Abstract
Background:
Exposure to environmental chemicals, including organophosphorus pesticides, is associated with behavioral disorders such as attention deficit hyperactivity disorder (ADHD). However, the impact of occupational pesticide exposure on ADHD development in adolescents has not been examined.
Objective:
We examined the association between exposure to chlorpyrifos and ADHD symptoms among adolescents in Egypt.
Methods:
Adolescent pesticide applicators and non-applicators, 12-21 years old, participated in a 10-month longitudinal study examining health effects from pesticide exposure. Repeated urine and blood samples were collected at various time points during the 10-months to assess biomarkers of chlorpyrifos exposure (urinary trichloro-2-pyridinol or TCPy) and effect (blood acetyl cholinesterase activity and butyryl cholinesterase activity). Parents from a subset of the cohort (N=64) completed the Short Form of Conners’ Parent Rating Scale – Revised. Poisson regressions were used to examine the associations between the number of ADHD symptoms and occupation and biomarkers.
Results:
Pesticide applicators had significantly more symptoms of ADHD than participants in the non-applicator group. Urinary TCPy levels were associated with increased symptoms, demonstrating a dose-response effect. Applicators with ADHD reported applying pesticides for more hours during the application season and had greater cumulative TCPy levels than participants without ADHD. One fourth of all applicators met the criteria for an ADHD diagnosis (having 6 or more reported symptoms).
Conclusions:
This study provides preliminary evidence of an association between occupational exposure to chlorpyrifos and ADHD symptoms among adolescent pesticide applicators in spite of its limited small sample size. There is a critical need to investigate the susceptibility of children and adolescents to repeated occupational and environmental exposures to pesticides because the developing brain may be uniquely sensitive to the neurotoxic effects of these agents.
Keywords: Organophosphate pesticides, ADHD, adolescent
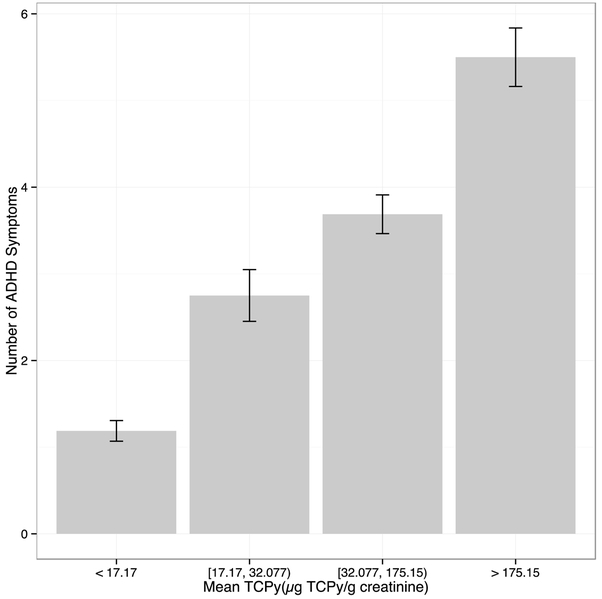
Graphical abstract
Exposure dependent increase in the number of parental reported ADHD symptoms in adolescent pesticide applicators by quartile of the average of participants’ TCPy levels. Errors bars represent one standard error.
1.1. INTRODUCTION
Exposures to environmental chemicals have an adverse impact on child neurodevelopment (Grandjean and Landrigan 2006, 2014). These outcomes include subtle deficits in attention and memory, decreased IQ, learning disabilities, autism spectrum disorder, and attention deficit hyperactivity disorder or ADHD (Rauh and Margolis 2016). Exposure during critical periods of development (i.e., in utero and early childhood), even at levels not harmful to adults, can have lasting impact on children’s brain development, productivity, and have financial impacts to society (Bellinger 2009; Rauh and Margolis 2016; Rice and Barone 2000). Recent studies have focused on the association between environmental exposures and ADHD, including exposure to organophosphate pesticides (Maryse F. Bouchard et al. 2010; Gonzalez-Alzaga et al. 2014; Polanska et al. 2013; Rauh et al. 2011; van Wendel de Joode et al. 2016; CJ Yu et al. 2016). ADHD is a behavioral disorder characterized by pervasive symptoms of inattention, impulsivity and hyperactivity (DSM-V) (APA 2000).
Both pre- and postnatal exposures to organophosphorus pesticides (OPs) have been associated with increased risk of ADHD. Cohort studies have found that maternal exposure to OPs during pregnancy, measured through either blood or urinary pesticide metabolites (i.e., trichloro-2-pyridinol, TCPy or dialkylphosphate metabolites, DAPs), was associated with increased risk of ADHD in children (elevated ORs of 1.3 to 6.5) (Marks et al. 2010; Rauh et al. 2006). Cross-sectional studies have examined exposure to OPs in children via urinary metabolite levels and found a similar increase in the risk of ADHD among more highly exposed children (M. F. Bouchard et al. 2010; Suarez-Lopez et al. 2013; van Wendel de Joode et al. 2016). Higher urinary pesticide metabolite levels were also found among children with a diagnosis of ADHD compared to children with no diagnosis (C-J Yu et al. 2016). Animal studies provide additional evidence of an association between exposure and ADHD symptoms (Dam et al. 2000; Eaton et al. 2008; Johnson et al. 1998; Moser and Padilla 1998; Slotkin 2005). However, the impact of occupational pesticide exposure among adolescents has not been examined. Adolescence is considered a critical developmental period when the pattern of synaptic connections is being formed (Giedd 2004; Rice and Barone 2000).
