι might also play critical roles in lung tumors independently of the KRAS pathway. Human LSCC often harbor the 3q26 amplification that includes the PRKCI gene (Regala et al., 2005b) (Box 2; Figures 2 and 3D), which results in higher levels of PKCλ/ι in LSCC cells, and serves to drive tumorigenesis (Balsara et al., 1997; Herbst et al., 2008; Regala et al., 2005b). Similar to the results in LUAD (Ali et al., 2016), PKCλ/ι inactivation in LSCC cell cultures enriched in stem-like cells harboring the 3q26 amplification resulted in reduced stem cell function in vitro and impaired growth of orthotopic tumors in the lungs of immunodeficient mice (Justilien et al., 2014). The interrogation of genes coamplified with PKCλ/ι led to the identification of SOX2 as a direct target of PKCλ/ι phosphorylation. This suggested that SOX2 could be a potential key downstream effector of the tumor-promoting functions of PKCλ/ι in LSCC TIC (Justilien et al., 2014) (Figure 2). Another co-amplified gene in the 3q26 amplicon is ECT2, a guanine nucleotide exchange factor (GEF) that targets Rho family members, such as CDC42 and RAC1. ECT2 had already been implicated in PKCλ/ι signaling (Justilien and Fields, 2009; Lin et al., 2000). Similarly to PKCλ/ι, ECT2 expression was equally upregulated in LUAD and LSCC, but only genetically amplified in LSCC, where it correlated with its gene copy number status (Justilien and Fields, 2009). Like in the case of SOX2, ECT2 activity was shown to be controlled by direct PKCλ/ι phosphorylation (Justilien et al., 2017), and to be important in mediating the downstream activation of MEK-ERK in LSCC cell lines (Justilien and Fields, 2009) (Figure 2). Nuclear ECT2 was shown to be key for ribosomal biogenesis through its interaction with RAC1 and nucleophosmin (NPM) at the promoter of ribosomal DNAs (Justilien et al., 2017) (Figure 2). The contribution of ECT2 to lung tumorigenesis was nicely demonstrated in vivo in a LUAD GEMM (LSL-KrasG12D;Trp53fl/fl +/− Ect2fl/fl) (Justilien et al., 2017). However, the functional impact of ECT2, as well as the contribution of PKCλ/ι-dependent ECT2 activity in vivo in the context of their amplification in LSCC, still needs to be addressed.
Compelling evidence demonstrated that the phosphorylation of SOX2 by PKCλ/ι promoted the expression of the Hedgehog Acyltransferase (HHAT), the limiting enzyme in the production of Sonic Hedgehog (SHH), one of the Hedgehog (Hh) ligands. Consistent with this, LSCC-derived oncospheres were more sensitive to Hh inhibition by SMO inhibitors, such as LDE225, than parental 2D cells. This is in agreement with the concept that PKCλ/ι is not required for adherent cell culture growth (Justilien et al., 2014). Intriguingly, however, exogenous Hh did not rescue the defective growth of SOX2-deficient oncospheres, suggesting that SOX2 controls Hh-independent pathways that are also required for the growth of LSCC TIC-like cells (Justilien et al., 2014). These interesting observations also uncovered a fundamental aspect of the context-dependent role of the tumor-promoting actions of PKCλ/ι. That is, while LSCC oncospheres relied on Hh signaling, LUAD oncospheres were dependent on NOTCH3 and were thus resistant to Hh targeting by a SMO inhibitor. This is consistent with their low expression of SOX2 and the fact that the Hh pathway is not altered upon PKCλ/ι inhibition in LUAD (Ali et al., 2016). Also, in agreement with these observations, NOTCH3 was not induced in LSCC oncospheres (Justilien et al., 2014). These data argue that TICs from LSCC and LUAD both require PKCλ/ι activity but rely on different downstream signaling pathways to maintain their tumor-initiating properties (Ali et al., 2016; Justilien et al., 2014) (Figure 2). Intriguingly, although both LUAD and LSCC express similarly high levels of PKCλ/ι mRNA and protein, the PRKCI gene amplification is predominantly found in LSCC, suggesting that still unidentified mechanisms drive PKCλ/ι expression in non-3q26 amplified NSCLC. Interestingly, basal cell carcinomas (BCC), which are the most frequently occurring form of skin cancer, also have a strong dependency on Hh signaling (Atwood et al., 2013; Mirza et al., 2017; Mirza et al., 2019). PKCλ/ι has been implicated in Hh activation in BCC (Atwood et al., 2013) (Figure 2). However, in contrast to the mechanism proposed in LSCC, PKCλ/ι controls Hh signaling through direct phosphorylation of GLI1, which in turn exerts positive feedback to promote PKCλ/ι expression (Atwood et al., 2013) (Figure 2). The requirement of PKCλ/ι for Hh signaling in BCC also led to test whether PKCλ/ι inhibition could potentially enhance the effect of Vorinostat, an HDAC inhibitor with potential clinical utility for the treatment of BCC but that required administration at very high doses (Mirza et al., 2017).
Summary of PKCλ/ι Pro-Tumorigenic Mechanisms and Pending Questions
PKCλ/ι protein expression is significantly increased and activated in all invasive breast cancer types (Paul et al., 2014). Also, there is some evidence showing that TGFβ and IL-1β activate NF-κB through PKCλ/ι to promote orthotopic tumor growth and invasion of breast cancer cells in immune-deficient mice (Paul et al., 2014; Rosse et al., 2014). However, there is currently no validation in physiologically relevant in vivo models with an intact immune system of the putative role of PKCλ/ι in this type of tumors.
Another important question that should be considered when ascribing a tumor promoter role for PKCλ/ι is that a fundamental attribute of a human oncogene is their ability to induce cell transformation. This can be achieved either alone, in immortalized cells, or in combination with other oncogenes or the inactivation of tumor suppressors in primary cell cultures (Vogelstein et al., 2013). In this regard, although the overexpression of caPKCλ/ι failed to induce demonstrable changes in proliferation or differentiation markers in the colonic epithelium (Murray et al., 2004), a more recent paper shows that the ectopic overexpression of PKCλ/ι in the fallopian tube secretory epithelium (FTSE), although not sufficient to drive tumorigenesis by itself, when combined with the simultaneous inactivation of PTEN and p53, gave rise to fallopian tube or ovarian tumors in about 40% of mutant mice (Sarkar et al., 2017). It remains to be determined how general this phenomenon is and whether it depends on a given overexpression threshold comparable to that found in human cancers. Also, it would be important to determine if there is a clear association in all human tumors between the degree of aggressiveness and the expression levels of PKCλ/ι in the context of the presence of other oncogenic mutations or the inactivation of tumor suppressors.
New Paradigms of Cell-Autonomous and Non-Autonomous Tumor Suppression by the aPKCs
Although compelling evidence in human cell lines and in some GEMM suggest that PKCλ/ι plays a pro-oncogenic role, several recent studies have challenged the universality of this paradigm. These include in vivo studies using physiologically relevant GEMM, the bioinformatics and biochemical characterization of PRKCI mutations, and the interrogation of human tumor samples. Overall, these data offer an improved perspective of the complexity of the role of aPKCs in cancer. An initial observation that might support a possible tumor-suppressive function of PKCλ/ι is the presence of inactivating somatic mutations (Box 2; Figure 3C). While low on average (1%), this incidence rises to 4.5%, 2.8% and 2.5% in endometrial carcinoma, non-melanoma skin cancer and CRC, respectively (Choi et al., 2018; Gao et al., 2013). Also, PRKCI mutations present in cancer cell lines are three times as prevalent (3%) as those found in human tumors on average, including a recognized hotspot mutation (R471C) that has been biochemically characterized and shown to contribute to loss of polarization capacity (Box 2; Figure 3C). This is potentially important because impaired polarity is strongly associated with increased tumor malignancy (Barretina et al., 2012; Chang et al., 2016; Gao et al., 2013; Linch et al., 2017) (Figure 4). Furthermore, a recent pan-cancer analysis of all PKCs suggested that truncations and indel mutations that affect large portions of the PKC catalytic domain would render the enzymes inactive (Antal et al., 2015). This is undoubtedly the case for the more prevalent frameshift insertions T276Qfs*7/Nfs*16, R272*/P in PRKCI, and mutations affecting highly conserved motifs required for its catalytic activity (Meharena et al., 2013; Newton and Brognard, 2017; Spitaler et al., 2000). Based on this evidence, it is reasonable to assume that these PRKCI mutations are present in the tumors because they offer a competitive advantage (Box 2; Figure 3C and 3D). Of particular relevance are the missense mutations found in the catalytic domain of PKCλ/ι in LSCC samples, all of which harbor the 3q26 amplification. The potential loss of function (LOF) of these mutations might create the paradoxical situation that these cancer cells may overexpress a potentially dominant-negative form of PKCλ/ι (Newton and Brognard, 2017). A more detailed analysis of the effects of 3q26 amplification and PRKCI mutations is required to clarify the functional contribution of these genetic alterations to tumorigenesis. Surprisingly, KRAS and PRKCI mutations have a significant tendency to cooccur (n = 35213 patients in 36382 samples in 155 studies, q value = 0.008) (Gao et al., 2013). This would suggest that the loss of PKCλ/ι activity is favored in KRAS mutant tumors, which needs to be reconciled with the proposed model that PKCλ/ι is universally required for KRAS-induced carcinogenesis.
Figure 4. Tumor Suppression by Polarity Control.
PRKCI mutations in the dibasic motif R480C (formerly R471C; red circle) contribute to a loss of substrate specificity that leads to loss of polarity and EMT. Additionally, polarity complexlocalized aPKC limits the pro-EMT actions of SNAI1. Finally, oncogenes such as ERBB2 can disrupt the PKCλ/ι-PAR3 complex and contribute to EMT.
Tumor Suppression by Polarity Control
Several of the mechanisms that maintain epithelial polarity have been reported to exert tumor-suppressive functions (Lee and Vasioukhin, 2008), and are significantly mutated in cancer (Choi et al., 2018; Gao et al., 2013). The aPKCs have been proposed as core components of the machinery that establishes cell polarity thanks to their ability to interact with PAR6 through their respective PB1 domains (Izumi et al., 1998; Lin et al., 2000; Linch et al., 2014; Muthuswamy and Xue, 2012; Rodriguez-Boulan and Macara, 2014; Suzuki et al., 2002) (Box 1; Figure 4). In this regard, it is widely accepted that both aPKCs have similar roles (Rodriguez-Boulan and Macara, 2014). This would explain why the loss of PKCλ/ι did not affect the highly polarized epithelia of the intestine (Murray et al., 2009; Nakanishi et al., 2016), as PKCζ could compensate for PKCλ/ι deficiency. The relative contribution of each aPKC to the tumor suppressor role of cell polarity has been recently addressed using primary mouse mammary epithelial organoids, which form a polarized 3D cell bilayer structure (Jung et al., 2019). In this system, induction of Snail 1 (SNAI1) promoted epithelial mesenchymal transition (EMT) in 2D, but not in 3D cultures, suggesting that some intrinsic property of polarized epithelial growth suppresses the ability of SNAI1 to induce EMT (Jung et al., 2019) (Figure 4). Interestingly, aPKCs, as part of the PAR complex, played a major role in destabilizing SNAI1 through direct phosphorylation and prevented EMT (Jung et al., 2019) (Figure 4). In this system, it was shown that inhibition of either PKCλ/ι or PKCζ was enough to induce SNAI1 stability and promote EMT, but the effect was additive when both aPKCs were simultaneously inhibited (Jung et al., 2019). This regulatory mechanism suggests that the tumor suppressor role of PKCλ/ι only becomes apparent when using adequate models that retain apical-basal polarity, such as epithelial organoids. Alternatively, other studies have suggested that disruption of homeostatic levels of aPKC, above or below a given threshold, can distort normal polarized morphogenesis in epithelia and contribute to tumorigenesis (Durgan et al., 2011; Linch et al., 2013). Disruption of the apical-basal polarity through the effect of oncogenes like ERBB2 (Aranda et al., 2006) or mutations like those in the hotspot dibasic motif of PKCλ/ι (Linch et al., 2013), offer additional examples of how the tumor suppression function of polarity is lost in malignancy and the potential implication of the aPKCs in that mechanism (Figure 4). In any case, data supporting a significant role of the aPKCs in the control of polarity in vivo in GEMM still needs to be produced.
