Abstract
Despite combination antiretroviral therapies making HIV a chronic rather than terminal condition for many people, the prevalence of HIV-associated neurocognitive disorders (HAND) is increasing. This is especially problematic for children living with HIV. Children diagnosed HAND rarely display the hallmark pathology of HIV encephalitis in adults, namely infected macrophages and multinucleated giant cells in the brain. This finding has also been documented in rhesus macaques infected perinatally with simian immunodeficiency virus (SIV). However, the extent and mechanisms of lack of susceptibility to encephalitis in perinatally HIV-infected children remain unclear. In the current study we compared brains of macaques infected with pathogenic strains of SIV at different ages to determine neuropathology, correlates of neuroinflammation and potential underlying mechanisms. Encephalitis was not found in the macaques infected within 24h of birth despite similar high plasma viral load and high monocyte turnover. Macaques developed encephalitis only when they were infected after 4 months of age. Lower numbers of CCR5-positive cells in the brain, combined with a less leaky blood-brain barrier, may be responsible for the decreased virus infection in the brain and consequently the absence of encephalitis in newborn macaques infected with SIV.
Keywords: HIV Encephalitis, Pediatric HIV Infection, Blood-Brain Barrier
Introduction
Over 36.7 million persons were living with HIV in 2016, with children (<15 years old) accounting for 2.1 million (AVERT 2018). In children infected with HIV surrounding birth (pre-, peri-, and post-parturition), there is a higher clinical incidence of neurologic disease and deficits than in adults infected with HIV; however, there is no pathological correlate in pediatric patients (Kure et al. 1991; Vazeux et al. 1992; Wilfert et al. 1994; Westmoreland et al. 1999; Mothi et al. 2011). In agreement, SIV-infected neonatal rhesus macaques have decreased levels of infected macrophages and multinucleated giant cells (MNGCs), a hallmark of HIV encephalitis (HIVE) and simian immunodeficiency virus encephalitis (SIVE), and viral RNA is rarely detected in those cells (Sharer et al. 1988; Lane et al. 1996b; Westmoreland et al. 1999). This decreased pathology following SIV infection could be attributed to an “immunosuppression of immaturity” in neonates (Lane et al. 1996b; Bowenkamp 2002).
HIV, and its closely related virus, SIV, enter the central nervous system (CNS) during primary infection utilizing the normal immune surveillance of monocytes and turnover of perivascular macrophages (PVMs) (Peluso et al. 1985; Ivey et al. 2009b). Once in the CNS, reservoirs of latent infection are formed (Clements et al. 2002; Burdo et al. 2010; Veenstra et al. 2017), including microglia and questionably astrocytes (Johnson et al. 1988; Sharer et al. 1988; Tornatore et al. 1994; Ivey et al. 2009a). Brain microvascular endothelial cells are also activated and the blood-brain barrier (BBB) is weakened by loss of tight junction expression (Ivey et al. 2009a, b). This can be measured by determining leakage of plasma proteins including fibrinogen into the brain parenchyma (Renner et al. 2012a).
The main goal of combination antiretroviral therapy (cART) is to decrease a patient’s viral load. However, despite decreased plasma viral loads with cART, patients still develop complications of HIV, including HIV-associated neurocognitive disorders (HAND), a collective term for HIV-related neurological impairments (Heaton et al. 2010). HAND includes HIV-encephalitis (HIVE), a usually chronic pathology characterized by the presence of infiltrating leukocytes, perivascular cuffs, and the presence of MNGCs (Heaton et al. 2010), formed by the fusion of macrophages in response to chronic inflammation (McNally and Anderson 2011; Milde et al. 2015; Delery and Maclean 2018). Due to their close genetic, anatomical and immunological proximity to humans, the rhesus macaque is considered as the premier animal model to study HIV infection and comorbidities.
Plasma viral load and monocyte turnover are predictive markers of HAND (Beck et al. 2015). We previously reported that increased turnover of monocytes was indicative of tissue macrophage infection and destruction by SIV in infected adult rhesus macaques. This was also observed in the brain in association with higher SIV tissue viral loads and increased rate of SIVE (Hasegawa et al. 2009; Burdo et al. 2010; Cai et al. 2015). In addition, those studies reported that the more rapid disease progression to AIDS in neonatal rhesus macaques correlated with earlier and sustained higher monocyte turnover following SIV infection (Merino et al. 2017; Sugimoto et al. 2017). This study attempts to unravel the paradox of a more rapid disease course, of high viral loads and yet no significant lesions in brains.
Materials and Methods
Animals:
A total of 130 Indian rhesus macaques (Macaca mulatta) including 8 uninfected animals from Tulane National Primate Research Center (TNPRC) were retrospectively selected for these studies. The 122 SIV-infected animals were divided into three groups based on their age at infection; neonates (0–3 months), juveniles (3 months–2 years) and adolescents/adults (>2 years), as is standard for rhesus macaque studies (Robillard, 2016). The inclusion/ exclusion criteria are as follows: Indian rhesus macaques, infected with SIV strains mac251, mac239, ΔB670, or −0302, and deceased with a complete histology report. Route of inoculum administration was not taken into consideration for this study, although only 2 animals were infected via the intrarectal route: all other animals were infected intravenously. We excluded animals that had received antiretroviral therapy (ART) for extended periods of time and responded to treatment (demonstrated by a decrease in plasma viral load). However, we included animals that had received ART for less than a week and “ART non-responders”: animals with elevated plasma viral load despite ART treatment. Eight uninfected animals were included in this study for CCR5 immunohistochemistry and analysis. Forty-eight hours prior to necropsy, either 5-bromo-2’-deoxyuridine (BrdU; Sigma Aldrich, St. Louis, MO) or 5-ethynyl-20-deoxyuridine (EdU; Molecular Biology, Carlsbad, CA) thymidine analogs, were intravenously administered to some of the animals. BrdU was intravenously injected at a concentration of 30 mg/kg for neonatal animals and 60 mg/kg for adult animals, while EdU was administered at a dose of 50 mg/kg for adult animals (Cai et al. 2015; Sugimoto et al. 2017). Twenty-four hours after BrdU or EdU injection, blood samples were obtained to determine monocyte turnover. Animals were housed and treated in accordance with “NIH Guide for the Care and Use of Laboratory Animals” (National Research Council, National Academic Press, Washington, DC, USA, 1996) and all treatments were pre-approved by the Tulane University Institution Animal Care and Use Committee (IACUC).
