Abstract
Loss of galactose-1 phosphate uridylyltransferase (GALT) activity in humans results in Classic Galactosemia, and the GalT-deficient (GalT−/−) mouse mimics the patient condition. GalT−/− ovaries display elevated endoplasmic reticulum (ER) stress marker, BiP, and downregulated canonical phosphatidylinositol 3-kinase (Pi3k)/protein kinase B (Akt) growth/pro-survival signaling. Numbers of primordial follicles are reduced in the mutants, recapitulating the accelerated ovarian aging seen in human patients. We previously found that oral administration of the compound Salubrinal (an eIF2α phosphatase inhibitor), resulted in reduction of ovarian BiP expression, rescued Pi3k/Akt signaling, and a doubling of primordial follicles in GalT−/− adults. Here, we further characterized galactosemic stress in GalT−/− mice versus wild-type (WT) controls, and examined whether Salubrinal treatment improved broader reproductive parameters. We assessed the expression levels of factors of the unfolded protein response (UPR), and found that BiP, phospho-Perk, and phospho-eIF2α were all elevated in GalT−/− ovaries. However, neither IKK activation (NFκB pathway) nor alternative Xbp1 splicing downstream of ER membrane protein Ire1α activation was induced, suggesting an Xbp1-independent UPR in galactosemic stress. Moreover, Salubrinal treatment significantly increased the number of ovulated eggs in mutant animals after gonadotrophic superovulation. Salubrinal treatment also normalized estrus cycle stage lengths and resulted in significantly larger litter sizes than vehicle-treated mutants. Overall, we show that Salubrinal protects against galactosemia-induced primordial follicle loss in a fashion that includes suppressing the de-phosphorylation of eIF2α, and that intervention in this way significantly improves and extends ovarian function, fertility, and fecundity.
Keywords: Classic Galactosemia, Galactosemic stress, Subfertility, Salubrinal, Perk branch of the Unfolded Protein Response (UPR), Pi3K/Akt signaling
1. Introduction
Classic Galactosemia, a secondary congenital disorder of glycosylation (CDG) [1–8], is an autosomal recessive disorder caused by deleterious mutations of the human galactose-1 phosphate uridylyltransferase (GALT) gene [9–13]. Despite the fact that the pathogenic mechanisms of the disease remain enigmatic, there is strong evidence that toxic metabolites accumulated in GALT-deficient cells over time play a major, albeit not sole, role in the pathophysiology of Classic Galactosemia [14–17]. Data from several investigators strongly supported a model where the accumulation of the toxic galactose metabolites induces aberrant protein glycosylation, which leads to cellular stress (galactosemic stress), activation of the unfolded protein response (UPR), and global changes in gene expression [18–22]. Early findings on GALT-deficient yeast and patient fibroblasts [18, 19], as well as subsequent studies by De-Souza and coworkers conclusively showed that gal-1P, a metabolite accumulated in GALT deficiency, is required to trigger the UPR [20]. The latter group definitively showed that concurrent deletion of the galactokinase-encoding gene GAL1 obliterated UPR activation and galactose toxicity in a GALT-deficient yeast model [20]. Since aberrant glycosylation and UPR activation are observed in patient cells and model organisms, the involvement of endoplasmic reticulum (ER) stress as a component of galactosemic stress has also been proposed. Yet, the precise ER stress response or UPR signaling pathways implicated have not been fully determined, nor have they been therapeutically targeted in GALT deficiency.
Recently, the GalT-deficient (GalT−/−) mouse model developed by our group revealed galactose sensitivity in mutant pups, impaired motor functions, growth restriction in both sexes, and subfertility in adult females, all features of Classic Galactosemia [23, 24]. Our on-going characterization of primary skin fibroblasts and tissues isolated from these mutant mice revealed a slower growth rate, the downregulation of pro-survival phosphoinositide 3 kinase/protein kinase B (Pi3k/Akt) signaling pathway [25, 26], and initial signs of UPR activation consistent with the abovementioned data. Because the significant health and well-being compromises faced by galactosemic girls and women are compounded when their ovaries fail, we are focusing on how the disease impacts the ovary, and whether modulation of the UPR might improve ovarian function in the GalT−/− mouse model [24].
Several studies have indeed reported that maintenance of ER homeostasis by the UPR is likely to be a key mechanism during folliculogenesis and oocyte maturation [27–30]. ER stress results in the activation of several signal transduction cascades collectively termed the UPR, which affects a wide variety of cellular functions [31]. One key aspect of the UPR is the activation of the transmembrane kinase Perk and subsequent phosphorylation of eIF2α, which leads to general translation attenuation. We previously demonstrated that when GalT−/−mice were treated with Salubrinal, which inhibits the dephosphorylation of eIF2α and thereby extends the consequences of Perk signaling [32–35], ER stress markers were reduced, and Pi3k/Akt signaling was upregulated to a level near that of wild-type (WT) mice in the whole ovary. Crucially, Salubrinal treatment was also shown to prevent the accelerated loss of primordial follicles seen in vehicle-treated GalT−/− controls, but the mechanism behind this protective effect was unclear [26].
Based on available knowledge and our own growing data, we hypothesize that accumulation of gal-1P in our mouse model induces a unique type of galactosemic stress response in the ovary, which accelerates ovarian aging by compromising the survival of the primordial follicle pool and, and perhaps affects ovarian function more broadly. Our earlier demonstration that Salubrinal (Fig. 1) can rescue numbers of primordial follicles [36] warranted additional experiments to see if fertility and/or fecundity were similarly improved. In this study, we further dissect UPR signaling pathways in GalT−/− ovaries, and evaluate how specific members of the UPR signaling cascade are impacted in the “rescuing” conditions of Salubrinal treatment. We go on to show that Salubrinal increases the number of mature eggs retrieved in gonadotrophic superovulation experiments, normalizes the length of estrus cycle stages, and enhances litter sizes (e.g., live birth rates) after breeding trials, without discernible gross effects upon offspring. These findings further open the door for understanding the mechanism that leads to the subfertility phenotype in the GalT−/− mouse model and may move us closer to novel therapeutic strategies to address the reproductive compromises faced by patients with Classic Galactosemia.
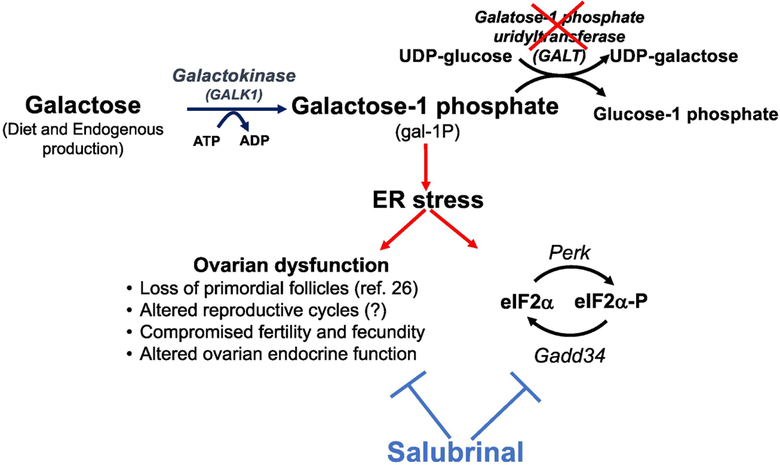
Figure 1. Schematic representation of GALT-deficiency induced Subfertility phenotype in the mouse model.
