Abstract
In order to develop a better understanding of the role environmental toxicants may play in the onset and progression of neurodegenerative diseases, it has become increasingly important to optimize sensitive methods for quickly screening toxicants to determine their ability to disrupt neuronal function. The nematode C. elegans can help with this effort. They have an integrated nervous system producing behavioral function, they provide easy access for molecular studies, they have rapid lifespans, and they are inexpensive models. This study focuses on methods of measuring neurodegeneration involving the dopaminergic system and the identification of compounds with actions that disrupt dopamine function in the model organism Caenorhabditis elegans. Several dopamine-mediated locomotory behaviors, Area Exploration, Body Bends, and Reversals, as well as Swimming-Induced Paralysis and learned 2-Nonanone Avoidance, were compared to determine the best behavioral method for screening purposes. These behavioral endpoints were also compared to morphological scoring of neurodegeneration in the dopamine neurons. We found that in adult worms, Area Exploration is more sensitive than the other behavioral methods for identifying DA-deficient locomotion and is comparable to neuromorphological scoring outputs. For larval stage worms, locomotion was an unreliable endpoint, and neuronal scoring appeared to be the best method. We compared the wild-type N2 strain to the commonly used dat-1p::GFP reporter strains BY200 and BZ555, and we further characterized the dopamine-deficient strains, cat-2 e1112 and cat-2 n4547. In contrast to published results, we found that the cat-2 strains slowed on food almost as much as N2s. Both showed decreased levels of cat-2 mRNA and DA content, rather than none, with cat-2 e1112 having the greatest reduction in DA content in comparison to N2. Finally, we compared and contrasted strengths, limitations, cost, and equipment needs for all primary methods for analysis of the dopamine system in C. elegans.
Keywords: C. elegans, Behavior, Neurodegeneration, Dopamine, cat-2, Locomotion
1. Introduction
As the population ages, a global increase in the occurrence of neurodegenerative and neurological diseases and disorders is expected over the next few decades. Neurodegenerative diseases are predicted to become the second leading cause of death by the year 2040 (WHO, 2004). As the etiology of these diseases is increasingly investigated, a common hypothesis that environmental toxicant exposure plays a role in onset and progression has emerged (Cannon and Greenamyre, 2011; Landrigan et al., 2005). One of the major neurodegenerative diseases thought to have a cause rooted in environmental factors is Parkinson’s disease (PD). Marked by dysfunction of the dopaminergic system in particular, PD results in the loss of dopamine (DA) neurons in the substantia nigra pars compacta and decreased control of motor function (Shulman et al., 2011). The development of PD is linked to familial factors at only ≈10% (Lesage and Brice, 2009), meaning the cause of ≈90% of cases cannot be explained. Another DA-related disease with possible links to environmental factors is Attention Deficit Hyperactivity Disorder (ADHD) (Banerjee et al., 2007; Swanson et al., 2000). ADHD manifests as hyperactivity and disruption of cognitive disorders, such as memory and inhibiting behaviors (Barkley, 1997). While ADHD is strongly linked to genetics at ≈80%, there is still a ≈20% occurrence from factors that remain largely unknown (Faraone et al., 2005).
The theory that environmental toxicant exposure is a risk factor for neurodegenerative disease is gaining empirical support (Chin-Chan et al., 2015; Cicchetti et al., 2009; Coppedè et al., 2006; Modgil et al., 2014; Monte, 2003). Several epidemiological studies have shown correlations between environmental toxicant exposure and dopaminergic neuronal disorders. Elevated serum levels of β-hexachlorocyclohexane (β-HCH) have been found in PD patients, high occupational exposure to pesticides and metals have been linked to an earlier age of PD onset, and organophosphates have been correlated to attention and ADHD in children (Marks et al., 2010; Ratner et al., 2014; Richardson et al., 2009). Environmental release of the pesticide glyphosate has also been correlated to the prevalence of both PD and ADHD (Samsel and Seneff, 2015; Seneff et al., 2015). In a case-control study completed by Tanner et al. (2011), a correlation was discovered between PD and usage of the pesticides rotenone and paraquat. In addition, laboratory research in model systems has confirmed that environmentally relevant toxicants, such as some pesticides, have the ability to disrupt the dopaminergic system (Betarbet et al., 2000; Caito et al., 2013; Jones and Miller, 2008; McCormack et al., 2002). The Betarbet et al. (2000) rat study found that exposure to the pesticide rotenone can induce dopaminergic neurodegeneration and lead to the development of multiple symptoms characteristic of PD. In another study, exposure to the pesticide paraquat was also able to induce dopaminergic neurodegeneration in mice (McCormack et al., 2002). Some of these same toxicants, as well as others, were found to induce morphologically detectable dopaminergic neurodegeneration in the nematode Caenorhabditis elegans (Caito et al., 2013; González-Hunt et al., 2014; Hartman et al., in press; Negga et al., 2012; Ray et al., 2014), and C. elegans is increasingly used in toxicological and neurotoxicological studies (Avila et al., 2012; Hunt, 2017; Leung et al., 2008). It may also be possible to investigate whether or not putative neurodegenerative compounds can elicit quantifiable behavioral defects in the worm. Behavioral studies in the C. elegans model have been well documented (Hart 2006); many of the behaviors have been extensively characterized and classified by their mediating neurotransmitter (Chase and Koelle, 2007). This study focuses solely on a subset of the behaviors mediated by DA, which has been shown to mediate basal slowing rate, egg-laying, swimming-induced paralysis, and odor avoidance (Hardaway et al., 2012; Kimura et al., 2010; McDonald et al., 2007; Sawin et al., 2000; Vidal-Gadea et al., 2012). Basal slowing rate (BSR), the dopamine-mediated decrease in locomotion that results from mechanosensory perception of a bacterial food source (Sawin et al., 2000), has generally been considered the most promising method for analyzing behavioral changes after toxicant exposure. The Sawin et al. (2000) study utilized the dopamine-deficient C. elegans mutant strain cat-2, which carries a mutation in the tyrosine hydroxylase necessary for DA synthesis (Lints and Emmons, 1999), to analyze behaviors characteristic of depleted DA levels. The authors found that the cat-2 mutant failed to slow forward motion on food. As the decrease in locomotion on food is a characteristic phenotype of wild-type N2 worms and a healthy dopaminergic system in C. elegans, our current study utilizes disruptions of this behavior as a proxy for DA dysfunction.
In addition to observation of changes to dopamine-mediated behaviors, a standard method for assessing dopaminergic alterations in C. elegans is visualization of morphological changes to DA neurons (Nass et al., 2002). While morphological and behavioral methods work relatively well, there are some caveats to both. To increase the utility of this model organism in neurotoxicological studies, we wanted to compare and contrast these approaches. Quantifying changes to neuronal morphology requires the use of specific transgenic worm strains expressing a fluorescent protein such as GFP in the DA neurons. With this morphological method, in order to test effects of toxicants in conjunction with genetic differences, one must either employ RNAi in the transgenic reporter strain, which can be complicated by the relative recalcitrance of neurons to RNAi knockdown in C. elegans, or use strains containing both the fluorescent transgene and the desired mutations, which would need to be created by crossing the transgenic strain with the mutant strain. In addition, there is some concern over whether the visually altered neuronal structure or loss of GFP in the neurons truly corresponds to neuronal degeneration as opposed, for example, to GFP degradation or aggregation. While the first paper to report the use of a transgenic strain to visualize neurodegeneration (Nass et al., 2002) did demonstrate that degeneration identified by fluorescence microscopy was comparable to that observed via transmission electron microscopy, it is not clear that this would be true for all environmental exposures and genetic manipulations. In addition, it is always possible that the transgenic changes to the dopaminergic cells could perturb the normal function of those neurons. Finally and perhaps most importantly, mechanisms of dopaminergic dysfunction that do not involve cellular degeneration would presumably not be detected by this method. With the behavioral method of DA system analysis, possible limitations include the necessity of reaching a threshold of degeneration before physical effects manifest (it is not clear whether behavior or morphological analysis would be more sensitive), uncertain specificity of the assays (i.e., are they controlled only by dopamine), and potential variability in the behaviors due to lab-to-lab variance of environmental factors. Conditions in the environment need to be carefully controlled for when completing behavioral analyses, as worms alter behaviors in response to laboratory conditions, such as light, temperature, and vibrations (Hart 2006).
In this study, we compare the use of morphological changes to the DA neurons and DA-mediated locomotory behaviors as tools for measuring DA system dysfunction in C. elegans, including consideration of sensitivity, specificity, efficiency, and cost-effectiveness. Our initial hypothesis was that behavior would be the most sensitive method for identifying DA dysfunction, because we suspected dopaminergic neurons most likely function at an inadequate level preceding outright morphologically detectable degeneration. We hoped to use the findings in this study to develop a screening tool for assessing the effects of environmental contaminants on the dopaminergic system that can be utilized as a first step prior to mammalian studies, which tend to be costly and time consuming. There is also potential for the assays in this study to be used for screening protective and therapeutic treatments of dopaminergic neurodegeneration. With neurodegenerative diseases increasing in prevalence, uncovering and regulating causative factors has become imperative. We hope that information in this study will inform efforts to discover environmental contributors to dopaminergic neurodegeneration.
2. Materials & Methods
2.1. Strains
Strains Bristol N2(WT), cat-2(e1112), cat-2(n4547), BZ555[egIs1(dat-1p::GFP)], tbh-1(n3247), tdc-1(n3420), cha-1(p503), tph-1(n4622), and UA57[baIs4(dat-1p::GFP + dat-1p::CAT-2)] were obtained from the Caenorhabditis Genetics Center (CGC). Strain BY200[vtIs1(dat-1p::GFP; rol-6)] was obtained from the Aschner Lab; studies used the strain in its original form [outcrossed four times: Nass et al. (2002)] or our lab’s 8X outcrossed population. For our locomotion studies, it is important to note that the rol-6 phenotype in BY200 is rarely present. We typically only see this rolling phenotype in severely stressed populations. Under normal conditions, BY200 worms move in a similar fashion to N2. Each experiment began with a synchronized population of L1 worms, obtained by sodium hydroxide/bleach lysis to harvest eggs and overnight liquid hatch in complete K-medium (Boyd et al., 2009). Worms were grown on K-agar OP50, bacterial food source, plates (Williams and Dusenbery, 1988) until the age specified in each experiment. Strains were maintained in 20°C incubators at all times, unless being used in experimental procedures or transferred at room temperature.