Worldwide prevalence rates of ADHD range from 3% to 9% among school-age children (Faraone et al. 2003; Froehlich et al. 2007). A number of studies have explored the prevalence of ADHD and risk factors among Egyptian school children (Aboul-Ata and Amin 2015; Attia et al. 2000; El-Nemr et al. 2015; El-Tallawy et al. 2005; Farahat et al. 2014; Saad et al. 2015; Soliman et al. 2010) and young adults (Abd El-Hay and El Sawy 2011). The prevalence of ADHD ranged from 6.0-7.5%, except for one study of school children living in a rural region which reported a prevalence of 20.5% (Aboul-Ata and Amin 2015). All studies reported a higher prevalence for males compared to females. Other risk factors identified included social and demographic variables such as low socioeconomic level, neonatal problems, and family history of psychiatric or medical conditions. None of these studies examined the relationships between ADHD and environmental or occupational exposures.
The objective of the current study is to examine the relationship between ADHD symptoms and exposure to chlorpyrifos among Egyptian adolescents. Specifically, to examine the association by occupational group (applicators vs. non-applicators), the study assessed the associations between TCPy and ADHD symptoms, and the associations between ADHD symptoms and cholinesterase activity (BChE and AChE).
2.1. METHODS
2.1.1. Participants
Adolescents, 12-21 years of age, working as pesticide applicators for the Ministry of Agriculture were recruited in 2010 to participate in a 10-month longitudinal study examining health effects from pesticide exposure across an application season (Crane et al. 2013; AA Ismail et al. 2017; Khan et al. 2014; Rohlman et al. 2016). Adolescents working as pesticide applicators at two field stations were invited to participate in the longitudinal study (N= 59). A convenience sample of adolescents living in the same communities, but not working for the Ministry of Agriculture, were recruited (N=39). As part of the larger longitudinal study, participants completed a neurobehavioral test battery and urine and blood samples were collected at various time points to assess biomarkers of exposure and effect. The Study was approved by the Institutional Review Board at Oregon Health and Science University in June 2009 and by the Medical Ethics committee of the Faculty of Medicine at Menoufia University in July 2009. Study procedures were described to both adolescents and parents. Written informed consent was obtained from participants who were 18 years or older and parents of youth under 18. Youth under 18 also provide written assent.
2.1.2. Occupational Pesticide Exposures
Pesticide application to the cotton crop is highly regulated by the Egyptian Ministry of Agriculture. The national government purchases and manufactures the country’s entire cotton production. Once farmers agree to plant cotton, applications of chemicals on those fields come under control of the Ministry of Agriculture. Thus, all pesticides, equipment and calibration procedures are standardized across the Governorate. Adolescents are hired by the Ministry as seasonal workers and may work for repeated seasons. Applicators work in teams of 3-4 with adult supervisors and may mix pesticides, hold flag markers in the field, or wear backpack sprayers and apply pesticides to the cotton fields. Limited personal protective equipment (PPE) is available or used. In addition, adolescents may also apply pesticides on family fields or work as private applicators. The application season begins in early June and continues through the end of August. Chlorpyrifos (CPF) is the primary pesticide applied to the cotton crop and is applied daily for up to 4 weeks. It is generally followed by an application of profenofos (PFF) another OP, then an application of either one or more pyrethorids, alpha-cypermethrin (αCM) or lambda-cyhalothrin (λCH). Finally, a second application of CPF is applied in August (Crane et al. 2013; Farahat et al. 2011; Singleton et al. 2014).
2.1.3. ADHD Questionnaire
Parents were asked to complete the Short Form of Conners’ Parent Rating Scale – Revised (CPRS-R-S; (Conners 2001). The Arabic version of the scale was validated among Egyptian children and adolescents (El-Defrawi et al. 1992) and has been used to assess attention deficit hyperactivity disorders associated with various types of health conditions among Arabic speaking populations (e.g. magnesium deficiency, (El Baza et al. 2016); serum iron deficiency, (Seleem et al. 2014)). The CPRS-R-S Scale consists of 27 items describing behaviors predictive for ADHD. Parents rated how often they observed each item during the last six months from 0 (never or rarely) to 3 (very often). An item was coded as “present” if rated a 2 or a 3, and “absent” if rated a 0 or 1. Odd number items are summed to form the inattention score, even number items are summed to form the hyperactivity-impulsivity score. The presence of six or more items in either domain is equivalent to the DSM-IV threshold of six symptoms for an ADHD diagnosis (APA 2013). Among the 98 participants in the longitudinal study, parents of 64 participants completed the ADHD questionnaire during the last test session in January, approximately four and half months after the application season ended.