PKCζ is a Versatile Tumor Suppressor
Initial studies have reported that PKCζ can be both downregulated and upregulated in human cancer, which has led to an overall confusing picture of its potential role as a tumor suppressor or oncogene (Garg et al., 2014). The fact that many studies have relied on the utilization of antibodies that were not isotype-specific for each aPKC had constituted a barrier that precluded a definitive conclusion on the specific role of PKCζ in cancer. Similar caveats exist for experiments using non-selective small molecule inhibitors or the over-expression of permanently active or kinase-dead mutants. Therefore, here we will review more definitive data that are based on the analysis of genetic tools that selectively delete PKCζ. In xenografts models, PKCζ has been shown to exert a pro-tumorigenic role in pancreatic cancer human cell lines (Butler et al., 2013), and more recently PKCζ has been proposed to promote lymphatic metastasis of human prostate cancer cells (Zang et al., 2019). However, the genetic in vivo evidence in an immunocompetent GEMM that PKCζ can be a tumor suppressor came from the analysis of PKCζ KO mice that had been crossed with a highly penetrant doxycycline-inducible KrasG12D model of lung adenocarcinoma (Galvez et al., 2009). These studies demonstrated that PKCζ deficiency led to increased tumorigenicity (Galvez et al., 2009). This effect was mediated through a cell-autonomous increase in the ability of PKCζ-deficient tumor cells to cope with nutrient deprivation (Galvez et al., 2009). This suggested that PKCζ loss conferred a growth advantage in nutrient-scarce microenvironments, such as those existing in poorly vascularized solid tumors (Galvez et al., 2009) (Figure 5). This concept of PKCζ as a critical regulator of the cancer cell response to nutrient deprivation was confirmed and extended by the analysis of its role in intestinal tumorigenesis. This study showed that the loss of PKCζ enabled CRC cells to undergo metabolic reprogramming in response to glucose deprivation by switching to glutamine utilization (Ma et al., 2013). This metabolic reprogramming helps to maintain tricarboxylic cycle activity and supports serine biosynthesis through the upregulation of phosphoglycerate dehydrogenase (PHGDH), the critical enzyme in the serine biosynthetic pathway (Ma et al., 2013) (Figure 5). Consistent with these observations, the deletion of Prkcz in mice with inactivated adenomatous polyposis coli (Apc), a common genetic event in many CRCs, showed enhanced intestinal tumorigenesis as compared with PKCζ-proficient APC-deficient tumors (Ma et al., 2013). These data are reminiscent of a series of earlier studies that showed that PKCζ was downregulated in AOM-induced colonic adenomas and can be considered an initial indication of its potential tumor suppressor activity (Kahl-Rainer et al., 1994; Mustafi et al., 2006; Wali et al., 1996). Intriguingly, another previous study had failed to detect an effect of total body PKCζ deficiency in intestinal tumorigenesis of ApcMin/+ mice (Oster and Leitges, 2006). One potential explanation is that this previous study used mice of 129S2/sv background, whereas Ma et al., (2013) used mice in a C57BL/6 background. In any case, it should be noted that an increase in intestinal tumorigenesis driven by APC deficiency was not only detected in the ApcMin/+ mouse model with total PKCζ KO (Ma et al., 2013), but also using Apc floxed mice crossed with a conditional Prkcz floxed line selectively KO in LGR5+ intestinal stem cells (ISC) (Llado et al., 2015).
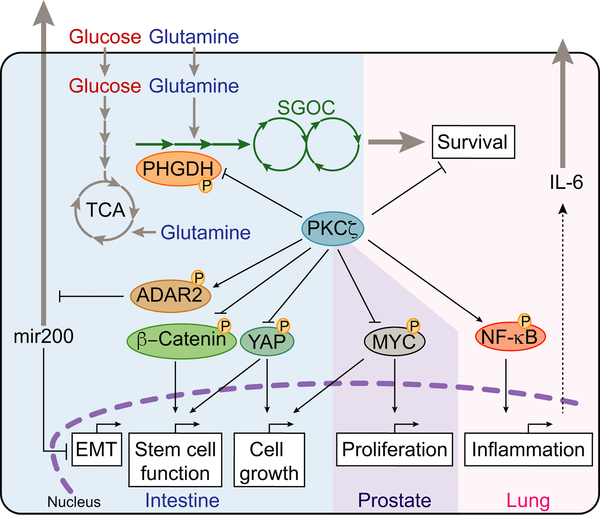
Figure 5. PKCζ is a Versatile Tumor Suppressor.
PKCζ inhibits PHGDH, MYC, YAP, and β-catenin by direct phosphorylation to limit EMT, stem cell function, cell growth, and proliferation. PKCζ regulates ADAR2 and NF-κB to limit EMT and inflammation, respectively.
Importantly, the work of Ma et al., (2013) established that metabolic reprogramming under nutrient stress is a crucial cell-autonomous mechanism through which PKCζ exerts its tumor suppressor activity (Ma et al., 2013). Consistent with this notion, another study demonstrated that PKCζ KO in ISC allowed them to cope with nutrient deprivation-induced stress and to maintain YAP/β-catenin signaling (Llado et al., 2015) (Figure 5). This finding has implications beyond cancer because PKCζ deficiency enables the intestinal epithelium to better regenerate after a damaging insult, such as radiation, due to their enhanced stemness (Llado et al., 2015). Further studies have shown that PKCζ is not just critical for regulating cancer metabolism but also plays a more versatile tumor suppressor role than previously anticipated. A recent study showed that PKCζ KO in CRC cells selectively reduced the expression of the miR200 family (Shelton et al., 2018) (Figure 5). This is particularly interesting because miR200s can regulate stemness and EMT, which is believed to be important during tumor metastasis (Burk et al., 2008). Thus, PKCζ loss promoted EMT and increased the metastatic potential of CRC cells in vitro and in vivo through the direct phosphorylation of ADAR2 (Shelton et al., 2018) (Figure 5). ADAR2 is an A-to-I deaminase that edits RNA molecules to modulate their stability and affinity for binding proteins. Mechanistic studies demonstrated that PKCζ deficiency reduced intracellular miR-200 levels by promoting their release into the extracellular milieu through an ADAR2-dependent process (Shelton et al., 2018) (Figure 5). This observation suggested that ADAR2-mediated editing of components of the vesicular secretory pathways might be a key event for the enhanced secretion of miR-200s in PKCζ-deficient cancer cells (Shelton et al., 2018) (Figure 5). Notably, interrogation of human patient datasets and liquid biopsies from metastatic and non-metastatic CRC patients revealed a correlation between low PKCζ expression and poor prognosis in CRC (Shelton et al., 2018). Of note, although the mRNA levels of PKCζ are slightly upregulated on average in TCGA colon adenocarcinoma (COAD) samples, as compared to normal colonic tissue (Figure 3A), PKCζ levels are downregulated when comparing the aggressive and metastatic CRC with more benign disease stages (Shelton et al., 2018).
Similar to the tumor-suppressive role of PKCζ in lung and intestinal cancer, loss of PKCζ in the context of prostate-specific PTEN deletion also increased tumorigenesis compared with PTEN deletion alone (Kim et al., 2013). PKCζ was shown to directly regulate MYC protein stability through phosphorylation of S373, a key mechanism for the control of its association with other transcription factors, such as MAX (Dang, 2012) (Kim et al., 2013) (Figure 5). Of particular relevance to the role of PKCζ in metabolic reprogramming in cancer cells (Ma et al., 2013), activation of MYC has also been shown to control, under nutrient stress conditions, the PHGDH-driven serine biosynthetic cascade (Gao et al., 2009; Nikiforov et al., 2002; Sun et al., 2015). Therefore, it is tempting to speculate that cancer cells with reduced PKCζ levels undergo metabolic reprogramming of the serine biosynthetic cascade via activation of MYC in response to nutrient-deprived tumor microenvironments.
Non-cell-autonomous Tumor Suppression by PKCλ/ι
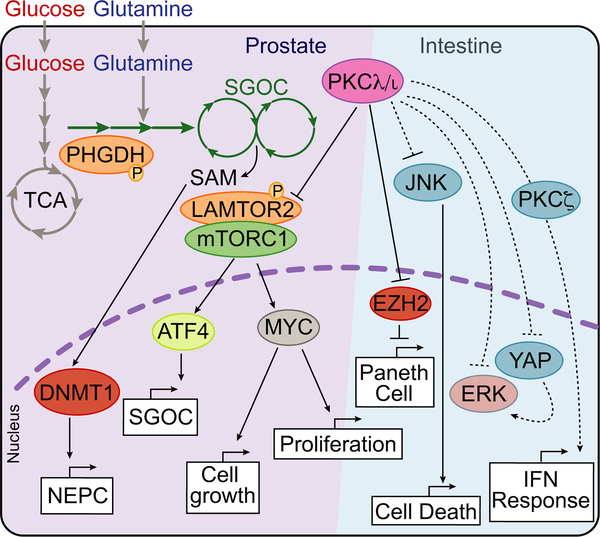
One striking observation that provided an important initial clue to the tumor-suppressive role of PKCλ/ι in CRC was that its levels are remarkably high in Paneth cells (Nakanishi et al., 2016). These are secretory cells located at the base of the intestinal crypt that are critical for the control of the intestinal barrier function and for the secretion of anti-microbial peptides to ensure a sterile environment (Clevers and Bevins, 2013). Interestingly, specific ablation of PKCλ/ι in intestinal epithelial cells (IECs), referred to hereon as LKO mice (Prkcif/f;Villin-cre), resulted in defective Paneth cell formation, increased IEC death, inflammation, and dysbiosis (Nakanishi et al., 2016). The inflammatory response associated to the loss of PKCλ/ι in IECs was previously reported, although that study did not analyze potential defects in Paneth cells (Calcagno et al., 2011). Mechanistic studies by Nakanishi et al., (2016) revealed that PKCλ/ι directly limits the levels of EZH2, the catalytic component of the Polycomb Repressor Complex 2 (PRC2) (Nakanishi et al., 2016). EZH2 represses the expression of ATOH1 and GF1, the two master transcriptional regulators of Paneth cell differentiation (Durand et al., 2012; Shroyer et al., 2005; Yang et al., 2001). Therefore, the loss of PKCλ/ι results in increased amounts of EZH2 in Paneth cells, which reduced the amount of ATOH1 and GF1, with the subsequent defects in Paneth cell differentiation (Durand et al., 2012; Nakanishi et al., 2016; Shroyer et al., 2005; Yang et al., 2001) (Figure 6). Consistent with these data, an additional study has also reported increased inflammation upon genetic deletion of PKCλ/ι in IECs, concomitant with a significant increase in intestinal barrier permeability and enhanced NF-κB activation (Forteza et al., 2016). This is relevant for human disease because Paneth cells are often found altered in patients with inflammatory bowel disease (IBD; ulcerative colitis [UC] and Crohn’s disease [CD]), who are also at higher risk of developing CRC (Kaser et al., 2010). Accordingly, PKCλ/ι expression was found to be downregulated in the intestine of CD and UC patients (Nakanishi et al., 2016; Wald et al., 2011). Therefore, the alterations in Paneth cells in LKO mice underlie enteritis and colitis that create a microenvironment conducive to transformation (Nakanishi et al., 2016). Notably, the inflammatory pro-tumorigenic environment created by PKCλ/ι loss in IECs is not in itself sufficient to drive tumor initiation (Nakanishi et al., 2016). However, a recent study demonstrated that the simultaneous IEC-specific deletion of both enzymes ( Prkcif/f;Prkczf/ff;Villin-cre, hereon DKO mice) resulted in highly aggressive and invasive intestinal carcinomas that faithfully phenocopied human serrated CRC (Nakanishi et al., 2018) (Figure 6). Serrated tumors account for about 30% of all CRC cases in humans and represent an alternative pathway to CRC from the conventional adenoma-carcinoma sequence (JE et al., 2015). Serrated CRC has characteristic histopathological features and molecular events, such as a lower incidence of activating mutations in the β-catenin pathway (JE et al., 2015). Transcriptomic analysis of tumors from DKO mice revealed a marked similarity with human serrated CRC, including the upregulation of key signaling pathways, such as the ERK cascade, and the complete exclusion of β-catenin from both precursor and carcinoma lesions in the DKO mice (Nakanishi et al., 2018). Importantly, serrated adenomas/polyps occur often associated with activation of the MAPK cascade, as a result of activating mutations in KRAS or BRAF (Bettington et al., 2013; Borowsky et al., 2018). Although undoubtedly important, these mutations are not always present in all serrated adenomas/polyps (JE et al., 2015). The DKO mouse model demonstrates that serrated CRC can initiate and progress without mutations in Kras or Braf (Nakanishi et al., 2018). Moreover, it has been shown in mouse models driven by the IEC-specific expression of Kras or Braf mutants that the additional inactivation of tumor suppressors, such as Cdkn2a (p16INK4a), Trp53, or Cdx2, is required for these tumors to progress to the advanced adenocarcinoma stage, suggesting the requirement of additional factors beyond activation of the MAPK pathway (Bennecke et al., 2010; Carragher et al., 2010; Sakamoto et al., 2017). Interestingly, the DKO model of serrated CRC does not require the additional genetic inactivation of tumor suppressors or the expression of additional oncogenes. This indicates that it is an adequate model of spontaneous tumorigenesis in vivo, recapitulating all the features of human serrated CRC. Of clinical relevance, a substantial reduction in aPKC protein abundance in human serrated adenomas correlated with reduced infiltration of CD8+ T cells (Nakanishi et al., 2018).