2.2. Video Acquisition
Locomotion videos, 10s each, were recorded using NIS-Elements BR software and a Nikon SMZ1500 stereomicroscope. Before video capture, each agar plate had the lid removed and was allowed to sit on the microscope stage for ≈30s to permit worm acclimation to placement. Lids were removed to avoid visual interference of internal lid condensation. Worms were allowed to acclimate to avoid video capture of tap-response or lid removal induced locomotion.
2.3. Behavioral Analyses
For this study, we developed a low-cost assay we term Area Exploration (AE). This assay functions to calculate the total area covered and measures each newly explored area only once; any areas explored more than once, mostly due to backward and forward motion in the same spot, are not counted as additionally explored areas. The rationale for measuring total AE as opposed to total movement is that worms with normal dopamine function make a large number of small movements within a given patch of food, but rarely leave the area with food until the food is depleted. Thus, if we count this small back-and-forth (in the same area) movement, we would reduce our ability to quantify the inappropriate, away-from-food movement mediated by dopamine deficiency. Therefore, instead, we measure total area explored, which increases as dopamine deficiency reduces the worms’ ability to sense food. AE was assessed using a macro (Figure S1A) created in Fiji (Schindelin et al., 2012) to construct pathways of worms throughout prerecorded 10s locomotion videos (Figure 1). The macro functions by first creating a pathway for each worm using the “Z-project...” function. Once pathways were completed, the “Analyze Particles...” function was used to quantify the area of each pathway. The individual sizes of the worms were also quantified using the same prerecorded videos and a similar macro (Figure S1B). The resulting size data was used for AE normalization purposes; we divided the area values from the AE macro by the correlating worm size values from the Size macro. Normalization to size was used to control for size differences due to treatment (since a growth-delayed/small worm must move more to cover the same area as a large worm), age, light source, and light bending due to the food status/thickness of agar plates.
Figure 1-.
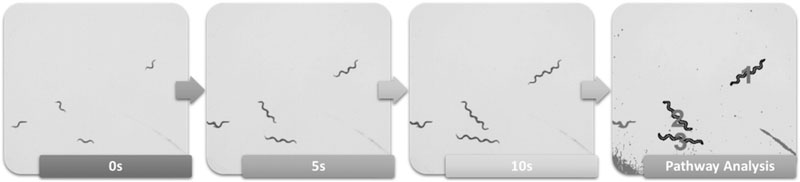
Depiction of Area Exploration Analysis Process. - Pathways are constructed from 10s locomotion videos and analyzed for total area coverage.
For adult strain comparison locomotion studies, age-synchronized populations of worms were grown until reaching the gravid adult stage and then recorded. To avoid any interference of transfer-induced stress, videos of these populations were recorded directly on the growth plates. Sample populations consisted of three biological replicates, defined as experiments performed on different days using worms obtained from different bouts of synchronization. For comparison of locomotion with and without a food source, synchronized gravid adult worms were washed from growth plates, gravity settled, and rinsed (supernatant was removed and replaced with fresh K-medium 2X) to remove any transferred bacteria. Sample populations were then divided between two new agar plates, one with 0P50 and one without any food source. Transferred worms were allowed to dry and acclimate to new plates for 30–60m at room temperature before video acquisition. Acclimation during this step is necessary to avoid video capture of increased movement observed after transfer and to ensure agar plates are dry; this increased movement disappears shortly after the liquid transferred along with the worms evaporates or is absorbed. Agar plates containing excessive surface moisture result in thrashing movements (swimming) and not body bends. Sample populations consisted of five biological replicates.
Body bends (BBs) and Reversals were manually quantified from identical worms using the same 10s locomotion videos as the AE assay. If movement resulted in forward motion and propagated a wave along the body of the worm, it was counted as one body bend. The initiation of backward motion from forward movement or a stopped position was counted as one reversal.
For the DA-mediated swimming induced paralysis (SWIP) (McDonald et al., 2007), ≈10 day 1 adult worms were picked into 300μl molecular grade water in 48 well plates with a platinum wire pick. All worms in a specific well were transferred all together and the start time on a stopwatch was recorded when worms were placed in the liquid. After a 30 minute incubation at room temperature, individual wells were observed under a stereomicroscope, and the number of worms paralyzed and the number of worms in total were recorded. The percentage of worms paralyzed was calculated per well. This experiment consisted of three biological replicates with three technical replicates each.
As another measure of dopaminergic function, the enhancement of odor avoidance to 2-nonanone by preconditioning was used as described (Kimura et al., 2010), with minor adaptations. ≈600–1000 day 1 adult worms were washed off 0P50 plates with K-medium into 15ml conical tubes, allowed to gravity settle, and the supernatant was removed and replaced with fresh K-medium. This process was repeated three times to remove most bacteria from The worms. The worms were then divided among three 60mm “pre-exposure” agar plates by transferring ring 10μl of pelleted worms to each plate. Each of these “pre-exposure” plates had six agar plugs n the lid (at the five points of a star with one plug in the center; Figure S2A). Once the liquid transferred with the worms had completely evaporated from the plates, we added 0.1μL 10% 2-nonanone in ethanol to each plug on one plate lid (“pre-exposed” group), 0.1 μL ethanol to each plug on a. other plate lid (“mock” group), and nothing on each plug of the final plate lid (“naïve” group, The worms were left on these plates for 60 minutes. Following this 60 minute incubation, they were washed off the plates and immediately plated on avoidance assay plates, 60mm agar plates divided vertically into six equal sections by lines drawn on the bottom using a 3D-printed stencil. The stencil included two dots, for odorant placement, spaced equidistant from the center in the second section and a dot, for worm placement, in the center of the plate (Figure S2B–C). The worms were transferred in a drop of liquid (5μL) to the center and allowed to dry (50–150 total worms per plate). When the worms began to crawl away from the center, 0.3μL 10% 2-nonanone in ethanol was added u each odorant dot and the plate was closed and inverted. After a 12 minute incubation, the plate was transferred to the −20°C freezer to prevent further worm movement. (Note: Incubation in the freezer was brief, and plates were not allowed to freeze completely.) After worms slowed further movement, plates were removed from the freezer, and the number of worms in each section was counted and used to calculate the avoidance index. [The avoidance index is a weighted index, which represents the number of worms in each section multiplied by a weight and then summed; the weighted sum is then divided by the total number of worms on the plate. The weights were −2.5 for section 1, −1.5 for section 2 (containing the spots of odorant), −0.5 for section 3, 0.5 for section 4, 1.5 for section 5, and 2.5. for section 6.] The experiments were done with three technical replicates (plates) per group and per pre-exposure condition for each of three biological replicates. For all replicates, the experimenter was blind to strain or treatment.
2.4. Neuronal Scoring
After video acquisition, ≈10 BY200 worms from each treatment group were picked with a platinum wire into one well, per group, of a 96-well plate containing 100μl K-medium and 10μl of a paralytic agent, either 300mM tetramisole hydrochloride (Sigma) or 10μl 200mM sodium azide (Sigma). Once paralysis was complete, fluorescence images of the DA neurons in the head region were captured on a Keyence BZ-X All-in-One Fluorescence Microscope. Using the captured images, the four cephalic (CEP) neurons of paralyzed worms were manually scored (Table 1) based on an adapted version of a previously described scoring system used in our laboratory (González-Hunt et al., 2014). Representative images illustrating morphological alterations used for scoring can be found in Supplemental Figure 3.
Table 1 -.
Neuron Scoring Categories - Explanation of each scoring category used for assessing neurodegeneration through morphological alterations in the transgenic BY200 strain.
| Neuron Scoring Categories |
|---|
| 0 | Normal |
| 1 | Slight Structural Changes (Thin/Crooked/Branched) |
| 2 | Blebs |
| 3 | Breaks |
| 4 | Only CEP Cell Bodies Present |
| 5 | Complete Absence of CEP Neuron |
2.5. cat-2 mRNA Analysis by Real-Time Reverse-Transcription PCR
RNA was isolated from frozen samples of 1000–5000 L4 worms using the Qiagen RNeasy Mini Kit with on-column DNase digestion using the Qiagen RNase-Free DNase Set. RNA concentration was quantified using . Nanodrop 1000 spectrophotometer (Thermo Scientific). Next, RNA was converted to cDNA with the Applied Biosystems High-Capacity cDNA Reverse Transcription Kit. The resulting cDNA samples were diluted .to 2ng/μl in Sigma water and expression levels were quantified using Real-Time PCR with the 7300 Real-time PCR System (Applied Biosystems), PowerSYBR Green PCR Master Mix (Applied Biosystems), and forward/reverse primers (Integrated DNA Technologies) for the cat-2 gene (Figure 2B). Primers were positioned downstream of the e1112 point mutation locus (Figure 2A); this location was chosen to amplify transcripts from all three curated isoforms of cat-2 in C. elegans. Gene expression fold change was calculated by normalizing the cycle threshold (Ct) values for cat-2 to the combined average expression of housekeeping genes cdc-42 and tba-1 (Figure 2B). Samples from five biological replicates were run in triplicate (n = 5, each sample assayed in technical triplicate).
Figure 2 -.