Dietary and Endogenously produced galactose is phosphorylated to galactose- 1 phosphate (gal-1P) by galactokinase (GALK1). In the presence of galactose–1 phosphate uridylyltransferase (GALT), gal-1P reacts with UDP- glucose to form UDP-galactose and glucose- 1 phosphate. Gal-1P accumulation causes ER stress and ovarian dysfunction. Salubrinal inhibits eIF2α dephophorylation, potentially ameliorating galactosemic stress, and normalizes the ovarian functions.
2. Materials and Methods
2.1. Chemicals and Reagents
Salubrinal was purchased from Sigma Inc. (St. Louis, MO). Antibodies against selected components of the Pi3k/Akt signaling pathway were included in the Phospho-Akt Pathway Antibody Sampler Kit (Catalog # 9916T, Cell Signaling Technology, Danvers, MA). Anti-mouse BiP/Grp78 (Catalog # 610979) was purchased from BD Bioscience (San Jose, CA). Anti-mouse Hsp90 (Catalog # 69703) was obtained from Santa Cruz Biotechnology Inc. (Dallas, TX). Anti-mouse Gapdh (Catalog #8795) was purchased from Sigma Inc. (St. Louis, MO). Antibody for phospho-Perk (Catalog # PA5–40294) was purchased from Thermo Fisher Scientific (Waltham, MA). Anti phospho-eIF2α (Catalog #3398) was obtained from Cell Signaling Technology, (Danvers, MA). Anti phospho-IkBα (Catalog # Sc8404) and anti IkBα (Catalog Sc1643) were obtained from Santa Cruz biotechnology Inc. (Dallas, TX). GSK2606414 (Catalog# 516535) and ISRIB (Catalog # SML0843) were obtained from Sigma Inc. (St. Louis, MO). The concentration of DMSO was <0.1% for all experiments to avoid cytotoxicity. Tunicamycin (Catalog# 11089-65-9) was obtained from Cayman chemicals (Ann Arbor, MI).
2.2. Cell culture
GalT-deficient fibroblasts strains were propagated in regular Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 15% fetal bovine serum (FBS), 1% penicillin and 1% streptomycin at 37 °C with 5% ambient CO2.
2.3. Animals and experimental groups
All animal studies were conducted in full compliance with the guidelines outlined in the guide for the care and use of laboratory animals and were approved by the University of Utah Institutional Animal Care and Use Committee. GalT−/− (GalT-deficient) mice used in this study were constructed as previously described and were fed with normal chow at all times since weaning [23]. Genetic background of all mice were confirmed by genotyping (molecular and biochemical) using previously published protocols [23].
2.4. Assessment of UPR in mutant mouse ovaries by Western blot analyses
Ovaries from WT and GalT-deficient mice were isolated and proteins were extracted as described before [24]. A total of 40–80 μg of protein were separated on 12% SDS/PAGE and transferred to nitrocellulose membrane using Mini-PROTEAN II Cell (Bio-Rad Laboratories, Hercules, CA). UPR proteins were detected using primary antibodies for protein kinase R (PKR)-like ER kinase (Perk), eukaryotic translation Initiation Factor 2 alpha (eIF2α) and BiP (also known as Grp78) at manufacturers’ recommended titers. Primary antibodies were detected with IR dye-conjugated secondary antibodies and visualized by Odyssey Image Analyzer (Li-Cor Biotechnology, Lincoln, NE). Quantitative analysis of the fluorescence signals was performed by Image Studio™ Lite software (Li-Cor Biotechnology, Lincoln, NE), and the results were normalized to the corresponding Gapdh abundance detected in the same samples. Student’s t-test was used to determine statistical significance of the results. We also assessed the NF-κB signaling in the samples by Western blot analyses using the respective antibodies.
2.5. Evaluation of UPR by Xbp1-splicing assay in GalT-deficient mice ovaries
Ovaries from 3-month-old WT and GalT-deficient mice were isolated as published before [24]. The samples were washed twice with PBS and total mRNA was isolated using the Quick RNA-TM Micro prep (Catalog # R1050, Zymo research, Irvine, CA). DNase I amplification grade (Invitrogen, Grand Island, NY) was used to remove genomic DNA. Total RNA concentration and purity was monitored and cDNA synthesis was performed with M-MLV Reverse Transcriptase (Catalog # M0253S, New England Biolabs, Ipswich, MA). PCR primers were designed to encompass the splicing sequences of mouse Xbp1 (sequences: AGAAGAGAACCACAAACTCCAG, GGGTCCAACTTGTCCAGAATGC). PCR products, amplified with annealing temperature at 55 °C, extension time 30 seconds for 30 cycles, were separated by electrophoresis on a 2% agarose gel for 2.5 hours. Spliced and unspliced Xbp1 band intensities were quantified using Image J. Software (NIH).
2.6. Evaluation of Perk-mediated eIF2α phosphorylation in mutant mouse fibroblasts
To evaluate the role of Perk in eIF2α phosphorylation in GalT-deficient cells, mutant fibroblasts at 70% confluence were treated with 1 μM GSK2606414 (PERK inhibitor) for 5 hours prior to Western blot analyses. Treated cells were harvested and lysed in cold hypotonic buffer [25 mM NaCl, 0.5 mM EDTA, 25 mM Tris HCl (pH 7.2)] with protease and phosphatase inhibitors at 4 °C. Clarified lysates were resolved by SDS-PAGE and transferred to nitrocellulose membrane as described above. Blots were incubated with antibodies to phospho-Perk, phospho-eIF2α, Atf4 and Chop. IRDye®800DX-labelled rabbit anti-mouse IgG (LICOR Biosciences, Lincoln, NE) were used as secondary antibody. Proteins were detected on the Odyssey™ Infrared Imager (LI-COR Biosciences, Lincoln, NE). Untreated cells and cells treated with 2 μg/ml tunicamycin were used as controls. To examine the effects of ISRIB (Integrated Stress Response Inhibitor) on Perk-mediated UPR in GalT-deficient cells, we incubated the mutant fibroblasts with 300nM of the compound for 20 hours before we harvested them for Western blot analysis.
2.7. Quantification of oocytes by superovulation
Untreated, vehicle treated and Salubrinal treated groups of WT and GalT-deficient females of 11 weeks of age (~20 – 23 g) were used for the study. We administered, both daily and orally (per os), 5 mg/kg Salubrinal to eight GalT-deficient and WT mice (n = 4 for the vehicle-treated group and n = 4 for the Salubrinal-treated group) for 35 days. At the end of the treatment, the mice were injected with 5 IU pregnant mare’s serum gonadotropin followed 48 hours later by 5 IU human chorionic gonadotropin to induce superovulation. Ovaries and oviducts were collected 14 hours post-hCG, and cumulus-oocyte-complexes were isolated from oviducts, while ovaries were flash-frozen in liquid N2 and stored until protein lysate preparation and Western blot analyses. Quantification of oocytes was performed manually by microscopy.
