Abstract
Human papillomavirus (HPV) infection is the major risk factor for anal dysplasia that may progress to squamous cell carcinoma of the anus (SCCA). We have previously shown that systemic administration of a PI3K/mTOR inhibitor (BEZ235), an autophagic inducer, results in decreased SCCA in our HPV mouse model. In this study, we investigate the impact of local, topical application of a BEZ235 on tumor-free survival, histopathology, PI3K/mTOR, and autophagy. The rationale for investigating a topical formulation is the localized nature of anal dysplasia/cancer and the goal for creating a clinically translatable formulation to decrease anal carcinogenesis. In this study, HPV transgenic mice were given no treatment, topical BEZ235, topical DMBA (carcinogen), or both topical DMBA+BEZ235. Mice were assessed for tumor development and treatment-related toxicities. Tissue was evaluated for histology, PI3K/mTOR inhibition (pS6 and pAkt), and autophagy (LC3β and p62). DMBA alone mice had an average of 16.9 weeks tumor-free survival, whereas mice receiving both DMBA+ topical BEZ235 had 19.3 weeks (P<0.000001). Histopathology revealed a significant decrease in dysplasia/carcinoma with the addition of topical BEZ235 to DMBA (P<0.000001). Comparing DMBA vs DMBA+BEZ235, topical BEZ235 resulted in a significant decrease in both pS6 and pAkt (P<0.001). Compared to no treatment mice, both BEZ235 and DMBA+BEZ235 treated mice had significantly higher LC3β expression, signifying autophagic induction (P<0.01), whereas DMBA, BEZ235, and DMBA+BEZ235 treated mice had significantly lower p62 expression, signifying active autophagy (P<0.0005). In conclusion, consistent with systemic delivery, topical application of BEZ235 shows decreased anal carcinogenesis through the activation of autophagy.
Keywords: anal cancer, autophagy, HPV, cancer prevention, carcinogenesis
Introduction
Anal dysplasia and anal cancer are ever-growing health problems (Leeds et al., 2016). Human papillomavirus (HPV) infection is a major risk factor for the development of anogenital dysplasia and cancer (Machalek et al., 2012; Silverberg et al., 2015; Engels et al., 2017; Wang et al., 2017). Even with the introduction of the HPV vaccine in 2007, the incidence of both anal dysplasia and anal cancer has continued to increase (Machalek et al., 2012). HPV infection, coupled with immunosuppression, heightens the risk for anal dysplasia and anal cancer development (Laga et al., 1992; Palefsky, 1999; Grulich et al., 2007; Collett et al., 2010; Engels et al., 2011; Engels et al., 2017).
Medical therapies for patients with anogenital dysplasia are available; however, each is associated with a high disease recurrence and many have adverse side effects (e.g., skin irritation, pain) that result in poor patient compliance. Such therapies include topical trichloroacetic acid (TCA) which has been investigated in high-risk HIV patients (Cranston et al., 2014). In this study recurrence rates were as high as 75% at 6 months. This therapy also requires that the clinician apply the drug frequently (monthly) under sedation due to discomfort during application (Singh et al., 2009). Twice-weekly topical 5-fluorouracil (5-FU) has also been investigated as a therapy for anal dysplasia (Richel et al., 2010). In an intention-to-treat analysis, 57% of patients showed a response at 6 months but 85% experienced side effects during the treatment period. As with TCA, no randomized controlled trials investigating 5-FU have been conducted. Finally, the most commonly utilized therapy is Imiquimod, a nonspecific immune modifier, also applied topically, which has been investigated in anal dysplasia patients (Kreuter et al., 2008). In a small, single-arm trial, the mean follow-up time was 30.3 months after treatment, and the mean time to recurrence was 24.6 months. Recurrence occurred in 26% of the study population (5 of 19 patients), and the majority of patients enrolled in this study had previously received Imiquimod treatment. In a prospective, placebo-controlled trial of Imiquimod in HIV-positive patients with high-grade dysplasia of the anal canal, 61% showed absence of high-grade lesions over 36 months of follow-up (Fox et al., 2010). Of note, 21% of the patients in this study were determined to have poor compliance, along with a “high rate of soreness”. Despite this being a more rigorous trial design with somewhat promising results, Imiquimod has not received FDA approval for intra-anal use in the setting of dysplasia, and, perhaps most importantly, prospective trials comparing either two or all three of the above therapies have not been pursued. As a result, we continue to lack a well-developed standard of care for anal dysplasia.
Given the lack of efficacy and significant side-effects of current therapies, novel strategies are needed to treat anal dysplasia and prevent anal cancer development. To better target these therapies, a more complete understanding of the molecular mechanism of anal carcinogenesis is required, since the above therapies are not specifically targeted to diseased tissue nor are they molecularly targeted. The molecular link between HPV infection and both cervical and anal dysplasia is well established. Inhibition of tumor suppressor proteins p53 and Rb occurs via binding of HPV oncoproteins (E6, E7), leading to degradation of these key regulators. In turn, this leads to the increased activity of potent cell growth and proliferation effectors such as mTOR, AKT and PI3K (Xi et al., 2015; Hoppe-Seyler et al., 2017). All of these pathways are upstream inhibitors of a pathway called autophagy. Autophagy is an evolutionarily well-conserved, intracellular catabolic process that identifies and degrades damaged proteins, DNA, and organelles (Guo et al., 2013). Under normal conditions, a functional autophagic mechanism maintains normal cellular health. Recently, we published results showing that perturbations in autophagy caused by the E6 and E7 HPV oncoproteins promote carcinogenesis (Carchman et al., 2016). E6 and E7 activate mTOR, which is an upstream inhibitor of autophagy, leading to the development of anal dysplasia and eventually SCCA (Li et al., 2015).