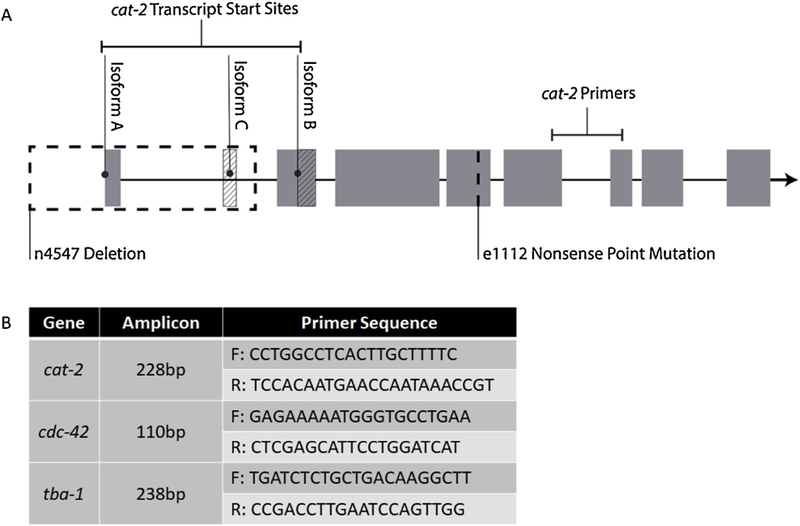
Information on cat-2 mRNA quantification. - (A) Schematic of cat-2 isoforms, locations of cat-2 strains’ mutations, and location of cat-2 primer set. (B) Size and sequence information for primers sets used in mRNA assay. All primers had an annealing temperature of 60°C.
2.6. ELISA Analysis of Dopamine Levels
Frozen samples of ≈2500–11000 L4 worms were thawed and sonicated in TE buffer using a Model 3000 Ultrasonic Homogenizer (Biologics, Inc.). Samples were sonicated at 10% power for 10s three times, and kept on ice between sonication periods. After sonication, 10μ aliquots of each samples were frozen for future protein quantification with Pierce BCA Protein Assay Kit (Thermo Scientific). The remaining sample volume was centrifuged, and the supernatant was used for DA quantification with the ABNOVA ultra-sensitive Dopamine ELISA Kit (KA3838). ELISA results were normalized to protein concentration levels. Samples from seven biological replicates were run in duplicate or triplicate.
2.7. 6-OHDA Dosing
For larval studies, 1000 synchronized L1 worms were incubated at 20°C for 1h in 0, 10, or 15mM 6-hydroxydopamine hydrochloride (6-OHDA). For adult studies, 300 synchronized L4 worms were incubated at 20°C for 1h in 0, 25, 50, or 100mM 6-OHDA. 6-OHDA (Sigma) doses were from a 100mM (or 200mM for high dosing experiment) stock solution dissolved in 20mM (or 40mM for high dosing experiment) ascorbic acid to prevent oxidation; all samples within individual experimental replicates were dosed in equivalent concentrations of ascorbic acid. Due to the rapid oxidation of 6-OHDA, worms were dosed in closed 1.7uL microcentrifuge tubes in the absence of bacteria. After 1h incubation, worms were gently centrifuged [2200 rcf (L1) or 1000 rcf (L4), 2m (L1) or 30s (L4)] and rinsed (aspirated to pellet, resuspended in ≈1mL K medium and re-centrifuged) six times to ensure removal of 6-OHDA. Worms were then plated and allowed to grow on agar plates. 1, 2, or 3d post-exposure, video of worm locomotion on the growth plates was captured. Immediately following video acquisition, on each day, worms were picked for neuron scoring. Larval studies consisted of sample populations from two biological replicates and adult studies consisted of three to nine biological replicates.
2.8. Statistical analysis
All experiments were analyzed using JMP Pro 13 or 14 for Windows (SAS Institute Inc., Cary, NC). Each statistical analysis began with a 1, 2, or 3-way ANOVA, except for Neuronal Scoring, which began with an Ordinal Logistics Chi Squared Whole Model Test. Statistical significance was set at a p-value less than 0.05. Post-hoc tests include Dunnett, Student’s t-test, and Tukey HSD. All bar graphs include error bars constructed using standard error of the mean.
3. Results
3.1. Time Course of Behavioral Changes and Neurodegeneration after 6-OHDA Larval Dosing
We created an analysis of “area explored,” in which we expected that the area covered by a worm on food would increase as dopamine deficiency reduces the worms’ ability to sense the food via mechanosensation. In order to compare sensitivity of AE and neuronal scoring, N2 and BY200 worms were treated with 6-OHDA to induce dopaminergic neurodegeneration. 6-OHDA was our toxicant of choice for inducing neurodegeneration, because it has been ‘hewn to selectively target the dopaminergic system (Nass et al., 2002). Worms were dosed at their first larval stage immediately after hatching and monitored over the course of 3 days for changes to locomotion and changes to neuron morphology. There were no detectable 6-OHDA -induced effects on locomotion on days 1 and 2 (Figure 3A). On day 3, a significant increase in AE can be seen at 10mM 6-OHDA in strain BY200. As expected based on our previous work (González Hunt et al., 2014), however, 10 and 15mM 6-OHDA induced significant dose-related changes to neuron morphology at all timepoints (Figure 3B). Locomotion changes in N2 after 6-OHDA were analyzed simultaneously with BY200. There were no detectable effects of 6-OHDA in N2s (Figure 3C). Plotting the same data in Figure 3C as absolute values rather than percent control (Figure 3D ) highlights the differences in locomotion between the two strains without regard to specific 6-OHlA effects. Strain differences in AE were observed at 0 and 15mM 6-OHDA on Day 3, with N2 moving significantly more than BY200.
Figure 3 -. Time course of behavioral changes and neurodegeneration after 6-OHDA larval dosing.
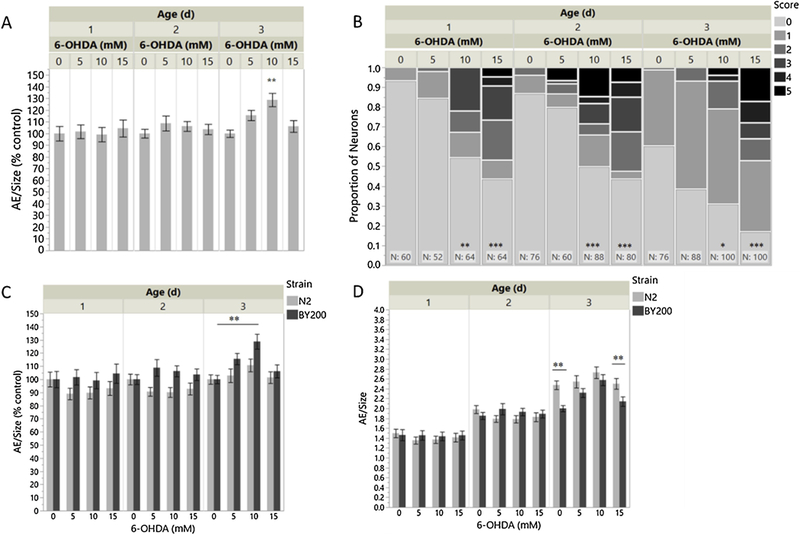
– (A) Area exploration normalized to size and (B) neurodegeneration scores over the course of 3 days following 1h L1 exposure to 6-OHDA in BY200. (C; D) Area exploration normalized to size in N2 and BY200 over the course of 3 days following 1h L1 exposure to 6-OHDA. “AE/Size (% control)” shows significance in comparison to strain-matched 0mM 6-OHDA control. “AE/Size” shows significance in comparison to dose-matched N2 control. All experiments were completed with two biological replicates. (A) n=777; 2-way ANOVA 6-OHDA*Age p=0.0006; Tukey HSD [10mM,3d vs. 0mM,3d p=0.0015]. (B) n=908 neurons/227 worms; 2-way ANOVA 6- OHDA*Age p<0.0001; Tukey HSD [15mM,3d vs. 0mM,3d p<0.0001; 15mM,2d vs. 0mM,2d p<0.0001; 10mM,2d vs. 0mM,2d p<0.0001; 15mM,1d vs. 0mM,1d p<0.0001; 10mM,1d vs. 0mM,1d p=0.0021; 10mM,3d vs. 0mM,3d p=0.0222]. (C) n=1489 3-way ANOVA 6-OHDA *Age*Strain p<0.0001; Tukey HSD [BY200,10mM,3d vs. BY200,0mM,3d p=0.0011]. (D) n=1489 3-way ANOVA 6- OHDA*Age*Strain p<0.0001; Student t-test [N2,0mM,3d vs. BY200,0mM,3d p=0.0003; N2,15mM,3d vs. BY200,15mM,3d p=0.0043].
3.2. Characterization of Locomotion in Adult Worms
We observed no 6-OHDA-induced behavioral changes in early larval stages, even when neurodegeneration was detectable. It may be that movement in larval stages is disrupted or too variable, possibly due to molting lethargus (Cassada and Russell, 1975) or strain-specific developmental rates, for reliable locomotion analysis. However, because comparable locomotion and neurodegeneration effects were seen in the BY200 strain in adults, we next analyzed AE in adult populations in more depth. To ensure we were capturing effects of DA deficiency, two DA deficient mutants were used as positive controls alongside N2 and BY200. The DA-deficient strains carry mutations in cat-2, the tyrosine hydroxylase involved in the production of DA. As locomotion is a DA-dependent behavior, these strains should exhibit altered movement in comparison to WT: specifically, they should exhibit a higher rate of movement in the presence of a food source. The nonsense point mutant cat-2 e1112 (“cat-2_point”) contains a stop codon in the middle of the gene (Figure 2A), has been the more widely used strain in previous locomotion studies, and is well characterized (Lints and Emmons, 1999; Sawin et al., 2000; Yao et al., 2010); the deletion mutant cat-2 n4547 (“cat-2_deletion”) contains a deletion eliminating two of the three transcriptional start sites (Figure 2A), and is a less well-studied strain (Omura et al., 2012). Observation of worms on growth plates revealed increased AE in all strains compared to WT, with the cat-2 point mutation mutant showing the greatest and most significant difference (Figure 4). To further characterize locomotion among adult worms and to compare our lab’s method of locomotion analysis to other established methods, we split the worms from the growth plates into two populations. One-half of the worms were transferred to plates without a food source and the other half were transferred to plates containing a lawn of OP50. All endpoints (AE, BBs, and reversals) were measured using the same video recordings of worm locomotion; BBs and Reversals use the exact same individual worms. In conditions of no food, the cat-2 point mutation strain exhibited a significantly higher rate of AE than WT (Figure 5A). In conditions with food, BY200 and both DA-deficient strains exhibit significantly higher rates of locomotion than WT. BSR [(On-Off)/Off], calculated using AE data on/off food, is significantly lower in BY200 and the cat-2 deletion strain compared to WT, but surprisingly not significantly different in the cat-2 point mutation strain (Figure 5B). In conditions of no food, BY200 exhibits fewer body bends in comparison to WT (Figure 5C). In on-food conditions, BY200 and the DA-deficient strains exhibit a greater number of body bends than WT. The BSR, calculated from BBs on/off food, is significantly less in BY200 and both DA-deficient strains (Figure 5D). In conditions of no food, BY200 exhibits a greater number of reversals than WT (Figure 5E). On food, BY200 and the DA-deficient strains exhibit a smaller number of reversals in comparison to WT, with the most significance seen in the DA-deficient strains.