Necropsy and collection of tissues:
Animals were humanely euthanized according to the standards set forth by the Office of Laboratory Animal Welfare (OLAW). Brain tissue was flash-frozen in liquid nitrogen or optimal cutting temperature (OCT) compound for brain tissue viral load, or formalin-fixed and paraffin-embedded (FFPE) for later immunofluorescent/immunohistochemical staining. SIVE was defined by the presence of multinucleated giant cells (MNGCs) on H&E stained sections of FFPE brain tissues examined by pathologists at TNPRC.
Determination of Monocyte Turnover:
Blood was collected at necropsy and centrifuged to collect plasma. The remaining blood was then layered over lymphocyte separation medium (Mediatech) and centrifuged at 2500 RPM for 20 min. Peripheral blood mononuclear cells (PBMCs) were collected and frozen using freezing media or stained for flow cytometry. For calculation of monocyte turnover within 48 h of necropsy, the PBMCs were stained using previously described methods and analyzed on an LSR II flow cytometer (BD Biosciences) to detect the surface markers of monoclonal antibodies and intracellular BrdU or EdU (Hasegawa et al. 2009; Cai et al. 2015; Sugimoto et al. 2017). Data was analyzed using FlowJo software (TreeStar).
Plasma Viral Load Determination:
Plasma viral load was measured by the TNPRC Pathogen Detection and Quantification Core (PDQC) from plasma collected and frozen at necropsy. The limit of detection of SIV RNA was 83 copies per milliliter of plasma (Monjure et al. 2014).
Brain Associated Viral Load:
Quantitative real time PCR (qPCR) assays were completed on fresh-frozen brain samples snap frozen in liquid nitrogen or OCT using previously published methodologies (Robichaux et al. 2016; Lee et al. 2016). Duplicates were run for sample reliability. The limit of detection per 1,000,000 cells was 50 copies for SIV RNA and 10 copies for SIV DNA (Robichaux et al. 2016; Lee et al. 2016). For statistical comparisons, samples with undetectable levels of SIV were reported as 25 for SIV RNA and 5 for SIV DNA, and all values were log-10 transformed (Hornung and Reed 1990).
Immunofluorescent and Immunohistochemical Staining:
For immunofluorescence (IF), sections were blocked with 5% normal goat serum for 30 min before the Glut-1 (SMP498, Invitrogen), diluted in phosphate-buffered saline containing 0.2% fish skin gelatin (PBS/FSG), was applied at room temperature for 1 h. After a PBS/FSG bath, secondary antibodies conjugated to Alexa Fluor 488 were diluted in PBS/FSG and applied for 1 h at room temperature. Subsequently fibrinogen (ab58207, Abcam) primary and Alexa Fluor 594 secondary antibodies were applied as described. After IF staining was complete, sections were washed before being soaked in a quenching solution of 10mM CuSO4 for 45 min. The sections were then washed with distilled water and mounted using a coverslip and Aqua-Mount aqueous mounting medium.
For immunohistochemistry, sections were incubated with 5% goat serum in tris-buffered saline (TBS) for 1 h followed immediately by CCR5 (Proteintech) incubation for 1 h at room temperature. After washing, sections were incubated with a biotinylated secondary antibody (Vector Laboratories) for 30 min. Dako Antibody Diluent (Dako, Carpinteria, CA) was used for both primary and secondary antibody dilutions. Following another wash, sections were incubated for 30 min with an avidin-biotin peroxidase complex (Vectastain ABC Elite kit, Vector Laboratories) and developed with diaminobenzidine (DAB; Dako) with Mayer’s Hematoxlyin (Dako) used as a nuclear counterstain. Sections were dehydrated and mounted using VectaMount (Vector Laboratories).
Quantification of CCR5 Expression:
Ten gray matter and 10 white matter images were randomly selected from each slide and imaged at 20x using a Nikon Coolscope light microscope, with uniform settings across images. CCR5+ cells within vessels were excluded from analyses. Positive staining was recorded and entered into GraphPad Prism 7.2 (GraphPad Software, La Jolla, CA) for graphical representation and statistical analysis.
Quantification of Fibrinogen Leakage:
The percent of vessels demonstrating fibrinogen extravasation was determined by running linear plot profiles on the green and red channels of individual vessels captured at 40x via ImageJ and graphing the resulting numerical data in GraphPad as dual overlay histograms. The histograms were then analyzed to determine whether the fibrinogen was above background levels outside of the two primary Glut-1 peaks; vessels that displayed this phenotype were considered to be extravasated. The number of extravasated vessels was divided by the total number of vessels measured to obtain the % of extravasated vessels. A total of 25 vessels were examined from each animal via random imaging, but were required to meet the selection criteria listed below. The criteria for vessel selection dictates that vessels must be less than 10μm in luminal diameter and that no single radius can be more than twice the length of the smallest luminal radius. This ensures that all vessels being imaged are nearly-horizontal cross sections therefore fibrinogen outside the luminal area is extravasated not running through the lumen on a vessel that has been cut at an angle.
Statistics:
All statistics were performed using GraphPad Prism 7.2. For length of infection a Student’s t-test was performed between juveniles with and without encephalitis and between adults with and without encephalitis. For plasma viral load and monocyte turnover a one-way ANOVA followed by Tukey’s multiple comparisons test was utilized. For brain viral load, the data was log10 transformed and analyzed using a one-way ANOVA with Tukey’s multiple comparisons test. CCR5 and fibrinogen data was analyzed using Welch’s two-tailed t-test. Statistical significance was defined as p<0.05.