To build on our anal carcinogenesis studies, we subsequently examined the impact of pharmacologic induction and inhibition of autophagy via the systemic administration of BEZ235 and chloroquine, respectively (Rademacher et al., 2017). BEZ235, a dual PI3K/mTOR inhibitor, led to autophagic induction and a corresponding increase in tumor-free survival, and decreased tumor incidence. On the other hand, inhibition of autophagosome/lysosome fusion with chloroquine significantly decreased tumor-free survival. Based on these data, we had strong preliminary evidence that autophagy plays an important role in preventing anal carcinogenesis. The current study evaluates the efficacy of BEZ235, when applied topically, in preventing anal carcinogenesis in our HPV mouse model of anal cancer.
In the ensuing study, we are examining the role of topical therapy for several reasons. First, under optimal conditions, immunosuppressed patients with anal dysplasia are taking several systemic drugs to treat their HIV or prevent organ rejection. In both cases, it is important avoid potential drug-drug interactions that would alter the efficacy of those therapies by introducing yet another systemic therapy. Second, anal dysplasia and anal cancer are localized disease processes, and therefore likely best treated with localized therapy. Moreover, unlike some current treatment options for anal dysplasia, administration of these topical therapies does not require a medical professional or specialized equipment. In general, topical therapies have several advantages over systemic treatments, including ease of administration, the ability to deliver the drug substance more selectively to a specific site, avoidance of fluctuations of drug levels, improved patient compliance, and enhanced suitability for self-administration. The ultimate goal is to develop an effective topical therapy with little to no local or systemic toxicity. The desired therapy will treat established anal dysplasia effectively, prevent anal dysplasia progression, and, most importantly, decrease anal cancer development.
Methods
Mice
K14E6/E7 mice were generated as previously described (Stelzer et al., 2010). This mouse model is the only preclinical HPV anal carcinogenesis model available and is a widely accepted model established by Dr. Paul Lambert. These mice express the HPV genotype 16 oncoproteins, E6 and E7, in their epithelium. When treated with a carcinogen, 7,12 dimethylbenz[a]anthracene (DMBA), these mice develop anal dysplasia that progresses into invasive squamous cell carcinoma, similar to that seen in humans. All mice were maintained in an American Association for Accreditation of Laboratory Animal Care-approved Wisconsin Institute for Medical Research (WIMR) Animal Care Facility. The experiments were performed in accordance with approved Institutional Animal Care and Use Committee protocol M005946.
7,12 dimethylbenz[a]anthracene (DMBA) treatment
Weekly topical application of 0.12μmole of DMBA (60% acetone/40% dimethylsulfoxide, DMSO) to the anus of K14E6/E7 mice was performed (Stelzer et al., 2010; Carchman et al., 2016). Mice were monitored weekly for tumor development and growth. Animals were sacrificed at 20 weeks post initiation of DMBA treatment. 20 weeks was the chosen time point given that in these mice 100% of mice will have overt anal cancer after 20 weeks of DMBA treatment. Control mice were age-matched K14E6/E7 mice not treated with DMBA.
Topical BEZ235
BEZ235 (LC Laboratories, Woburn, MA) was dissolved in PEG-300 at various concentrations along a gradient curve of 0.5%, 1%, 2.5% and 5%, and applied to five mice per group for two weeks. The lowest concentration that resulted in a decrease in pAkt and pS6, 1% w/v (data not shown), was then used for all subsequent treatments. BEZ235 then was applied topically to the anus of 40 K14E6/E7 mice five days a week (Monday-Friday) for 20 weeks. Of these forty mice, twenty mice were treated with DMBA along with topical BEZ235 while the remaining 20 mice were treated only topical BEZ235. Pharmacologic controls were K14E6/E7 mice that received no BEZ235, with and without DMBA treatments.
Immunofluorescence (IF) and immunohistochemistry (IHC)
Paraffin sections were deparaffinized, rehydrated, subjected to antigen retrieval, permeabilized, and blocked with 10% donkey serum per standard protocols (Barth et al., 2010). To examine autophagy, the sections were stained with monoclonal rabbit antibody against LC3β (1:50 in 10% donkey serum in PBS; Santa Cruz Biotechnology) or monoclonal mouse antibody for p62 (1:200 in 10% donkey serum in PBS; Abcam) overnight at 4° Celsius. Sections were then washed and stained with donkey anti-rabbit Fluor 488 and donkey anti-mouse Fluor 594 (1:500 in 10% donkey serum in PBS; Life Technologies) for one hour in the dark at room temperature. Slides were then counterstained with DAPI. Slides were imaged using the Ziess Axio Imager M2 imaging system. Images at 200x magnification were obtained. Each 200x image was imported into Image J version 2.0.0 (Fiji distribution) and underwent the follow processing. Images were split in the three channels (488, 594 and DAPI). All images were thresholded using the default dark background. The areas of interest, the anorectal transition zone (ATZ), were manually selected and then RawIntDen measured for the region of interest to measure the intensity of the fluorescent signal. The RawIntDen was then normalized for the area of the region selected (RawIntDen/Area).
The protein expression of pAkt and pS6 were assessed using immunohistochemistry, as they are downstream products of PI3K and mTOR activation, respectively. Paraffin-embedded sections were deparaffinized, rehydrated, subjected to citrate buffer antigen retrieval, permeabilized with 2N HCl, and then blocked with 5% horse serum. Sections were then stained with a monoclonal rabbit antibody against pAkt (1:50 in 5% horse serum in PBS; antibody #3787, Cell Signaling Technology) or a polyclonal rabbit antibody against pS6 (1:50 in 5% horse serum in PBS; antibody #2211, Cell Signaling Technology) overnight at 4° Celsius. Each slide was then washed and incubated with VectaStain universal secondary antibody (1:50 in 5% horse serum in PBS; Vector Laboratories). Slides were then subjected to R.T.U. VectaStain ABC reagent (Vector Laboratories). Finally, sections were incubated with 3,3’-diaminobenzidine (DAB) substrate (DAB Peroxidase (HRP) Substrate; Vector Laboratories), counterstained with hematoxylin, dehydrated, and mounted. Light microscopy was performed and images at 200x magnification were acquired using the Ziess Axio Imager M2 imaging system. Images were then analyzed with ImageJ and analyzed as described above.