Figure 4 -.
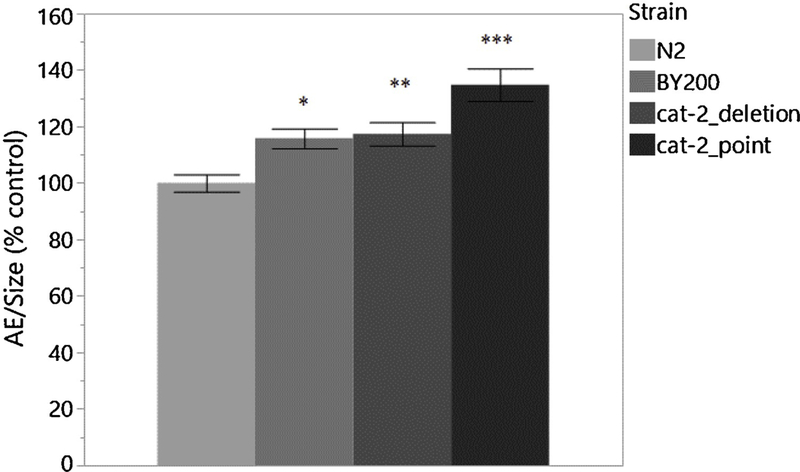
Area exploration in adult worms. - Comparison of AE in WT, BY200, and DA deficient strains after reaching adulthood normalized to N2 control. Experiment was completed with 3 biological replicates. n=4061-way ANOVA Strain p=<0.0001; Dunnett [BY200 vs. N2 p=0.0280; cat-2_deletion vs. N2 p=0.0080; cat-2_point vs. N2 p<0.0001].
Figure 5 -.
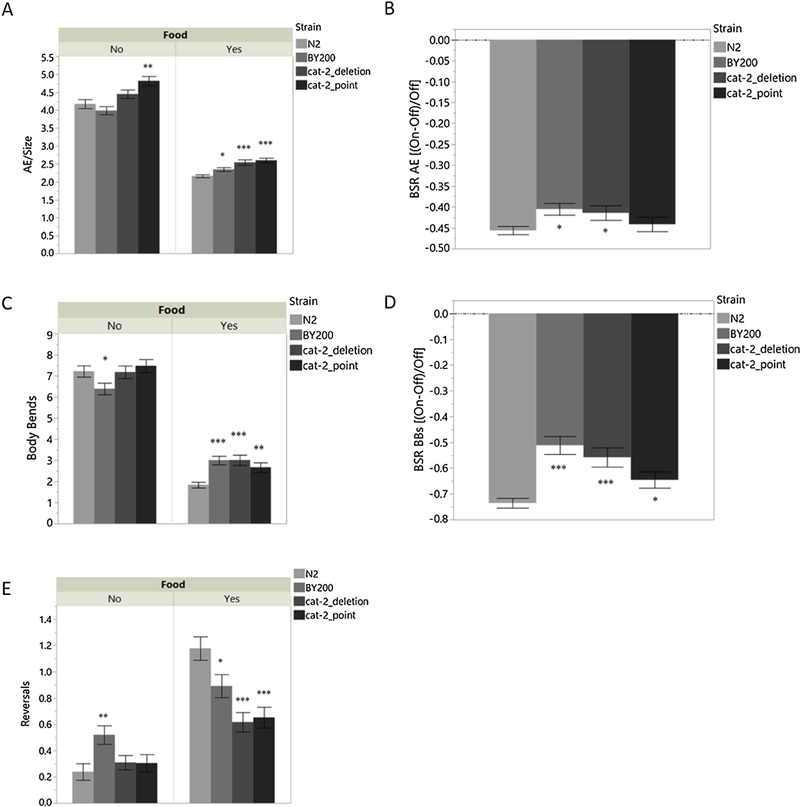
Characterization of locomotion in adult worms. – (A) AE of adult strains On/Off food source. (B) BSR based on AE values. (C) BBs of adult strains On/Off food source. (D) BSR calculated using BBs values. (E) Reversals in adult strains On/Off food source. All analyses methods utilized the same sets of videos. BBs and Reversals utilized the same exact worms. All experiments were completed with 5 biological replicates. (A) n=862 1-way ANOVA Strain [Food=No p<0.0001; Food=Yes p< 0.0001]; Student t-test [(Food=No: cat-2_point vs. N2 p=0.0003) (Food=Yes: cat-2_point vs. N2 p<0.0001; cat-2_deletion vs. N2 p<0.0001; BY200 vs. N2 p=0.0257)]. (B) n=862 1-way ANOVA Strain p=0.0447; Student t-test [BY200 vs. N2 p=0.0123; cat-2_deletion vs. N2 p=0.0380]. (C) n=6511-way ANOVA Strain [Food=No p =.0469; Food=Yes p=< 0.0001]; Student t-test [(Food=No: N2 vs. BY200 p=0.0492) (Food=Yes: cat-2_deletion vs. N2 p<0.0001; BY200 vs. N2 p=0.0001; cat-2_point vs. N2 p=0.0056)]. (D) n=6511-way ANOVA Strain p=<0.0001; Student t-test [BY200 vs. N2 p<0.0001; cat-2_deletion vs. N2 p<0.0001; cat-2_point vs. N2 p=0.0473]. (E) n=6511-way ANOVA Strain [Food=No p=0.0122; Food=Yes p< 0.0001]; Student t-test [(Food=No: BY200 vs. N2 p=0.0025) (Food=Yes: N2 vs. cat-2_deletion p<0.0001; N2 vs. cat-2_point p<0.0001; N2 vs. BY200 p=0.0168)].
In order test the specificity of the AE assay to DA deficiency, we tested other neuronal mutants using this endpoint. We compared basal locomotion in the presence of food between several strains containing mutations in the cholinergic (cha-1), serotonergic (tph-1), tyraminergic (tdc-1), octopaminergic (tdc-1 and tbh-1), and dopaminergic (cat-2_point) neurons. We also included WT and strain UA57, which overexpresses cat-2, to this study. The results of this study show that the AE assay has the ability to detect deficiencies in DA and serotonin production pathways (Figure 6).
Figure 6 -.
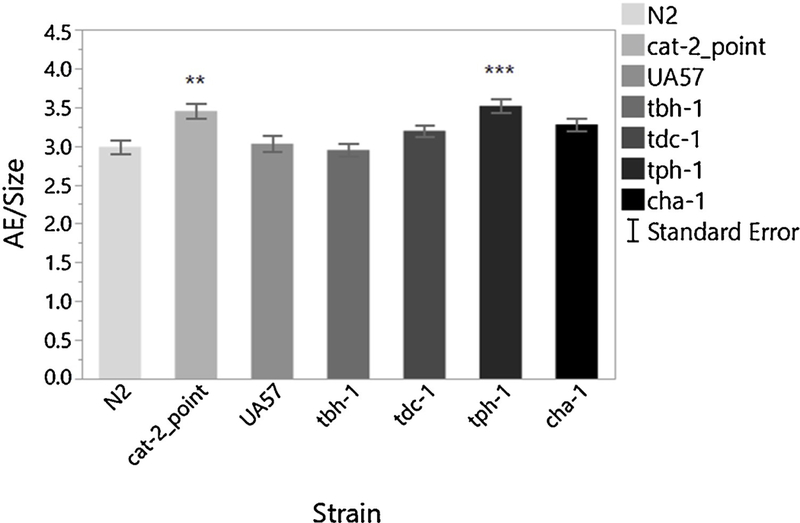
Area Exploration in mutant strains deficient in neurotransmitter production. – The Area Exploration assay is sensitive to decreases in dopamine (cat-2) and serotonin (tph-1). n=778 worms 1-way ANOVA Strain p<0.0001; Dunnett [N2 vs. cat− 2_point p=0.0006; N2 vs. tph-1 p<0.0001].
In the search for a more DA specific assay, we conducted supplementary experiments analyzing other assays that have been described as dopaminergic-dependent: SWIP (McDonald et al., 2007) and learned 2-nonanone avoidance (Kimura et al., 2010). For SWIP analysis, we compared WT, cat-2_point, UA57, tbh-1, and tph-1. We chose to include UA57 and tbh-1 in this assay, because both mutants showed increased SWIP in our preliminary assays, and previous studies have shown SWIP is regulated by increased DA function (Hardaway et al., 2012); tph-1 was chosen due to the results of the neuronal mutation strain comparison AE assay. This assay was able to identify a deficiency in the octopamine synthesis pathway and an upregulation in the DA synthesis pathway, but was not able to identify a difference between cat-2_point and tph-1 (Figure S4). For 2-nonanone avoidance strain comparisons, we chose to compare WT, cat-2_point, and tph-1. The results indicate an enhanced avoidance index after pre-exposure in the WT strain, but neither mutant strain altered their avoidance after pre-exposure to 2-nonanone (Figure S5B). Effects to WT avoidance were also not seen after 50mM 6-OHDA exposure (Figure S5A). It is important to note, several variable methods of analyzing nonanone or nonanol exist (Bargmann et al., 1993; Kaur et al., 2012; Salim et al., 2018; Sammi et al., 2018), such as immediate odor avoidance. However, we decided to use the Kimura et al. (2010) method for assessing DA-mediated learning, because the dopaminergic neurons (which are not chemosensory) play only a supplementary role in immediate avoidance behavior, precluding specificity to dopamine function.