Results
Age-Dependent Incidence of SIVE:
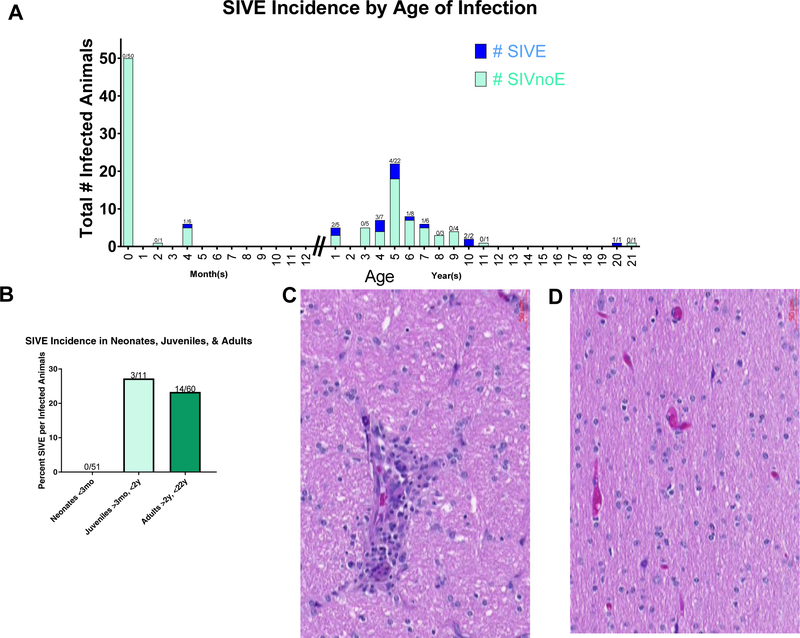
Previous studies have noted the lack of evidence of neonatal encephalitis as defined by the presence of MNGCs (Sharer et al. 1986; Kure et al. 1991; Vazeux et al. 1992; Lane et al. 1996a; Westmoreland et al. 1999). We first set out to examine retrospectively the incidence of SIVE in the TNPRC archives. Out of 51 neonates infected at, or near, birth, none developed encephalitis (Figure 1A, B). The first case of encephalitis found in the TNPRC archives was observed when an animal was infected at 4 months of age (Figure 1A). Approximately 23% of adults (infected >2y & <15y) and 27% juveniles (infected >3mo & <2y) developed encephalitis compared to zero neonate (infected <3mo) (Figure 1B). A representative image of an SIV-infected adult with an MNGC (Figure 1C), and an SIV-infected neonate without encephalitis (Figure 1D) are included.
Figure 1. Incidence of SIV infection in neonates, juveniles and adults.
The archive of CNS tissues from macaques infected with SIV was examined. No macaques developed encephalitis when infected as neonates (A). The earliest observed case of encephalitis was four months post birth. After one year of age, approximately 25% of animals developed encephalitis (B). SIVE was defined by the presence of multinucleated giant cells (C), which is noticeable absent from neonatally infected macaques (D).
Adults were noted to have increased rates of other reported brain pathologies compared to neonates and juveniles (Table 1). Overall, almost half of all SIV-infected adults or juveniles experienced some form of brain pathology, while only 10% of SIV-infected neonates developed any kind of brain pathology, and most were due to secondary infections.
Table 1.
Increased rates of reported brain pathologies in adults compared to neonates and juveniles
| Neonates-51 | Juveniles-11 | Adults-60 | |
|---|---|---|---|
| Encephalitis: | 0 | 3 | 14 |
| Other Brain Pathology: | 6 | 2 | 13 |
|
|
Septicemia | Gliosis | Meningoencephalitis |
| Perivascular Inflammation | Cerebral Necrosis | Perivascular Inflammation | |
| Bacterial Meningoencephalitis |
|
Perivascular Inflammation Meninges | |
| Acute inflammation Lesions-Herpes B | Lymphoplasmacytic Infiltration & Necrosis | ||
| Choroid Plexus Inflammation | Neutrophilic Meninigitis | ||
| Gliosis | Necrosis & Hemorrhage | ||
| CMV Meningeal Infection | |||
| Subacute Inflammation |
Peripheral indicators of encephalitis:
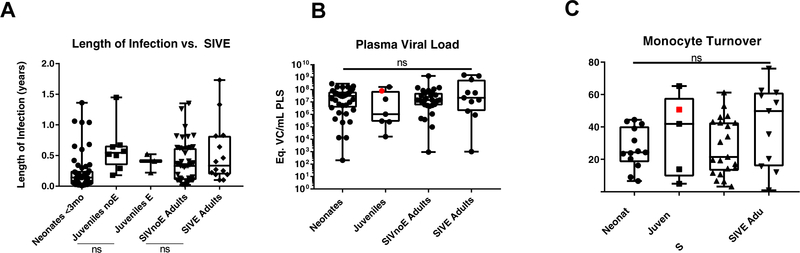
Next, we sought to examine physiological markers that have been previously published as being correlates of encephalitis. These include length of infection, plasma viral load, and monocyte turnover. Rapid disease progression (less than 150 days post infection) has been associated with encephalitis in adults (Westmoreland et al. 1999). Compared to adults, infected neonates develop AIDS more rapidly (Li et al. 2007; Wang et al. 2010). Conversely, it appears that rapid disease progression in perinatally infected macaques does not lead to development of encephalitis. To investigate this inconsistency, we compared the length of infection between SIV-infected juvenile rhesus macaques with and without encephalitis (SIVE and SIVnoE, respectively) and SIV-infected adult rhesus macaques with and without encephalitis. Length of infection was not significantly correlated with development of encephalitis in either of the age groupings (Figure 2A, p=0.3712, p=0.1566). We note here that many of the neonatal animals were euthanized earlier due to the study design and therefore pose a potential confounding variable. We were also limited by the number of juvenile animals available as they are not of a widely-studied age range.
Figure 2. Peripheral indicators of encephalitis.
The interval from day of infection until necropsy for each animal was determined from the TNPRC records system. There was no significant difference between the animal groupings based on length of infection (A, neonates n=51, juveniles with no encephalitis n=8, juveniles with encephalitis n=3, SIVnoE adults n=46, SIVE adults n=14). The plasma viral load was determined for animals with available plasma collected at necropsy. There was no significant difference between the groups (B, neonates n=39, juveniles n=7, SIVnoE adults n=29, SIVE adults n=11; p=0.6005). There was also no statistical difference between the groups for monocyte turnover, based on % BrdU-labeled CD14+ cells (C, p=0.2440). The red point indicates the one juvenile animal with encephalitis that had both plasma samples available for testing.
Next, we examined the plasma viral load of SIV-infected neonates, juveniles, adults without encephalitis (SIVnoE), and adults with encephalitis (SIVE). There was no significant statistical difference between the means of the four groups (Figure 2B, p=0.6005). The red point indicates the one juvenile animal with encephalitis.
Increased monocyte turnover is a predictive indicator of progression to AIDS (Hasegawa et al. 2009; Beck et al. 2015). There was no significant difference between the BrdU-labeled CD14+ monocytes across the groups examined (Figure 2C, p=0.2440). The red point indicates the one juvenile macaque that developed encephalitis. Thus, SIV-infected neonatal rhesus macaques have a decreased incidence of SIV encephalitis despite having similar monocyte turnover and plasma viral loads to SIVnoE and SIVE adults.