Figure 6. Tumor Suppression by PKCλ/ι.
PKCλ/ι blocks NEPC differentiation, cell growth and proliferation through inhibition of mTORC1-dependent metabolic reprogramming by directly phosphorylating LAMTOR2. Also, PKCλ/ι promotes Paneth cell differentiation, prevents cell death and interferon response by modulating the levels of JNK, ERK, YAP, and directly phosphorylating EZH2.
An important question arising from these observations is: what is the specific and “unique” contribution of each aPKC to serrated cancer initiation and progression in the DKO mouse model and in human serrated tumors? The most salient transcriptional phenotype of PKCζ-KO IECs is a profound shutdown of the interferon (IFN) signaling pathways (Nakanishi et al., 2018). This suggests that this cell-autonomous role of PKCζ underlies spontaneous tumorigenesis in the intestinal epithelium of DKO mice (Figure 6). Importantly, whereas the loss of PKCλ/ι alone led to massive recruitment of CD8+ T cells that blunted tumor initiation, loss of both aPKCs abolished CD8+ T cell infiltration, thereby allowing tumor initiation and generation of not only adenomas but also aggressive invasive carcinomas (Nakanishi et al., 2018). These observations establish a paradigm for the cooperation between the two aPKCs as tumor suppressors by impinging in unique and complementary cascades (Nakanishi et al., 2018) (Figure 7). Thus, PKCζ loss contributes to the DKO cancer phenotype predominantly via the impairment of the IFN pathway (Nakanishi et al., 2018). This is important because a functional IFN cascade is required for the activation of the immunosurveillance triggered by the IEC-specific loss of PKCλ/ι that results in the infiltration of CD8+ T cells (Nakanishi et al., 2018) (Figure 7). Thus, there is a synergistic cooperation between the two aPKCs to prevent intestinal tumorigenesis. On the one hand, PKCλ/ι’s role in the intestinal epithelium is to maintain an antiinflammatory environment that restrains inflammation-induced tumorigenesis. On the other hand, the role of PKCζ is to transmit the IFN signals required for the activation of the immunosurveillance activity put in motion as a consequence of PKCλ/ι is loss in IECs. Identifying the critical steps in these two processes will open up the possibility of inhibiting immune evasion in serrated CRC by modulating targets downstream of PKCλ/ι.
Figure 7. Targetable Vulnerabilities of aPKC-Deficient Tumors.
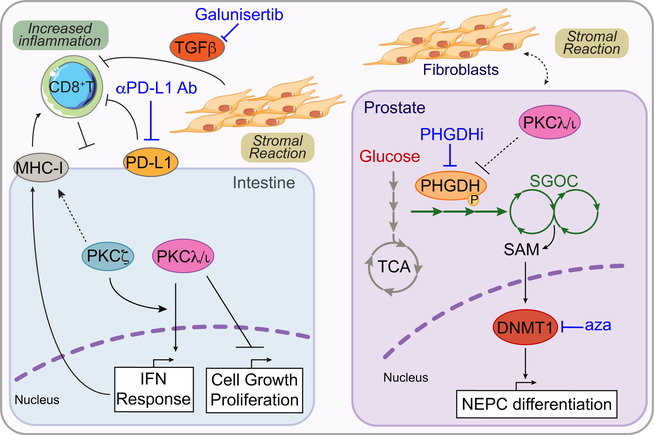
TGFβ inhibition with Galunisertib combined with anti PD-L1 antibodies (Ab) exploits a vulnerability created in highly mesenchymal CRC, like those from the serrated origin, by alleviating the stromal activation and boosting the antitumor response. Also, inhibition of PHGDH and/or DNMT activity with azacytidine (aza) can exploit a vulnerability created in therapy-resistant PCa to block NEPC differentiation and tumorigenesis.
An interesting twist to the role of PKCλ/ι in the control of immunosurveillance came from the mouse model mentioned above in which PKCλ/ι is ectopically overexpressed in the FTSE (Sarkar et al., 2017). This model results in immunosuppression through the recruitment of myeloid-derived suppressor cells (MDSCs) (Sarkar et al., 2017). In broader terms, these studies are in good agreement with the results of Nakanishi et al., (2018) showing that the conditional loss of PKCλ/ι in the intestinal epithelium results in increased immunosurveillance. It would be interesting to determine if the loss of PKCλ/ι in FTSE or its ectopic induction in the intestinal epithelium results in enhanced immunosurveillance and increase immunosuppression in each tissue, respectively. The precise mechanism whereby the loss or the ectopic expression of PKCλ/ι in either system affects the immune response is less clear. Thus, whereas the loss of PKCλ/ι in the intestinal epithelium leads to increased immunological cell death and the activation of the interferon pathways that promote MHC-I cross-presentation and CD8+ infiltration (Nakanishi et al., 2018), in the case of the overexpression of PKCλ/ι in the FTSE that results in immunosuppression through the activation of YAP (Sarkar et al., 2017). Interestingly, the loss of PKCλ/ι in IECs also results in the activation of YAP, although likely through a noncell-autonomous mechanism, as discussed below (Nakanishi et al., 2018) (Figure 6). In any case, the concept that upregulation of YAP by PKCλ/ι overexpression in FTSE accounts for the immunosuppressive phenotype of these mutant mice is at odds with recent evidence demonstrating that the activation of YAP by the KO of its upstream negative regulator, LATS1/2, promotes the IFN pathway in several cell systems that results in the activation of anti-cancer immunity (Moroishi et al., 2016). This evidence would agree with the phenotype of PKCλ/ι loss in the intestinal epithelium. Further studies should clarify this important question by generating genetic models of YAP inhibition or activation by the KO of LATS1/2 in PKCλ/ι-deficient or PKCλ/ι overexpressed systems. In any case, how PKCλ/ι in cancer cells might impinge in the immunological landscape of the tumor microenvironment seems to be context-dependent because the loss of PKCλ/ι simultaneously with that of PKCζ in the intestine conditional DKO mice promotes an immunosuppressive environment associated with a strong desmoplastic response (Nakanishi et al., 2018). It would be interesting to know if the loss of PKCλ/ι, as opposed to its ectopic overexpression, gives a similar phenotype in the context of PTEN and p53 inactivation in the FTSE system.
Relevance of PKCλ/ι and PKCζ in Human Colorectal Cancer
PRKCZ mRNA abundance was found to be most downregulated in the colon cancer subtype (CCS) 2 and CCS3 as compared with the CCS1 group (Nakanishi et al., 2018). This is particularly important because the CCS3 group also showed downregulation of PRKCI. This subtype is characterized by having elements of serrated origin, being highly mesenchymal and associated with the lowest survival rate (Guinney et al., 2015). In agreement with this notion, analysis of human patient samples demonstrated that protein expression of both aPKCs were significantly downregulated in tubular adenomas and even more prominently in sessile serrated adenomas/polyps (SSA/Ps) (Nakanishi et al., 2018). Gene set enrichment analysis of patients stratified according to the median expression of PRKCI and PRKCZ, demonstrated a significant positive enrichment in EMT and TGFβ signaling as well as serrated gene signatures in the PRKCllowPRKCZlow group, as compared with the PRKCIhighPRKCZhigh (Nakanishi et al., 2018). Consistently, the PRKCIlowPRKCZlow patients had a significantly worse prognosis than the PRKCIhighPRKCZhigh group (Nakanishi et al., 2018). Thus, albeit the slight average upregulation of PRKCZ and PRKCI in patients in the COAD dataset (Figure 3A), the detailed stratification of patients in the different subtypes of CRC demonstrated a negative correlation with aggressiveness and survival (Llado et al., 2015; Nakanishi et al., 2018; Shelton et al., 2018).
Cell-Autonomous Tumor Suppression by PKCλ/ι in Colorectal Cancer
Although PKCλ/ι plays a non-cell-autonomous role in CRC, the study by Nakanishi et al. (2018) also provided evidence that this kinase can act in a cell-autonomous manner to suppress cancer. Thus, LKO mice treated with an anti-CD8 antibody fully developed aggressive serrated CRC indistinguishable from the lesions in the DKO mice (Nakanishi et al., 2018). This indicates that PKCλ/ι KO alone in IECs cannot drive cancer by itself because the simultaneous activation of immunosurveillance prevents tumor initiation. However, removal of immunosurveillance by anti-CD8 treatment or concomitant KO of PKCζ in the epithelium allows the PKCλ/ι-deficient IECs to become cancer cells (Nakanishi et al., 2018). PKCλ/ι, therefore, emerges as a cell-autonomous tumor suppressor because its loss is sufficient to activate the anabolic and growth signaling pathways required for tumorigenesis. The oncogenic mechanisms activated by the loss of PKCλ/ι in CRC are less clear and most likely determined by the microenvironment of the inflammatory intestinal tissue. Notably, the careful inspection of the IEC-specific LKO intestinal tissues suggests a potent activation of the ERK, EGFR and KRAS pathways (Nakanishi et al., 2018) (Figure 6). How the loss of PKCλ/ι activates the EGFR-KRAS-ERK cascade is not yet understood. However, a potential hint may come from the observation that the YAP pathway is also highly activated by PKCλ/ι loss (Nakanishi et al., 2018). This is interesting because previous reports have linked the activation of YAP to the synthesis of several EGF family members (Gregorieff et al., 2015). How YAP is activated in the context of the LKO intestinal tissue might be related to the generation of IL-6 by the mutant tumor microenvironment (Figure 6), as shown previously (Taniguchi et al., 2015).