2.1.4. Biomarkers of Pesticide Exposure
From April 2010 to January 2011, urine samples were collected at 35 time points. The samples were collected at the field station at the beginning of work shift and stored in a cooler with wet ice until transported to the laboratory at Menoufia University at the end of the test session. In the laboratory, the samples were divided into two 5 ml cryovials; one to be shipped to the University at Buffalo for analysis and one to be stored at −20 °C at Menoufia University laboratory. The shipped cryovials were then express mailed to the University at Buffalo laboratory for analysis of pesticide metabolites. The method for analysis of urinary TCPy was described elsewhere (Crane et al. 2013; Farahat et al. 2011), where, urinary TCPy was measured using negative-ion chemical ionization gas chromatography-mass spectrometry (GC-MS) using 13C-15N-3,5,6-TCPy as an internal standard. The within run imprecision of this assay is very low, as shown by a < 2% coefficient of variation and an intraclass correlation coefficient of 0.997. Colorimetric analysis of creatinine was done by the Jaffe reaction (Fabiny and Ertingshausen 1971) and urine TCPy concentrations are expressed as μg TCPy/g creatinine. Cumulative TCPy levels were calculated for each participant by calculating the area under the curve over the 10 months study period (Khan et al. 2014). Cumulative TCPy values were then combined into 4 categories based on their first quartile, median, and the third quartile which were 17.17, 32.08, and 175.15, respectively. There were 16 participants in each category.
2.1.5. Biomarkers of Cholinesterase Inhibition
Blood samples were collected at 5 time points to be analyzed for AChE and BChE activity, a biomarker of effect for OP exposure, across the 10 months of the study. The first two samples were collected prior to the first application cycle (April 11 and June 1), the third sample was collected at the end of the first application cycle of CPF (June 16), the fourth sample was collected at the end of the application season (August 31), and the fifth sample was collected 4 months after pesticide application ended (January 1, 2011). The blood samples were collected into 10-mL lavender top (EDTA) Vacutainer tubes through venipuncture. The tubes were immediately kept in wet ice and transferred to Menoufia University. The samples were then analyzed in duplicate for AChE and BChE using the EQM Test-Mate kit (EQM Research, Cincinnati, OH, USA). This method is based on the Ellman method (Ellman et al. 1961), and described in details in a previous publication (Crane et al. 2013; Farahat et al. 2011).
For the purpose of this study, the maximum change in both AChE and BChE activity were calculated by subtracting the lowest value from the two blood samples collected during the application cycle (time 3 or time 4) from a baseline level. Blood samples from time 1 or time 2, which were collected prior to the first application cycle, or time 5, which was collected 4 months after the end of the application cycle were used as the baseline measure. This change in cholinesterase activity level was used to examine the relationship between the changes in cholinesterase activity and ADHD symptoms.
3.1. STATISTICAL ANALYSIS
The group differences between applicators and non-applicators regarding sociodemographic characteristics, occupational history and biomarkers were tested using the appropriate statistical tests. T-test was used to examine the differences in quantitative variables e.g. age, education, and years worked in pesticide application between applicators and non-applicators. The likelihood of being classified as having ADHD (i.e., having six or more symptoms present) among adolescent pesticide applicators in relation to non-applicators was evaluated using Fisher’s exact test. Fisher’s exact test was also used to examine the differences between types of ADHD diagnosis (i.e., inattention, hyperactivity, mixed). Poisson regression was used to investigate the association between the number of ADHD symptoms and the biomarkers of exposure (TCPy) and effect (AChE and BChE) after controlling for job status (applicator vs. non-applicator) and age.
4.1. RESULTS
There was no difference between the applicators working for the Ministry of Agriculture and the non-applicators on age or years of education (Table 1). The applicators reported working for the Ministry of Agriculture an average of 2.7 years. Both applicators (76%) and non-applicators (52%) reported applying pesticides at home. Applicators had significantly higher, 14-fold greater cumulative urinary TCPy level compared to non-applicators, and a significantly greater decrease in both BChE (p < 0.001) and AChE (p < 0.01) activity compared to non-applicators.The correlation between percent change in BChE and log(TCPy) was −0.91 (p < 0.0001) and the correlation between percent change in AChE and log(TCPy) was −0.63 (p < 0.0001).
Table 1.
Demographic characteristics, chlorpyrifos biomarker levels and ADHD symptoms among applicators and non-applicators.
| Non Applicators N=25 |
Applicators N=39 |
p-value | |
|---|---|---|---|
| Age in years (mean ± sd) | 16.2 ± 2.3 | 15.8 ± 1.5 | 0.4 |
| Years of Education (mean ± sd) | 9.6 ± 1.8 | 9.5 ± 1.9 | 0.9 |
| Years worked as applicator | -- | 2.7 ± 1.4 | |
| Pesticide Use at home or the garden, % (count) | 52.0 % (13) | 76.3 % (29) | 0.06 |
| Cumulative TCPy levels (μg TCPy/g creatinine) | 2,259 ± 3,417 | 32,115 ± 56,968 | <0.001 |
| Average TCPy levels (μg TCPy/g creatinine) | 35.92 ± 49.83 | 258.32 ± 323.54 | <0.001 |
| Log (Average TCPy levels) | 3.17 ± 0.77 | 4.59 ± 1.53 | <0.001 |
| BChE (percent of change) | −14.91 ± 11.62 | −45.95 ± 36.62 | <0.001 |
| AChE (percent of change) | −5.25 ± 4.50 | −9.78 ± 8.26 | 0.018 |
| Number of ADHD symptoms | 1.44 ± 2.18 | 4.46 ± 4.95 | < 0.001 |
| ADHD criteria for diagnosis, % (count) | 100% (25) | 74.4% (29) | 0.0047 |
| No | 0.0 (0) | 25.6% (10) | |
| Yes | |||
| Types of ADHD disorders, % (count) | |||
| Normal | 100% (25) | 74.4% (29) | 0.0522 |
| Inattention | -- | 7.7% (3) | |
| Hyperactivity | -- | 10.3% (4) | |
| Mixed* | -- | 7.7% (3) |
Values represent the mean ± SE
The presence of 6 or more symptoms in both categories is scored as mixed.