2.8. Breeding trial
5 weeks old GalT-deficient female mice were used for this experiment. Mice were treated with: (1) DMSO (0.6%) in Soymilk as vehicle group, (2) 5mg/kg Salubrinal in 0.6% DMSO in soymilk as treatment group per os daily for 5 weeks. For the last two weeks prior to breeding, estrus cycle was evaluated for all groups. Females were moved to breeder cages where two females were paired with one WT male. Females were continuously mated from 11 weeks of age to 6 months of age or until three litters were achieved. Animals within the breeder cage were fed a maintenance chow diet (Harlan Laboratories, Indianapolis, IN). Females remained within the breeder cage until they showed visual or palpable signs of pregnancy, at which point they were separated and maintained on the same diet until parturition.
Following delivery, pups were counted, weighed regularly, and euthanized on post-natal day 21 and the females were returned to the breeder cage. The organs from pups were isolated and histological analysis was carried out to check any signs of toxicity in the pups born from each group. The breeding trial was carried out with 5 – 7 mice per group. Data for survival analysis, pup weights, and litter sizes were included for analysis at the intervals for which the dam was present in the trial. Animals were monitored by frequent visual and physical examinations of each animal with regard to food intake, normal activity, movement, grooming, failure to thrive, weight loss, and any signs of discomfort or infection.
2.9. Statistical analysis
All measurements were reported as mean ± s.e.m. Statistical significance was determined as indicated, by using Student’s t-test or two-way ANOVA followed by Tukey’s HSD test for pairwise comparison using optionally i) GraphPad Prism version 7.01 for Windows (GraphPad Software) or ii) the statistical programming environment R. A p-value of less than 0.05 was considered statistically significant.
3. Results
3.1. UPR in GalT-deficient adult mouse ovaries is characterized by Perk activation in the apparent absence of Ire1α activation
Previously, we demonstrated that in GalT deficiency, the major ER chaperone BiP/Grp78 is over-expressed [19, 24–26]. Under non-stressed conditions, BiP associates with two of the major sensors of ER stress, the ER membrane proteins Perk and Ire1α [37]. ER stress results in the dissociation of BiP from these membrane proteins and their subsequent activation by phosphorylation. The activated Perk (phospho-Perk) then phosphorylates eIF2α, resulting in the attenuation of general protein translation. Ire1α activation (after ER stress induced BiP dissociation), results in the cleavage and splicing of the mRNA encoding the transcription factor Xbp1, and enhanced activity of the NFκB mediator Inhibitor of Kappa B Kinase complex (via subunit IKKβ). To dissect BiP-associated events that occur in the ovaries of our GalT-deficient mouse model, we evaluated the expression of these factors and/or their downstream targets.
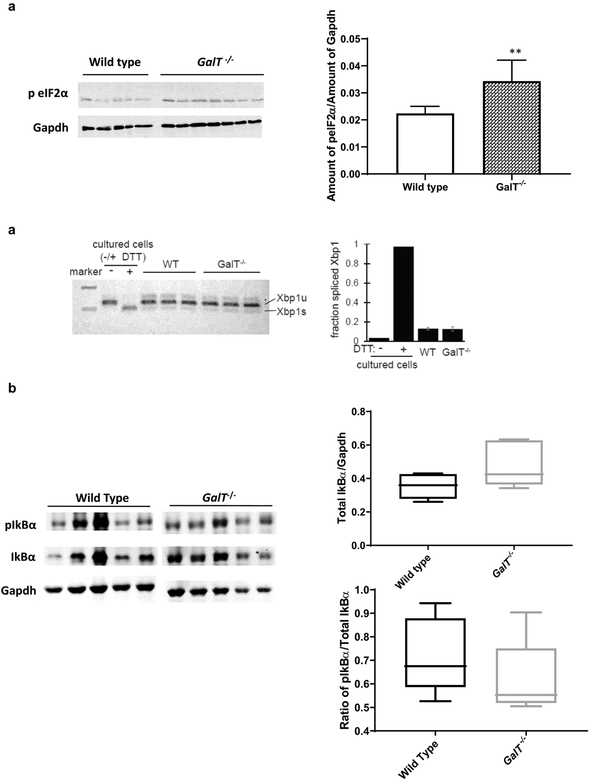
Here, protein expression levels of UPR genes in the ovaries of two-month-old mutant mice (n = 7) were compared with those of WT control (n = 5) mice. We noted enhanced expression of phospho-eIF2α protein in mutant ovaries (Fig. 2a). In contrast, we found that neither readout of Ire1α activation, Xbp1 splicing (Fig. 2b), nor IKKβ activity (Fig. 2c), were elevated in GalT-deficient mice ovaries. To evaluate IKKβ activity, we examined the expression of its target protein Inhibitor of kappa B alpha (IkBα), and its IKKβ–phosphorylated form known to be associated with its degradation and “activated” NFκB (p-IkBα). Unlike phospho-eIF2α, we did not see any significant difference in the expression of IkBα or p-IkBα in mutant ovaries compared to controls (Fig.2c).
Figure 2. Galactosemic stress induced eIF2α.phosphorylation in GalT- deficient mouse ovaries.
a) Protein expression levels of UPR marker peIF2α in WT and GalT-deficient mice ovaries (n=5 for WT and n=7 for GalT−/−. P values are derived from Student’s t-test analyses, **P = 0.008).
b) Comparison of level of unspliced (Xbp1u) spliced (Xbp1s)) Xbp1 in the ovaries of mutant and WT controls. First two lanes contain PCR products from mouse cells +/− ER stress inducer (2 mM DTT) as controls.
c) Comparison of protein levels of pIkB α and IkBα in mutant and WT ovaries (n=4 in each group). P values are derived from Student’s t-test analyses, P = 0.103 for Total IkBα and P = 0.345 for pIkBα).
3.2. Activation of eIF2α phosphorylation in GalT deficient cells is mediated by Perk
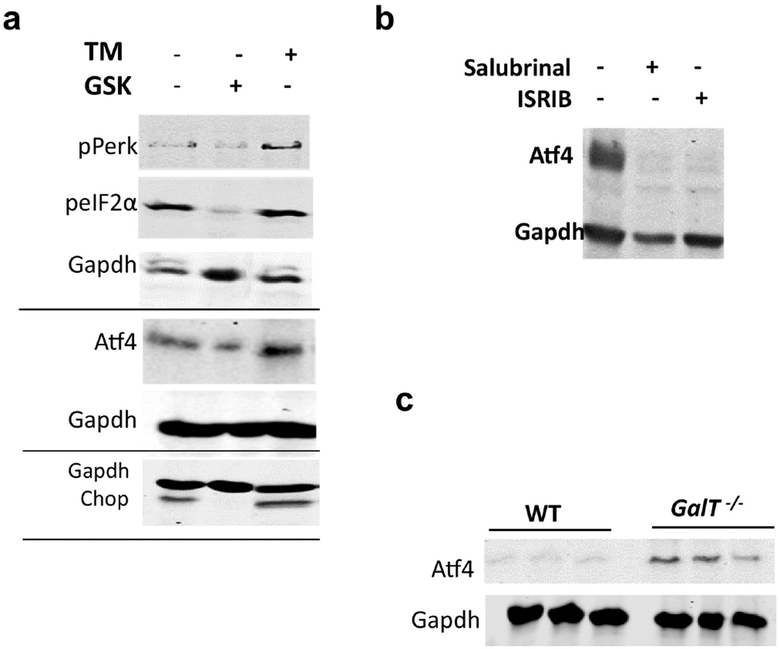
It has been known that eIF2α can be phosphorylated by other protein kinases apart from Perk such as HRI, GCN2 and PKR under different stresses such as amino acid deprivation (GCN2), the presence of dsRNA (PKR) (viral infections) or heme deficiency (HRI) (see [38] for review). To support the notion that the increase in eIF2α phosphorylation found in GalT-deficient cells is mediated through the Perk branch of UPR, we treated the GalT−/− fibroblasts in the presence of Perk inhibitor GSK2606414. As shown in lanes 1 & 2 of Fig. 3a, the addition of Perk inhibitor, we saw a concomitant reduction of Perk and eIF2α phosphorylations (50% and 80% respectively) in the GalT-deficient fibroblasts. More importantly, the reduction of Perk and eIF2α led to diminished levels of downstream effectors Atf4 and Chop. As positive control of UPR activation, addition of tunicamycin to the cultured mutant cells resulted in up-regulation of pPerk, phospho-eIF2α, Atf4 proteins (lane 3, Fig. 3a).