Cell-Autonomous Tumor Suppression by PKCλ/ι in Neuroendocrine Prostate Cancer
The definitive proof that PKCλ/ι can be a tumor suppressor in a cell-autonomous manner, at least in very aggressive cancers, came from the study of the signaling pathways that control lineage plasticity during the emergence of castration-resistant prostate cancer (PCa) with neuroendocrine phenotype (NEPC) (Reina-Campos et al., 2019) (Figure 6). Advanced variants of PCa, of which NEPC is the most aggressive, are currently incurable due to limited or transient responses to available therapies. NEPC often results from differentiation of androgen receptor (AR)-positive luminal cells to AR-negative small cells that express an array of stem cell, basal, neuronal, and endocrine markers (Rickman et al., 2017). Interestingly, prostate-specific deletion of PKCλ/ι in the context of PTEN loss promoted the emergence of aggressive prostate carcinoma with basal and NEPC features (Reina-Campos et al., 2019). Transcriptomic analysis of PCa cell lines with PKCλ/ι KO and interrogation of PTEN/PKCλ/ι-KO tumors revealed that mTORC1 activation is the main downstream consequence of PKCλ/ι loss (Reina-Campos et al., 2019). mTORC1 is a central hub of nutrient signaling and cell anabolism (Kim and Guan, 2019; Zoncu et al., 2011). Of note, an unbiased screen of PKCλ/ι interactors based on the proximity-dependent biotinylation assay BioID2 (Kim et al., 2016) identified LAMTOR2 as a PKCλ/ι interactor protein. LAMTOR2 is a subunit of the Ragulator complex, which participates in the recruitment of the machinery that docks mTORC1 to the lysosomal membrane where it is activated (de Araujo et al., 2017; Su et al., 2017). LAMTOR2 is also involved in lysosomal localization, representing a second mechanism by which it controls the spatial localization of mTORC1 and its coactivators (Teis et al., 2006; Walton et al., 2018). These data showed that PKCλ/ι-dependent phosphorylation of LAMTOR2 makes a crucial contribution to mTORC1 activation and the acquisition of NEPC features (Reina-Campos et al., 2019) (Figure 6). The link between PKCλ/ι and mTORC1 may partially explain the increased tumor growth and proliferation, but it is insufficient to explain the effects on NEPC differentiation. In this regard, ATF4 was identified as an upstream regulator of the transcriptional changes resulting from PKCλ/ι KO (Reina-Campos et al., 2019). Interestingly, ATF4 has previously been shown to mediate the anabolic effects of mTORC1 activation via regulation of the serine glycine and one-carbon pathway (SGOCP) (Ben-Sahra et al., 2016; Locasale, 2013). Coordinated activation of this metabolic branch diverts glucose-derived carbons and imports glutamine-derived nitrogen into the synthesis of serine and glycine. The interconversion of these amino acids donates one-carbon unit to the salvage pathway of methionine synthesis, thus increasing the pool of nucleotide precursors and S-adenosyl methionine (SAM) (Locasale, 2013). SAM is the universal donor for all methylation reactions in the cell, including DNA and chromatin methylation by DNA methyltransferases (DNMTs) and protein methyltransferases, respectively (Reid et al., 2017). Epigenetic control of gene expression by DNA and chromatin methylation is especially important during tumor progression and acquisition of therapy resistance, as occurs during differentiation towards the NEPC phenotype (Beltran et al., 2016). Cell plasticity allows tumor cells to profoundly change their transcriptomic landscape and become cell types that are insensitive, or become better adapted, to therapeutic pressure (Rickman et al., 2017). However, while most epigenetic research has focused on the enzymatic control of DNA and histone modification by writers, readers, and erasers, less attention has been paid to the mechanisms that “supply the ink”. In this regard, inhibition of either mTORC1 or ATF4 has been shown to reduce cell proliferation and reverse NEPC differentiation markers in vitro concomitant with a reduction in intracellular SAM concentration (Reina-Campos et al., 2019) (Figure 6). Importantly, PKCλ/ι deficiency was shown to be key to a serine-dependent increase in the incorporation of 13C-labeled methionine carbons (source of SAM) into the DNA (Reina-Campos et al., 2019). Inhibition of this process by either suppressing the synthesis of SAM or inhibiting DNMT activity reverted NEPC differentiation in vitro and in vivo (Reina-Campos et al., 2019). Therefore, PKCλ/ι controls the ability of tumor cells to generate the SAM necessary to undergo epigenetically regulated cell specification programs. This has high clinical relevance because loss of PKCλ/ι expression has been observed in both de novo NEPC and treatment-induced NEPC differentiation (Reina-Campos et al., 2019). While no PKCλ/ι deletions or amplifications have been reported in NEPC (Reina-Campos et al., 2019), a potential LOF mutation in PRKCI has been found in one NEPC sample (Beltran et al., 2016). Intriguingly, PKCλ/ι has been previously shown to be upregulated in PCa adenocarcinoma in the context of AR overactivation and to exert a tumor promoter role (Ishiguro et al., 2009; Win and Acevedo-Duncan, 2008; Win and Acevedo-Duncan, 2009). However, while this is somewhat in agreement with the correlation of AR and PKCλ/ι levels observed in PCa patients (Reina-Campos et al., 2019), previous studies suggesting the tumor-promoting role of PKCλ/ι were limited to in vitro models (Ishiguro et al., 2009; Win and Acevedo-Duncan, 2008; Win and Acevedo-Duncan, 2009).
Beyond PCa, several other tumor types have been shown to use similar cell differentiation mechanisms to escape targeted therapy. In fact, the tumor suppressor mechanism of PKCλ/ι in PCa might also be at play in lung cancer because NSCLC tumors carrying mutant EGFR are able to undergo NE differentiation and acquire the small cell lung carcinoma phenotype (SCLC) upon treatment with an EGFR inhibitor (Niederst et al., 2015; Oser et al., 2015). Although the existence of a role for PKCλ/ι in SCLC still needs to be demonstrated, the findings in NEPC suggest that lung cancer that silence PKCλ/ι expression might be able to have an increased ability to undergo NE differentiation, thus increasing their metastatic and malignant behavior.
Direct Therapeutic Targeting of the PKCs
Over the years, multiple PKC inhibitors have been developed with a broad spectrum of potency and selectivity towards all or specific PKC isozymes. Although these inhibitors could represent valuable research tools, their efficacy in cancer is yet to be established (Mochly-Rosen et al., 2012; Newton and Brognard, 2017). Indeed, not only have most clinical trials of such inhibitors failed to demonstrate efficacy (Mackay and Twelves, 2007), but some have even reported worsened outcomes, as highlighted by a meta-analysis of five clinical trials with more than 1,000 NSCLC patients (Zhang et al., 2015). It is of great interest to see if any of the currently active clinical trials testing PKC inhibitors as anti-cancer therapeutics show any therapeutic benefit.
A critical issue that needs to be considered when targeting any PKC is the difficulty of generating truly isozyme-selective compounds. For example, midostaurin (PKC412), which has been widely used as a “PKCα-specific” inhibitor, also targets PKCβ1, PKCβ2, PKCγ, PKCδ, and PKCε (Manley et al., 2018). Moreover, the tumor suppressor roles of PKCλ/ι and PKCζ, at least in CRC and NEPC, should be taken into consideration when considering aPKC inhibitors as potential anti-cancer drugs. In this context, preclinical results obtained with some purportedly aPKC-specific inhibitors should also be viewed with caution. For example, the “selective” PKCζ inhibitor PZ09 inhibits not only both aPKCs but also most of the 37 kinases tested in the original study (Trujillo et al., 2009). Therefore, the interpretation of these experiments should take into consideration the potential off-target effects of these compounds (Kusne et al., 2014). We should also have a better understanding of how the PKC isozymes function in normal cell types in the tissue microenvironment, which play a prominent role in controlling tumorigenesis (Nakanishi et al., 2018), before considering the use of aPKC inhibitors in the laboratory and, especially, in the clinic. Several PKC inhibitors currently being tested as anti-cancer agents were previously used as immunosuppressants for inflammatory diseases. Immunosuppression in the context of cancer would be detrimental for the patient. Curiously, PKC inhibitors such as AEB071, which affects early T cell activation (Evenou et al., 2009), is currently in clinical trials for uveal melanoma in combination with a phosphoinositide 3-kinase inhibitor (BYL719) (NCT02273219). Another immunosuppressive PKC inhibitor, gold sodium thiomalate, has been proposed for the treatment of advanced NSCLC based on the preclinical data discussed above (Regala et al., 2005b). A good approach to clarify the on-target effect of PKCλ/ι selective compounds could be to generate a drug-resistant PKCλ/ι mutant that retained WT function. A complete understanding of the role of the aPKCs in the immune system will help to design studies that should include strategies aimed at mitigating the immune side effects of these treatments.
Targetable Vulnerabilities of aPKC-deficient Tumors
An alternative approach to the direct inhibition of aPKCs is to target the uncovered vulnerabilities downstream of these kinases. For example, targeting mTORC1 could be an attractive strategy in NEPC. However, targeting specific downstream effectors of mTORC1 could provide an alternative approach. These include metabolic enzymes of the SGOCP, which may play a fundamental role in tumorigenesis in CRC and PCa (Reina-Campos et al., 2019). PHGDH that was found to be the rate-limiting step of the SGOCP during the emergence of NE features in CRPC, could represent a potential vulnerability in PKCλ/ι-deficient PCa tumors (Reina-Campos et al., 2019) in a manner analogous to that shown for PKCζ by Ma et al. (2013) (Figure 7). Efforts to inhibit PHGDH, which is also amplified in breast cancer and upregulated in several other tumors (Locasale, 2013), have yielded promising results in preclinical studies, including the finding that PHGDH knockdown strongly inhibited the growth of an aggressive PCa cell line in xenografts (Reina-Campos et al., 2019). Since the SGOCP pathway plays a critical role in supplying methyl groups for histone and DNA methylation, one potential strategy to treat NEPC is inhibition of DNMTs; for example, with decitabine (5-aza-2′-deoxycytidine), which is FDA approved for myelodysplastic syndrome. In support of this, efficacy with decitabine was recently shown in PCa xenograft experiments (Reina-Campos et al., 2019), and a clinical trial of enzalutamide and decitabine in metastatic CRPC has just been initiated (NCT03709550).
In keeping with the concept that PKCλ/ι-dependent epigenetic regulation of lineage plasticity processes may provide a therapeutic opportunity in NEPC, FDA-approved EZH2 inhibitors have shown promising effects in preclinical NEPC models (Ku et al., 2017; Mu et al., 2017). Of note, EZH2 is a target of PKCλ/ι and is essential for the regulation of Paneth cell biology and, most likely, for the inflammatory phenotype of PKCλ/ι-deficient intestinal epithelium (Nakanishi et al., 2016) (Figure 6). This observation suggests that inhibitors of the catalytic function of EZH2 might also be effective in IBD through the rescue of Paneth cell function and reduction of inflammation and colitis-associated dysbiosis caused by loss of the intestinal barrier integrity. However, EZH2 also plays developmental and, in some contexts, tumor suppressor roles (Kim and Roberts, 2016). The results of ongoing clinical trials assessing the toxicity and efficacy of these drugs will be of great interest.
The aPKCs in the Therapeutic Targeting of the Immunosuppressive Microenvironment
As noted above, development of invasive intestinal tumors in DKO mice is accompanied by massive activation of the stromal response and a profound immunosuppression, characterized by exclusion of CD8+ T cells from the tumor area and the recruitment to the tumor of programmed death-ligand 1 (PD-L1)-positive myeloid-derived suppressor cells (Nakanishi et al., 2018). This is of high clinical relevance because one of the four consensus molecular signatures of CRC includes the CMS4 group, which is characterized by increased expression of stromal markers and the poorest prognosis (Guinney et al., 2015). Therefore, the aPKCs emerge as signaling kinases that regulate not only tumor initiation but also the acquisition of the stromal and immunosuppressive phenotype that drives cancer cells to more malignant states (Figure 7). However, while the simultaneous loss of both aPKCs results in a very aggressive cancer, it also creates two vulnerabilities that can be exploited therapeutically. Thus, although the reduction of the stromal response in vivo with an inhibitor of the TGFβ receptor does not block tumorigenesis because it is unable to restore the infiltration of CD8+ T cells, it blunts the progression of adenomas to carcinomas (Nakanishi et al., 2018). This is an important point when considering potential treatment options for aggressive forms of CRC and other highly desmoplastic tumors. In contrast, treatment of the DKO mice with an anti-PD-L1 antibody has no effect on the most aggressive and desmoplastic tumors. This is consistent with the fact that aggressive CRC in humans responds poorly to immune-checkpoint inhibitors and suggests that additional therapies targeting the tumor stroma will be required to sensitize highly desmoplastic immunosuppressed cancers to immunological reactivation (Puccini and Lenz, 2018). In keeping with this notion, the data from Nakanishi et al., (2018) demonstrated that the simultaneous inhibition of the TGFβ receptor allows anti-PD-L1 immuno-checkpoint therapy to dramatically inhibit tumor formation (Nakanishi et al., 2018) (Figure 7). These findings are in keeping with a recent study showing the same effect of the combination therapy for the treatment of CRC metastasis (Tauriello et al., 2018). Importantly, these studies establish the simultaneous loss of both aPKCs as a potential diagnostic biomarker for serrated CRC and offer guidance for the treatment of this tumor and others that display a strong stromal/immunosuppressive phenotype.