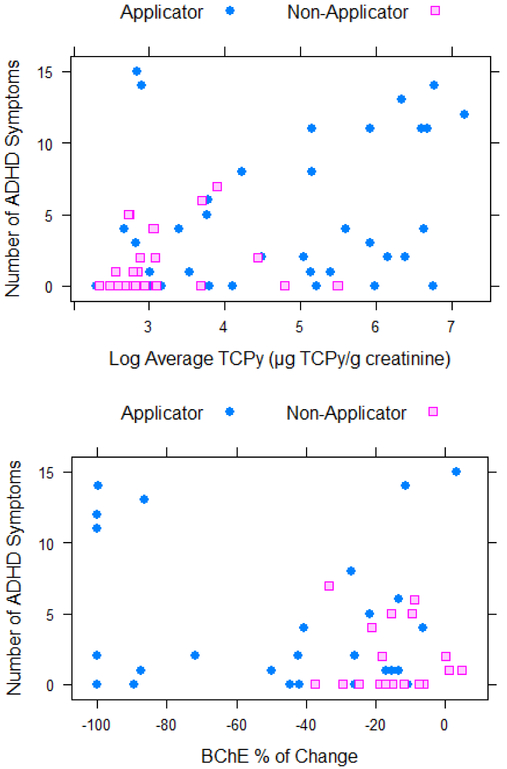
Applicators had significantly more parent reported ADHD symptoms compared to the nonapplicators (p < 0.001; Table 1). Cumulative TCPy levels for all participants were divided into quartiles and a dose-response effect was observed for TCPy levels and number of symptoms (Figure 1). After controlling for job category and age, Poisson regression with log-link demonstrated a significant association between the number of symptoms and the log of TCPy (regression coefficient is 0.24 with standard error 0.049, p < 0.001) after controlling for age and applicator status (Figure 2a). A significant association was also observed between the percent change in AChE (regression coefficient is −4.22 with standard error 0.810, p < 0.001) and percent change in BChE (regression coefficient is −0.597 with standard error 0.23, p = 0.011) after controlling for age, applicator status, and applying pesticides at home (Figure 2b and 2c).
Figure 1.
Exposure dependent increase in the number of parental reported ADHD symptoms by quartile of the average of participants’ TCPy levels. Errors bars represent one standard error.
Figure 2.
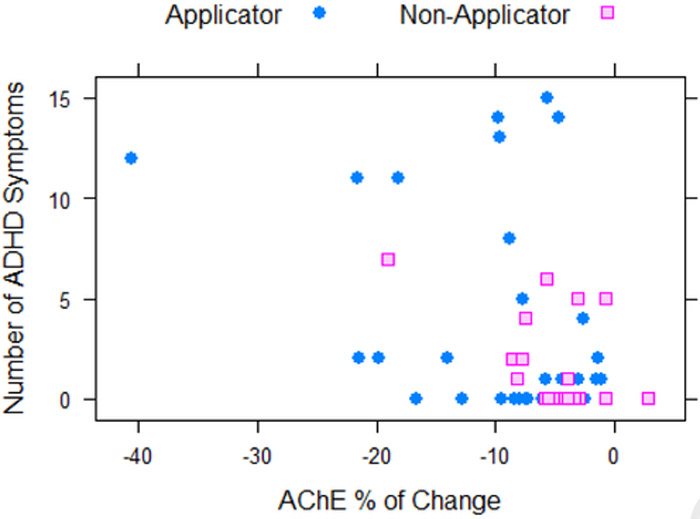
Correlations between number of ADHD symptoms and log cumulative TCPy (2a), BChE percent of change (2b), and AChE percent of change (2c) for Applicators and Non-Applicators.
The Connors’ Rating Scale utilizes a six-item cut-off score, where participants with six or more reported symptoms are equivalent to the DSM-IV threshold of 6 symptoms required for an ADHD diagnosis. Among the 64 adolescents that were included in this analysis, approximately one fourth of the applicators met the criteria of having ADHD disorder (had scores above the six symptoms cut-off; Table 1). None of the non-applicator participants met this threshold. An examination of demographic characteristics and work behaviors between the applicators meeting the criteria for ADHD and those who do not found that there was no difference between the groups on age, years of education, or years worked as an applicator for the Ministry of Agriculture (Table 2). However, applicators meeting the criteria for an ADHD diagnosis had significantly more symptoms (p < 0.001) and had higher average TCPy levels (p < 0.05) compared to the applicators not meeting these criteria. Although not significant, applicators meeting the criteria of ADHD worked more hours during the application season and reported applying more pesticides at home, compared to applicators not meeting the criteria.
Table 2.