Figure 3. Role of active Perk in eIF2α phosphorylation in GalT-deficient mouse fibroblasts.
a) Quantification of phospho-eIF2α and its downstream targets Atf4 and Chop in GalT−/− fibroblasts in the presence/absence of 1 μM GSK2606414 or 2 μg/ml tunicamycin. (Representative image from three independent experiments using three different cell lines that yielded identical results.)
b) Expression level of Atf4 in GalT−/− fibroblasts in the presence/absence of 50 μM Salubrinal or 300 nM ISRIB.
c) Protein expression levels of Atf4 in WT and GalT−/− mice fibroblasts (n=3 in each group).
To further demonstrate the involvement of phospho-eIF2α in UPR under galactosemic stress conditions, we treated the mutant fibroblasts with ISRIB (Integrated Stress Response Inhibitor), an experimental drug which reverses the effects of eIF2α phosphorylation [39] and found reversal of Atf4 up-regulation in the presence of the inhibitor (lane 3, Fig. 3b). As control, we showed that Salubrinal treatment prevented the up-regulation of Atf4 (lane 2, Fig. 3b).
Fig. 3c shows the up-regulated Perk pathway in the GalT-deficient cells corresponds to increased levels of the downstream effector Atf4 in the mutant fibroblasts compared to WT controls.
3.3. Effects of Salubrinal on UPR and survival signaling in the GalT-deficient ovary
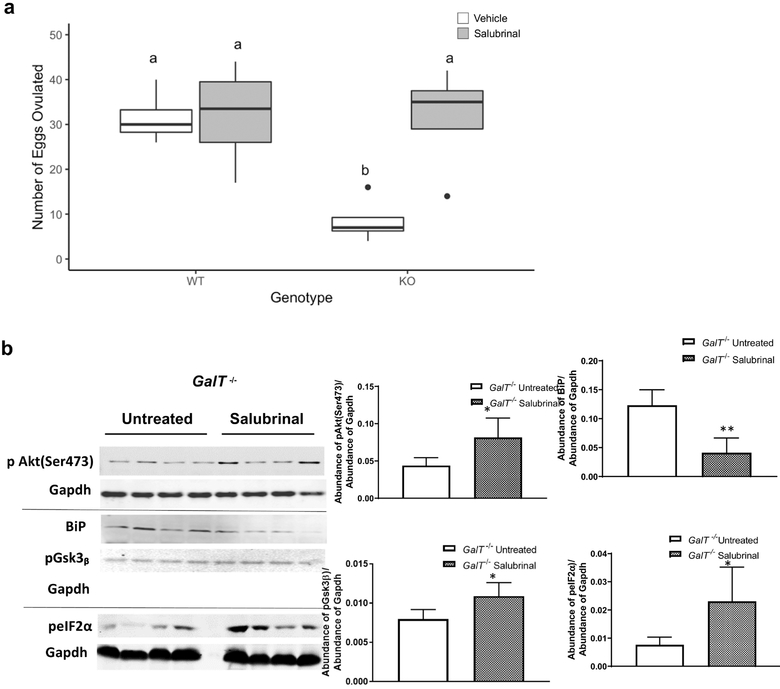
Having generated more information about the nature of ovarian ER stress response induced by GalT-deficiency, we returned to our “rescue” model, the application of Salubrinal. As mentioned, Salubrinal administration for GalT-deficient mice was associated with a near-doubling of primordial follicles compared to untreated or vehicle-treated control animals [26]. In this study, Salubrinal treatment was associated with a significant increase in the number of ovulated eggs in mutant animals after gonadotrophin “superovulation” [untreated vs. treated (8.5±2.3 vs 31.5±6.1) p<0.05] (average count from four mice in each group) (Fig. 4a). Salubrinal treatment was not associated with any change in the number of ovulated eggs in WT animals after hormonal stimulation [untreated vs treated (31.5 ± 3.0 vs 32 ± 5.9)] (average of four mice from each group).
Figure 4. Salubrinal treatment resulted in normalizing the aberrant pro survival & UPR signaling and more mature follicles in GalT- deficient mouse ovaries.
a) Salubrinal improved the ovulation in GalT-deficient mice (WT=Wild type, KO=GalT−/−) (n=4 for each group). P values are derived from two-way ANOVA followed by Tukey HSD, *the data are significant (p < 0.05).
b) Salubrinal reversed the aberrant Pi3K/Akt and UPR signaling in ovaries from gonadotrophin-stimulated GalT-deficient mouse. Graphical representations on the right (n=4 for each group. P values are derived from Student’s t-test analyses, *p ≤ 0.05, **p ≤ 0.005).
Salubrinal treatment to this point corresponded to the reduction in ovarian ER stress markers and greatly enhanced numbers of primordial follicles in treated mice [26], and now, significantly increased numbers of eggs retrieved after superovulation. Next, Western blot analyses were performed on lysates from ovaries from the same Salubrinal- or vehicle-treated GalT−/− animals for the following ER stress-related targets. We detected an approximate 66% reduction in BiP in ovaries from Salubrinal-treated animals compared to vehicle-treated animals (Fig. 4b), which we interpret as a reduction in galactosemic stress by the treatment. Accordingly, the level of phosphorylated eIF2α was significantly higher (+188%) with Salubrinal treatment, consistent with Salubrinal’s known mechanism of action - inhibition of the dephosphorylation of eIF2α - which further favors attenuating protein translation. Salubrinal treatment also corresponded to increased phosphorylation of Akt at Ser473 and its downstream target pGsk3β (Fig. 4b). Phosphorylation was increased by approximately 88.4% and 38%, for pAkt and pGsk3β respectively. We did not detect any significant effects on the levels of heat shock protein 90 (Hsp90) or pAkt (Thr308) phosphorylation in ovaries from GalT−/− animals treated with Salubrinal (data not shown). Further characterization of the impact of Salubrinal upon GalT−/− female reproductive performance followed.