Central Nervous System-Associated Virus:
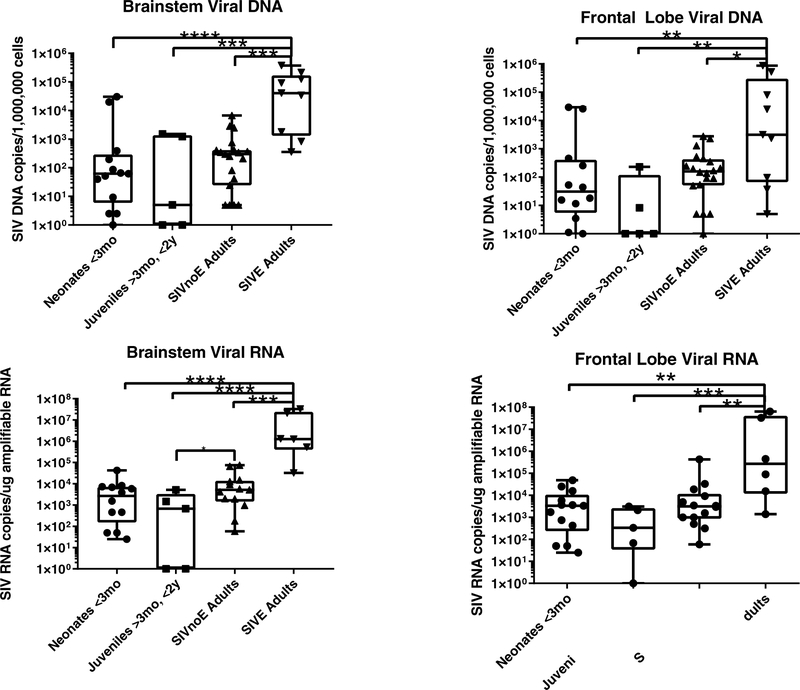
As there were no definitive lesions by histology, and none of our peripheral indicators of encephalitis was positive, we next determined if virus had entered the CNS. Positive DNA viral load indicates that virus has incorporated into the host DNA, while detectable viral RNA indicates that there is viral gene expression. Viral DNA was found in the brainstem and frontal lobes in all groups (Figure 3A, B) and viral RNA was also found in all groups (Figure 3C. D). Viral DNA & RNA levels were consistently higher in the brainstem versus the frontal lobe for all groups (Figure 3A–D). As could be anticipated, animals with encephalitis had significantly higher viral DNA and RNA levels than every other group (Figure 3A–D, p<0.05). It was also noted that SIV-infected adults without encephalitis had significantly higher brainstem RNA viral levels compared to juveniles (Figure 3C, p=0.043). However, juveniles also had the widest range of viral DNA and RNA levels out of all the groups indicating a possible developmental difference which could be examined further.
Figure 3. Cell-associated viral load.
Animals with encephalitis had higher levels of viral DNA in both the brainstem (A, neonates n=13, juveniles n=5, SIVnoE adults n=19, SIVE adults n=9; p<0.0001) and frontal lobe (B, neonates n=12, juveniles n=5, SIVnoE adults n=20, SIVE adults n=9; p<0.0013) than any of the other groups of animals. There was no significant difference between the non-encephalitic groups. To determine if there was ongoing viral replication, viral RNA was determined. Encephalitic animals had higher RNA levels than the other groups in both brainstem (C, neonates n=12, juveniles n=5, SIVnoE adults n=13, SIVE adults n=6; p<0,0001) and frontal lobe (D, neonates n=13, juveniles n=5, SIVnoE n=14, SIVE adults n=6; p<0.0004). There was also higher viral RNA in adults, even without encephalitis, than juvenile animals (C).
Quantification of Virus Susceptible Cells in Neonates and Adults:
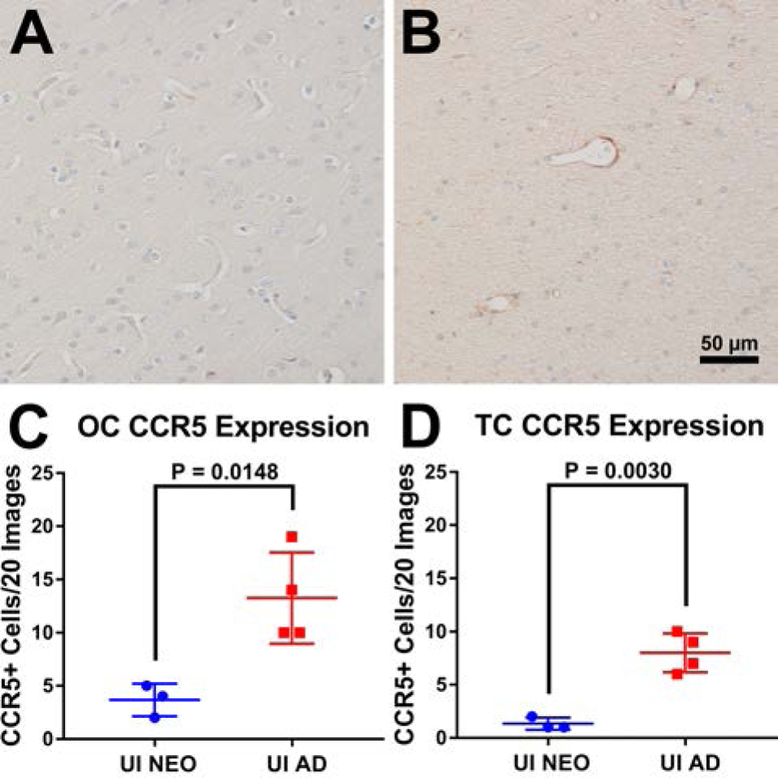
CCR5 is a major co-receptor necessary for HIV/SIV virus binding and fusion on T-cells, monocytes, and macrophages. Due to this, we sought to examine expression of CCR5 within neonatal and adult macaque brains. Using immunohistochemistry, little to no CCR5 expression was observed within temporal and occipital cortical tissues of uninfected neonates (Figure 4A) with uninfected adults showing more expression (Figure 4B), especially around vessels. This indicates that even in the uninfected brain environment adults have more cells that are susceptible to SIV infection than neonates which could explain the lack of encephalitis seen in this age group (Figure 1B). To verify these observations, ten random images of white matter and ten random images of grey matter were taken of each macaque and total CCR5+ cell counts were obtained. Adults were found to have significantly more CCR5+ cells within the brain compared to neonates (Figure 4C, p=0.0148). The observed lack of encephalitis of neonatal macaques may be in part due to fewer CCR5+ cells being present within the brain compared to adult macaques, leading to the chances of SIV being able to take up residence within the cells of the brain being lower.