Histology
Anal tissue was collected and fixed in 4% paraformaldehyde for 24 hours, and then placed in 70% ethanol. After fixation, the tissues were processed, embedded in paraffin, and serially sectioned at 5μm thickness. Every fourth section was stained with hematoxylin and eosin (H&E) and evaluated by a gastrointestinal pathologist for evidence of dysplasia (low-grade versus high-grade), carcinoma in situ, or invasive carcinoma.
Statistical analysis
In order to detect at least a fifty percent difference in tumor incidence with a Type I error rate of 5% and a Type II error rate of 20% (80% power) between the treated and untreated groups, 12 mice per group were needed. Fisher’s two-sided exact t-test was used to determine differences between treatment groups in tumor incidence at 20 weeks. A Kaplan-Meier analysis with Log-Rank testing was applied to determine differences in time to tumor onset. The comparisons between treatment groups of average histologic grade and expression of LC3β, p62, pAkt, and pS6 were made using a one-way ANOVA with Tukey post-hoc testing. SPSS version 24 (IBM, Armonk, North Castle, NY) was utilized to perform each of these analyses. Statistical significance was defined as P ≤ 0.05.
Results
Immunohistochemistry and immunofluorescence
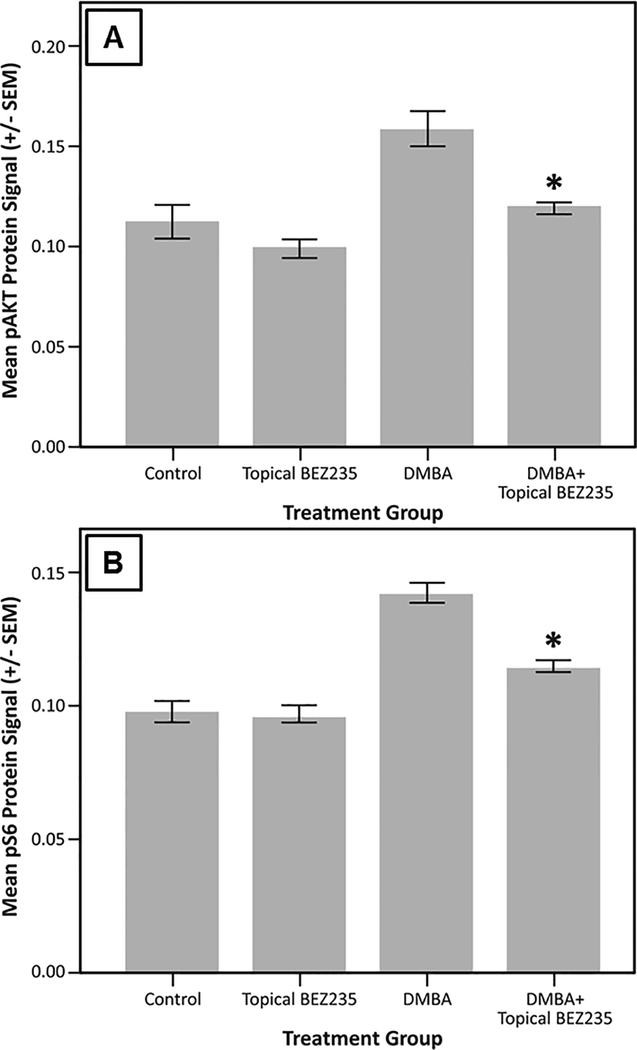
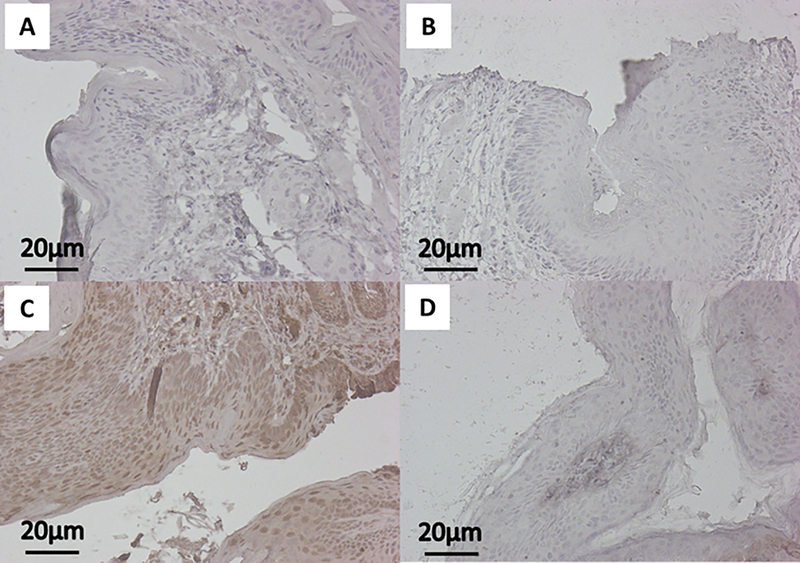
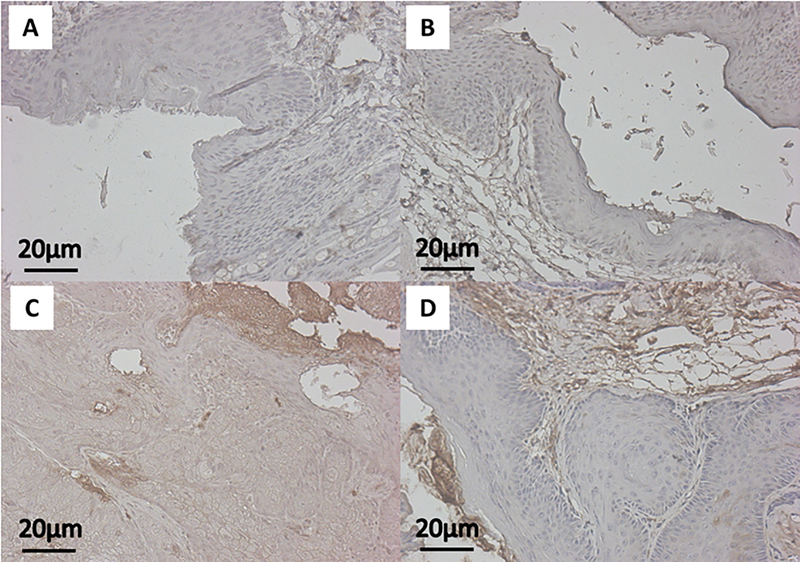
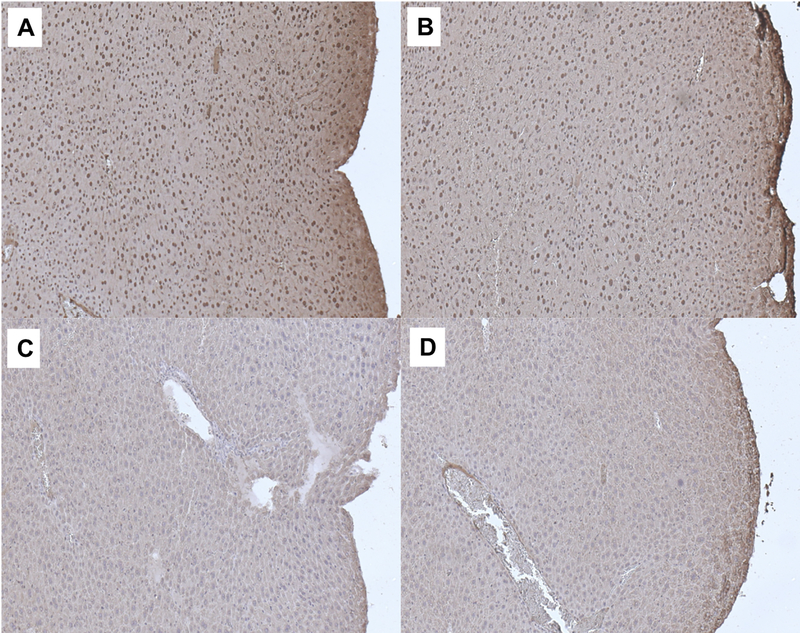
Using IHC staining for downstream effectors within the PI3K/mTOR pathway, namely pAkt and pS6, we assessed the on-target effects of BEZ235 when applied topically to the anus. We did not see a significant difference in pAkt between no treatment mice and those treated with topical BEZ235 alone (Fig. 1A; mean ± SEM; 0.112 ± 0.008 vs 0.099 ± 0.005; P = 0.40). We did, however, see a significant reduction in pAkt expression comparing DMBA mice with those treated with both DMBA and topical BEZ235 (Fig. 1A; mean ± SEM; 0.159 ± 0.008 vs 0.119 ± 0.002; P = 0.00023). Similar findings were seen with pS6, with no treatment and topical BEZ235 treated mice not differing (Fig. 1B; mean ± SEM; 0.098 ± 0.004 vs 0.097 ± 0.003; P = 1.0), whereas, mice treated with DMBA and topical BEZ235 saw a reduction in pS6 compared to those treated with DMBA alone (Fig. 1B; mean ± SEM; 0.115 ± 