Conclusions and Perspectives
While PKCζ has been established as a versatile tumor suppressor in cancer, establishing universal rules on the role and mechanisms of action of PKCλ/ι has proven more complicated due to its pleiotropic role and context-specific functions. Nonetheless, after more than 30 years of aPKC research, a pattern has begun to emerge. For example, the tumor-promoting role of PKCλ/ι is most apparent in situations of oncogene-induced stress to maintain TIC-like populations. Thus, high levels of PKCλ/ι favor early survival of tumor cells transformed by potent oncogenes such as KRAS, or BCR-ABL, by mediating pro-survival signaling through RAC1-MEK-ERK, NFκB, NOTCH, and Hh pathways. On the other hand, loss of PKCλ/ι might be favored as a late event that develops in cellular contexts under conditions of changes in lineage plasticity due to therapy resistance, like in NEPC. Also, the tumor suppressor role of PKCλ/ι seems determinant under conditions of inflammation and complex immune responses in the tumor microenvironment such as those found in CRC. Together, this preliminary set of rules indicates that the tumor suppressor actions of PKCλ/ι might override its tumor-promoting activity, at least during cancer progression. In that model, the trade-off of losing PKCλ/ι involves unrestrained growth and proliferation, immune suppression, and increased cell plasticity, which drastically fuel tumorigenesis. However, many questions remain to be answered; especially those aimed at establishing a unifying understanding of how PKCλ/ι works in different types of cancers.
ACKNOWLEDGMENTS
Research in the authors’ laboratories was supported by grants from the National Institutes of Health (R01CA192642 and R01CA218254 to M.T.D.-M.; R01DK108743, R01CA207177 and R01CA211794 to J.M.). We thank Juan F. Linares for comments and critical reading of the manuscript.
Footnotes
DECLARATION OF INTERESTS
M.R.-C., M.T.D.-M., and J.M. are inventors on a patent application related to this work, filed by Sanford Burnham Prebys Medical Discovery Institute. M.T.D.-M. and J.M. report receiving a commercial research grant from Halozyme Therapeutics.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Ali SA, Justilien V, Jamieson L, Murray NR, and Fields AP (2016). Protein Kinase Ciota Drives a NOTCH3-dependent Stem-like Phenotype in Mutant KRAS Lung Adenocarcinoma. Cancer Cell 29, 367–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antal CE, Hudson AM, Kang E, Zanca C, Wirth C, Stephenson NL, Trotter EW, Gallegos LL, Miller CJ, Furnari FB, et al. (2015). Cancer-associated protein kinase C mutations reveal kinase’s role as tumor suppressor. Cell 160, 489–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aranda V, Haire T, Nolan ME, Calarco JP, Rosenberg AZ, Fawcett JP, Pawson T, and Muthuswamy SK (2006). Par6-aPKC uncouples ErbB2 induced disruption of polarized epithelial organization from proliferation control. Nat Cell Biol 8, 1235–1245. [DOI] [PubMed] [Google Scholar]
- Atwood SX., Li M., Lee A., Tang JY., and Oro AE. (2013). GLI activation by atypical protein kinase C iota/lambda regulates the growth of basal cell carcinomas. Nature 494, 484–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin RM, Garratt-Lalonde M, Parolin DA, Krzyzanowski PM, Andrade MA, and Lorimer IA (2006). Protection of glioblastoma cells from cisplatin cytotoxicity via protein kinase Ciota-mediated attenuation of p38 MAP kinase signaling. Oncogene 25, 2909–2919. [DOI] [PubMed] [Google Scholar]
- Balsara BR, Sonoda G, du Manoir S, Siegfried JM, Gabrielson E, and Testa JR (1997). Comparative genomic hybridization analysis detects frequent, often high-level, overrepresentation of DNA sequences at 3q, 5p, 7p, and 8q in human non-small cell lung carcinomas. Cancer Res 57, 2116–2120. [PubMed] [Google Scholar]
- Barretina J, Caponigro G, Stransky N, Venkatesan K, Margolin AA, Kim S, Wilson CJ, Lehar J, Kryukov GV, Sonkin D, et al. (2012). The Cancer Cell Line Encyclopedia enables predictive modelling of anticancer drug sensitivity. Nature 483, 603–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedi A, Barber JP, Bedi GC, el-Deiry WS, Sidransky D, Vala MS, Akhtar AJ, Hilton J, and Jones RJ (1995). BCR-ABL-mediated inhibition of apoptosis with delay of G2/M transition after DNA damage: a mechanism of resistance to multiple anticancer agents. Blood 86, 1148–1158. [PubMed] [Google Scholar]
- Beltran H, Prandi D, Mosquera JM, Benelli M, Puca L, Cyrta J, Marotz C, Giannopoulou E, Chakravarthi BV, Varambally S, et al. (2016). Divergent clonal evolution of castration-resistant neuroendocrine prostate cancer. Nat Med 22, 298–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Sahra I, Hoxhaj G, Ricoult SJH, Asara JM, and Manning BD (2016). mTORC1 induces purine synthesis through control of the mitochondrial tetrahydrofolate cycle. Science 351, 728–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennecke M, Kriegl L, Bajbouj M, Retzlaff K, Robine S, Jung A, Arkan MC, Kirchner T, and Greten FR (2010). Ink4a/Arf and oncogene-induced senescence prevent tumor progression during alternative colorectal tumorigenesis. Cancer Cell 18, 135–146. [DOI] [PubMed] [Google Scholar]
- Bettington M, Walker N, Clouston A, Brown I, Leggett B, and Whitehall V (2013). The serrated pathway to colorectal carcinoma: current concepts and challenges. Histopathology 62, 367–386. [DOI] [PubMed] [Google Scholar]
- Bjorkoy G, Perander M, Overvatn A, and Johansen T (1997). Reversion of Ras- and phosphatidylcholine-hydrolyzing phospholipase C-mediated transformation of NIH 3T3 cells by a dominant interfering mutant of protein kinase C lambda is accompanied by the loss of constitutive nuclear mitogen-activated protein kinase/extracellular signal-regulated kinase activity. J Biol Chem 272, 11557–11565. [DOI] [PubMed] [Google Scholar]
- Borowsky J, Dumenil T, Bettington M, Pearson SA, Bond C, Fennell L, Liu C, McKeone D, Rosty C, Brown I, et al. (2018). The role of APC in WNT pathway activation in serrated neoplasia. Mod Pathol 31, 495–504. [DOI] [PubMed] [Google Scholar]
- Brass N, Ukena I, Remberger K, Mack U, Sybrecht GW, and Meese EU (1996). DNA amplification on chromosome 3q26.1-q26.3 in squamous cell carcinoma of the lung detected by reverse chromosome painting. Eur J Cancer 32A, 1205–1208. [DOI] [PubMed] [Google Scholar]
- Burk U, Schubert J, Wellner U, Schmalhofer O, Vincan E, Spaderna S, and Brabletz T (2008). A reciprocal repression between ZEB1 and members of the miR-200 family promotes EMT and invasion in cancer cells. EMBO Rep 9, 582–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butle AM, Scotti Buzhard ML, L S, Smit KE, Field AP, and Murra NR (2013). Protein kinase C zeta regulates human pancreatic cancer cell transformed growth and invasion through a STAT3-dependent mechanism. PLoS One 8, e72061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calcagno SR, Li S, Shahid MW, Wallace MB, Leitges M, Fields AP, and Murray NR (2011). Protein kinase C iota in the intestinal epithelium protects against dextran sodium sulfate-induced colitis. Inflamm Bowel Dis 17, 1685–1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carragher LA, Snell KR, Giblett SM, Aldridge VS, Patel B, Cook SJ, Winton DJ, Marais R, and Pritchard CA (2010). V600EBraf induces gastrointestinal crypt senescence and promotes tumour progression through enhanced CpG methylation of p16INK4a. EMBO Mol Med 2, 458–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, Jacobsen A, Byrne CJ, Heuer ML, Larsson E, et al. (2012). The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov 2, 401–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang JT, Palanivel VR, Kinjyo I, Schambach F, Intlekofer AM, Banerjee A, Longworth SA, Vinup KE, Mrass P, Oliaro J, et al. (2007). Asymmetric T lymphocyte division in the initiation of adaptive immune responses. Science 315, 1687–1691. [DOI] [PubMed] [Google Scholar]
- Chang MT, Asthana S, Gao SP, Lee BH, Chapman JS, Kandoth C, Gao J, Socci ND, Solit DB, Olshen AB, et al. (2016). Identifying recurrent mutations in cancer reveals widespread lineage diversity and mutational specificity. Nat Biotechnol 34, 155–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, and Zhang M (2013). The Par3/Par6/aPKC complex and epithelial cell polarity. Exp Cell Res 319, 1357–1364. [DOI] [PubMed] [Google Scholar]
- Choi EJ, Lee JH, Kim MS, Song SY, Yoo NJ, and Lee SH (2018). Intratumoral Heterogeneity of Somatic Mutations for NRIP1, DOK1, ULK1, ULK2, DLGAP3, PARD3 and PRKCI in Colon Cancers. Pathol Oncol Res 24, 827–832. [DOI] [PubMed] [Google Scholar]
- Clevers HC, and Bevins CL (2013). Paneth cells: maestros of the small intestinal crypts. Annu Rev Physiol 75, 289–311. [DOI] [PubMed] [Google Scholar]
- Coghlan MP, Chou MM, and Carpenter CL (2000). Atypical protein kinases Clambda and -zeta associate with the GTP-binding protein Cdc42 and mediate stress fiber loss. Mol Cell Biol 20, 2880–2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang CV (2012). MYC on the path to cancer. Cell 149, 22–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Araujo MEG, Naschberger A, Furnrohr BG, Stasyk T, Dunzendorfer-Matt T, Lechner S, Welti S, Kremser L, Shivalingaiah G, Offterdinger M, et al. (2017). Crystal structure of the human lysosomal mTORC1 scaffold complex and its impact on signaling. Science 358, 377–381. [DOI] [PubMed] [Google Scholar]
- Desai TJ, Brownfield DG, and Krasnow MA (2014). Alveolar progenitor and stem cells in lung development, renewal and cancer. Nature 507, 190–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Meco MT, and Moscat J (2012). The atypical PKCs in inflammation: NF-kappaB and beyond. Immunol Rev 246, 154–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duran A, Diaz-Meco MT, and Moscat J (2003). Essential role of RelA Ser311 phosphorylation by zetaPKC in NF-kappaB transcriptional activation. EMBO J 22, 3910–3918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durand A, Donahue B, Peignon G, Letourneur F, Cagnard N, Slomianny C, Perret C, Shroyer NF, and Romagnolo B (2012). Functional intestinal stem cells after Paneth cell ablation induced by the loss of transcription factor Math1 (Atoh1). Proc Natl Acad Sci U S A 109, 8965–8970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durga J., Kaj N., Ji D., and Hal A. (2011). Par6B and atypical PKC regulate mitotic spindle orientation during epithelial morphogenesis. J Biol Chem 286, 12461–12474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eblen ST, Slack JK, Weber MJ, and Catling AD (2002). Rac-PAK signaling stimulates extracellular signal-regulated kinase (ERK) activation by regulating formation of MEK1-ERK complexes. Mol Cell Biol 22, 6023–6033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eramo A, Lotti F, Sette G, Pilozzi E, Biffoni M, Di Virgilio A, Conticello C, Ruco L, Peschle C, and De Maria R (2008). Identification and expansion of the tumorigenic lung cancer stem cell population. Cell Death Differ 15, 504–514. [DOI] [PubMed] [Google Scholar]