3.4. Salubrinal treatment normalizes estrus cycle stage length, increases litter sizes in GalT-deficient female mice without altering gross offspring parameters
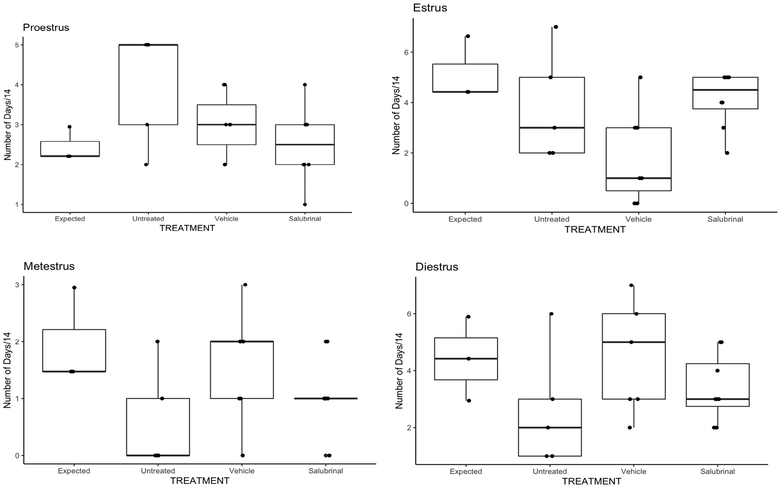
Earlier, Tang et al. reported that GalT-deficient mice had significantly smaller litters and a significantly longer time-to-pregnancy than their WT littermates [23]. Since Salubrinal prevented the loss of primordial follicles and normalizes numbers of ovulated oocytes after superovulation, it became essential to determine the efficacy of Salubrinal in correcting female reproductive function more broadly. We went on to monitor estrus cycle stage lengths of treated versus vehicle control animals (Fig. 5). The lengths of each cycle stage were calculated from published data [40], and plotted versus those determined for our treatment groups. Remarkably, Salubrinal effectively normalized the lengths of individual cycle stages to lengths that would be expected in young wild-type mice [40]. In contrast, lengths of estrus and metestrus were more variable in vehicle and untreated animals than in the Salubrinal group. (Compare median lengths of n= 3, 4 or 5 animals between untreated, vehicle-treated, and Salubrinal-treated animals to “Expected”).
Figure 5. Salubrinal effectively normalized reproductive cycle in GalT-deficient mice.
Salubrinal normalized he lengths of individual cycle stages to lengths that would be expected in young wild-type mice.
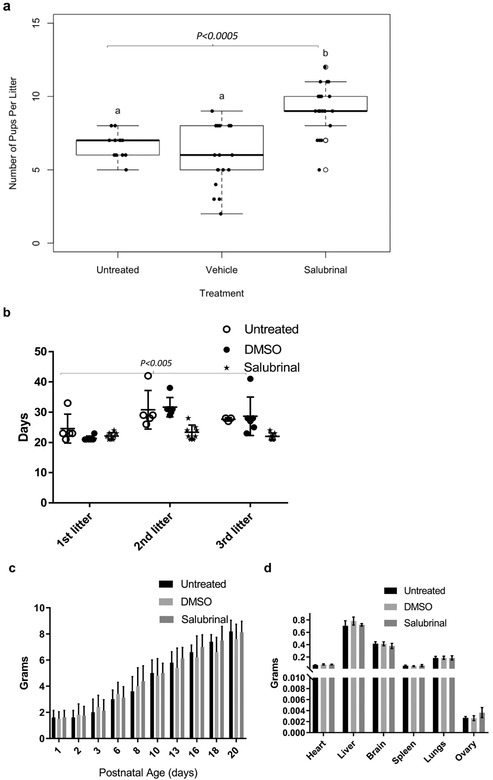
An extensive breeding trial followed, with treated and untreated GalT−/− females placed with males of proven fertility after the 5 weeks of treatment. We evaluated the impact of prior Salubrinal treatment upon litter size for up to three litters. The combined litter size (Fig. 6a) was significantly increased with Salubrinal treatment (p <0.0005). We additionally monitored the number of days between litters during this experiment, and Fig. 6b shows that Salubrinal significantly reduces the time to pregnancy of the second and third litters (p <0.005) in GalT-deficient mice compared to those of untreated and vehicle-treated GalT-deficient mice.
Figure 6. Salubrinal normalized the Time to pregnancy and improved litter size in GalT-deficient mice.
a) Impact of Salubrinal treatment on litter size.
b) Effects of Salubrinal treatment on Time to Pregnancy (TTP) (n=5 in untreated, n=6 in vehicle treated and n=7 in Salubrinal treated. (P values are derived from one-way ANOVA analysis using GraphPad Prism, *p ≤ 0.0005,)
c) Impact of Salubrinal treatment on body weights of the pups at pre-weaning age.
d) Effects of Salubrinal treatment on organ weights of the pups at post weaning age.
To determine whether Salubrinal might negatively affect offspring development, we monitored pup weights daily from their day of birth until postnatal day 20. No differences in body weight were seen between animals treated with Salubrinal and the control groups (Fig. 6c). A subset of pups was sacrificed on postnatal day 21 for organ necropsy and weighing. No visible alterations in organ characteristics were noted for any organ in any treatment group. Further, Salubrinal treatment had no effect on heart, liver, brain, ovaries, kidneys and spleen organ weights. This initial evaluation, along with increased total pup numbers, did not favor gross compromise for offspring of mothers treated with Salubrinal (Fig. 6d).
4. Discussion
The unfolded protein response (UPR) plays crucial roles during oocyte and follicle development, including the meeting of increased protein demand by oocytes to ensure proper protein synthesis, folding, modification and trafficking [41–43]. In mouse cumulus-oocyte complexes, fatty acid-induced ER stress impairs protein secretion and mitochondrial activity resulting in abnormal embryo development, which is reversed by Salubrinal [27]. In addition, Wu and coworkers [44] revealed that conception in obese mice is compromised by ER stress. That group demonstrated a relationship between obesity and ER stress in cumulus-oocyte complexes associated with reduced mitochondrial membrane potential, high autophagy levels and high intracellular lipid levels. Pre-ovulatory administration of Salubrinal in their hands restored oocyte quality at least in part by increasing levels of the mitochondrial replication factors mitochondrial transcription factor A (TFAM) and dynamin related protein 1 (DRP1). Accordingly, increased mtDNA was found in oocytes treated with Salubrinal [44]. Harada et al. investigated the roles of UPR signaling in granulosa and cumulus cells during follicular growth and maturation in the mouse ovary [45]. During early embryonic development, BiP/GRP78 (HSPA5) regulates UPR signaling, and this can protect inner cell mass cells from apoptosis during early embryonic development [46]. What has been less clear is whether disease states that compromise egg or embryo development, and possibly fertility and fecundity overall, involve ER stress. Our data suggest that GALT deficiency compromises oocyte and follicle survival in an UPR-dependent fashion, which can be rescued by altering the stress response by applying the experimental drug Salubrinal.