Figure 4. Quantitation of viral susceptible cells in the CNS.
Single-label immunohistochemistry for CCR5 counterstained with hematoxylin showed expression within neonatal (A) and adult (B) macaque brain. When the total counts of CCR5+ staining in 20 random images per macaque were compared between neonatal and adult groups, adults were found to express significantly more CCR5+ cells than neonates in occipital cortical sections (C, p = 0.0148; two-tailed t-test; neonates n = 3; adults n = 4) and temporal cortical sections (D, p = 0.0030; two-tailed t-test; neonates n = 3; adults n = 4).
Analysis of Blood Brain Barrier Permeability:
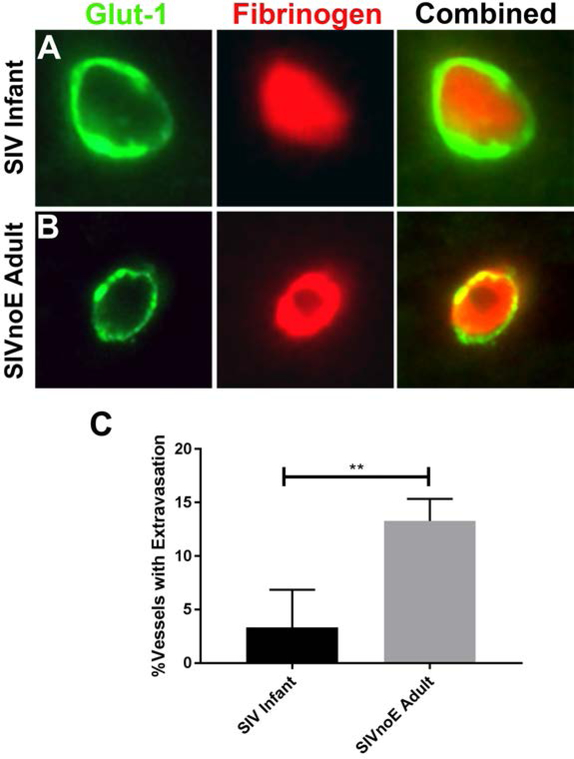
To further evaluate why macaques infected as neonates vs adults do not develop SIVE, we measured the degree of BBB breakdown after SIV infection in both groups. To examine this, we performed double immunofluorescent stains using antibodies against Glucose transporter 1 (Glut-1), an endothelial marker and fibrinogen, a serum protein. As described in the materials and methods, any fibrinogen that was determined to be outside of the luminal space was considered to be extravasated. Six adult and six neonatal SIVnoE animals were examined to determine the average percent of vessels demonstrating fibrinogen extravasation for each group (Figure 5A, B). Interestingly, while perinatally infected pediatric animals showed less than 2% of vessels with fibrinogen extravasation on average, adult SIVnoE animals averaged above 12% (Figure 5C). This significant difference in BBB permeability may indicate an increased risk of viral neuroinvasion in adults that is not present in pediatric animals suggesting that the more competent neonatal BBB may play a role in their decreased brain tissue viral loads.
Figure 5. Permeability of blood-brain barrier.
The endothelial cells of the CNS (green) prevented most of the leakage of fibrinogen (red) into the parenchyma of pediatric animals infected with SIV (A). In contrast, fibrinogen could be observed in more blood vessels of adults infected with SIV (B, as overlap of red and green staining). The proportion of vessels with measurable leakage of fibrinogen was significantly higher in adults than infants (C).
Discussion
The results presented here elaborate on an interesting phenomenon: the lack of pathological encephalitis in rhesus macaques infected with SIV as neonates. Previous reports had demonstrated a lack of encephalitis in neonatal rhesus macaques infected within 24 h of birth, despite having elevated plasma viral loads and neurological deficits (Westmoreland et al. 1999). This included fewer and harder to detect SIV-infected cells in the CNS. This phenomenon had been hypothesized to be due to maturation-dependent host factors or an “immunosuppression of immaturity” (Westmoreland et al. 1999; Bowenkamp 2002). The current study proposes that maturation-dependent host factors in the BBB, such as permeability, and levels of virus-susceptible cells in the CNS, could account for this phenomenon.
We did not observe MNGCs, the defining factor for encephalitis, in the CNS of any of the 51 neonatal Indian rhesus macaques examined. The first case of encephalitis was not seen until an animal was infected after 4 months of age, and juveniles had a similar incidence of encephalitis as adults at approximately 25% (3 out of 11 juveniles compared with 14 of 60 adults). Neonatal animals infected during the first 3 months of age also had lower levels of other brain pathologies than any other groups, with approximately 10% of neonates noted to have any CNS pathology (6 of 51). This compared to 20% for juveniles (2 out of 11), and 25% for adults (13 out of 60). Once encephalitis is included, this approaches 50% for juveniles (5 out of 11), and adults (27 out of 60).
Our previous studies have observed distinct differences in plasma viral load and monocyte turnover in macaques infected with SIV as neonates compared with adults (Hasegawa et al. 2009; Merino et al. 2017; Sugimoto et al. 2017). It should be noted that those studies examined dynamic changes across the stages of SIV infection: acute, chronic or AIDS. In this study, we divided our animals into the age ranges neonate, juvenile and adults, regardless of stage of infection. We also further split our adult groups into adults with and without encephalitis, a distinction we did not include in the previous studies. The current study, which did not separate terminal AIDS from scheduled necropsy, also reports the last plasma viral load determined, and only the monocyte turnover immediately before necropsy. Our observation of no significant difference in monocyte turnover in rhesus macaques with or without encephalitis corroborates a report in pig-tailed macaques (Beck et al. 2015). Finally, our previous studies included animals that had received ART and whose infection was controlled on ART. These variances within groups could potentially be due to the larger sample sizes, representation of developmental changes, or the result of brain damage and subsequent responses to viral infection as the animals were at different stages of disease progression.