Comparison of demographics, biomarkers, and work characteristics between applicators meeting the criteria for an ADHD diagnosis and those not meeting the criteria.
| Applicators without ADHD diagnosis N=29 |
Applicators with ADHD diagnosis N = 10 |
|
|---|---|---|
| Age, years | 15.7 ± 1.6 | 16.1 ± 1.3 |
| Education, years | 9.4 ± 1.9 | 9.8 ± 1.8 |
| Years worked as an applicator | 2.8 ± 1.5 | 2.7 ± 1.2 |
| Cumulative hours worked during the season | 29.5 ± 19.9 | 39.3 ± 25.7 |
| Pesticide Use at home or the garden % (count) | 69.0% (20) | 90.0 % (9) |
| Number of ADHD symptoms | 1.86 ± 2.13 | 12.0 ± 2.05* |
| Average TCPy levels (μg TCPy/g creatinine) | 173.89 ± 235.05 | 503.17 ± 424.87** |
| Log of average TCPy levels (μg TCPy/g creatinine) | 4.26 ± 1.39 | 5.55 ± 1.56 |
| BChE (percent of change) | −38.97 ± 31.27 | −65.15 ± 45.25 |
| AChE (percent of change) | −7.94 ± 5.79 | −14.87 ± 11.92 |
p < 0.001
p < 0.05
5.1. DISCUSSION
As part of a longitudinal study investigating occupational pesticide exposure among adolescents we examined the association between exposure to chlorpyrifos, an organophosphate pesticide, and ADHD symptoms. Biomarkers of exposure and effect, urinary TCPy, a specific metabolite of chlorpyrifos, and cholinesterase activity were used to characterize exposure. Adolescents working as pesticide applicators in Egypt have more symptoms of ADHD than non-applicators. Elevated urinary TCPy levels were associated with a greater number of symptoms and demonstrate a dose-response effect (Figure 1). Furthermore, adolescents working as pesticide applicators were 18 times more likely to be above the threshold for ADHD diagnosis than applicators. Although there was no difference in years worked as a pesticide applicator, adolescents with an ADHD diagnosis reported working significantly more hours than those without an ADHD diagnosis and more of them also reported applying pesticides outside of work.
Previous studies have reported an association between prenatal exposure (Marks et al. 2010; Rauh et al. 2006) and postnatal exposure to organophosphate pesticides and ADHD (M. F. Bouchard et al. 2010; Suarez-Lopez et al. 2013; van Wendel de Joode et al. 2016). However, no study has examined adolescents occupationally exposed to pesticides. Adolescence is a time of rapid development. In addition to the hormonal and physiological changes associated with puberty, there are also significant developmental changes in the brain, primarily in the prefrontal cortex (Crews et al. 2007; Spear 2010; Steinberg 2008) that may make the adolescent brain more vulnerable to environmental toxins. In addition, adolescents working as pesticide applicators have repeated exposures throughout the application season that are generally higher than exposures through diet or environment.
This study expands our previous work with this cohort which has demonstrated an increase in urinary TCPy levels and decreased blood cholinesterase activity during the application season followed by recovery to baseline levels several weeks after application ends (Crane et al. 2013; AA Ismail et al. 2017). This pattern of exposure is found in both applicators and non-applicators, although the applicators show significantly greater changes from baseline. In addition, we have found increased symptoms among applicators during the season and decrements in neurobehavioral performance that accumulate and persist over time (AA Ismail et al. 2017; Khan et al. 2014; Rohlman et al. 2016). This study provides evidence that exposure to chlorpyrifos is associated with increased ADHD symptoms.
Members of the research team have been working with the Ministry of Agriculture to examine the impact of pesticide exposure on adolescent health (Callahan et al. 2014; Crane et al. 2013; A Ismail et al. 2017; Rohlman et al. 2016). Recognizing the risk to adolescents, we have also focused on identifying ways to reduce exposure. Through observation of the application process and analysis of work and hygiene behaviors contributing to urinary metabolite levels (Callahan et al. 2017), we have identified specific behaviors associated with increased exposure. This information has been presented to the adolescents, their parents, and the Ministry of Agriculture. We have also used this information to develop and deliver a training with specific recommendations on ways to reduce exposure among this population. Findings from this study can be used to promote changes in policy and legislation at both the local and national level. These could include the adoption of work practices that reduce exposure, the use of alternative pesticides, and restriction on activities for adolescents.
A limitation of the study is that we rely only on parental report and do not use self-rated symptoms from adolescents. Although adolescent self-report of internalizing (e.g., depression, anxiety) can be more valid, content, criterion and construct validity indicate that parental report (and teacher/observer report) has more validity than self-report among youth (Du Rietz et al. 2017; Hope et al. 1999; Smith et al. 2000). While a new version of the Connors Rating Scale is available (Conners 2008), we chose to use the previous version of the scale that has been used and validated in this population (El-Defrawi et al. 1992). Another limitation is the small sample size which may contribute to the large confidence interval. However, the study is strengthened by the use of a tool that has been validated and used in other populations in Egypt. Although one could hypothesize that participants with ADHD may have been more likely to become pesticide applicators, the mean number of reported symptoms among applicator was 4.5 and the majority of applicators (74%) were below the diagnostic threshold. Among the applicators there was no difference between age, education, or years worked as an applicator for those with symptoms falling above and below the threshold. The primary difference between these groups was the amount of recent exposure (hours worked during the season) reported by the applicators; applicators meeting the criteria for ADHD worked more hours during the season (39 hours) and more reported applying more pesticides at home (90%) than applicators not meeting the criteria (working 29 hours during the season and 69% report applying pesticides at homes). This indicates that in the current study recent exposure, and not past exposure, is likely associated with increased symptoms. This is confirmed by the dose-response association between TCPy levels and symptoms. However, there is a need to replicate these findings. Furthermore, if these symptoms are associated with recent exposures, then there is a need to examine changes over time to determine whether these symptoms persist or if they decrease after exposure ends.