The UPR involves upregulation of key response genes and activation of post-translational signal transduction cascades [37, 47–50]. This includes the phosphorylation and activation of stress sensor proteins, such as IRE1 and PERK, and the downstream induction of many UPR genes. Our first biochemical assessment under galactosemic conditions demonstrated an increase in the levels of BiP, and the expression of ATF4 and CHOP in the fibroblasts of GALT-deficient patients [19, 25]. In another study, we showed that the UPR is activated in the ovaries and cerebella of GalT-deficient mouse model, which was associated with a downregulation of pro-survival Pi3k/Akt signaling in those tissues [26]. We also demonstrated that treatment with Salubrinal resulted in an upregulation of Pi3k/Akt signaling to near the level seen in WT control ovary and cerebellum. This was accompanied by significantly decreased loss of primordial follicles in the ovaries, and significantly decreased loss of Purkinje cells in the cerebella of mutant mice [26]. The next logical steps were a) to more precisely define the UPR signaling pathways activated in the ovaries of GalT−/− mice, and b) to determine whether Salubrinal treatment also associated with the survival of greater numbers of mature ovarian follicles, greater numbers of surviving eggs, and improved fecundity in GalT-deficient mice.
We found here that expression levels of phospho-eIF2α were higher in GalT-deficient ovaries compared to WT controls (Fig 2a). In contrast, we did not detect splicing of Xbp1 associated with the Ire1-UPR in ovaries from GalT-deficient mice (Fig. 2b). Interestingly, De-Souza and co-workers showed that when they challenged GALT-deficient yeasts with galactose, it resulted in activation of UPR through the Ire1 signaling pathway and HAC1 (yeast equivalent of Xbp1) splicing [20]. However, it should be noted that Ire1p is the only UPR sensor in yeast [51, 52]. Normally, Ire1α initiates the unconventional splicing of Xbp1 transcript so that an active Xbp1 transcription factor is produced [53, 54]. Under non-stressed circumstances, unspliced Xbp1 protein is produced that lacks transactivation function, and can instead act as a dominant-negative of the active form that results from splicing. Xbp1 splicing often favors cell survival during embryo and fetal development by allowing the execution of a complete response to unfolded protein and ER stress [55–57]. In addition, we did not detect an increase in IKK/NFκB activity, as measured by phospho-IκBα levels. As IKK is known to be activated downstream of Ire1, this observation further indicates an absence of Ire1 signaling [49]. While the “missing” Ire1α response in the presence of Perk activation and eIF2α phosphorylation needs further study, a lack of ostensibly pro-survival Xbp1 splicing and NFκB activation in ovaries of GalT-deficient mice may be related to the follicle loss seen in the disease model. Conceptually, different tissue types might elicit different UPR signaling pathways in responding to ER stress. This could be due to the different tissue-specific expression among the three UPR sensors. In fact, accumulating evidence suggest that engagement of IRE1α-XBP1 pathways are particularly relevant to variety of neurological disorders involving the misfolding and deposition of abnormal protein aggregates in human brain [58–60].
As eIF2α could be phosphorylated by other kinases such as HRI, GCN2 and PKR under different stresses (see [38] for review), we further examined the effects of GSK2606414 (a PERK inhibitor) on the expression level of eIF2α phosphorylation and its downstream targets Atf4 and Chop in the mutant mouse fibroblasts. Fig. 3a revealed a significant inhibitory effect on the expression level of phospho-Perk, phsopho-eIF2α, Atf4, and Chop. These data further explain that eIF2α phosphorylation in GalT-deficiency is mediated through the Perk branch of UPR. In hindsight, the involvement of Perk rather than other eIF2α kinases is expected because of the aberrant glycosylation detected in the patient cells [61]. Besides, other kinases act under different stresses, which are not relevant to what we know about GALT deficiency to-date. Nevertheless, our revealing of the involvement of Perk branch, but not the Ire1 branch of UPR in galactosemic stress said little about the role of the ATF6 branch in GALT deficiency, which is currently under investigation.
The presence of Perk/eIF2α activation in our mutant mouse model further support our original hypothesis that GalT deficiency causes ER stress as a component of an overall galactosemic stress response. In the ovary, this is associated with follicle loss, altered reproductive cycles, and compromised fertility and fecundity (Fig. 1). We validated Salubrinal’s known action as an eIF2α dephosphorylation inhibitor by showing that whole ovaries from Salubrinal-treated animals exhibit higher levels of phospho-eIF2α than vehicle-treated ovaries (Fig. 4b). Salubrinal treatment has been consistently shown to modulate the expression of Atf4 downstream target genes depends on the context [62–64]. By way of example, Salubrinal can induce up- or downregulation of Chop and BiP expression depending upon the cell or tissue type evaluated and the nature of the stress, indicating the existence of additional, albeit not fully characterized components of regulation [62–65]. In the ovary, we detect reduced BiP in whole ovary in response to Salubrinal, and this associates with the improvements in follicle survival, egg, and offspring production (below).
Our latest data favor a more refined hypothesis where galactosemic stress in the ovaries of GalT-deficient mice triggers the activation of Perk-UPR and enhanced expression of the downstream effectors like Atf4 and Chop. This is associated with an accelerated primordial follicle loss, which in young animals corresponds to reduced ovulation and subfertility, and hastened ovarian demise. However, more work is needed in the future to identify how the Perk-UPR is activated by toxic galactose metabolites, and specifically how the over-expression of Atf4/Chop diminishes the survival of the primordial follicle pool. In addition, it will be important to determine if the Atf6-UPR signaling pathway plays a role in the subfertility phenotype.
The results from the current study of the GalT−/− mouse model have significant implications to patients with Classic Galactosemia. Classic Galactosemia is a CDG [1–8] and therefore, it is not surprising to see signaling pathways closely tied to ER stress response being perturbed in patients [21]. In fact, the MAPK signaling pathway, which has been shown to play a major role in ER stress response [66], is among the most dysregulated in the patient studies [21]. As ER stress response signaling pathways play crucial roles during oocyte and follicle development [41–43], it is conceivable that additional perturbations to ER homeostasis in the disease state would be especially detrimental to ovarian function and development in human patients, as seen in the mouse model.
Finally, here we showed that Salubrinal treatment resulted in an apparent normalization of estrus cycle stage lengths, significantly increased numbers of eggs retrieved after superovulation, and significantly larger litter sizes in young mice, while normalizing the time between litters. Each of these improvements is in addition to our previous demonstration that primordial follicle numbers are significantly larger in animals treated with the drug [26]. Crucially, our initial assessment of offspring of Salubrinal-treated galactosemic mice did not reveal any gross abnormalities, nor alterations in organ weights after necropsy. Thus, Salubrinal treatment in a time window leading up to attempted conception, but removal of the drug before the time windows of successive breeding attempts, can support ovarian function, fertility, and fecundity in an extended fashion. Salubrinal is currently an experimental tool and has not been approved for human consumption. Nevertheless, our proof-of-concept studies suggest that a drug with same mode of action and a proven safety profile could be taken by patients to protect the ovarian reserve, and then withdrawn prior to attempts to conceive. Considering the paramount importance of safety during and after pregnancy, and the need to maximize the health of offspring, our data represent very early support of an attractive future treatment paradigm for Galactosemic women.
5. Conclusions
We found the activation of Perk but not Ire1α in the ovaries of GalT-deficient mice. These findings might unravel additional mechanisms that explain how the UPR contributes to the pathogenesis of subfertility in GalT deficiency. Our findings further support the concept that extension of pro-survival UPR signaling is a potential therapeutic target for patients afflicted with Classic Galactosemia.
Highlights.
Elevated eIF2α phosphorylation is seen in ovaries and fibroblasts of GalT−/− mice.
The Perk branch of the UPR, but not the Ire1α branch, is activated in ovaries and fibroblasts of GalT−/− mice.