Despite the lack of encephalitis, the neonatal brain may still be a reservoir for SIV (Westmoreland et al. 1999; Clements et al. 2002; Burdo et al. 2010). Although DNA & RNA viral loads in neonates and juveniles had a wider range and were significantly lower than adults with encephalitis, the presence of viral DNA indicates that virus had successfully incorporated into cells in the brain. Further, measurable viral RNA indicates an ongoing, albeit low level, productive infection. It has been postulated that S/HIV can enter the CNS through the choroid plexus, which is located inside the lateral, 3rd, and 4th ventricles and is often part of the FFPE brainstem block. This could be one reason why virus is so often found in the caudal region of the brain (Falangola et al. 1995), and why we observed higher levels of viral DNA and RNA levels in the brainstem than in the frontal lobe (Figure 3). However, this does not explain the lack of encephalitis in neonatal SIV infection.
After the initial peak of virus during acute infection, there is lower viral load in the CNS (Clements et al. 2002). Thus, the lower levels seen in our neonates and juveniles could be due to several of them undergoing scheduled necropsy during the chronic phase of infection. DNA levels were reported to remain the same throughout asymptomatic and acute infection. The high levels of DNA and RNA in the SIVE adults indicates that virus had established and was in active viral replication, which could lead to increases in DNA viral loads as the viral infection spreads across the brain.
HIV-1 requires the presence of chemokine co-receptors CCR5 and/or CXCR4 in order to enter T-cells and macrophages, and increased levels of CCR5 receptors is associated with an increased risk of transmission (Berger et al. 1999; Wilen et al. 2012; Shaw and Hunter 2012). As primary infection most frequently requires CCR5+ cells (Harbison et al. 2014; Joseph et al. 2015), and as the CNS viral reservoir is established shortly after infection (Clements et al. 2002; Soulas et al. 2011; Sturdevant et al. 2015; Veenstra et al. 2017), there may be innate differences between neonates and adults, including levels of susceptible cells, as well as the tightness of the BBB. That uninfected adult macaques have higher counts of CCR5+ cells in both gray and white matter than uninfected neonates could potentially explain why SIV-infected adults have higher incidence of encephalitis than SIV-infected neonates. Future studies are warranted where the levels of CCR5+ cells in the CNS are reduced prior to infection.
SIVE development in adult rhesus macaques is correlated with decreased expression of zonularoccludens-1, a tight junction protein (Renner et al. 2012a). This is interesting in the context of our results because we demonstrated low levels of blood vessel leakage in SIV-infected neonatal macaques, which would indicate a tighter BBB. It has been posited, but not conclusively demonstrated, that neonatal rhesus macaques lack an intact, functional BBB at birth, however data only exists for murine and human models. It is therefore hard to argue whether the virus entered the brains of the neonates more easily because they lacked the BBB and therefore the Trojan horse model may not be necessary. While it is widely accepted that virus enters the brain within 14 days, recent studies using orally infected neonatal animals have detected viral RNA in the brain of an animal, 96 h after initial exposure (Amedee et al. 2018). However, 40% of infants had detectable viral DNA in at least one tissue 48 h after exposure, and more at 72 and 96 hours respectively, demonstrating the wide variability of animal and infectious differences in neonatal rhesus macaques. Future studies are needed to examine BBB, and blood-CSF, barrier development in rhesus macaques.
While this study focuses on infiltrating myeloid cells, we acknowledge that resident macrophages in the brain are also important to HIV pathology. Microglia are permissive for infection with HIV (Cenker et al. 2017) and may become viral reservoirs (Castellano et al. 2017). In late-stage disease, microglia can express CD163 and upregulate IBA-1, both classical markers for macrophages (Borda et al. 2008; Bortell et al. 2018). Viral proteins induce microglial activation, including cytokine secretion (Renner et al. 2012b), and infection has led to aging-associated dysfunction (Chen et al. 2017). This “inflamm-aging” could be mediated through Sirt-1, a chromatic-modifying protein, which regulates numerous pathways including inflammation and aging (Bortell et al. 2018). The effects of aging in microglial responses is therefore also of importance. There are distinct morphological changes in microglia as macaques age (Robillard et al. 2016). There are increased complexity and altered cytokine responses between neonatal and adult microglia (Harry and Kraft 2012; Robillard et al. 2016). There is increased activation of both microglia and astrocytes as primates age, even in eugeric aging (Robillard et al. 2016). This is likely linked to priming and increased activation with age and could underlie a lack of resolution of inflammation (Norden and Godbout 2013). Thus, it is likely that microglia are chronically activated following HIV/SIV infection, and could represent a source of inflamm-aging.
This research is significant to the field of HIV/AIDS research, as well as developmental neuroimmunology, as it highlights differences in neurological disease progression between neonates and adults. If this effect extends beyond SIV infections, the implications are significant because it demonstrates an innate protection to neonatal brains that should be examined. Once the mechanisms of this protection are elucidated, they could be manipulated in order to protect the brain from further damage, or in new treatment designs.
Acknowledgements:
1. Real-Time PCR expertise provided by the PDQC at TNPRC.
2. Nedra Lacour in Angela Amedee’s lab for technical support.
3. Division of comparative pathology, especially prosectors, pathologists, and histology core.
Funding: This research was supported by PHS grants: P51-OD011104 (formerly RR00164) for the Tulane National Primate Research Center base grant, R21-MH108458 (W-K.K.), R01-MH107333 (W-K.K.), R01-AI097059 (M.J.K.), R01-HL125054 (M.J.K.), R33-AI110163 (M.J.K.), R21-AI116198 (M.J.K.), R21-DA041017 (M.J.K.), R01-NS104016 (A.G.M.), R21-MH113517 (A.G.M.)
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Author Disclosure Statement: No competing financial interests exist.