3.3. Molecular Characterization of BY200 and DA-Deficient Strains
Previous experiments in this study identified a difference in locomotion between the DA-deficient strains. One explanation for this is a difference in DA production between the strains. As previously mentioned, one strain carries a nonsense point mutation presumably resulting in nonsense-mediated decay of any mRNA produced, and one carries a deletion mutation. While the point mutation (e1112) affects all three isoforms of the cat-2 gene, the deletion mutant (n4547) only interfere, with two of the curated isoforms. Based on the nature of these mutations and the locomotion observations, in which locomotion was generally more altered in the point mutant, we hypothesized the cat-2 point mutant would have less cat-2 production than the cat-2 deletion mutant and as a result less DA production, making the cat-2 point mutant the optimal strain for representation of a true DA deficiency. In order to identify the strain with the greatest DA deficiency, we first tested potential transcript-level changes by quantifying cat-2 mRNA. This experiment shows expression of cat-2. mRNA is significantly lower in both cat-2 mutant strains, but contrary to our expectations, the decrease in expression was more pronounced in the deletion mutant (Figure 7A). This result suggests that the cat-2 deletion mutant should have lower DA production than the point motion. To try to understand why our locomotion observations were not as expected, based on mRNA content, we directly measured the DA content of each strain. As expected, DA content was decreased in the DA deficient strains (Figure 7B). Contrary to cat-2 expression patterns but consistent with behavioral results, the cat-2 point mutant has somewhat less DA than the deletion mutant.
Figure 7 -.
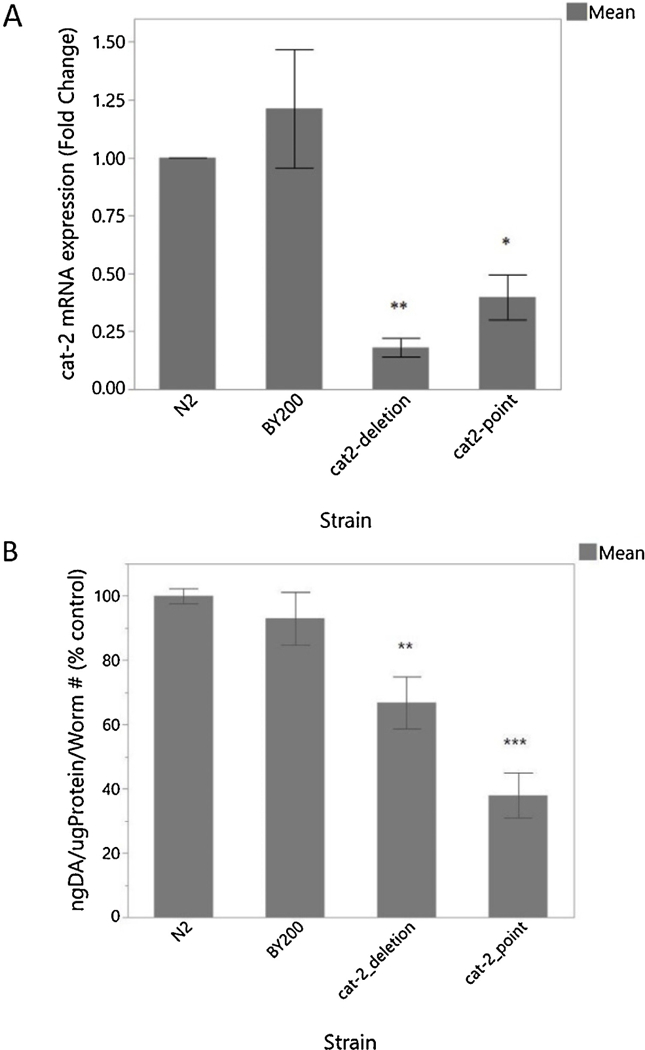
Molecular characterization of BY200 and DA-deficient strains. – (A) RT-PCR mRNA quantification of cat-2 gene expression. (B) ELISA quantification of DA content. RT-PCR was completed with 5 biological replicates and ELISA was completed with 4 biological replicates. (A) n=201-way ANOVA Strain p=0.0002; Student t-test [N2 vs.cat-2_deletion p=0.0007; N2 vs. cat− 2_point p=0.0073]. (B) n=59 1-way ANOVA Strain p<0.0001; Student t-test [N2 vs. cat-2_point p<0.0001; N2 vs. cat-2_deletion p=0.0043].
The apparent trends towards higher cat-2 mRNA and DA content in the BY200 were not statistically significant; at least in the case of the mRNA, this may have been in part because expression was highly variable among biological replicates.
3.4. AE and Neurodegeneration in Adult Worms after 6-OHDA L4 Dosing
Our results so far indicate that AE seems to be the most sensitive method of locomotion analysis in adult worms; AE results also align better with expected results among the DA-deficient strains, based on molecular observations and mutant characteristics. To further test this assay’s sensitivity, we compared AE to neurodegeneration in adult worms after toxicant exposure. For this purpose, we dosed N2, BY200, BZ555, and the cat-2 point mutant with 25 and 50mM 6-OHDA. We did not use the deletion mutant since it showed weaker phenotypes than did the point mutant. We exposed L4s instead of L1s, because development of DA neurons is not complete until the L2 stage when the two posterior deirid neurons, PDE, are formed (Altun et al., 2002–2018), and we wished to ensure exposure of all dopaminergic neurons. However, we found that these neurons do not seem to be affected by 6-OHDA at these concentrations (data not shown); therefore, scoring reflects CEP neurons only, as they are the primary targets of 6-OHDA exposure. AE and neuronal scoring assays were both able to identify significant differences after 6-OHDA treatment in the BY200 strain (Figure 8A–B). BZ555 showed a significant response to 6-OHDA treatment in the neuronal scoring assay (Figure 8B) but did not exhibit any behavioral changes (Figure 8A). There were no significant dose dependent changes to N2 or cat-2 e1112 behavior (Figure 8A).
Figure 8 -.
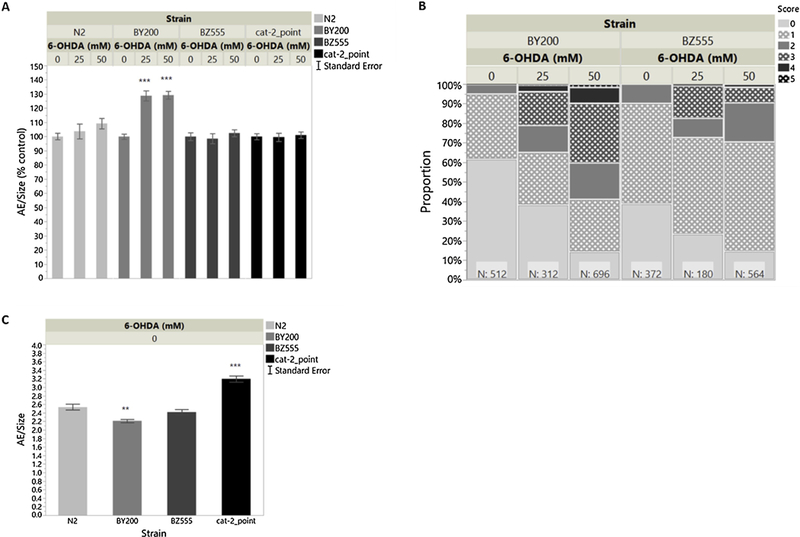
AE and neurodegeneration in adult worms after 1h L4 exposure to 6-OHDA. - (A) Area Exploration/Size normalized to strain matched 0mM 6-OHDA control. “AE/Size (% control)” shows significance in comparison to strain-matched 0mM 6-OHDA control. n= 1970 worms 2-way ANOVA Strain*6-OHDA p<0.0001; Tukey HSD [BY200,25mM vs. BY200,0mM p<0.0001; BY200,50mM vs. BY200,0mM p<0.0001]. (B) Neurodegeneration scores of BY200 and BZ555. n=2636 neurons/659 worms Ordinal Logistics Chi Squared Whole Model Test p<0.0001; Effect Likelihood Ratio Chi Squared Tests [Strain p=0.0234; 6-OHDA p<0.0001; Strain*6-OHDA p<0.0001]. (C) Basal Area Exploration/Size in N2, BY200, BZ555 and the cat-2 point mutant following 1h L4 exposure to 0mM 6-OHDA; these strain underwent the 6-OHDA protocol and were exposure to ascorbic acid only. “AE/Size” shows significance in comparison to dose-matched N2 control. n=759 worms 1-way ANOVA Strain p<0.0001; Dunnett [N2 vs. BY200 p=0.0041; N2 vs. cat-2_point p<0.0001]. Note: All graphs in this figure are a combination of 3 separate experiments, each completed with 3 biological replicates.
4. Discussion
4.1. Area Exploration vs. Neurodegeneration
Significant neurodegeneration was seen during larval stages, but effects of 6-OHDA on locomotion were not easily detected until worms reached adulthood. While the cause of this discrepancy is not currently known, we believe there could be several factors involved. One possibility is that the larvae are not moving much due to molting stage lethargus around the timepoints chosen in this experiment; although, we cannot exclude other possibilities, such as less innate DA signaling mediated behavior at this life stage. Because strains or treatment could result in differential developmental rates and lethargus at different times, AE analysis in larvae could lead to possible false positive or negative results.