0.002 vs 0.142 ± 0.004; P = 0.000014). Representative images of pAkt IHC for treatment groups are displayed in Figure 2; those mice treated with DMBA alone showed a diffuse pAkt signal, which is not seen in mice treated with DMBA and topical BEZ235. Evaluation of pS6 protein expression gave corresponding results (Fig. 3), with mice treated with DMBA alone show a diffuse pS6 signal that is significantly reduced with the addition of topical BEZ235. IHC analysis of pS6 and pAkt expression of in the liver of treated and control mice was undertaken to evaluate for first-pass effects and evidence of systemic absorption. Our analyses showed no change in expression of these proteins, indicating little to no systemic absorption of BEZ235 (Fig. 4).
Figure 1. Mean pAkt and pS6 protein expression.
K14E6/E7 mice were given no treatment (Control), topical BEZ235 alone (Topical BEZ235), DMBA alone (DMBA), or DMBA and topical BEZ235 concurrently (DMBA+ Topical BEZ235). To evaluate the on-target effect of topical BEZ235 treatment, fixed tissue sections were stained for pAkt and pS6 using immunohistochemistry. Compared to mice treated with DMBA alone, panel A shows the addition of topical BEZ235 treatment to DMBA significantly decreased pAkt protein expression (mean ± SEM; 0.159 ± 0.008 vs 0.119 ± 0.002; *P = 0.00023). In panel B, the addition of topical BEZ235 treatment to DMBA treatment significantly decreased pS6 compared to DMBA controls (mean ± SEM; 0.115 ± 0.002 vs 0.142 ± 0.004; *P = 0.000014).
Figure 2: Represenatative immunohistochemistry images of pAkt expression.
Images of anal tissue from K14E6/E7 mice were stained for pAkt (brown) protein expression with hematoxylin counterstaining (blue). The panels correspond with the following groups: A) No treatment (Control), B) Topical BEZ235, C) DMBA, and D) DMBA+Topical BEZ235.
Figure 3: Represenatative immunohistochemistry images of pS6 expression.
Images show anal tissue from K14E6/E7 mice stained for pS6 (brown) protein expression with hematoxylin counterstaining (blue). The panels correspond with the following groups: A) No treatment (Control), B) Topical BEZ235, C) DMBA, and D) DMBA+Topical BEZ235.
Figure 4: Represenatative immunohistochemistry images of pAkt and pS6 expression in livers of mice with and without topical BEZ235 treatment.