- Evenou JP, Wagner J, Zenke G, Brinkmann V, Wagner K, Kovarik J, Welzenbach KA, Weitz-Schmidt G, Guntermann C, Towbin H, et al. (2009). The potent protein kinase Cselective inhibitor AEB071 (sotrastaurin) represents a new class of immunosuppressive agents affecting early T-cell activation. J Pharmacol Exp Ther 330, 792–801. [DOI] [PubMed] [Google Scholar]
- Forteza R, Figueroa Y, Mashukova A, Dulam V, and Salas PJ (2016). Conditional knockout of polarity complex (atypical) PKCiota reveals an anti-inflammatory function mediated by NF-kappaB. Mol Biol Cell 27, 2186–2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvez AS, Duran A, Linares JF, Pathrose P, Castilla EA, Abu-Baker S, Leitges M, Diaz-Meco MT, and Moscat J (2009). Protein kinase Czeta represses the interleukin-6 promoter and impairs tumorigenesis in vivo. Mol Cell Biol 29, 104–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO, Sun Y, Jacobsen A, Sinha R, Larsson E, et al. (2013). Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal 6, pl1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao P, Tchernyshyov I, Chang TC, Lee YS, Kita K, Ochi T, Zeller KI, De Marzo AM, Van Eyk JE, Mendell JT, and Dang CV (2009). c-Myc suppression of miR-23a/b enhances mitochondrial glutaminase expression and glutamine metabolism. Nature 458, 762–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg R, Benedetti LG, Abera MB, Wang H, Abba M, and Kazanietz MG (2014). Protein kinase C and cancer: what we know and what we do not. Oncogene 33, 5225–5237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregorieff A, Liu Y, Inanlou MR, Khomchuk Y, and Wrana JL (2015). Yap-dependent reprogramming of Lgr5(+) stem cells drives intestinal regeneration and cancer. Nature 526, 715–718. [DOI] [PubMed] [Google Scholar]
- Griner EM, and Kazanietz MG (2007). Protein kinase C and other diacylglycerol effectors in cancer. Nat Rev Cancer 7, 281–294. [DOI] [PubMed] [Google Scholar]
- Guinney J, Dienstmann R, Wang X, de Reynies A, Schlicker A, Soneson C, Marisa L, Roepman P, Nyamundanda G, Angelino P, et al. (2015). The consensus molecular subtypes of colorectal cancer. Nat Med 21, 1350–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafson WC, Ray S, Jamieson L, Thompson EA, Brasier AR, and Fields AP (2004). Bcr-Abl regulates protein kinase Ciota (PKCiota) transcription via an Elk1 site in the PKCiota promoter. J Biol Chem 279, 9400–9408. [DOI] [PubMed] [Google Scholar]
- Habegger KM, Matzke D, Ottaway N, Hembree J, Holland J, Raver C, Mansfeld J, Muller TD, Perez-Tilve D, Pfluger PT, et al. (2012). Role of adipose and hepatic atypical protein kinase C lambda (PKClambda) in the development of obesity and glucose intolerance. Adipocyte 1, 203–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagerstrand D., Tong A., Schumache SE., Ilic N., Shen RR., Cheung HW., Vazquez F., Shrestha Y., Ki SY., Giacomell AO., et al. (2013). Systematic interrogation of 3q26 identifies TLOC1 and SKIL as cancer drivers. Cancer Discov 3, 1044–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellbert K, Kampfer S, Maly K, Hochholdinger F, Mwanjewe J, Baier G, Uberall F, and Grunicke HH (2000). Implication of atypical protein kinase C isozymes lambda and zeta in Ras mediated reorganization of the actin cytoskeleton and cyclin D1-induction. Adv Enzyme Regul 40, 49–62. [DOI] [PubMed] [Google Scholar]
- Herbst RS, Heymach JV, and Lippman SM (2008). Lung cancer. N Engl J Med 359, 1367–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong Y (2018). aPKC: the Kinase that Phosphorylates Cell Polarity. F1000Res 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang RP, Wu JX, Fan Y, and Adamson ED (1996). UV activates growth factor receptors via reactive oxygen intermediates. J Cell Biol 133, 211–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiguro H, Akimoto K, Nagashima Y, Kojima Y, Sasaki T, Ishiguro-Imagawa Y, Nakaigawa N, Ohno S, Kubota Y, and Uemura H (2009). aPKClambda/iota promotes growth of prostate cancer cells in an autocrine manner through transcriptional activation of interleukin-6. Proc Natl Acad Sci U S A 106, 16369–16374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumi Y, Hirose T, Tamai Y, Hirai S, Nagashima Y, Fujimoto T, Tabuse Y, Kemphues KJ, and Ohno S (1998). An atypical PKC directly associates and colocalizes at the epithelial tight junction with ASIP, a mammalian homologue of Caenorhabditis elegans polarity protein PAR-3. J Cell Biol 143, 95–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson EL, Willis N, Mercer K, Bronson RT, Crowley D, Montoya R, Jacks T, and Tuveson DA (2001). Analysis of lung tumor initiation and progression using conditional expression of oncogenic K-ras. Genes Dev 15, 3243–3248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamieson L, Carpenter L, Biden TJ, and Fields AP (1999). Protein kinase Ciota activity is necessary for Bcr-Abl-mediated resistance to drug-induced apoptosis. J Biol Chem 274, 3927–3930. [DOI] [PubMed] [Google Scholar]
- JE IJ, Vermeulen L, Meijer GA, and Dekker E (2015). Serrated neoplasia-role in colorectal carcinogenesis and clinical implications. Nat Rev Gastroenterol Hepatol 12, 401–409. [DOI] [PubMed] [Google Scholar]
- Jin Z, Xin M, and Deng X (2005). Survival function of protein kinase C{iota} as a novel nitrosamine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone-activated bad kinase. J Biol Chem 280, 16045–16052. [DOI] [PubMed] [Google Scholar]
- Jung HY, Fattet L, Tsai JH, Kajimoto T, Chang Q, Newton AC, and Yang J (2019). Apical-basal polarity inhibits epithelial-mesenchymal transition and tumour metastasis by PAR-complex-mediated SNAI1 degradation. Nat Cell Biol 21, 359–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justilien V, Ali SA, Jamieson L, Yin N, Cox AD, Der CJ, Murray NR, and Fields AP (2017). Ect2-Dependent rRNA Synthesis Is Required for KRAS-TRP53-Driven Lung Adenocarcinoma. Cancer Cell 31, 256–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justilien V, and Fields AP (2009). Ect2 links the PKCiota-Par6alpha complex to Rac1 activation and cellular transformation. Oncogene 28, 3597–3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justilien V, Walsh MP, Ali SA, Thompson EA, Murray NR, and Fields AP (2014). The PRKCI and SOX2 oncogenes are coamplified and cooperate to activate Hedgehog signaling in lung squamous cell carcinoma. Cancer Cell 25, 139–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahl-Rainer P, Karner-Hanusch J, Weiss W, and Marian B (1994). Five of six protein kinase C isoenzymes present in normal mucosa show reduced protein levels during tumor development in the human colon. Carcinogenesis 15, 779–782. [DOI] [PubMed] [Google Scholar]
- Kampfer S., Windegger M., Hochholdinger F., Schwaiger W., Pestell RG., Baier G., Grunicke HH., and Uberall F. (2001). Protein kinase C isoforms involved in the transcriptional activation of cyclin D1 by transforming Ha-Ras. J Biol Chem 276, 42834–42842. [DOI] [PubMed] [Google Scholar]
- Kaser A, Zeissig S, and Blumberg RS (2010). Inflammatory bowel disease. Annu Rev Immunol 28, 573–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khosravi-Far R, Solski PA, Clark GJ, Kinch MS, and Der CJ (1995). Activation of Rac1, RhoA, and mitogen-activated protein kinases is required for Ras transformation. Mol Cell Biol 15, 6443–6453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim CF, Jackson EL, Woolfenden AE, Lawrence S, Babar I, Vogel S, Crowley D, Bronson RT, and Jacks T (2005). Identification of bronchioalveolar stem cells in normal lung and lung cancer. Cell 121, 823–835. [DOI] [PubMed] [Google Scholar]
- Kim DI, Jensen SC, Noble KA, Kc B, Roux KH, Motamedchaboki K, and Roux KJ (2016). An improved smaller biotin ligase for BioID proximity labeling. Mol Biol Cell 27, 1188–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, and Guan KL (2019). mTOR as a central hub of nutrient signalling and cell growth. Nat Cell Biol 21, 63–71. [DOI] [PubMed] [Google Scholar]
- Kim JY, Valencia T, Abu-Baker S, Linares J, Lee SJ, Yajima T, Chen J, Eroshkin A, Castilla EA, Brill LM, et al. (2013). c-Myc phosphorylation by PKCzeta represses prostate tumorigenesis. Proc Natl Acad Sci U S A 110, 6418–6423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KH, and Roberts CW (2016). Targeting EZH2 in cancer. Nat Med 22, 128–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoblich JA (2008). Mechanisms of asymmetric stem cell division. Cell 132, 583–597. [DOI] [PubMed] [Google Scholar]
- Ku SY, Rosario S, Wang Y, Mu P, Seshadri M, Goodrich ZW, Goodrich MM, Labbe DP, Gomez EC, Wang J, et al. (2017). Rb1 and Trp53 cooperate to suppress prostate cancer lineage plasticity, metastasis, and antiandrogen resistance. Science 355, 78–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusne Y, Carrera-Silva EA, Perry AS, Rushing EJ, Mandell EK, Dietrich JD, Errasti AE, Gibbs D, Berens ME, Loftus JC, et al. (2014). Targeting aPKC disables oncogenic signaling by both the EGFR and the proinflammatory cytokine TNFalpha in glioblastoma. Sci Signal 7, ra75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M, and Vasioukhin V (2008). Cell polarity and cancer--cell and tissue polarity as a noncanonical tumor suppressor. J Cell Sci 121, 1141–1150. [DOI] [PubMed] [Google Scholar]
- Leitges M, Sanz L, Martin P, Duran A, Braun U, Garcia JF, Camacho F, Diaz-Meco MT, Rennert PD, and Moscat J (2001). Targeted disruption of the zetaPKC gene results in the impairment of the NF-kappaB pathway. Mol Cell 8, 771–780. [DOI] [PubMed] [Google Scholar]
- Lin D, Edwards AS, Fawcett JP, Mbamalu G, Scott JD, and Pawson T (2000). A mammalian PAR-3-PAR-6 complex implicated in Cdc42/Rac1 and aPKC signalling and cell polarity. Nat Cell Biol 2, 540–547. [DOI] [PubMed] [Google Scholar]
- Lin M, Smith LT, Smiraglia DJ, Kazhiyur-Mannar R, Lang JC, Schuller DE, Kornacker K, Wenger R, and Plass C (2006). DNA copy number gains in head and neck squamous cell carcinoma. Oncogene 25, 1424–1433. [DOI] [PubMed] [Google Scholar]
- Linch M., Goh G., Hiley C., Shanmugabavan Y., McGranahan N., Rowan A., Wong YNS., King H., Furness A., Freeman A., et al. (2017). Intratumoural evolutionary landscape of high-risk prostate cancer: the PROGENY study of genomic and immune parameters. Ann Oncol 28, 2472–2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linch M, Sanz-Garcia M, Rosse C, Riou P, Peel N, Madsen CD, Sahai E, Downward J, Khwaja A, Dillon C, et al. (2014). Regulation of polarized morphogenesis by protein kinase C iota in oncogenic epithelial spheroids. Carcinogenesis 35, 396–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linch M, Sanz-Garcia M, Soriano E, Zhang Y, Riou P, Rosse C, Cameron A, Knowles P, Purkiss A, Kjaer S, et al. (2013). A cancer-associated mutation in atypical protein kinase Ciota occurs in a substrate-specific recruitment motif. Sci Signal 6, ra82. [DOI] [PubMed] [Google Scholar]