Reduced Pi3k/Akt signaling occurs in the ovaries of gonadotrophin-treated GalT−/− mice.
Salubrinal enhances eIF2α phosphorylation and Pi3k/Akt signaling in the ovaries of GalT−/− mice.
Acknowledgement
Research support from NIH grant 1 R01 HD074844, a generous gift from the Dershem Family (Race 4 Jase), and a K2R2R grant award from the Primary Children’s Hospital Foundation (Intermountain Healthcare) to Kent Lai. Studies performed in the Johnson Laboratory were supported by University of Colorado-Denver Department of OB/GYN Research Funds.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Coss KP, et al. , N-Glycan abnormalities in children with Galactosemia. Journal of Proteome Research, 2014. 13: p. 384–394. [DOI] [PubMed] [Google Scholar]
- 2.Liu Y, et al. , N- and O-linked glycosylation of total plasma glycoproteins in galactosemia. Mol Genet Metab, 2012. 106(4): p. 442–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maratha A, et al. , Classical Galactosaemia and CDG, the N-Glycosylation Interface. A Review. JIMD Rep, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maratha A, et al. , Classical galactosemia: novel insights in IgG N-glycosylation and N-glycan biosynthesis. European Journal of Human Genetics, 2016. 24(7): p. 976–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Charlwood J, et al. , Defective galactosylation of serum transferrin in galactosemia. Glycobiology, 1998. 8(4): p. 351–7. [DOI] [PubMed] [Google Scholar]
- 6.Dobbie JA, Holton JB, and Clamp JR, Defective galactosylation of proteins in cultured skin fibroblasts from galactosaemic patients. Ann Clin Biochem, 1990. 27 (Pt 3): p. 274–5. [DOI] [PubMed] [Google Scholar]
- 7.Ornstein KSME, M.E., Berry GT, Roth S, Segal S, Abnormal galactosylation of complex carbohydrates in cultured fibroblasts from patients with galactose-1-phosphate uridyltransferase deficiency. Pediatric Research, 1992. May 31((5)): p. 508–511. [DOI] [PubMed] [Google Scholar]
- 8.Sturiale L, et al. , Hypoglycosylation with increased fucosylation and branching of serum transferrin N-glycans in untreated galactosemia. Glycobiology, 2005. 15: p. 1268–1276. [DOI] [PubMed] [Google Scholar]
- 9.Bosch AM, Classical galactosemia revisited. Journal of Inherited Metabolic Disorders, 2006. 29(4): p. 516–525. [DOI] [PubMed] [Google Scholar]
- 10.Berry GT, Is prenatal myo-inositol deficiency a mechanism of CNS injury in galactosemia?. Journal of Inherited Metabolic Disorders 2011. 34(2): p. 345–355. [DOI] [PubMed] [Google Scholar]
- 11.Isselbacher KJ, Galactose metabolism and Galactosemia. American Journal of medicine, 1959. 26(5): p. 715–723. [DOI] [PubMed] [Google Scholar]
- 12.Kalckar HM, Anderson EP, and Isselbacher KJ, Galactosemia, a Congenital Defect in a Nucleotide Transferase - a Preliminary Report. Proceedings of the National Academy of Sciences of the United States of America, 1956. 42(2): p. 49–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Isselbacher KJ, et al. , Congenital galactosemia, a single enzymatic block in galactose metabolism. Science, 1956. 123(3198): p. 635–6. [DOI] [PubMed] [Google Scholar]
- 14.Gitzelmann R and Bosshard NU, Partial Deficiency of Galactose-1-Phosphate Uridyltransferase. European Journal of Pediatrics, 1995. 154(7): p. S40–S44. [DOI] [PubMed] [Google Scholar]
- 15.Gitzelmann R, Galactose-1-Phosphate in the Pathophysiology of Galactosemia. European Journal of Pediatrics, 1995. 154(7): p. S45–S49. [DOI] [PubMed] [Google Scholar]
- 16.Gitzelmann R, Curtius HC, and Schneller I, Galactitol and Galactose-1-Phosphate in Lens of a Galactosemic Infant. Experimental Eye Research, 1967. 6(1): p. 1-+. [DOI] [PubMed] [Google Scholar]
- 17.Gitzelmann R, Formation of Galactose-1-Phosphate from Uridine Diphosphate Galactose in Erythrocytes from Patients with Galactosemia. Pediatric Research, 1969. 3(4): p. 279-+. [DOI] [PubMed] [Google Scholar]
- 18.Slepak T, et al. , Intracellular galactose-1-phosphate accumulation leads to environmental stress response in yeast model. Molecular Genetics and Metabolism, 2005. 86(3): p. 360–371. [DOI] [PubMed] [Google Scholar]
- 19.Slepak TI, et al. , Involvement of endoplasmic reticulum stress in a novel Classic Galactosemia model. Molecular Genetics and Metabolism, 2007. 92(1–2): p. 78–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De-Souza EA, et al. , The unfolded protein response has a protective role in yeast models of classic galactosemia. Dis Model Mech, 2014. 7(1): p. 55–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Coman DJ, et al. , Galactosemia, a single gene disorder with epigenetic consequences. Pediatr Res, 2010. 67(3): p. 286–92. [DOI] [PubMed] [Google Scholar]
- 22.Gibney PA, et al. , Common and divergent features of galactose-1-phosphate and fructose-1-phosphate toxicity in yeast. Mol Biol Cell, 2018. 29(8): p. 897–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tang M, et al. , Subfertility and growth restriction in a new galactose-1phosphate uridylyltransferase (GALT) – deficient mouse model. European Journal of Human Genetics, 2014. 22: p. 1172–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen W, et al. , Assessment of ataxia phenotype in a new mouse model of galactose-1 phosphate uridylyltransferase (GALT) deficiency. Journal of Inherited Metabolic Disease, 2017. 40(1): p. 131–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Balakrishnan B, et al. , Galactose-1 phosphateuridylyltransferase (GalT) gene: a novel positive regulator of the PI3K/Akt signaling pathway in mouse fibroblasts. Biochemistry and Biophysics Research Communications 2016. 470: p. 205–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Balakrishnan B, et al. , Reversal of aberrant PI3K/Akt signaling by Salubrinal in a GalT-deficient mouse model. Biochim Biophys Acta, 2017. 1863(12): p. 3286–3293. [DOI] [PubMed] [Google Scholar]
- 27.Wu LL, et al. , Endoplasmic reticulum (ER) stress in cumulus-oocyte complexes impairs pentraxin-3 secretion, mitochondrial membrane potential (DeltaPsi m), and embryo development. Mol Endocrinol, 2012. 26(4): p. 562–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Latham KE, Endoplasmic Reticulum Stress Signaling in Mammalian Oocytes and Embryos: Life in Balance. International Review of Cell and Molecular Biology, Vol 316, 2015. 316: p. 227–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang JY, et al. , Inhibition of Endoplasmic Reticulum Stress Improves Mouse Embryo Development. Plos One, 2012. 7(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang JY, et al. , Effect of Endoplasmic Reticulum Stress on Porcine Oocyte Maturation and Parthenogenetic Embryonic Development In Vitro. Biology of Reproduction, 2012. 86(4). [DOI] [PubMed] [Google Scholar]
- 31.Ron D and Walter P, Signal integration in the endoplasmic reticulum unfolded protein response. Nature Reviews Molecular Cell Biology, 2007. 8(7): p. 519–529. [DOI] [PubMed] [Google Scholar]