References:
- Amedee AM, Phillips B, Jensen K, et al. (2018) Early Sites of Virus Replication After Oral SIVmac251 Infection of Infant Macaques: Implications for Pathogenesis. AIDS Res Hum Retroviruses 34:286–299. doi: 10.1089/aid.2017.0169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- AVERT (2018) Children, HIV and AIDS In: AVERT Glob. Inf. Educ. HIV AIDS. https://www.avert.org/professionals/hiv-social-issues/key-affected-populations/children Accessed 6 Jul 2018 [Google Scholar]
- Beck SE, Queen SE, Witwer KW, et al. (2015) Paving the path to HIV neurotherapy: predicting SIV CNS disease. Eur J Pharmacol 759:303–12. doi: 10.1016/j.ejphar.2015.03.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger EA, Murphy PM, Farber JM (1999) Chemokine Receptors as HIV-1 Co-Receptors: Roles in Viral Entry, Tropism, and Disease. Annu Rev Immunol 17:657–700. doi: 10.1146/annurev.immunol.17.1.657 [DOI] [PubMed] [Google Scholar]
- Borda JT, Alvarez X, Mohan M, et al. (2008) CD163, a marker of perivascular macrophages, is up-regulated by microglia in Simian immunodeficiency virus encephalitis after haptoglobin-hemoglobin complex stimulation and is suggestive of breakdown of the blood-brain barrier. Am J Pathol 172:725–737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortell N, Basova L, Najera JA, et al. (2018) Sirtuin 1-Chromatin-Binding Dynamics Points to a Common Mechanism Regulating Inflammatory Targets in SIV Infection and in the Aging Brain. J Neuroimmune Pharmacol 13:163–178. doi: 10.1007/s11481-017-9772-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowenkamp KE (2002) Central nervous system immune activation and the neuropathogenesis of pediatric acquired immune deficiency syndrome in the Simian immunodeficiency virus-infected rhesus macaque (Macaca mulatta). University of Connecticut [Google Scholar]
- Burdo TH, Soulas C, Orzechowski K, et al. (2010) Increased monocyte turnover from bone marrow correlates with severity of SIV encephalitis and CD163 levels in plasma. PLoS Pathog 6:1–13. doi: 10.1371/journal.ppat.1000842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Y, Sugimoto C, Arainga M, et al. (2015) Preferential destruction of interstitial macrophages over alveolar macrophages as a cause of pulmonary disease in SIV-infected rhesus macaques HHS Public Access. J Immunol 15:4884–4891. doi: 10.4049/jimmunol.1501194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellano P, Prevedel L, Eugenin EA (2017) HIV-infected macrophages and microglia that survive acute infection become viral reservoirs by a mechanism involving Bim. Sci Rep 7:12866. doi: 10.1038/s41598-017-12758-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cenker JJ, Stultz RD, McDonald D (2017) Brain Microglial Cells Are Highly Susceptible to HIV-1 Infection and Spread. AIDS Res Hum Retroviruses 33:1155–1165. doi: 10.1089/AID.2017.0004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen NC, Partridge AT, Sell C, et al. (2017) Fate of microglia during HIV-1 infection: From activation to senescence? Glia 65:431–446. doi: 10.1002/glia.23081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements JE, Babas T, Mankowski JL, et al. (2002) The Central Nervous System as a Reservoir for Simian Immunodeficiency Virus (SIV): Steady-State Levels of SIV DNA in Brain from Acute through Asymptomatic Infection. J Infect Dis 186:905–913. doi: 10.1086/343768 [DOI] [PubMed] [Google Scholar]
- Delery EC, Maclean AG (2018) Chronic Viral Neuroinflammation: Speculation on Underlying Mechanisms. Viral Immunol 00:1–8. doi: 10.1089/vim.2018.0093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falangola MF, Hanly A, Galvao-Castro B, Petito CK (1995) HIV infection of human choroid plexus: a possible mechanism of viral entry into the CNS. J Neuropathol Exp Neurol 54:497–503 [DOI] [PubMed] [Google Scholar]
- Harbison C, Zhuang K, Gettie A, et al. (2014) Giant cell encephalitis and microglial infection with mucosally transmitted simian-human immunodeficiency virus SHIV SF162P3N in rhesus macaques. J Neurovirol 20:62–72. doi: 10.1007/s13365-013-0229-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harry GJ, Kraft AD (2012) Microglia in the developing brain: A potential target with lifetime effects. Neurotoxicology 33:191–206. doi: 10.1016/j.neuro.2012.01.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa A, Liu H, Ling B, et al. (2009) The level of monocyte turnover predicts disease progression in the macaque model of AIDS. Immunobiology 114:2917–2925. doi: 10.1182/blood-2009-02-204263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Clifford DB, Franklin DR, et al. (2010) HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology 75:2087–96. doi: 10.1212/WNL.0b013e318200d727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornung RW, Reed LD (1990) Estimation of Average Concentration in the Presence of Nondetectable Values. Appl Occup Environ Hyg 5:46–51. doi: 10.1080/1047322X.1990.10389587 [DOI] [Google Scholar]
- Ivey NS, Maclean AG, Lackner AA (2009a) Acquired immunodeficiency syndrome and the blood-brain barrier. J Neurovirol 15:111–122. doi: 10.1080/13550280902769764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivey NS, Renner NA, Moroney-Rasmussen T, et al. (2009b) Association of FAK activation with lentivirus-induced disruption of blood-brain barrier tight junction-associated ZO-1 protein organization. J Neurovirol 15:312–23. doi: 10.1080/13550280902998413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson RT, Mcarthur J, Narayan O (1988) The neurobiology of human immunodeficiency virus infection. FASEB J 2:2970–2981. doi: 10.1096/fasebj.2.14.2846395 [DOI] [PubMed] [Google Scholar]
- Joseph SB, Arrildt KT, Sturdevant CB, Swanstrom R (2015) HIV-1 target cells in the CNS. J Neurovirol 21:276–89. doi: 10.1007/s13365-014-0287-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kure K, Llena JF, Lyman WD, et al. (1991) Human immunodeficiency virus-1 infection of the nervous system: An autopsy study of 268 adult, pediatric, and fetal brains. Hum Pathol 22:700–710. doi: 10.1016/0046-8177(91)90293-X [DOI] [PubMed] [Google Scholar]
- Lane JH, Sasseville VG, Smith MO, et al. (1996a) Neuroinvasion by simian immunodeficiency virus coincides with increased numbers of perivascular macrophages/microglia and intrathecal immune activation. J Neurovirol 2:423–32 [DOI] [PubMed] [Google Scholar]