In BY200 adults, the effects of 6-OHDA were detectable using either AE or neuronal scoring There was similar dose response using both endpoints. This result, along with the basal AE observed in cat-2 mutants, makes a strong case for AE being a dopamine-mediated behavior. In comparison to BY200, neurodegeneration in BZ555 seemed to respond in a similar, although statistically different, dose dependent manner to 6-OHDA treatment but had no detectable behavioral response The difference in sensitivity, detected by neuronal scoring, between BY200 and BZ555 could be attributed to their genetic difference, as BZ555 does not carry the rol-6 gene. The lack of behavioral response in AE by BZ555 is not as easily explained, as there was detectable neurodegeneration at levels shown to coincide with increased locomotion in BY200. Interestingly, while cat-2 mutants move significantly more than WT under control conditions (as expected), increasing 6-OHDA trea/me.t had no further effect on movement, suggesting that despite the residual DA we detected by ELISA, DA deficiency-mediated increased AE may already be maximal in the cat-2 point mutant.
4.2. Possible Non-Monotonic Dose-Response in Movement
In larvae, we observed an effect of 6-OHDA at 10mM but not 15mM 6-OHDA. This seemingly counterintuitive finding may be explained by processes that have opposite effects on our outcome: decreasing DA function leads to increasing AE, but increasing general toxicity may eventually overcome this increased movement. We were u. able to provide concrete proof that this effect is occurring. We attempted several high dose experiments with L4 dosing and reached a 6-OHDA solubility limit before being able to detect whole organism effects of 6-OHDA. While we do not have proof that this is the case in our experiments, we highlight this possibility to alert other researchers about using exposure levels that are too high, particularly with chemicals with greater acute organismal toxicity than 6-OHDA. Bypassing lower doses that could elicit increased locomotion, could lead to toxicants not being identified by chemical screens. It is thus important to use a range of doses and identify doses that are optimal in terms of detecting an effect.
4.3. AE vs. BBs vs. Reversals
All of the tested methods of analyzing basal locomotion on agar plates were adequate for identifying DA deficiency in on-food conditions. However, AE was the sole method, in this study, for identifying DA deficiency off food. While looking at on-food and off-food conditions individually, AE trends aligned more with expected results, based on measured strain DA content. When comparing BSR, which takes into consideration locomotion in both food conditions, the BSR calculated using BBs seems to be a better identifier of DA deficiency. However, neither method of calculating BSR correlated to DA content. It is important to note that these endpoints were obtained using the same videos/population of worms. Whether the rate of movement of the cat-2 point mutant is higher or lower in comparison to the other strains differs depending on analysis method; the cat-2 point mutant has a significantly higher AE rate than BY200 and the cat-2 deletion mutant on food, but fewer BBs in the same conditions.
Understanding the basis of the differences between AE and BBs would require further analyses of subtle non-speed related behavioral changes, such as bending angle, bend amplitude, and wavelength, may need to be completed to get a clearer idea of the locomotion differences among strains (Angstman et al., 2016).
4.4. Molecular Differences in the DA System among Strains
Published results indicate that cat-2 mutants should not have a difference in locomotion when on food compared to when off food (Sawin et al., 2000). This was not what we observed, perhaps due to differences between lab environments, transfer methods, or the type of bacteria used as a food source. Whatever the reason, we found that the cat-2 mutants slowed when on food almost as much as did N2 worms. However, despite this rather mild phenotype, the two mutant strains were statistically dissimilar to each other as well. This finding led us to inquire about the differences in cat-2 expression among the strains, in order to identify the strain with the greater DA deficiency to use as a positive control for future studies.
In contrast to some previous descriptions (Lints and Emmons, 1999; Omura et al., 2012), neither cat-2 mutant is a null mutant. Of the two mutants, the point mutant shows a stronger phenotype. This is as expected, given that the deletion mutation leaves one of the three isoforms of the cat-2 gene intact. While we chose ELISA determination of DA levels for this study previous HPLC analysis has also shown that the cat-2 point mutant retains about 40% DA levels relative to WT (Sanyal et al., 2004). We hypothesize that the residual DA production could be explained by the presence of tyrosinases, which can bypass the need for cat-2 in the DA production pathway (Rios et al., 1999). In addition to the unexpected DA quantification results, the relatively high level of cat-2 mRNA in the point mutant is also surprising, since this nonsense mutation would be expected to cause nonsense-mediated decay of the transcript. We speculate that the detected mRNA may reflect non-functional transcripts that escape nonsense-mediated decay. Laser ablation or other methods to selectively destroy dopamine-producing cells might permit further determination of the behavioral consequences of complete loss of dopamine function.
4.5. Differences between WT, BY200, and BZ555
A concerning finding in .his study was that strain BY200 seems to be differentially affected by 6-OHDA treatment in comparison to the WT strain. In fact, BY200 was the only strain that showed a behavioral change after 6-OHDA treatment at our standard doses [at a higher concentration, 100mM 6-OHDA, we were able to elicit a response from WT (Figure S6)]. It is unclear if BY200 is more or less sensitive than WT, as we could have missed the optimal effect dose for behavioral changes in the other strains, but this differential response needs to be taken into consideration when identifying compounds as neurodegenerative. In addition to this difference in treatment response, all three methods of locomotion analysis identified a difference in locomotory behavior between N2 and BY200, which may mean BY200 should not be regarded as the “same as WT” when using it for neuronal scoring experiments. The explanation for these differences is unclear, as BY200 was constructed from the N2 strain (Nass et al., 2002). Potential explanations include, but are not limited to, general interference due to the overexpression of the transgene (or its components, pdat-1:GFP and rot-6), such as disruption by the transgene of expression of other RNAs, or an interference of cellular function by copious non-native expression of genetic product.
In an attempt to identify a DA neuron reporter strain more similar to WT, we also tested the BZ555 strain. BZ555 also contains a dat-1p::GFP transgene but does not have the rol-6 marker and is listed on the CGC website (https://cgc.umn.edu/strain/BZ555) as less sensitive to 6-OHDA than BY200. We tested this strain in our 6-OHDA AE studies in conjunction with N2, BY200, and cat-2_point. We found BZ555 does not have a behavioral response to 6-OHDA treatment. This suggests the rol-6 gene mutation, only present in BY200, may be linked to the differential behavioral response. The rol-6 gene mutation leads to a cuticle collagen defect (Kramer et al., 1990), and we speculate it could possibly increase cuticle permeability to toxicant exposure. As previously stated, the reasoning behind the differential behavioral response between BY200 and BZ555 is potentially a little more complex than that, because BZ555 was shown to respond to treatment through neuronal scoring at similar levels of neurodegeneration that induced behavioral changes in BY200. In addition, we observed that BY200 displayed brighter GFP fluorescence than BZ555, and BZ555 neurons tend to appear much thinner in comparison, which is why our scoring system, developed using the bright BY200 strain, detected more minor damage in the control BZ555 groups than BY200.
4.6. Dopamine-and Serotonin-Mediated Behavior
In this study, we used several commonly employed DA-mediated behaviors to attempt to identify one that was truly specific to DA deficiency. AE has been our main behavioral endpoint and readily identified the DA-deficiency in the cat-2 mutant strains through the decrease in basal slowing in the presence of a food source. To test whether this phenotype was specific to DA deficiency, we compared the locomotion of several neurotransmitter deficient strains using the AE assay and found that deficiencies in serotonin were also detectable. This was surprising because the Sawin et al. (2000) study found that the serotonin-mediated form of BSR is the enhanced slowing rate and is only detectable after the worms have undergone conditions of starvation. Trying to tease apart these two neurotransmitter deficiencies has proven to be difficult. Neither the SWIP assay nor the 2-nonanone avoidance assay were able to identify differential phenotypes between cat-2_point and tph-1. Thus, the behavioral assays currently available could serve as a first-tier test of potential dopaminergic dysfunction, but additional research would be required to confirm dopaminergic involvement.
4.7. Comparisons among DA System Function Analyses Methods used in this Study
Table 2 offers comparisons of the primary methods addressed in this study. It highlights significant points to consider when choosing a method of analyzing the dopaminergic system in C. elegans after toxicant exposure. Neuron scoring is generally a low cost, straightforward method of assessing morphological changes to the DA system. In cases where a lab may not have easy access to a fluorescence microscope, the cost would increase significantly. One downside to this assay is the necessity of using strains with transgenic reporter constructs such as the commonly used pdat-1::GFP, which could possibly affect the sensitivity or resistance of the strains to toxicant exposure. Area Exploration, Body Bends, and Reversals all analyze locomotion in a similar manner with slightly different effort requirements. The biggest advantages of one of these locomotion analyses over the others is ease of use and increased automation. AE relies on computer-generated data, thereby relatively minimizing user error and increasing throughput, which is ideal for a screening tool. BBs requires manual counting of user defined bends, which can take a considerable amount of time and vary greatly from researcher to researcher based on their definition of what constitutes a body bend. Reversals are not as automated as AE, but identification and manual counting is simple and straightforward and therefore less subject to user differences. Unfortunately, reversals are not as numerous as forward bends per individual worm, resulting in lower output values and a potentially smaller dynamic range. The last two methods described in Table 2, cat-2 mRNA quantification using RT-PCR and DA content analysis using ELISA, may be less useful as screening tools, considering they both require somewhat costly reagent kits, a high “n,” and a considerable amount of time to complete. However, as these two methods analyze molecular function of the DA system, they can be quite useful for more thorough mechanistic studies of the effects that toxicants, already identified through a screening process, may have on the overall dopaminergic system in C. elegans.
Table 2 -.