Images show liver tissue from K14E6/E7 mice stained for pAkt and pS6 (brown) protein expression with hematoxylin counterstaining (blue). The panels correspond with the following groups: A) DMBA without Topical BEZ235 for pAkt, B) Topical BEZ235 with DMBA for pAkt, C) DMBA without Topical BEZ235 for pS6, and D) Topical BEZ235 with DMBA for pS6.
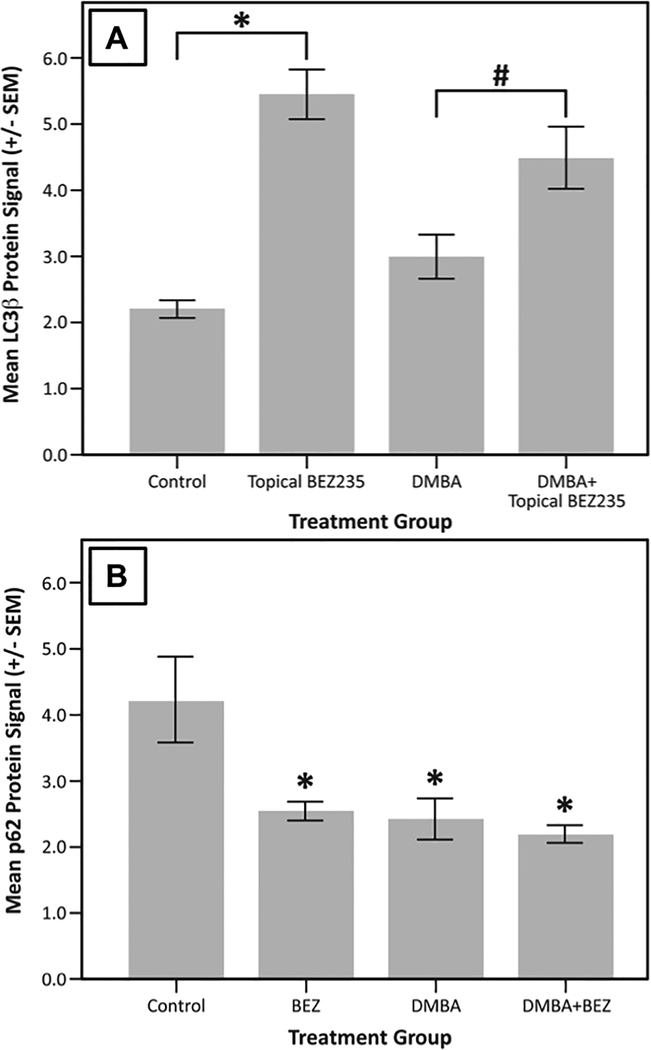
To assess autophagic induction, LC3β protein expression was measured using IF staining. Mice receiving topical BEZ235 alone or DMBA and topical BEZ showed, on average, the highest levels of LC3β protein expression (Fig. 5A). Compared with no treatment mice, mice treated with topical BEZ235 alone had significantly higher LC3β protein expression (Fig 5A; mean ± SEM; 5.46 ± 0.37 vs 2.20 ± 0.13; P < 1e-7). Mice treated with both DMBA and topical BEZ235 also had significantly higher mean LC3β protein expression compared to the no treatment group and those treated with DMBA alone, (Fig. 5A; mean ± SEM; 4.50 ± 0.48 vs 2.20 ± 0.13, P = 0.00032; 4.50 ± 0.48 vs 3.00 ± 0.33, P = 0.016). Overall, as seen in Figure 5A, induction of LC3β protein expression is highest in those mice treated with topical BEZ235 alone or with a combination of topical BEZ235 and DMBA.
Figure 5. Mean LC3β and p62 protein expression.
K14E6/E7 mice were given no treatment (Control), topical BEZ235 alone (Topical BEZ235), DMBA alone (DMBA), or DMBA and topical BEZ235 concurrently (DMBA+ Topical BEZ235). LC3β protein expression at the anal transition zone (ATZ) was measured via immunofluorescent staining. In panel A, mice treated with topical BEZ235 alone showed higher LC3β expression than untreated controls (mean ± SEM; 5.46 ± 0.37 vs 2.20 ± 0.13; * P < 1e-7). Furthermore, those mice treated with topical BEZ235 and DMBA showed, on average, significantly higher levels of LC3β expression than those treated with DMBA alone (mean ± SEM; 4.50 ± 0.48 vs 3.00 ± 0.33, # P = 0.016). As shown in panel B, mice treated with topical BEZ235, DMBA, or DMBA+ topical BEZ235 had, on average, significantly lower mean p62 protein expression than the Control group (*P < 0.001 for all comparisons), signifying active autophagy in each of the treatment groups.
We then measured p62 protein expression using IF to assess autophagic function in our treatment groups, as p62 is degraded solely via the autophagic pathway. Mice treated with topical BEZ235, DMBA, and DMBA plus topical BEZ235 all showed, on average, reductions in p62 protein expression, signifying completion of the autophagic pathway (Fig. 5B; P < 0.05 for all comparisons with the no treatment group).