6.1. CONCLUSIONS
This study provides preliminary evidence of an association between occupational exposure to chlorpyrifos and ADHD symptoms among adolescent pesticide applicators in spite of its limited small sample size. Although OPs are increasingly restricted for use in the United States (US), many of these pesticides are still being produced and used in low and middle income countries. There is a critical need to investigate the susceptibility of children and adolescents to repeated occupational and environmental exposures to pesticides because the developing brain may be uniquely sensitive to the neurotoxic effects of these agents. These findings should be used to support changes in policy and legislation. Furthermore, it is recognized that ADHD may persist into adulthood and have long-term outcomes, impacting performance at school, work, and within relationships (Fredriksen et al. 2014; Hodgkins et al. 2011; Rosler et al. 2010). This is the first study to demonstrate an exposure dependent association of ADHD and chlorpyrifos exposure in adolescents, however, there is a need to prospectively examine the association between ADHD symptoms and exposure and to determine if these symptoms reduce after exposure ends.
HIGHLIGHTS.
Pesticide exposure is associated with attention deficit hyperactivity disorder (ADHD)
Adolescent pesticide applicators had more symptoms of ADHD than non-applicators
Biomarkers were associated with increased symptoms, showing a dose-response effect
Need to investigate the susceptibility of adolescents to occupational pesticide exposure
ACKNOWLEDGMENTS:
We thank the Egyptian Ministry of Agriculture, the adolescents and their parents for their participation in the study. We would like to thank Mahmoud Ismail, Tameem Abou Eleinin and Mohammed Fouaad, and other members of the Research Team at Menoufia University for their assistance with data collection. The work was supported by the Fogarty International Center and the National Institute of Environmental Health Sciences (R21 ES017223 and R01 ES022163).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
COMPETING FINANCIAL INTERESTS DECLARATION: No conflict of interest.
7.1 REFERENCES
- Abd El-Hay M, El Sawy H. 2011. Attention deficit hyperactivity disorder among a sample of students from tanta university. Middle East Current Psychiatry 18:138–143. [Google Scholar]
- Aboul-Ata MA, Amin FA. 2015. The prevalence of adhd in fayoum city (egypt) among school-age children: Depending on a dsm-5-based rating scale. J Atten Disord. [DOI] [PubMed] [Google Scholar]
- APA. 2000. Diagnostic and statistical manual of mental disorders: Dsm-iv-tr. Washington, DC. [Google Scholar]
- Attia M, Tayel K, Mounier G, Ahmed M, Abo-Rass N. 2000. Adhd-part i prevalence and some socio-demographic parameters. Alexandria Journal od Pediatrics 14:1–8. [Google Scholar]
- Bellinger DC. 2009. Interpreting epidemiologic studies of developmental neurotoxicity: Conceptual and analytic issues. Neurotoxicology and teratology 31:267–274. [DOI] [PubMed] [Google Scholar]
- Bouchard MF, Bellinger DC, Wright RO, Weisskopf MG. 2010. Attention-deficit/hyperactivity disorder and urinary metabolites of organophosphate pesticides. Pediatrics 125:e1270–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchard MF, Bellinger DC, Wright RO, Weisskopf MG. 2010. Attention-deficit/hyperactivity disorder and urinary metabolites of organophosphate pesticides. Pediatrics 125:1270–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callahan CL, Al-Batanony M, Ismail AA, Abdel-Rasoul G, Hendy O, Olson JR, et al. 2014. Chlorpyrifos exposure and respiratory health among adolescent agricultural workers. International journal of environmental research and public health 11:13117–13129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callahan CL, Hamad LA, Olson JR, Ismail AA, Abdel-Rasoul G, Hendy O, et al. 2017. Longitudinal assessment of occupational determinants of chlorpyrifos exposure in adolescent pesticide workers in egypt. International journal of hygiene and environmental health 220:1356–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conners C 2001. Development of the crs-r In: Conner' rating scales-revised, (Conners C, ed). North Tonawabda, NY:Mutli-Health Systems, 83–98. [Google Scholar]
- Conners CK. 2008. Conners 3rd edition manual. Toronto, Ontario, Canada:Mult-Health Systems. [Google Scholar]
- Crane AL, Abdel Rasoul G, Ismail AA, Hendy O, Bonner MR, Lasarev MR, et al. 2013. Longitudinal assessment of chlorpyrifos exposure and effect biomarkers in adolescent egyptian agricultural workers. Journal of exposure science & environmental epidemiology 23:356–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews F, He J, Hodge C. 2007. Adolescent cortical development: A critical period of vulnerability for addiction. Pharmacol Biochem Behav 86:189–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dam K, Seidler FJ, Slotkin TA. 2000. Chlorpyrifos exposure during a critical neonatal period elicits gender-selective deficits in the development of coordination skills and locomotor activity. Brain Res Dev Brain Res 121:179–187. [DOI] [PubMed] [Google Scholar]