Methods of analyzing dopaminergic system function. – This table lists the endpoints used in this study to examine different aspects of the DA system, from a molecular level to behavioral phenotypes. This purpose of this table is to provide insight into the options available and their advantages and disadvantages when assessing the state of the dopaminergic system. Price range depends on availability of assay specific equipment.
| Method | Positives | Negatives | Cost | Assay Specific Reagents |
Assay Specific Equipment |
|---|---|---|---|---|---|
| Neuron Scoring | • Direct visualization of neurons | • Must use strain with transgene insert pdat-1::GFP • Only identifies morphologically-based dopaminergic dysfunction |
Low-Very High | • Paralytic agent ($) | • Fluorescence Scope ($ $ $ $) |
| Area Exploration | • Less prone to user error | • Slightly time-consuming • Difficult to use for multi-strain comparisons at larval stages |
Low - High | • None | • Stereomicroscope with Camera and Video Acquisition Software ($ $ $) |
| Body Bends | • Published standard method | • Very time-consuming • Identification of BBs can be difficult • Difficult to use for multi-strain comparisons at larval stages |
Low - High | • None | • Stereomicroscope with Camera and Video Acquisition Software ($ $ $) |
| Reversals | • Easy to quantify | • Low output values • Difficult to use for multi-strain comparisons at larval stages |
Low- High | • None | • Stereomicroscope with Camera and Video Acquisition Software ($ $ $) |
| cat-2 mRNA Quantification | • Detection of changes at the molecular level | • Requires high number of worms | Moderate - High | • RNeasy Kit with DNase Digestion ($) • cat-2 primers ($) • Power SYBR Green MM ($ $) |
• Real-time PCR System ($ $ $) • Thermocycler ($ $) |
| DA ELISA | • Directly quantifies neurotransmitter changes | • Very time-consuming • Requires high number of worms |
Moderate - High | • ABNOVA DA ELISA Kit ($ $) • BCA Protein Assay Kit ($) |
• Microplate Reader ($ $ $) |
5. Conclusion
While locomotion analysis is a promising tool, it is quite variable and currently not diagnostic for definitive proof of dopaminergic dysfunction. Analyzing behaviors is a quick and easy screening method, but further analyses would be needed to definitively say which specific neuronal system is disrupted. Morphological neurodegeneration appears to be a less variable and a more reliable method, but requires the use of strains containing a specific transgene for visualization. There are many strengths and limitations to consider when choosing between the two methods; locomotion analysis is effective for screening for behavioral changes, but is probably best used either before or in conjunction with neuronal scoring.
In the process of comparing locomotion and neurodegeneration, we found that quantification of cat-2 mRNA content by RT-PCR and quantification of DA content by ELISA also show promise as methods of analyzing DA system function; it remains to be tested whether they will be responsive to chemical challenge. The choice of tool may depend on other aspects of experimental design and logistical constraints (Table 2), and for more in-depth mechanistic analysis, all of the methods taken together may tell a more informative story about what is happening to the DA system after toxicant exposure.
Supplementary Material
Highlights.
Larval stages are inconsistent for locomotion analysis; neuron scoring is more apt.
Dopamine deficient strains retain some basal slowing.
The cat-2 mutants (e1112 and n4547) have differing levels of DA and cat-2 mRNA.
Neither of the cat-2 mutants (e1112 and n4547) is a null strain.
BY200 locomotion is more affected by 6-OHDA than wild-type; BZ555 is unaffected.
Acknowledgments
We would like to thank the Meyer Lab, WormBase, and the Aschner Lab. We thank Dr. Ed Levin for guidance in this research and comments on this manuscript. We would like to thank Dr. Andrey Massarsky for his help in adapting the ELISA assay for use with C. elegans. We would also like to thank Jolán von Plutzner for 3D printing the stencil used in our nonanone avoidance assays. Some strains were provided by the CGC, which is funded by NIH Office of Research Infrastructure Programs (P40 0D010440). This work was supported by the National Institute of General Medical Sciences [(LLS) grant number 5T32GM007105–43] and the National Institutes of Health [(JNM) grant numbers R01ES017540, P42ES010356; (JHH) grant number F32ES027306].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Altun ZF, Herndon LA, Wolkow CA, Crocker C, Lints R, Hall DH, 2002-2018. WormAtlas. http://www.wormatlas.org.
- Angstman NB, Frank H-G, Schmitz C, 2016. Advanced Behavioral Analyses Show that the Presence of Food Causes Subtle Changes in C. elegans Movement. Frontiers in Behavioral Neuroscience 10(60). 10.3389/fnbeh.2016.00060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avila D, Helmcke K, Aschner M, 2012. The Caenorhabiditis elegans model as a reliable tool in neurotoxicology. Human & experimental toxicology 31(3), 236–243. 10.1177/0960327110392084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee TD, Middleton F, Faraone SV, 2007. Environmental risk factors for attention-deficit hyperactivity disorder. Acta Paediatrica 96(9), 1269–1274. doi: 10.1111/j.1651-2227.2007.00430.x [DOI] [PubMed] [Google Scholar]
- Bargmann CI, Hartwieg E, Horvitz HR, 1993. Odorant-selective genes and neurons mediate olfaction in C. elegans. Cell 74(3), 515–527. [DOI] [PubMed] [Google Scholar]
- Barkley RA, 1997. Behavioral inhibition, sustained attention, and executive functions: Constructing a unifying theory of ADHD. Psychological Bulletin 121(1), 65–94. 10.1037/0033-2909.121.1.65 [DOI] [PubMed] [Google Scholar]
- Betarbet R, Sherer TB, MacKenzie G, Garcia-Osuna M, Panov AV, Greenamyre JT, 2000. Chronic systemic pesticide exposure reproduces features of Parkinson’s disease. Nat Neurosci 3(12), 1301–1306. [DOI] [PubMed] [Google Scholar]
- Boyd WA, Smith MV, Kissling GE, Rice JR, Snyder DW, Portier CJ, Freedman JH, 2009. Application of a mathematical model to describe the effects of chlorpyrifos on Caenorhabditis elegans development. PloS one 4(9), e7024–e7024. 10.1371/journal.pone.0007024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caito SW, Valentine WM, Aschner M, 2013. Dopaminergic neurotoxicity of S-ethyl N,N-dipropylthiocarbamate (EPTC), molinate, and S-methyl-N,N-diethylthiocarbamate (MeDETC) in Caenorhabditis elegans. Journal of neurochemistry 127(6), 10.1111/jnc.12349.10.1111/jnc.12349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon JR, Greenamyre JT, 2011. The Role of Environmental Exposures in Neurodegeneration and Neurodegenerative Diseases. Toxicological Sciences 124(2), 225–250. 10.1093/toxsci/kfr239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassada RC, Russell RL, 1975. The dauerlarva, a post-embryonic developmental variant of the nematode Caenorhabditis elegans. Developmental Biology 46(2), 326–342. 10.1016/0012-1606(75)90109-8 [DOI] [PubMed] [Google Scholar]
- Chase DL, Koelle MR, 2007. Biogenic amine neurotransmitters in C. elegans. WormBook. 10.1895/wormbook.1.132.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin-Chan M, Navarro-Yepes J, Quintanilla-Vega B, 2015. Environmental pollutants as risk factors for neurodegenerative disorders: Alzheimer and Parkinson diseases. Frontiers in Cellular Neuroscience 9(124). 10.3389/fncel.2015.00124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchetti F, Drouin-Ouellet J, Gross RE, 2009. Environmental toxins and Parkinson’s disease: what have we learned from pesticide-induced animal models? Trends in Pharmacological Sciences 30(9), 475–483. https://doi.org/10.10167i.tips.2009.06.005 [DOI] [PubMed] [Google Scholar]
- Coppedè F, Mancuso M, Siciliano G, Migliore L, Murri L, 2006. Genes and the Environment in Neurodegeneration. Bioscience Reports 26(5), 341–367. 10.1007/s10540-006-9028-6 [DOI] [PubMed] [Google Scholar]
- Faraone SV, Perlis RH, Doyle AE, Smoller JW, Goralnick JJ, Holmgren MA, Sklar P, 2005. Molecular Genetics of Attention-Deficit/Hyperactivity Disorder. Biological Psychiatry 57(11), 1313–1323. 10.1016/i.biopsych.2004.11.024 [DOI] [PubMed] [Google Scholar]
- González-Hunt CP, Leung MCK, Bodhicharla RK, McKeever MG, Arrant AE, Margillo KM, Ryde IT, Cyr DD, Kosmaczewski SG, Hammarlund M, Meyer JN, 2014. Exposure to Mitochondrial Genotoxins and Dopaminergic Neurodegeneration in Caenorhabditis elegans. PLoS ONE 9(12), e114459. 10.1371/journal.pone.0114459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardaway JA, Hardie SL, Whitaker SM, Baas SR, Zhang B, Bermingham DP, Lichtenstein AJ, Blakely RD, 2012. Forward genetic analysis to identify determinants of dopamine signaling in Caenorhabditis elegans using swimming-induced paralysis. G3 (Bethesda, Md.) 2(8), 961–975. 10.1534/g3.112.003533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart AC, 2006. Behavior, in: Community, T.C.e.R. (Ed.) WormBook. WormBook; 10.1895/wormbook.1.87.1 [DOI] [Google Scholar]
- Hartman JH, Gonzalez-Hunt C, Hall SM, Ryde I, Caldwell KA, Caldwell GA, Meyer JN, in press. Genetic defects in mitochondrial dynamics in Caenorhabditis elegans impact ultraviolet C radiation-and 6-hydroxydopamine-induced neurodegeneration. International Journal of Molecular Sciences. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt PR, 2017. The C. elegans model in toxicity testing. Journal of applied toxicology: JAT 37(1), 50–59. 10.1002/jat.3357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DC, Miller GW, 2008. The effects of environmental neurotoxicants on the dopaminergic system: A possible role in drug addiction. Biochemical Pharmacology 76(5), 569–581. 10.1016/Lbcp.2008.05.010 [DOI] [PubMed] [Google Scholar]