- Llado V, Nakanishi Y, Duran A, Reina-Campos M, Shelton PM, Linares JF, Yajima T, Campos A, Aza-Blanc P, Leitges M, et al. (2015). Repression of Intestinal Stem Cell Function and Tumorigenesis through Direct Phosphorylation of beta-Catenin and Yap by PKCzeta. Cell Rep 10, 740–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locasale JW (2013). Serine, glycine and one-carbon units: cancer metabolism in full circle. Nat Rev Cancer 13, 572–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Jamieson L, Brasier AR, and Fields AP (2001). NF-kappaB/RelA transactivation is required for atypical protein kinase C iota-mediated cell survival. Oncogene 20, 4777–4792. [DOI] [PubMed] [Google Scholar]
- Ma L, Tao Y, Duran A, Llado V, Galvez A, Barger JF, Castilla EA, Chen J, Yajima T, Porollo A, et al. (2013). Control of nutrient stress-induced metabolic reprogramming by PKCzeta in tumorigenesis. Cell 152, 599–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay HJ, and Twelves CJ (2007). Targeting the protein kinase C family: are we there yet? Nat Rev Cancer 7, 554–562. [DOI] [PubMed] [Google Scholar]
- Mainardi S, Mijimolle N, Francoz S, Vicente-Duenas C, Sanchez-Garcia I, and Barbacid M (2014). Identification of cancer initiating cells in K-Ras driven lung adenocarcinoma. Proc Natl Acad Sci U S A 111, 255–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manley PW, Caravatti G, Furet P, Roesel J, Tran P, Wagner T, and Wartmann M (2018). Comparison of the Kinase Profile of Midostaurin (Rydapt) with That of Its Predominant Metabolites and the Potential Relevance of Some Newly Identified Targets to Leukemia Therapy. Biochemistry 57, 5576–5590. [DOI] [PubMed] [Google Scholar]
- Martin P, Duran A, Minguet S, Gaspar ML, Diaz-Meco MT, Rennert P, Leitges M, and Moscat J (2002). Role of zeta PKC in B-cell signaling and function. EMBO J 21, 4049–4057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin PM, and Hussaini IM (2005). PKC eta as a therapeutic target in glioblastoma multiforme. Expert Opin Ther Targets 9, 299–313. [DOI] [PubMed] [Google Scholar]
- Meharena HS, Chang P, Keshwani MM, Oruganty K, Nene AK, Kannan N, Taylor SS, and Kornev AP (2013). Deciphering the structural basis of eukaryotic protein kinase regulation. PLoS Biol 11, e1001680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metz PJ, Lopez J, Kim SH, Akimoto K, Ohno S, and Chang JT (2016). Regulation of Asymmetric Division by Atypical Protein Kinase C Influences Early Specification of CD8(+) T Lymphocyte Fates. Sci Rep 6, 19182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirza AN, Fry MA, Urman NM, Atwood SX, Roffey J, Ott GR, Chen B, Lee A, Brown AS, Aasi SZ, et al. (2017). Combined inhibition of atypical PKC and histone deacetylase 1 is cooperative in basal cell carcinoma treatment. JCI Insight 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirza AN., McKellar SA., Urman NM., Brown AS., Hollmig T., Aasi SZ., and Oro AE. (2019). LAP2 Proteins Chaperone GLI1 Movement between the Lamina and Chromatin to Regulate Transcription. Cell 176, 198–212 e115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochly-Rosen D, Das K, and Grimes KV (2012). Protein kinase C, an elusive therapeutic target? Nat Rev Drug Discov 11, 937–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moroishi T, Hayashi T, Pan WW, Fujita Y, Holt MV, Qin J, Carson DA, and Guan KL (2016). The Hippo Pathway Kinases LATS1/2 Suppress Cancer Immunity. Cell 167, 1525–1539 e1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscat J, and Diaz-Meco MT (2000). The atypical protein kinase Cs. Functional specificity mediated by specific protein adapters. EMBO Rep 1, 399–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscat J, and Diaz-Meco MT (2009). p62 at the crossroads of autophagy, apoptosis, and cancer. Cell 137, 1001–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscat J, Diaz-Meco MT, Albert A, and Campuzano S (2006). Cell signaling and function organized by PB1 domain interactions. Mol Cell 23, 631–640. [DOI] [PubMed] [Google Scholar]
- Moscat J, Diaz-Meco MT, and Wooten MW (2009). Of the atypical PKCs, Par-4 and p62: recent understandings of the biology and pathology of a PB1-dominated complex. Cell Death Differ 16, 1426–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscat J, Karin M, and Diaz-Meco MT (2016). p62 in Cancer: Signaling Adaptor Beyond Autophagy. Cell 167, 606–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu P, Zhang Z, Benelli M, Karthaus WR, Hoover E, Chen CC, Wongvipat J, Ku SY, Gao D, Cao Z, et al. (2017). SOX2 promotes lineage plasticity and antiandrogen resistance in TP53- and RB1-deficient prostate cancer. Science 355, 84–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray NR, and Fields AP (1997). Atypical protein kinase C iota protects human leukemia cells against drug-induced apoptosis. J Biol Chem 272, 27521–27524. [DOI] [PubMed] [Google Scholar]
- Murray NR, Jamieson L, Yu W, Zhang J, Gokmen-Polar Y, Sier D, Anastasiadis P, Gatalica Z, Thompson EA, and Fields AP (2004). Protein kinase Ciota is required for Ras transformation and colon carcinogenesis in vivo. J Cell Biol 164, 797–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray NR, Kalari KR, and Fields AP (2011). Protein kinase Ciota expression and oncogenic signaling mechanisms in cancer. J Cell Physiol 226, 879–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray NR, Weems J, Braun U, Leitges M, and Fields AP (2009). Protein kinase C betaII and PKCiota/lambda: collaborating partners in colon cancer promotion and progression. Cancer Res 69, 656–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustafi R, Cerda S, Chumsangsri A, Fichera A, and Bissonnette M (2006). Protein Kinase-zeta inhibits collagen I-dependent and anchorage-independent growth and enhances apoptosis of human Caco-2 cells. Mol Cancer Res 4, 683–694. [DOI] [PubMed] [Google Scholar]
- Muthuswamy SK, and Xue B (2012). Cell polarity as a regulator of cancer cell behavior plasticity. Annu Rev Cell Dev Biol 28, 599–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi Y, Duran A, L’Hermitte A, Shelton PM, Nakanishi N, Reina-Campos M, Huang J, Soldevila F, Baaten BJG, Tauriello DVF, et al. (2018). Simultaneous Loss of Both Atypical Protein Kinase C Genes in the Intestinal Epithelium Drives Serrated Intestinal Cancer by Impairing Immunosurveillance. Immunity 49, 1132–1147 e1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi Y., Reina-Campos M., Nakanishi N., Llado V., Elmen L., Peterson S., Campos A., De SK., Leitges M., Ikeuchi H., et al. (2016). Control of Paneth Cell Fate, Intestinal Inflammation, and Tumorigenesis by PKClambda/iota. Cell Rep 16, 3297–3310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayak RC, Hegde S, Althoff MJ, Wellendorf AM, Mohmoud F, Perentesis J, Reina-Campos M, Reynaud D, Zheng Y, Diaz-Meco MT, et al. (2019). The signaling axis atypical protein kinase C lambda/iota-Satb2 mediates leukemic transformation of B-cell progenitors. Nat Commun 10, 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton AC, and Brognard J (2017). Reversing the Paradigm: Protein Kinase C as a Tumor Suppressor. Trends Pharmacol Sci 38, 438–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niederst MJ, Sequist LV, Poirier JT, Mermel CH, Lockerman EL, Garcia AR, Katayama R, Costa C, Ross KN, Moran T, et al. (2015). RB loss in resistant EGFR mutant lung adenocarcinomas that transform to small-cell lung cancer. Nat Commun 6, 6377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niessen MT, Scott J, Zielinski JG, Vorhagen S, Sotiropoulou PA, Blanpain C, Leitges M, and Niessen CM (2013). aPKClambda controls epidermal homeostasis and stem cell fate through regulation of division orientation. J Cell Biol 202, 887–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikiforov MA, Chandriani S, O’Connell B, Petrenko O, Kotenko I, Beavis A, Sedivy JM, and Cole MD (2002). A functional screen for Myc-responsive genes reveals serine hydroxymethyltransferase, a major source of the one-carbon unit for cell metabolism. Mol Cell Biol 22, 5793–5800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono Y, Fujii T, Ogita K, Kikkawa U, Igarashi K, and Nishizuka Y (1988). The structure, expression, and properties of additional members of the protein kinase C family. J Biol Chem 263, 6927–6932. [PubMed] [Google Scholar]
- Ono Y, Fujii T, Ogita K, Kikkawa U, Igarashi K, and Nishizuka Y (1989). Protein kinase C zeta subspecies from rat brain: its structure, expression, and properties. Proc Natl Acad Sci U S A 86, 3099–3103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oser MG, Niederst MJ, Sequist LV, and Engelman JA (2015). Transformation from non-small-cell lung cancer to small-cell lung cancer: molecular drivers and cells of origin. Lancet Oncol 16, e165–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oster H, and Leitges M (2006). Protein kinase C alpha but not PKCzeta suppresses intestinal tumor formation in ApcMin/+ mice. Cancer Res 66, 6955–6963. [DOI] [PubMed] [Google Scholar]
- Paul A, Gunewardena S, Stecklein SR, Saha B, Parelkar N, Danley M, Rajendran G, Home P, Ray S, Jokar I, et al. (2014). PKClambda/iota signaling promotes triple-negative breast cancer growth and metastasis. Cell Death Differ 21, 1469–1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puccini A, and Lenz HJ (2018). Colorectal cancer in 2017: Practice-changing updates in the adjuvant and metastatic setting. Nat Rev Clin Oncol 15, 77–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racz A, Brass N, Heckel D, Pahl S, Remberger K, and Meese E (1999). Expression analysis of genes at 3q26-q27 involved in frequent amplification in squamous cell lung carcinoma. Eur J Cancer 35, 641–646. [DOI] [PubMed] [Google Scholar]
- Regala RP, Davis RK, Kunz A, Khoor A, Leitges M, and Fields AP (2009). Atypical protein kinase C{iota} is required for bronchioalveolar stem cell expansion and lung tumorigenesis. Cancer Res 69, 7603–7611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regala RP, Weems C, Jamieson L, Copland JA, Thompson EA, and Fields AP (2005a). Atypical protein kinase Ciota plays a critical role in human lung cancer cell growth and tumorigenicity. J Biol Chem 280, 31109–31115. [DOI] [PubMed] [Google Scholar]
- Regala RP., Weems C., Jamieson L., Khoor A., Edell ES., Lohse CM., and Fields AP. (2005b). Atypical protein kinase C iota is an oncogene in human non-small cell lung cancer. Cancer Res 65, 8905–8911. [DOI] [PubMed] [Google Scholar]
- Reid MA, Dai Z, and Locasale JW (2017). The impact of cellular metabolism on chromatin dynamics and epigenetics. Nat Cell Biol 19, 1298–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reina-Campos M, Linares JF, Duran A, Cordes T, L’Hermitte A, Badur MG, Bhangoo MS, Thorson PK, Richards A, Rooslid T, et al. (2019). Increased Serine and One-Carbon Pathway Metabolism by PKClambda/iota Deficiency Promotes Neuroendocrine Prostate Cancer. Cancer Cell 35, 385–400 e389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reina-Campos M, Shelton PM, Diaz-Meco MT, and Moscat J (2018). Metabolic reprogramming of the tumor microenvironment by p62 and its partners. Biochim Biophys Acta Rev Cancer 1870, 88–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren R (2005). Mechanisms of BCR-ABL in the pathogenesis of chronic myelogenous leukaemia. Nat Rev Cancer 5, 172–183. [DOI] [PubMed] [Google Scholar]