- Du Rietz E, Kuja-Halkola R, Brikell I, Jangmo A, Sariaslan A, Lichtenstein P, et al. 2017. Predictive validity of parent- and self-rated adhd symptoms in adolescence on adverse socioeconomic and health outcomes. European child & adolescent psychiatry 26:857–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton DL, Daroff RB, Autrup H, Bridges J, Buffler P, Costa LG, et al. 2008. Review of the toxicology of chlorpyrifos with an emphasis on human exposure and neurodevelopment. Crit Rev Toxicol 38 Suppl 2:1–125. [DOI] [PubMed] [Google Scholar]
- El Baza F, AlShahawi H, Zahra S, AbdelHakim R. 2016. Magnesium supplementation in children with attention deficit hyperactivity disorder. Egypt J Med Hum Genet 17:63–70. [Google Scholar]
- El-Defrawi M, Mahfouz R, Ragab L. 1992. A reliability and validity study of a rating scale for attention deficit hyperactivity disorder in egyptian children and adolescents. Egypt J Psychiat 15:38–44. [Google Scholar]
- El-Nemr F, Badr H, Salem M. 2015. Prevalence of attention deficit hyperactiity disorder in children. Science Journal of Public Health 3:274–280. [Google Scholar]
- El-Tallawy H, Hassan W, EA E-B, Shehata G. 2005. Prevalence of attention deficit hyperactivity disorder among elementary schools children in assiut city-egypt. Egypt J Neurol Psychiat Neurosurg 42:517–526. [Google Scholar]
- Ellman GL, Courtney KD, Andres V Jr., Feather-Stone RM. 1961. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88–95. [DOI] [PubMed] [Google Scholar]
- Fabiny DL, Ertingshausen G. 1971. Automated reaction-rate method for determination of serum creatinine with the centrifichem. Clin Chem 17:696–700. [PubMed] [Google Scholar]
- Farahat FM, Ellison CA, Bonner MR, McGarrigle BP, Crane AL, Fenske RA, et al. 2011. Biomarkers of chlorpyrifos exposure and effect in egyptian cotton field workers. Environ Health Perspect 119:801–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farahat T, Alkot M, Rajab A, Anbar R. 2014. Attention-deficit hyperactive disorder among primary school children in menoufia governorate, egypt. Int J Family Med 2014:257369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faraone SV, Sergeant J, Gillberg C, Biederman J. 2003. The worldwide prevalence of adhd: Is it an american condition? World Psychiatry 2:104–113. [PMC free article] [PubMed] [Google Scholar]
- Fredriksen M, Dahl AA, Martinsen EW, Klungsoyr O, Faraone SV, Peleikis DE. 2014. Childhood and persistent adhd symptoms associated with educational failure and long-term occupational disability in adult adhd. Attention deficit and hyperactivity disorders 6:87–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froehlich TE, Lanphear BP, Epstein JN, Barbaresi WJ, Katusic SK, Kahn RS. 2007. Prevalence, recognition, and treatment of attention-deficit/hyperactivity disorder in a national sample of us children. Arch Pediatr Adolesc Med 161:857–864. [DOI] [PubMed] [Google Scholar]
- Giedd JN. 2004. Structural magnetic resonance imaging of the adolescent brain. Ann N Y Acad Sci 1021:77–85. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Alzaga B, Lacasana M, Aguilar-Garduno C, Rodriguez-Barranco M, Ballester F, Rebagliato M, et al. 2014. A systematic review of neurodevelopmental effects of prenatal and postnatal organophosphate pesticide exposure. Toxicology letters 230:104–121. [DOI] [PubMed] [Google Scholar]
- Grandjean P, Landrigan PJ. 2006. Developmental neurotoxicity of industrial chemicals. Lancet 368:2167–2178. [DOI] [PubMed] [Google Scholar]
- Grandjean P, Landrigan PJ. 2014. Neurobehavioural effects of developmental toxicity. Lancet Neurol 13:330–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkins P, Arnold LE, Shaw M, Caci H, Kahle J, Woods AG, et al. 2011. A systematic review of global publication trends regarding long-term outcomes of adhd. Frontiers in psychiatry 2:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hope TL, ADams C, Reynolds L, Powers D, Perez RA, Kelley ML. 1999. Parent vs. Self-report: Contributions toward diagnosis of adolescent psychopathology. Journal of Psychopathology and Behavioral Assessment 21:349–363. [Google Scholar]
- Ismail A, Wang K, Olson J, Bonner MR, Hendy O, Abdel Rasoul G, et al. 2017. The impact of repeated organophosphorus pesticide exposure on biomarkers and neurobehavioral outcomes among adolescents. In Process. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismail AA, Bonner MR, Hendy O, Abdel Rasoul G, Wang K, Olson JR, et al. 2017. Comparison of neurological health outcomes between two adolescent cohorts exposed to pesticides in egypt. PloS one 12:e0172696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson DE, Seidler FJ, Slotkiin TA. 1998. Early biochemical detection of delayed neurotoxicity resulting from developmental exposure to chlorpyrifos. Brain research bulletin 45:143–147. [DOI] [PubMed] [Google Scholar]