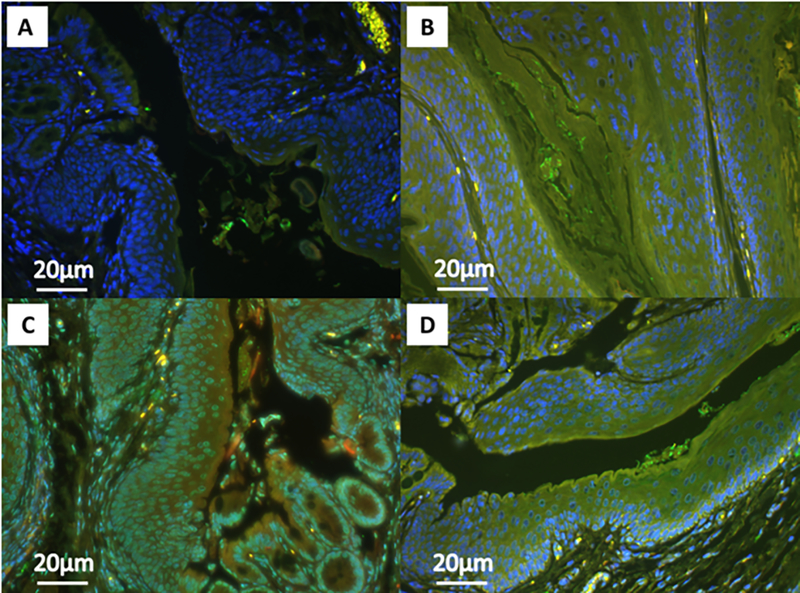
Figure 6 displays representative images from our IF analysis of LC3β and p62 expression. LC3β expression is shown in green, p62 in red, and DAPI nuclear staining in blue. Panels B, C, and D show significantly more LC3β signal with DMBA and/or topical BEZ235 treatment, and significantly lower p62 signal, representing autophagic induction and autophagic degradation, respectively.
Figure 6: Represenatative immunoflourescence images of LC3β and p62 expression.
Images show anal tissue from K14E6/E7 mice stained for LC3β (green) and p62 (red) protein expression with DAPI nuclear staining (blue). Structures appearing yellow indicate co-localization of the LC3β and p62 signals. The panels correspond with the following groups: A) No treatment (Control), B) Topical BEZ235, C) DMBA, and D) DMBA+Topical BEZ235.
Histopathology
Tissue from the anal transition zone (ATZ) was histopathologically evaluated for the presence or absence of squamous cell carcinoma of the anus in the different treatment groups. The distribution of mice without cancer versus those with cancer is displayed in Table 1. A Fisher’s Exact Test comparing the no treatment and topical BEZ235 groups yielded no significant difference (P = 1.00), however, a comparison of the DMBA and DMBA plus topical BEZ235 yielded a significant difference with the DMBA alone group having significantly more tumors at 20 weeks (P < 0.0001).
Table 1:
Tumor incidence between treatment groups
| Histopathology | |||||
|---|---|---|---|---|---|
| Genotype | Treatment | Total Mice (N) | No SCCA | SCCA | Pa |
| K14E6/E7 | Control | 24 | 24 | 0 | 1.00 |
| Topical BEZ235 | 20 | 20 | 0 | ||
| DMBA | 25 | 0 | 25 | <0.0001 | |
| DMBA+Topical BEZ235 | 20 | 12 | 8 | ||
Fisher’s Exact Test
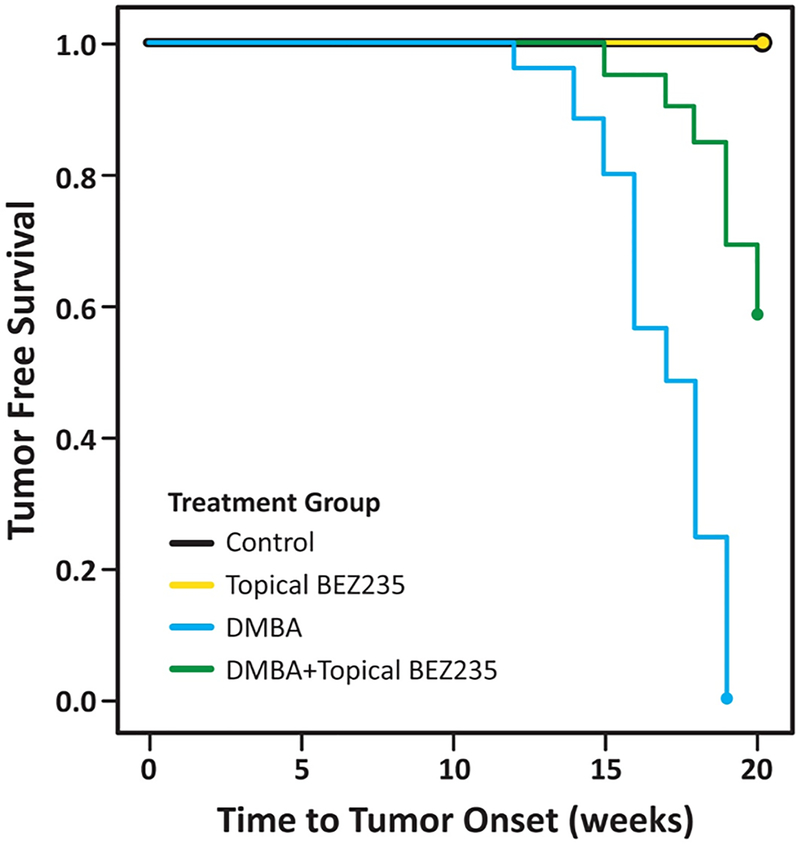
Tumor-free survival
Tumor-free survival (Fig. 7) was significantly prolonged in K14E6/E7 mice given topical BEZ235 in addition to DMBA, as compared with those that received DMBA alone (16.9 weeks to 19.3 weeks; P < 0.000001). No treatment mice receiving no therapy and those receiving topical BEZ235 alone did not develop tumors over the 20-week study period.
Figure 7. Tumor free survival in K14E6/E7 mice.
K14E6/E7 mice were given no treatment (Control), topical BEZ235 alone (topical BEZ235), DMBA alone (DMBA), or DMBA and topical BEZ235 concurrently (DMBA+ topical BEZ235). Mice were evaluated weekly, then euthanized at 20 weeks. The DMBA+BEZ235 group survived, on average, 19.3 weeks prior to tumor onset, whereas the DMBA alone group survived, on average, 16.9 weeks. This difference in tumor free survival was significant at P < 0.000001.