- 32.Boyce M, et al. , A selective inhibitor of eIF2alpha dephosphorylation protects cells from ER stress. Science, 2005. 307(935–937). [DOI] [PubMed] [Google Scholar]
- 33.Sokka AL, et al. , Endoplasmic reticulum stress inhibition protects against excitotoxic neuronal injury in the rat brain. J Neurosci, 2007. 27(4): p. 901–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nakajima S, et al. , eIF2alpha-Independent Inhibition of TNF-alpha-Triggered NF-kappaB Activation by Salubrinal. Biol Pharm Bull, 2015. 38(9): p. 1368–74. [DOI] [PubMed] [Google Scholar]
- 35.Kuo TF, et al. , Free fatty acids induce transglutaminase 2-dependent apoptosis in hepatocytes via ER stress-stimulated PERK pathways. J Cell Physiol, 2012. 227(3): p. 1130–7. [DOI] [PubMed] [Google Scholar]
- 36.Balakrishnan B, et al. , Reversal of aberrant PI3K/Akt signaling by Salubrinal in a GalT-deficient mouse model. Biochim Biophys Acta, 2017. [DOI] [PubMed] [Google Scholar]
- 37.Schroder M and Kaufman RJ, ER stress and the unfolded protein response. Mutat Res, 2005. 569(1–2): p. 29–63. [DOI] [PubMed] [Google Scholar]
- 38.Donnelly N, et al. , The eIF2alpha kinases: their structures and functions. Cell Mol Life Sci, 2013. 70(19): p. 3493–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sidrauski C, et al. , The small molecule ISRIB reverses the effects of eIF2alpha phosphorylation on translation and stress granule assembly. Elife, 2015. 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Caligioni CS, Assessing reproductive status/stages in mice. Curr Protoc Neurosci, 2009. Appendix 4: p. Appendix 4I. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee MT, Bonneau AR, and Giraldez AJ, Zygotic genome activation during the maternal-to-zygotic transition. Annu Rev Cell Dev Biol, 2014. 30: p. 581–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li L, Zheng P, and Dean J, Maternal control of early mouse development. Development, 2010. 137(6): p. 859–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gosden RG, Oogenesis as a foundation for embryogenesis. Mol Cell Endocrinol, 2002. 186(2): p. 149–53. [DOI] [PubMed] [Google Scholar]
- 44.Wu LL, et al. , Mitochondrial dysfunction in oocytes of obese mothers: transmission to offspring and reversal by pharmacological endoplasmic reticulum stress inhibitors. Development, 2015. 142(4): p. 681–91. [DOI] [PubMed] [Google Scholar]
- 45.Harada M, et al. , Evidence of the activation of unfolded protein response in granulosa and cumulus cells during follicular growth and maturation. Gynecol Endocrinol, 2015. 31(10): p. 783–7. [DOI] [PubMed] [Google Scholar]
- 46.Luo S, et al. , GRP78/BiP is required for cell proliferation and protecting the inner cell mass from apoptosis during early mouse embryonic development. Mol Cell Biol, 2006. 26(15): p. 5688–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Back SH, et al. , ER stress signaling by regulated splicing: IRE1/HAC1/XBP1. Methods, 2005. 35(4): p. 395–416. [DOI] [PubMed] [Google Scholar]
- 48.Bravo R, et al. , Endoplasmic reticulum and the unfolded protein response: dynamics and metabolic integration. Int Rev Cell Mol Biol, 2013. 301: p. 215–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schmitz ML, et al. , The Crosstalk of Endoplasmic Reticulum (ER) Stress Pathways with NF-kappaB: Complex Mechanisms Relevant for Cancer, Inflammation and Infection. Biomedicines, 2018. 6(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gardner BM, et al. , Endoplasmic Reticulum Stress Sensing in the Unfolded Protein Response. Cold Spring Harbor Perspectives in Biology, 2013. 5(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wu H, Ng BS, and Thibault G, Endoplasmic reticulum stress response in yeast and humans. Biosci Rep, 2014. 34(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ma YJ and Hendershot LM, The unfolding tale of the unfolded protein response. Cell, 2001. 107(7): p. 827–830. [DOI] [PubMed] [Google Scholar]
- 53.Pavitt GD and Ron D, New insights into translational regulation in the endoplasmic reticulum unfolded protein response. Cold Spring Harb Perspect Biol, 2012. 4(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Margariti A, et al. , XBP1 mRNA splicing triggers an autophagic response in endothelial cells through BECLIN-1 transcriptional activation. J Biol Chem, 2013. 288(2): p. 859–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Clauss IM, et al. , In situ hybridization studies suggest a role for the basic region-leucine zipper protein hXBP-1 in exocrine gland and skeletal development during mouse embryogenesis. Dev Dyn, 1993. 197(2): p. 146–56. [DOI] [PubMed] [Google Scholar]
- 56.Michalak M and Gye MC, Endoplasmic reticulum stress in periimplantation embryos. Clin Exp Reprod Med, 2015. 42(1): p. 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sha HB, et al. , The IRE1 alpha-XBP1 Pathway of the Unfolded Protein Response Is Required for Adipogenesis. Cell Metabolism, 2009. 9(6): p. 556–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hetz C, et al. , Unfolded protein response transcription factor XBP-1 does not influence prion replication or pathogenesis. Proceedings of the National Academy of Sciences of the United States of America, 2008. 105(2): p. 757–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Toda H, et al. , Behavioral stress and activated serotonergic neurotransmission induce XBP-1 splicing in the rat brain. Brain Research, 2006. 1112: p. 26–32. [DOI] [PubMed] [Google Scholar]
- 60.Aufenberg C, et al. , Spinal cord trauma activates processing of xbp1 mRNA indicative of endoplasmic reticulum dysfunction. Journal of Neurotrauma, 2005. 22(9): p. 1018–1024. [DOI] [PubMed] [Google Scholar]
- 61.Wang Q, Groenendyk J, and Michalak M, Glycoprotein Quality Control and Endoplasmic Reticulum Stress. Molecules, 2015. 20(8): p. 13689–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Logsdon AF, et al. , Altering endoplasmic reticulum stress in a model of blast-induced traumatic brain injury controls cellular fate and ameliorates neuropsychiatric symptoms. Front Cell Neurosci, 2014. 8: p. 421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Matsuoka M and Komoike Y, Experimental Evidence Shows Salubrinal, an eIF2alpha Dephosphorylation Inhibitor, Reduces Xenotoxicant-Induced Cellular Damage. Int J Mol Sci, 2015. 16(7): p. 16275–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dey S, et al. , Both transcriptional regulation and translational control of ATF4 are central to the integrated stress response. J Biol Chem, 2010. 285(43): p. 33165–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hetz C, The unfolded protein response: controlling cell fate decisions under ER stress and beyond. Nat Rev Mol Cell Biol, 2012. 13(2): p. 89–102. [DOI] [PubMed] [Google Scholar]
- 66.Darling NJ and Cook SJ, The role of MAPK signalling pathways in the response to endoplasmic reticulum stress. Biochim Biophys Acta, 2014. 1843(10): p. 2150–63. [DOI] [PubMed] [Google Scholar]