- Khan K, Ismail AA, Abdel Rasoul G, Bonner MR, Lasarev MR, Hendy O, et al. 2014. Longitudinal assessment of chlorpyrifos exposure and self-reported neurological symptoms in adolescent pesticide applicators. BMJ Open 4:e004177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks AR, Harley K, Bradman A, Kogut K, Barr DB, Johnson C, et al. 2010. Organophosphate pesticide exposure and attention in young mexican-american children: The chamacos study. Environmental health perspectives 118:1768–1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser VC, Padilla S. 1998. Age- and gender-related differences in the time course of behavioral and biochemical effects produced by oral chlorpyrifos in rats. Toxicol Appl Pharmacol 149:107–119. [DOI] [PubMed] [Google Scholar]
- Polanska K, Jurewicz J, Hanke W. 2013. Review of current evidence on the impact of pesticides, polychlorinated biphenyls and selected metals on attention deficit / hyperactivity disorder in children. International journal of occupational medicine and environmental health 26:16–38. [DOI] [PubMed] [Google Scholar]
- Rauh V, Arunajadai S, Horton M, Perera F, Hoepner L, Barr DB, et al. 2011. Seven-year neurodevelopmental scores and prenatal exposure to chlorpyrifos, a common agricultural pesticide. Environmental health perspectives 119:1196–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauh VA, Garfinkel R, Perera FP, Andrews H, Hoepner L, Barr DB, et al. 2006. Impact of prenatal chlorpyrifos exposure on neurodevelopment in the first 3 years of life among inner-city children. Pediatrics 118:e1845–1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauh VA, Margolis AE. 2016. Research review: Environmental exposures, neurodevelopment, and child mental health - new paradigms for the study of brain and behavioral effects. J Child Psychol Psychiatry 57:775–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice D, Barone S Jr. 2000. Critical periods of vulnerability for the developing nervous system: Evidence from humans and animal models. Environ Health Perspect 108 Suppl 3:511–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohlman DS, Ismail AA, Rasoul GA, Bonner MR, Hendy O, Mara K, et al. 2016. A 10-month prospective study of organophosphorus pesticide exposure and neurobehavioral performance among adolescents in egypt. Cortex 74:383–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosler M, Casas M, Konofal E, Buitelaar J. 2010. Attention deficit hyperactivity disorder in adults. World J Biol Psychiatry 11:684–698. [DOI] [PubMed] [Google Scholar]
- Saad K, Elserogy Y, Abdel Rahman AA, Al-Atram AA, Mohamad IL, ElMelegy TT, et al. 2015. Adhd, autism and neuroradiological complications among phenylketonuric children in upper egypt. Acta Neurol Belg 115:657–663. [DOI] [PubMed] [Google Scholar]
- Seleem M, El-Gohary T, EId M, Sroor E. 2014. Serum ferritin is negatively correlated with inattention in a sample of egyptian children with attention deficit hyperactivity disorder. The Arab Journal of Psychiatry 25:96–106. [Google Scholar]
- Singleton ST, Lein PJ, Farahat FM, Farahat T, Bonner MR, Knaak JB, et al. 2014. Characterization of alpha-cypermethrin exposure in egyptian agricultural workers. International journal of hygiene and environmental health 217:538–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA. 2005. Developmental neurotoxicity of organophosphates: A case study of chlorpyrifos In: Toxicity of organophosphate and carbamate pesticides, (Gupta RC, ed). San Diego:Elsevier Academic Press, 293–314. [Google Scholar]
- Smith BH, Pelham WE, Gnagy E, Molina B, Evans S. 2000. The reliability, validity, and unique contributions of self-report by adolescents receiving treatment for attention-deficit/hyperactivity disorder. Journal of consulting and clinical psychology 68:489–499. [DOI] [PubMed] [Google Scholar]
- Soliman G, Afify M, Yehia M, Abdel-Naem E, Abd Alkarim S. 2010. "Attention deficit hyperactivity disorders"an epidemiological study of preschool and primary school children in minia city. El-Minia Med Bul 21:171–179. [Google Scholar]
- Spear LP. 2010. The behavioral neuroscience of adolesence. New York:W.W. Norton & Company. [Google Scholar]
- Steinberg L 2008. A social neuroscience perspective on adolescent risk-taking. Dev Rev 28:78–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez-Lopez JR, Jacobs DR Jr., Himes JH, Alexander BH. 2013. Acetylcholinesterase activity, cohabitation with floricultural workers, and blood pressure in ecuadorian children. Environmental health perspectives 121:619–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wendel de Joode B, Mora AM, Lindh CH, Hernandez-Bonilla D, Cordoba L, Wesseling C, et al. 2016. Pesticide exposure and neurodevelopment in children aged 6–9 years from talamanca, costa rica. Cortex 85:137–150. [DOI] [PubMed] [Google Scholar]
- Yu C-J, Du J-C, Chiou H-C, Yang S-H, Liao K-W, Yang W, et al. 2016. Attention deficit/hyperactivity disorder and urinary nonylphenol levels: A case-control study in taiwanese children. PLoS ONE 11:e0149558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu CJ, Du JC, Chiou HC, Chung MY, Yang W, Chen YS, et al. 2016. Increased risk of attention-deficit/hyperactivity disorder associated with exposure to organophosphate pesticide in taiwanese children. Andrology 4:695–705. [DOI] [PubMed] [Google Scholar]