Discussion
Autophagy is an evolutionarily well-conserved, intracellular catabolic process that maintains cellular health by limiting the pro-carcinogenic effects of disease processes such as oncovirus infection (Zhou et al., 2009). HPV infection and the ensuing oncoprotein expression leads to inhibition of normal autophagic function, which is adaptive to allow for viral persistence, replication, and spread (Griffin et al., 2013; Surviladze et al., 2013). Through several mechanisms, both E6 and E7 oncoproteins have been demonstrated to inhibit autophagy (Spangle et al., 2010; Hanning et al., 2013), causing an accumulation of dysfunctional proteins and organelles that result in genomic and cellular damage, creating an environment that promotes cancer development.
We have previously shown, using the same HPV mouse model as the current study, that autophagic dysregulation occurs early in anal carcinogenesis (Carchman et al., 2016). Furthermore, pharmacologic induction of autophagy with systemic treatment (PI3K/mTOR inhibition) yielded decreases in tumor rates with induction of autophagy (Rademacher et al., 2017). Consistent with published data, our studies showed higher levels of pAkt and pS6 expression in the setting of DMBA treatment, signifying activation of the PI3K/mTOR pathway (Abba et al., 2016). Our results in this study confirm that BEZ235, when applied topically, significantly reduces these phosphorylated effector proteins downstream of PI3K and mTOR, pAkt and pS6, leading to induction of the autophagic pathway and a marked reduction in tumor incidence.
In the setting of DMBA treatment, we show a reduction in the number of mice with SCCA with the addition of topical BEZ235. This provides encouraging evidence that autophagic induction can overcome the inhibitory effects of the HPV oncoproteins with respect to autophagic function, resulting dysplasia development and carcinogenesis progression. To further characterize the ability of this therapy to treat already established anal dysplasia, future experiments will examine this therapy in mice with established dysplasia and monitor for anal cancer development and anal dysplasia progression/regression. Furthermore, we do not know the degree to which the PI3K/mTOR pathway is responsible for the effect seen in this study. With this in mind, we have initiated studies evaluating the efficacy of agents targeting PI3K alone, mTOR alone, or both, in mice with and without established dysplasia. Once this data is obtained, we will be able to more thoughtfully pursue other important pathways should our results require further investigation.
With respect to the concentration of topical BEZ235 chosen for these studies, the primary intent was not to evaluate toxicity. However, we habitually evaluated the perianal skin during our drug treatments. With toxicity in mind, we observed no grossly detectable skin breakdown, hair loss, or erythema in any of the mice, including all mice that received topical BEZ235 alone and the mice that were initially used for dose determination (up to 5% w/v of topical BEZ235). In terms of future clinical studies, we anticipate higher patient compliance and fewer local side effects compared those topical therapies currently in use. The authors would like to note that for this indication BEZ235 is investigational and thus not FDA approved.
In conclusion, the above results show a targeted inhibitory effect of topical BEZ235 on the PI3K/mTOR pathway that leads to a robust induction of functional autophagy. This increase in autophagy is not only significant, but able to overcome HPV oncoprotein suppression of autophagic function. With restored autophagic function, anal carcinogenesis is significantly limited, thus leading to a decrease in anal dysplasia and increased tumor-free survival with no noted side-effects from therapy. Given that various other HPV- associated cancers (cervix, head and neck, etc.) likely go through the same molecular changes during carcinogenesis, one may expect that this therapy may be effective in cancer prevention for these other HPV-associated disease processes. Given the ease of topical application to the anus, examination of the use of these topical agents on HPV-associated anal carcinogenesis is a good first step to later examining the effectiveness of these agents against cancer development. This preclinical evidence provides the basis for future clinical translation studies that will further examine this effective, well-tolerated, local therapy to prevent anal carcinogenesis and potentially other HPV-associated cancers.
Acknowledgements
We would like to acknowledge Dr. Paul Lambert for his generous support, and for providing the mice utilized in these experiments. We would also like to acknowledge the University of Wisconsin Carbone Cancer Center (UWCCC) Experimental Pathology Laboratory for paraffin-embedding and serial sectioning of pathological specimens.
Grant Support: This work is supported by NIH/NCI to the UW Carbone Cancer Center (grant number - P30 CA14520), the Society for Surgery of the Alimentary Tract (E.H.C., grant number - PRJ96MZ) and the National Cancer Institute of the National Institutes of Health (B.L.R., grant number - T32CA090217).
Footnotes
Conflicts of Interest: The authors declare no potential conflicts of interest.