- Rickman DS, Beltran H, Demichelis F, and Rubin MA (2017). Biology and evolution of poorly differentiated neuroendocrine tumors. Nat Med 23, 1–10. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Boulan E, and Macara IG (2014). Organization and execution of the epithelial polarity programme. Nat Rev Mol Cell Biol 15, 225–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosse C, Lodillinsky C, Fuhrmann L, Nourieh M, Monteiro P, Irondelle M, Lagoutte E, Vacher S, Waharte F, Paul-Gilloteaux P, et al. (2014). Control of MT1-MMP transport by atypical PKC during breast-cancer progression. Proc Natl Acad Sci U S A 111, E1872–1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajan MP, Jurzak MJ, Samuels VT, Shulman GI, Braun U, Leitges M, and Farese RV (2014). Impairment of insulin-stimulated glucose transport and ERK activation by adipocyte-specific knockout of PKC-lambda produces a phenotype characterized by diminished adiposity and enhanced insulin suppression of hepatic gluconeogenesis. Adipocyte 3, 19–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto N, Feng Y, Stolfi C, Kurosu Y, Green M, Lin J, Green ME, Sentani K, Yasui W, McMahon M, et al. (2017). BRAF(V600E) cooperates with CDX2 inactivation to promote serrated colorectal tumorigenesis. Elife 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar S, Bristow CA, Dey P, Rai K, Perets R, Ramirez-Cardenas A, Malasi S, Huang-Hobbs E, Haemmerle M, Wu SY, et al. (2017). PRKCI promotes immune suppression in ovarian cancer. Genes Dev 31, 1109–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scotti ML, Bamlet WR, Smyrk TC, Fields AP, and Murray NR (2010). Protein kinase Ciota is required for pancreatic cancer cell transformed growth and tumorigenesis. Cancer Res 70, 2064–2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengupta A, Duran A, Ishikawa E, Florian MC, Dunn SK, Ficker AM, Leitges M, Geiger H, Diaz-Meco M, Moscat J, and Cancelas JA (2011). Atypical protein kinase C (aPKCzeta and aPKClambda) is dispensable for mammalian hematopoietic stem cell activity and blood formation. Proc Natl Acad Sci U S A 108, 9957–9962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah SP, Roth A, Goya R, Oloumi A, Ha G, Zhao Y, Turashvili G, Ding J, Tse K, Haffari G, et al. (2012). The clonal and mutational evolution spectrum of primary triple-negative breast cancers. Nature 486, 395–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelton PM, Duran A, Nakanishi Y, Reina-Campos M, Kasashima H, Llado V, Ma L, Campos A, Garcia-Olmo D, Garcia-Arranz M, et al. (2018). The Secretion of miR-200s by a PKCzeta/ADAR2 Signaling Axis Promotes Liver Metastasis in Colorectal Cancer. Cell Rep 23, 1178–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shroyer NF., Wallis D., Venken KJ., Bellen HJ., and Zoghbi HY. (2005). Gfi1 functions downstream of Math1 to control intestinal secretory cell subtype allocation and differentiation. Genes Dev 19, 2412–2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh B, Stoffel A, Gogineni S, Poluri A, Pfister DG, Shaha AR, Pathak A, Bosl G, Cordon-Cardo C, Shah JP, and Rao PH (2002). Amplification of the 3q26.3 locus is associated with progression to invasive cancer and is a negative prognostic factor in head and neck squamous cell carcinomas. Am J Pathol 161, 365–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soloff RS, Katayama C, Lin MY, Feramisco JR, and Hedrick SM (2004). Targeted deletion of protein kinase C lambda reveals a distribution of functions between the two atypical protein kinase C isoforms. J Immunol 173, 3250–3260. [DOI] [PubMed] [Google Scholar]
- Spitaler M, Villunger A, Grunicke H, and Uberall F (2000). Unique structural and functional properties of the ATP-binding domain of atypical protein kinase C-iota. J Biol Chem 275, 33289–33296. [DOI] [PubMed] [Google Scholar]
- Su MY, Morris KL, Kim DJ, Fu Y, Lawrence R, Stjepanovic G, Zoncu R, and Hurley JH (2017). Hybrid Structure of the RagA/C-Ragulator mTORC1 Activation Complex. Mol Cell 68, 835–846 e833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L, Song L, Wan Q, Wu G, Li X, Wang Y, Wang J, Liu Z, Zhong X, He X, et al. (2015). cMyc-mediated activation of serine biosynthesis pathway is critical for cancer progression under nutrient deprivation conditions. Cell Res 25, 429–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland KD, Song JY, Kwon MC, Proost N, Zevenhoven J, and Berns A (2014). Multiple cells-of-origin of mutant K-Ras-induced mouse lung adenocarcinoma. Proc Natl Acad Sci U S A 111, 4952–4957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki A, Ishiyama C, Hashiba K, Shimizu M, Ebnet K, and Ohno S (2002). aPKC kinase activity is required for the asymmetric differentiation of the premature junctional complex during epithelial cell polarization. J Cell Sci 115, 3565–3573. [DOI] [PubMed] [Google Scholar]
- Tang Z, Li C, Kang B, Gao G, Li C, and Zhang Z (2017). GEPIA: a web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res 45, W98–W102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi K, Wu LW, Grivennikov SI, de Jong PR, Lian I, Yu FX, Wang K, Ho SB, Boland BS, Chang JT, et al. (2015). A gp130-Src-YAP module links inflammation to epithelial regeneration. Nature 519, 57–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tauriello DVF, Palomo-Ponce S, Stork D, Berenguer-Llergo A, Badia-Ramentol J, Iglesias M, Sevillano M, Ibiza S, Canellas A, Hernando-Momblona X, et al. (2018). TGFbeta drives immune evasion in genetically reconstituted colon cancer metastasis. Nature 554, 538–543. [DOI] [PubMed] [Google Scholar]
- Teis D, Taub N, Kurzbauer R, Hilber D, de Araujo ME, Erlacher M, Offterdinger M, Villunger A, Geley S, Bohn G, et al. (2006). p14-MP1-MEK1 signaling regulates endosomal traffic and cellular proliferation during tissue homeostasis. J Cell Biol 175, 861–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trujillo JI, Kiefer JR, Huang W, Thorarensen A, Xing L, Caspers NL, Day JE, Mathis KJ, Kretzmer KK, Reitz BA, et al. (2009). 2-(6-Phenyl-1H-indazol-3-yl)-1H-benzo[d]imidazoles: design and synthesis of a potent and isoform selective PKC-zeta inhibitor. Bioorg Med Chem Lett 19, 908–911. [DOI] [PubMed] [Google Scholar]
- Uberall F., Hellbert K., Kampfer S., Maly K., Villunger A., Spitaler M., Mwanjewe J., Baier-Bitterlich G., Baier G., and Grunicke HH. (1999). Evidence that atypical protein kinase C-lambda and atypical protein kinase C-zeta participate in Ras-mediated reorganization of the F-actin cytoskeleton. J Cell Biol 144, 413–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogelstein B, Papadopoulos N, Velculescu VE, Zhou S, Diaz LA Jr., and Kinzler KW (2013). Cancer genome landscapes. Science 339, 1546–1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorhagen S, and Niessen CM (2014). Mammalian aPKC/Par polarity complex mediated regulation of epithelial division orientation and cell fate. Exp Cell Res 328, 296–302. [DOI] [PubMed] [Google Scholar]
- Wald FA, Forteza R, Diwadkar-Watkins R, Mashukova A, Duncan R, Abreu MT, and Salas PJ (2011). Aberrant expression of the polarity complex atypical PKC and non-muscle myosin IIA in active and inactive inflammatory bowel disease. Virchows Arch 459, 331–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wali RK, Bissonnette M, Khare S, Aquino B, Niedziela S, Sitrin M, and Brasitus TA (1996). Protein kinase C isoforms in the chemopreventive effects of a novel vitamin D3 analogue in rat colonic tumorigenesis. Gastroenterology 111, 118–126. [DOI] [PubMed] [Google Scholar]
- Walton ZE, Patel CH, Brooks RC, Yu Y, Ibrahim-Hashim A, Riddle M, Porcu A, Jiang T, Ecker BL, Tameire F, et al. (2018). Acid Suspends the Circadian Clock in Hypoxia through Inhibition of mTOR. Cell 174, 72–87 e32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Qian J, Hoeksema MD, Zou Y, Espinosa AV, Rahman SM, Zhang B, and Massion PP (2013). Integrative genomics analysis identifies candidate drivers at 3q26–29 amplicon in squamous cell carcinoma of the lung. Clin Cancer Res 19, 5580–5590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Win HY, and Acevedo-Duncan M (2008). Atypical protein kinase C phosphorylates IKKalphabeta in transformed non-malignant and malignant prostate cell survival. Cancer Lett 270, 302–311. [DOI] [PubMed] [Google Scholar]
- Win HY, and Acevedo-Duncan M (2009). Role of protein kinase C-iota in transformed non-malignant RWPE-1 cells and androgen-independent prostate carcinoma DU-145 cells. Cell Prolif 42, 182–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood LD, Parsons DW, Jones S, Lin J, Sjoblom T, Leary RJ, Shen D, Boca SM, Barber T, Ptak J, et al. (2007). The genomic landscapes of human breast and colorectal cancers. Science 318, 1108–1113. [DOI] [PubMed] [Google Scholar]
- Xu X, Rock JR, Lu Y, Futtner C, Schwab B, Guinney J, Hogan BL, and Onaitis MW (2012). Evidence for type II cells as cells of origin of K-Ras-induced distal lung adenocarcinoma. Proc Natl Acad Sci U S A 109, 4910–4915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JQ, Leitges M, Duran A, Diaz-Meco MT, and Moscat J (2009). Loss of PKC lambda/iota impairs Th2 establishment and allergic airway inflammation in vivo. Proc Natl Acad Sci U S A 106, 1099–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Q, Bermingham NA, Finegold MJ, and Zoghbi HY (2001). Requirement of Math1 for secretory cell lineage commitment in the mouse intestine. Science 294, 2155–2158. [DOI] [PubMed] [Google Scholar]
- Yang YL, Chu JY, Luo ML, Wu YP, Zhang Y, Feng YB, Shi ZZ, Xu X, Han YL, Cai Y, et al. (2008). Amplification of PRKCI, located in 3q26, is associated with lymph node metastasis in esophageal squamous cell carcinoma. Genes Chromosomes Cancer 47, 127–136. [DOI] [PubMed] [Google Scholar]
- Yin N, Liu Y, Khoor A, Wang X, Thompson A, Leitges M, Justilien V, Weems C, Murray N, and Fields A (2019). Protein Kinase Ci and Wnt/β-catenin signaling: Alternative pathways to Kras/Trp53 driven lung adenocarcinoma. Cancer Cell. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zang G, Mu Y, Gao L, Bergh A, and Landstrom M (2019). PKCzeta facilitates lymphatic metastatic spread of prostate cancer cells in a mice xenograft model. Oncogene 38, 4215–4231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang LL., Cao FF., Wang Y., Meng FL., Zhang Y., Zhong DS., and Zhou QH. (2015). The protein kinase C (PKC) inhibitors combined with chemotherapy in the treatment of advanced non-small cell lung cancer: meta-analysis of randomized controlled trials. Clin Transl Oncol 17, 371–377. [DOI] [PubMed] [Google Scholar]
- Zoncu R, Efeyan A, and Sabatini DM (2011). mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol 12, 21–35. [DOI] [PMC free article] [PubMed] [Google Scholar]