- Lane JH, Tarantal AF, Pauley D, et al. (1996b) Localization of simian immunodeficiency virus nucleic acid and antigen in brains of fetal macaques inoculated in utero. Am J Pathol 149:1097–104 [PMC free article] [PubMed] [Google Scholar]
- Lee M, Kim W-K, Kuroda MJ, et al. (2016) Development of real-time PCR for quantitation of simian immunodeficiency virus 2-LTR circles. J Med Primatol 45:215–221. doi: 10.1111/jmp.12244 [DOI] [PubMed] [Google Scholar]
- Li J, Bentsman G, Potash MJ, Volsky DJ (2007) Human immunodeficiency virus type 1 efficiently binds to human fetal astrocytes and induces neuroinflammatory responses independent of infection. BMC Neurosci 8:31. doi: 10.1186/1471-2202-8-31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNally AK, Anderson JM (2011) Macrophage fusion and multinucleated giant cells of inflammation In: Dittmar T, Zanker K (eds) Cell Fusion in Health and Disease: Advances in Experimental Medicine and Biology. Springer, Dordrecht, pp 97–111 [DOI] [PubMed] [Google Scholar]
- Merino KM, Allers C, Didier ES, Kuroda MJ (2017) Role of Monocyte/Macrophages during HIV/SIV Infection in Adult and Pediatric Acquired Immune Deficiency Syndrome. Front Immunol 8:1693. doi: 10.3389/fimmu.2017.01693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milde R, Ritter J, Tennent GA, et al. (2015) Multinucleated giant cells are specialized for complement-mediated phagocytosis and large target destruction. Cell Rep 13:1937–48. doi: 10.1016/j.celrep.2015.10.065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monjure CJ, Tatum CD, Panganiban AT, et al. (2014) Optimization of PCR for quantification of simian immunodeficiency virus genomic RNA in plasma of rhesus macaques (Macaca mulatta) using armored RNA. J Med Primatol 43:31–43. doi: 10.1111/jmp.12088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mothi SN, Karpagam S, Swamy VHT, et al. (2011) Paediatric HIV--trends & challenges. Indian J Med Res 134:912–9. doi: 10.4103/0971-5916.92636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norden DM, Godbout JP (2013) Review: microglia of the aged brain: primed to be activated and resistant to regulation. Neuropathol Appl Neurobiol 39:19–34. doi: 10.1111/j.1365-2990.2012.01306.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peluso R, Haase A, Stowring L, et al. (1985) A Trojan Horse mechanism for the spread of visna virus in monocytes. Virology 147:231–6 [DOI] [PubMed] [Google Scholar]
- Renner NA, Redmann RK, Moroney-Rasmussen T, et al. (2012a) S100β as a novel and accessible indicator for the presence of monocyte-driven encephalitis in AIDS. Neuropathol Appl Neurobiol 38:162–74. doi: 10.1111/j.1365-2990.2011.01200.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renner NA, Sansing HA, Morici LA, et al. (2012b) Microglia activation by SIV-infected macrophages: Alterations in morphology and cytokine secretion. J Neurovirol 18:213–221. doi: 10.1007/s13365-012-0100-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robichaux S, Lacour N, Bagby GJ, Amedee AM (2016) Validation of RPS13 as a reference gene for absolute quantification of SIV RNA in tissue of rhesus macaques. J Virol Methods 236:245–251. doi: 10.1016/j.jviromet.2016.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robillard KN, Lee KM, Chiu KB, MacLean AG (2016) Glial cell morphological and density changes through the lifespan of rhesus macaques. Brain Behav Immun 55:60–69. doi: 10.1016/j.bbi.2016.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharer LR, Baskin GB, Cho ES, et al. (1988) Comparison of simian immunodeficiency virus and human immunodeficiency virus encephalitides in the immature host. Ann Neurol 23 Suppl:S108–12. doi: 10.1002/ANA.410230727 [DOI] [PubMed] [Google Scholar]
- Sharer LR, Eptein LG, Cho E-S, et al. (1986) Pathologic features of AIDS encephalopathy in children: Evidence for LAV/HTLV-III infection of brain. Hum Pathol 17:271–284. doi: 10.1016/S0046-8177(83)80220-2 [DOI] [PubMed] [Google Scholar]
- Shaw GM, Hunter E (2012) HIV transmission. Cold Spring Harb Perspect Med 2:. doi: 10.1101/cshperspect.a006965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soulas C, Conerly C, Kim W-K, et al. (2011) Recently Infiltrating MAC387+ Monocytes/Macrophages: A Third Macrophage Population Involved in SIV and HIV Encephalitic Lesion Formation. Am J Pathol 178:2121–2135. doi: 10.1016/J.AJPATH.2011.01.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturdevant CB, Joseph SB, Schnell G, et al. (2015) Compartmentalized Replication of R5 T Cell-Tropic HIV-1 in the Central Nervous System Early in the Course of Infection. PLOS Pathog 11:e1004720. doi: 10.1371/journal.ppat.1004720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto C, Merino KM, Hasegawa A, et al. (2017) Critical Role for Monocytes/Macrophages in Rapid Progression to AIDS in Pediatric Simian Immunodeficiency Virus-Infected Rhesus Macaques. J Virol 91:JVI.00379–17. doi: 10.1128/JVI.00379-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tornatore C, Chandra R, Berger JR, Major EO (1994) HIV-1 infection of subcortical astrocytes in the pediatric central nervous system. Neurology 44:481–7 [DOI] [PubMed] [Google Scholar]
- Vazeux R, Lacroix-Ciaudo C, Blanche S, et al. (1992) Low levels of human immunodeficiency virus replication in the brain tissue of children with severe acquired immunodeficiency syndrome encephalopathy. Am J Pathol 140:137–44 [PMC free article] [PubMed] [Google Scholar]
- Veenstra M, León-Rivera R, Li M, et al. (2017) Mechanisms of CNS Viral Seeding by HIV+ CD14+ CD16+ Monocytes: Establishment and Reseeding of Viral Reservoirs Contributing to HIV-Associated Neurocognitive Disorders. MBio 8:. doi: 10.1128/mBio.01280-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Xu H, Pahar B, et al. (2010) Simian immunodeficiency virus selectively infects proliferating CD4+ T cells in neonatal rhesus macaques. Blood 116:4168–74. doi: 10.1182/blood-2010-03-273482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westmoreland SV, Williams KC, Simon MA, et al. (1999) Neuropathogenesis of simian immunodeficiency virus in neonatal rhesus macaques. Am J Pathol 155:1217–28. doi: 10.1016/S0002-9440(10)65224-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilen CB, Tilton JC, Doms RW (2012) HIV: Cell Binding and Entry. Cold Spring Harb Perspect Med 2:a006866. doi: 10.1101/cshperspect.a006866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilfert CM, Wilson C, Luzuriaga K, Epstein L (1994) Pathogenesis of Pediatric Human Immunodeficiency Virus Type 1 Infection. Source J Infect Dis 170:286–292 [DOI] [PubMed] [Google Scholar]