References
- Abba MC, Zhong Y, Lee J, Kil H, Lu Y, Takata Y et al. (2016). DMBA induced mouse mammary tumors display high incidence of activating Pik3ca H1047 and loss of function Pten mutations. Oncotarget 7:64289–64299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barth S, Glick D, Macleod KF (2010). Autophagy: assays and artifacts. J Pathol 221:117–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carchman EH, Matkowskyj KA, Meske L, Lambert PF (2016). Dysregulation of Autophagy Contributes to Anal Carcinogenesis. PLOS ONE 11:1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collett D, Mumford L, Banner NR, Neuberger J, Watson C (2010). Comparison of the Incidence of Malignancy in Recipients of Different Types of Organ: A UK Registry Audit. American Journal of Transplantation 10:1889–1896. [DOI] [PubMed] [Google Scholar]
- Cranston RD, Baker JR, Liu Y, Wang L, Elishaev E, Ho KS (2014). Topical Application of Trichloroacetic Acid Is Efficacious for the Treatment of Internal Anal High-Grade Squamous Intraepithelial Lesions in HIV-Positive Men. Sexually Transmitted Diseases 41:420–426. [DOI] [PubMed] [Google Scholar]
- Engels EA, Yanik EL, Wheeler W, Gill JM, Shiels MS, Dubrow R et al. (2017). Cancer-Attributable Mortality Among People With Treated Human Immunodeficiency Virus Infection in North America. Clinical Infectious Diseases 65:636–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engels EA, Pfeiffer RM, Fraumeni JF, Kasiske BL, Israni AK, Snyder JJ et al. (2011). Spectrum of Cancer Risk Among US Solid Organ Transplant Recipients. JAMA 306:1891–1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox PA, Nathan M, Francis N, Singh N, Weir J, Dixon G et al. (2010). A double-blind, randomized controlled trial of the use of imiquimod cream for the treatment of anal canal high-grade anal intraepithelial neoplasia in HIV-positive MSM on HAART, with long-term follow-up data including the use of open-label imiquimod. AIDS 24:2331–2335. [DOI] [PubMed] [Google Scholar]
- Griffin LM, Cicchini L, Pyeon D (2013). Human papillomavirus infection is inhibited by host autophagy in primary human keratinocytes. Virology 437:12–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grulich AE, van Leeuwen MT, Falster MO, Vajdic CM (2007). Incidence of cancers in people with HIV/AIDS compared with immunosuppressed transplant recipients: a meta-analysis. The Lancet 370:59–67. [DOI] [PubMed] [Google Scholar]
- Guo J, Xia B, White E (2013). Autophagy-Mediated Tumor Promotion. Cell 155:1216–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanning JE, Saini HK, Murray MJ, Caffarel MM, van Dongen S, Ward D et al. (2013). Depletion of HPV16 early genes induces autophagy and senescence in a cervical carcinogenesis model, regardless of viral physical state. The Journal of pathology 231:354–366. [DOI] [PubMed] [Google Scholar]
- Hoppe-Seyler K, Bossler F, Braun JA, Herrmann AL, Hoppe-Seyler F (2017). The HPV E6/E7 Oncogenes: Key Factors for Viral Carcinogenesis and Therapeutic Targets. Trends in Microbiology 26:158–168. [DOI] [PubMed] [Google Scholar]
- Kreuter A, Potthoff A, Brockmeyer NH, Gambichler T, Stücker M, Altmeyer P et al. (2008). Imiquimod Leads to a Decrease of Human Papillomavirus DNA and to a Sustained Clearance of Anal Intraepithelial Neoplasia in HIV-Infected Men. Journal of Investigative Dermatology 128:2078–2083. [DOI] [PubMed] [Google Scholar]
- Laga M, Icenogle JP, Marsella R, Manoka A, Nzila N, Ryder RW et al. (1992). Genital papillomavirus infection and cervical dysplasia—opportunistic complications of hiv infection. International Journal of Cancer 50:45–48. [DOI] [PubMed] [Google Scholar]
- Leeds IL, Fang SH (2016). Anal cancer and intraepithelial neoplasia screening: A review. World journal of gastrointestinal surgery 8:41–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Gong Z, Zhang L, Zhao C, Zhao X, Gu X et al. (2015). Autophagy knocked down by high-risk HPV infection and uterine cervical carcinogenesis. Int J Clin Exp Med 8:10304–10314. [PMC free article] [PubMed] [Google Scholar]
- Machalek DA, Poynten M, Jin F, Fairley CK, Farnsworth A, Garland SM et al. (2012). Anal human papillomavirus infection and associated neoplastic lesions in men who have sex with men: a systematic review and meta-analysis. The Lancet Oncology 13:487–500. [DOI] [PubMed] [Google Scholar]
- Palefsky JM (1999). Anal squamous intraepithelial lesions: relation to HIV and human papillomavirus infection. Journal of acquired immune deficiency syndromes (1999) 21:42–48. [PubMed] [Google Scholar]
- Rademacher BL, Matkowskyj KA, Meske LM, Romero A, Sleiman H, Carchman EH (2017). The role of pharmacologic modulation of autophagy on anal cancer development in an HPV mouse model of carcinogenesis. Virology 507:82–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richel O, Wieland U, Vries HJC, Brockmeyer NH, Noesel VC, Potthoff A et al. (2010). Topical 5‐fluorouracil treatment of anal intraepithelial neoplasia in human immunodeficiency virus‐positive men. British Journal of Dermatology 163:1301–1307. [DOI] [PubMed] [Google Scholar]
- Silverberg MJ, Lau B, Achenbach CJ, Jing Y, Althoff KN, D’Souza G et al. (2015). Cumulative Incidence of Cancer Among Persons With HIV in North America. Annals of Internal Medicine 163:507–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh JC, Kuohung V, Palefsky JM (2009). Efficacy of trichloroacetic acid in the treatment of anal intraepithelial neoplasia in HIV-positive and HIV-negative men who have sex with men. Journal of acquired immune deficiency syndromes (1999) 52:474–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spangle JM, Munger K (2010). The human papillomavirus type 16 E6 oncoprotein activates mTORC1 signaling and increases protein synthesis. J Virol 84:9398–9407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stelzer MK, Pitot HC, Liem A, Schweizer J, Mahoney C, Lambert PF (2010). A mouse model for human anal cancer. Cancer Prev Res (Phila) 3:1534–1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surviladze Z, Sterk RT, DeHaro SA, Ozbun MA (2013). Cellular entry of human papillomavirus type 16 involves activation of the phosphatidylinositol 3-kinase/Akt/mTOR pathway and inhibition of autophagy. J Virol 87:2508–2517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C-cJ, Sparano J, Palefsky JM (2017). Surgical Oncology Clinics of North America. Surgical oncology clinics of North America 26:17–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi R, Pan S, Chen X, Hui B, Zhang L, Fu S et al. (2015). HPV16 E6-E7 induces cancer stem-like cells phenotypes in esophageal squamous cell carcinoma through the activation of PI3K/Akt signaling pathway in vitro and in vivo. Oncotarget 7:57050–57065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Münger K (2009). Expression of the human papillomavirus type 16 E7 oncoprotein induces an autophagy-related process and sensitizes normal human keratinocytes to cell death in response to growth factor deprivation. Virology 385:192–197. [DOI] [PMC free article] [PubMed] [Google Scholar]