- Kaur S, Sammi SR, Jadiya P, Nazir A, 2012. RNAi of cat-2, a putative tyrosine hydroxylase, increases alpha synuclein aggregation and associated effects in transgenic C. elegans. CNS & neurological disorders drug targets 11(4), 387–394. [DOI] [PubMed] [Google Scholar]
- Kimura KD, Fujita K, Katsura I, 2010. Enhancement of odor avoidance regulated by dopamine signaling in Caenorhabditis elegans. The Journal of neuroscience : the official journal of the Society for Neuroscience 30(48), 16365–16375. 10.1523/jneurosci.6023-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer JM, French RP, Park EC, Johnson JJ, 1990. The Caenorhabditis elegans rol-6 gene, which interacts with the sqt-1 collagen gene to determine organismal morphology, encodes a collagen. Molecular and Cellular Biology 10(5), 2081–2089. 10.1128/mcb.10.5.2081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landrigan PJ, Sonawane B, Butler RN, Trasande L, Callan R, Droller D, 2005. Early Environmental Origins of Neurodegenerative Disease in Later Life. Environmental health perspectives 113(9), 1230–1233. 10.1289/ehp.7571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesage S, Brice A, 2009. Parkinson’s disease: from monogenic forms to genetic susceptibility factors. Human Molecular Genetics 18(R1), R48–R59. 10.1093/hmg/ddp012 [DOI] [PubMed] [Google Scholar]
- Leung MCK, Williams PL, Benedetto A, Au C, Helmcke KJ, Aschner M, Meyer JN, 2008. Caenorhabditis elegans: an emerging model in biomedical and environmental toxicology. Toxicological sciences : an official journal of the Society of Toxicology 106(1), 5–28. 10.1093/toxsci/kfn121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lints R, Emmons SW, 1999. Patterning of dopaminergic neurotransmitter identity among Caenorhabditis elegans ray sensory neurons by a TGFbeta family signaling pathway and a Hox gene. Development 126(24), 5819–5831. [DOI] [PubMed] [Google Scholar]
- Marks AR, Harley K, Bradman A, Kogut K, Barr DB, Johnson C, Calderon N, Eskenazi B, 2010. Organophosphate pesticide exposure and attention in young Mexican-American children: the CHAMACOS study. Environmental health perspectives 118(12), 1768–1774. 10.1289/ehp.1002056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack AL, Thiruchelvam M, Manning-Bog AB, Thiffault C, Langston JW, Cory-Slechta DA, Di Monte DA, 2002. Environmental Risk Factors and Parkinson’s Disease: Selective Degeneration of Nigral Dopaminergic Neurons Caused by the Herbicide Paraquat. Neurobiology of Disease 10(2), 119–127. 10.1006/nbdi.2002.0507 [DOI] [PubMed] [Google Scholar]
- McDonald PW, Hardie SL, Jessen TN, Carvelli L, Matthies DS, Blakely RD, 2007. Vigorous Motor Activity in Caenorhabditis elegans Requires Efficient Clearance of Dopamine Mediated by Synaptic Localization of the Dopamine Transporter DAT-1. The Journal of Neuroscience 27(51), 14216–14227. 10.1523/jneurosci.2992-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modgil S, Lahiri DK, Sharma VL, Anand A, 2014. Role of early life exposure and environment on neurodegeneration: implications on brain disorders. Translational Neurodegeneration 3(1), 9 10.1186/2047-9158-3-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monte DAD, 2003. The environment and Parkinson’s disease: is the nigrostriatal system preferentially targeted by neurotoxins? The Lancet Neurology 2(9), 531–538. 10.1016/S1474-4422(03)00501-5 [DOI] [PubMed] [Google Scholar]
- Nass R, Hall DH, Miller DM, Blakely RD, 2002. Neurotoxin-induced degeneration of dopamine neuron in Caenorhabditis elegans. Proceedings of the National Academy of Sciences 99(5), 3264–3269. 10.1073/pnas.042497999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negga R, Stuart JA, Machen ML, Salva J, Lizek AJ, Richardson SJ, Osborne AS, Mirallas O, McVey KA, Fitsanakis VA, 2012. Exposure to Glyphosate-and/or Mn/Zn-Ethylene-bis-Dithiocarbamate-Containing Pesticides Leads to Degeneration of γ-Aminobutyric Acid and Dopamine Neurons in Caenorhabditiselegans. Neurotoxicity Research 21(3), 281–290. 10.1007/s12640-011-9274-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omura DT, Clark DA, Samuel ADT, Horvitz HR, 2012. Dopamine Signaling Is Essential for Precise Rates of Locomotion by C. elegans. PLOS ONE 7(6), e38649. 10.1371/journal.pone.0038649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratner MH, Farb DH, Ozer J, Feldman RG, Durso R, 2014. Younger age at onset of sporadic Parkinson’s disease among subjects occupationally exposed to metals and pesticides. Interdisciplinary toxicology 7(3), 123–133. 10.2478/intox-2014-0017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray A, Martinez BA, Berkowitz LA, Caldwell GA, Caldwell KA, 2014. Mitochondrial dysfunction, oxidative stress, and neurodegeneration elicited by a bacterial metabolite in a C. elegans Parkinson’s model. Cell Death & Amp; Disease 5, e984 10.1038/cddis.2013.513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson JR, Shalat SL, Buckley B, Winnik B, O’Suilleabhain P, Diaz-Arrastia R, Reisch J, German DC, 2009. Elevated serum pesticide levels and risk of Parkinson disease. Archives of neurology 66(7), 870–875. 10.1001/archneurol.2009.89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rios M, Habecker B, Sasaoka T, Eisenhofer G, Tian H, Landis S, Chikaraishi D, Roffler-Tarlov S, 1999. Catecholamine Synthesis is Mediated by Tyrosinase in the Absence of Tyrosine Hydroxylase. The Journal of Neuroscience 19(9), 3519–3526. 10.1523/jneurosci.19-09-03519.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salim C, Thadathil N, Muralidhara M, Rajini PS, 2018. Insights on the age dependent neurodegeneration induced by Monocrotophos, (an organophosphorous insecticide) in Caenorhabditis elegans fed high glucose: Evidence in wild and transgenic strains. Comparative biochemistry and physiology. Toxicology & pharmacology : CBP 211, 15–24. 10.1016/j.cbpc.2018.05.002 [DOI] [PubMed] [Google Scholar]
- Sammi SR, Agim ZS, Cannon JR, 2018. From the Cover: Harmane-Induced Selective Dopaminergic Neurotoxicity in Caenorhabditis elegans. Toxicol Sci 161(2), 335–348. 10.1093/toxsci/kfx223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samsel A, Seneff S, 2015. Glyphosate, pathways to modern diseases III: Manganese, neurological diseases, and associated pathologies. Surgical Neurology International 6, 45 10.4103/2152-7806.153876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanyal S, Wintle RF, Kindt KS, Nuttley WM, Arvan R, Fitzmaurice P, Bigras E, Merz DC, Hébert TE, van der Kooy D, Schafer WR, Culotti JG, Van Tol HHM, 2004. Dopamine modulates the plasticity of mechanosensory responses in Caenorhabditis elegans. The EMBO journal 23(2), 473–482. 10.1038/sj.emboj.7600057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawin ER, Ranganathan R, Horvitz HR, 2000. C. elegans Locomotory Rate Is Modulated by the Environment through a Dopaminergic Pathway and by Experience through a Serotonergic Pathway. Neuron 26(3), 619–631. 10.1016/S0896-6273(00)81199-X [DOI] [PubMed] [Google Scholar]
- Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez J-Y, White DJ, Hartenstein V, Eliceiri K, Tomancak P, Cardona A, 2012. Fiji: an open-source platform for biological-image analysis. Nature Methods 9, 676 10.1038/nmeth.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seneff S, Swanson N, Li C, 2015. Aluminum and Glyphosate Can Synergistically Induce Pineal Gland Pathology: Connection to Gut Dysbiosis and Neurological Disease. Agricultural Sciences Vol.06No.01, 29 10.4236/as.2015.61005 [DOI] [Google Scholar]
- Shulman JM, Jager PLD, Feany MB, 2011. Parkinson’s Disease: Genetics and Pathogenesis. Annual Review of Pathology: Mechanisms of Disease 6(1), 193–222. doi: 10.1146/annurev-pathol-011110-130242 [DOI] [PubMed] [Google Scholar]
- Swanson JM, Flodman P, Kennedy J, Spence MA, Moyzis R, Schuck S, Murias M, Moriarity J, Barr C, Smith M, Posner M, 2000. Dopamine genes and ADHD. Neuroscience & Biobehavioral Reviews 24(1), 21–25. 10.1016/S0149-7634(99)00062-7 [DOI] [PubMed] [Google Scholar]
- Tanner CM, Kamel F, Ross GW, Hoppin JA, Goldman SM, Korell M, Marras C, Bhudhikanok GS, Kasten M, Chade AR, Comyns K, Richards MB, Meng C, Priestley B, Fernandez HH, Cambi F, Umbach DM, Blair A, Sandler DP, Langston JW, 2011. Rotenone, paraquat, and Parkinson’s disease. Environmental health perspectives 119(6), 866–872. 10.1289/ehp.1002839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal-Gadea AG, Davis S, Becker L, Pierce-Shimomura JT, 2012. Coordination of behavioral hierarchies during environmental transitions in Caenorhabditis elegans. Worm 1(1), 5–11. 10.4161/worm.19148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO, 2004. Atlas : Country resources for neurological disorders 2004. Neurology Atlas, 51. [Google Scholar]
- Williams PL, Dusenbery DB, 1988. Using the Nematode Caenorhabditis Elegans To Predict Mammalian Acute Lethality To Metallic Salts. Toxicology and Industrial Health 4(4), 469–478. 10.1177/074823378800400406 [DOI] [PubMed] [Google Scholar]
- Yao C, El Khoury R, Wang W, Byrd TA, Pehek EA, Thacker C, Zhu X, Smith MA, Wilson-Delfosse AL, Chen SG, 2010. LRRK2-mediated neurodegeneration and dysfunction of dopaminergic neurons in a Caenorhabditis elegans model of Parkinson’s disease. Neurobiology of Disease 40(1), 73–81. 10.1016/j.nbd.2010.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


